Abstract
Phosphorus (P) is an essential nutrient for crop production, and animal manures are rich in P. When using animal manures as alternatives to synthetic fertilizers, it is important to know the kinetics of P release from different animal manures and the forms, amounts, and dynamics of P in manure-treated soils. We chose four types of manure, viz., pig manure (PM), chicken manure (CM), dairy manure (DM), and commercial organic compost (OM), and evaluated the P release rate and availability in water solution and flooded/upland paddy soils. The WEP/total P (TP) and the water-extractable P (WEP) concentrations are highest for OM with the order: OM > PM > CM > DM. An increase in soil Olsen-P concentration was observed for the addition of manure with a varying application rate of P from low to moderate to high. The release capacity of Olsen-P in flooded conditions was higher than that in upland conditions. Under the flooded soil, PM and OM have faster release rates than CM and OM in the upland soil. Moreover, PM significantly increased available P by 29% in the flooded paddy soil while moderately inorganic P increased by 17% in the upland paddy soil. Olsen-P has a significant linear relationship with available P (Resin-P + NaHCO3-Pi; R2 = 0.104; P < 0.01) and moderately inorganic P (NaOH-Pi + HCl-P; R2 = 0.286; P < 0.01). The structural equation model showed that the organic input was beneficial to the conversion of moderately inorganic P to available P. Our results indicate that PM amendment promotes the release of available P in paddy soil.
1. Introduction
Phosphorus (P) is a necessary macroelement for crop growth and development.1 However, the increasing overapplication of chemical fertilizers has intensified soil P accumulation and increased the risk of P loss to surface water. In recent years, using animal manures as an alternative P source in farmland has been recognized as an effective method to reduce the negative impact of chemical fertilizers on the environment. Animal manures could improve soil total phosphorus (TP) concentration, combined with the increase in total carbon (TC), total nitrogen (TN), and other nutrients needed by crops.2 Furthermore, the addition of manures could mitigate the soil acidity and improve the soil microbial activity.3−5
Compared to other nutrients, soil P existed in a variety of forms, which are differed in their availability to crops. Therefore, it is essential to understand the effect of animal manures on soil P availability and a number of studies have been reported on this topic. For instance, Agbenin and Igbokwe6 indicated that dairy manure increases soil Resin-P and, however, contributes less to NaHCO3-P and NaOH-P. Chicken manure increases the concentration of root P and the labile P (H2O + NaHCO3-Pi), respectively, by 37 and 59% higher than no fertilization.7 Long-term poultry manure amendment increased the content of the readily available inorganic P (Pi) in andisols by 56–286% than unfertilized control.8 The cattle manure application resulted in a higher content of TP and organic P (Po) and a greater presence of Ca-P fraction (ranging from 364.4 to 482.8 mg kg–1) than those found in soils that received no fertilizer (control) or mineral fertilizer.9 A meta-analysis of 774 comparisons from 141 published studies found that manure application increased available P by an average of 66.2% compared to mineral fertilizer.10 However, given the complex effects of animal manures on the change of soil P forms and availability, the release of P from animal manures was vital to understand the role of animal manure in the soil P pools.
The P release rate of animal manure also depends on animal types (e.g., ruminant or nonruminant), animal feed, and manure treatment methods. The form and concentration of P from animal manures were affected by animal types (e.g., ruminant or nonruminant animal), food sources, and treatment methods. Only a limited of studies that provide detailed information of the P release rate from manures have been published. There were literature reports that they obtained 3.0 g kg–1 (ca. 20.7%) of 1 h water-extractable P (WEP) from poultry manure (14.5 g of P kg–1).11 The WEP of dairy manure, pig manure, and chicken manure accounted for 39, 22, and 32% of TP, respectively.12 When the manures were added into soil, Agbenin and Igbokwe6 found that animal manures increased the TP content first (0–40 days) and then decreased (40–120 days) in sandy clay loam soil and suggested that the decrease was likely due to the fixation (or mineralization) of P by soil microorganisms.13 Garg and Bahl14 applied poultry manure in Samana sandy loam soil and found that soil Olsen-P increased by 0.23 μg g–1 day–1 compared with green manure and crop residue.
Paddy soil as the largest arable wetland on earth is the world’s most important anthrosol for food production.15 Alternation between flooded and upland conditions results in much more complex changes in P forms in paddy soil. Numerous studies reported that long-term mid-level swine manure amendments enhanced the P composition (mainly as orthophosphate and/or myo-IHP) but only in the plough layer (20 cm layer) of the paddy soil.16 Swine manure application into the paddy soil presented significantly (P < 0.05) higher contents of NaOH-Pi and TP than those in the soil amended with only chemical fertilizer.17 However, some other studies also found that Po content increased by adding manures into paddy soil.18 Studies have shown that 551 megatons (Mt) (dry weight base) of manure were generated in 2014, which contained 5.2 Mt of P. Scenario analysis suggests that by 2020, up to 38% of manure from pig and dairy farm could be recycled directly into the field.19 Rice paddy fields can be utilized as natural wetlands for treating manure.20 For example, swine manure is a relatively inexpensive form of organic fertilizer and is known to be the most commonly applied fertilizer in organic rice production in developed agricultural regions, such as the Taihu Lake region of southeastern China.21 There is still a lack of information on P release from animal manures in paddy soil. However, due to the complex dependence of P availability on manure type, soil property, and management practice, further exploration of the transformation of P in paddy soil treated with different kinds of manures is essential to the rational utilization of manure and reduction of environmental pollution.
Hence, in this study, we aim to investigate (i) the release rate and kinetics of P from four different types of animal manures in water and paddy soils, (ii) the release capacity of different amounts of manure in flooded and upland paddy soils, and (iii) how those animal manures affect soil P fraction change in flooded and upland soils.
2. Results
2.1. Dissolution Kinetics of Water-Extractable P from Four Manures
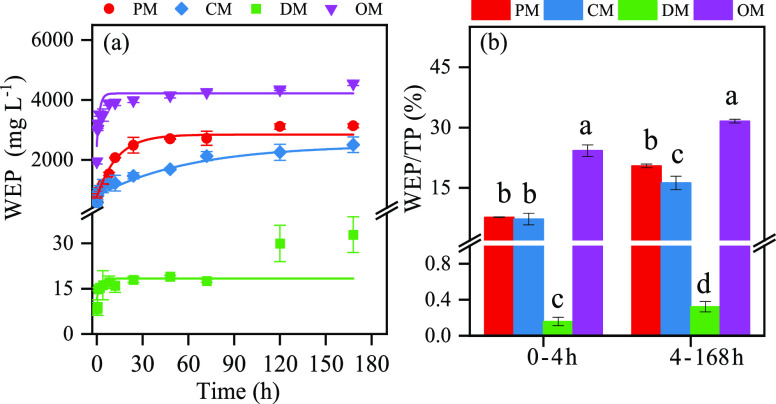
The release dynamics of WEP from different animal manures PM, CM, DM, and OM are shown in Figure 1. The curves were best fitted to an exponential function of WEP: Yt = Y0 + A e–kt , with parameters defined in Table S2 note and values of Y0, A, and k listed in Table S2. WEP rises quickly in the first few hours (0–4 h) and then slowly becomes flat (4–168 h) (Figure 1a). The water-extractable P (WEP)/total P (TP) applied in animal manures after a certain period of incubation was calculated as follows
| 1 |
Figure 1.
Dissolution kinetics of water-extractable P (a) and the release rate WEP/TP (b) in four different animal manures. PM, pig manure; CM, chicken manure; DM, dairy manure; and OM, commercial organic compost. The value was the average value, and the error bar (n = 3) represents the standard deviation.
The release rates of WEP/TP from PM, CM, DM, and OM were 7.75, 7.29, 0.159, and 24.3%, respectively, at 4th hour and 20.5, 16.2, 0.322, and 31.6%, respectively, at 168th hour (Figure 1b). The release rate constants (k) of the four manures were given below in an increasing order: CM (0.016 mg kg–1 h–1; R2 = 0.912), PM (0.076 mg kg–1 h–1; R2 = 0.986), DM (0.451 mg kg–1 h–1; R2 = 0.715), and OM (0.571 mg kg–1 h–1; R2 = 0.887) (Table S2).
2.2. Dissolution Kinetics of Olsen-P in Flooded or Upland Soil Treated with Manure
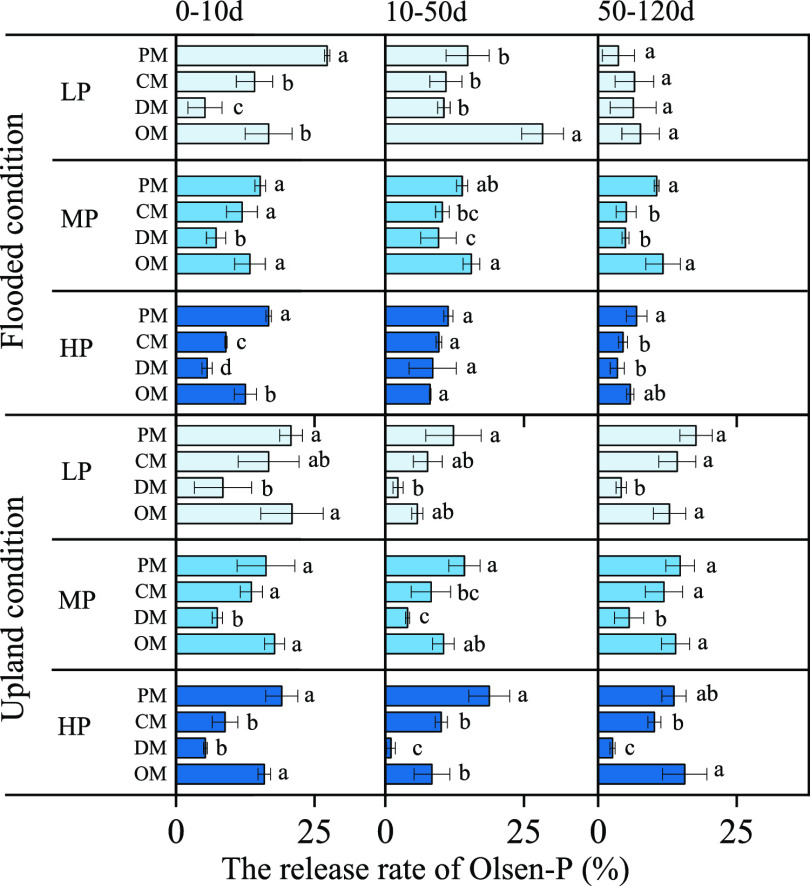
The release rates of Olsen-P in flooded and upland soils treated with different animal manures are shown in Figure 2. Compared to the control, manure addition by the rates of approximately 60 (LP), 120 (MP), and 200 (HP) kg of P2O5 ha–1 increased soil Olsen-P concentration (Figure S1) and there was no significant difference in the effect of the manure amount (LP, MP, and HP) on Olsen-P. Taking the LP addition as an example, we found that animal manures significantly (P < 0.05) increased the release rate of Olsen-P with the order: PM ≈ OM > CM > DM (Figure 2). The release rate of Olsen-P applied in animal manures after a certain period of incubation was calculated as follows
| 2 |
Figure 2.
Release rate of Olsen-P under different animal manure levels under flooded and upland conditions in paddy soil. LP, 60 kg of P2O5 ha–1; MP, 120 kg of P2O5 ha–1; HP, 200 kg of P2O5 ha–1. PM, pig manure; CM, chicken manure; DM, dairy manure; and OM, commercial organic compost. The value was the average value, and the error bar (n = 3) represents the standard deviation.
The release rate of Olsen-P was higher (0–10 and 10–50 days) and later plateaued gradually (50–120 days) under flooded conditions; however, the release rate of Olsen-P was higher (0–10 days) and later plateaued gradually (10–120 days) under upland conditions (Figure 2). The P release rates were 24.88, 9.07, 9.36, and 32.19% on the 10th day for PM-, CM-, DM-, and OM-treated flooded soils, respectively; the corresponding rates were 27.33, 15.73, 11.47, and 33.65% under the upland condition, respectively (Figure S2). The data for LP were best fitted to the same exponential equation shown in Section 2.1, and the k values for PM, CM, DM, and OM were found to be 0.3460 (R2 = 0.706), 0.0714 (R2 = 0.661), 0.0472 (R2 = 0.643), and 0.2591 (R2 = 0.558) mg kg–1 day–1, respectively, for flooded conditions (Table S3). For the upland conditions, the corresponding values were 0.1080 (R2 = 0.530), 0.2137 (R2 = 0.863), 0.1572 (R2 = 0.805), and 0.1965 (R2 = 0.797) mg kg–1 day–1 (Table S3). PM and OM showed a faster release rate under the flooded condition; however, the release rate was higher for CM and OM in the upland soil.
2.3. Change of Soil P Fractions in Flooded or Upland Soils Treated with Manures
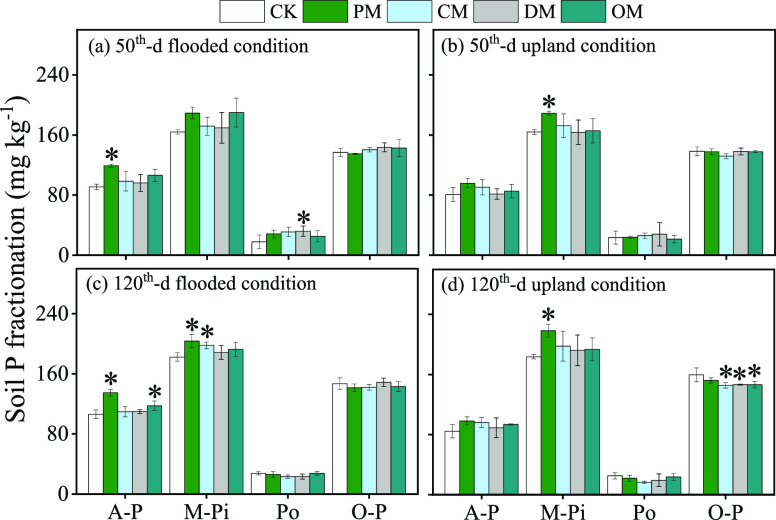
We further analyzed soil P fractions change on the 50th and 120th days of incubation (Figure 3). Compared to the control, in the flooded soil (Figure 3a,c), the addition of PM increased A-P by 30.81 and 27.02%, respectively, on the 50th and 120th days notably (P < 0.05) and then enhanced M-Pi concentration significantly by 11.64% (P < 0.05) on the 120th day; meanwhile, OM and CM significantly increased A-P and M-Pi by 10.51 and 8.54%, respectively, on the 120th day (P < 0.05). In the upland soil (Figure 3b,d), PM increased M-Pi notably by 15.30 and 18.87% on the 50th and 120th days (P < 0.05), and O-P declined by 8.71, 8.21, and 8.21% with the addition of CM, DM, and OM, respectively (P < 0.05). Our results showed that the PM of LP enhanced the P composition (mainly as the moderately inorganic P and/or available P).
Figure 3.
Sequentially extracted soil P fractions of the manure-treated flooded soil samples after 50 days (a) and 120 days (c) of incubation and the manure-treated upland soil samples after 50 days (b) and 120 days (d) of incubation. PM, pig manure; CM, chicken manure; DM, dairy manure; and OM, commercial organic compost. The value was the average value, and the error bar (n = 3) represents the standard deviation. The asterisk symbols denote significant differences in CK and animal manures. A-P = available P = Resin-P + NaHCO3-Pi; M-Pi = moderately inorganic P = NaOH-Pi + HCl-P; Po = Organic-P = NaOH-Po + NaHCO3-Po; O-P = Occluded-P = Residual-P. *p < 0.05.
2.4. Relationship between Olsen-P and P Fractions under Flooded and Upland Conditions
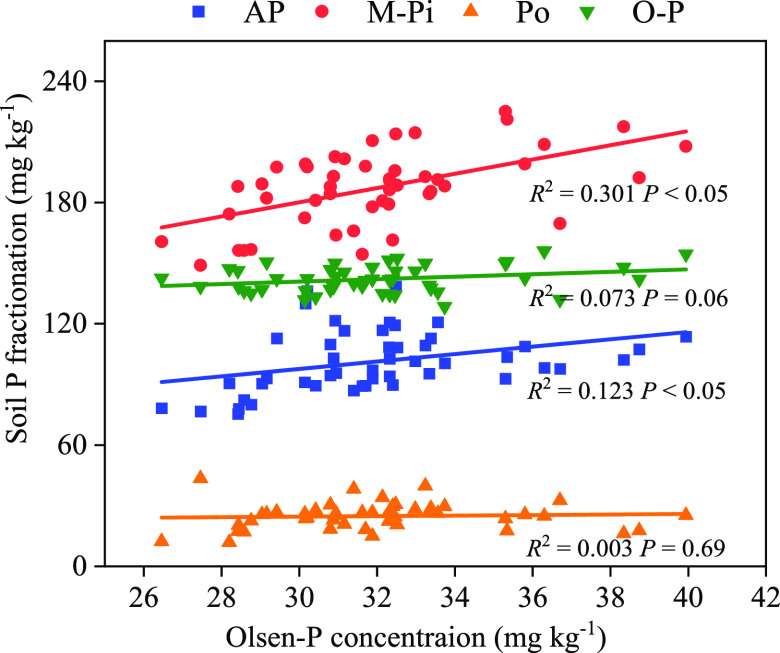
The relationship between Olsen-P and P fractions in the paddy soils amended with manures under flooded and upland conditions are shown in Figure 4. Soil Olsen-P was closely correlated with M-Pi (R2 = 0.286; P < 0.01) and A-P (R2 = 0.104; P < 0.01), while no significant correlation was found between the fractions of O-P and Po (Figure 4).
Figure 4.
Correlation analysis of Olsen-P and P fractions on the 50th and 120th days of incubation of flooded and upland soils mixed with different manures. PM, pig manure; CM, chicken manure; DM, dairy manure; and OM, commercial organic compost. A-P = Available P = Resin-P + NaHCO3-Pi; M-Pi = Moderately Inorganic P = NaOH-Pi + HCl-P; Po = Organic-P = NaOH-Po + NaHCO3-Po; and O-P = Occluded-P = Residual-P.
2.5. Transformation of Soil P Pools Evaluated by SEM under Flooded and Upland Conditions
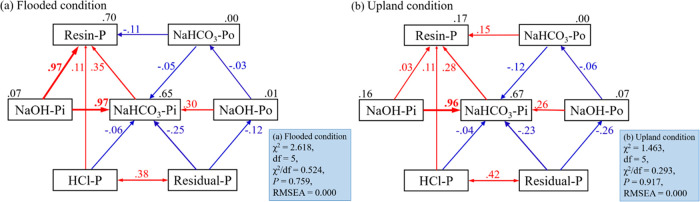
To further explore how animal manure applications affect soil P pools in paddy soil, SEM was used, as shown in Figure 5. The SEM model explained 65 and 70% of the variation of Resin-P and NaHCO3-Pi under flooded soil, respectively (χ2 = 2.618, df = 5, χ2/df = 0.524, P = 0.759, and RMSEA = 0.000). In general, the NaOH-Pi had a positive effect on Resin-P (path coefficient = 0.97) and NaHCO3-Pi (path coefficient = 0.97), the NaHCO3-Pi had a positive effect on Resin-P (path coefficient = 0.35), and however, the NaHCO3-Po had a negative effect on Resin-P (path coefficient = −0.11) (Figure 5a). Under upland conditions, the SEM model explained 67% of the variation of NaHCO3-Pi (χ2 = 1.463, df = 5, χ2/df = 0.293, P = 0.917, and RMSEA = 0.000). The NaOH-Pi had a positive effect on NaHCO3-Pi (path coefficient = 0.96), and the Resin-P had a positive effect by NaOH-Pi (path coefficient = 0.03), NaHCO3-Pi (path coefficient = 0.28), and NaHCO3-Po (path coefficient = 0.15) (Figure 5b).
Figure 5.
Structural equation model (SEM) analysis of the transformation of different P components with the addition of animal manures in paddy soil under the flooded condition (a) and upland condition (b). Optimal model fitting results under the flooded condition (a): χ2 = 2.618, df = 5, χ2/df = 0.524, P = 0.759, and RMSEA = 0.000; optimal model fitting results under the upland condition (b): χ2 = 1.463, df = 5, χ2/df = 0.293, P = 0.917, and RMSEA = 0.000. The number on the arrow represents the standardized path coefficient, the red and blue arrows represent the positive and negative effects, respectively, and the thickness of the arrow represents the size of the impact effect.
3. Discussion
In this study, it was found that the difference of P content in different organic fertilizers follows the order of PM ≈ CM > OM > DM (Table 1). Water extraction of P from animal manures is fast at 0–4 h (Figure 1a,b), and the maximum WEP of animal manures follows the order of OM > PM > CM > DM (Figure 1 and Table S2). The low P content and WEP in DM may be attributed to the different management systems adopted in raising cows.13 The pig and chicken were raised in an intensively managed system where all the nutritional needs were met, while cows’ main food was grassed with little nutrient supplement.22 For example, phytate, which is present in seeds and therefore in manures of livestock fed on grains, has been considered to be available in soils.23 The WEP/TP ratios of OM (31.6%), PM (20.5%), and CM (16.2%) are significantly higher than that of DM (0.32%) (Table S2). The high WEP/TP ratio of PM may be attributed to the observation that the enzymes produced by pigs in their intestines could mineralize Po, so the P in pig manure was mainly labile P (orthophosphate).16 Compared with cattle, chicken ingested high P level forage while having weak digestion capacity,23 which results in higher soluble P in CM.24 In addition to substrate, OM also contains a small amount of chemical fertilizer, resulting in a higher proportion of WEP/TP. With a higher proportion of WEP, when added to the soil, manures can lose a significant portion of its P quickly to surface runoff if not absorbed by plants or retained by the soil, thus reducing fertilization efficiency, impairing surface and ground water quality, and potentially exacerbating eutrophication.25 In addition, more than 28% of all the P in poultry manure was released to water in the first hour. Our results about WEP indicated that the application of PM and CM was more conducive to the effectiveness of P.
Table 1. Selected Basic Physical and Chemical Properties of Four Kinds of Animal Manurea.
| manure | TC (g kg–1) | TN (g kg–1) | TK (g kg–1) | TP (g kg–1) |
|---|---|---|---|---|
| PM | 187 ± 5.56 b | 19.6 ± 0.96 b | 32.7 ± 1.01 a | 15.3 ± 0.09 a |
| CM | 249 ± 8.01 a | 29.2 ± 1.08 a | 17.6 ± 0.54 b | 15.4 ± 0.17 a |
| DM | 154 ± 5.77 c | 19.0 ± 0.98 b | 10.0 ± 0.66 d | 10.2 ± 0.10 c |
| OM | 146 ± 6.02 c | 21.3 ± 1.37 b | 12.8 ± 0.79 c | 14.4 ± 0.09 b |
TP, total phosphorus; TC, total carbon; TN, total nitrogen; TK, total potassium. PM, pig manure; CM, chicken manure; DM, dairy manure; and OM, commercial organic compost.
With the analysis of soil Olsen-P change in flooded and upland paddy soils amended with different application rates of four manures, we found that the addition of manures increased soil Olsen-P significantly, and the release rate rose to maximum exponentially (Figure 2 and Figure S1). This is mainly because part of the P in manures is soluble, which increases the Olsen-P in the paddy soil.16 Olsen-P of the control in the flooded condition was higher than that in the upland condition. This is due to the increase in the solubility and availability of P caused by the reduction of Fe3+ to Fe2+ under anaerobic conditions and the increase in the hydrolysis of Fe-P and Al-P caused by the increase in pH value in acidic soil.26 We also found that there was no significant difference in the effect of the manure amount (LP, MP, and HP) on Olsen-P (Figure 2 and Table S3). This may be due to excessive application of animal manures to provide P and organic matter that could not be recontacted and degraded by microorganisms,16 which showed that there was a negative correlation between the amount of manure and the release rate of P. Excessive manure amendment of soil is a waste of natural resources and is detrimental to the environment.27 Therefore, the low rates of manure (LP) used in this study could be a suitable level of animal manures for the amendment of paddy soil.
Manure application has been reported to increase soil concentrations of both total and soluble P as well as concentrations of specific P forms.28−30 From the results of the detailed P pool change, we found that PM has a positive effect, which increased soil available P on the 50th and 120th days notably (P < 0.05) under flooded conditions and enhanced the moderately-Pi concentration significantly on the 50th day (Figure 3b) and 120th day (Figure 3c,d). The results showed that the effect of PM on soil moderately-Pi was more significant than other manures in the long-term manure amendment. Due to the management system, food sources, and animal types (ruminant animal), P in pig manure was more easily absorbed by plants.31 Moreover, TP losses to pig production increased by a factor of 95 during the last 5 decades, from 8.7 Gg in 1960 to 829 Gg in 2010. In the business as usual scenario, the TP loss was projected to increase by 55% between 2010 and 2030, respectively.32 Therefore, increasing the utilization of pig manure at a judicially chosen application rate could not only reduce the pressure of chemical phosphate fertilizer but also reduce the pollution of pig manure to the environment. However, the addition of CM, DM, and OM lowers O-P (P < 0.05) under the upland condition (Figure 3d). The result indicates that the application of animal manures improves the activation of steady P in soil. Similar reports have shown that CM reduced stable Ca-P, increased Fe-P and inositol hexaphosphate,33 and increased availability of NaOH-P and HCl-P.7 However, there was also some literature, which showed that resin recovery increased, whereas NaHCO3-P and NaOH-P decreased with increasing dung application.6 The inconsistency might be due to the different effects of different soil incubation conditions on adsorption capacity and recovery rate of phosphate in the soil.
Conceptual models of the soil P cycle usually assume that soluble Pi mediates the transformations between most other P pools in soil.34 In (semi)natural ecosystems, plant uptake could deplete soil soluble Pi (represented by Resin-P fraction).35 When soluble Pi was depleted, it could be replenished by solid-phase P (NaHCO3-P, NaOH-P, and HCl-P) by a combination of abiotic and biotic processes, which could transfer P from the solid phase to liquid phase.36 Our study of the transformation of soil P pools revealed that NaHCO3-Pi and NaOH-Pi could be directly transferred to Resin-P (Figure 5), NaOH-Pi had direct positive effects on NaHCO3-Pi under upland conditions (Figure 5b), and NaOH-Pi had direct positive effects on Resin-P and NaHCO3-Pi under flooded conditions (Figure 5a). When Resin-P was consumed, NaHCO3-Pi could rapidly transform to Resin-P under upland conditions, while NaHCO3-Pi and NaOH-Pi could rapidly transform to Resin-P under flooded conditions. This may be because the flooded condition was conducive to the conversion of moderately inorganic P to available P.26 The lack of direct influences from Occluded-P suggests that available P cannot be directly transformed from low-soluble P pools (Figure 5), which was consistent with the conceptual model.34 Soil Olsen-P was significantly correlated with M-Pi and A-P, especially for NaOH-Pi (Figure 4), suggesting that NaOH-Pi has a central role in mediating P transformations in soils (Figure 5). The strong direct influence of NaOH-Pi on NaHCO3-Pi was consistent with the notion that moderately inorganic P could rapidly exchange with available P and could act as a short-term, plant-available P pool.37 Soil Resin-P was a very dynamic P pool that could be greatly affected by the amount of soil solution and by the short-term changes in plant and soil microbial activities and leaching,38 which probably also explains the relatively weak relations of soluble Pi with other P pools. In conclusion, through the structural equation model, we found that manure treatments were conducive to the activation of soil P pools, especially the transformation from NaOH-Pi to NaHCO3-Pi. Compared with the upland condition, the flooded condition was more conducive to the activation of moderately inorganic P.
4. Conclusions
In this study, we observed that the P availability of PM and CM was higher than that of OM and DM. Both the flooded and upland soils amended with a low rate of manure ([LP], 60 kg P2O5 ha–1) contain levels of Olsen-P that are not significantly lower than those obtained with MP and HP amendment. The finding may serve as a guideline for the rational application of manures to reduce the loss of resources and environmental pollution. In the flooded soil, the application of PM and CM increased soil available P and/or moderately inorganic P, while PM increased the content of moderately inorganic P in the upland condition, among which NaOH-Pi played a key role in the transformation of P pools in paddy soil. Our results indicated that the application of PM and CM in flooded and upland conditions was beneficial to the utilization of soil P. This study provides a better understanding of the effect of animal manures on P availability from the simulation in the laboratory. The next research direction will be to conduct field experiments to investigate the forms of P in field soil with different kinds of animal manures.
5. Materials and Methods
5.1. Preparation of Soil and Animal Manures
The soil samples used in the experiment were collected from Yixing Agro-Environment Research Base, the National Agroecosystem Observatory and Research Station of Changshu (31°16′ N, 119°54′ E). The research base is in the subtropical zone with an annual average temperature of 16.5 °C and an annual average rainfall of 1516 mm. The rice–wheat rotation is currently practiced in this region. Surface (0–20 cm) soil samples were air-dried and passed through a 2 mm sieve for the incubation study and analysis. The following properties were obtained for the soil samples: pH 6.2; TC, 11.83 g kg–1; TN, 2.92 g kg–1; TP, 0.492 g kg–1; soil organic carbon (SOC), 7.47 g kg–1; available potassium (AK), 80 mg kg–1; and Olsen-P, 23.59 mg kg–1.
Four kinds of animal manures were selected, including pig manure (PM), chicken manure (CM), dairy manure (DM), and commercial organic compost (OM). The commercial organic compost (OM) was made mainly from cow manure and mushroom residue. PM, CM, and DM were collected from a ranch at Jiaxing City, Zhejiang Province, and the OM was collected from a ranch at Wuxi City, Jiangsu Province. Manures were air-dried and sieved (<2 mm) before analysis. Their basic physical and chemical properties are given in Table 1.
5.2. Sample Preparation
5.2.1. Water Extraction of P from the Four Manure Samples
One gram of animal manure (PM, CM, DM, and OM) and 30 mL of deionized water were added to a 50 mL centrifugal tube. The sample in the tube was incubated in a constant temperature oscillator (180 rpm, 25 °C). Three samples were taken out at each of the following incubation durations (0, 0.5, 1, 2, 4, 8, 12, 24, 48, 72, 120, and 168 h), and each sample was filtered by a 0.45 μm membrane filter to obtain the liquid supernatant.
5.2.2. Incubation of Paddy Soil with Different Manures
The incubation was conducted under simulated flooded and upland conditions and was lasted 120 days from February 27, 2019 to June 26, 2019. Five soil treatments were conducted, including no addition of animal manure (CK) and with addition of PM, CM, DM, and OM. Each manure was applied at three rates, namely, LP (low P, equivalent to approximately 60 kg of P2O5 ha–1), MP (moderate P, equivalent to approximately 120 kg of P2O5 ha–1), and HP (high P, equivalent to approximately 200 kg of P2O5 ha–1) (soil bulk density = 1.2 g cm–3 in the 0–20 cm soil depth). The detail is shown in Table S1. Triplicate samples of 350 g of dry soil were transferred into 500 mL reagent bottles, weighed, and mixed with animal manures. To the bottle that simulates the upland condition, distilled water of the amount equivalent to 60% of the water holding capacity was added; to the bottle that simulates the flooded condition, distilled water was added to a level 2 cm above the soil surface. The reagent bottles were sealed and incubated at 25 °C for 120 days. Water was added to the bottles every 3 days to compensate for the water lost to evaporation. The soil Olsen-P analysis was performed after 1, 5, 10, 25, 50, 75, 100, and 120 days of incubation, and soil P fractions of LP were determined after 50 and 120 days of incubation.
5.3. Sample Analysis
Soil and animal manure samples were sieved by 2 mm after air-drying. TC and total TN in the sample were determined by the combustion method using a vario MAX CNS elemental analyzer (vario MACRO CN, Elementar Analysensysteme GmbH, Germany). TP and TK were heat-digested using concentrated H2SO4–H2O2 and measured by ultraviolet spectrophotometric molybdenum blue colorimetry (UVmini-1240) and flame photometry, respectively.39 Soil pH was measured in a 1:2.5 (w/v) soil/water solution using a pH meter. WEP was analyzed using a molybdenum blue spectrophotometer (UVmini-1240).40 Olsen-P was extracted using sodium bicarbonate (0.5 mol L–1 NaHCO3, pH = 8.5) for 0.5 h.40 AK was extracted using 1 M NH4OAc in a ratio of 10 mL of solution per 1 g of soil and analyzed by atomic absorption spectrophotometry.41
Soil P fractionation was performed using the sequential extraction method described by Tiessen and Moir42 on 0.5 g of air-dried soil samples in the following sequential steps: (1) Resin-P was extracted with deionized water and one resin strip (Sinopharm Chemical Reagent Co., Ltd.); (2) NaHCO3-Pi and NaHCO3-Po were extracted with 30 mL of 0.5 M NaHCO3 (pH 8.5); (3) NaOH-Pi and NaOH-Po were extracted with 30 mL of 0.5 M NaOH; (4) HCl-P was extracted with 30 mL of 1 M HCl; and (5) Residual-P was digested by H2SO4–H2O2 at 360 °C. After adding the solution in each step, the soil samples were subjected to shaking in a reciprocating shaker for 16 h (180 rpm, 24 °C) and then centrifuged at 0 °C for 10 min (8000 rpm). The supernatant was filtered through a 0.45 μm membrane filter. The NaHCO3 and NaOH extracts were divided into two aliquots to measure the total P (10 mL of supernatant + 0.9 M H2SO4 and 0.5 g of ammonium persulphate at 330 °C) and Pi. Soil Po in each extract was calculated from the difference between TP from digestion and Pi. Resin-P and NaHCO3-Pi were considered to be available P (A-P); NaOH-Pi and HCl-P as moderately inorganic P (M-Pi); NaOH-Po and NaHCO3-Po as Po; and Residual-P as Occluded-P (O-P). The content of P in the supernatant was determined with an ultraviolet spectrometer (UV-2500 Japan) and analyzed by the ascorbic acid molybdenum blue method.43
5.4. Statistical Analysis
The effect of animal manure on soil P was analyzed by one-way ANOVA and the Duncan test at the P < 0.05 level. The relationships between soil Olsen-P and P fractions were analyzed by linear regression. P < 0.05 was considered statistically significant. The structural equation model (SEM) was used to study the interaction and transformation of different P components in soil34 using IBM SPSS AMOS 22.0 (IBM Corporation 2013). Root-mean-square-error of approximation (RMSEA) (<0.08), chi-square (χ2) (χ2/df < 2), and the P value of χ2 (P > 0.05) were used to evaluate the model fitting. In this study, SPSS 18.0 software was used for statistical analysis.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (no. 41671304) and the National Key Research and Development Program of China (no. 2017YFD0200206).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c05748.
Detailed tables and figures, the application rates of animal manure, parameters of the exponential equation, dynamic change of Olsen-P, and the release rate of Olsen-P (PDF)
Author Contributions
§ G.-L.C. and L.X. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Yang X.; Chen X.; Yang X. Effect of organic matter on phosphorus adsorption and desorption in a black soil from Northeast China. Soil Tillage Res. 2019, 187, 85–91. 10.1016/j.still.2018.11.016. [DOI] [Google Scholar]
- Qaswar M.; Jing H.; Ahmed W.; Dongchu L.; Shujun L.; Lu Z.; Cai A.; Lisheng L.; Yongmei X.; Jusheng G.; Huimin Z. Yield sustainability, soil organic carbon sequestration and nutrients balance under long-term combined application of manure and inorganic fertilizers in acidic paddy soil. Soil Tillage Res. 2020, 198, 104569. 10.1016/j.still.2019.104569. [DOI] [Google Scholar]
- Hu Y.; Xia Y.; Sun Q.; Liu K.; Chen X.; Ge T.; Zhu B.; Zhu Z.; Zhang Z.; Su Y. Effects of long-term fertilization on phoD-harboring bacterial community in Karst soils. Sci. Total Environ. 2018, 628-629, 53–63. 10.1016/j.scitotenv.2018.01.314. [DOI] [PubMed] [Google Scholar]
- Luo G.; Li L.; Friman V.-P.; Guo J.; Guo S.; Shen Q.; Ling N. Organic amendments increase crop yields by improving microbe-mediated soil functioning of agroecosystems: A meta-analysis. Soil Biol. Biochem. 2018, 124, 105–115. 10.1016/j.soilbio.2018.06.002. [DOI] [Google Scholar]
- Mi W.; Sun Y.; Xia S.; Zhao H.; Mi W.; Brookes P. C.; Liu Y.; Wu L. Effect of inorganic fertilizers with organic amendments on soil chemical properties and rice yield in a low-productivity paddy soil. Geoderma. 2018, 320, 23–29. 10.1016/j.geoderma.2018.01.016. [DOI] [Google Scholar]
- Agbenin J. O.; Igbokwe S. O. Effect of soil–dung manure incubation on the solubility and retention of applied phosphate by a weathered tropical semi-arid soil. Geoderma. 2006, 133, 191–203. 10.1016/j.geoderma.2005.07.006. [DOI] [Google Scholar]
- Waldrip H. M.; He Z.; Erich M. S. Effects of poultry manure amendment on phosphorus uptake by ryegrass, soil phosphorus fractions and phosphatase activity. Biol. Fertil. Soils 2011, 47, 407–418. 10.1007/s00374-011-0546-4. [DOI] [Google Scholar]
- Poblete-Grant P.; Suazo-Hernández J.; Condron L.; Rumpel C.; Demanet R.; Malone S. L.; Mora M. d. L. L. Soil available P, soil organic carbon and aggregation as affected by long-term poultry manure application to Andisols under pastures in Southern Chile. Geoderma Reg. 2020, 21, e00271 10.1016/j.geodrs.2020.e00271. [DOI] [Google Scholar]
- Milić S.; Ninkov J.; Zeremski T.; Latković D.; Šeremešić S.; Radovanović V.; Žarković B. Soil fertility and phosphorus fractions in a calcareous chernozem after a long-term field experiment. Geoderma. 2019, 339, 9–19. 10.1016/j.geoderma.2018.12.017. [DOI] [Google Scholar]
- Du Y.; Cui B.; Zhang Q.; Wang Z.; Sun J.; Niu W. Effects of manure fertilizer on crop yield and soil properties in China: A meta-analysis. Catena 2020, 193, 104617. 10.1016/j.catena.2020.104617. [DOI] [Google Scholar]
- Kleinman P. J. A.; Wolf A. M.; Sharpley A. N.; Beegle D. B.; Saporito L. S. Survey of Water-Extractable Phosphorus in Livestock Manures. Soil Sci. Soc. Am. J. 2005, 69, 701–708. 10.2136/sssaj2004.0099. [DOI] [Google Scholar]
- Li G.; Li H.; Leffelaar P. A.; Shen J.; Zhang F. Characterization of phosphorus in animal manures collected from three (dairy, swine, and broiler) farms in China. PLoS One 2014, 9, e102698 10.1371/journal.pone.0102698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azeez J. O.; Van Averbeke W. Fate of manure phosphorus in a weathered sandy clay loam soil amended with three animal manures. Bioresour. Technol. 2010, 101, 6584–6588. 10.1016/j.biortech.2010.03.073. [DOI] [PubMed] [Google Scholar]
- Garg S.; Bahl G. S. Phosphorus availability to maize as influenced by organic manures and fertilizer P associated phosphatase activity in soils. Bioresour. Technol. 2008, 99, 5773–5777. 10.1016/j.biortech.2007.10.063. [DOI] [PubMed] [Google Scholar]
- Huang X.; Tang H.; Kang W.; Yu G.; Ran W.; Hong J.; Shen Q. Redox interface-associated organo-mineral interactions: A mechanism for C sequestration under a rice-wheat cropping system. Soil Biol. Biochem. 2018, 120, 12–23. 10.1016/j.soilbio.2018.01.031. [DOI] [Google Scholar]
- Liang X.; Jin Y.; He M.; Liu Y.; Hua G.; Wang S.; Tian G. Composition of phosphorus species and phosphatase activities in a paddy soil treated with manure at varying rates. Agric. Ecosyst. Environ. 2017, 237, 173–180. 10.1016/j.agee.2016.12.033. [DOI] [Google Scholar]
- Yan X.; Wei Z.; Hong Q.; Lu Z.; Wu J. Phosphorus fractions and sorption characteristics in a subtropical paddy soil as influenced by fertilizer sources. Geoderma. 2017, 295, 80–85. 10.1016/j.geoderma.2017.02.012. [DOI] [Google Scholar]
- Yin Y.; Liang C. H. Transformation of phosphorus fractions in paddy soil amended with pig manure. J. Soil Sci. Plant Nutr. 2013, 13, 809–818. 10.4067/S0718-95162013005000064. [DOI] [Google Scholar]
- Jia W.; Qin W.; Zhang Q.; Wang X.; Ma Y.; Chen Q. Evaluation of crop residues and manure production and their geographical distribution in China. J. Cleaner Prod. 2018, 188, 954–965. 10.1016/j.jclepro.2018.03.300. [DOI] [Google Scholar]
- Yan X.; Gong W. The role of chemical and organic fertilizers on yield, yield variability and carbon sequestration— results of a 19-year experiment. Plant Soil 2010, 331, 471–480. 10.1007/s11104-009-0268-7. [DOI] [Google Scholar]
- Li L.; Liang X.; Ye Y.; Zhao Y.; Zhang Y.; Jin Y.; Yuan J.; Chen Y. Effects of repeated swine manure applications on legacy phosphorus and phosphomonoesterase activities in a paddy soil. Biol. Fertil. Soils. 2015, 51, 167–181. 10.1007/s00374-014-0956-1. [DOI] [Google Scholar]
- Shafqat M. N.; Pierzynski G. M.; Xia K. Phosphorus Source Effects on Soil Organic Phosphorus: A31P NMR Study. Commun. Soil Sci. Plant Anal. 2009, 40, 1722–1746. 10.1080/00103620902895821. [DOI] [Google Scholar]
- Peirce C. A. E.; Smernik R. J.; McBeath T. M. Phosphorus availability in chicken manure is lower with increased stockpiling period, despite a larger orthophosphate content. Plant Soil 2013, 373, 359–372. 10.1007/s11104-013-1807-9. [DOI] [Google Scholar]
- Toor G. S.; Cade-Menun B. J.; Sims J. T. Establishing a linkage between phosphorus forms in dairy diets, feces, and manures. J. Environ. Qual. 2005, 34, 1380–1391. 10.2134/jeq2004.0232. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Lin Y.; Chiu P. C.; Imhoff P. T.; Guo M. Phosphorus release behaviors of poultry litter biochar as a soil amendment. Sci. Total Environ. 2015, 512-513, 454–463. 10.1016/j.scitotenv.2015.01.093. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Zhao X.; Wang L.; Wang Y.; Li W.; Wang S.; Xing G. The regime and P availability of omitting P fertilizer application for rice in rice/wheat rotation in the Taihu Lake Region of southern China. J. Soils Sediments 2015, 15, 844–853. 10.1007/s11368-014-1047-5. [DOI] [Google Scholar]
- Yan Z.; Liu P.; Li Y.; Ma L.; Alva A.; Dou Z.; Chen Q.; Zhang F. Phosphorus in China’s Intensive Vegetable Production Systems: Overfertilization, Soil Enrichment, and Environmental Implications. J. Environ. Qual. 2013, 42, 982–989. 10.2134/jeq2012.0463. [DOI] [PubMed] [Google Scholar]
- Ylivainio K.; Uusitalo R.; Turtola E. Meat bone meal and fox manure as P sources for ryegrass (Lolium multiflorum) grown on a limed soil. Nutr. Cycling Agroecosyst. 2008, 81, 267–278. 10.1007/s10705-007-9162-y. [DOI] [Google Scholar]
- Waldrip-Dail H.; He Z.; Erich S. M.; Honeycutt W. C. Soil Phosphorus Dynamics in Response to Poultry Manure Amendment. Soil Sci. 2009, 174, 195–201. 10.1097/SS.0b013e31819cd25d. [DOI] [Google Scholar]
- Ma Q.; Wen Y.; Ma J.; Macdonald A.; Hill P. W.; Chadwick D. R.; Wu L.; Jones D. L. Long-term farmyard manure application affects soil organic phosphorus cycling: A combined metagenomic and 33P/14C labelling study. Soil Biol. Biochem. 2020, 149, 107959. 10.1016/j.soilbio.2020.107959. [DOI] [Google Scholar]
- Audette Y.; O’Halloran I. P.; Evans L. J.; Martin R. C.; Voroney R. P. Kinetics of phosphorus forms applied as inorganic and organic amendments to a calcareous soil II: effects of plant growth on plant available and uptake phosphorus. Geoderma. 2016, 279, 70–76. 10.1016/j.geoderma.2016.06.002. [DOI] [Google Scholar]
- Bai Z. H.; Ma L.; Qin W.; Chen Q.; Oenema O.; Zhang F. S. Changes in pig production in China and their effects on nitrogen and phosphorus use and losses. Environ. Sci. Technol. 2014, 48, 12742–12749. 10.1021/es502160v. [DOI] [PubMed] [Google Scholar]
- Yan Z.; Chen S.; Dari B.; Sihi D.; Chen Q. Phosphorus transformation response to soil properties changes induced by manure application in a calcareous soil. Geoderma. 2018, 322, 163–171. 10.1016/j.geoderma.2018.02.035. [DOI] [Google Scholar]
- Hou E.; Chen C.; Kuang Y.; Zhang Y.; Heenan M.; Wen D. A structural equation model analysis of phosphorus transformations in global unfertilized and uncultivated soils: P transformations in global soils. Global. Biogeochem. Cycles 2016, 30, 1300–1309. 10.1002/2016GB005371. [DOI] [Google Scholar]
- Peñuelas J.; Poulter B.; Sardans J.; Ciais P.; van der Velde M.; Bopp L.; Boucher O.; Godderis Y.; Hinsinger P.; Llusia J.; Nardin E.; Vicca S.; Obersteiner M.; Janssens I. A. Human-induced nitrogen-phosphorus imbalances alter natural and managed ecosystems across the globe. Nat. Commun. 2013, 4, 2934. 10.1038/ncomms3934. [DOI] [PubMed] [Google Scholar]
- Frossard E.; Condron L. M.; Oberson A.; Sinaj S.; Fardeau J. C. Processes governing phosphorus availability in temperate soils. J. Environ. Qual. 2000, 29, 15–23. 10.2134/jeq2000.00472425002900010003x. [DOI] [Google Scholar]
- Yang X.; Post W. M. Phosphorus transformations as a function of pedogenesis: A synthesis of soil phosphorus data using Hedley fractionation method. Biogeosciences. 2011, 8, 2907–2916. 10.5194/bg-8-2907-2011. [DOI] [Google Scholar]
- Turner B. L.; Haygarth P. M. Phosphorus forms and concentrations in leachate under four grassland soil types. Soil Sci. Soc. Am. J. 2000, 64, 1090–1099. 10.2136/sssaj2000.6431090x. [DOI] [Google Scholar]
- Thomas R. L.; Sheard R. W.; Moyer J. R. Comparison of conventional and automated procedures for nitrogen, phosphorus, and potassium analysis of plant material using a single digest. Agron. J. 1967, 59, 240–243. 10.2134/agronj1967.00021962005900030010x. [DOI] [Google Scholar]
- Olsen S. R.; Cole C. V.; Watanabe F. S.; Dean L.. A Estimation of available phosphorus in soils by extraction with sodium bicarbonate. In: USDA Circular.1954, (939. pp.1–19).
- David D. J. The determination of exchangeable sodium, potassium, calcium and magnesium in soils by atomic-absorption spectrophotometry. Analyst 1960, 85, 495–503. 10.1039/an9608500495. [DOI] [Google Scholar]
- Tiessen H.; Moir J. O. Characterization of available P by sequential extraction, in Carter, M. R. (ed.): Soil sampling and methods of analysis. Can. J. Soil Sci. 1993, 75–86. [Google Scholar]
- Hedley M. J.; Stewart J. W. B.; Chauhan B. S. Changes in Inorganic and Organic Soil Phosphorus Fractions Induced by Cultivation Practices and by Laboratory Incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. 10.2136/sssaj1982.03615995004600050017x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.