Abstract

Mussel-inspired surface chemistry is recognized as a simple, efficient, and mild surface modification method and has become a research hotspot in many fields. In this study, polyethylenimine/dopamine was coated on the surface of SBA-15 using a co-deposition method, making it possible to immobilize naringinase with high activity and operation stability. The optimal modification and immobilization conditions as well as enzyme properties were investigated. The naringinase activity can reach up to 753.78 U/g carrier, which was much higher than those of the previous works. Besides, the residual naringinase activity still kept 78.91% of the initial activity after one month of storage and maintained 60.79% after 8 cycles. Therefore, the strategy of mussel-inspired enzyme immobilization could be recognized as a promising and universal enzyme immobilization method, with the advantages of high relative enzyme activity, enzyme carrying rate, enzyme activity recovery rate, and good reusability and storage stability.
1. Introduction
Naringin is a dihydroflavonoid compound with a certain bitter taste and is widely found in citrus fruits. In order to avoid the bad taste of juice products, naringinase is usually used in the industry for debittering treatment,1 which is an enzyme complex with α-l-rhamnosidase (E.C.3.2.1.40) and β-d-glucosidase (E.C.3.2.1.21) activities. Naringinase can be used to completely hydrolyze the bitter substance naringin in two steps: in the first step, α-l-rhamnosidase hydrolyzes naringin into rhamnose and prunin, of which the bitterness is only one third of the naringin; in the second step, the prunin is hydrolyzed into naringenin and glucose by β-d-glucosidase.2 Up to now, naringinase had played a huge role in the debittering of grapefruit juice, rhamnose production, wine flavoring, pharmaceutical industry, and so on.3−6 However, considering the high price of naringinase and the difficulty of recovering free naringinase from fruit juice,7 immobilized naringinase is welcomed owing to its advantages of high stability and ease of recycling, which can reduce the industrial process costs.8,9
Nowadays, several kinds of materials were used for the immobilization of naringinase, including nanofibers and magnetic carrier. Huang et al.10 successfully immobilized naringinase in electrospun cellulose acetate nanofibers using a layer-by-layer self-assembly technique, and the final enzyme activity reached 0.122 U/g. Bodakowska-Boczniewicz et al.11 immobilized naringinase on a magnetic carrier activated by polyethylenimine (PEI), and 16.40 U/g of enzyme activity was obtained. It could be seen that the enzyme activity was still very low in the present works. Mesoporous silica is recognized as the most widely used mesoporous materials, with the advantages of mature preparation route, high specific surface area, large pore volume, good thermal stability, and chemical stability.12,13 Compared with the free enzyme, the immobilized enzyme in the pores of mesoporous silica can not only maintain its high-efficiency, specific and mild enzyme catalytic reaction characteristics but also improve the stability of the enzyme.14 Additionally, the pore size and pore volume of mesoporous silica are relatively larger than that of the other immobilized enzyme carriers. Therefore, mesoporous silica can load more enzyme molecules, which improves the enzyme loading capacity and immobilization efficiency of the immobilized enzyme.15,16
Besides, in order to make the enzyme and the mesoporous material carrier bond firmly and improve the operational stability, the modification of the carrier surface is essential, and some active groups such as amino groups or aldehyde groups are usually introduced on the surface of the carriers. Mussel-inspired surface chemistry is a simple, efficient, and mild reaction condition material surface modification method, which has the characteristics of forming super adhesion to the surface of any solid material. The strong adhesion component in the foot silk gland fluid of marine mussels is Mytilus foot protein 5, in which the amino acid sequence contains up to 30% l-3-(3,4-dihydroxyphenyl)alanine (DOPA) and 15% lysine residues. Dopamine (DA) has both the catechol group of l-DOPA and the amino functional group of lysine.17 Under oxidizing conditions, DA can polymerize spontaneously, and the polymerized product also shows strong adhesion,18,19 which can adhere to the surface of organic or inorganic solid materials. Therefore, the adhesion coating on the surfaces of mesoporous carriers would be a good choice to prepare novel immobilization carriers.
However, simple self-assembly of DA always suffered from several problems such as slow deposition speed, uneven deposition blocking of the carrier pores, and poor solvent stability in strong acids, strong bases, and strong polar solvents.17 In order to address these problems, another component such as PEI was always added into the solution, which could co-deposit with DA.20 In the process of the co-deposition of PEI/polydopamine (PDA), the catechol group of PDA could first interact with the carrier to form an anchor point,21 and then the DA itself could form a covalent bond through an oxidative coupling reaction. PDA molecules could form a noncovalent cross-linked structure through hydrogen bonding and π–π stacking, and further underwent the Michael addition reaction with PEI. The introduction of PEI disrupted the noncovalent interactions in PDA aggregates and effectively inhibited the formation of particles,22 which made the coating on the surface of the material more uniform, more stable, denser, and hydrophilic. If the PEI/PDA coating mentioned above was used for the naringinase immobilization, not only the strong adhesion surface property could be utilized but also the covalent connection would be formed between PEI and naringinase by using cross-linking agents. To the best of our knowledge, there was no study on the modification of the immobilized enzyme carrier by using this novel strategy.
In this work, a novel strategy of mussel-inspired enzyme immobilization using the PEI/DA co-deposition method was proposed and applied for the immobilization of naringinase with high activity and operational stability. The mesoporous material of SBA-15 was selected as the carrier and used for the coating of PEI/DA. The surface PEI/PDA coating modification conditions, immobilization conditions, and enzyme properties would be studied.
2. Materials and Methods
2.1. Materials
Aspergillus niger FFCC uv-11 was from our laboratory’s self-preservation, and the naringinase fermentation broth was produced via this strain, with a naringinase activity of 838.22 U/mL. Naringin (mass fraction ≥ 98%) was purchased from Baoji Fang Sheng Biological development Co., Ltd, China. Mesoporous molecular sieve SBA-15, DA hydrochloride and PEI, sodium hydroxide, anhydrous ethanol, citric acid, disodium hydrogen phosphate, diethylene glycol, glutaraldehyde (50% by mass), hydrochloric acid, and tris(hydroxymethyl)methyl aminomethane were obtained from Sinopharm Chemical Reagent Co., Ltd.
2.2. Preparation of Immobilized Naringinase on the Mussel-Inspired Functional Carrier
Immobilized naringinase on the mussel-inspired functional carrier was prepared, and the synthesis steps are shown in Scheme 1.
Scheme 1. Schematic Diagram of the Preparation Process and Mechanism for the Immobilized Naringinase on the Mussel-Inspired Functional Carrier.
2.2.1. Mussel-Inspired Modification of the Immobilization Material
The PEI/PDA-coated SBA-15 was prepared by the co-deposition method according to the previous work.23 In brief, 1 g of SBA-15 was first dissolved in 20 mL of Tris-HCl buffer (pH: 8.5, 0.05 M), and ultrasonic dispersion for 10 min. Subsequently, a certain proportion and concentration of DA and PEI was added into the solution, and the mixture was kept stirring for a certain period of time at room temperature. The modified SBA-15 was obtained after centrifugation, washing, and vacuum drying at 60 °C, which was labeled PEI/PDA-SBA-15. If there was a need for specifying the PEI/PDA-SBA-15, for example, the concentration of DA used in the experiment was 1.5 mg/mL, then the modified SBA-15 carrier was labeled 1.5PEI/PDA-SBA-15. Similarly, 2.5PEI/PDA-SBA-15 meant the concentration of DA used in the experiment was 2.5 mg/mL, and 3.5PEI/PDA-SBA-15 meant the concentration of DA used in the experiment was 3.5 mg/mL.
The weighing method was used to calculate the deposition rate by the following equation
| 1 |
wherein, m0 and m1 represent the input mass and modified mass of SBA-15, respectively.
2.2.2. Preparation of Immobilized Naringinase
An amount of 0.2 g of PEI/PDA-SBA-15 was dispersed into 8 mL of naringinase solution with certain activity (the pH value of the mixture was adjusted by citric acid buffer), and 1 mL of a certain concentration of glutaraldehyde was added. The mixture was shaken for some time at a constant temperature, and the immobilized naringinase was collected by centrifugation at 4000 rpm for 10 min. Additionally, the pelleted immobilized naringinase was washed with deionized water three times, and dried by vacuum at 40 °C for further use. The immobilized enzyme was finally labeled naringinase-PEI/PDA-SBA-15.
2.3. Characterization of Materials
The morphology of the samples was determined by scanning electron microscopy (SEM, JEOL JSM-6700F, Japan) and high-resolution transmission electron microscopy (HRTEM, JEOL JEM-2100, Japan). Fourier transforms infrared spectra of the samples were recorded on a spectrophotometer (FT-IR, Bruker T27, Germany) with the wavenumber range of 4000–400 cm–1. The nitrogen adsorption/desorption isotherms were measured on a QuantaChrome Quadrasorb SI analyzer (USA) after vacuum degassing at 120 °C for 6 h. The specific surface areas and the pore volumes were calculated using the Brunauer–Emmett–Teller (BET) method. The pore size distributions were obtained from the adsorption branches of the isotherms using the Barrett–Joyner–Halenda (BJH) model. The elemental analysis (C, N, and H) of samples was carried out using an Elementar Vario EL III analyzer (Germany).
2.4. Study on Enzymatic Characteristics
2.4.1. Optimization of Enzymatic Hydrolysis Reaction Conditions
The effects of naringinase-PEI/PDA-SBA-15 enzymatic hydrolysis temperature and pH on enzyme activity were studied. In addition, the storage stability and repeated experiments of the immobilized naringinase were investigated by measuring the enzyme activity after 30 days and 8 times of repeated use, respectively.
2.4.2. Determination of Naringinase Activity
The naringinase activity was determined by the modified Davis method:24−27 0.8 mL of 0.8 g/L naringin solution was added into a flask containing 30 mg of immobilized naringinase or 0.2 mL of free naringinase, and the flask was then placed in a water bath at 55 °C for 30 min. Then, 0.1 mL of the naringinase reaction solution was added into 5 mL of diethylene glycol (90%, v/v) and 0.1 mL of 4 mol/L NaOH solution. The mixture was left to stand for 15 min and then the absorbance of the solution was measured at 420 nm. Definition of naringinase activity (U): the amount of naringinase required to degrade 1 mg of naringin per minute at pH 7.5 and 55 °C is one unit of naringinase activity. Definition of the specific activity of naringinase (U/g carrier or U/mL enzyme solution): naringinase activity demonstrated by 1 g of immobilized naringinase (or 1 mL free naringinase) at pH 7.5 and 55 °C.
2.6. Calculation of Naringinase-Carrying Rate and Naringinase Activity Recovery
The immobilized naringinase-carrying rate (B) was calculated by the following equation28
| 2 |
wherein, B represents the naringinase carrying rate, A0 refers to the total naringinase activity in the naringinase solution before immobilization, and A1 refers to the total naringinase activity remaining in the supernatant after immobilization.
Immobilized naringinase activity recovery (R) was calculated by the following equation28
| 3 |
wherein, R represents the recovery rate of the naringinase, A0 and A1 are the same as in eq 1, and A2 refers to the total activity of immobilized naringinase in the enzymatic reaction that was used to measure total naringinase activity.
Relative naringinase activity (Q) was calculated by the following equation
| 4 |
wherein, Q represents the relative enzyme activity, P refers to the enzyme activity under certain conditions, and K refers to the highest enzyme activity under the same conditions.
2.9. Study of Kinetic Parameters
PEI/PDA-SBA-15-immobilized naringinase (30 mg) was added into 0.8 mL of naringin solution with different concentrations (0.5, 1.0, 1.5, 2.0, and 2.5 mg/mL) and reacted at pH 7.5 and 55 °C. Free naringinase (0.2 mL) was added into 0.8 mL of naringin solution with different concentrations (0.5, 1.0, 1.5, 2.0, and 2.5 mg/mL) and reacted at pH 4.5 and 50 °C. The maximum reaction velocity (Vmax) and apparent Michaelis constant (Km) values were calculated by the Lineweaver–Burk equation described as follows, and the turnover constant (Kcat) and catalytic coefficient (Kcat/Km) were then determined.29
| 5 |
| 6 |
3. Results and Discussion
3.1. Characterization of Materials
The surface morphology of the composites was examined by SEM. As shown in Figure 1A, the mesoporous silica SBA-15 formed a short rod-like structure. After being co-deposited by PDA and PEI on the carrier, the rod-like structure of PEI/PDA-SBA-15 was similar to that of SBA-15 (Figure 1B), indicating that the thin PDA film did not alter the surface morphology of supporting mesopores. To further validate the formation of PDA and PEI coating, TEM was used to record the morphologies of the obtained materials. As shown in Figure 1C, the as-prepared SBA-15 sample possessed a high ordered hexagonal mesostructure. After the co-deposition modification of PDA and PEI, the ordered mesoporous structure of SBA-15 was retained with a visible thin film of PEI/PDA on the mesopore surface (Figure 1D).
Figure 1.
SEM images of SBA-15 (A), PEI/PDA-SBA-15 (B), HRTEM images of SBA-15 (C), and PEI/PDA-SBA-15 (D), and the scale bar of (A,B) were 100 nm, and the scale bar of (C,D) were 500 nm.
Figure 2 shows the FT-IR spectra of SBA-15 and PEI/PDA-SBA-15. The characteristic peaks of SBA-15, 1090 and 3430 cm–1 were presented in the FT-IR spectrum, corresponding to Si–OH (stretching vibration) and Si–O–Si bonds. From the FT-IR spectrum of PEI/PDA-SBA-15, the peak of 2926 cm–1 was the characteristic of the −CH2– bond, and two peaks at 3430 and 1464 cm–1 were also observed, which were corresponding to the bending vibration of −OH and −NH– bonds, respectively. Besides, the vibration of the −NH2 bond appeared at 1630 cm–1.30 These results indicated that the PEI and PDA were successfully coated on the surface of SBA-15. In addition, the element contents of the SBA-15, PDA-SBA-15, PEI/PDA-SBA-15, and naringinase-PEI/PDA-SBA-15 were determined by an elemental analyzer. As shown in Table 1, it was clear that the contents of C, N, and H elements in PDA-SBA-15 increased slightly compared with those in SBA-15, and the PEI/PDA-SBA-15 exhibited higher element contents than PDA-SBA-15 and SBA-15, which indicated that the addition of PEI could shorten the deposition time and form a more uniform and stable hydrophilic coating. In addition, when the carrier was used for the naringinase immobilization, the element content increased remarkably, especially the C content was raised up to 23.16%, which was attributed to the presence of a large number of amino acids and water molecules on naringinase. Therefore, the PEI and PDA were successfully co-deposited on SBA-15 and had good performance for immobilizing naringinase.
Figure 2.
FT-IR spectra of SBA-15 and PEI/PDA-SBA-15.
Table 1. Element Composition of the SBA-15, PDA-SBA-15, PEI/PDA-SBA-15, and Naringinase-PEI/PDA-SBA-15.
| sample | N (%) | C (%) | H (%) |
|---|---|---|---|
| SBA-15 | 0.00 | 0.73 | 2.15 |
| PDA-SBA-15 | 0.14 | 1.78 | 2.21 |
| PEI/PDA-SBA-15 | 2.68 | 6.49 | 3.03 |
| naringinase-PEI/PDA-SBA-15 | 2.91 | 23.16 | 4.14 |
The mesostructural parameters of the mussel-inspired functionalized carrier were derived from the nitrogen adsorption/desorption isotherms with their pore size distribution from BJH model. As shown in Figure 3A,B, the SBA-15, 1.5 mg/mL DA and PEI-modified SBA-15 (referred to as 1.5PEI/PDA-SBA-15, the same below), as well as 3.5PEI/PDA-SBA-15 exhibited type IV isotherms with a H1-type hysteresis loops, which were the characteristics of mesoporous materials. It was obviously indicated that the pore size of SBA-15 gradually decreased as the concentration of PDA and PEI increased during the modification process: 11.37 nm for SBA-15, 10.422 nm for as-prepared 1.5PEI/PDA-SBA-15, 9.694 nm for as-prepared 2.5PEI/PDA-SBA-15, and 9.473 nm for as-prepared 3.5PEI/PDA-SBA-15. Accordingly, the BET surface area and pore volume of SBA-15 decreased because of the PDA and PEI coating on the mesoporous silica support (Table 2). The mesostructural parameters of the immobilized enzyme on the mussel-inspired functional carrier are shown in Figure 3C,D. The SBA-15, PEI/PDA-SBA-15, and naringinase-PEI/PDA-SBA-15 all showed type IV and H1-type hysteresis loops, and the pore size distribution curves exhibited a narrow size range and gradually shifted to a low value with the co-deposition of DA and PEI and immobilized naringinase, and the pore sizes of SBA-15, PEI/PDA-SBA-15, and naringinase-PEI/PDA-SBA-15 were 11.37, 9.694, and 6.91 nm, respectively. In addition, the coating of PDA and PEI and the connection of naringinase reduced the BET surface area and pore volume of SBA-15 (Table 2). It was indicated that not only PDA and PEI were successfully encapsulated in the mesopores but also naringinase was immobilized as well.
Figure 3.

(A,C) Pore size distribution from BJH desorption of SBA-15, 1.5PEI/PDA-SBA-15, 2.5PEI/PDA-SBA-15, 3.5PEI/PDA-SBA-15. (B,D) Pore size distribution from BJH desorption of SBA-15, PEI/PDA-SBA-15, and naringinase-PEI/PDA-SBA-15.
Table 2. Mesoporous Properties of SBA-15, 1.5PEI/PDA-SBA-15, 2.5PEI/PDA-SBA-15, 3.5PEI/PDA-SBA-15, and Naringinase-PEI/PDA-SBA-15.
| sample | BJH pore size (nm) | specific surface area (m2/g) | total pore volume (cm3/g) |
|---|---|---|---|
| SBA-15 | 11.370 | 710.108 | 1.123 |
| 1.5PEI/PDA-SBA-15 | 10.422 | 473.739 | 1.042 |
| 2.5PEI/PDA-SBA-15 | 9.694 | 474.636 | 0.890 |
| 3.5PEI/PDA-SBA-15 | 9.473 | 475.778 | 0.839 |
| naringinase-PEI/PDA-SBA-15 | 6.910 | 346.305 | 0.418 |
3.2. Optimization of the Mussel-Inspired Functional Process
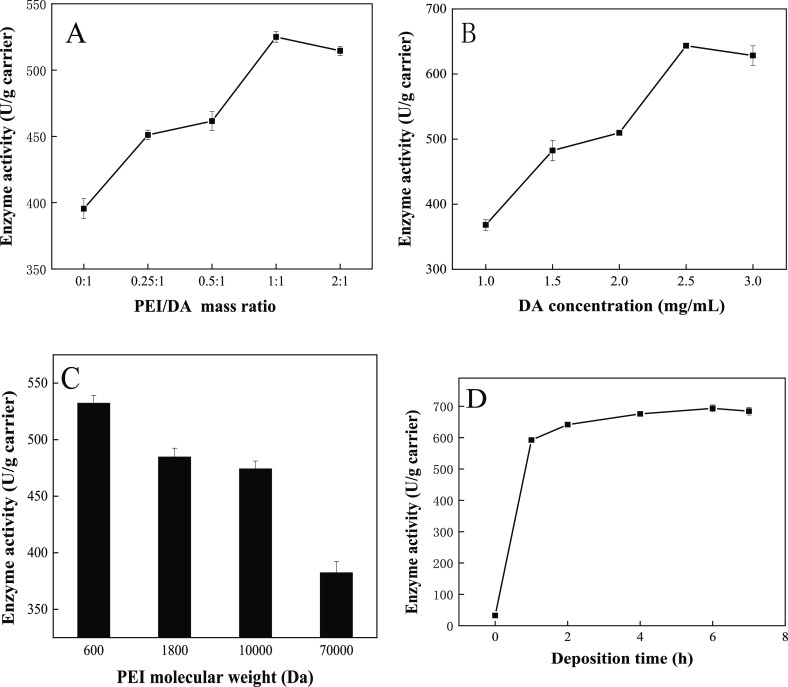
As shown in Figure 4, it was shown that different PEI/DA mass ratios, DA concentrations, PEI molecular weights, and deposition times had an impact on the material-immobilized naringinase.
Figure 4.
Effects of different PEI/DA mass ratio (A), DA concentration (B), PEI molecular weight (C), and deposition time (D)on immobilized naringinase activity.
3.2.1. Effect of the PEI/DA Mass Ratio on Immobilization
First, the effect of the PEI/DA mass ratio on the enzyme activity of the immobilized naringinase is investigated, and the results are shown in Figure 4A. When the naringinase was immobilized by PDA-coated SBA-15, the enzyme activity was 395.45 U/g carrier. In contrast, the enzyme activity could raise to 524.97 U/g carrier using PEI/PDA-coated SBA-15 as carriers. When the mass ratio of PEI/DA was 1:1, the enzyme activity reached the maximum. The obvious increase in enzyme activity was because of the Michael addition reaction between PEI and DA. The introduction of PEI disrupted the noncovalent interactions in PDA aggregates and effectively inhibited the formation of particles,22 which made the coating on the surface of the material more uniform, more stable, denser, and hydrophilic, which was beneficial to the enzyme connection. However, when the PEI/DA mass ratio increased from 1:1 to 2:1, the deposition rate decreased from 2.30 to 1.76% and the activity of the immobilized enzyme decreased. It might be for this reason that the ratio of PEI was too high, and a large number of amino groups of PEI would consume more functional groups of DA, which subsequently reduced the number of sites on the DA molecules that could be combined with the surface of the carrier, thereby hindering the deposition of PDA coating on the surface of the carrier.17 Therefore, the PEI/DA mass ratio of 1:1 was selected as the optimal mass ratio for the subsequent experiments.
3.2.2. Effect of the DA Concentration on Immobilization
Keeping the mass ratio of DA/PEI at 1:1, the influence of the concentration of DA in Tris-HCl buffer on the enzyme activity of the immobilized enzyme was investigated. As shown in Figure 4B, as the concentration of DA increased, the enzyme activity rose and reached the highest enzyme activity at a concentration of 2.5 mg/mL. When the DA concentration further increased to 3.0 mg/mL, the enzyme activity decreased slightly to 628.35 U/g carrier. When the DA concentration was lower than 2.5 mg/mL, there would be less coating deposited on the surface of the material, resulting in insufficient sites that could be connected to the surface of naringinase. When the concentration reached 2.5 mg/mL, the surface coating of the SBA-15 material remained saturated and enzyme activity was the best. When the feed concentration of DA exceeded 2.5 mg/mL, the enzymatic activity was found to slightly decrease. It might be the reason that the higher concentration of the DA solution would cause serious quinonation reaction, resulting in a black quinonization product,31 which was directly deposited on the surface of the substrate before subsequent polymerization, and was not conducive to the polymerization reaction of DA. Therefore, a DA concentration of 2.5 mg/mL was selected for the following experiments.
3.2.3. Effect of the PEI Molecular Weight on Immobilization
Figure 4C shows the effects of different molecular weights of PEI on the activity of immobilized enzymes. From the figure, it could be seen that the increase of the molecular weight of PEI would significantly inhibit the deposition behavior of DA/PEI, which was not conducive to the co-deposition process. As the molecular weight of PEI increased, on the one hand, the distance between the anchor points increased significantly, resulting in a decrease in the relative density of the coating that might be formed,20 covalent bond formed by oxidative coupling and noncovalent reduction formed by the hydrogen bond, and π–π stacking were reduced in the unit area; on the other hand, DA or PDA was more likely to be formed in the solution as terminal branched molecules rather than cross-linked networks. In addition, the high-molecular weight PEI would also cause the hydrophilic end of the “surfactant-like” formed by the two to be too large, and it was difficult to stably adsorb on the surface of the carrier. Therefore, the PEI molecular weight of 600 Da was used for the subsequent studies.
3.2.4. Effect of Deposition Time on Immobilization
Figure 4D shows the effect of deposition time on the activity of the immobilized enzyme. The immobilized enzyme activity shown by the carrier increased initially with the increase of the deposition time. The highest enzyme activity was obtained at 6 h of deposition time and tended to be invariable when the deposition time exceeded 6 h. It might be the reason that PDA and PEI could still be deposited on the surface of the coating when the number of active amino groups in the surface coating reached saturation. Therefore, the deposition time of 6 h was selected as the optimal time.
In summary, the optimal conditions for mussel-inspired technology to modify SBA-15 were concluded as follows: PEI/DA mass ratio of 1:1, DA concentration of 2.5 mg/mL, modification time of 6 h, and PEI molecular weight of 600 Da, and used for the subsequent studies.
3.3. Optimization of Immobilization Conditions
3.3.1. Effect of Temperature on Immobilization
The immobilization temperature had a critical influence on the immobilized enzyme. Too low temperature would affect the immobilization rate of the enzyme.32 In addition, the spatial structure of naringinase was easy to be destroyed at high temperatures, resulting in the reduction of enzyme activity.33 As shown in Figure 5A, the activity of immobilized naringinase increased with the increase of the temperature. When the temperature rose to 35 °C, the activity of immobilized naringinase reached a maximum value of 705.38 U/g carrier. The temperature rose from 35 to 45 °C, the enzyme activity of immobilized naringinase decreased, which might be because of the temperature sensitivity of naringinase. Therefore, 35 °C was the optimum immobilization temperature.
Figure 5.
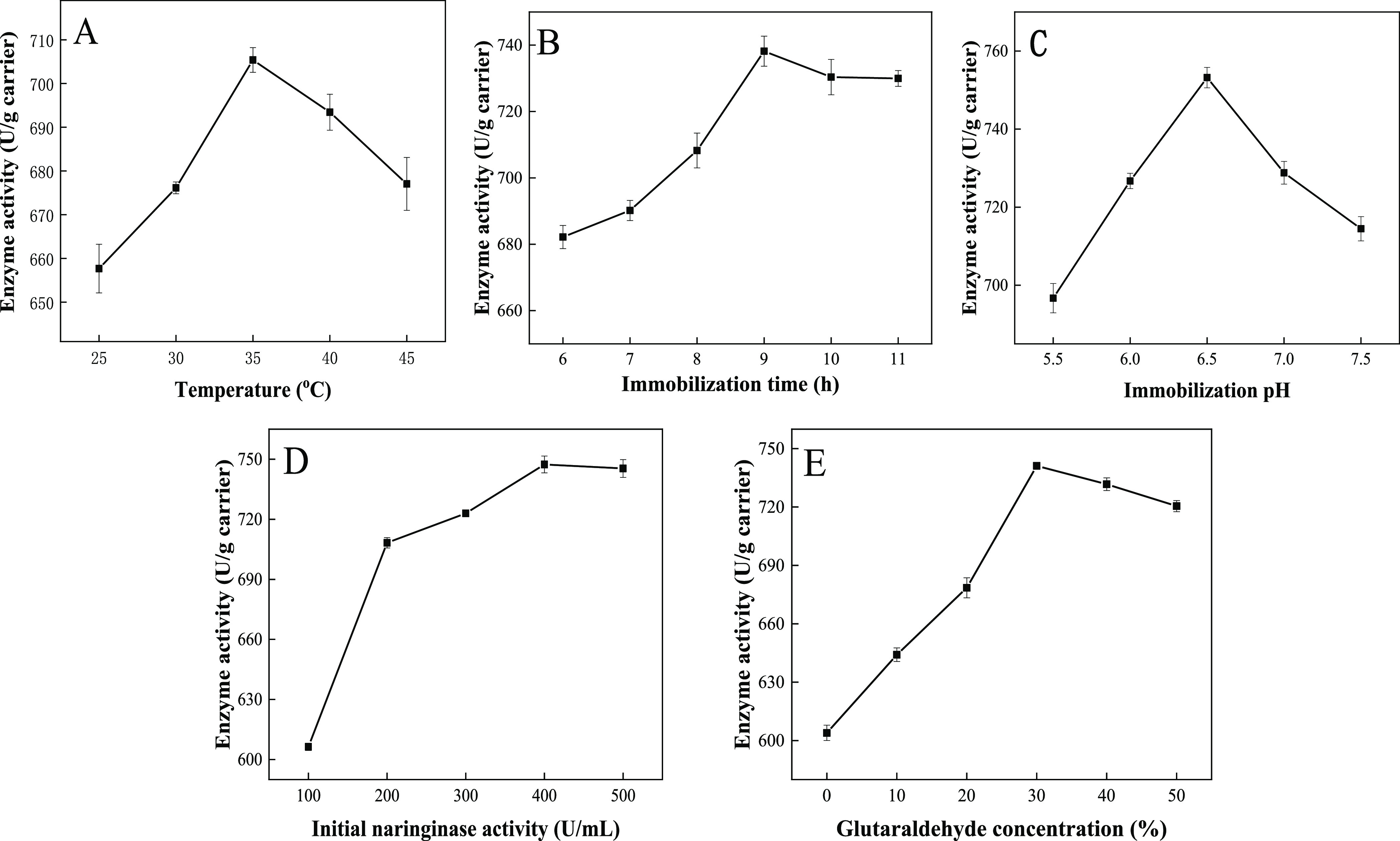
Effects of immobilization temperature (A), immobilization pH (C), immobilization time (B), initial naringinase activity (D), and glutaraldehyde concentration (E) on immobilized naringinase activity.
3.3.2. Effect of the Immobilization Time on Immobilization
As shown in Figure 5B, with the increase of the immobilization time, more enzyme molecules could be immobilized on the PEI/PDA-SBA-15 and the activity of the immobilized enzyme gradually increased, owing to the fact that the combination of mussel-inspired functional materials and naringinase required sufficient time. The maximum enzyme activity of 738.17 U/g carrier was achieved at 9 h. However, when the immobilization time was above 9 h, more naringinase might be attached to the surface or pores of the material, and the active center of the enzyme was covered, which increased the steric hindrance effect between naringinase molecules and naringin and led to the decrease of immobilized naringinase activity. Therefore, the immobilization time of 9 h was selected for the following experiments.
3.3.3. Effect of Immobilization pH on Immobilization
Figure 5C shows the effect of immobilized pH on immobilized naringinase. The enzyme activity of the immobilized naringinase reached the maximum at an immobilized pH value of 6.5, with the enzyme activity of 736.3 U/g carrier. When the pH value was higher than 6.5, the enzyme activity of immobilized naringinase decreased slightly, maintaining 95.2% of the maximum enzyme activity. Because the PDA, PEI, and SBA-15 were mostly connected through noncovalent interactions, and the coating stability in strong acids, strong bases, and strong polar solvents was poor,21 the DA/PEI coating preferred to immobilize enzymes near the neutral pH range. Therefore, the immobilization pH of 6.5 was used for the subsequent studies.
3.3.4. Effect of the Initial Naringinase Activity on Immobilization
As shown in Figure 5D, the enzyme activity of immobilized naringinase increases with the increase of the initial dosage of the enzyme. When the dosage of enzyme was 400 U/mL, the enzyme activity of immobilized naringinase achieved the maximum. When the dosage kept increasing, a large number of enzymes were bound to the carrier, which increased steric hindrance and diffusion restriction,34 so that the enzyme and the substrate could not be fully contacted and the enzyme activity increased slowly or even decreased. Therefore, the initial naringinase activity of 400 U/mL was selected for the following studies.
3.3.5. Effect of the Glutaraldehyde Concentration on Immobilization
It could be seen from Figure 5E that a high enzyme activity of 603.95 U/g carrier is achieved without adding glutaraldehyde, suggesting that the strong adhesion interactions (including hydrogen interaction, electrostatic interaction, and so on) are suitable for the immobilization of the enzyme. When the volume fraction of glutaraldehyde increased from 10 to 30%, the enzyme activity increased but further increasing caused the relative enzyme activity to decrease. Glutaraldehyde served as not only a cross-linking agent for the PEI/PDA coating and immobilization reaction but also a denaturant for enzymes. As the volume fraction of glutaraldehyde increased, the free aldehyde groups of SBA-15 increased, which was conducive to the covalent connection between the material and the enzyme; but when the volume fraction of glutaraldehyde exceeded a certain amount, the enzyme underwent a large number of intramolecular or intermolecular strong cross-linking reactions, which greatly reduced the activity of the enzyme. Therefore, a glutaraldehyde volume fraction of 30% was appropriate for the immobilization because of the presence of strong physical and chemical interactions between the PEI/PDA coating and the naringinase, including the adhesion interaction (including hydrogen interaction, electrostatic interaction, and so on) and the covalent connection formed between PEI and naringinase.
As mentioned above, the optimized parameters of naringinase immobilization were optimized as follows: temperature of 35 °C, pH value of 7.5, time of 9 h, initial naringinase concentration of 400 U/mL, and glutaraldehyde concentration of 30%. The optimized naringinase activity increased to 741.14 U/g carrier.
3.4. Hydrolysis Properties of the Immobilized Naringinase
3.4.1. Optimum Reaction Conditions of Immobilized Naringinase
As shown in Figure 6A, the relative enzyme activity of free naringinase at pH 3–4 was less than 20% of the highest enzyme activity, which was obtained at pH 4.5 and dropped rapidly at pH 5.0–8.5. In contrast, the immobilized naringinase maintained more than 50% relative enzyme activity between pH 3.0 and 8.5 and reached the maximum relative enzyme activity at pH 7.5. Because the enzyme activity of the free enzyme was mainly affected by the pH of the main reaction phase. In strong acids and strong bases, the conformation of the binding site of the enzyme and the substrate was destroyed, which affects the effective binding of the substrate, and was manifested as a significant decrease in enzyme activity. As for the immobilized enzyme because the enzyme molecule was attached to the carrier, the charge environment on the carrier surface also had an important influence on the enzyme activity of the immobilized enzyme.35 Because of the protection of the carrier, the binding site conformation was relatively stable. The degree of damage by acid and alkali was reduced, and the ability to resist acid and alkali was enhanced.
Figure 6.
Effects of reaction temperature (A) and reaction pH (B) on free naringinase and immobilized naringinase activity.
The best reaction temperature for free enzyme and immobilized enzyme were 50 and 55 °C, respectively (Figure 6B). The immobilized enzyme still maintained more than 85% relative enzyme activity between 40 and 60 °C. The relative enzyme activity of free enzymes decreased rapidly when the temperature was lower than 45 °C and higher than 50 °C. The immobilization of the enzyme on the carrier often limited its conformational changes, stabilizing the three-dimensional structure of the protein,36 because of which the enzyme showed higher resistance to high temperatures,37 which was confirmed by the authors of several studies. It was indicated that immobilization could remarkably improve the acid resistance and temperature stability of naringinase.
As discussed above, PEI/PDA-SBA-15-immobilized naringinase had a wide pH range and good thermal stability. The optimal enzymatic hydrolysis conditions were: temperature of 55 °C and pH of 7.5. The enzyme activity of immobilized naringinase was increased to 753.78 U/g carrier. The enzyme loading rate, enzyme activity recovery rate were 93.91 and 97.28%, respectively, which was much higher than that of Luo et al.38 It should be the reason of the strong physical and chemical interactions between the PEI/PDA coating and the naringinase, including the adhesion interaction (including hydrogen interaction, electrostatic interaction, and so on), and the covalent connection formed between PEI and naringinase when the glutaraldehyde was served as a cross-linking agent.
The activities of PEI/PDA-SBA-15 and other carriers immobilized naringinase are summarized in Table 3. The value of activity were given under the same unit. Compared with the previous literature studies (Table 3), the immobilized naringinase activity in this work was much higher, suggesting that the PEI/PDA-SBA-15 had an excellent performance of immobilized naringinase.
Table 3. Comparison of Enzyme Activity of Naringinase Immobilized on Different Materials.
3.4.2. Reusability of the Immobilized Naringinase
The repeated experiments of immobilized naringinase were performed in this study. Figure 7A shows the reusability of the immobilized naringinase, and it was clear that the enzyme activity remained 60.79% of the initial activity after 8 cycles, which was much higher than that of Lei et al.39 This result indicated that the naringinase immobilized on PEI/PDA-SBA-15 exhibited good operational stability and had great development potential in debittering juice and improving product quality. There would be a small amount of loss and leakage of immobilized enzyme during recycling. However, the activity of immobilized naringinase decreased by 40% of the initial activity after 8 cycles of circulation, mainly because the active center of the enzyme could no longer be used after binding to the substrate, resulting in the inactivation of the enzyme.40
Figure 7.
Recycling of immobilized naringinase (A) and storage stability of free naringinase and immobilized naringinase (B).
3.4.3. Storage Stability of Immobilized Naringinase
The storage stability of immobilized naringinase and free naringinase is shown in Figure 7B. It could be seen that the immobilized naringinase maintained more than 75% of the relative naringinase activity within 30 days of storage. In contrast, the activity of free naringinase decreased rapidly after 15 days of storage and only 30% relative enzyme activity was left after 30 days of storage. These results brought out that with the advantages of excellent storage stability, the immobilized naringinase had better application potential than free naringinase, especially when the enzyme needed long distance transportation and storage after production.
In summary, the enzyme activities of the immobilized naringinase after 8 times of repeated use and storage for 30 days were 433.88 U/g carrier and 572.18 U/g carrier, respectively, suggesting that the immobilized naringinase exhibited good reusability and storage stability.
3.4.4. Kinetic Parameters
The double reciprocal curves of the enzymatic hydrolysis reaction of free naringinase (i.e., fermentation broth) and PEI/PDA-SBA-15-immobilized naringinase are shown in Figure 8. The Km value of PEI/PDA-SBA-15 immobilized naringinase for naringin degradation was 1.59 g/L, which was lower the Km (1.84 g/L) of fermentation broth to degrade naringin, indicating that the immobilized naringinase had a higher affinity for the substrate, which was consistent with the result of Homa et al.41 The decrease in the Km value on immobilization displayed that the interaction between the enzyme and substrate may have been strengthened by a suitable orientation of the enzyme active site toward the substrate and the matrix structure caused lesser steric limitations, thus, the substrate was free to interact with PEI/PDA-SBA-15. Besides, the reduction in mass transfer limitation might be due to the ordered mesoporous structure of PEI/PDA-SBA-15, which improved the binding efficiency between the immobilized enzyme and substrate.42 The maximum reaction rate Vmax (0.044 g/(L·min)) of the substrate degradation reaction of PEI/PDA-SBA-15-immobilized naringinase was less than the Vmax value of the fermentation broth degradation substrate reaction (0.058 g/(L·min)), it might be because of the steric hindrance effect of the immobilized enzyme, which made it difficult for the immobilized enzyme to act on the substrate and reduced the enzymatic reaction rate. In addition, the Kcat of the immobilized naringinase and fermentation broth degradation substrate was calculated by substituting the formula, and the results were 0.126 and 0.032 s–1, respectively. The ratio of Kcat/Km was 0.079 and 0.017 M–1 s–1, respectively, indicating that PEI/PDA-SBA-15-immobilized naringinase had a higher catalytic efficiency.
Figure 8.
Lineweaver–Burk plot of hydrolysis of naringin by free naringinase and immobilized naringinase.
4. Conclusions
In this work, the preparation of mussel-inspired functional materials, the characteristics of immobilized naringinase, as well as enzymatic hydrolysis conditions were studied. In addition, the optimal modification and immobilization conditions as well as enzyme properties were investigated. The naringinase activity was much higher than that of the previous works. As a novel enzyme immobilization material, PEI/PDA co-deposition-modified SBA-15 had achieved significant progress in enzyme activity enhancement, reusability, and storage stability and made it possible for the application of mussel-inspired technology in the field of immobilized enzymes.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 31601411, no. 21804129 and no. 31771914), Scientific Research Project of Liaoning Provincial Department of Education (no. J2020043 & no. J2020096) and Key Projects of Liaoning Natural Science Foundation Program (no. 20180540130). We are grateful for the test analysis support from Analysis Center of Dalian Polytechnic University.
Author Contributions
X.Z.: data curation, writing—original draft. J.T.: writing—review and editing. H.Z.: writing—review and editing. C.Y.: data curation. S.W.: data curation. Q.L. and X.S.: conceptualization, funding acquisition, writing—review and editing.
The authors declare no competing financial interest.
References
- Cavia-Saiz M.; Muñiz P.; Ortega N.; Busto M. D. Effect of enzymatic debittering on antioxidant capacity and protective role against oxidative stress of grapefruit juice in comparison with adsorption on exchange resin. Food Chem. 2011, 125, 158–163. 10.1016/j.foodchem.2010.08.054. [DOI] [Google Scholar]
- Ribeiro M. H. Naringinases: occurrence, characteristics, and applications. Appl. Microbiol. Biotechnol. 2011, 90, 1883. 10.1007/s00253-011-3176-8. [DOI] [PubMed] [Google Scholar]
- González-Pombo P.; Fariña L.; Carrau F.; Batista-Viera F.; Brena B. M. Aroma enhancement in wines using co-immobilized Aspergillus niger glycosidases. Food Chem. 2014, 143, 185–191. 10.1016/j.foodchem.2013.07.107. [DOI] [PubMed] [Google Scholar]
- Liu Q.; Lu L.; Xiao M. Cell surface engineering of α-l-rhamnosidase for naringin hydrolysis. Bioresour. Technol. 2012, 123, 144–149. 10.1016/j.biortech.2012.05.083. [DOI] [PubMed] [Google Scholar]
- Soria F.; Ellenrieder G.; Oliveira G. B.; Cabrera M.; Carvalho L. B. α-l-rhamnosidase of Aspergillus terreus immobilized on ferromagnetic supports. Appl. Microbiol. Biotechnol. 2011, 93, 1127–1134. 10.1007/s00253-011-3469-y. [DOI] [PubMed] [Google Scholar]
- Šimčíková D.; Kotik M.; Weignerová L.; Halada P.; Pelantová H.; Adamcová K.; Křen V. α-L-rhamnosyl-β-D-glucosidase (rutinosidase) from Aspergillus niger: characterization and synthetic potential of a novel diglycosidase. Adv. Synth. Catal. 2014, 357, 107–117. 10.1002/adsc.201400566. [DOI] [Google Scholar]
- Chen Y.; Ni H.; Chen F.; Cai H.; Li L.; Su W. Purification and characterization of a naringinase from Aspergillus aculeatus JMUdb058. J. Agric. Food Chem. 2013, 61, 931–938. 10.1021/jf303512q. [DOI] [PubMed] [Google Scholar]
- Makas Y. G.; Kalkan N. A.; Aksoy S.; Altinok H.; Hasirci N. Immobilization of laccase in κ-carrageenan based semi-interpenetrating polymer networks. J. Biotechnol. 2010, 148, 216–220. 10.1016/j.jbiotec.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Zang L.; Qiu J.; Wu X.; Zhang W.; Sakai E.; Wei Y. Preparation of magnetic chitosan nanoparticles as support for cellulase immobilization. Ind. Eng. Chem. Res. 2014, 53, 3448–3454. 10.1021/ie404072s. [DOI] [Google Scholar]
- Huang W.; Zhan Y.; Shi X.; Chen J.; Deng H.; Du Y. Controllable immobilization of naringinase on electrospun cellulose acetate nanofibers and their application to juice debittering. Int. J. Biol. Macromol. 2017, 98, 630–636. 10.1016/j.ijbiomac.2017.02.018. [DOI] [PubMed] [Google Scholar]
- Bodakowska-Boczniewicz J.; Garncarek Z. Immobilization of naringinase from Aspergillus Niger on a magnetic polysaccharide carrier. Molecules 2020, 25, 2731. 10.3390/molecules25122731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X.; Zhang L.; Zhang Y.; Yang G.; Yan Z. Amine-modified SBA-15: Effect of pore structure on the performance for CO2 capture. Ind. Eng. Chem. Res. 2011, 50, 3220–3226. 10.1021/ie101240d. [DOI] [Google Scholar]
- Diao X. Synthesis and characterization of large pore size and highly ordered mesoporous molecular sieve SBA-15. Adv. Mater. Res. 2012, 554–556, 620–623. 10.4028/www.scientific.net/amr.554-556.620. [DOI] [Google Scholar]
- Li S.; Wu Z.; Lu M.; Wang Z.; Li Z. Improvement of the enzyme performance of trypsin via adsorption in mesoporous silica SBA-15: hydrolysis of BAPNA. Molecules 2013, 18, 1138–1149. 10.3390/molecules18011138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D.; Streb C.; Hartmann M. Covalent anchoring of chloroperoxidase and glucose oxidase on the mesoporous molecular sieve SBA-15. Int. J. Mol. Sci. 2010, 11, 762–778. 10.3390/ijms11020762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou B.; Song C.; Xu X.; Xia J.; Huo S.; Cui F. Enhancing stabilities of lipase by enzyme aggregate coating immobilized onto ionic liquid modified mesoporous materials. Appl. Surf. Sci. 2014, 311, 62–67. 10.1016/j.apsusc.2014.04.210. [DOI] [Google Scholar]
- Yang H.-C.; Luo J.; Lv Y.; Shen P.; Xu Z.-K. Surface engineering of polymer membranes via mussel-inspired chemistry. J. Membr. Sci. 2015, 483, 42–59. 10.1016/j.memsci.2015.02.027. [DOI] [Google Scholar]
- Tang J.; Shi Z.; Berry R. M.; Tam K. C. Mussel-inspired green metallization of silver nanoparticles on cellulose nanocrystals and their enhanced catalytic reduction of 4-nitrophenol in the presence of β-cyclodextrin. Ind. Eng. Chem. Res. 2015, 54, 3299–3308. 10.1021/acs.iecr.5b00177. [DOI] [Google Scholar]
- Liu G.; Xiang J.; Xia Q.; Li K.; Yan H.; Yu L. Fabrication of durably antibacterial cotton fabrics by robust and uniform immobilization of silver nanoparticles via mussel-inspired polydopamine/polyethyleneimine coating. Ind. Eng. Chem. Res. 2020, 59, 9666–9678. 10.1021/acs.iecr.9b07076. [DOI] [Google Scholar]
- Yang H.-C.; Wu M.; Li Y.; Chen Y.; Wan L.; Xu Z. Effects of polyethyleneimine molecular weight and proportion on the membrane hydrophilization by codepositing with dopamine. J. Appl. Polym. Sci. 2016, 133, 43792. 10.1002/app.43792. [DOI] [Google Scholar]
- Kinugawa S.; Wang S.; Taira S.; Tsuge A.; Kaneko D. Single-molecule interaction force measurements of catechol analog monomers and synthesis of adhesive polymer using the results. Polym. J. 2016, 48, 715–721. 10.1038/pj.2015.140. [DOI] [Google Scholar]
- Cao X.; Luo J.; Woodley J. M.; Wan Y. Mussel-inspired co-deposition to enhance bisphenol A removal in a bifacial enzymatic membrane reactor. Chem. Eng. J. 2018, 336, 315–324. 10.1016/j.cej.2017.12.042. [DOI] [Google Scholar]
- Lee H.; Dellatore S. M.; Miller W. M.; Messersmith P. B. Mussel-Inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodakowska-Boczniewicz J.; Garncarek Z. Immobilization of naringinase from penicillium decumbens on chitosan microspheres for debittering grapefruit juice. Molecules 2019, 24, 4234. 10.3390/molecules24234234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defaei M.; Taheri-Kafrani A.; Miroliaei M.; Yaghmaei P. Improvement of stability and reusability of α-amylase immobilized on naringin functionalized magnetic nanoparticles: A robust nanobiocatalyst. Int. J. Biol. Macromol. 2018, 113, 354. 10.1016/j.ijbiomac.2018.02.147. [DOI] [PubMed] [Google Scholar]
- Nunes M. A. P.; Vila-Real H.; Fernandes P. C. B.; Ribeiro M. H. L. Immobilization of naringinase in PVA-alginate matrix using an innovative technique. Appl. Microbiol. Biotechnol. 2010, 160, 2129–2147. 10.1007/s12010-009-8733-6. [DOI] [PubMed] [Google Scholar]
- Vila-Real H.; Alfaia A. J.; Rosa M. E.; Calado A. R.; Ribeiro M. H. L. An innovative sol–gel naringinase bioencapsulation process for glycosides hydrolysis. Process Biochem. 2010, 45, 841–850. 10.1016/j.procbio.2010.02.004. [DOI] [Google Scholar]
- Cui P.; Li J.; Xiao Z.; Tan C. Immobilization of penicillium sp.naringinase on epoxy resin. Food Ferment. Ind. 2014, 40, 87–92. [Google Scholar]
- Zhu Y.; Jia H.; Xi M.; Li J.; Yang L.; Li X. Characterization of a naringinase from Aspergillus oryzae 11250 and its application in the debitterization of orange juice. Process Biochem. 2017, 62, 114–121. 10.1016/j.procbio.2017.07.012. [DOI] [Google Scholar]
- Lv Y.; Yang H.-C.; Liang H.-Q.; Wan L.-S.; Xu Z.-K. Nanofiltration membranes via co-deposition of polydopamine/polyethylenimine followed by cross-linking. J. Membr. Sci. 2015, 476, 50–58. 10.1016/j.memsci.2014.11.024. [DOI] [Google Scholar]
- Ye Q.; Zhou F.; Liu W. Bioinspired catecholic chemistry for surface modification. Chem. Soc. Rev. 2011, 40, 4244–4258. 10.1039/c1cs15026j. [DOI] [PubMed] [Google Scholar]
- Abdel-Naby M. A.; Sherif A. A.; El-Tanash A. B. Immobilization of Aspergillus oryzae tannase and properties of the immobilized enzyme. J. Appl. Microbiol. 1999, 87, 108–114. 10.1046/j.1365-2672.1999.00799.x. [DOI] [Google Scholar]
- Donato L.; Algieri C.; Rizzi A.; Giorno L. Kinetic study of tyrosinase immobilized on polymeric membrane. J. Membr. Sci. 2014, 454, 346–350. 10.1016/j.memsci.2013.12.029. [DOI] [Google Scholar]
- Diab M. A.; El-Sonbati A. Z.; Bader D. M. D.; Zoromba M. S. Thermal stability and degradation of chitosan modified by acetophenone. J. Polym. Environ. 2011, 20, 29–36. 10.1007/s10924-011-0330-4. [DOI] [PubMed] [Google Scholar]
- Foresti M. L.; Valle G.; Bonetto R.; Ferreira M. L.; Briand L. E. FTIR, SEM and fractal dimension characterization of lipase B from Candidaantarctica immobilized onto titania at selected conditions. Appl. Surf. Sci. 2010, 256, 1624–1635. 10.1016/j.apsusc.2009.09.083. [DOI] [Google Scholar]
- Elnashar M. M. M.; Hassan M. E. Novel epoxy activated hydrogels for solving lactose intolerance. BioMed Res. Int. 2014, 2014, 1–9. 10.1155/2014/817985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonowicz A.; Sarkar J. M.; Bollag J.-M. Improvement instability of an immobilized fungal laccase. Appl. Microbiol. Biotechnol. 1988, 29, 129–135. 10.1007/bf00251691. [DOI] [Google Scholar]
- Luo J.; Li Q.; Sun X.; Tian J.; Fei X.; Shi F.; Zhang N.; Liu X. The study of the characteristics and hydrolysis properties of naringinase immobilized by porous silica material. RSC Adv. 2019, 9, 4514–4520. 10.1039/c9ra00075e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei S.; Xu Y.; Fan G.; Xiao M.; Pan S. Immobilization of naringinase on mesoporous molecular sieve MCM-41 and its application to debittering of white grapefruit. Appl. Surf. Sci. 2011, 257, 4096–4099. 10.1016/j.apsusc.2010.12.003. [DOI] [Google Scholar]
- Wang H.; Zhang W.; Zhao J.; Xu L.; Zhou C.; Chang L.; Wang L. Rapid decolorization of phenolic azo dyes by immobilized laccase with Fe3O4/SiO2 nanoparticles as support. Ind. Eng. Chem. Res. 2013, 52, 4401–4407. 10.1021/ie302627c. [DOI] [Google Scholar]
- Homa T.; Mikani M. Kinetic and thermodynamic features of nanomagnetic cross-linked enzyme aggregates of naringinase nanobiocatalyst in naringin hydrolysis. Int. J. Biol. Macromol. 2018, 119, 717–725. 10.1016/j.ijbiomac.2018.08.005. [DOI] [PubMed] [Google Scholar]
- He S.; Song D.; Chen M.; Cheng H. Immobilization of lipases on magnetic collagen fibers and its applications for short-chain ester synthesis. Catalysts 2017, 7, 178. 10.3390/catal7060178. [DOI] [Google Scholar]