SUMMARY
Neuroinflammation as a result of immune cell recruitment into the central nervous system (CNS) is a key pathogenic mechanism of multiple sclerosis (MS). However, current anti-inflammatory interventions depleting immune cells or directly targeting their trafficking into the CNS can have serious side effects, highlighting a need for better immunomodulatory strategies. We detected increased Reelin concentrations in the serum of MS patients, resulting in increased endothelial permeability to leukocytes through increased NF-κB mediated expression of vascular adhesion molecules. We thus investigated the prophylactic and therapeutic potential of Reelin immunodepletion in experimental autoimmune encephalitis (EAE) and further validated the results in Reelin KO mice. Removal of plasma Reelin by either approach significantly protected against neuroinflammation and largely abolished the neurological consequences by reducing endothelial permeability and immune cell accumulation in the CNS. Our findings identify Reelin depletion as a mechanistically novel therapeutic approach with a potentially superior inherent safety margin for the treatment of MS and other diseases where leukocyte extravasation is a major driver of pathogenicity.
Keywords: Multiple sclerosis, MS, experimental autoimmune encephalomyelitis, EAE, chronic inflammation, neuroinflammation, leukocyte, monocyte, endothelial permeability, E-Selectin, ICAM-1, Reelin, antibody
One-sentence summary
A novel therapeutic approach that selectively targets endothelial adhesion of leukocytes, blocking neuroinflammation and encephalitic neuropathology.
INTRODUCTION
Inflammatory responses are generally protective in cases of acute infection or tissue damage but become deleterious when they enter a chronic state. Recruitment of circulating leukocytes is a general mechanism and hallmark of many pathological disorders including multiple sclerosis (MS) (1). Microglia (resident cells) and myelomonocytes (myeloid cells derived from leukocytes) are involved in the initiation and continuation of inflammation resulting in the demyelination of white matter (2-4), and their respective roles have been unraveled in elegant studies (1). In a murine model of experimental autoimmune encephalitis (EAE) used to study human MS, the inhibition of monocyte recruitment to the spinal cord is sufficient to block myelin degradation and paralysis (1). An extensive histopathological assessment of MS lesions revealed that macrophages are abundantly present in all brains of patients with this disorder (5). This finding has opened a powerful approach to treat MS by inhibiting monocyte infiltration.
Immunotherapeutic interventions are now widely used in MS to reduce the relapse rate and the accumulation of new brain lesions. Although substantial progress has been made in the development of treatments for relapsing-remitting MS, effective therapies are lacking for progressive forms of MS (6, 7). Moreover, interventions with biologics that target immune cells directly to prevent T-cell infiltration into the nervous system are difficult to regulate and have narrow therapeutic bandwidth. They result for instance in increased risk for progressive multifocal leukoencephalopathy (PML), a viral infection of the brain that is often fatal (8). Therefore, identifying optimal, immunomodulatory therapies for all forms of MS continues to constitute a major unmet need for patients (9).
In two independent genome-wide association studies (GWAS) (10, 11), Reelin single-nucleotide polymorphisms were found to be associated with MS severity score. However, these association studies did not shed light on the biological function of Reelin in MS. Initially recognized only for its role in guiding neurons during brain development and as a synaptic homeostatic regulator (12-15), we recently reported a novel non-neuronal function for systemically circulating Reelin in the vasculature (16) where Reelin regulates the NF-κB mediated expression of several vascular adhesion molecules. Endothelial adhesion is an obligate step that precedes leukocyte extravasation, which leads to excessive inflammation in autoimmune syndromes such as MS. Here we show that circulating Reelin is increased during MS relapse compared to health individuals and to patients in remission, thus correlating with disease state and severity. Genetic reduction or immunodepletion of Reelin in a pre-clinical EAE mouse model reduces endothelial adhesion and monocyte extravasation, thereby preventing neuroinflammation, demyelination, and paralysis. This proof-of-concept study opens the door to a new class of immunotherapeutics, selectively dampening NF-κB activation only at the endothelial barrier while avoiding direct T-cell suppression.
RESULTS
Identification of circulating Reelin as a potential biomarker for MS.
Progression of MS relies on myeloid cell extravasation and infiltration into the central nervous system (1, 17, 18). Based on our previous findings (16), we hypothesized that this mechanism might be regulated by Reelin and consequently evaluated whether circulating Reelin is increased in the pro-inflammatory context of MS. ELISA detected elevated Reelin concentrations in the circulation of relapsing-remitting MS patients during relapse (Table 1). Relapsing-remitting MS patients in remission had Reelin concentrations similar to the control group suggesting that circulating Reelin levels correlate with MS severity and stage and that lowering of Reelin in plasma might be a novel approach to treat MS.
Table 1.
Reelin concentration in serum is increased in patient with relapsing-remitting MS during the relapse phase.
| Control | RRMS in relapse |
RRMS in remission |
p | |
|---|---|---|---|---|
| n (total) | 18 | 19 | 24 | |
| n (female) | 12 | 16 | 21 | |
| n (male) | 6 | 3 | 3 | |
| Age | 37.4 ± 4.2 | 33.3 ± 2.2 | 34.4 ± 1.3 | |
| Reelin (ng/ml) | 12.79 ± 1.62 | 23.88 ± 4.86 | 12.31 ± 1.15 | *CT vs Rel; *Rel vs Rem |
| Medication | ||||
| None (n=5) | 22.23 ± 7.85 | |||
| Not reported (n=3) | 34.55 ± 22.37 | |||
| Avonex (n=2) | 24.96 ± 2.74 | |||
| Copaxone (n=2) | 11.53 ± 0.08 | |||
| Plex (n=2) | 10.03 ± 0.38 | |||
| Duplicate (n=1) | 74.05 | |||
| Fingolimod (n=1) | 21.68 | |||
| Cytoxan (n=1) | 9.08 | |||
| Rituxan (n=1) | 20.38 | |||
| Tysabri (n=1) | 20.65 |
Concentrations for Reelin are shown per group and detailed per treatment (below medication) in the “RRMS in relapse” group. RRMS, relapsing-remitting MS; CT, Control; Rel, Relapse; Rem, Remission
p<0.05 (ANOVA, Tukey).
Reelin promotes monocyte adhesion to human endothelial cells in vitro and in mice in vivo.
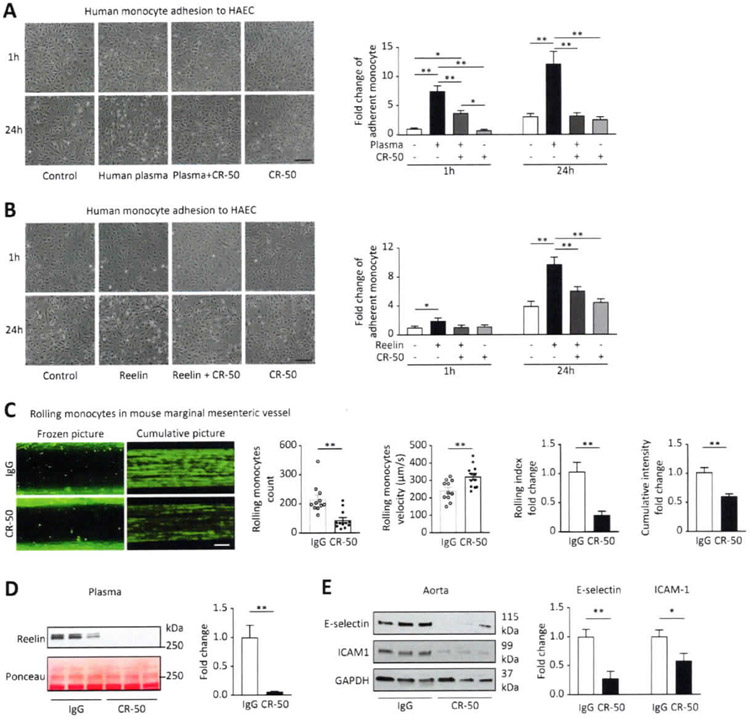
To investigate the role of Reelin and its therapeutic potential in human MS, we used a “human” in vitro system consisting of human aortic endothelial cells incubated with human plasma (Figure 1A) or recombinant Reelin (Figure 1B). In both stimulations, the Reelin-neutralizing monoclonal antibody (CR-50) (12, 19) rapidly (within 1 hr) inhibited human monocyte adhesion to endothelial cells in a sustained (24h) manner.
Figure 1. CR-50 inhibits Reelin-dependent adhesion of monocytes to endothelial cells.
(A) Human aortic endothelial cells (HAEC) were starved and stimulated with human plasma, plasma+CR-50 or CR-50 only (0.15 μg/ml) for 1 or 24 h. After washing, the cells were incubated with human monocytes (U937) for 45 min, washed and fixed. Adherent monocytes were counted, and representative pictures are shown (n=3). (B) Using the same protocol, HAEC were stimulated with Reelin (20nM), Reelin+CR-50 or CR-50 only (0.15 μg/ml) for 1 or 24 h (n=3). (C) 4-week-old Cx3cr1-GFP male and female mice were injected intraperitoneally with 100μg lgG (n=13) or CR-50 (n=12). Intravital microscopy was performed 3 days after injections to record numbers and speed of rolling monocytes on the marginal mesenteric vessels. 6 different vessels / mouse were recorded for 10 sec and analyzed; cumulative pictures represent individual images (100 frames/second) integrated over a 10 sec period and stacked; rolling index = monocyte number / velocity. (D,E) After intravital microscopy, immunoblotting was performed on plasma and aorta protein extracts. *p<0.05 and **p<0.01 (t-test or ANOVA).
Using an in vivo mouse model with genetically GFP-labelled monocytes (Cx3cr1-GFP), we tested the ability of CR-50 to reduce monocyte rolling on the vessel wall using intravital microscopy (Movie S1). Three days after a single CR-50 intraperitoneal injection (100μg), the number of rolling monocytes attached to the endothelial surface was greatly reduced and rolling velocity was increased compared to control (mouse IgG; Figure 1C). This resulted in decreased monocyte interaction with the vascular wall as shown by decreased rolling index and cumulative intensity (illustrated by representative cumulative pictures; Figure 1C). We did not observe gender differences in the monocyte adhesion or velocity (Figure S1A), white blood cell number and the vessel area measured by intravital microscopy was similar in both groups (Figure S1B).
Reelin was below the detection limit in the plasma of CR-50-treated mice (Figure 1D) and this correlated with decreased expression of rolling (E-selectin) and adhesion (ICAM1) markers in the aorta (Figure 1E). This is consistent with our previously published findings showing that Reelin increases E-selectin and ICAM-1 expression in endothelial cells (16). These data support Reelin as a therapeutic target for the inhibition of monocyte recruitment and the reduction of perivascular cellular inflammation.
Genetic Reelin deletion protects from paralysis in mice.
To explore the role of Reelin in MS, we used experimental autoimmune encephalomyelitis (EAE) as a mouse model used to study human MS, which closely mimics the inflammation and demyelination of the central nervous system seen in humans. For this purpose, we have crossed Cx3cr1-GFP with genetically GFP-labelled monocytes to ubiquitous Reelin conditional KO mice (Cag-Cre−/+ Relnfl/fl). Cx3cr1-GFP; Relnfl/fl mice carrying or not carrying the Cag-Cre transgene were treated with tamoxifen for 5 days to obtain WT (Cx3cr1-GFP; Cag-Cre− Relnfl/fl) and Reelin cKO (Cx3cr1-GFP; Cag-Cre+ Relnfl/fl) littermates (Figures 2A,B). One week after the first tamoxifen injection, EAE (20) was induced by Myelin Oligodendrocyte Glycoprotein immunization using a standard protocol (Figure 2A). As previously described (20), WT mice developed progressive paralysis starting at the tail at day 13 and reaching a plateau (with slower progression after the exponential phase) at day 16 (Figures 2C,D). Blinded clinical scoring of EAE was paralleled by the weight loss (Figure 2E) and by a decreased upside-down hanging time on a grid (Figure 2F), independently reflecting both paresis and paralysis. EAE index (Figure 2D) was calculated by numerical addition of the EAE clinical score for each mouse, representing the area under the curve. The same calculation was done for the hanging time index (Figure 2F). Based on the same principle, a weight loss index (Figure 2E) was calculated as the numerical addition over the days of the weight loss. For each day, the weight loss itself is defined as the average weight before EAE (from day 7 to 12) minus the mouse weight.
Figure 2. Reelin cKO mice are protected from EAE.
(A) Cx3cr1-GFP; Cag-Cre+or− Relnfl/fl male mice were treated with tamoxifen for 5 days as described (51) to obtain WT (Cx3cr1-GFP; Cag-Cre− Relnfl/fl; n=10) or Reelin cKO (Cx3cr1-GFP; Cag-Cre+ Relnfl/fl; n=9). One week after the first tamoxifen injection, EAE was induced by Myelin Oligodendrocyte Glycoprotein immunization using standard published procedures (20). (B) 21 days after EAE induction the experiment was terminated and plasma Reelin was measured by immunoblotting. (C-G) EAE severity was evaluated daily from day 10 by (D) EAE clinical score (from 0= unaffected to 10=dead), (E) weight loss and (F) hanging test (for a maximum time of 180 sec). (D-F) EAE severity, weight loss and hanging time indexes were calculated for each animal by integrating daily scores over the course of the experiment and represent the area under the curve (AUC). (G) Relative plasma Reelin expression in WT mice (n=9) was correlated to EAE severity index. *p<0.05 and **p<0.01 (2-way ANOVA, t-test or linear correlation with Pearson’s r).
Compared to WT, the Reelin cKO mice were protected from paralysis. The EAE onset and plateau were delayed by 3 and 4 days, respectively, and the severity was greatly reduced in the absence of an exponential progression phase (Figures 2C,D) resulting in no weight loss (Figure 2E) or decreased hanging time (Figure 2F). Intriguingly, variable plasma Reelin expression in the WT group was correlated with EAE index (Figure 2G, plasma collected at day 21). Taking together, these results demonstrate that low plasma Reelin levels correlate with reduced EAE severity and the total absence of Reelin protects from or substantially mitigates initial paralysis and its progression.
Genetic Reelin deletion prevents monocyte extravasation to the central nervous system.
Next, we sought to validate that Reelin deletion protects from EAE by reduction of monocyte recruitment to the CNS and dampened subsequent inflammation. Adhesion proteins were analyzed in the spinal cord, revealing that vascular E-selectin and ICAM-1 protein expression was decreased in Reelin cKO mice (Figures 3A,B). mRNA and protein levels of the Reelin receptor Apoer2 were not affected (Figures S2A,B). Reduced expression of adhesion proteins in Reelin cKO mice was associated with reduced accumulation of inflammatory cells in the spinal cord as shown by Cx3cr1-GFP fluorescence (Figure 3C). Both resident microglia and infiltrating monocytes express Cx3cr1-GFP, therefore the microglia-specific marker lba1 was used to discriminate between the two populations. Reelin cKO mice showed a significant (p<0.05) reduction of total inflammatory cell number (positive for Cx3cr1-GFP, regardless of lba1 expression) and infiltrating monocytes (positive for Cx3cr1-GFP, negative for lba1; Figure 3D). Monocyte infiltration is minimal during EAE-stage 0-1 and rises sharply around stage 3 (1). Remarkably, the Reelin cKO mice were protected from monocyte infiltration at all stages (Figure 3D). Reelin deletion had a moderate effect on microglia activation, as it induced a non-significant reduction of microglia count (Figure S2C). Since infiltrating monocytes are necessary to develop neuroinflammation during EAE (1), our data suggest that Reelin deletion prevents myelin degradation by decreasing monocyte rolling/adhesion on the vascular wall and extravasation into the CNS.
Figure 3. Reduced CNS inflammation in Reelin cKO mice.
(A-D) Cx3cr1-GFP; Cag-Cre+or− Relnfl/fl male mice were treated with tamoxifen for 5 days as described (51) to obtain WT (Cx3cr1-GFP; Cag-Cre− Relnfl/fl; n=10) or Reelin cKO (Cx3cr1-GFP; Cag-Cre+ Relnfl/fl; n=9). One week after the first tamoxifen injection, EAE was induced by Myelin Oligodendrocyte Glycoprotein immunization using standard procedures (20). At 21 days after EAE induction, the experiment was terminated and expression of the indicated proteins was determined in the spinal cord. (A) E-selectin and ICAM-1 were quantified by immunoblotting. (B) ICAM-1 was visualized by immunofluorescence (scale = 100μm). (C) Cx3cr1-GFP fluorescence area was quantified in spinal cord (scale = 200μm). (D) In the Cx3cr1-GFP-positive cell population, the total number of inflammatory cells (Cx3cr1-GFP-positive), monocytes (Cx3cr1-GFP-positive, lba-1-negative; indicated by the arrows) and microglia (Cx3cr1-GFP and lba1 double positive) were visualized by immunofluorescence (scale = 50μm). Bar graphs represent the average cell population per group and per group sorted by EAE score (at 21 days). *p<0.05 and **p<0.01 (t-test or ANOVA).
Prophylactic Reelin inhibition protects from paralysis.
In light of the dramatic effect of genetic Reelin deletion, we reasoned that therapeutic Reelin depletion could be a novel approach to prevent the onset and possibly progression of EAE. For this purpose, we first used the Reelin function blocking antibody CR-50 or mouse lgG (as control) to evaluate its potential use as a prevention treatment in Cx3cr1-GFP mice starting one week before EAE induction (Figures 4A,B). As detailed in Figures 1C-E, CR-50 treatment (intraperitoneal injection of 100μg) effectively cleared Reelin from the circulation, decreasing endothelial adhesion marker expression and strongly reducing monocyte adhesion to the vascular wall. Next, we intraperitoneally injected 100μg of CR-50 and control lgG, twice per week, followed by EAE induction. In naïve mice, CR-50 treatment alone did not affect mouse weight or any other EAE parameters compared to lgG alone (independent of EAE; Figure S3A). Importantly, CR-50 antibody treatment did not detectably decrease Reelin protein amount in the CNS (spinal cord or brain) compared to lgG (Figure S3B,C), owing to the intrinsically low ability of the antibodies to cross the blood-brain barrier. This makes unlikely unintended possible cognitive side effects as a result of disrupted synaptic homeostasis by suppression of Reelin function in the CNS.
Figure 4. Prophylactic Anti-Reelin treatment protects from EAE.
(A) Cx3cr1-GFP male mice were injected intraperitoneally with 100μg of lgG (n=10) or CR-50 (n=9) twice per week. One week after the first injection, EAE was induced by Myelin Oligodendrocyte Glycoprotein immunization using standard procedures (20). (B) 21 days after EAE induction plasma Reelin was measured by immunoblotting. (C-G) EAE severity was evaluated daily starting at day 10 by scoring (D) survival, (E) EAE clinical score (from 0=unaffected to 10=dead), (F) weight loss and (G) grid hanging endurance (for a maximum time of 180 sec). (C-G) EAE severity, weight loss and hanging time indices are calculated for each animal by integrating daily scores over the course of the experiment and represent the area under the curve (AUC). (H) Relative plasma Reelin expression in control lgG injected mice (n=9) was correlated to EAE severity, weight loss and hanging time indexes. *p<0.05 and **p<0.01 (Mantel-Cox test, 2-way ANOVA, t-test or linear correlation with Pearson’s r).
As expected, lgG-treated mice responded to EAE induction and developed progressive paralysis starting at day 12 and reaching a plateau at day 15 (Figures 4C,E). This observer-blinded EAE scoring was confirmed by the weight loss (Figure 4F) and by a decreased hanging time on a grid (Figure 4G), reflecting both paresis and paralysis. Compared to the lgG-treated mice, the CR-50 treatment reduced EAE progression and severity. EAE onset and plateau were delayed by 4 and 5 days, respectively, and the severity was reduced by more than 50% as judged by the EAE index (Figures 4C,E). Accordingly, weight loss (trend statistically non-significant; Figure 4F) and muscle force loss (reflected by hanging time; Figure 4G) were delayed in the CR-50-treated mice. In addition, survival after 21 days was 100% in the CR-50 group compared to 70% in the control group (Figure 4D).
We confirmed the protective effect of CR-50 treatment in a pilot study using a clinically milder mode of EAE induction (by using 50% of the regular dose of Complete Freund’s Adjuvant), which induced only moderate paresis and paralysis with no respiratory depression or death. After 21 days, none of the CR-50 treated mice presented EAE symptoms, as judged by EAE clinical score, weight loss and hanging time, unlike the lgG group, which was clinically affected (Figures S4A-D). This model also showed that both incidence and severity of EAE were reduced by CR-50 treatment.
Finally, as observed with the WT mice in Figure 2G, plasma Reelin expressions in the lgG-treated group correlated with all of the above EAE parameters (EAE index, weight loss index and hanging time index; Figure 4H). Taking together, these results confirm our previous conclusions with the cKO model and establish that plasma Reelin concentrations correlate with EAE severity and that a Reelin-blocking strategy protects from paralysis progression.
Prophylactic Reelin inhibition prevents monocyte extravasation to the central nervous system.
Next, we analyzed the expression of endothelial cell adhesion markers. CR-50 treatment reduced the expression of E-selectin and ICAM-1 in the spinal cord vasculature compared to the lgG group (Figures 5A,B). Consequently, accumulation of inflammatory cells in the spinal cord was reduced as shown by Cx3cr1-GFP fluorescence (Figure 5C). As established in the Reelin cKO model, we used lba1 staining of the spinal cord to discriminate infiltrating monocytes (positive for Cx3cr1-GFP only) from the resident microglia (Cx3cr1-GFP and IBA1 double positive). CR-50 treatment induced a significant (p<0.05) reduction of total inflammatory cell number, driven by a reduction of infiltrating monocytes at all EAE stages (Figure 5D).
Figure 5. Reduced CNS inflammation in anti-Reelin antibody-treated mice.
(A-D) Cx3cr1-GFP male mice were injected intraperitoneally with 100μg of irrelevant lgG (n=10) or CR-50 (n=9) twice per week. One week after the first injection, EAE was induced by Myelin Oligodendrocyte Glycoprotein immunization using standard procedures (20). (A) E-selectin and ICAM-1 protein expression in spinal cord was quantified by immunoblotting. (B) ICAM-1 expression in spinal cord was visualized by immunohistochemistry (scale = 100μm). (C) Cx3cr1-GFP fluorescence area was quantified in spinal cord (scale = 200μm). (D) In the Cx3cr1-GFP-positive cell population, the total number of inflammatory cells (Cx3cr1-GFP-positive), monocytes (Cx3cr1-GFP-positive, lba-1-negative; indicated by the arrows) and microglia (Cx3cr1-GFP and lba1 double positive) were visualized by immunofluorescence (scale = 50μm). Bar graphs represent the cell population average per group and per group sorted by EAE score (at 21 days). *p<0.05 and **p<0.01 (t-test or ANOVA).
Therapeutic Reelin inhibition reduces paralysis severity and promotes recovery.
To test whether anti-Reelin strategies can be translationally applied to human MS, we performed another series of experiments in which we initiated anti-Reelin treatment after the onset of EAE symptoms. Starting at the first day of clinical signs of paralysis (determined individually for each mouse), the Reelin function blocking antibody CR-50 or mouse control lgG were injected twice per week by intraperitoneal injection (100μg; Figure 6A). lgG or CR-50 treatment was randomly assigned among littermates at day 0 and mice showing paralysis starting after day 14 were excluded to maintain consistent groups with similar treatment duration. As expected, CR-50 effectively reduced plasma Reelin concentration (Figure 6B), while the in the lgG group Reelin increased during the course of EAE. This result is in agreement with the elevated Reelin concentrations we observed during MS in relapsing patients (Table 1).
Figure 6. Therapeutic Anti-Reelin treatment alleviates EAE progression.
(A) Cx3cr1-GFP male littermates were randomly assigned in lgG or CR-50 group and EAE was induced by Myelin Oligodendrocyte Glycoprotein immunization using standard procedures (20). Starting from the first day of paralysis, mice were injected intraperitoneally with 100μg of lgG (n=16) or CR-50 (n=14) twice per week. (B) 21 days after EAE induction plasma Reelin was measured by immunoblotting. (C-F) EAE severity was evaluated daily starting at day 10 by scoring (C) survival, (D) EAE clinical score (from 0=unaffected to 10=dead), (E) weight loss and (F) grid hanging endurance (for a maximum time of 180 sec). (D-F) EAE severity, weight loss and hanging time indexes are calculated for each animal by integrating daily scores over the course of the experiment and represent the area under the curve (AUC). EAE, weight and hanging time recovery are calculated for each mouse as the difference between the maximum (for EAE score) or minimum (for weight and hanging time) and day 21. (G) Fluorescence-activated cell sorting (FACS) was performed on spinal cord (n=8 lgG and n=8 CR-50 mice) to determine infiltration of monocytes (CD11+), B cells (CD19+), T helper cells (CD4+) and T cells (CD8+). *p<0.05 and **p<0.01 (Mantel-Cox test, 2-way ANOVA or t-test).
While lgG-treated mice responded to EAE induction and developed progressive paralysis (Figures 6C-F) with 75% survival (Figure 6C), mirroring the results in our previous prevention study, all the CR-50-treated mice survived (Figure 6C) and showed greatly mitigated paralysis severity (Figure 6D). Weight loss (trend statistically non-significant; Figure 6E) and hanging time (p<0.05, Figure 6F) were also improved by the CR-50 treatment. Importantly, CR-50 significantly improved the recovery, as judged by EAE score, weight and hanging time recovery calculated for each mouse as the difference between the maximum (for EAE score) or minimum (for weight and hanging time) and day 21 (Figures 6D-F).
Finally, in agreement with the histology performed in the Reelin cKO (Figures 3C,D) and the prophylactic treatment study (Figure 5C,D), fluorescence-activated cell sorting (FACS, Figure 6G, S5G) confirmed that CR-50 treatment decreased the infiltration of monocytes (CD11+) into the CNS, as well as B cells (CD19+), T helper cells (CD4+) and T cells (CD8+) (trends not reaching statistical significance).
Taken together, we have shown that genetic, prophylactic or therapeutic depletion of circulating Reelin prevents or mitigates EAE in mice by diminishing vascular adhesion protein expression, thereby potently reducing monocyte rolling on the vascular wall, infiltration into the CNS and myelin degradation.
DISCUSSION
In this study, we have shown that genetic and therapeutic neutralization of circulating Reelin can prevent chronic inflammatory conditions that depend upon monocyte extravasation, such as multiple sclerosis, by modulating endothelial permeability (Figure 7). For the first time, we have established that circulating Reelin in humans is increased during MS relapse and reduced to control levels in remitting patients (Table 1). We show that human Reelin promotes monocyte adhesion to endothelial cells and that a Reelin blocking antibody can prevent this adhesion. This result was confirmed using intravital microscopy, where anti-Reelin antibody-treated mice displayed a significant reduction of monocyte adhesion to the vascular wall, accompanied by decreased expression of adhesion molecules (ICAM-1, E-selectin) in the aorta (Figure 1). In a mouse EAE model, both genetic and pharmacologic (antibody-mediated) Reelin depletion reduced paralysis progression (Figures 2 and 4). Ex vivo analysis revealed that Reelin-depleted mice (genetic or antibody-mediated) had reduced adhesion marker expression in the vasculature of the spinal cord accompanied by a decrease of inflammatory cell numbers in the grey and white matter (Figures 3 and 5). This was mainly due to decreased monocyte infiltration in the absence of Reelin. Finally, in a preclinical proof-of-concept study, we have demonstrated disease protection by application of a Reelin neutralizing antibody where CR-50 was given at the onset of EAE symptoms, resulting in diminished neuroinflammation, paralysis and improved recovery (Figure 6). Taken together with the human and intravital microscopy data, the efficacy of anti-Reelin antibody intervention in the standard mouse EAE model indicates that Reelin depletion acts by reducing monocyte infiltration, thereby preventing neuroinflammation and thus progression of paralysis.
Figure 7. Reelin depletion protects from multiple sclerosis by reducing monocyte extravasation and inflammation.
This mechanistic model incorporates the findings described in this article and the literature to date, as discussed. Reelin promotes the expression of vascular adhesion proteins, thereby increasing monocyte adhesion / extravasation, inflammation and consequently demyelination. Both genetic or therapeutic Reelin depletion prevent this inflammatory cascade and thus paralysis in a murine EAE model. We surmise that the same mechanism is conserved in humans where Reelin also promotes monocyte adhesion to vascular endothelial cells in vitro.
Recruitment of circulating leukocytes is a general mechanism of tissue inflammation which proceeds along a series of well-defined steps (21-23): 1) chemoattraction upon cytokine and chemokine release, 2) transient (rolling) adhesion to endothelial cells mediated by carbohydrate/receptor recognition, such as E-selectin, 3) tight adhesion through adhesion proteins, such as integrins, ICAM-1 or VCAM-1, and 4) endothelial transmigration (=diapedesis). We have shown that Reelin depletion decreases adhesion protein expression on endothelial cells, resulting in decreased monocyte/endothelium adhesion and diminished monocyte extravasation. This is mediated by the binding of Reelin to its receptor Apoer2/Lrp8 which increases NF-κB target gene expression (16) in endothelial cells and integrin β1 in multiple myeloma cells (24, 25).
Under normal conditions, the blood–brain barrier effectively regulates the passage of immune cells into the CNS. However, under pathological conditions, monocytes infiltrate the CNS where they, in concert with activated microglia, promote inflammatory demyelination (26) resulting in demyelination and paralysis (1, 27, 28). Targeted microglia inactivation of TAK1, while sparing infiltrating monocytes, prevents both initial and relapsing paralysis (29). Recently, single-cell profiling confirmed context-dependent subsets of CNS-resident macrophages, while monocyte-derived cells were highly diverse and the primary antigen presenters (30). Taken together, these studies imply that both lineages are required, probably at different stages, with early microglia activation followed by monocyte infiltration, to drive neurological damage through demyelination and progression of paralysis (27). This proposed mechanism agrees well with our results, where Reelin depletion specifically blocks monocyte infiltration, yet is sufficient to inhibit EAE progression.
In our control groups, we observed a striking correlation between plasma Reelin concentration and EAE severity score. Interestingly, a Reelin SNP is associated with MS severity and age of the onset in two GWAS analyses (10, 11), but how this SNP affects Reelin function has yet to be revealed. Although the primary source for peripheral Reelin by far is the liver where Reelin is secreted by the hepatic stellate cells (31, 32), we cannot exclude a minor contribution from other organs (such as kidney). Regardless of the origin, the effect of Reelin would be expected to manifest itself equally on the vasculature. Another limitation of this study is the use of the murine EAE model to study human MS, as this model reflects only some aspects and mechanisms of MS. This study is thus a first pre-clinical step towards additional clinical studies before translational application to human diseases can proceed.
Functions of Reelin in coagulation have also been reported (33, 34) and fibrinogen production modulates CNS autoimmunity, demyelination and MS (35-37) raising the possibility that platelet-derived Reelin might locally alter endothelial permeability. A role for CNS-produced Reelin during EAE is unlikely, as the peripheral antibody-mediated depletion does not affect CNS Reelin levels to any measurable extent (Figure S3B,C), yet potently protects against paralysis and disease progression. Preserving Reelin expression in the CNS is important as Reelin is not only essential for neuronal migration and positioning in the developing brain (13-15) but also in the adult brain where it modulates synaptic plasticity (38, 39), migration of neuroblasts (40), as well as dendrite (41) and dendritic spine (42) formation.
Currently, there are several disease-modifying agents (DMA) approved for relapsing forms of MS. Alpha4-integrin is an adhesion molecule that is widely expressed on all leukocytes (43-45). It is recognized by natalizumab, a humanized monoclonal antibody that potently blocks alpha4-integrin and thereby prevents the interaction with its main ligands, Vascular Cell Adhesion Molecule (VCAM)-1 and fibronectin (46). Natalizumab is difficult to titrate and thus tends to indiscriminately abolish the ability of immune cells to enter the brain and spinal cord to perform their physiological surveillance functions (47-49). This excessive immunosuppression greatly increases the risk for progressive multifocal leukoencephalopathy (PML), a viral infection of the brain that is often fatal (8). Since anti-Reelin interventions target a different mode of action, its predicted broader with more balanced effects on CNS immune surveillance could conceivably mitigate such complications and potentially reduce PML incidence. As our studies have shown (Figure S3G,H), Reelin depletion normalizes, but does not completely abolish, the expression of vascular adhesion markers, thereby preserving protective physiological diapedesis functions while dampening excessive inflammation.
In conclusion, in the current study we have exploited a function of Reelin in the regulation of vascular permeability for leukocyte extravasation to devise a novel strategy for combating autoimmunity driven neuroinflammation in MS. Our study identifies Reelin as a novel therapeutic target for reducing the extravasation of monocytes and - more generally - leukocytes, thereby establishing an alternative endothelial-specific tunable immunomodulatory approach for the treatment of MS by selectively reducing endothelial permeability and infiltration of inflammatory cells into the CNS. Patients with progressive forms of MS, for which there currently are no effective treatment options (6, 7), represent more than half of the 2.3 million individual affected with MS worldwide (50). Therefore, identifying effective therapeutic interventions for all forms of MS represents a significant unmet need (9). Beyond MS, anti-Reelin strategies would be expected to be similarly effective for the treatment of other chronic inflammatory syndromes that depend upon excessive leukocyte extravasation such as atherosclerosis, arthritis or Crohn’s disease.
MATERIALS AND METHODS
Study design
The purpose of this study was to explore a novel therapeutic approach targeting endothelial adhesion and permeability to prevent monocyte extravasation instead of directly targeting the immune system. We hypothesized that genetic and therapeutic neutralization of circulating Reelin would reduce endothelium/monocyte adhesion and diapedesis, thus preventing chronic inflammatory syndromes that depend upon monocyte extravasation such as MS. These objectives were addressed using a human MS cohort, HAEC and U937 cultures, mouse models for intravital microscopy and EAE. Power analysis was used to determine a range for the sample size and no outliers were excluded. Animals were randomized and EAE was evaluated by two blinded operators. Biological replicates are specified in each figure legend.
Human MS cohort.
Serum of anonymous patients with relapsing-remitting MS (RRMS) in remission, RRMS during an acute relapse and healthy controls were obtained from UT Southwestern Medical Center MS tissue repository (authorization #STU022011-211, Dallas, Texas). The study presented here has been approved by the “Human Research Protection Program Office” as “Not Human Research” which doesn’t require IRB approval.
Cx3cr1-GFP mice.
Cx3cr1-GFP mice (B6.129P-Cx3cr1tm1Litt/J) were purchased from the Jackson Laboratories (Stock No. 005582). These mice express EGFP in monocytes, dendritic cells, NK cells, and brain microglia under the control of the endogenous Cx3cr1 locus. Cx3cr1-GFP monocytes downregulate GFP expression upon differentiation into macrophages.
For intra-vital microscopy, 4-week-old Cx3cr1-GFP males and females received one injection (100μg, intra-peritoneally) of lgG or CR-50 antibodies. For EAE, 7-week-old Cx3cr1-GFP male mice were injected (intraperitoneally) with 100μg of lgG or CR-50 twice per week.
Cx3cr1-GFP; Cag-Cre Relnfl/fl mice.
Cx3cr1-GFP and Cag-Cre Relnfl/fl mice were crossed to obtain Reelin conditional KO (cKO) with monocyte labeled with GFP. Cag-Cre Relnfl/fl mice were obtain previously by crossing mice carrying the loxP-targeted Reln gene with the Cag-Cre mice from the Jackson Laboratories (Stock No. 004682).
To induce Cre-mediated DNA recombination, 7-week-old Cx3cr1-GFP; Cag-Cre Relnfl/fl mice (with or without the Cag-Cre transgene) were intraperitoneally injected with tamoxifen (0.135 mg/g) dissolved in sunflower oil for five consecutive days to obtain WT and Reelin cKO mice (51).
Statistics.
For cell culture, each condition was tested at least in duplicate (unless specified differently), and all experiments were repeated at least 3 times at different passages. For animals, the “n” values are specified in each legend. The software GraphPad Prism was used to run all the statistical analysis. Values from multiple experiments are expressed as mean±SEM. Normality was tested using the Kolmogorov-Smirnov test. Statistical significance was determined for multiple comparisons using one-way analysis of variance (ANOVA) followed by Turkey multiple comparison (for normal distribution) or Kruskal-Wallis (for non-normal distribution) test. Student’s t-test was used for comparisons of two groups. To test the different evolution between 2 groups over time, a 2-way ANOVA was used (the asterisk on the curves represents significative difference for the treatment or genotype). The correlations were calculated by linear regression (Pearson r). The survival curves were tested with Log-rank (Mantel-Cox test). p<0.05 was considered significant.
Supplementary Material
Supplementary Figures S1. Intravital microscopy in anti-Reelin antibody-treated mice (refers to Figure 1).
Supplementary Figure 2. Apoer2 (Reelin receptor) expression in spinal cord (refers to Figure 3).
Supplementary Figure 3. Anti-Reelin antibody-treated mice are protected from EAE (refers to Figure 4).
Supplementary Figure 4. Anti-Reelin antibody-treated mice are protected from moderate EAE (refers to Figure 4).
Supplementary Figure 5. Flow-based immuno-phenotyping gating strategy (refers to Figure 6).
Supplementary Movie 1. CR-50 inhibits Reelin-dependent monocyte rolling on endothelium (refers to Figure 1).
ACKNOWLEDGEMENTS
We thank Anna Middleton, Tamara Terrones, Alisa Rodriguez, Alexander Brennan, Sharvari Ghayal and Rehana Hussain for their technical assistance. We also thank Dr. Katsuhiko Mikoshiba for originally providing the CR-50 hybridoma.
Funding
L.C. was supported by postdoctoral fellowship grants from DFG (CA 1303/1-1). P.W.S. and C.M. were supported by grants from NIH (R01-HL131597 and R01-HL109604 respectively). O.S. was supported by a grant from Sanofi Genzyme. J.H. was supported by grants from the NHLBI (R37 HL063762), NIA (RF AG053391), the NINDS and NIA (R01 NS093382), BrightFocus A2016396S, the Bluefield Project to Cure FTD and a Harrington Scholar Innovator Award (2019).
Footnotes
Declaration of interests
L.C., M.Z.K. and J.H. are shareholders of Reelin Therapeutics, Inc.
L.C. and J.H. are co-inventors of a patent related to anti-Reelin strategies (application number 15/763,047 and publication number 20180273637).
REFERENCES
- 1.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FMV, Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat. Neurosci 14, 1142–1149 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Benveniste EN, Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J. Mol. Med 75, 165–173 (1997). [DOI] [PubMed] [Google Scholar]
- 3.Bauer J, Sminia T, Wouterlood FG, Dijkstra CD, Phagocytic activity of macrophages and microglial cells during the course of acute and chronic relapsing experimental autoimmune encephalomyelitis. J. Neurosci. Res 38, 365–375 (1994). [DOI] [PubMed] [Google Scholar]
- 4.Dendrou CA, Fugger L, Friese MA, Immunopathology of multiple sclerosis. Nat. Rev. Immunol 15, 545–558 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H, Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann. Neurol 47, 707–717 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Feinstein A, Freeman J, Lo AC, Treatment of progressive multiple sclerosis: what works, what does not, and what is needed. Lancet Neurol. 14, 194–207 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Ontaneda D, Fox RJ, Progressive multiple sclerosis. Curr. Opin. Neurol 28, 237–243 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cutter GR, Stüve O, Does risk stratification decrease the risk of natalizumab-associated PML? Where is the evidence? Mult. Scler 20, 1304–1305 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Thompson AJ, A much-needed focus on progression in multiple sclerosis. Lancet Neurol. 14, 133–135 (2015). [DOI] [PubMed] [Google Scholar]
- 10.International Multiple Sclerosis Genetics Consortium, Genome-wide association study of severity in multiple sclerosis. Genes Immun. 12, 615–625 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baranzini SE, Wang J, Gibson RA, Galwey N, Naegelin Y, Barkhof F, Radue E-W, Lindberg RLP, Uitdehaag BMG, Johnson MR, Angelakopoulou A, Hall L, Richardson JC, Prinjha RK, Gass A, Geurts JJG, Kragt J, Sombekke M, Vrenken H, Qualley P, Lincoln RR, Gomez R, Caillier SJ, George MF, Mousavi H, Guerrero R, Okuda DT, Cree BAC, Green AJ, Waubant E, Goodin DS, Pelletier D, Matthews PM, Hauser SL, Kappos L, Polman CH, Oksenberg JR, Genome-wide association analysis of susceptibility and clinical phenotype in multiple sclerosis. Hum. Mol. Genet 18, 767–778 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Arcangelo G, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, Curran T, Reelin is a secreted glycoprotein recognized by the CR-50 monoclonal antibody. J. Neurosci 17, 23–SI (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herz J, Chen Y, Reelin, lipoprotein receptors and synaptic plasticity. Nat. Rev. Neurosci 7, 850–859 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Tissir F, Goffinet AM, Reelin and brain development. Nat. Rev. Neurosci 4, 496–505 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J, Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 97, 689–701 (1999). [DOI] [PubMed] [Google Scholar]
- 16.Ding Y, Huang L, Xian X, Yuhanna IS, Wasser CR, Frotscher M, Mineo C, Shaul PW, Herz J, Loss of Reelin protects against atherosclerosis by reducing leukocyte-endothelial cell adhesion and lesion macrophage accumulation. Science Signaling. 9, ra29–ra29 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, Noelle RJ, Becher B, Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat. Med 11, 328–334 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Hickey WF, Kimura H, Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 239, 290–292 (1988). [DOI] [PubMed] [Google Scholar]
- 19.Ogawa M, Miyata T, Nakajima K, Yagyu K, Seike M, Ikenaka K, Yamamoto H, Mikoshiba K, The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron. 14, 899–912 (1995). [DOI] [PubMed] [Google Scholar]
- 20.Bittner S, Afzali AM, Wiendi H, Meuth SG, Myelin oligodendrocyte glycoprotein (MOG35-55) induced experimental autoimmune encephalomyelitis (EAE) in C57BL/6 mice. J Vis Exp (2014), doi: 10.3791/51275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schnoor M, Alcaide P, Voisin M-B, van Buul JD, Crossing the Vascular Wall: Common and Unique Mechanisms Exploited by Different Leukocyte Subsets during Extravasation. Mediators Inflamm. 2015, 946509 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore KJ, Tabas I, Macrophages in the pathogenesis of atherosclerosis. Cell. 145, 341–355 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan SY, Shen Q, Rigor RR, Wu MH, Neutrophil transmigration, focal adhesion kinase and endothelial barrier function. Microvasc. Res 83, 82–88 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin L, Yan F, Zhao D, Lv M, Liang X, Dai H, Qin X, Zhang Y, Hao J, Sun X, Yin Y, Huang X, Zhang J, Lu J, Ge Q, Reelin promotes the adhesion and drug resistance of multiple myeloma cells via integrin β1 signaling and STAT3. Oncotarget. 7, 9844–9858 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin L, Zhang X, Cao L, An Q, Hao J, Zhang Y, Jin R, Chang Y, Huang X, Lu J, Ge Q, Reelin promotes adhesion of multiple myeloma cells to bone marrow stromal cells via integrin β1 signaling. J Cancer. 8, 2212–2222 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falcãio AM, van Bruggen D, Marques S, Meijer M, Jäkel S, Agirre E, null Samudyata, Floriddia EM, Vanichkina DP, Ffrench-Constant C, Williams A, Guerreiro-Cacais AO, Castelo-Branco G, Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat. Med 24, 1837–1844(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Epelman S, Lavine KJ, Randolph GJ, Origin and functions of tissue macrophages. Immunity. 41, 21–35 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ginhoux F, Jung S, Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol 14, 392–404 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Goldmann T, Weghofer P, Müller PF, Wolf Y, Varol D, Yona S, Brendecke SM, Kierdorf K, Staszewski O, Datta M, Luedde T, Heikenwalder M, Jung S, Prinz M, A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat. Neurosci 16, 1618–1626 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Jordão MJC, Sankowski R, Brendecke SM, null Sagar, Locatelli G, Tai Y-H, Tay TL, Schramm E, Armbruster S, Hagemeyer N, Groß O, Mai D, Çiçek Ö, Falk T, Kerschensteiner M, Grün D, Prinz M, Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science. 363 (2019), doi: 10.1126/science.aat7554. [DOI] [PubMed] [Google Scholar]
- 31.Smalheiser NR, Costa E, Guidotti A, Impagnatiello F, Auta J, Lacor P, Kriho V, Pappas GD, Expression of reelin in adult mammalian blood, liver, pituitary pars intermedia, and adrenal chromaffin cells. Proc. Natl. Acad. Sci. U.S.A 97, 1281–1286 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carotti S, Perrone G, Amato M, Vespasiani Gentilucci U, Righi D, Francesconi M, Pellegrini C, Zalfa F, Zingariello M, Picardi A, Onetti Muda A, Morini S, Reelin expression in human liver of patients with chronic hepatitis C infection. Eur J Histochem. 61, 2745 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tseng W-L, Chen T-H, Huang C-C, Huang Y-H, Yeh C-F, Tsai H-J, Lee H-Y, Kao C-Y, Lin S-W, Liao H-R, Cheng J-C, Tseng C-P, Impaired thrombin generation in Reelin-deficient mice: a potential role of plasma Reelin in hemostasis. J. Thromb. Haemost 12, 2054–2064 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Gowert NS, Krüger I, Klier M, Donner L, Kipkeew F, Gliem M, Bradshaw NJ, Lutz D, Köber S, Langer H, Jander S, Jurk K, Frotscher M, Korth C, Bock HH, Elvers M, Loss of Reelin protects mice against arterial thrombosis by impairing integrin activation and thrombus formation under high shear conditions. Cell. Signal 40, 210–221 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Ryu JK, Petersen MA, Murray SG, Baeten KM, Meyer-Franke A, Chan JP, Vagena E, Bedard C, Machado MR, Rios Coronado PE, Prod’homme T, Charo IF, Lassmann H, Degen JL, Zamvil SS, Akassoglou K, Blood coagulation protein fibrinogen promotes autoimmunity and demyelination via chemokine release and antigen presentation. Nat Commun. 6, 8164 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nave K-A, Ehrenreich H, A bloody brake on myelin repair. Nature. 553, 31–32 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Petersen MA, Ryu JK, Chang K-J, Etxeberria A, Bardehle S, Mendiola AS, Kamau-Devers W, Fancy SPJ, Thor A, Bushong EA, Baeza-Raja B, Syme CA, Wu MD, Rios Coronado PE, Meyer-Franke A, Yahn S, Pous L, Lee JK, Schachtrup C, Lassmann H, Huang EJ, Han MH, Absinta M, Reich DS, Ellisman MH, Rowitch DH, Chan JR, Akassoglou K, Fibrinogen Activates BMP Signaling in Oligodendrocyte Progenitor Cells and Inhibits Remyelination after Vascular Damage. Neuron. 96, 1003–1012.e7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weeber EJ, Beffert U, Jones C, Christian JM, Forster E, Sweatt JD, Herz J, Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J. Biol. Chem 277, 39944–39952 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Beffert U, Weeber EJ, Durudas A, Qiu S, Masiulis I, Sweatt JD, Li W-P, Adelmann G, Frotscher M, Hammer RE, Herz J, Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron. 47, 567–579 (2005). [DOI] [PubMed] [Google Scholar]
- 40.Gong C, Wang T-W, Huang HS, Parent JM, Reelin regulates neuronal progenitor migration in intact and epileptic hippocampus. J. Neurosci 27, 1803–1811 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niu S, Renfro A, Quattrocchi CC, Sheldon M, D’Arcangelo G, Reelin promotes hippocampal dendrite development through the VLDLR/ApoER2-Dab1 pathway. Neuron. 41, 71–84 (2004). [DOI] [PubMed] [Google Scholar]
- 42.Niu S, Yabut O, D’Arcangelo G, The Reelin signaling pathway promotes dendritic spine development in hippocampal neurons. J. Neurosci 28, 10339–10348 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frenette PS, Wagner DD, Adhesion molecules--Part 1. N. Engl. J. Med 334, 1526–1529 (1996). [DOI] [PubMed] [Google Scholar]
- 44.Frenette PS, Wagner DD, Adhesion molecules--Part II: Blood vessels and blood cells. N. Engl. J. Med 335, 43–45 (1996). [DOI] [PubMed] [Google Scholar]
- 45.Mastorakos P, McGavem D, The anatomy and immunology of vasculature in the central nervous system. Sci Immunol. 4 (2019), doi: 10.1126/sciimmunol.aav0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shirani A, Stüve O, Natalizumab for Multiple Sclerosis: A Case in Point for the Impact of Translational Neuroimmunology. J. Immunol 198, 1381–1386 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Stüve O, Marra CM, Jerome KR, Cook L, Cravens PD, Cepok S, Frohman EM, Phillips JT, Arendt G, Hemmer B, Monson NL, Racke MK, Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann. Neurol 59, 743–747 (2006). [DOI] [PubMed] [Google Scholar]
- 48.Stüve O, Marra CM, Bar-Or A, Niino M, Cravens PD, Cepok S, Frohman EM, Phillips JT, Arendt G, Jerome KR, Cook L, Grand’Maison F, Hemmer B, Monson NL, Racke MK, Altered CD4+/CD8+ T-cell ratios in cerebrospinal fluid of natalizumab-treated patients with multiple sclerosis. Arch. Neurol 63, 1383–1387 (2006). [DOI] [PubMed] [Google Scholar]
- 49.del Pilar Martin M, Cravens PD, Winger R, Frohman EM, Racke MK, Eagar TN, Zamvil SS, Weber MS, Hemmer B, Karandikar NJ, Kleinschmidt-DeMasters BK, Stüve O, Decrease in the numbers of dendritic cells and CD4+ T cells in cerebral perivascular spaces due to natalizumab. Arch. Neurol 65, 1596–1603 (2008). [DOI] [PubMed] [Google Scholar]
- 50.Browne P, Chandraratna D, Angood C, Tremlett H, Baker C, Taylor BV, Thompson AJ, Atlas of Multiple Sclerosis 2013: A growing global problem with widespread inequity. Neurology. 83, 1022–1024 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lane-Donovan C, Philips GT, Wasser CR, Durakoglugil MS, Masiulis I, Upadhaya A, Pohlkamp T, Coskun C, Kotti T, Steller L, Hammer RE, Frotscher M, Bock HH, Herz J, Reelin protects against amyloid β toxicity in vivo. Sci Signal. 8, ra67 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures S1. Intravital microscopy in anti-Reelin antibody-treated mice (refers to Figure 1).
Supplementary Figure 2. Apoer2 (Reelin receptor) expression in spinal cord (refers to Figure 3).
Supplementary Figure 3. Anti-Reelin antibody-treated mice are protected from EAE (refers to Figure 4).
Supplementary Figure 4. Anti-Reelin antibody-treated mice are protected from moderate EAE (refers to Figure 4).
Supplementary Figure 5. Flow-based immuno-phenotyping gating strategy (refers to Figure 6).
Supplementary Movie 1. CR-50 inhibits Reelin-dependent monocyte rolling on endothelium (refers to Figure 1).