Abstract
Background
Rheumatoid arthritis‐associated interstitial lung disease (RA‐ILD) is an irreversible pathologic condition of unknown cause, commonly involving the joint and the lung with variable amounts of fibrotic change. In contrast to rheumatoid arthritis or other chronic interstitial lung diseases such as interstitial pulmonary fibrosis, there is so far no extensively accepted or implemented animal model for this disease.
Aims
To provide guidance for those who are investigating the pathogenesis of RA‐ILD with animal models.
Materials and Methods
An analysis of papers from PubMed during 1978‐2020.
Results
We outline the present status quo for animal models of RA‐ILD about their modeling methods and pathogenesis, compare their pros and cons with respect to their ability to mimic the clinical and histological features of human disease and discuss their applicability for future research.
Discussion
There is no doubt that these animal models do provide valuable information relating to the pathogenesis of RA‐ILD and the development of effective therapeutic drugs. Nevertheless, these animal models can not entirely recapitulate clinical pathology and have some limitations in experimental research application. Therefore, it should be emphasized that we should improve and explore animal models in more accordance with the pathogenesis and clinical characteristics of human RA‐ILD.
Conclusion
These established animal models of the disease can significantly progress our understanding of the etiology of RA‐ILD, the fundamental mechanisms of its pathogenesis and the identification of new bio‐markers, and can contribute to the development and implementation of novel treatment strategies.
Keywords: animal models, autoimmune disease, interstitial lung disease, lung inflammation, rheumatoid arthritis
The manuscript summarizes the characteristics of animal models about rheumatoid arthritis‐associated interstitial lung disease (RA‐ILD), which can provide reference for those researchers who are interested in investigating the pathogenesis of RA‐ILD.
1. INTRODUCTION
Rheumatoid arthritis‐associated interstitial lung disease (RA‐ILD) is a systematic autoimmune disease characterized by joint lesions 1 and diffuse interstitial pulmonary changes, 2 accompanying poor prognosis. 3 , 4 , 5 The possibility of developing RA‐ILD increases over time after diagnosis of rheumatoid arthritis (RA) and the median survival of RA‐ILD is about 2.6–3.5 years. 4 , 6 Usual interstitial pneumonitis (UIP) and nonspecific interstitial pneumonitis (NSIP), these two subtypes predominate in RA‐ILD. 7 , 8 , 9 It includes other relatively rare subtypes: desquamative interstitial pneumonitis (DIP), respiratory bronchiolitis‐associated interstitial lung disease (RB‐ILD), organizing pneumonia (OP), lymphocytic interstitial pneumonitis, pleuroparenchymal fibroelastosis, and diffuse alveolar damage, according to its histological features. 7 , 10 The UIP pattern featuring heterogeneity tends to display fibrotic disease process with subpleural and paraseptal matrix deposition, 11 , 12 , 13 , 14 while the NSIP pattern featuring homogeneity trends to appear inflammatory infiltration in nature with alveolar wall thickening. 12 , 13 , 15 , 16
In patients of RA‐ILD, RA usually develops before onset of ILD. 9 Yet, ILD precedes onset of RA 17 , 18 , 19 , 20 and both conditions occur simultaneously in some patients of RA‐ILD. 9 The pathogenesis of RA‐ILD is still unclear and there is a lack of effective treatment. Therefore, it is of great importance to establish appropriate experimental animal models of RA‐ILD.
At present, common methods to induce RA‐ILD include adjuvant, Collagen Type II (CII), combination of organic dust extract (ODE) and CII, zymosan, aberrant expression of specific genes through transgenic systems or gene mutation. In this review, we have a relatively comprehensive introduction for present animal models of RA‐ILD to provide reference for the basic research, drug screening and efficacy evaluation of RA‐ILD.
2. ANIMAL MODELS OF RA‐ILD
2.1. Animal model of RA‐ILD induced by immune stimulants
2.1.1. Complete Freund's adjuvant
Freund's adjuvant is the common adjuvant, which is widely used to induce onset arthritis in animal models. It includes Freund's complete adjuvant (FCA) and Freund's incomplete adjuvant (FIA). The difference between FCA and FIA is that FCA contains Mycobacterium tuberculosis in the form of inactivated or dead BCG vaccine. 21 It also means that FCA is comprised of FIA and M. tuberculosis.
Adult Wistar rats were given a single injection of 0.1 ml FCA into the right rear paw. 22 , 23 After the injection of adjuvant, the first joint swelling appeared from 12 h to Day 3. Likely, body weight increased until Day 17 and then decreased until the termination of experiment. The secondary joint swelling occurred on Day 18 until the end Day of 28. After 20 days, the FCA‐treated rats began to manifest erythema and paw swelling at the contralateral joint. The lung of FCA‐treated rats developed the thickened alveolar walls and inflammatory cell infiltration comprised by lymphocytes, eosinophilic granulocytes, and macrophages on Day 21. Furthermore, the rats displayed apparent deposition of collagen fibers and pleural thickening in the lung on Day 28 with increased serum and pulmonary levels of pro‐inflammatory cytokines such as tumor necrosis factor‐α (TNF‐α), interleukin‐6 (IL‐6), and IL‐1β, in addition to high pulmonary transforming growth factor (TGF)‐β1 expression. 22 , 23
The histopathological pattern of this animal model is similar to the clinical characteristics of RA‐ILD. The mechanism in this animal model is that FCA may elicit inflammation in joints and lung due to macrophage phagocytosis and circulation. 21 Merit is that this model is characterized by easy operation, short modeling time and low experiment cost. In addition, inflammation and fibrosis were mainly distributed in the pleural and subpleural area, which was similar to the pathological distribution of clinical RA‐ILD especially RA associated with UIP. The demerit is that this animal model of the disease has a certain self‐limitation. 24
2.1.2. Bovine Collagen Type II
CII is extensively used to induce onset of arthritis in RA animal models. 25 , 26 Presumably, anti‐CII antibodies react with the CII that is a major protein in cartilage, causing joint inflammation and bone erosion. Several investigators have found that anti‐citrullinated protein antibodies (ACPAs), reacting with various citrullinated proteins, can not only be generated at joints, but also in the lung 27 and ACPAs are closely related to RA‐ILD. 28 , 29 However, the relevant pulmonary manifestation after CII immunization has scarcely been reported. Therefore, the researcher conducted this experiment and found it suitable for bovine Collagen Type II (bCII) to induce onset of RA‐ILD in DBA/1 mice.
On Day 0, the DBA/1 mice were given an injection of 50 μg of bCII emulsified in CFA into the base of the tail. 30 On Day 21,105 and 161, the mice were given booster injections of 50 μg of bCII emulsified in IFA. On Day 7, the CII‐treated mice began to develop inflammatory cell aggregates in the subpleural region accompanying increasing anti‐cyclic citrullinated peptide (CCP) antibodies associated with ACPAs. Anti‐CII antibodies began to develop and inflammatory arthritis occurred on Day 14. Thus, this indicated that lung inflammation preceded the onset of arthritis in CII‐treated mice. On Day 49, the mice developed an increase in ankle thickness. There were increased inflammatory cytokines of IL‐1β, IL‐6, IFN‐γ, TNF‐α levels, IL‐4, IL‐17, CCL3, and CCL4, and high levels of CD4+ T cells CD8+ T cells, Th1 cells, Th2 cells, Th17 cells, CD4+CD25+Foxp3+ regulatory T cells, and macrophages in the lungs of CIA mice. Of note, CD11bhi macrophages, regarded as interstitial macrophages, 31 , 32 , 33 dominated the inflammatory cellular infiltration. In addition, ACPAs and Complement 3 (C3) were deposited in the subpleural region. However, on Day 182 or 189, lung inflammation could be reversed and there is no indication of pulmonary fibrosis.
The merit about this model is that it can be used for initiation phase of RA‐ILD to understand how citrullination and ACPAs interacts. In addition, it can reflect the inflammation phase of RA‐ILD due to inflammation area is mainly distributed in subpleural area, which is similar to the pathological distribution of preclinical RA‐associated UIP. Breach of tolerance and generation of autoantibodies toward self and collagen make the model the gold standard for studies. 34 However, there is no fibrosis and inflammation is self‐limiting in the lung. Moreover, this model cannot replicate the characteristics of chronic and irreversible progress of human RA‐ILD.
2.1.3. Chicken CII and ODE
Environmental factors play an important role in RA‐ILD development, especially in long‐term and repetitive inhalation relevant to occupational exposure of farmers and workers 35 who were inevitably faced with OD. Notably, components of OD were trace metals, predominance of gram‐positive bacteria (98%), and high muramic acid, 36 , 37 which can cause macrophage dysfunction, airway, and lung parenchymal inflammation. 36 Simultaneously, lots of research has shown that CII can induce onset of inflammatory arthritis in the murine. Considering these, combination of the ODE and chicken CII resulted in specific establishment of an animal model of RA‐ILD.
On Day 1, DBA/1J strain mice were given a subcutaneous injection of 2 mg/ml of chick CII emulsified in FCA. 38 At Week 3, the mice were given the booster injection of chick CII emulsified in FIA. At the same time, the mice received daily treatment with intranasal inhalation of 12.5% ODE for 5 weeks continuously (weekends excluded).
Inflammatory arthritis began at Week 3 and became severely obvious at Week 5. CII + ODE‐treated mice displayed a decline of bone mineral density (BMD) in both the proximal tibia and calcaneus compared with CII‐treated mice. In addition, CII + ODE‐treated mice demonstrated a reduction of bone volume, trabecular thickness, and polar moment of inertia in the calcaneus compared with CII‐treated mice. In short, CIA + ODE (coexposure) treatment resulted in trabecular bone loss and deterioration. Moreover, the coexposure group developed more severe arthritis symptom than ODE‐treated group.
After 5 weeks, the lung in coexposure mice displayed increased lymphoid aggregates, bronchiolar, and alveolar inflammation comprised of lung neutrophils and exudative macrophages, with collagen deposition and increased hyaluronan, fibronectin accompanying high levels of pentraxin‐2, anti‐CCP IgG antibody, Type II collagen IgG, amphiregulin, anti‐MAA IgG antibody, immunoglobulin (IgG, IgM, and IgA), TNF‐α, IL‐6, and neutrophil chemoattractants (CXCL1 and CXCL2). Nevertheless, there was less inflammation infiltration in alveolar compartment and more extracellular matrix protein deposition in the CII + ODE group versus the ODE group. In summary, lung inflammation was shifted to pulmonary interstitial changes in the coexposure treated mice compared with ODE‐treated mice. Interestingly, the female mice manifested suppressed inflammatory cell and arthritis than male mice under the CII + ODE treatment.
The merit is that this novel animal model vividly mimics what influence the environmental factors have on RA‐ILD individuals and the interplay between arthritis and lung inflammatory disease that repetitive inhalant exposure to an environmental inflammatory agent rich in microbe aggravates articular inflammation, bone destruction, as well as cartilage erosion and conversely arthritis elicitation appears to impact the inflammatory/interstitial lung processes. CII and inhalation induced arthritis and fibrosis, which can simulate the pathological process of human RA‐ILD in the environment. This preclinical animal model could be further exploited to develop potential interventional strategies to mitigate arthritis and lung disease such as through respiratory protection and environmental improvement. Also, this animal model can be used to investigate biological sex differences in morbidity and mortality of human RA‐ILD. However, the operation on this established model is relatively complex, because it is necessary to ODE and have the mouse going through specific inhalation every day to better simulate certain circumstances where human are.
2.2. Animal model of RA‐ILD induced by genetic engineering
2.2.1. Animal model of RA‐ILD by gene mutation
The SKG mice have a spontaneous point mutation in the second SH2 domain of ZAP70 relevant to tyrosine‐phosphorylated immunoreceptor tyrosine‐based activation motifs of TCR‐ζ and CD3 chains, as a consequence of decreased T‐cell receptor signaling in thymocytes, which impaired positive and negative CD4+ T cell selection in the thymus enabling autoreactive CD4+T cells to escape into the periphery. 39 , 40 Furthermore, it consequently displays the phenotype of immunological diseases and might be crucial to the development of RA‐ILD.
SKG mice failed to develop RA‐ILD under specific‐pathogen‐free (SPF) conditions despite the fact that thymic production of autoimmune CD4+T cells persisted in the periphery, but intraperitoneal injection of zymosan especially β‐glucans can cause the development of an RA‐ILD clinical phenotype in the same condition above mentioned. 41 Zymosan came from crude yeast cell wall extract, the main constituents of which were β‐glucans. 41 Interestingly, SKG mice spontaneously began to manifest chronic arthritis about 2 months of age and developed the RA‐ILD disease about 6 months of age in a conventional environment containing certain microbial compounds, but not under SPF conditions. 41 This suggested that a specific combination of aberrant expression in thymus CD4+ T cell and exposure to particular microbes was of vital importance to the development of RA‐ILD, but either one was not.
SKG mice were given a single intraperitoneal injection of 5 mg zymosan to provoke severe arthritis and advance onset of ILD under SPF condition. 42 , 43 , 44 The mice began to display symmetrical joint swelling and developed inflammatory arthritis of the wrists, ankles, and digits characterized by synovial inflammation with moderate cartilage damage and narrowing of the joint space at 2–3 weeks after zymosan injection. Symmetrical joint swelling began in small joints of the digits and progressed to larger joints, accompanying severe synovitis with massive subsynovial infiltration of neutrophils, lymphocytes (mainly CD4+ T cells), macrophages, and plasma cells. Likewise, it presented villus proliferation of synoviocytes with pannus formation and neovascularization, and neutrophil‐rich exudates in the joint cavity with progression of synoviocyte proliferation. It was predicted that this unique combination of high self‐reactivity of T cells and susceptibility of synovial cells to inflammatory stimuli leads to predominant development of erosive arthritis in SKG mice. Moreover, the severity of joints swelling gradually increased before peaking in severity at 6–10 weeks after zymosan injection and then declined but remained elevated in mice 16 weeks after zymosan injection. Destruction and fusion of the subchondral bones, joint dislocation, and osteoporosis appeared at 8–12 months of age. Also, SKG mice developed high titers of rheumatoid factor (RF) and other autoantibodies in the circulation with increased pro‐inflammatory cytokines of IL‐1, IL‐6, and TNF‐a as well as incremental chemical mediators that destroy the surrounding cartilage and bone.
At 12–16 weeks after zymosan injection, arthritic SKG mice developed a patchy subpleural and peribronchovascular mixed inflammation formed by CD4+ T cells, CD8+ T cells, B220+ B cells, CD11b+ macrophages, and neutrophils, with variable amounts of collagen but not large areas of excessive collagen deposition in the areas of accumulated cells. There was also a small increase in the number of lavaged cells: macrophages, neutrophils, and lymphocytes. The increased fibrosis seen in zymosan‐injected SKG mice was associated with active TGF‐β signaling in the lung parenchyma. Likewise, there was a decline in lung static compliance. The lung inflammation can involve up to 89% of the mice injected with zymosan. However, neither exposure to cigarette smoke nor bleomycin resulted in the onset of arthritis in SKG mice. 42 Furthermore, anti‐cyclic citrullinated antibodies were present in the sera of arthritic SKG mice that were injected with zymosan, but not detected in the sera of the mice exposed to cigarette smoke.
SKG mice injected with zymosan closely mirroring human RA‐ILD have their own advantages and disadvantages. SKG mice can represent a useful model that will allow the investigation of the immune and inflammatory mechanisms contributing to the development of interstitial pneumonia in the setting of autoimmune arthritis. The pulmonary manifestation of SKG mice following intraperitoneal zymosan injection strongly resembles NSIP pathology to that seen in human RA‐ILD. However, SKG mice did not develop a fibrotic UIP phenotype while there was increased deposition of collagen in the lung after 18 weeks post zymosan injection, 42 which is not consistent with the course of RA‐ILD. Just lung involvement is insufficient to induce onset of arthritis in SKG mice. 42 Furthermore, while the SKG mice with identical genetic background undergo the same circumstances, they can show various disease phenotype of joint and lung.
2.3. Transgenic animal models of RA‐ILD
2.3.1. The D1CC transgenic mouse
The human class II transactivator (CIITA) gene was linked to the rat CII promoter and enhancer in DBA/1 mice through plasmid construction, which can result in aberrant expression of major histocompatibility complex Class II (MHC Class II). 45 These transgenic DBA/1 mice were called D1CC transgenic mice. The D1CC transgenic mice treated by small amounts of bCII in adjuvant can induce onset of RA‐ILD in the D1CC transgenic mice.
The D1CC mice were given the first intradermal injection of 10 µg of bCII emulsified in an equal volume of CFA into the base of the tail on Day 0. 45 , 46 Mice received booster injections of bCII in the same location using IFA on Days 21, 42, and 63. 45 , 46 The erosive destruction of joints started in the early stages of inflammation for 2 months after the second booster injection. Eighty‐nine percent of D1CC mice developed articular symptom of joint swelling, redness, and heat after immunization. 45 The mice displayed infiltration of inflammatory cells, proliferation of synoviocytes with pannus formation characterized by accumulation of an abundance of granulocytes, and erosion of bone. At the terminal stage of chronic inflammatory arthritis, it revealed the decline of bone mineral density and joint space narrowing and erosions at the patella, distal femur, proximal tibia as well as fibula, and severe ankylosis at the proximal interphalangeal and metacarpophalangeal joints, but without the appearance of osteophytes. Fifteen months after the second boost, the mice manifested the complete destruction of all joints and shortening of phalanges together with the high levels of anti‐CCP antibodies.
The infiltrating inflammatory cells in the lung damage were granulocytes, lymphocytes, T cells and macrophages with pneumocyte hyperplasia and fibrotic changes. Mixed cellular inflammation was distributed in the peribronchial and perivascular areas with fibrosis, deposition of newly synthesized elastic fibers, and increased anti‐cyclic citrullinated peptide antibodies for 6 months after the second boost. Serum SP‐D levels were significantly increased approximately 10 months after the first injection of bCII, which can be a suitable marker of lung damage in RA‐ILD. TNF‐α expression in macrophages, BAX expression in reactive pneumocytes, TGF‐β expression in fibroblastic cells, IL‐6 expression in plasmacytoid cells, and soluble collagen expression was high in the lung lesions in DICC mice 10 months following the first bCII injection. 46 The number of 8‐OHdG‐positive epithelial and inflammatory cells was significantly increased in DICC mice at 10 months after bCII injection. These molecules play an important role in the pathophysiology of RA and fibrosis in humans during inflammation, apoptosis, and extracellular matrix production. 46
Merit to this model is that it can be used in evaluation of biomarkers and general pathophysiology of chronic disease process in resembling human RA‐ILD, which can well mimic the chronic and progressive course of RA‐ILD. Demerit to this model is that this model needs long time to get molded and could not completely replicate the relevant pulmonary pathological manifestations of UIP the type of which is predominant in the RA‐ILD.
2.3.2. The 3647 line of the tumor necrosis factor transgenic (TNF‐Tg) mouse
The 3647 line of TNF‐Tg (Tg3647)mouse strains carrying a single copy of human TNF transgene with a modified 3ʹ‐untranslated region (3ʹ‐UTR) exchanging for the 3ʹ‐modified β‐globin UTR, 47 , 48 began to appear the swollen ankle joints around 2 months of age and developed inflammatory erosive arthritis at the age of 3 months without any stimuli. The TNF‐Tg mouse strains, overexpressing the human TNF‐α gene, are usually evaluated at cross‐sectional time points of 3, 4, 5.5, and 12 months. 49 , 50 , 51 , 52
The Tg3647 mice developed knee and ankle arthritis with dysfunction of the joint draining lymph nodes featuring a progressive increase in their popliteal lymph node volumes in which activated monocytes, conventional dendritic cells, and CD21hi/CD23+ B cells accumulated, due to the increase in lymphatic trafficking from the synovial space. Furthermore, grip strength decreased as well. Grip strength and the total volume of the lymph node reflected disease progression and activity in inflammatory arthritis.
The most notable patterns of lungs in Tg3647 mice included interstitial cellular infiltrates, thickened alveolar septums, perivascular, and peribronchiolar inflammatory infiltrates as well as the formation of follicle‐like structures lacking a germinal center with no apparent interstitial fibrosis. There was a significant increase of CD11b+/CD11c+ double‐positive cells and apoptosis in the lung tissue in the Tg3647 mouse line, concomitant with other increased populations of monocytes and dendritic cells. In addition to this, there was also a significant increase in the serum levels of human TNF‐α, IL‐17, interferon‐γ–inducible protein 10, monocyte chemoattractant protein 1, leukemia inhibitory factor, and keratinocyte chemoattractant.
The merit to Tg3647 murine model is that it mimics a cellular NSIP pattern dominated by an interstitial accumulation of inflammatory cells with a small amount of fibrotic change. A prior inflammatory state of this animal model could be used to investigate into inflammation phase of RA‐ILD. In addition, it could be used to assess anti‐TNF therapies but can also be used for testing other biologics and small molecules could be reversed with anti‐inflammatory therapies. 48 Moreover, the female Tg3647 mice manifested the earlier symptoms of arthritis, and developed the more severe pathology of interstitial lung disease, which resulted in shorter lifespans and more mortality than their male counterparts. 52 It could be used as a suitable model to better understand the mechanisms underlying sexual dimorphism. However, the demerit to this model is that it is relatively unitary in nature for the reason that the process of human RA‐ILD is due to the interaction of multiple genes and multiple factors. 53 In addition, this model takes a long time to establish and the experiment cost of it is high.
3. CONCLUDING REMARKS
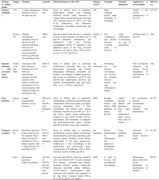
So far, we have focused our review on the pathophysiological and histomorphological characteristics of preclinical disease models, in addition to their practical aspects including reproducibility and feasibility simulating human pathology in RA‐ILD (summarized in Table 1). There is no doubt that they do provide valuable information relating to the pathogenesis of RA‐ILD and the development of effective therapeutic drugs. Nevertheless, these animal models can not entirely recapitulate clinical pathology and have some limitations in experimental research application. Therefore, it should be emphasized that we should improve and explore animal models in more accordance with the pathogenesis and clinical characteristics of human RA‐ILD.
Table 1.
Animal models of RA‐ILD: Pathogenic triggers, features of human disease, subtypes, strengths, and limitations
| Strategy of animal model | Trigger | Methods | Animals | Mimicked features of RA‐ILD | Subtype of ILD | Strengths | Potential limitations | Applications or novel insights | References |
|---|---|---|---|---|---|---|---|---|---|
| Immune stimulants | CFA | A single subcutaneous injection of CFA at the right rear paw | Wistar rats | Onset of arthritis before pulmonary manifestation; double joint swelling; thickened alveolar walls, deposition of collagen fibers, pleural thickening in the lung with increased levels of TNF‐α, IL‐6, and IL‐1β, inflammatory cell infiltration: lymphocytes, eosinophilic granulocytes, and macrophages | UIP | Short modeling period, rapid replication of the disease | Similar to the clinical characteristics of RA‐ILD | Song et al. 22 and Yang et al. 23 | |
| Bovine CII | Multiple subcutaneous Injections at base of the tail: the first injection with 50 μg of bovine CII emulsified in CFA; booster injections with 50 μg of bovine CII emulsified in IFA | DBA/1 mice | Lung involvement prior the onset of arthritis; increase in ankle thickness with high levels of anti‐CII antibodies; inflammatory cell infiltration(T cells and CD11bhi macrophages), ACPAs, C3 deposited in the subpleural region of the lung, increased cytokines of IL‐1β, IL‐6, IFN‐γ, TNF‐α levels, IL‐4, IL‐17, CCL3, and CCL4 | Similar to UIP | How citrullination and ACPAs interacts involving onset of RA‐ILD | Lung inflammation is self‐limited, no sign of lung fibrosis | Initiation phase of RA‐ILD | Sato et al. 30 | |
| Immune stimulants with environmental factors | Chicken CII and ODE | Coexposure with daily intranasal inhalation of 12.5% ODE and subcutaneous injections: the first injection with 2 mg/ml of chick CII emulsified in CFA; a booster injection with 2 mg/ml of chick CII emulsified in IFA | DBA/1J mice | Onset of arthritis before pulmonary manifestation; trabecular bone loss and deterioration; bronchiolar and alveolar inflammation infiltration: neutrophils and exudative macrophages; collagen deposition, high levels of pentraxin‐2, anti‐CCP IgG, anti‐CII IgG, amphiregulin, anti‐MAA IgG antibody, immunoglobulin (IgG, IgM, IgA), TNF‐α, IL‐6, CXCL1, and CXCL2 | Not mentioned | Mimicking the link between environment and RA‐ILD; the interplay between arthritis and lung inflammatory disease | How environment influences the disease. Sexual dimorphism in manifestation of RA‐ILD | Poole et al. 38 | |
| Gene mutation | Zymosan | A single intraperitoneal injection of 5 mg zymosan | SKG mice for BALB/c mice background | Onset of arthritis before pulmonary manifestation; symmetrical joint swelling and inflammation of the wrists, ankles, and digits, synovitis: neutrophils, CD4+ T cells, macrophages and plasma cells, pannus formation; increased levels of RF, IL‐1, IL‐6, and TNF‐a, patchy mixed inflammation formed by T cells, B220+ B cells, CD11b+ macrophages, and neutrophils in subpleural and peribronchovascular region, collagen deposition, and decline in lung static compliance | NSIP | Strongly resembling cellular and fibrotic NSIP pathology of human RA‐ILD | Cannot develop a fibrotic UIP phenotype, lung injury and inflammation alone cannot elicit onset of arthritis | the mechanisms between environmental exposures, immunity and inflammation | Keith et al. 42 and Redente et al. 43 |
| Transgenic systems | Bovine CII | Intradermal injections at base of the tail: the first injection with 5–10 μg of bovine CII emulsified in CFA; a booster injection with 5–10 μg of bovine CII emulsified in IFA | D1CC mice for DBA/1 mice background | Onset of arthritis before pulmonary manifestation; chronic arthritis: uxtaarticular demineralization, joint space narrowing, joint erosions, pannus formation; mixed cellular inflammation of the lung: granulocytes, lymphocytes, T cells, macrophages in the peribronchial and perivas‐cular areas, increased anti‐CCP antibodies, TNF‐α, BAX, TGF‐β, IL‐6, LPO, 8‐OHdG, and SP‐D. | Not mentioned | Chronic, immunologically inflammatory and progressive development of RA‐ILD | Long modeling period | evaluation of biomarkers, chronic RA‐ILD model | Kanazawa et al. 45 and Terasaki et al. 46 |
| None | Spontaneous generation without any inducers | 3647 line of TNF‐Tg mice for C57BL/6 mice ground | Knee and ankle arthritis: monocytes, dendritic cells, and CD21hi/CD23+ B cells; increased PLN volumes, decreased grip strength; thickened alveolar septums, follicle‐like structures, interstitial cellular infiltrates: CD11b+/CD11c+ double‐positive cells, monocytes and dendritic cells; apoptosis in the lung tissue, increased human TNF‐α, IL‐17, IP‐10, MCP‐1, LIF, and KC | NSIP | It could be reversed with anti‐inflammatory therapies | Inflammation phase of RA‐ILD, sexual dimorphism in manifestation of RA‐ILD | Wu et al. 49 , Fehrenbach et al. 50 , Wu et al. 51 , and Bell et al. 52 |
Abbreviations: CFA,complete Freund's adjuvant; CII, collagen type II; IFA, incomplete Freund's adjuvant; IP‐10, interferon‐γ–inducible protein 10; KC, keratinocyte chemoattractant; LIF, leukemia inhibitory factor; MCP‐1, monocyte chemoattractant protein 1; ODE, organic dust extract; PLN, popliteal lymph node; TNF‐Tg, tumor necrosis factor transgenic.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
Li Xiong read a number of relevant literatures and wrote the manuscript as well as concluded the table. Liang Xiong revised grammar mistakes and made expression of language more native. Hong Ye ensured the language had its fluency and logic. Wan‐Li Ma did the final proofreading to get the manuscript more completeness.
ACKNOWLEDGMENT
This study was supported in part by grants from the National Natural Science Foundation of China (No. 81873401 to HY; No. 81973991 to WLM).
Xiong L, Xiong L, Ye H, Ma W. Animal models of rheumatoid arthritis‐associated interstitial lung disease. Immun Inflamm Dis. 2021;9:37–47. 10.1002/iid3.377
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting this review are available within the paper and on request from the corresponding author.
REFERENCES
- 1. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388:2023–2038. [DOI] [PubMed] [Google Scholar]
- 2. Hurd ER. Extraarticular manifestations of rheumatoid arthritis. Semin Arthritis Rheum. 1979;8:151–176. [DOI] [PubMed] [Google Scholar]
- 3. Olson AL, Swigris JJ, Sprunger DB, et al. Rheumatoid arthritis‐interstitial lung disease‐associated mortality. Am J Respir Crit Care Med. 2011;183:372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bongartz T, Nannini C, Medina‐Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population‐based study. Arthritis Rheum. 2010;62:1583–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim EJ, Elicker BM, Maldonado F, et al. Usual interstitial pneumonia in rheumatoid arthritis‐associated interstitial lung disease. Eur Respir J. 2010;35:1322–1328. [DOI] [PubMed] [Google Scholar]
- 6. Hakala M. Poor prognosis in patients with rheumatoid arthritis hospitalized for interstitial lung fibrosis. Chest. 1988;93:114–118. [DOI] [PubMed] [Google Scholar]
- 7. Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yoshinouchi T, Ohtsuki Y, Fujita J, et al. Nonspecific interstitial pneumonia pattern as pulmonary involvement of rheumatoid arthritis. Rheumatol Int. 2005;26:121–125. [DOI] [PubMed] [Google Scholar]
- 9. Lee HK, Kim DS, Yoo B, et al. Histopathologic pattern and clinical features of rheumatoid arthritis‐associated interstitial lung disease. Chest. 2005;127:2019–2027. [DOI] [PubMed] [Google Scholar]
- 10. Tansey D, Wells AU, Colby TV, et al. Variations in histological patterns of interstitial pneumonia between connective tissue disorders and their relationship to prognosis. Histopathology. 2004;44:585–596. [DOI] [PubMed] [Google Scholar]
- 11. Yousem SA, Colby TV, Carrington CB. Lung biopsy in rheumatoid arthritis. Am Rev Respir Dis. 1985;131:770–777. [DOI] [PubMed] [Google Scholar]
- 12. Veeraraghavan S, Nicholson AG, Wells AU. Lung fibrosis: new classifications and therapy. Curr Opin Rheumatol. 2001;13:500–504. [DOI] [PubMed] [Google Scholar]
- 13. Katzenstein AL, Myers JL. Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am J Respir Crit Care Med. 1998;157:1301–1315. [DOI] [PubMed] [Google Scholar]
- 14. Carrington CB, Gaensler EA, Coutu RE, FitzGerald MX, Gupta RG. Natural history and treated course of usual and desquamative interstitial pneumonia. N Engl J Med. 1978;298:801–809. [DOI] [PubMed] [Google Scholar]
- 15. Katzenstein AL, Fiorelli RF. Nonspecific interstitial pneumonia/fibrosis histologic features and clinical significance. Am J Surg Pathol. 1994;18:136–147. [PubMed] [Google Scholar]
- 16. Katzenstein AL, Myers JL. Nonspecific interstitial pneumonia and the other idiopathic interstitial pneumonias: classification and diagnostic criteria. Am J Surg Pathol. 2000;24:1–3. [DOI] [PubMed] [Google Scholar]
- 17. Nielen MMJ, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380–386. [DOI] [PubMed] [Google Scholar]
- 18. Kolfenbach JR, Deane KD, Derber LA, et al. Autoimmunity to peptidyl arginine deiminase type 4 precedes clinical onset of rheumatoid arthritis. Arthritis Rheum. 2010;62:2633–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brusca SB, Abramson SB, Scher JU. Microbiome and mucosal inflammation as extra‐articular triggers for rheumatoid arthritis and autoimmunity. Curr Opin Rheumatol. 2014;26:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Willis VC, Demoruelle MK, Derber LA, et al Sputum autoantibodies in patients with established rheumatoid arthritis and subjects at risk of future clinically apparent disease. Arthritis Rheum. 2013;65:2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schurgers E, Mertens F, Vanoirbeek JA, Put S, Mitera T. De Langhe, E. , Billiau, A. et al, Pulmonary inflammation in mice with collagen‐induced arthritis is conditioned by complete Freund's adjuvant and regulated by endogenous IFN‐gamma. Eur J Immunol. 2012;42:3223–3234. [DOI] [PubMed] [Google Scholar]
- 22. Song LN, Kong XD, Wang HJ, Zhan LB. Establishment of a rat adjuvant arthritis‐interstitial lung disease model. BioMed Res Int. 2016;2016:2970783–2970786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang G, Lyu L, Wang X, Bao L, Lyu B, Lin Z. Systemic treatment with resveratrol alleviates adjuvant arthritis‐interstitial lung disease in rats via modulation of JAK/STAT/RANKL signaling pathway. Pulm Pharmacol Ther. 2019;56:69–74. [DOI] [PubMed] [Google Scholar]
- 24. Vidal B, Cascão R, Finnilä MAJ, et al. Effects of tofacitinib in early arthritis‐induced bone loss in an adjuvant‐induced arthritis rat model. Rheumatology (Oxford). 2018;57:1461–1471. [DOI] [PubMed] [Google Scholar]
- 25. Stuart JM, Townes AS, Kang AH. Collagen autoimmune arthritis. Annu Rev Immunol. 1984;2:199–218. [DOI] [PubMed] [Google Scholar]
- 26. Brand DD, Latham KA, Rosloniec EF. Collagen‐induced arthritis. Nat Protoc. 2007;2:1269–1275. [DOI] [PubMed] [Google Scholar]
- 27. Reynisdottir G, Olsen H, Joshua V, et al. Signs of immune activation and local inflammation are present in the bronchial tissue of patients with untreated early rheumatoid arthritis. Ann Rheum Dis. 2016;75:1722–1727. [DOI] [PubMed] [Google Scholar]
- 28. Kelly CA, Saravanan V, Nisar M, et al. Rheumatoid arthritis‐related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics‐‐a large multicentre UK study. Rheumatology (Oxford). 2014;53:1676–1682. [DOI] [PubMed] [Google Scholar]
- 29. Correia CS, Briones MR, Guo R, Ostrowski RA. Elevated anti‐cyclic citrullinated peptide antibody titer is associated with increased risk for interstitial lung disease. Clin Rheumatol. 2019;38:1201–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sato T, Satooka H, Ichioka S, Maruo Y, Hirata T. Citrullinated fibrinogen is a target of auto‐antibodies in interstitial lung disease in mice with collagen‐induced arthritis. Int Immunol. 2020;32:533–545. [DOI] [PubMed] [Google Scholar]
- 31. Misharin AV, Morales‐Nebreda L, Mutlu GM, Budinger GR, Perlman H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am J Respir Cell Mol Biol. 2013;49:503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Byrne AJ, Maher TM, Lloyd CM. Pulmonary Macrophages: A New Therapeutic Pathway in Fibrosing Lung Disease? Trends Mol Med. 2016;22:303–316. [DOI] [PubMed] [Google Scholar]
- 33. McCubbrey AL, Barthel L, Mohning MP, et al. Deletion of c‐FLIP from CD11b(hi) macrophages prevents development of bleomycin‐induced lung fibrosis. Am J Respir Cell Mol Biol. 2018;58:66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Asquith DL, Miller AM, McInnes IB, Liew FY. Animal models of rheumatoid arthritis. Eur J Immunol. 2009;39:2040–2044. [DOI] [PubMed] [Google Scholar]
- 35. Too CL, Muhamad NA, Ilar A, et al. Occupational exposure to textile dust increases the risk of rheumatoid arthritis: results from a Malaysian population‐based case‐control study. Ann Rheum Dis. 2016;75:997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Poole JA, Wyatt TA, Oldenburg PJ, Elliott MK, West WW, Sisson JH. Von Essen, S. G.et al, Intranasal organic dust exposure‐induced airway adaptation response marked by persistent lung inflammation and pathology in mice. Am J Physiol Lung Cell Mol Physiol. 2009;296:L1085–L1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Poole JA, Alexis NE, Parks C, et al. Repetitive organic dust exposure in vitro impairs macrophage differentiation and function. J Allergy Clin Immunol. 2008;122(375‐382):e374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Poole JA, Thiele GM, Janike K, et al. Combined collagen‐induced arthritis and organic dust‐induced airway inflammation to model inflammatory lung disease in rheumatoid arthritis. J Bone Miner Res. 2019;34:1733–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li W, Moore MJ, Vasilieva N, et al. Altered thymic T‐cell selection due to a mutation of the ZAP‐70 gene causes autoimmune arthritis in mice. Nature. 2003;426:450–454. [DOI] [PubMed] [Google Scholar]
- 40. Wiest DL, Ashe JM, Howcroft TK, et al. A spontaneously arising mutation in the DLAARN motif of murine ZAP‐70 abrogates kinase activity and arrests thymocyte development. Immunity. 1997;6:663–671. [DOI] [PubMed] [Google Scholar]
- 41. Yoshitomi H, Sakaguchi N, Kobayashi K, et al. A role for fungal {beta}‐glucans and their receptor Dectin‐1 in the induction of autoimmune arthritis in genetically susceptible mice. J Exp Med. 2005;201:949–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Keith RC, Powers JL, Redente EF, et al. A novel model of rheumatoid arthritis‐associated interstitial lung disease in SKG mice. Exp Lung Res. 2012;38:55–66. [DOI] [PubMed] [Google Scholar]
- 43. Redente EF, Aguilar MA, Black BP, et al. Nintedanib reduces pulmonary fibrosis in a model of rheumatoid arthritis‐associated interstitial lung disease. Am J Physiol Lung Cell Mol Physiol. 2018;314:L998–L1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Keith RC, Sokolove J, Edelman BL, et al. Testosterone is protective in the sexually dimorphic development of arthritis and lung disease in SKG mice. Arthritis Rheum. 2013;65:1487–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kanazawa S, Ota S, Sekine C, et al. Aberrant MHC class II expression in mouse joints leads to arthritis with extraarticular manifestations similar to rheumatoid arthritis. Proc Natl Acad Sci U S A. 2006;103:14465–14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Terasaki Y, Terasaki M, Kanazawa S, et al. Effect of H2 treatment in a mouse model of rheumatoid arthritis‐associated interstitial lung disease. J Cell Mol Med. 2019;23:7043–7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li P, Schwarz EM. The TNF‐alpha transgenic mouse model of inflammatory arthritis. Springer Semin Immunopathol. 2003;25:19–33. [DOI] [PubMed] [Google Scholar]
- 48. Keffer J, Probert L, Cazlaris H, et al. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991;10:4025–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wu EK, Henkes ZI, McGowan B, et al. TNF‐induced interstitial lung disease in a murine arthritis model: accumulation of activated monocytes, conventional dendritic cells, and CD21+/CD23−B cell follicles is prevented with Anti‐TNF Therapy. J Immunol. 2019;203:2837–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fehrenbach H, Bell RD, Rudmann C, Wood RW, Schwarz EM, Rahimi H. Longitudinal micro‐CT as an outcome measure of interstitial lung disease in TNF‐transgenic mice. PLoS One. 2018;13:e0190678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wu EK, Eliseeva S, Rahimi H, Schwarz EM, Georas SN. Restrictive lung disease in TNF‐transgenic mice: correlation of pulmonary function testing and micro‐CT imaging. Exp Lung Res. 2019;45:175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bell RD, Wu EK, Rudmann CA, et al. Selective sexual dimorphisms in musculoskeletal and cardiopulmonary pathologic manifestations and mortality incidence in the tumor necrosis factor‐transgenic mouse model of rheumatoid arthritis. Arthritis Rheumatol. 2019;71:1512–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang D, Zhang J, Lau J, et al. Mechanisms of lung disease development in rheumatoid arthritis. Nat Rev Rheumatol. 2019;15:581–596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting this review are available within the paper and on request from the corresponding author.


