Abstract
Objective:
To characterize the contemporary efficacy and utilization patterns of coronary artery bypass grafting (CABG) in specific cancer types.
Methods:
We leveraged the data from the National-Inpatient-Sample and plotted trends of utilization and outcomes of isolated CABG (with no other additional surgeries during the same hospitalization) procedures from January 1, 2003 through September 1, 2015. Propensity-score matching was used to assess for potential differences in outcomes, by type of cancer-status, among contemporary (2012–2015) patients.
Results:
Overall, the utilization of CABG decreased over time (250,677 in 2003 vs. 134,534 in 2015, P<.001). However, the proportion of those with comorbid cancer increased (7% vs. 12.6%, P<.001). Over time, in-hospital mortality associated with CABG use in cancer remained unchanged (.9% v. 1.0%, P=.72); yet, cancer patients saw an increase in associated major bleeding (4.5% v. 15.3%, P<.001) and rate of stroke (.9% v. 1.5%, P<.001) over time. In-hospital cost-of-care associated with CABG-use in cancer also increased over time ($29,963 v. $33,636, P<.001). When stratified by cancer types, in-hospital mortality was not higher in breast lung, prostate, colon cancer and lymphoma vs. non-cancer CABG patients (all P>.05). However, there was a significantly higher prevalence of major bleeding but not stroke in patients with breast and prostate cancer only compared to non-cancer CABG patients (P<.01). Discharge dispositions were not found to be different between cancer sub-groups and non-cancer patients (P>.05;) except breast cancer patients who had lower home, but higher skilled care disposition (P<.001).
Conclusion:
Among those undergoing CABG, the prevalence of co-morbid cancer has steadily risen. Outside of major bleeding, these patients appear to share similar outcomes to those without cancer indicating that CABG utilization should be not be declined in cancer patients when otherwise indicated. Further research into the factors underlying the decision to pursue CABG in specific cancer sub-groups are needed.
Keywords: PCI, CABG, cancer, national inpatient sample
Introduction:
Coronary artery disease (CAD) remains the leading cause of cardiovascular disease (CVD) death, accounting for nearly 50% of CVD deaths1. However, recent data has suggested a significant shift in the representation of those with CAD, with a higher prevalence of patients with a concurrent cancer diagnosis, a condition for which a new generation of novel immune-based and targeted therapies have altered and dramatically prolonged life-expectancies. Despite these improved outcomes, cancer patients face nearly twice the risk of CAD, including acute coronary syndromes, within months of a cancer diagnosis2. This increased risk is even more compounded by recent advances in cancer treating therapies, including radiation treatment which have dramatically prolonged life-expectancies in cancer, but are often associated with an increased CVD risk1. Many of these patients present with increasingly complex CAD and challenging clinical scenarios. Although available data suggest a potential uptake in the use of percutaneous coronary interventions (PCI) in this population, the proportional use of coronary artery bypass graft (CABG) remains largely unknown1.
Available evidence suggests cancer patients undergoing PCI have increased complications, such as in-hospital mortality and bleeding. This has been largely attributed to the adverse CVD effects of contemporary anti-cancer therapies, as well as alterations in coagulation profile seen in cancer3, 4. However, there are limited data on clinical outcomes after CABG in patients with a coexistent diagnosis of cancer. Additionally, most randomized controlled trials of CVD care and outcomes exclude patients with active malignancy and treatment. Moreover, there is even more sparse data on clinical outcomes after CABG in specific cancer types, or the presence of metastases.
In this analysis, we examine temporal trends, in-hospital outcomes, complications and dispositions associated with CABG use by type of cancer diagnosis and presence of metastases between 2003–2015.
Methods:
Data Source:
The National Inpatient Sample (NIS) is an inpatient database in the United States (US)3 developed by the Agency for Healthcare Research and Quality (AHRQ). In the present study, we used NIS data from January 1, 2003 through September 30, 2015. The details of the dataset are mentioned in the Supplemental Methods.
Study Population and Variables:
We used ICD-9-CM codes to identify all hospitalized adults (≥18 years), who had a procedure code of (PR1-PR15 of NIS) of CABG (36.1x). The discharge diagnoses and procedures were recoded using the clinical classification of diseases software (CCS) into broad categories, available as separate variables within the NIS data set. We used the CCS coded discharge diagnoses to further define our initial cohort, where we identified CABG exclusively using the code 44 (PRCCS1–15 only). Since this study studied isolated CABG5, concomitant other major vascular or valvular surgeries were excluded (excluded ICD-9 procedure codes 00.66, 36.01, 36.02, 36.05, 36.03, 36.04, 34.06, 34.07 and CCS codes 43, 45, 49, 63, 51, 52, 55, 56). In this constructed cohort, we then identified cancer patients using DXCCS codes (DXCCS1-DXCCS30) 11–45. NIS provides 29 co-morbidities (also known as Elixhauser’s Comorbidity measures) based on ICD-9 CM diagnoses, and the diagnosis-related group in effect on the date of discharge. These co-morbidities are not directly related to the principal diagnosis or the main reason for admission, and are likely to have originated before the hospital stay6. Hospitalizations with the co-morbidities of cancer (CM_TUMOR, CM_LYMPH and CM_METS), were included in the cancer cohort. All patients who did not have either the DXCCS codes listed above, or the listed specific co-morbidities, were considered non-cancer patients7.
NIS variables included in the study were demographic characteristics (age, sex, race), income quartile, insurance status, number of bypass grafts used during CABG, hospital level characteristics, and co-morbidities. In 2015, the Healthcare Cost and Utilization Project (HCUP) State Inpatient Database was used to create an index based on 29 co-morbidity measures designed to predict in-hospital mortality8, which was calculated for our cohort as well.
Outcomes:
NIS provided data on specific outcomes of interest, including hospitalization charges, length of stay (LoS), in-hospital mortality and discharge disposition. Actual cost of hospitalization was obtained using methodology described in a prior manuscript7. Charges and costs were inflation-adjusted to 20159. In addition, outcomes of major bleeding, ischemic stroke, pulmonary complications, and cardiac complications defined using ICD-9 CM diagnosis or procedure codes were also studied. The procedure codes associated with the complications were confirmed to be on the day of CABG or after. However, the pure ICD-9 CM diagnosis code-based outcomes were assumed to be not present preceding the CABG or present on arrival as alternate diagnosis. All ICD-9 CM codes, CCS codes and comorbidity codes used in defining the cohort, comorbidities and outcomes are listed in Supplemental Table 1.
Statistical Analysis:
Annual variance analysis for NIS datasets was performed using the DOMAIN method for all years10. We followed the recommendations from AHRQ for analysis using survey data11. Survey-specific statements with hospital and patient-level weights were used to obtain national estimates. The Rao-Scott Chi-Square test was used to compare categorical variables, and a survey specific t-test was used for continuous variables. We used the Cochrane Armitage test of trend for categorical variables and survey-specific linear regression for continuous variables. Hospital charges and LoS were log-transformed because they were not normally distributed, and geometric mean was presented12, 13.
Modeling for outcomes were performed using a propensity score matched design wherein 1 cancer hospitalization for CABG was matched with 2 non-cancer hospitalizations for CABG (1:2 match). The propensity score is a number which represents the relationship between multiple characteristics and the dependent variable as a single characteristic14. First, non-parsimonious logistic regression model with cancer as the dependent variable was performed using aforementioned variables to generate propensity scores. The models were screened for missingness of greater than 20%. It was determined that there was no requirement for multiple imputation since missingness was < 5%15. NIS weights were used in the propensity estimation model14. Next, the propensity score is used to generate the 1-to-2 cancer-to-non-cancer matched dataset using Parson’s digit-based greedy matching16. This algorithm matches a case to control at the 8th, 7th, 6th … decimal point, using a greedy matching algorithm. Multiple matched datasets were used for analysis: 1) first model included all the years from 2003 to 2015 for cancer and non-cancer and was used for trends analysis of outcomes, 2) another contemporary model was made to account for time bias wherein cancer and non-cancer cohorts were used from 2012–2015, and 3) five matched datasets similar to one in (2) were created for breast cancer, lung cancer, colon cancer, prostate cancer and lymphoma where each of these cancers were matched to 2 non-cancer controls undergoing CABG. The specific details of the model, reason for model selection and its performance was presented in Supplemental Methods and Supplemental Table 2. Propensity matched trends using the first specified model was used to study in-hospital mortality, stroke, major bleeding and cost-of-care trends across all year. Specific outcomes in those undergoing CABG across individual cancers, all cancers in comparison with non-cancer is presented using the propensity matched models specified in points (2) and (3) above (Supplemental Figure 1).
Certain subgroup analysis, namely those undergoing radiation therapy and those with metastatic cancer, were felt to be a higher risk category. The outcomes in these categories were compared to cancer patients without these diagnoses using Survey-specific logistic and linear regression techniques (PROC SURVEYLOGISTIC and PROC SURVEYREG). The outcomes were adjusted for age, sex, race, income quartile, insurance status, number of Elixhauser’s co-morbidity, hospital bed size, hospital location, discharge weight and baseline comorbidities of atrial fibrillation, hypertension, chronic renal disease, anemia as well as coagulopathy. Finally, a sensitivity analysis was performed using subgroups of model (2) who survived the hospitalization.
All analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, North Carolina) and the description of methodology is presented in graphical form in Supplemental Figure 1A.
Results:
A total of 2,126,331 patients with CABG admissions were identified between 2003–2015 from NIS. Of these, 183,185 (8.6%) were found to have co-morbid cancer and 14,266 (.67%) had metastatic cancer. Baseline demographics, comorbidities, surgical characteristics and hospital level details of those undergoing CABG from 2012–2015 in specific types compared to the non-cancer cohort are shown in Table 1. It is noted that cancer patients undergoing CABG are older, predominantly white, are more likely to have Medicare as their primary insurance and had higher Elixhauser’s mortality score (all P<.001). Four vessel revascularization prevalence for breast (8%) and lung cancer (6%) was lower compared to non-cancer (10.5%; both P<.001). This trend was not seen in those with prostate cancer, colon cancer and lymphoma. (Table 1).
Table 1:
CABG demographics (patient level, financial and hospital level) from 2012–2015 in cancer vs. non-cancer patients.
| Variable | Breast Cancer (n=5,000) a | Lung Cancer (n=2,420) a | Colon Cancer (n = 4,125) a | Prostate Cancer (n = 15,500) a | Lymphoma (n = 3,390) a | Non-Cancer (n=442, 410) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient Characteristics | |||||||||||
| Age, years (mean ± SE) | 70.7±0.3 | <.001 | 69.6±0.4 | <.001 | 70.5±0.3 | <.001 | 72.4±0.1 | <.001 | 64.9±0.5 | <.001 | 63.5+.1 |
| ≥ 65 years (%) | 78.9 | <.001 | 75.6 | <.001 | 77.5 | <.001 | 86.9 | <.001 | 60.5 | <.001 | 51.1 |
| Women, % | 97.3 | <.001 | 30.0 | .03 | 25.5 | .94 | 23.5 | .19 | 25.6 | ||
| Race, % | .0004 | <.001 | .008 | <.001 | <.001 | ||||||
| White | 80.3 | 87.4 | 83.2 | 83.1 | 86.3 | 77.9 | |||||
| Black | 9.7 | 6.1 | 6.1 | 7.3 | 5.2 | 7.5 | |||||
| Hispanic | 5.0 | 2.8 | 5.0 | 4.5 | 4.4 | 7.4 | |||||
| Asian or Pacific Islander | 1.6 | 1.5 | 3.1 | 2.0 | 1.1 | 3.0 | |||||
| Native American | 0.3 | 0.9 | .4 | 0.3 | 0.2 | .6 | |||||
| Other | 3.1 | 1.3 | 2.3 | 2.8 | 3.0 | 3.7 | |||||
| Income quartiles | .64 | .08 | .02 | <.001 | <.001 | ||||||
| 0–25 | 28.3 | 25.0 | 29.8 | 25.2 | 21.5 | 29.5 | |||||
| 26–50 | 26.7 | 32.1 | 24.4 | 24.9 | 28.8 | 27.6 | |||||
| 51–75 | 25.5 | 24.2 | 23.1 | 26.0 | 25.8 | 24.2 | |||||
| 76–100 | 19.5 | 18.7 | 22.7 | 23.9 | 23.9 | 18.7 | |||||
| Payment source (%) | <.001 | <.001 | <.001 | <.001 | <.001 | ||||||
| Medicare | 73.5 | 72.3 | 72.9 | 77.3 | 58.7 | 50.6 | |||||
| Medicaid | 5.1 | 5.0 | 2.9 | 1.1 | 3.9 | 7.5 | |||||
| Private | 19.2 | 19.0 | 21.1 | 18.7 | 34.0 | 34.3 | |||||
| Self-Pay | 1.1 | 1.0 | 1.2 | 0.7 | 1.8 | 4.2 | |||||
| No Charge | 0.1 | 0 | 0.4 | 0.1 | 0.2 | .5 | |||||
| Others | 1.0 | 2.7 | 1.6 | 2.1 | 1.5 | 2.9 | |||||
| Comorbidities (%) | |||||||||||
| Traditional Cardiovascular | |||||||||||
| Cardiomyopathy | 5.2 | .07 | 4.1 | .93 | 5.1 | .13 | 3.4 | .06 | 8.3 | <.001 | 4.1 |
| Prior Myocardial Infarction | 13.4 | .02 | 19.8 | .03 | 18.8 | .04 | 16.7 | .49 | 14.8 | .33 | 16.2 |
| Prior Percutaneous Coronary Intervention | 16.9 | .67 | 14.9 | .14 | 17.5 | .98 | 17.9 | .46 | 18.6 | .43 | 17.4 |
| Prior Coronary Bypass Grafting | 1.5 | .95 | 1.4 | .96 | 1.8 | .41 | 1.2 | .25 | 1.3 | .75 | 1.5 |
| Peripheral Vascular Disease | 16.0 | .24 | 27.7 | <.001 | 15.8 | .40 | 15.1 | .54 | 12.7 | .15 | 14.7 |
| Prior TIA/Stroke | 8.6 | .30 | 11.0 | .008 | 8.9 | .24 | 8.3 | .26 | 6.6 | .29 | 7.7 |
| Atrial Fibrillation | 29.3 | .009 | 33.7 | <.001 | 34.1 | <.001 | 36.4 | <.001 | 28.6 | .08 | 25.6 |
| Hypertension | 88.3 | .001 | 83.5 | .56 | 85.7 | .32 | 86.5 | .002 | 74.8 | <.001 | 84.4 |
| Diabetes | 58.9 | .03 | 45.3 | <.001 | 57.7 | .20 | 48.9 | <.001 | 51.8 | .05 | 55.5 |
| Obesity | 25.0 | .53 | 15.1 | <.001 | 23.5 | .12 | 17.9 | <.001 | 22.4 | .04 | 25.9 |
| Dyslipidemia | 79.7 | .18 | 73.4 | .02 | 74.4 | .02 | 82.1 | <.001 | 73.9 | .01 | 78.0 |
| Non-Traditional | |||||||||||
| Anemia | 19.5 | .13 | 22.3 | .008 | 25.2 | <.001 | 18.7 | .14 | 18.0 | .84 | 17.7 |
| Arthritis and Collagen Vascular disease | 3.3 | .005 | 2.5 | .48 | 2.7 | .20 | 1.7 | .14 | 3.1 | .05 | 2.0 |
| Chronic renal disease | 13.8 | .29 | 17.8 | .09 | 18.6 | .005 | 18.9 | <.001 | 21.4 | <.001 | 15.0 |
| Chronic lung disease | 24.0 | .09 | 57.0 | <.001 | 19.5 | .12 | 18.2 | <.001 | 15.6 | <.001 | 21.8 |
| Coagulation disorder | 15.7 | .85 | 17.2 | .46 | 17.6 | .20 | 18.7 | <.001 | 26.3 | <.001 | 15.9 |
| Smoker | 38.2 | <.001 | 66.3 | <.001 | 44.1 | .33 | 46.2 | .72 | 37.2 | <.001 | 45.8 |
| Total Elixhauser’s comorbidities | <.001 | <.001 | <.001 | .008 | <.001 | ||||||
| 0 | 1.2 | 1.5 | 2.1 | 2.7 | 2.1 | 3.2 | |||||
| 1 | 7.2 | 7.2 | 11.0 | 13.8 | 8.6 | 14.3 | |||||
| 2 | 21.5 | 16.5 | 17.8 | 25.9 | 16.5 | 23.5 | |||||
| ≥ 3 | 70.1 | 74.8 | 69.1 | 57.7 | 72.9 | 59.0 | |||||
| Elixhauser’s mortality score (mean ± SE) | 5.5±.3 | <.001 | 9.8±0.5 | <.001 | 6.0±0.3 | <.001 | 6.1±0.2 | <.001 | 10.1±0.4 | <.001 | 4.1±.1 |
| Surgical Details (%)b | |||||||||||
| One vessel bypass | 18.9 | .12 | 23.4 | .0003 | 18.6 | .26 | 15.0 | .003 | 15.9 | .42 | 17.3 |
| Two vessel bypass | 40.0 | .08 | 42.2 | .03 | 39.9 | .12 | 39.0 | .04 | 40.3 | .10 | 39.3 |
| Three vessel bypass | 26.8 | .12 | 22.7 | .002 | 26.3 | .08 | 29.7 | .48 | 28.5 | .71 | 28.0 |
| Four vessel bypass | 8.0 | .0001 | 6.0 | <.001 | 10.2 | .12 | 11.5 | .53 | 10.6 | .28 | 10.5 |
| One internal mammary artery use | 84.3 | <.001 | 82.0 | <.001 | 89.9 | .16 | 89.4 | .09 | 88.9 | .60 | 88.4 |
| Two internal mammary artery use | 2.5 | .0007 | 2.5 | .02 | 3.4 | .07 | 3.2 | .002 | 3.1 | .05 | 3.5 |
| Teaching hospital (%) | 70.4 | .13 | 69.4 | .54 | 67.8 | .82 | 68.5 | .72 | 70.8 | .14 | 68.1 |
| Bed size, (%) | .66 | .17 | .70 | .007 | .32 | ||||||
| Small | 8.6 | 6.8 | 8.5 | 8.0 | 7.5 | 9.2 | |||||
| Medium | 22.9 | 22.9 | 23.2 | 22.3 | 23.5 | 23.8 | |||||
| Large | 68.5 | 70.3 | 68.4 | 69.7 | 69.0 | 67.1 | |||||
| Region (%) | .003 | .22 | .005 | <.001 | .02 | ||||||
| Northeast | 19.9 | 16.1 | 18.1 | 18.8 | 19.9 | 15.8 | |||||
| Midwest | 23.3 | 24.8 | 26.6 | 23.7 | 23.5 | 22.8 | |||||
| South | 42.0 | 46.9 | 40.5 | 41.0 | 41.7 | 45.9 | |||||
| West | 14.8 | 12.2 | 14.9 | 16.5 | 14.9 | 15.5 | |||||
P-values presented in column next to the values for each cancer is versus non-cancer, the values of which is presented in last column
these don’t add up to 100% but representative of % of total number of CABG in each group. If a surgery had one vessel bypass with only internal mammary artery it will be counted twice.
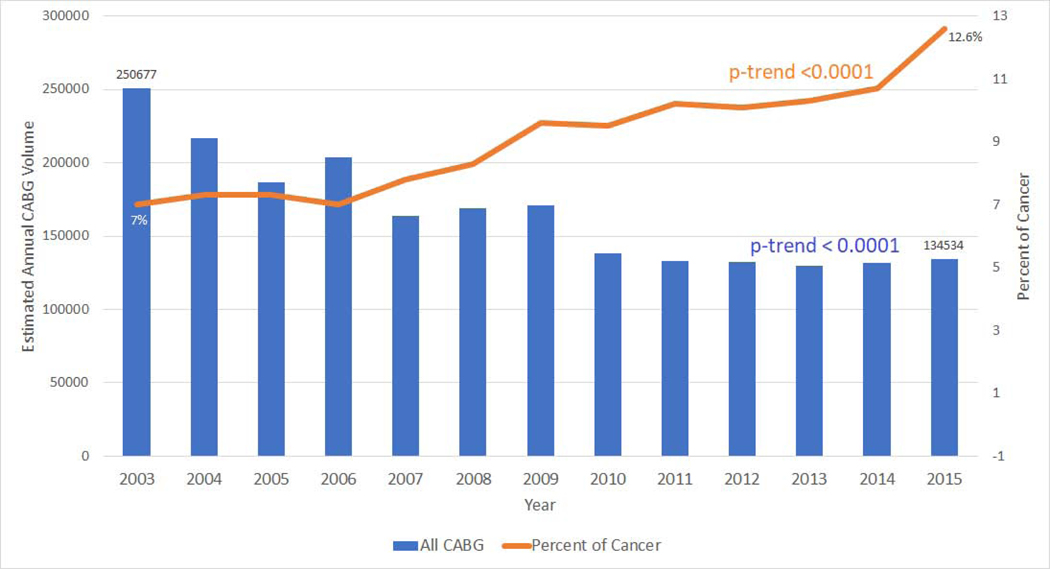
Overall, CABG use in the US fell over time 250,677 in 2003 vs. 134,534 in 2015, P<.001; Figure 1). However, over the same period, the percentage/proportion of cancer patients undergoing CABG use increased over time (7% in 2003 to 12.6% in 2015, P<.001; Figure 1). The cancer sub-groups comprised of prostate cancer (30%) followed by breast cancer (10%), colon cancer (8%) and lymphoma (6%) (Supplemental Figure 1B). On stratification by cancer types, there was an absolute reduction in CABG use among breast, lung, colon, prostate and lymphoma patients over time (Supplemental Figure, 2A-E). Yet, among cancer patients, no change in the relative proportion of CABG use was seen when stratified by presence of metastases (P=.12; Supplemental Figure 2F).
Figure 1: Coronary artery bypass graft use and cancer.
Overall, coronary artery bypass graft use in the United States fell over time (250,677 in 2003 vs. 134,534 in 2015, P<.001; first Y axis). However, over the same period, the percentage of cancer patients undergoing coronary artery bypass graft use increased over time (7% in 2003 to 12.6% in 2015, P<.001; second Y axis).
Propensity matched trends in complications, outcomes, and disposition associated with CABG use in cancer vs. non-cancer (2003–2015)
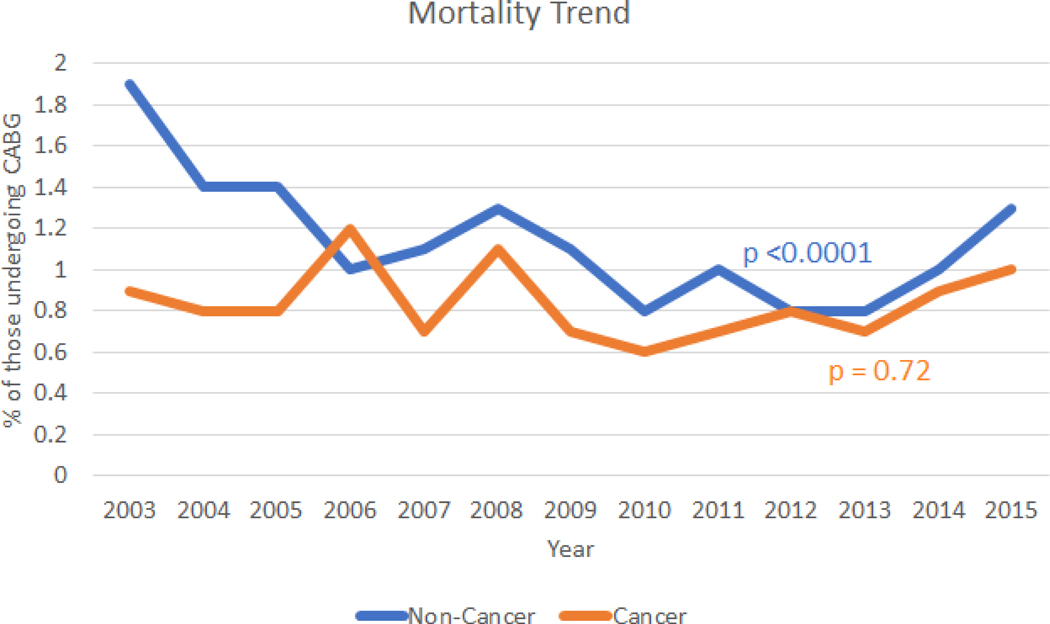
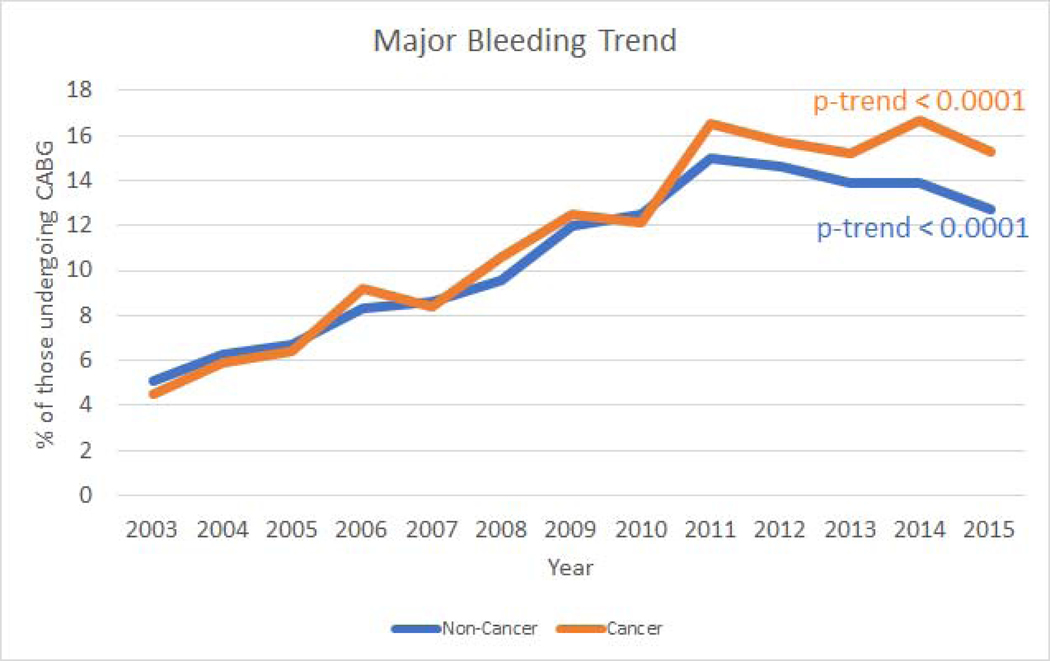
Over time (2003–2015) we saw a decreasing trend of in-hospital mortality associated with CABG use in non-cancer (P<.001) which was not seen in the cancer population (P=.72; Figure 2); however, we saw an increasing trend in associated major bleeding trend in both cancer and non-cancer (P<.001 for both; Figure 3). Additionally, we also saw an increasing trend in stroke associated with CABG use in both cancer and non-cancer (P<.001 for both; Supplemental Figure 3A). Furthermore, consistent with increasing CABG use in cancer, we also observed an increasing trend in in-hospital cost of care associated with CABG use (P<.001) for both cancer and non-cancer, Supplemental Figure 3B).
Figure 2: Propensity matched in-hospital mortality trends and coronary artery bypass graft use in cancer and non-cancer.
Trends in in-hospital mortality per 100 coronary artery bypass graft utilizations in cancer and non-cancer patients from 2003–2015. (P-trends = .72 for cancer and <.001 for non-cancer).
Figure 3: Propensity matched in-hospital major bleeding trends and coronary artery bypass graft use in cancer and non-cancer.
Trends in in-hospital bleeding per 100 coronary artery bypass graft utilizations in cancer and non-cancer patients from 2003–2015. (P<.001 for both trends).
Complications, outcomes, and disposition associated with CABG use in cancer types vs. non-cancer (propensity matched, 2012–2015)
Outcomes, complications as well as dispositions associated with cancer types are shown in Table 2, 3 and Supplemental Table 3. While in-hospital mortality was comparable in breast lung, colon cancer and lymphoma vs. non-cancer CABG patients (all P>.05), however prostate cancer patients undergoing CABG had lower in-hospital mortality compared to non-cancer CABG patients (P=.01). Moreover, there was a significantly higher prevalence of major bleeding in patients with breast and prostate cancer vs. non-cancer CABG patients (P<.01) but not in lung, colon cancer and lymphoma vs. non-cancer CABG patients (P>.05). In addition, CABG in breast cancer and lymphoma was associated with similar rates of stroke, pulmonary/cardiac complications, LoS, as well as total hospital costs (P>.05) compared to their counterparts. Additionally, in a sensitivity analyses we compared the analysis of length of stay and cost of care among those propensity matched for cancer vs non-cancer including only those hospitalizations that did not suffer in-hospital mortality and found consistent results.
Table 2:
Propensity matched (1 cancer: 2 non-cancer, model 2) in-hospital and disposition outcome of those undergoing CABG from the years 2012- September 2015 in breast cancer and lung cancer. The propensity matching was done on variables of age, gender, race, income quartiles, insurance, total Elixhauser’s comorbidities, hospital size and geographic region, discharge weight and comorbidities of atrial fibrillation, hypertension, diabetes, anemia, chronic renal disease and coagulation disorder. C-statistic for propensity fit was 0.7 for both cohort’s indicative of good match. In breast cancer gender was not used for matching since >99.7% cases were female.
| Variable | Breast Cancer (n = 5,000) | Matched Non-Cancer (n = 10,000) | P-value | Lung Cancer (n=2,295) | Matched Non-Cancer (n = 4,600) | P-value |
|---|---|---|---|---|---|---|
| In-Hospital Outcomes (%) | ||||||
| In-hospital mortality | 1.3 | .9 | .31 | 1.3 | 1.3 | >.99 |
| Major bleeding | 20.6 | 13.9 | <.001 | 16.5 | 13.8 | .19 |
| Ischemic Stroke | 2.6 | 2.3 | .56 | 2.0 | 1.4 | .43 |
| Pulmonary complications | 9.5 | 9.1 | .69 | 11.8 | 9.2 | .14 |
| Cardiac complications | 9.1 | 10.3 | .31 | 11.1 | 11.6 | .77 |
| Length of stay (median ± confidence interval, days) | 7.5±.1 | 7.2±.1 | .22a | 7.2±.2 | 7.3±.1 | .49 a |
| Total hospital costs (median ± confidence interval, US$) b | 34,219±699 | 33,713±467 | .24a | 34,483±697 | 32,163±721 | .04 a |
| Disposition (%) | <.001 | .47 | ||||
| Home | 30.0 | 37.1 | 33.3 | 37.0 | ||
| Short term hospital | .4 | .7 | 1.3 | .8 | ||
| Skilled care facility | 33.7 | 26.3 | 26.8 | 25.6 | ||
| Home health care | 35.9 | 35.9 | 38.6 | 36.6 |
Log transformed means were compared using Survey specific linear regression due to skewed nature of data
Using HCUP cost-to-charge, wage index adjustment along with inflation adjustment
Table 3:
Propensity matched (1 cancer: 2 non-cancer, model 2) in-hospital and disposition outcome of those undergoing CABG from the years 2012- September 2015 in colon cancer, and prostate cancer. The propensity matching was done on variables of age, gender, race, income quartiles, insurance, total Elixhauser’s comorbidities, hospital size and geographic region, discharge weight and comorbidities of atrial fibrillation, hypertension, diabetes, anemia, chronic renal disease and coagulation disorder. C-statistic for propensity fit was 0.7 indicative of good match. For prostate cancer gender was not used since all patient with prostate cancer were male.
| Variable | Colon Cancer (n = 3,930) | Matched Non-Cancer (n = 7,865) | P-value | Prostate Cancer (n = 14,335) | Matched Non-Cancer (n = 28,675) | P-value |
|---|---|---|---|---|---|---|
| In-Hospital Outcomes (%) | ||||||
| In-hospital mortality | .6 | 1.2 | .19 | 3.9 | .9 | .01 |
| Major bleeding | 15.9 | 12.9 | .053 | 14.8 | 14.2 | .45 |
| Ischemic Stroke | 1.5 | 2.6 | .08 | 1.1 | 2.0 | .003 |
| Pulmonary complications | 7.0 | 10.2 | .01 | 7.7 | 9.9 | .001 |
| Cardiac complications | 11.6 | 12.1 | .69 | 12.0 | 12.4 | .58 |
| Length of stay (median ± confidence interval, days) | 6.8±.1 | 7.2±.1 | <.001 a | 6.8±.1 | 7.1±.1 | <.001 a |
| Total hospital costs (median ± confidence interval, US$) b | 33,014±637 | 32,793±501 | .63 a | 33,380±378 | 34,099±312 | <.001 a |
| Disposition (%) | .50 | .25 | ||||
| Home | 35.9 | 35.5 | 37.0 | 37.5 | ||
| Short term hospital | 1.2 | .6 | .4 | .8 | ||
| Skilled care facility | 26.0 | 25.2 | 23.4 | 23.9 | ||
| Home health care | 36.9 | 38.7 | 39.2 | 37.8 |
Log transformed means were compared using Survey specific linear regression due to skewed nature of data
Using HCUP cost-to-charge, wage index adjustment along with inflation adjustment
Further, lung cancer had higher associated total hospital costs (P=.04). It was noted that colon cancer had lower rates of stroke, pulmonary complications and LoS (P<.05) and prostate cancer had a lower total hospital costs (P<.001). Finally, discharge dispositions were not found to be different between cancer types and non-cancer patients (P>.05;) except breast cancer patients who had lower home, but higher skilled care disposition compared to their counterparts (P<.001).
Additionally, we also observed comparable in-hospital mortality and higher prevalence of major bleeding in metastatic cancer than non-metastatic cancer (Supplemental Table 4). Finally, while in-hospital outcomes and discharge disposition were not associated with previous radiotherapy use, there was a significantly higher total hospital costs, but lower length of stay associated with previous radiotherapy use in CABG patients (Supplemental Table 4).
Discussion:
In this large contemporary population-based analysis, we found that even though the overall number of annual CABG procedures has decreased over time, the concurrent relative proportion of those with co-morbid cancer has increased. Among patients undergoing CABG with co-morbid cancer, in-hospital mortality did not change over time. However, the proportion of these patients with complicating major bleeding, or ischemic stroke increased over time. Similarly, the cost-of-care in those with co-morbid cancer undergoing CABG trended up as well. Moreover, cancer patients saw no elevated risk associated with CABG use compared to non-cancer patients except breast and prostate cancer that had higher prevalence of major bleeding associated with CABG use. These findings suggest that among select patients; utilization of CABG may still be efficacious even in the presence of a cancer diagnosis.
The reduction in number of CABG patients observed over time in our study could be reflective of the increased use of PCI procedures in cancer. In fact, a prior paper demonstrated that between 2004 and 2014, there was a modest increase in the number of PCI procedures performed in patients with cancer over time4. The prognostic impacts of cancer on PCI outcomes have been previously described17, 18. A recent study demonstrated that patients with cancer undergoing PCI in the US have worse short-term clinical outcomes compared to non-cancer patients1, 4. Importantly, cancer patients undergoing PCI are known to be at elevated risk for potential peri-procedural or in-hospital adverse events, such as increased in-hospital mortality and bleeding. This is only compounded by the potential off-target adverse CVD effects of many contemporary anti-cancer therapies, as well as alterations in coagulation profile seen in cancer3, 4. Finally, given the increasing incidence of complex CAD in the setting of cancer, efficacious revascularization strategies to optimize outcomes, particularly in those not eligible for PCI, are needed4.
An alternate revascularization strategy less explored in cancer is CABG19. Cancer patients with complex CAD, as well as those with contraindications for PCI, such as patients not able to tolerate dual anti-platelet therapy, often warrant potential CABG for revascularization1. Additionally, among patients opting for CABG20–23, improved mortality has been reported during the past few years24, 25. Our study demonstrated comparable mortality, complication and disposition rates between cancer and non-cancer patients; however, breast and prostate cancer had higher prevalence of major bleeding compared to non-cancer patients following CABG. Our findings are in line with past studies that have shown an increase in the rate of post-operative bleeding associated with CABG use over time26. This may have been due to introduction of newer anti-coagulation and anti-fibrinolytic drugs over recent years, which may mitigate ischemic risk but increases risk of bleeding in certain cancers1, 27. Second, breast and prostate cancers may represent patients with very advanced cancer stage and thus have higher bleeding risk associated with CABG compared with other cancer types. Finally, the use of anti-cancer therapy specific to breast and prostate cancer such as selective estrogen receptor modulator and androgen deprivation therapy respectively may in part explain the higher bleeding risk associated with breast and prostate cancers1, 27.
The in-hospital outcomes, complications and dispositions not being different between majority of cancer types and non-cancer, further suggests of selective efficacy among this population. Different cancer types represent completely different risk profiles and thus more data are required to test the impact of selective interventions by cancer types. However, surgical revascularization may remain a strong option in those with complex CAD and cancer. Our findings will help move towards personalized treatment by accurately risk stratifying different cancer types and accordingly selecting the best form of revascularization. These findings have important implications for the practicing surgeon since these findings will enhance his decision process behind type of revascularization to be used appropriately in this growing population.
Several limitations of this study warrant consideration. Because of reliance on ICD-9-CM codes, we could not determine previous cancer diagnosis (active or historical) or the duration of a cancer diagnosis or CABG use. Unfortunately, NIS data being an administrative dataset does not allow for precise staging of cancer beyond the presence of advanced or metastatic disease, nor is there provision for timing or type of anti-cancer therapy (i.e. radiation vs. chemotherapy vs. recent cancer surgery), or code status. Prior use of radiotherapy, although coded in NIS may be underreported and thus results should be interpreted as such. Although we used a matched propensity score design to account for indication bias, important unmeasured clinical characteristics that may be predictors of outcomes were not available, and therefore these findings may be subject to confounding. Notably, given the datasets used, this study represents associations with no mechanistic data included to imply causal relationships. We could not capture code status and the impact of patient-physician discussion of prognosis and shared decision making. In addition, due to the administrative nature of data, we were unable to distinguish co-morbidities from complications of hospitalization. Moreover, as the NIS is a deidentified database, long-term outcomes and complications occurring after the initial CABG hospitalization could not be assessed. Furthermore, it is also not possible to follow-up patients in NIS dataset thus precluding us from performing any survival analyses. Finally, despite the performance of extensive analyses and the overall comprehensive nature of the NIS dataset employed, the precise factors underlying post-operative bleeding, such as use of active or recent treatments (i.e. surgery, anti-coagulant therapies, etc.) could not be determined and require prospective validation.
Conclusion:
In summary, despite general declines in the number of annual CABG procedures, the proportional representation of those with cancer has increased over time. The presence of specific types of cancer does not appear to associate with increased mortality or limiting complications, except higher bleeding risk for breast and prostate cancer, even after accounting for comorbid disease burden. Given the prevalence of complex coronary disease, and the potential challenges of prolonged anticoagulation in the presence of cancer, consistent consideration of the efficacy of CABG is warranted depending on cancer type, thus helping the surgeon chose the best form of revascularization procedure. Moreover, further research into the factors underlying the decision to pursue CABG in cancer patients, the impact of patient-physician perceptions of cancer prognosis, and the factors associated with survival after CABG among those with cancer are needed.
Supplementary Material
Acknowledgments
FUNDING: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Dr. Gumina is supported by NIH NHLBI grant R01HL127442. Dr. Addison was supported by an NCI K12CA133250 grant.
ABBREVIATIONS LIST:
- CABG –
Coronary artery bypass graft
- PCI –
Percutaneous coronary intervention
- NIS-
National inpatient sample
- CVD:
Cardiovascular disease
Footnotes
DISCLOSURES: All other authors declare no conflicts of interests in relation to the work presented in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Guha A, Dey AK, Jneid H, Addison D. Acute Coronary Syndromes in Cancer Patients. European heart journal. 2019;40:1487–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Navi BB, Reiner AS, Kamel H, et al. Arterial thromboembolic events preceding the diagnosis of cancer in older persons. Blood. 2019;133:781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Hawwas M, Tsitlakidou D, Gupta N, Iliescu C, Cilingiroglu M, Marmagkiolis K. Acute Coronary Syndrome Management in Cancer Patients. Curr Oncol Rep. 2018;20:78. [DOI] [PubMed] [Google Scholar]
- 4.Potts JE, Iliescu CA, Lopez Mattei JC, et al. Percutaneous coronary intervention in cancer patients: a report of the prevalence and outcomes in the United States. European heart journal. 2019;40:1790–1800. [DOI] [PubMed] [Google Scholar]
- 5.Kilic A, Shah AS, Conte JV, et al. Understanding variability in hospital-specific costs of coronary artery bypass grafting represents an opportunity for standardizing care and improving resource use. J Thorac Cardiovasc Surg. 2014;147:109–115. [DOI] [PubMed] [Google Scholar]
- 6.Overview of Disease Severity Measures Disseminated with the Nationwide Inpatient Sample (NIS) and Kids’ Inpatient Database (KID). Vol 2018 Rockville, MD2005. [Google Scholar]
- 7.Guha A, Dey AK, Armanious M, et al. Health care utilization and mortality associated with heart failure-related admissions among cancer patients. ESC Heart Fail. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying Increased Risk of Readmission and In-hospital Mortality Using Hospital Administrative Data: The AHRQ Elixhauser Comorbidity Index. Med Care. 2017;55:698–705. [DOI] [PubMed] [Google Scholar]
- 9.CPI Inflation Calculator. Vol 2018. Online2018. [Google Scholar]
- 10.Houchens R, Ross D, Elixhauser A. Final Report on Calculating National Inpatient Sample (NIS) Variances for Data Years 2012 and Later. HCUP Methods Series Report # 2015–09. Vol 2018. ONLINE2015. [Google Scholar]
- 11.Yoon F, Sheng M, Jiang HJ, Steiner CA, Barrett ML. Calculating Nationwide Readmissions Database (NRD) Variances. HCUP Methods Series Report # 2017–01 ONLINE. Vol 2018: U.S. Agency for Healthcare Research and Quality.; 2017. [Google Scholar]
- 12.Bland JM, Altman DG. Transformations, means, and confidence intervals. BMJ. 1996;312:1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilbert BD, Horstman JM. Captain’s LOG: Taking Command of SAS® Logarithm Functions. Vol 20182014. [Google Scholar]
- 14.Dugoff EH, Schuler M, Stuart EA. Generalizing observational study results: applying propensity score methods to complex surveys. Health Serv Res. 2014;49:284–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kontopantelis E, White IR, Sperrin M, Buchan I. Outcome-sensitive multiple imputation: a simulation study. BMC Med Res Methodol. 2017;17:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parsons LS. Performing a 1: N case-control match on propensity score. proceedings of the 29th Annual SAS users group international conference: SAS Institute; 2004:165–129. [Google Scholar]
- 17.Tabata N, Sueta D, Yamamoto E, et al. Outcome of current and history of cancer on the risk of cardiovascular events following percutaneous coronary intervention: a Kumamoto University Malignancy and Atherosclerosis (KUMA) study. European heart journal. Quality of care & clinical outcomes. 2018;4:290–300. [DOI] [PubMed] [Google Scholar]
- 18.Hess CN, Roe MT, Clare RM, et al. Relationship Between Cancer and Cardiovascular Outcomes Following Percutaneous Coronary Intervention. Journal of the American Heart Association. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moazzami K, Dolmatova E, Maher J, et al. In-Hospital Outcomes and Complications of Coronary Artery Bypass Grafting in the United States Between 2008 and 2012. Journal of cardiothoracic and vascular anesthesia. 2017;31:19–25. [DOI] [PubMed] [Google Scholar]
- 20.Epstein AJ, Polsky D, Yang F, Yang L, Groeneveld PW. Coronary revascularization trends in the United States, 2001–2008. JAMA. 2011;305:1769–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cornwell LD, Omer S, Rosengart T, Holman WL, Bakaeen FG. Changes over time in risk profiles of patients who undergo coronary artery bypass graft surgery: the Veterans Affairs Surgical Quality Improvement Program (VASQIP). JAMA surgery. 2015;150:308–315. [DOI] [PubMed] [Google Scholar]
- 22.Nallamothu BK, Young J, Gurm HS, Pickens G, Safavi K. Recent trends in hospital utilization for acute myocardial infarction and coronary revascularization in the United States. The American journal of cardiology. 2007;99:749–753. [DOI] [PubMed] [Google Scholar]
- 23.ElBardissi AW, Aranki SF, Sheng S, O’Brien SM, Greenberg CC, Gammie JS. Trends in isolated coronary artery bypass grafting: an analysis of the Society of Thoracic Surgeons adult cardiac surgery database. J Thorac Cardiovasc Surg. 2012;143:273–281. [DOI] [PubMed] [Google Scholar]
- 24.Reston JT, Tregear SJ, Turkelson CM. Meta-analysis of short-term and mid-term outcomes following off-pump coronary artery bypass grafting. The Annals of thoracic surgery. 2003;76:1510–1515. [DOI] [PubMed] [Google Scholar]
- 25.Culler SD, Kugelmass AD, Brown PP, Reynolds MR, Simon AW. Trends in coronary revascularization procedures among Medicare beneficiaries between 2008 and 2012. Circulation. 2015;131:362–370; discussion 370. [DOI] [PubMed] [Google Scholar]
- 26.Shiomi H, Morimoto T, Furukawa Y, et al. Comparison of Five-Year Outcome of Percutaneous Coronary Intervention With Coronary Artery Bypass Grafting in Triple-Vessel Coronary Artery Disease (from the Coronary Revascularization Demonstrating Outcome Study in Kyoto PCI/CABG Registry Cohort-2). The American journal of cardiology. 2015;116:59–65. [DOI] [PubMed] [Google Scholar]
- 27.Mangano DT, Miao Y, Vuylsteke A, et al. Mortality associated with aprotinin during 5 years following coronary artery bypass graft surgery. JAMA. 2007;297:471–479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.