Abstract
Outbreaks of COVID-19 (coronavirus disease 2019) have been reported in workers in fish farms and fish processing plants arising from person-to-person transmission, raising concerns about aquatic animal food products' safety. A better understanding of such incidents is important for the aquaculture industry's sustainability, particularly with the global trade in fresh and frozen aquatic animal food products where contaminating virus could survive for some time. Despite a plethora of COVID-19-related scientific publications, there is a lack of reports on the risk of contact with aquatic food animal species or their products. This review aimed to examine the potential for Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) contamination and the potential transmission via aquatic food animals or their products and wastewater effluents. The extracellular viability of SARS-CoV-2 and how the virus is spread are reviewed, supporting the understanding that contaminated cold-chain food sources may introduce SAR-CoV-2 via food imports although the virus is unlikely to infect humans through consumption of aquatic food animals or their products or drinking water; i.e., SARS-CoV-2 is not a foodborne virus and should not be managed as such but instead through strong, multifaceted public health interventions including physical distancing, rapid contact tracing, and testing, enhanced hand and respiratory hygiene, frequent disinfection of high-touch surfaces, isolation of infected workers and their contacts, as well as enhanced screening protocols for international seafood trade.
Keywords: SARS-CoV-2, Aquaculture, Aquatic food products, Foodborne viruses, Transmission, Zoonotic
Graphical abstract
1. Description of foodborne viruses
The early reports suggested that the seafood market's exceptionally wide contamination, such as seafood tanks, air contamination by live animals from various sources for sale, or rodent infestation, might explain the initiation of the SARS-CoV-2 outbreak (Jalava, 2020; Ceylan et al., 2020), but this virus is characteristically not foodborne (Li et al., 2020a). Most recently, the transmission of SARS-CoV-2 via contaminated cold-chain food sources has been linked to two re-emergent outbreaks of COVID-19 in Beijing, China (Han et al., 2020; Pang et al., 2020), but the route of infection was not established. This nuance can be categorized as a “non-traditional” transmission mechanism (Fisher et al., 2020). In this review, foodborne viruses refer to human and animal viruses infecting humans via food consumption and drinking water. These viruses normally infect humans upon ingestion of food contaminated in one of three main ways: (1) by infected food handlers, (2) by food that has been in contact with animal body fluids (zoonotic transmission), human feces or vomit, or aerosols from an infected person or contaminated materials, and (3) by food products (e.g., pork and liver) originating from infected animals (zoonotic transmission) (Meng et al., 1997; Acha and Szyfres, 2003; Tei et al., 2003; Koopmans and Duizer, 2004; FAO/WHO Food and Agriculture Organization of the United Nations/World Health Organization, 2008; Vasickova et al., 2005; Lewis et al., 2010; EFSA European Food Safety AuthorityHAZ, 2011; Velebit et al., 2019; Desdouits et al., 2020; Carraturo et al., 2020). Infections with these viruses are common causes of human disease (Havelaar et al., 2015; Petrović and D'Agostino, 2016; Desdouits et al., 2020).
The viruses, sometimes referred to as enteric viruses (Fabiszewski de Aceituno et al., 2013; Miranda and Schaffner, 2019), are shed in feces (Koopmans and Duizer, 2004), resulting in fecal-oral transmission (Li et al., 2020a; Miranda and Schaffner, 2019) or they are shed in vomitus and transmitted by the ingestion of aerosolized vomitus particles (Miranda and Schaffner, 2019). The list of viruses, which is summarized in Table 1 , is very long (Fabiszewski de Aceituno et al., 2013) and includes viruses primarily transmitted via food or drinking water, such as enteroviruses, hepatitis A virus, hepatitis E virus, norovirus, and rotavirus (Vasickova et al., 2005; Bosch et al., 2018; Velebit et al., 2019). Humans infected with these viruses often shed large amounts of virus particles in the diarrheal feces (~105 to >1012 infectious particles per ml or g) (Gerba, 2000; Bishop, 1996) while the infective dose is relatively low (~101 to 102 infectious particles) which easily leads to infection upon ingestion of the contaminated food (Anderson and Weber, 2004; Todd et al., 2008). The Table 1 list also includes some emerging zoonotic viruses capable of being transmitted via food (Koopmans and Duizer, 2004), such as SARS-CoV, Nipah virus (Luby et al., 2006), and highly pathogenic avian influenza (HPAI) virus (FAO/WHO Food and Agriculture Organization of the United Nations/World Health Organization, 2008) that can replicate in the human gastrointestinal tract and have animal reservoirs and are therefore a permanent threat for pandemics (Bosch et al., 2018). However, this transmission route is rare and is probably restricted to few situations, for example, HPAI virus in poultry or eggs and Nipah virus in date palm sap. The potential for various food products, including meat and meat products, dairy products, bread, fruits, vegetables, and ready-to-eat foods, to serve as carriers for transmission of SARS-CoV-2 has been reviewed (Yekta et al., 2020); no direct link has been established between SARS-CoV-2 infection and food consumption. To date, no zoonotic fish viruses have been reported (Boylan, 2011; Woolhouse et al., 2012; Bondad-Reantaso et al., 2020), although fish is recognized as a reservoir host for the San Miguel sea lion virus (or Vesicular exanthema of swine virus) that has been associated with human infection and vesicular lesions (Smith et al., 1998).
Table 1.
Viruses transmitted to humans through food consumption and drinking water.
| Virus common name (abbreviation and/serotype) | Virus family | Food commodity | Clinical disease produced | Reference |
|---|---|---|---|---|
| Hepatitis A virus (HAV)⁎, 1 | Picornaviridae | Bivalve molluscan shellfish (including oysters, clams, cockles and mussels); fresh produce; prepared foods | Hepatitis | Lowry et al., 1989; Cliver, 1997; Bidawid et al., 2000; Sattar et al., 2000; Vasickova et al., 2005; FAO & WHO, 2008; Velebit et al., 2015, Velebit et al., 2019; Miranda and Schaffner, 2019 |
| Norovirus (NoV)⁎, 1 | Caliciviridae | Bivalve molluscan shellfish (including oysters, clams, cockles and mussels); fresh produce; prepared foods | Gastroenteritis | Cliver, 1997; Koopmans and Duizer, 2004; Vasickova et al., 2005; FAO & WHO, 2008; Baert et al., 2011; Rodriguez-Manzano et al., 2014; Velebit et al., 2015, Velebit et al., 2019; Miranda and Schaffner, 2019 |
| Sapovirus | Caliciviridae | Salad; river water; oysters | Gastroenteritis | Koopmans and Duizer, 2004; Vasickova et al., 2005; Miranda and Schaffner, 2019; Yekta et al., 2020 |
| Human rotavirus (HRV) (group A-C)⁎ | Reoviridae | Water used for drinking, ice production, or for food preparation/processing | Gastroenteritis | Koopmans and Duizer, 2004; Vasickova et al., 2005; Velebit et al., 2015, Velebit et al., 2019; Miranda and Schaffner, 2019 |
| Enterovirus (e.g., poliovirus, Coxsackie A, B virus) | Picornaviridae | Oysters; contaminated water or food | Associated with a range of symptoms including neurological symptoms | Koopmans and Duizer, 2004; Vasickova et al., 2005; Bosch et al., 2018 |
| Hepatitis E virus (HEV)⁎ | Hepeviridae | raw or undercooked meat of pig or wild boar or Sika deer; unpasteurized milk, shellfish and ethnic foods; contaminated water | Hepatitis | Meng et al., 1997; Tei et al., 2003; Vasickova et al., 2005; FAO & WHO, 2008; Lewis et al., 2010; Velebit et al., 2015, Velebit et al., 2019; Miranda and Schaffner, 2019; Yekta et al., 2020 |
| Astrovirus | Astroviridae | transmission is fecal-oral via food or water (<1% of astrovirus infections are considered foodborne (Glass et al., 1996) | Gastroenteritis | Koopmans and Duizer, 2004; Vasickova et al., 2005 |
| Human parvovirus | Parvoviridae | Shellfish | Erythema infectiosum | Yekta et al., 2020 |
| Human adenovirus (HAdv) (types 40 and 41) | Adenoviridae | Shellfish | Gastroenteritis | Koopmans and Duizer, 2004; Vasickova et al., 2005; Rodriguez-Manzano et al., 2014. |
| Rodent arenaviruses | Vasickova et al., 2005 | |||
| Tick-borne encephalitis virus (TBE) | Flaviviridae | raw (unpasteurized) cow's or goat's or sheep's milk and raw milk cheeses | Encephalitis |
Dumpis et al., 1999; Acha and Szyfres, 2003; Vasickova et al., 2005 |
| Hantavirus | Contamination of food or water with saliva or urine, or through the dust of feces from infected wild rodents | Hantavirus pulmonary syndrome (HPS) & Hemorrhagic fever with renal syndrome | Acha and Szyfres, 2003; Vasickova et al., 2005 | |
| FMDV | Picornaviridae | Raw cow milk | malaise, fever, vomiting, oral ulcers & skin blisters | Vasickova et al., 2005 |
| Aichi virus | Picornaviridae | Oysters and seafood | Gastroenteritis | Koopmans and Duizer, 2004; Vasickova et al., 2005 |
| Human coronavirus | Coronaviridae | Gastroenteritis & common cold | Koopmans and Duizer, 2004 | |
| Bovine coronavirus | Coronaviridae | Gastroenteritis | MacLachlan and Dubovi, 2017 | |
| Severe Acute Respiratory Syndrome-Coronavirus (SARS-CoV)⁎ | Coronaviridae | FAO & WHO, 2008; Petrović and D'Agostino, 2016 | ||
| Nipah virus⁎ | Fruit | Luby et al., 2006; FAO & WHO, 2008; Petrović and D'Agostino, 2016; Velebit et al., 2015, Velebit et al., 2019 | ||
| Highly pathogenic avian influenza (HPAI) virus⁎ | Poultry | FAO & WHO, 2008; Petrović and D'Agostino, 2016 |
denotes foodborne viruses of main concern (Koopmans and Duizer, 2004; FAO & WHO, 2008).
the two most important foodborne viruses; primarily associated with food-handler transmission and sewage-contaminated foods (Velebit et al., 2019): “NoV is most significant by virtue of sheer number of cases”, and “HAV because it causes a more severe disease” (Fabiszewski de Aceituno et al., 2013).
Coronaviruses that infect humans are mostly transmitted via the respiratory route. It is not established that they are also transmitted via the fecal-oral route like foodborne viruses to cause infection in the human gastrointestinal tract (Li et al., 2020a). Table 2 compares the sources of transmission between enteric viruses, respiratory viruses, and SARS-CoV-2. While SARS-CoV-2 is shed in feces and the viral nucleic acids (RNA) can be detected in sewage-polluted water (Randazzo et al., 2020; Ahmed et al., 2020; Orive et al., 2020; La Rosa et al., 2020; Wurtzer et al., 2020; Medema et al., 2020; Wu et al., 2020a, Wu et al., 2020b, Wu et al., 2020c; Rimoldi et al., 2020; Ampuero et al., 2020), there is no proof that it is transmitted through food consumption (Desai and Aronoff, 2020; EFSA, 2020). Nonetheless, concerns have been raised about the presence of SARS-CoV-2 on frozen aquatic food animal species or their products, including their packaging materials and storage environments (Bondad-Reantaso et al., 2020; Caiyu and Hui, 2020; Han et al., 2020), necessitating better information on the associated risk of virus spread through the consumption of aquatic food products.
Table 2.
Virus sources of transmission for enteric viruses, respiratory viruses, and SARS-CoV-2.
| Source | Enteric viruses1 | Respiratory viruses2 | SARS-CoV-2 |
|---|---|---|---|
| Drinking water | +4 | − | ? |
| Foods3 | + | ? | ?* |
| Person-to-person | + | + | + |
| Fomites | + | + | +** |
| Wastewater | + | ? | ? |
The enteric viruses are listed in Table 1.
Respiratory viruses include Influenza A and B viruses, respiratory syncytial virus (RSV), rhinovirus, parainfluenza viruses, adenovirus, human bocavirus and coronaviruses, and emerging zoonotic viruses including avian influenza viruses, Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS) coronaviruses).
The term foods is used to include fresh, frozen, prepared or processed food in general.
+ denotes confirmed (**although several studies have shown that SARS-CoV-2 can survive on fomites, transmission through contaminated surfaces is “not thought to be a significant risk” (Lewis, 2021); ? denotes not yet known (*although long-range transport of SARS-CoV-2 has been linked to contaminated cold-chain food sources, “no direct link has been established between COVID-19 infection and foodborne transmission” (Han et al., 2020); − denotes not known to occur.
2. Coronavirus taxonomy
Coronaviruses are classified in the order Nidovirales, which derives its name from the Latin word “nidus”, meaning “nest”, in reference to the unique strategy of genome expression of nidoviruses during replication. The virus particles have an envelope and a large single-stranded RNA genome of positive sense (ranging from 26 to 32 kb) with a 5′ cap and 3′ poly-A tail and replicate in the cytoplasm of the host cell and bud into the Golgi apparatus, passing through the cytoplasm in smooth-walled vesicles and are then released through exocytosis (MacLachlan and Dubovi, 2017). These virions, which are pleomorphic but often spherical or rod-shaped, range from 120 to 220 nm in diameter, with distinctive and large (20 nm) club-shaped glycoprotein spikes protruding from the viral envelope. These viruses affect a wide range of vertebrate hosts, frequently targeting respiratory or enteric epithelial cells (MacLachlan and Dubovi, 2017). In 2018, the International Committee for the Taxonomy of Viruses (ICTV, 2019) approved changes to the nidovirus taxonomy based on genomic analyses undertaken by Gulyaeva et al. (2017); the family Coronaviridae now has two subfamilies, Orthocoronavirinae and Letovirinae.
The subfamily Letovirinae has one genus, Alphaletovirus, containing two species, Microhyla alphaletovirus 1 of the ornamented pygmy frog Microhyla fissipes (Bukhari et al., 2018), and Pacific salmon nidovirus (PsNV) discovered in diseased farmed Chinook salmon Oncorhynchus tshawytscha off the coast of Vancouver Island-Canada (Mordecai et al., 2019).
The subfamily Orthocoronavirinae has four genera, Alphacoronavirus (αCoV), Betacoronavirus (βCoV), Gammacoronavirus (γCoV), and Deltacoronavirus (δCoV) (Cui et al., 2019; Walker et al., 2019). The genus Betacoronavirus consists of four species of coronaviruses whose spike (S) glycoprotein is cleaved into subunits S1 and S2 and have a hemagglutinin-esterase (HE) glycoprotein: Betacoronavirus 1 (human coronavirus OC43, bovine coronavirus, porcine HEV, canine respiratory coronavirus, and equine coronavirus); SARS-related coronavirus; Middle East Respiratory Syndrome coronavirus (MERS-CoV)-related coronavirus; and Rousettus bat coronavirus HKU9. SARS-CoV and MERS-CoV cause severe respiratory diseases in humans, whereas human coronavirus (HCoV) OC43 and HKU1, together with HCoV 229E and NL63 of genus Alphacoronavirus, cause the common cold in humans.
2.1. Human coronaviruses
The seven HCoV infections are zoonotic. These viruses can infect different mammalian hosts and establish zoonotic-reverse zoonotic cycles generating novel mutant viruses with viral genes derived from human and animal CoVs, which are then trafficked back into human populations at a later date (Huynh et al., 2012). HCoVs 229E, NL63, SARS-CoV, and MERS-CoV originated from bats where they are non-pathogenic (Pyrc et al., 2006; Pfefferle et al., 2009; van Boheemen et al., 2012; Huynh et al., 2012; Hu et al., 2015; Cui et al., 2019; Ravelomanantsoa et al., 2020; Ye et al., 2020). The intermediate host of 229E is suspected to be camelids - alpaca (Corman et al., 2016); for SARS-CoV, it is palm civets (Tu et al., 2004); and for MERS-CoV, it is dromedary camels (Raj et al., 2014). The parental viruses of OC43 and HKU1 have been found in rodents (Cui et al., 2019). The intermediate host of OC43 is suspected to be bovines (Vijgen et al., 2005; Cui et al., 2019). Antigenically related bovine coronavirus variants have been isolated from humans with diarrhea (MacLachlan and Dubovi, 2017). It is now believed that OC43 and 229E are undergoing antigenic evolution or antigenic drift in the S protein that enables them to escape recognition by the immune system, similarly to the antigenic drift that occurs in the haemagglutinin protein of seasonal influenza viruses (Kister and Bedford, 2021; Smith et al., 2004).
Acute pneumonia associated with the 2019 novel coronavirus that began in Wuhan city, Hubei province, China, was named COVID-19 (coronavirus disease 2019) by the World Health Organization (WHO), who characterized it as a pandemic (i.e., a global disease outbreak for which the human population has no prior exposure) (Joseph, 2020); the first pandemic caused by a coronavirus (Piret and Boivin, 2021). Sequence analysis of the earliest full-length genomes of the 2019 novel coronavirus showed it to be closest to SARS-CoV (Zhou et al., 2020), which is carried by Chinese horseshoe bats (Rhinolophus sinicus) (Hu et al., 2017). The ICTV named it SARS-CoV-2, and it belongs to the genus Betacoronavirus, subgenus Sarbecovirus within the species SARS-related coronavirus (Gorbalenya et al., 2020).
2.2. Aquatic animal nidoviruses
Fish nidoviruses were previously all placed in the family Coronaviridae, subfamily Torovirinae, and genus Bafinivirus (de Groot et al., 2012a, de Groot et al., 2012b; Schütze, 2016). Following the 2018 ICTV approved changes to nidovirus taxonomy (ICTV, 2019), the subfamily Torovirinae, was moved to a new family Tobaniviridae. Thus, fish nidoviruses now belong to two families, Coronaviridae and Tobaniviridae. The fish nidovirus Pacific salmon nidovirus (PsNV) is in the family Coronaviridae, subfamily Letovirinae, and genus Alphaletovirus (Mordecai et al., 2019). Coronaviruses found in marine aquatic habitats were reviewed by Mordecai and Hewson (2020). None of these coronaviruses found in aquatic animals are considered zoonotic and are not closely related to those in humans and therefore would not cross the species barrier.
The original bafiniviruses are now classified in the family Tobaniviridae, subfamily Piscanivirinae, comprising two genera: genus Bafinivirus containing White bream virus (WBV) and Fathead minnow nidovirus (FHMNV) (Batts et al., 2012), and genus Oncotshavirus containing Chinook salmon bafinivirus (CSBV), Atlantic salmon bafinivirus (ASBV), Yellow catfish bacilliform virus (YCBV), and Crucian carp nidovirus (CCNV) (Gulyaeva et al., 2017; Kibenge et al., 2016; Zhang et al., 2019; Xiao-Yu et al., 2019). These viruses exhibit ultrastructural features of bacilliform morphology (Schütze, 2016). Virions similar in morphology have been described for genus Okavirus, family Roniviridae (Yellow head virus (YHV) and Gill-associated virus, GAV) (Schütze, 2016), the nidoviruses of most importance to aquaculture where they cause disease in Penaeid shrimp (Penaeus monodon; Litopenaeus vannamei) farmed throughout the Eastern Hemisphere (Cowley, 2016). None of these aquatic animal nidoviruses are considered to be zoonotic.
3. Routes of transmission of SAR-CoV-2
Despite the importance of SARS-CoV-2, our current knowledge of its transmission remains incomplete. The SARS-CoV-2 was proposed to originate from bats (Paraskevis et al., 2020; Ji et al., 2020; Lau et al., 2020; Zhou et al., 2020). It was suspected of infecting humans following direct contact with intermediate host animals (Day et al., 2020). The initial acute pneumonia outbreak caused by SARS-CoV-2 was epidemiologically linked to the Huanan Seafood Wholesale Market in Wuhan City, China, where poultry, bats, snakes, frogs, hedgehogs, marmots, and other exotic wildlife live animals were also sold (Ma et al., 2020; Chen et al., 2020a; Lu et al., 2020; Ji et al., 2020; Jalava, 2020). As the outbreak progressed to a pandemic, spread via direct contact between humans became the main route of exposure (Shereen et al., 2020). With the subsequent detection of SARS-CoV-2 on imported frozen foods and their packaging materials that was linked to two re-emergent outbreaks of COVID-19 in Beijing, China (Pang et al., 2020), it has been hypothesized that contaminated cold-chain food sources may present a risk for SARS-CoV-2 transmission between countries and regions (Han et al., 2020). However, no direct link has been established between SARS-CoV-2 infection and food consumption (Desai and Aronoff, 2020; EFSA, 2020).
3.1. Zoonotic transmission of SARS-CoV-2
The exact intermediate animal host that transmitted SARS-CoV-2 to humans remains uncertain even though it has been reported that SARS-CoV-2 uses the same cell entry receptor angiotensin 1 converting enzyme 2 (ACE2), as SARS-CoV (Zhou et al., 2020; Letko et al., 2020; Wan et al., 2020), which suggests that SARS-CoV-2 may have the same host range as SARS-CoV. The highly trafficked Malayan pangolins (Manis javanica) (Liu et al., 2019) that were sampled in Guangdong province, China, were suggested as a possible intermediate animal host because of (a) the strong similarity of pangolin-associated coronaviruses with SARS-CoV-2 in the receptor-binding domain (RBD) of the S glycoprotein (Lam et al., 2020; Andersen et al., 2020) that interacts with ACE2, (b) the isolation of SARS-CoV-2 related coronavirus from Malayan pangolins (Xiao et al., 2020b), and (c) the high sequence similarity (>80%) with SARS-CoV-2 homologous sequences in metaviromes of lungs of dead pangolins (Liu et al., 2019; Wahba et al., 2020; Wong et al., 2020). However, there is no evidence that Malayan pangolins facilitate SARS-CoV-2 adaptation to humans (Boni et al., 2020; Tang et al., 2020; Liu et al., 2020; Li et al., 2020b).
Presence of virus receptors on host cells at the virus entry sites and in target tissues and organs determine the host range, tissue tropism, and pathogenesis of the virus infection. SARS-CoV-2 enters host cells via binding of the virus S glycoprotein to the cell receptor ACE2 (Hoffmann et al., 2020), followed by the cleavage of S protein by transmembrane serine protease 2 (TMPRSS2) (Matsuyama et al., 2010), releasing the fusion peptide and allowing for host-cell entry (Millet and Whittaker, 2015). This viral entry mediated by the interaction of ACE2 with the S protein is the major constraint to the interspecies transmission of SARS-CoV-2 (Zhao et al., 2020). Damas et al. (2020), using in-silico analysis, studied ACE2 sequences from 410 vertebrate species, including mammals, birds, fishes, reptiles, and amphibians, for their propensity to bind to the SARS-CoV-2 S protein and found only mammals to have binding scores that fell into the medium to very high categories. That analysis predicted that the ACE2 proteins of birds, fishes, reptiles, and amphibians are not likely to bind SARS-CoV-2 S protein, indicating that vertebrate classes other than mammals are not likely to be an intermediate host or reservoir for SARS-CoV-2 (Damas et al., 2020). Chen et al. (2020b) screened 11 representative animal species among pet animals, livestock, poultry, and wildlife for SARS-CoV-2 target cells (i.e., cells coexpressing ACE2 and TMPRSS2). The study found the cat to have the highest number of target cells among the animal species investigated; these cells were widely distributed in gastrointestinal, respiratory, and urinary systems, suggesting that cats can be infected via multiple routes and maybe intermediate hosts in the current pandemic (Chen et al., 2020b). Target cells for SARS-CoV-2 were also found in pig kidney and lung, suggesting pigs could become intermediate hosts in future coronavirus outbreaks (Chen et al., 2020b). However, current evidence shows that pigs are not susceptible (Schlottau et al., 2020). Chen et al. (2020c) analyzed the structure of the ACE2 receptor in different animals, and while ACE2 was found to be widely expressed and the structure highly conserved in the animal kingdom, those of snake, frog, and fish had only 61%, 60%, and 59% sequence identity, respectively, to that of the human ACE2 receptor (Chen et al., 2020c). Such low sequence similarity in these animals suggests that SARS-CoV-2 is unlikely to successfully infect them (Bondad-Reantaso et al., 2020). Ji et al. (2020) compared the relative synonymous codon usage bias between SARS-CoV-2 and different animal species. They found that SARS-CoV-2 and snakes from China have similar synonymous codon usage bias (Ji et al., 2020), suggesting snakes as a possible intermediate host. However, this is unlikely as animal reservoirs for human viruses are mainly mammals and birds (de Jesus, 2020). Guo et al. (2020), using a deep learning algorithm to predict potential virus hosts, indicated that mink might be an intermediate host of SARS-CoV-2. The recent human-to-mink-to-mink-to-human cycles of SARS-CoV-2 transmission (Zhou and Shi, 2021) further implicate mink as a potential intermediate host. Interestingly, the binding score for the mink ACE2 to the SARS-CoV-2 S protein was ranked very low in the study by Damas et al. (2020). Moreover, the analysis by Boni et al. (2020) showed that SARS-CoV-2-related viruses have been circulating in Rhinolophus spp. bats for a long time, with abundant recombination events (Banerjee et al., 2019).
Coronaviruses circulate in mammals and birds (Cui et al., 2019; Li et al., 2020b) and aquatic animals (Mordecai and Hewson, 2020). SARS-CoV-2 is a zoonotic virus that likely has a wide mammalian host-range (Shi et al., 2020; Chen et al., 2020b; Tiwari et al., 2020; Mahdy et al., 2020). The OIE considers SAV-CoV-2 an emerging pathogen, and therefore member countries must report confirmed infections in animals in their countries to the OIE (OIE, 2019, OIE, 2020a). As listed in Table 3 , some of these animals may serve as reservoirs once the COVID-19 pandemic is over (Santini and Edward, 2020), and therefore pause veterinary public health concerns (Mahdy et al., 2020). SARS-CoV-2 has been reported to cross the species barrier and exhibit reverse zoonosis in farmed minks, domestic cats, dogs, and captive tigers, puma, and lions (Sharun et al., 2020; Mahdy et al., 2020). Minks are of particular concern as they are farmed on a large scale in many countries (Wikipedia, 2021). It is now well established that minks are highly susceptible to the SARS-CoV-2 virus and are readily infected through the transmission of the virus from infected humans coming in contact with mink (ProMED International Society for Infectious Diseases, 2020a, ProMED International Society for Infectious Diseases, 2020c; Oreshkova et al., 2020; Santini and Edward, 2020; OIE, 2020b), and mink-to-mink transmission is very efficient (Sharun et al., 2020; Shuai et al., 2020). Moreover, a mink-unique variant of SARS-CoV-2 has been reported in Denmark and the Netherlands, providing evidence of mink-to-human transmission of SARS-CoV-2 within mink farms (i.e., reverse anthroponosis) (Sharun et al., 2020; Oude Munnink et al., 2021), although the risk of spread from animals to humans is generally considered to be low (CanCovid, 2020). To date, SARS-CoV-2 infection in farmed minks has been documented in the USA, the Netherlands, Sweden, Italy, Denmark, France, Canada, Greece, Lithuania, and Spain (Sharun et al., 2020), and Poland (OIE, 2021), with humans as the only source of introduction of the virus to minks. Fearing the possibility that minks may serve as a reservoir during and once the COVID-19 pandemic is over (Santini and Edward, 2020), the affected mink farms were depopulated (ProMed, 2020c; Oreshkova et al., 2020; Maestro and Spary, 2020; Enserink, 2020) to eliminate the risk of SARS-CoV-2 becoming enzootic in the mink population or worse changing the SARS-CoV-2 pandemic into a panzootic (Gollakner and Capua, 2020). Most recently, a wild mink who had contracted the virus, apparently from contact with farmed mink, was identified in Utah, USA - the first free-ranging, native wild animal confirmed with SARS-CoV-2 (https://www.koin.com/news/wild-mink-in-utah-tests-positive-for-sars-cov-2/). This finding indicates the potential for wild mustelids to become a permanent reservoir of infection for other animal species (Manes et al., 2020), as occurred with rabies in raccoons and skunks (Rupprecht et al., 1995). Other animal species in the case of SARS-CoV-2 would include cervids because white-tailed deer have been identified by experimental infection as a susceptible wild animal species to the virus (Palmer et al., 2021). The prospect of SARS-CoV-2 spilling into farmed and wild terrestrial animals like mustelids and cervids is a major concern.
Table 3.
Susceptibility of different animals to SARS-CoV-2 infection1.
| Animals susceptible2 | Animals with discordant susceptibility3 | Animals not susceptible |
|---|---|---|
| Chinese horseshoe bat (Rhinolophus sinicus) | Dog | Poultry (chicken, duck, turkey, goose, pigeon)4 |
| Himalayan palm civet (Paguma larvata) | Pig | Mouse4 |
| Egyptian fruit bat (Rousettus aegyptiacus)5 | Rat4 | |
| Domestic cat5 | Racoon dog | |
| Farmed mink5, 6 | Hedgehog | |
| Ferret5 | Platypus | |
| Golden Syrian hamster5 | Guinea pig | |
| Racoon | Elephant | |
| Squirrels | Kangaroo rat | |
| Rabbit | Meerkat | |
| Sheep | Aquatic food animals (finfish, crustaceans, mollusks, amphibians)4 | |
| Cattle | ||
| Horse | ||
| Stoat | ||
| Polecat | ||
| Orangutan | ||
| Common marmoset | ||
| Pangolin | ||
| Macaques (Macaca fascicularis and Macaca mulatta) | ||
| Captive tigers and lions5 | ||
| Apes |
Compiled from Shi et al. (2020); Lakdawala and Menachery (2020); Chen et al., 2020b, Chen et al., 2020c; Xiao et al., 2020b; Zhang et al., 2020a; Halfmann et al., 2020; Sit et al., 2020; Sia et al., 2020; Bosco-Lauth et al., 2020; Cohen, 2020; Andersen et al., 2020; Wan et al., 2020; Santini and Edward, 2020; Kim et al., 2020; Luan et al., 2020; Zhai et al., 2020; Munster et al., 2020; Bondad-Reantaso et al., 2020; OIE, 2020b; USDA [United States Department of Agriculture], 2020; CVMA, 2020; Bao et al., 2020; Schlottau et al., 2020; Richard et al., 2020; and ProMED 2020a,b). Animal species not listed do not yet have any evidence available (CVMA, 2020).
At-risk animals that may serve as reservoirs once the COVID-19 pandemic is over or as animal models for SARS-CoV-2 infections.
Conflicting experimental studies have been reported for these animals: dogs (Chen et al., 2020b) and pigs (Santini and Edward, 2020) (e.g., Shi et al. (2020) did not detect SARS-CoV-2 in infected pigs and Schlottau et al. (2020) confirmed that pigs are not susceptible, but computational model predictions of infectivity in wild boar (Luan et al., 2020) and pigs (Chen et al., 2020b; Wan et al., 2020; Zhou et al., 2020; Zhai et al., 2020) indicated pigs to be susceptible to SARS-CoV-2. Chen et al. (2020b) reported dogs have very rare co-expression of ACE2 and TMPRSS2, but computational model predictions of infectivity indicated dogs to be susceptible to SARS-CoV-2.
These animals have no co-expression of the entry receptor ACE2 and entry activator TMPRSS2 in lung cells (e.g., poultry, Chen et al., 2020b) or the ACE2 receptor is not used by SARS-CoV-2 (e.g., mouse and rat, Wan et al., 2020), or the ACE2 receptor has very low sequence identity (≤61%) compared to the human ACE2 receptor (e.g., snake, frog, fish, Chen et al., 2020c).
These animals show transmission between other animals of the same species under experimental infections (Shi et al., 2020; CVMA, 2020; OIE, 2020).
These animals transmit to humans (i.e., reverse anthroponosis, Oreshkova et al., 2020; Santini and Edward, 2020; CVMA, 2020; OIE, 2020).
Aquatic animals are cold-blooded (poikilothermic) and are naturally resistant to mammalian and avian viruses, which replicate at ≥37 °C. Snakes, which are also cold-blooded, were shown to have a similar synonymous codon usage bias as SARS-CoV-2, which may allow homologous recombination to occur and contribute to the SARS-CoV-2 cross-species transmission (Ji et al., 2020). However, an expert FAO report found “no evidence to suggest that SARS-CoV-2 can infect aquatic food animals (e.g., finfish, crustaceans, mollusks, amphibians)”. It concluded that “these animals do not play an epidemiological role in spreading COVID-19 to humans” (Bondad-Reantaso et al., 2020). V’kovski et al. (2020) investigated the replication kinetics of SARS-CoV-2 and SARS-CoV at 33 °C and 37 °C, mimicking the ambient temperatures of the human upper and lower respiratory tract, respectively. While both viruses replicated to similar titers at 37 °C, SARS-CoV-2, in contrast to SARS-CoV, replicated more efficiently at 33 °C (had 10-fold higher titer than at 37 °C), and the fraction of SARS-CoV-2 infected cells increased significantly at 33 °C compared to 37 °C and SARS-CoV. The enhanced replication of SARS-CoV-2 at 33 °C supports its increased replication in the upper respiratory tract and transmissibility compared to SARS-CoV. Another coronavirus, Mouse hepatitis virus (MHV), was shown to replicate equally between 32 °C and 40 °C (Deng et al., 2019). However, there are no published data on the minimum temperature for in vitro replication of SARS-COV-2 or related viruses. Such data would be a very strong argument to exclude any chance for SARS-CoV-2 replication in ectothermic aquatic animals, except perhaps in the hottest regions of the world.
3.2. Person-to-person transmission of SAR-CoV-2
The transmission routes for SARS-CoV-2 from human-to-human may be in one of three ways:
-
•
Direct person-to-person transmission through close contact.
-
•
Fomite transmission (contact transmission).
-
•
Transmission via fecal-oral route (Khan et al., 2020).
3.2.1. Direct person-to-person transmission of SARS-CoV-2 through close contact
SARS-CoV-2 primarily spreads person-to-person through close contact with symptomatic and asymptomatic individuals (Chu et al., 2020; Wiersinga et al., 2020). COVID-19 cases had very high shedding of the virus in pharyngeal samples during the first week of symptoms (Wölfel et al., 2020), having peaked about two to three days before the onset of symptoms (He et al., 2020). The current criterion considers a patient completely recovered and not infectious after two consecutive negative RT-qPCR test results on respiratory samples (Mesoraca et al., 2020); several studies have shown negative virus isolation results for SARS-CoV-2 eight days after symptom onset (Wiersinga et al., 2020). Zou et al. (2020) analyzed both symptomatic and asymptomatic individuals. They observed that the SARS-CoV-2 RNA shedding pattern of infected individuals resembles that for the influenza A virus (Tsang et al., 2015) and appears different from that of SARS-CoV (Peiris et al., 2003). The SARS-CoV-2 viral load in the nasal and throat swabs of symptomatic and asymptomatic individuals was similar, suggesting asymptomatic individuals' transmission potential (Zou et al., 2020). Current estimates suggest that 15% of infected individuals do not develop symptoms at all (i.e., excluding pre-symptomatic individuals) (Day et al., 2020; Byambasuren et al., 2020), but <10% of new infections originate from asymptomatic individuals (Day et al., 2020; Buitrago-Garcia et al., 2020). However, these reports may grossly underestimate the number of asymptomatic individuals; one report estimated the proportion of asymptomatic infections to range from 18% to 81% (Nikolai et al., 2020). As many as 40% of cases were thought to be asymptomatic based on a seroprevalence study in 10 cities in the US (Havers et al., 2020). A decision analytical model by CDC showed that 59% of all transmission came from asymptomatic individuals - 35% from presymptomatic individuals and 24% from persons who never developed symptoms (Johansson et al., 2021).
Direct person-to-person transmission occurs primarily through the air via respiratory droplets produced by coughing, sneezing, talking, yelling, laughing, singing, or normal breathing from an infected individual (Phan et al., 2020; Fineberg, 2020). The droplet-mediated transmission consists of droplets measuring >5 μm, which generally travel a short distance (~ 2 m) from the infected individual as they drop out from the air (CanCOVID, 2020). Less common transmission through the air is the airborne transmission (also called aerosol transmission) that can occur following medical procedures such as airway intubation, ventilation, and some dental procedures that create aerosols - particles measuring ≤5 μm that rapidly evaporate in the air, leaving behind droplet nuclei (Klompas et al., 2020); these can remain suspended in the air for a longer time (Arslan et al., 2020) (similarly to pollen), spreading further than droplets (WHO, 2020a; CanCOVID, 2020). Airborne transmission can also occur in confined environments where aerosols may be moved farther when the air is mechanically moved (e.g., by fans or air conditioners) (CanCOVID, 2020). Current scientific understanding from studies and investigations of outbreaks suggests that most of the transmissions occur through direct inhalation by people in close contact by droplet-mediated and airborne transmissions (Lewis, 2021). Both droplet-mediated and airborne transmissions can be prevented by the physical distancing of 2 m or more, N95 respirators and face masks, eye protection, and other basic measures such as enhanced hygiene (Chu et al., 2020).
SARS-CoV-2 has also been detected in non-respiratory bodily fluids, including feces (Wu et al., 2020a; Wang et al., 2020c; Xu et al., 2020a, Chen et al., 2020d), blood (Chen et al., 2020a), ocular secretions (Wu et al., 2020b), saliva (To et al., 2020), milk (WHO, 2020b), urine (Guan et al., 2020), and semen samples (Li et al., 2020c). However, the role of these biological materials in the transmission is uncertain.
3.2.1.1. Transmission in crowded and confined indoor spaces
There are several reports of efficient transmission of SARS-CoV-2 in crowded, confined indoor spaces such as long-term care facilities (McMichael et al., 2020), workplaces including factories, churches, restaurants, ski resorts, shopping centers, worker dormitories, cruise ships, and vehicles, or social events occurring indoor (Chan et al., 2020; Leclerc et al., 2020). In long-term care facilities, COVID-19 outbreaks were, in part, ascribed to the health care personnel being able to move between facilities in the region (McMichael et al., 2020). In a negative pressure isolation ward in a non-intensive care unit, fomite transmission was the primary route of virus exposure (Wei et al., 2020). In other congregated areas, the transmission could be linked with activities characterized by increased production of respiratory droplets and aerosols (Hamner et al., 2020). Clusters have been seen in several places where crowding occurs, including meat-processing factories in England and Wales (Leclerc et al., 2020; Thompson, 2020; ProMed, 2020b) and Germany (Tidey, 2020), meat and poultry processing facilities in the USA (Dyal et al., 2020; Waltenburg et al., 2020), and abattoirs in Australia (Anonymous, 2020; Davis and Burns, 2020). In Chile, there was anecdotal evidence of an increasing number of SARS-CoV-2 positive workers in fish processing plants and salmon farm sites in the XI region (El Magallánico, 2020). However, most of the workers were infected or had traveled from another region (M. Godoy, personal communication). According to the Centres for Disease Control and Prevention (CDC), workers in seafood processing are not exposed to SARS-CoV-2 through the fish and other seafood products they handle but rather from having close, and often, extended contact with coworkers and supervisors (CDC, 2020).
3.2.2. Fomite transmission (contact transmission) of SARS-CoV-2
Fomite transmission, such as infection via fomite to hand contamination (e.g., if a person touches a contaminated inanimate material and then transfers the infectious virus to mucous membranes in the eyes, nose, or mouth) (Kabir et al., 2020; Kitajima et al., 2020), occurs through indirect contact with surfaces that have been contaminated by an infected person (Ong et al., 2020; WHO, 2020a; ECDC, 2020). There are several scenarios of contaminated surfaces, but this transmission route appears to be less common (CanCOVID, 2020). The amount of infectious virus transferred in this case may not be sufficient to infect (Dowell et al., 2004; CanCOVID, 2020; Goldman, 2020). Other scenarios are only considered a theoretical risk; for example, an infected person touches/pets a domestic animal. The virus remains on the animal's hair coat, fur, or feathers long enough to transmit to another person (CVMA, 2020). However, a real risk has become evident with the transmission of SARS-CoV-2 via contaminated cold-chain food sources. The survival period and transmission distance of the virus could be prolonged (Han et al., 2020; Pang et al., 2020; Fisher et al., 2020). This “non-traditional” transmission mechanism (Fisher et al., 2020) has been linked to the COVID-19 resurgence in Beijing, China (Han et al., 2020; Pang et al., 2020).
3.2.2.1. SARS-CoV-2 survival in the environment
Factors such as temperature, pH, relative humidity, and the virus (i.e., naked or enveloped particle) influence the stability of a virus in the environment (Otter et al., 2016). Kampf et al. (2020) reviewed the literature on the persistence of coronaviruses (enveloped viruses) on inanimate surfaces and their inactivation by chemical disinfection. Human coronaviruses such as SARS-CoV and MERS-CoV, and HCoV persisted on the surfaces for up to 9 days. They were still effectively inactivated with 62–71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 min (Kampf et al., 2020). Taylor et al. (2020) studied the stability of cultured SARS-CoV-2 and SARS-CoV in aerosols and on various surfaces (stainless steel, copper, and cardboard) at 21–23 °C and 40% relative humidity over seven days. Both viruses were viable in aerosols for at least 3 h. For both viruses, the infectious virus survived 72 h after application on the plastic and stainless steel surfaces (Taylor et al., 2020). No viable SARS-CoV-2 was detected on cardboard after 24 h, and no viable SARS-CoV was detected after 8 h (van Doremalen et al., 2020; Taylor et al., 2020). On copper, no viable SARS-CoV-2 was found after 4 h, and no viable SARS-CoV was found after 8 h (Taylor et al., 2020). Pastorino et al. (2020) noted the presence of proteins to prolong infectivity. Overall, these studies demonstrated the potential for aerosol and fomite transmission of SARS-CoV-2 since it remained viable and infectious in aerosols for hours and on surfaces up to days (van Doremalen et al., 2020; Pastorino et al., 2020). Infectious SARS-CoV-2 was not detectable in nasal mucus and sputum after 48 h, although viral RNA could be detected for seven days (Matson et al., 2020).
3.2.2.2. SARS-CoV-2 survival in cold storage or transport
Dai et al. (2020) investigated the survival of SARS-CoV-2 (~104.5 log10 TCID50/ml) attached to pieces of salmon stored at 4 °C or 25 °C. The salmon-attached SARS-CoV-2 remained viable at 4 °C and 25 °C for 8 and 2 days, respectively, demonstrating that SARS-CoV-2 can survive for more than a week at 4 °C - the temperature in refrigerators or cold rooms for the temporary storage of fish (Dai et al., 2020). Independently, Fisher et al. (2020) studied the stability of cultured SARS-CoV-2 (~106 log10 TCID50/ml) spiked in pieces of salmon, chicken, and pork stored at three different temperatures (4 °C, -20 °C, and -80 °C) over 21 days. The viral titers remained unchanged (i.e., there was neither viral replication nor viral inactivation) for the duration of the study in both the refrigerated (4 °C) and frozen (-20 °C and -80 °C) samples (Fisher et al., 2020). These two studies demonstrated that SARS-CoV-2 could survive the time and temperatures associated with transportation and storage conditions associated with international seafood trade (Dai et al., 2020; Fisher et al., 2020), further supporting the speculation that contaminated cold-chain food sources initiated the COVID-19 resurgence in Beijing (Pang et al., 2020).
3.2.2.3. Risk of transmission of SARS-CoV-2 via aquatic food animals or their products
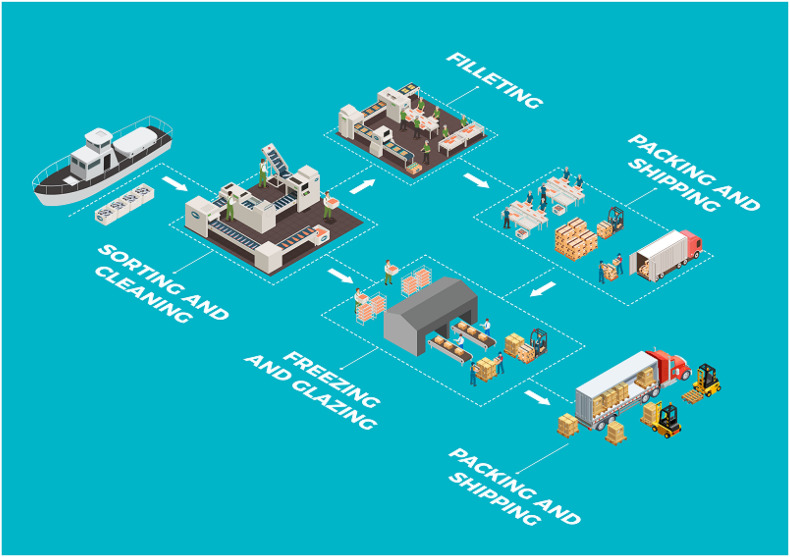
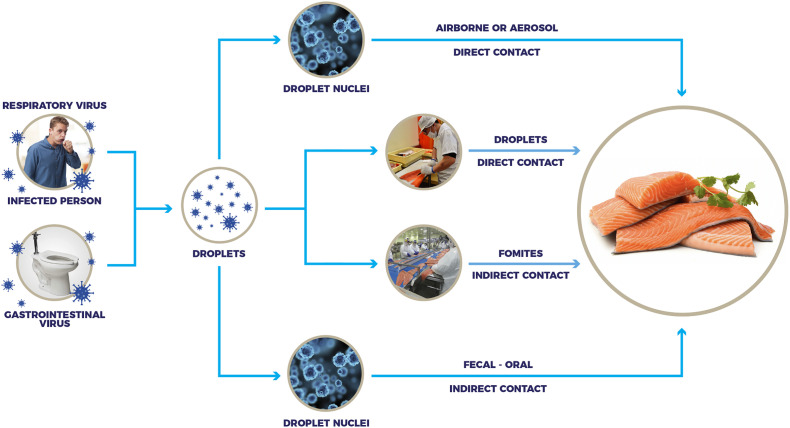
Fig. 1 illustrates the typical layout of a fish processing plant to demonstrate potential points of possible contamination with SARS-CoV-2, and Fig. 2 illustrates the potential risk of transmission of the virus via contaminated aquatic food products, particularly when handled by infected workers (Bondad-Reantaso et al., 2020; Taylor et al., 2020). The global trade in fresh and frozen aquatic animal food products is favorable for the contaminating virus to survive and be transported over long distances (Han et al., 2020). Temperature and relative humidity further influence the virus' survival in the environment (Lee et al., 2015; van Doremalen et al., 2013, van Doremalen et al., 2020; Fisher et al., 2020). The Global Aquaculture Alliance (GAA) provided a guidance document for seafood processing facilities seeking best practices to keep their employees and community healthy and limit the spread of COVID-19 (GAA, 2020).
Fig. 1.
Typical layout of a fish processing plant to demonstrate potential points of possible contamination with SARS-CoV-2.
Fig. 2.
Illustration of the potential risk of transmission of the virus via contaminated aquatic food products, particularly when handled by infected workers.
Although the initial COVID-19 cases were in people who had visited the Huanan seafood wholesale market in Wuhan city, Hubei province, China (Jiang et al., 2020; Chen et al., 2020a; Zhou et al., 2020), there is no evidence that the seafood and fish from the animal market were associated with the outbreak (Lu et al., 2020). Low temperatures favoring viral survival and high humidity have been suggested to explain why seafood markets in China could be sources of COVID-19 outbreaks (Caiyu and Hui, 2020; Jalava, 2020). A recent resurgence of COVID-19 cases in Beijing, China, has been linked to the massive Xinfadi Market (Pang et al., 2020). Of the earliest 53 people testing positive for SARS-CoV-2, 48 had worked, and three had shopped at the seafood market (Wang and Yu, 2020). Among the environmental samples tested at the same seafood market, 40 samples were positive for SARS-CoV-2, including samples taken from chopping boards used to process imported salmon (Caiyu, 2020; Caiyu and Hui, 2020; Wang and Yu, 2020). Further investigation of the 14 booths in the Xinfadi Market trading hall identified booth #S14 as the virus' source; salmon was the only imported commodity sold at this booth (Pang et al., 2020). Upon examination of all salmon (3582 in total) in the original sealed package in the cold storage at the Xinfadi Market, six were positive for SARS-CoV-2 RNA, and five of these fish were from the company that supplied the salmon to booth #14 on May 30, 2020 (Pang et al., 2020). Genome sequencing of the virus identified it as a European SARS-CoV-2 virus strain (Pang et al., 2020). The authors suggest this case to be the origin of the COVID-19 resurgence in Beijing linked to a contaminated cold-chain food source (Pang et al., 2020), although they did not establish the route of infection. The more virus consumed in a food, the more likely an illness will result (Todd et al., 2008). Foodborne virus infections often result in shedding large amounts of virus particles in the diarrheal feces or vomitus (~105 to >1012 infectious particles per ml or g) (Gerba, 2000; Bishop, 1996) that easily lead to infection upon ingestion of the contaminated food (Anderson and Weber, 2004; Todd et al., 2008). Since viruses do not replicate in non-living cells, the amount of infectious SARS-CoV-2 transferred this way would have to be massive to be sufficient to infect.
Approximately 80,000 tons of chilled and frozen salmon is imported by China each year from the major salmon producing countries (Chile, Norway, Faroe Islands-Denmark, Australia, and Canada) (Campbell, 2020). The Government aquaculture authority in Chile, Sernapesca, reiterated that salmon processing plants in Chile are HACCP (Hazard Analysis Critical Control Points) compliant, and also, because of the COVID-19 pandemic, companies have implemented additional biosafety protocols to reduce or avoid contagion among operators and in turn ensure that there is no contamination of the products made, following the recommendations of the WHO and the Ministry of Health of Chile (Sernapesca, 2020), thereby guaranteeing the safety of the aquatic products exported. Sernapesca will also implement regular supervision of salmon processing plants using online checks (Sernapesca, 2020).
While screening for viruses in food is not mandatory, it is recommended; Regulation (EC) No 178/2002 of the European Parliament regarding food law, Article 14, states that “Food shall not be placed on the market if it is unsafe” (Food Safety Authority of Ireland, 2019). After detecting SAR-CoV-2 on chopping boards used to process imported salmon (Caiyu, 2020; Caiyu and Hui, 2020; Wang and Yu, 2020), China began testing all frozen food imports for SARS-CoV-2 RNA and suspended shrimp imports from three producers from Ecuador after traces of the virus were found on the outer packaging of six samples taken from the shipment (Korban and Welling, 2020). An additional case of SARS-CoV-2 RNA on Ecuadorian shrimp packaging was reported a week later (Korban and Sapin, 2020). Additional incidents have been reported across the country where SARS-CoV-2 was detected on imported foods, mostly on their packaging materials (Han et al., 2020). In one case, the virus was also detected on the interior of a shipping container (Han et al., 2020).
The FAO has published a qualitative assessment of the likelihood of exposure to SARS-CoV-2 from wild, livestock, companion, and aquatic animals in COVID-19 affected areas (El Masry et al., 2020). The likelihood of humans or animals getting exposed to SARS-CoV-2 in COVID-19 affected areas through contact with aquatic animals (namely fish, amphibians, mollusks, and crustaceans) is considered negligible (i.e., extremely unlikely to occur/result in exposure) (El Masry et al., 2020). The fact is that SARS-CoV-2 does not replicate in aquatic animals as they are “cold-blooded” and have a different ACE2 cell receptor, and therefore, would not cross the species barrier. The in-silico analysis conducted by Damas et al. (2020) predicted that the ACE2 proteins of birds, fishes, reptiles, and amphibians are not likely to bind the SARS-CoV-2 S protein, indicating that vertebrate classes other than mammals are not likely to be an intermediate host or reservoir for SARS-CoV-2. The likelihood of exposure is low (i.e., unlikely to occur/result in exposure) through handling or consumption of raw products originating from aquatic animal species processed and sold in markets or retail shops in conditions not meeting the Codex Alimentarius food hygiene standards (CAC, 2009) where cross-contamination occurred; the likelihood drops to negligible for sufficiently heat-treated products (El Masry et al., 2020). The likelihood of infection, post-exposure, was not assessed (El Masry et al., 2020).
3.2.3. Transmission via fecal-oral route and presence of SARS-CoV-2 in wastewater
Several papers report the detection of SARS-CoV-2 RNA in wastewater (Ahmed et al., 2020; Holshue et al., 2020; Lodder and de Roda Husman, 2020; Randazzo et al., 2020; Chen et al., 2020a; Gao et al., 2020; Wang et al., 2020a, Wang et al., 2020b, Wang et al., 2020c; Xiao et al., 2020a; Ling et al., 2020; Kitajima et al., 2020), and it has been suggested that this is a sensitive surveillance system and early warning tool for COVID-19, as was previously shown for poliovirus (Lodder et al., 2012) and Aichi virus (Lodder et al., 2013). Kitajima et al. (2020) recently reviewed the potential of wastewater surveillance for understanding the epidemiology of COVID-19. SARS-CoV-2 in wastewater can enter aquatic ecosystems, particularly during poor sanitation, and infect many people (Wartecki and Rzymski, 2020), for example, via aerosolization. The concern for the role of wastewater as a potential source of SARS-CoV-2 has been heightened by three lines of evidence supporting the possibility that SARS-CoV-2 can replicate in enterocytes of the gastrointestinal tract (Kitajima et al., 2020; Singh et al., 2020):
1. Reports of COVID-19 patients with diarrhea and with the virus in feces (Chen et al., 2020a; Gao et al., 2020; Wang et al., 2020a, Wang et al., 2020b, Wang et al., 2020c; Kitajima et al., 2020). A systematic review and meta-analysis of such studies found that 12% of COVID-19 patients have gastrointestinal symptoms, and 40.5% of patients with confirmed SARS-CoV-2 infection passed the virus in feces (Parasa et al., 2020). Another meta-analysis on COVID-19 patients found fecal samples from 48.1% of the patients positive for viral RNA, and of these, 70.3% were positive even after their respiratory samples tested negative (Cheung et al., 2020).
2. Reports showing that SARS-CoV-2 infects gastrointestinal glandular epithelial cells (Xiao et al., 2020a) and gut enterocytes (Lamers et al., 2020), and the ACE2 cell receptor for SARS-CoV-2 (Yan et al., 2020) is expressed in the small intestine, lung and oral mucosa (Kitajima et al., 2020; Hamming et al., 2004; Xu et al., 2020b; Liang et al., 2020). Furthermore, ACE2 and the cellular serine protease TMPRSS2 were found to be coexpressed not only in lung alveolar type 2 cells but also in esophageal upper epithelial and gland cells, ileum and colon (Zhang et al., 2020b).
3. Report by Singh et al. (2020) predicting enterocytes and goblet cells of the small intestines and colon, and gallbladder basal cells to be susceptible to SARS-CoV-2 based on their expression of SARS-CoV-2 and coronavirus-associated receptors and factors (SCARFs).
In contrast, although a positive fecal test is as accurate as a pharyngeal swab test for laboratory diagnosis of COVID-19, patients with a positive fecal test did not have gastrointestinal symptoms (Zhang et al., 2020c). Moreover, while the virus is readily isolated from throat and lung samples, there is only one report on the isolation of SARS-CoV-2 from a single fecal sample (Holshue et al., 2020) – despite high concentrations of viral RNA (Wölfel et al., 2020). Besides, viral RNA detection does not equate to the infectious virus (Cevik and Bamford, 2020) - “The viral RNA is the equivalent of the corpse of the virus,” (Emanuel Goldman quoted by Lewis (2021)); while RT-PCR could detect SARS-CoV RNA in untreated wastewater from two hospitals, the virus could not be isolated using Vero E6 cell culture (Wang et al., 2005a). The stability of SARS-CoV in feces, urine, and water and chemical inactivation of the virus in wastewater were studied by Wang et al. (2005b). The intact virus was reported to persist for two days (viral RNA for seven days) in hospital or domestic sewage or tap water; three days in feces; 14 days in PBS; and 17 days in urine at 20 °C (Silverman and Boehm, 2020). The virus persisted longer at 4 °C: 14 days in wastewater and 17 days in feces or urine (Wang et al., 2005b). It is also unknown if SARS-CoV-2 could survive passage through the stomach (Ng and Tilg, 2020), and how long it remains infective in wastewater remains to be determined (Wartecki and Rzymski, 2020).
In experimental studies of SARS-CoV-2 infection in cats published to date (Shi et al., 2020; Halfmann et al., 2020; Bosco-Lauth et al., 2020), and where fecal samples were tested, viral RNA was either not detected in the feces of virus-inoculated cats or was detected. However, the virus was not recovered from the viral RNA-positive small intestines. Experimental studies using ferrets showed them to be highly susceptible to SARS-CoV-2 infection and transmitted the virus through direct and indirect contact similar to humans (Kim et al., 2020; Richard et al., 2020; Schlottau et al., 2020). However, the infectious virus could not be recovered from the trachea, kidney, and intestine tissues (Kim et al., 2020) or was isolated from the throat and nasal swabs but not from rectal swabs (Richard et al., 2020). In the experimental study with minks, which developed the more severe disease, infectious virus was detected in the nasal washes of all three animals on days 2 and 4 post-inoculation (p.i) but not from the concha swabs or rectal swabs of any animals at any time points (Shuai et al., 2020). White-tailed deer experimentally inoculated intranasally with SARS-CoV-2 developed a subclinical infection, and infected animals shed infectious virus in their nasal secretions (Palmer et al., 2021). Although viral RNA was detected in nasal secretions of all inoculated and indirect contact animals between 2 and 21 days p.i, viral RNA from feces was detected only intermittently and transiently through days 6–7 p.i; infectious SARS-CoV-2 shedding was detected by virus isolation in nasal secretions of all inoculated and indirect contact animals between days 2 and 7 p.i, whereas shedding in feces was only detected in inoculated animals and only on day 1 p.i (Palmer et al., 2021). Thus, the SARS-CoV-2 material detected in wastewater may not be infectious, and wastewater may not move the viable virus to an aquatic environment (Wartecki and Rzymski, 2020). However, it is still possible for the ingested virus to migrate to the respiratory tract (Li et al., 2020a).
Wartecki and Rzymski (2020) reviewed the potential survival of coronaviruses in aquatic environments and wastewater and observed that coronavirus survival likely depends on four key conditions:
-
1.
Water temperature – higher temperature decreases survivability.
-
2.
Light availability – UV-B light decreased SARS-CoV titer.
-
3.
Level of organic matter – adsorption of virus particles to the suspended organic matter may be protective, whereas the presence of antagonistic microorganisms may inactivate the virus.
-
4.
Predation – certain protozoa graze on viruses (Feichtmayer et al., 2017).
In organic matter, for example, transmissible gastroenteritis virus (TGEV), a diarrheal pathogen of swine and surrogate for SARS-CoV-2, at 25 °C, survived for 22 days in reagent-grade water. In contrast, in wastewater (lake water), it survived for only nine days (Casanova et al., 2009). Thus, coronavirus survival in treated wastewater (Carducci et al., 2020) is significantly different from survival in untreated wastewater that is known to contain microorganisms (protozoa, ciliates, flagellates, bacteria), which decrease the presence of viable viruses (Feichtmayer et al., 2017; Wartecki and Rzymski, 2020).
4. Consideration for the aquaculture industry
The impacts of the COVID-19 pandemic on the fisheries and aquaculture sector are wide-ranging (FAO/WHO Food and Agriculture Organization of the United Nations/World Health Organization, 2008). The concerns about the safety of aquatic animal food products have directly impacted the aquaculture industry. This review aims to better understand the potential for SARS-CoV-2 contamination and its potential transmission via aquatic food animals or their products to curtail these direct impacts. The industry also faces global economic impacts by changing consumer demands, access to international markets, and problems with transport and border restrictions (FAO/WHO Food and Agriculture Organization of the United Nations/World Health Organization, 2008) that may be longer-lasting, making the COVID-19 pandemic one of the most economically devastating diseases to affect the whole aquaculture value chain. This review supports the understanding that contaminated cold-chain food sources may introduce SAR-CoV-2 via food imports (Dai et al., 2020; Fisher et al., 2020), although the virus is unlikely to infect humans through consumption of aquatic food animals or their products or drinking water, i.e., SAR-CoV-2 is not a foodborne virus (Li et al., 2020a) and should not be managed as such but instead through the implementation of strong, multifaceted public health interventions such as physical distancing, rapid contact tracing, and testing, enhanced hand and respiratory hygiene, frequent disinfection of high-touch surfaces, and isolation of infected workers and their contacts, as advocated by the GAA (GAA [Global Aquaculture Alliance], 2020). The “non-traditional” transmission of SAR-CoV-2 via cold-chain food contamination calls for enhanced screening protocols used in international seafood trade to prevent re-introducing SAR-CoV-2 in importing countries and regions.
5. Conclusions
We provide critical information about how aquatic food does not present the big danger to the human population as was initially feared due to the association of early outbreaks to seafood markets and indicate areas needing more research. SARS-CoV-2 is not a foodborne virus and should not be managed as such. This virus can contaminate surfaces, including food handled by an infected person or coming in contact with contaminated material. Although SARS-CoV-2 has low stability on fomites at 21-23 °C (room temperature), it has been demonstrated that the virus can survive the time and temperatures associated with transportation and storage conditions associated with international food trade, thereby presenting a “non-traditional” transmission mechanism requiring enhanced screening protocols for the international seafood trade. While mostly viral RNA has been found on aquatic animals' products or surfaces in contact with aquatic animal products, a recent COVID-19 resurgence in Beijing, China, was linked to contaminated cold-chain food sources. However, a direct link between SARS-CoV-2 infection and food consumption remains to be documented.
Funding
No funding was provided for writing the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The continued funding of FSBK’s laboratory by the Natural Sciences and Engineering Research Council (NSERC) of Canada has made this review possible. We thank the anonymous reviewers who agreed to review this manuscript for the journal.
References
- Acha P.N., Szyfres B. 3rd ed. Pan American Health Organization; Washington: 2003. Zoonoses and Communicable Diseases Common to Man and Animals. [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., et al. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: A proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ampuero M., Valenzuela S., Valiente-Echeverria F., Soto-Rifo R., Barriga G.P., Chnaiderman J., et al. 2020. SARS-CoV-2 Detection in Sewage in Santiago, Chile-Preliminary Results. medRxiv preprint. (this version posted July 3, 2020) [DOI] [Google Scholar]
- Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E.J., Weber S.G. Rotavirus infection in adults. Lancet Infect. Dis. 2004;4:91–99. doi: 10.1016/S1473-3099(04)00928-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous Coronavirus shutdown at Cedar Meats over as abattoir resumes full operations. 2020. https://www.abc.net.au/news/2020-05-27/coronavirus-shutdown-melbourne-cedar-meats-workers-return/12289970 Available at:
- Arslan M.A., Xu B., El-Din M.G. Transmission of SARS-CoV-2 via fecal-oral and aerosols-borne routes: environmental dynamics and implications for wastewater management in underprivileged societies. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baert L., Mattison K., Loisy-Hamon F., Harlow J., Martyres A., Lebeau B., Stals A., Van Coillie E., Herman L., Uyttendaele M. Review: Norovirus prevalence in Belgian, Canadian and French fresh produce: A threat to human health? Int. J. Food Microbiol. 2011;151:261–269. doi: 10.1016/j.ijfoodmicro.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Banerjee A., Kulcsar K., Misra V., Frieman M., Mossman K. Bats and coronaviruses. Viruses. 2019;11:41. doi: 10.3390/v11010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L., Deng W., Huang B., Gao H., Liu J., Ren L., et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020;583:830–833. doi: 10.1038/s41586-020-2312-y. [DOI] [PubMed] [Google Scholar]
- Batts W.N., Goodwin A.E., Winton J.R. Genetic analysis of a novel nidovirus from fathead minnows. J Gen Virol. 2012;93:1247–1252. doi: 10.1099/vir.0.041210-0. [DOI] [PubMed] [Google Scholar]
- Bidawid S., Farber J.M., Sattar S.A. Contamination of foods by food handlers: experiments on hepatitis A virus transfer to food and its interruption. Appl. Environ. Microbiol. 2000;66:2759–2763. doi: 10.1128/AEM.66.7.2759-2763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop R.F. Natural history of human rotavirus infection. Arch. Virol. 1996;12((Suppl):119–128. doi: 10.1007/978-3-7091-6553-9_14. [DOI] [PubMed] [Google Scholar]
- Bondad-Reantaso M.G., MacKinnon B., Bin H., Jie H., Tang-Nelson K., Surachetpong W., et al. Viewpoint: SARS-CoV-2 (The cause of COVID-19 in Humans) is not known to infect aquatic food animals no contaminate their products. Asian Fisheries Science. 2020;33:74–78. 10.33997/j.afs.2020.33.1.009 Asian Fisheries Society ISSN: 0116–6514 E-ISSN: 2073–3720. [Google Scholar]
- Boni M.F., Lemey P., Jiang X., Lam T.T.-Y., Perry B., Castoe T., Rambaut A., Robertson D.L. Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. BioRxiv. 2020 doi: 10.1101/2020.03.30.015008. [Preprint]. bioRxiv. [DOI] [PubMed] [Google Scholar]
- Bosch A., Gkogka E., Le Guyader F.S., Loisy-Hamon F., Lee A., et al. Foodborne viruses: detection, risk assessment, and control options in food processing. Int. J. Food Microbiol. 2018;285:110–128. doi: 10.1016/j.ijfoodmicro.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco-Lauth A.M., Hartwig A.E., Porter A.M., Gordy P.W., et al. Pathogenesis, transmission and response to re-exposure of SARS-CoV-2 in domestic cats. bioRxiv. 2020 doi: 10.1101/2020.05.28.120998. preprint. [DOI] [Google Scholar]
- Boylan S. Veterinary Clinics of North America - Exotic Animal Practice. Vol. 14. 2011. Zoonoses associated with fish; pp. 427–438. [DOI] [PubMed] [Google Scholar]
- Buitrago-Garcia D.C., Egli-Gany D., Counotte M.J., Hossmann S., Imeri H., Salanti G., Low N. The role of asymptomatic SARS-CoV-2 infections: rapid living systematic review and meta-analysis. medRxiv preprint doi: https://doi.org/10.1101/2020.04.25.20079103. this version posted May 24, 2020. 2020 doi: 10.1371/journal.pmed.1003346. https://www.medrxiv.org/content/early/2020/04/29/2020.04.25.20079103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari K., Mulley G., Gulyaeva A.A., Zhao L., Shu G., Jiang J., Neuman B.W. Description and initial characterization of metatranscriptomic nidovirus-like genomes from the proposed new family Abyssoviridae, and from a sister group to the Coronavirinae, the proposed genus Alphaletovirus. Virology. 2018;524:160–171. doi: 10.1016/j.virol.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byambasuren O., Cardona M., Bell K., Clark J., McLaws M., Glasziou P. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. MedRxiv. 2020 doi: 10.1101/2020.05.10.20097543. Preprint posted June 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAC [Codex Alimentarius Commission] 2009. Food hygiene (Basic texts), Fourth edition. Codex Alimentarius Commission. (ISBN 978-92-5-105913-5) [Google Scholar]
- Caiyu L. 2020. Beijing supermarkets stop selling salmon after wholesalers test positive for coronavirus.https://www.globaltimes.cn/content/1191462.shtml Available at: (Accessed July 08, 2020) [Google Scholar]
- Caiyu L., Hui Z. 2020. Virologists rebuke seafood markets becoming suspicious COVID-19 hot spots after cases test positive in Beijing market.https://www.globaltimes.cn/content/1191478.shtml Available at: (Accessed July 08, 2020) [Google Scholar]
- Campbell C. 2020. Should the world be worried about the “explosive” new outbreak of coronavirus in Beijing?https://time.com/5854112/china-beijing-coronavirus-covid19-second-wave/ Available at. (Accessed July 16, 2020) [Google Scholar]
- CanCovid . 06.10.2020: SARS-CoV-2 Viral transmission. 2020. SARS-CoV-2 viral transmission. CanCOVID state of the science report: Volume 3. [Google Scholar]
- Carducci A., Federigi I., Liu D., Thompson J.R. Making waves: coronavirus detection, presence and persistence in the water environment: state of the art and knowledge needs for public health. Water Res. 2020;179 doi: 10.1016/j.watres.2020.115907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraturo F., Del Giudice C., Morelli M., Cerullo V., Libralato G., Galdiero E., Guida M. Persistence of SARS-CoV-2 in the environment and COVID-19 transmission risk from environmental matrices and surfaces. Environ. Pollut. 2020;265 doi: 10.1016/j.envpol.2020.115010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova L., Rutala W.A., Weber D.J., Sobsey M.D. Survival of surrogate coronaviruses in water. Water Res. 2009;43:1893–1898. doi: 10.1016/j.watres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC [Centers for Disease Control and Prevention] Coronavirus Disease 2019 (COVID-19). Protecting seafood processing workers from COVID-19. 2020. https://www.cdc.gov/coronavirus/2019-ncov/community/guidance-seafood-processing.html Available at.
- Cevik M., Bamford C.G.G. COVID-19 pandemic – a focuses review for clinicians. Clin. Microbiol. Infect. 2020;26:842–847. doi: 10.1016/j.cmi.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceylan Z., Meral R., Cetinkaya T. Relevance of SARS-CoV-2 in food safety and food hygiene: potential preventive measures, suggestions and nanotechnological approaches. Virus Dis. 2020;31:154–160. doi: 10.1007/s13337-020-00611-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J., Yuan S., Kok K., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet. 2020 doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Sun J., Zhu J., et al. Single-cell screening of SARS-CoV-2 target cells in pets, livestock, poultry and wildlife. bioRxiv. 2020 doi: 10.1101/2020.06.13.149690. 2020.06.13.149690. [DOI] [Google Scholar]
- Chen Y., Guo Y., Pan Y., Zhao Z.J. Structure analysis of the receptor binding of 2019-nCoV. Biochem. Biophys. Res. Commun. 2020;525:135–140. doi: 10.1016/j.bbrc.2020.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Lan Y., Yuan X., Deng X., Li Y., Cai X., Li L., He R., Tan Y., Deng X., Gao M., Tang G., Zhao L., Wang J., Fan Q., Wen C., Tong Y., Tang Y., Hu F., Li F., Tang X. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerging Microbes & Infections. 2020;9:469–473. doi: 10.1080/22221751.2020.1732837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K.S., Hung I.F., Chan P.P., Lung K.C., Tso E., Liu R., et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology. 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D.K., Akl E.A., Duda S., Yaacoub S., Schünemann H.J., et al. Physical distancing, face masks and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliver D.O. Virus transmission via food. World Health Statistics Quarterly. 1997;50:90–101. [PubMed] [Google Scholar]
- Cohen J. From mice to monkeys, animals studied for coronavirus answers. Science. 2020;368(6488):221–222. doi: 10.1126/science.368.6488.221. https://science.sciencemag.org/content/368/6488/221 [DOI] [PubMed] [Google Scholar]
- Corman V.M., Eckerle I., Memish Z.A., Liljander A.M., Dijkman R., Jonsdottir H., et al. Link of a ubiquitous human coronavirus to dromedary camels. Proc. Natl. Acad. Sci. U. S. A. 2016;113:9864–9869. doi: 10.1073/pnas.1604472113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley J.A. Nidoviruses of fish and crustaceans. In: Kibenge, F.S.B., and Godoy, M.M. (Eds.), aquaculture virology. Elsevier, Amsterdam. Chapter. 2016;32:443–472. doi: 10.1016/B978-0-12-801573-5.00032-2. [DOI] [Google Scholar]
- Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nature Reviews. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CVMA (Canadian Veterinary Medical Association) 2020. COVID-19 and animals – frequently asked questions for veterinarians.https://www.canadianveterinarians.net/documents/updated-covid-19-and-animals-frequently-asked-questions-for-veterinarians (June 19, 2020). Available at. (Accessed on June 29, 2020) [Google Scholar]
- Dai M., Li H., Yan N., Huang J., Zhao L., Xu S., Wu J., Jiang S., Pan C., Liao M. Long-term survival of SARS-CoV-2 on salmon as a source for international transmission. The Journal of Infectious Diseases. 2020:jiaa712. doi: 10.1093/infdis/jiaa712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damas J., Hughes G.M., Keough K.C., Painter C.A., Persky N.S., Corbo M., Hiller M., Koepfli K.-P. Broad host range of SARS-CoV-2 predicted by comparative and structural analysis of ACE2 in vertebrates. Proc. Natl. Acad. Sci. 2020;117:22311–22322. doi: 10.1073/pnas.2010146117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J., Burns A. 2020. Why was there a COVID-19 outbreak in Colac's abattoir, but not one in Warrnambool?https://www.abc.net.au/news/2020-08-06/covid-19-abattoir-outbreak-raises-questions-about-dhhs-response/12526816 Available at. (Accessed on January 18, 2021) [Google Scholar]
- Day T., Gandon S., Lion S., Otto S.P. On the evolutionary epidemiology of SARS-CoV-2. Curr. Biol. 2020 doi: 10.1016/j.cub.2020.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot R.J., Baker S.C., Baric R., Enjuanes L., Gorbalenya A.E., Holmes K.V., et al. In: Virus Taxonomy, Classification and Nomenclature of Viruses, Ninth Report of the International Committee on Taxonomy of Viruses. King A.M.Q., Adams M.J., Carstens E.B., Lefkowitz E.J., editors. Elsevier; Amsterdam: 2012. Family Coronaviridae; pp. 806–828. [Google Scholar]
- de Groot R.J., Cowley J.A., Enjuanes L., Faaberg K.S., Perlman S., Rottier P.J.M., et al. In: Virus Taxonomy, Classification and Nomenclature of Viruses, Ninth Report of the International Committee on Taxonomy of Viruses. King A.M.Q., Adams M.J., Carstens E.B., Lefkowitz E.J., editors. Elsevier; Amsterdam: 2012. Order Nidovirales; pp. 785–795. [Google Scholar]
- de Jesus E.G. 2020. No, snakes probably aren’t the source of that new coronavirus in China.https://www.sciencenews.org/article/snakes-probably-not-sourcespread-new-coronavirus-outbreak-china Available at. (Accessed on July 22, 2020) [Google Scholar]
- Deng X., Mettelman R.C., O’Brien A., Thompson J.A., O’Brien T.E., Baker S.C. Analysis of coronavirus temperature-sensitive mutants reveals an interplay between the macrodomain and papain-like protease impacting replication and pathogenesis. J. Virol. 2019;93 doi: 10.1128/JVI.02140-18. e02140-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A.N., Aronoff D.M. Food safety and COVID-19. JAMA. 2020;323:1982. doi: 10.1001/jama.2020.5877. [DOI] [PubMed] [Google Scholar]
- Desdouits M., de Graaf M., Strubbia S., Oude Munnink B.B., Kroneman A., Le Guyader F.S., Koopmans M.P.G. Novel opportunities for NGS-based one health surveillance of foodborne viruses. One Health Outlook. 2020;2:14. doi: 10.1186/s42522-020-00015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell S.F., Simmerman J.M., Erdman D.D., Wu J.S., Chaovavanich A., Javadi M., Yang J.Y., Anderson L.J., Tong S., Ho M.S. Severe acute respiratory syndrome coronavirus on hospital surfaces. Clinical infectious diseases. 2004;39:652–657. doi: 10.1086/422652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumpis U., Crook D., Oksi J. Tick-borne encephalitis. Clin. Infect. Dis. 1999;28:882–890. doi: 10.1086/515195. [DOI] [PubMed] [Google Scholar]
- Dyal J.W., Grant M.P., Broadwater K., et al. COVID-19 among workers in meat and poultry processing facilities—19 states, April 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69:557–561. doi: 10.15585/mmwr.mm6918e3. [DOI] [PubMed] [Google Scholar]
- ECDC (European Centre for Disease Prevention and Control) Transmission of COVID-19. 2020. https://www.ecdc.europa.eu/en/covid-19/latest-evidence/transmission Available at. (Accessed June 30,2020)
- EFSA [European Food Safety Authority] 2020. Coronavirus: no evidence that food is a source or transmission route.http://www.efsa.europa.eu/en/news/coronavirus-no-evidence-food-source-or-transmission-route Available at. [Google Scholar]
- EFSA [European Food Safety Authority]HAZ) EFSA Panel on Biological Hazards (BIOHAZ); Scientific Opinion on An Update on the present knowledge on the occurrence and control of foodborne viruses. EFSA Journal. 2011;9(7) doi: 10.2903/j.efsa.2011.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Masry I., von Dobschuetz S., Plee L., Larfaoui F., Yang Z., Song J., Pfeiffer D., Calvin S., Roberts H., Lorusso A., Barton-Behravesh C., Zheng Z., Kalpravidh W., Sumption K. FAO; Rome: 2020. Exposure of Humans or Animals to SARS-CoV-2 from Wild, Livestock, Companion and Aquatic Animals: Qualitative Exposure Assessment. FAO Animal Production and Health, Paper; p. 181. [DOI] [Google Scholar]
- Enserink M. Coronavirus rips through Dutch mink farms, triggering culls. Science. 2020;368(6496):1169. doi: 10.1126/science.368.6496.1169. [DOI] [PubMed] [Google Scholar]
- Fabiszewski de Aceituno A.M., Rocks J.J., Jaykus L.-E., Leon J.S. In: Foodborne viruses. Guide to foodborne pathogens. R.B. Labbe, Garcia S., editors. Wiley Blackwell; Oxford UK: 2013. pp. 352–376. [Google Scholar]
- FAO/WHO [Food and Agriculture Organization of the United Nations/World Health Organization] In: Microbiological Risk Assessment Series No. 13. ISBN 978-92-5-106117-6 Available at http://www.fao.org/tempref/docrep/fao/011/i0451e/i0451e00.pdf (Accessed February 4, 2021). Lawrence T, editor. Sales and Marketing Group, Communication Division Food and Agriculture Organization of the United Nations; Rome: 2008. Viruses in food: scientific advice to support risk management activities: Meeting report. (73pp) [Google Scholar]
- Feichtmayer J., Deng L., Griebler C. Antagonistic microbial interactions: contributions and potential applications for controlling pathogens in the aquatic systems. Front. Microbiol. 2017;8:2192. doi: 10.3389/fmicb.2017.02192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg H.V. National Academies of Science, Engineering, and Medicine; 2020. Rapid Expert Consultation on SARS-CoV-2 Viral Shedding and Antibody Response for the COVID-19 Pandemic (April 8, 2020)https://www.nap.edu/download/25774 ISBN 978-0-309-67654-0. (Accessed January 21, 2021) [Google Scholar]
- Fisher D., Reilly A., Zheng A., Cook A., Anderson D. Seeding of outbreaks of COVID-19 by contaminated fresh and frozen food. BioRxiv. 2020 doi: 10.1101/2020.08.17.255166. preprint. (This version posted August 18, 2020) [DOI] [Google Scholar]
- Food Safety Authority of Ireland General Principles of Food Law; EU Legislation. 2019. https://www.fsai.ie/legislation/food_legislation/general_principles_of_food_law.html Available at.
- GAA [Global Aquaculture Alliance] Protecting Employees at Seafood Facilities During COVID-19. 2020. https://www.aquaculturealliance.org/blog/covid-19-update/ Available at. (Accessed July 24, 2020)
- Gao Q.Y., Chen Y.X., Fang J.Y. 2019 novel coronavirus infection and gastrointestinal tract. J. Dig. Dis. 2020;1(2) doi: 10.1111/1751-2980.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C. Assessment of enteric pathogen shedding by bathers during recreational activity and its impact on water quality. Quant. Microbiol. 2000;2:55–64. doi: 10.1023/A:1010000230103. [DOI] [Google Scholar]
- Goldman E. Exaggerated risk of transmission of COVID-19 by fomites. Lacent. 2020;20:892–893. doi: 10.1016/S1473-3099(20)30561-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollakner R., Capua I. Is COVID-19 the first pandemic that evolves into a panzootic? Vet. Ital. 2020;56:7–8. doi: 10.12834/VetIt.2246.12523.1. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., et al. Severe acute respiratory syndrome-related coronavirus: The species and its viruses – A statement of the coronavirus study group. bioRxiv. 2020 doi: 10.1101/2020.02.07.937862. preprint. [DOI] [Google Scholar]
- Guan W., Ni Z-y., Hu Y., et al. China medical treatment expert Group for Covid-19. Clinical characteristics of 2019 novel coronavirus infection in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyaeva A.A., Dmitry L.C.S.V., et al. Proceedings for the XIVth International Nidovirus Symposium, S4. P-05, Kansas City, MO, USA, June 4–9, 2017. 2017. Evolutionary based classification of genomic diversity of nidoviruses connects metagenomics and experimental research. [Google Scholar]
- Guo Q., Li M., Wang C., Wang P., Fang Z., Tan J., et al. Host and infectivity prediction of Wuhan 2019 novel coronavirus using deep learning algorithm. bioRxiv preprint doi: https://doi.org/10.1101/2020.01.21.914044; this version posted August 23, 2020 (Accessed February 4, 2021). 2020 doi: 10.1101/2020.01.21.914044. [DOI] [Google Scholar]
- Halfmann P.J., et al. Transmission of SARS-CoV-2 in domestic cats. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2013400. NEJMc2013400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.V., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamner L., Dubbel P., Capron I., Ross A., Jordan A., Lee J., et al. 2020. High SARS-CoV-2 Attack Rate Following Exposure at a Choir Practice - Skagit County, Washington, March 2020. MMWR Morbidity and mortality weekly report. May 69; pp. 606–610. [DOI] [PubMed] [Google Scholar]
- Han J, Zhang X, He S, Jia P. Can the coronavirus disease be transmitted from food? A review of evidence, risks, policies and knowledge gaps. Environmental Chemistry Letters. 2020 doi: 10.1007/s10311-020-01101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelaar A.H., Kirk M.D., Torgerson P.R., Gibb H.J., Hald T., Lake R.J., et al. World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havers F.P., Reed C., Lim T., et al. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23–May 12, 2020. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.4130. Published online July 21, 2020. [DOI] [PubMed] [Google Scholar]
- He X., Lau E.H.Y., Wu P., et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181 doi: 10.1016/j.cell.2020.02.052. 271-280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., et al. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Zeng L.-P., Yang X.-L., Ge X.-Y., Zhang W., Li B., et al. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog. 2017;13(11) doi: 10.1371/journal.ppat.1006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh J., Li S., Yount B., Smith A., Sturges L., Olsen J.C., et al. Evidence supporting a zoonotic origin of human coronavirus strain NL63. J. Virol. 2012;86:12816–12825. doi: 10.1128/JVI.00906-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Committee on Taxonomy of Viruses . 2019. ICTV Master Species List 2019 v2. Checklist dataset doi:10.15468/i4jnfv accessed via GBIF.org on 2020-07-03. [Google Scholar]
- Jalava K. First respiratory transmitted food borne outbreak? Int. J. Hyg. Environ. Health. 2020;226 doi: 10.1016/j.ijheh.2020.113490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W., Wang W., Zhao X., Zai J., Li X. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J. Med. Virol. 2020;92:433–440. doi: 10.1002/jmv.25682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F., Deng L., Zhang L., Cai Y., Cheung C.W., Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19) J. Gen. Intern. Med. 2020;2019 doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M.A., Quandelacy T.M., Kada S., et al. SARS-CoV-2 transmission from people without COVID-19 symptoms. JAMA Netw. Open. 2021;4(1) doi: 10.1001/jamanetworkopen.2020.35057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph A. 2020. Disease caused by the novel coronavirus officially has a name: Covid-19.https://www.statnews.com/2020/02/11/disease-caused-by-the-novel-coronavirus-has-name-covid-19/ (accessed February 14, 2020) [Google Scholar]
- Kabir M.T., Uddin M.S., Hossain M.F., Abdulhakim J.A., Alam M.A., Ashraf G.M., Bungau S.G., Bin-Jumah M.N., Abdel-Daim M.M., Aleya L. nCOVID-19 pandemic: from molecular pathogenesis to potential investigational therapeutics. Front. Cell Dev. Biol. 2020;8:616. doi: 10.3389/fcell.2020.00616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Liu J., Xue M. Transmission of SARS-CoV-2, required developments in research and associated public health concerns. Front. Med. 2020;7:310. doi: 10.3389/fmed.2020.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibenge F.S.B., Kibenge M.J.T., Morton A. 2016. A novel salmonid bafinivirus with a wide in-vitro host range. (GenBank Accession number KY130432) [Google Scholar]
- Kim Y., Kim S.-G., Kim S.-M., et al. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe. 2020;27:704–709. doi: 10.1016/j.chom.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kister K.E., Bedford T. Evidence for adaptive evolution in the receptor-binding domain of seasonal coronaviruses OC43 and 229E. eLife. 2021;10 doi: 10.7554/eLife.64509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M., Ahmed W., Bibby K., Carducci A., et al. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Environ. 2020;739:139076. doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klompas M., Baker M.A., Rhee C. Airborne transmission of SARS-CoV-2: theoretical considerations and available evidence. JAMA. 2020;324:441–442. doi: 10.1001/jama.2020.12458. [DOI] [PubMed] [Google Scholar]
- Koopmans M., Duizer E. Foodborne viruses: An emerging problem. Int. J. Food Microbiol. 2004;90:23–41. doi: 10.1016/S0168-1605(03)00169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korban D., Sapin R. 2020. China’s Chongqing detects new case of COVID-19 on ecuadorian shrimp packaging.https://www.intrafish.com/coronavirus/chinas-chongqing-detects-new-case-of-covid-19-on-ecuadorian-shrimp-packaging/2-1-843632?utm_source=Intrafish%20Breaking%20News&utm_campaign=1372f95992-EMAIL_CAMPAIGN_2020_07_15_03_25&utm_medium=email&utm_term=0_7d6d2b593c-1372f95992-244403057 Available at. (Accessed July 16, 2020) [Google Scholar]
- Korban D., Welling D. 2020. China suspends Ecuadorian shrimp imports over COVID-19 traces.https://www.intrafish.com/coronavirus/china-suspends-ecuadorian-shrimp-imports-over-covid-19-traces/2-1-841412?%20Breaking%20News=&utm_term=0_7d6d2b593c-4f5bba15d7-244403057 Available at. [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Ferraro G.B., Veneri C., Bonadonna L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736(2020):139652. doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakdawala S.S., Menachery V.D. The search for a COVID-19 model: A comparison of SARS-CoV-2 replication, transmission, and disease in mice to monkeys. Science. 2020;368:942–943. doi: 10.1126/science.abc6141. [DOI] [PubMed] [Google Scholar]
- Lam T.T., Jia N., Zhang Y.W., et al. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature. 2020;583(7815):282–285. doi: 10.1038/s41586-020-2169-0. https://doi:10.1038/s41586-020-2169-0 [DOI] [PubMed] [Google Scholar]
- Lamers M.M., Beumer J., van der Vaart J., et al. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369:50–54. doi: 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S.K.P., Luk H.K.H., Wong A.C.P., Li K.S.M., Zhu L., He Z., Fung J., Chan T.T.Y., Fung K.S.C., Woo P.C.Y. Possible bat origin of severe acute respiratory syndrome coronavirus 2. Emerg. Infect. Dis. 2020;26:1542–1547. doi: 10.3201/eid2607.200092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc Q.L., Fuller N.M., Knight L.E., et al. What settings have been linked to SARS-CoV-2 transmission clusters? Wellcome Open Research. 2020 doi: 10.12688/wellcomeopenres.15889.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.J., Si J., Yun H.S., Ko G. Effect of temperature and relative humidity on the survival of foodborne viruses during food storage. Appl. Environ. Microbiol. 2015;2015(81):2075–2081. doi: 10.1128/AEM.04093-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for lineage B -coronaviruses, including 2019-nCoV. Nat. Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. COVID-19 rarely infects through surfaces. So why are we still deep cleaning? Nature. 2021;590:26–28. doi: 10.1038/d41586-021-00251-4. [DOI] [PubMed] [Google Scholar]
- Lewis H.C., Wichmann O., Duizer E. Transmission routes and risk factors for autochthonous hepatitis E virus infection in Europe: a systematic review. Epidemiol. Infect. 2010;138:145–166. doi: 10.1017/S0950268809990847. [DOI] [PubMed] [Google Scholar]
- Li D., Zhao M.Y., Tan T.H.M. What makes a foodborne virus: comparing coronaviruses with human noroviruses. Curr. Opin. Food Sci. 2020;42:1–7. doi: 10.1016/j.cofs.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zai J., Zhao Q., Nie Q., Li Y., Chaillon A. Evolutionary history, potential intermediate animal host, and cross-species analyses of SARS-CoV-2. J. Med. Virol. 2020;92:602–611. doi: 10.1002/jmv.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Jin M., Bao P., Zhao W., Zhang S. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw. Open. 2020;3(5) doi: 10.1001/jamanetworkopen.2020.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W., Feng Z., Rao S., et al. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut. 2020;69:1141–1143. doi: 10.1136/gutjnl-2020-320832. [DOI] [PubMed] [Google Scholar]
- Ling Y., Xu S.B., Lin Y.X., Tian D., Zhu Z.Q., Dai F.H., Wu F., Song G.-Z., Huang W., Chen J., Hu B.J., Wang S., Mao E.Q., Zhu L., Zhang W.H., Lu H.Z. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin. Med. J. 2020;133:1039–1043. doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Chen W., Chen J.P. Viral metagenomics revealed Sendai virus and coronavirus infection of Malayan pangolins (Manis javanica) Viruses. 2019;11:979. doi: 10.3390/v11110979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Jiang J.-Z., Wan X.-F., Hua Y., Li L., Zhou J., et al. Are pangolins the intermediate host of the 2019 novel coronavirus (SARS-CoV-2)? PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder W., de Roda Husman A.M. SARS-CoV-2 in wastewater: potential health risk, but also data source. The Lancet. Gastroenterol. Hepatol. 2020;5(6):533–534. doi: 10.1016/S2468-1253(20)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder W.J., Buisman A.M., Rutjes S.A., Heijne J.C., Teunis P.F., de Roda Husman A.M. Feasibility of quantitative environmental surveillance in poliovirus eradication strategies. Appl. Environ. Microbiol. 2012;78:3800–3805. doi: 10.1128/AEM.07972-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder W.J., Rutjes S.A., Takumi K., de Roda Husman A.M. Aichi virus in sewage and surface water, the Netherlands. Emerg. Infect. Dis. 2013;19:1222–1230. doi: 10.3201/eid1908.130312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry P.W., Levine R., Stroup D.F., Gunn R.A., Wilder M.H., Konigsberg C., Jr. Hepatitis A outbreak on a floating restaurant in Florida. Am. J. Epidemiol. 1989;129:155–164. doi: 10.1093/oxfordjournals.aje.a115104. [DOI] [PubMed] [Google Scholar]
- Lu H., Stratton C.W., Tang Y.-W. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol. 2020;92:401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan J., Lu Y., Jin X., Zhang L. Spike protein recognition of mammalian ACE2 predicts the host range and an optimized ACE2 for SARS-CoV-2 infection. Biochem Biophys Res Commun. 2020;526:165–169. doi: 10.1016/j.bbrc.2020.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby S.P., Rahman M., Hossain M.J., Blum L.S., Husain M.M., Gurley E., et al. Foodborne transmission of Nipah virus, Bangladesh. Emerg. Infect. Dis. 2006;12:1888–1894. doi: 10.3201/eid1212.060732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:1–7. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLachlan N.J., Dubovi E.J. In: In Fenner's Veterinary Virology. Fifth Edition. MacLachlan N. James, Dubovi Edward J.…, editors. Academic Press; 2017. p. 393. [Google Scholar]
- Maestro L.P., Spary S. CNN; 2020. Spain orders cull of nearly 100,000 farmed mink after animals test positive for COVID-19.https://www.cnn.com/2020/07/17/europe/spain-culls-mink-scli-intl/index.html Available at. [Google Scholar]
- Magallánico El. 2020. https://translate.google.com/translate?sl=es&tl=en&u=https%3A%2F%2Fwww.aqua.cl%2F2020%2F06%2F15%2Fmagallanes-autoridades-anuncian-mano-dura-contra-responsable-del-denominado-brote-de-las-pesqueras%2F
- Mahdy M.A.A., Younis W., Ewaida Z. An overview of SARS-CoV-2 and animal infection. Front. Vet. Sci. 2020;7:596391. doi: 10.3389/fvets.2020.596391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes C., Gollakner R., Capua I. Could Mustelids spur COVID-19 into a panzootic? Veterinaria Italiana. 2020;56:65–66. doi: 10.12834/VetIt.2375.13627.1. [DOI] [PubMed] [Google Scholar]
- Matson M.J., Kwe Yinda C., Seifert S.N., Bushmaker T., Fischer R.J., van Doremalen N., et al. Effect of environmental conditions on SARS-CoV-2 stability in human nasal mucus and sputum. Emerg. Infect. Dis. 2020;26:2276–2278. doi: 10.3201/eid2609.202267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., et al. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the Transmembrane protease TMPRSS2. J. Virol. 2010;84:12658–12664. doi: 10.1128/JVI.01542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael T.M., Currie D.W., Clark S., et al. Public health–Seattle and King County; Evergreen health; CDC COVID-19 investigation team. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382 doi: 10.1056/NEJMoa2005412. 20052011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Environmental Science & Technology Letters. 2020. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. [DOI] [PubMed] [Google Scholar]
- Meng X.J., Purcell R.H., Halbur P.G., Lehman J.R., Webb D.M., et al. A novel virus in swine is closely related to the human hepatitis E virus. Proc. Natl. Acad. Sci. U. S. A. 1997;94:9860–9865. doi: 10.1073/pnas.94.18.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesoraca A., Margiotti K., Viola A., Cima A., Sparacino D., Giorlandino C. Evaluation of SARS-CoV-2 viral RNA in fecal samples. Virol. J. 2020;17:86. doi: 10.1186/s12985-020-01359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet J.K., Whittaker G.R. Host cell proteases: critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015;202:120–134. doi: 10.1016/j.virusres.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R.C., Schaffner D.W. Virus risk in the food supply chain. Curr. Opin. Food Sci. 2019;30:43–48. doi: 10.1016/j.cofs.2018.12.002. [DOI] [Google Scholar]
- Mordecai G.J., Hewson I. Coronaviruses in the sea. Front. Microbiol. 2020;11:1795. doi: 10.3389/fmicb.2020.01795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordecai G.J., Miller K.M., Di Cicco E., Schulze A.D., Kaukinen K.H., Ming T.J., Li S., Tabata A., Teer A., Patterson D.A., et al. Endangered wild salmon infected by newly discovered viruses. eLife. 2019;8:e47615. doi: 10.7554/eLife.47615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster V.J., Feldmann F., Williamson B.N., et al. Respiratory disease in rhesus macaques inoculated with SARS-CoV-2. Nature. 2020;585:268–272. doi: 10.1038/s41586-020-2324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S.C., Tilg H. COVID-19 and the gastrointestinal tract: more than meets the eye. Gut. 2020;69:973–974. doi: 10.1136/gutjnl-2020-321195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolai L.A., Meyer C.G., Kremsner P.G., Velavan T.P. Asymptomatic SARS coronavirus 2 infection: invisible yet invincible. Int. J. Infect. Dis. 2020;100:112–116. doi: 10.1016/j.ijid.2020.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE SARS-CoV-2, Poland. Available at: https://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review?page_refer=MapFullEventReport&reportid=38036 (Accessed February 4, 2021) 2021 [Google Scholar]
- OIE Terrestrial Animal Health Code. Chapter 1.1. 2019. https://www.oie.int/index.php?id=169&L=0&htmfile=chapitre_notification.htm Available at: (Accessed on June 29, 2020)
- OIE Questions and Answers on 2019-nCoV Acute Respiratory Disease. 2020. https://www.oie.int/en/scientific-expertise/specific-information-and-recommendations/questions-and-answers-on-2019novel-coronavirus/ Available at. (Accessed July 27, 2020)
- OIE OIE Technical Factsheet: Infection with SARS-CoV-2 in Animals. 2020. https://www.oie.int/fileadmin/Home/eng/Our_scientific_expertise/docs/pdf/COV-19/A_Factsheet_SARS-CoV-2.pdf Available at.
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323(16):1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreshkova N., Molenaar R.-J., Vreman S., Harders F., Munnink B.B.O., Hakze R., Gerhards N., et al. SARS-CoV2 infection in farmed mink, Netherlands, April 2020. bioRxiv. 2020 doi: 10.1101/2020.05.18.101493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orive G., Lertxundi U., Barcelo D. Early SARS-CoV-2 outbreak detection by sewage-based epidemiology. Sci. Total Environ. 2020;732(2020):139298. doi: 10.1016/j.scitotenv.2020.139298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter J.A., Donskey C., Yezli S., Douthwaite S., Goldenberg S.D., Weber D.J. 2016. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92:235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oude Munnink B.B., Sikkema R.S., Nieuwenhuijse D.F., Molenaar R.J., Munger E., Molenkamp R., van der Spek A., Tolsma P., et al. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science. 2021;371:172–177. doi: 10.1126/science.abe5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer M.V., Martins M, Falkenberg S, Buckley A, Caserta L.C., et al. bioRxiv; 2021. Susceptibility of white-tailed deer (Odocoileus virginianus) to SARS-CoV-2. bioRxiv preprint doi: https://doi.org/10.1101/2021.01.13.426628; this version posted January 14, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang X., Ren L., Wu S., Ma W., Yang J., Di L., Li J., Xiao Y., Kang L., Du S., et al. Cold-chain food contamination as the possible origin of COVID-19 resurgence in Beijing. Natl. Sci. Rev. 2020;7:1861–1864. doi: 10.1093/nsr/nwaa264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasa S., Desai M., et al. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with Coronavirus Disease 2019. A systematic review and meta-analysis. JAMA Netw. Open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskevis D., Kostaki E.G., Magiorkinis G., Panayiotakopoulos G., Sourvinos G., Tsiodras S. Full-genome evolutionary analysis of the novel corona virus (20109-nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. Infection, Genetics and Evolution. 2020;79(2020):104212. doi: 10.1016/j.meegid.2020.104212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorino B., Touret F., Gilles M., de Lamballerie X., Charrel R.N. Prolonged infectivity of SARS-CoV-2 in fomites. Emerg. Infect. Dis. 2020;26:2256–2257. doi: 10.3201/eid2609.201788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Hung I.F., Poon L.L., Law K.I., Tang B.S., Hon T.Y., Chan C.S., Chan K.H., Ng J.S., Zheng B.J., Ng W.L., Lai R.W., Guan Y., Yuen K.Y., HKU/UCH SARS Study Group Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet (London, England) 2003;361(9371):1767–1772. doi: 10.1016/s0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrović T., D'Agostino M. Antimicrobial Food Packaging. Academic Press; 2016. Viral contamination of food; pp. 65–79. [Google Scholar]
- Pfefferle S., Oppong S., Drexler J.F., Gloza-Rausch F., Ipsen A., Seebens A., et al. Distant relatives of severe acute respiratory syndrome coronavirus and close relatives of human coronavirus 229E in bats, Ghana. Emerg Infect Dis. 2009;15:1377–1384. doi: 10.3201/eid1509.090224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan L.T., Nguyen T.V., Luong Q.C., Nguyen T.V., Nguyen H.T., Le H.Q., et al. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N. Engl. J. Med. 2020;382 doi: 10.1056/NEJMc2001272. 872–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piret J, Boivin G. Pandemics throughout history. Frontiers in Microbiology. 2021;11:1–16. doi: 10.3389/fmicb.2020.631736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ProMED International Society for Infectious Diseases Coronavirus disease 2019 Update (146): Netherlands (North Brabant) Animal, Farmed mink, Epidemiology. 2020. https://promedmail.org/promed-post/?id=20200501.7286113 Archive Number: 20200501.7286113.
- ProMED International Society for Infectious Diseases . 2020. Coronavirus disease 2019 Update (274): meat plants, immunity, global.https://promedmail.org/promed-post/?id=7492011 WHO. Archive Number: 20200621.7492011. (Accessed June 21, 2020) [Google Scholar]
- ProMED International Society for Infectious Diseases Coronavirus disease 2019 update (266): Denmark (ND) animal, farmed mink, 1st rep Archive Number: 20200617.7479510. 2020. https://promedmail.org/promed-post/?id=20200617.7479510https://promedmail.org/promed-post/?id=20200617.7479510 (Accessed July 17, 2020)
- Pyrc K., Dijkman R., Deng L., Jebbink M.F., Ross H.A., Berkhout B., van der Hoek L. Mosaic structure of human coronavirus NL63, one thousand years of evolution. J. Mol. Biol. 2006;364:964–973. doi: 10.1016/j.jmb.2006.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj V.S., Farag E.A., Reusken C.B., Lamers M.M., Pas S.D., Voermans J., et al. Isolation of MERS coronavirus from a dromedary camel, Qatar, 2014. Emerg. Infect. Dis. 2014;20:1339–1342. doi: 10.3201/eid2008.140663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181(2020):115942. doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravelomanantsoa N.A.F., Guth S., Andrianiaina A., Andry S., Gentles A., et al. The zoonotic potential of bat-borne coronaviruses. Emerg Top Life Sci. 2020;4:365–381. doi: 10.1042/ETLS20200097. [DOI] [PubMed] [Google Scholar]
- Richard M., Kok A., de Meulder D., Bestebroer T.M., Lamers M.M., Okba N.M.A., et al. SARS-CoV-2 is transmitted via contact and via the air between ferrets. Nat. Commun. 2020;11:3496. doi: 10.1038/s41467-020-17367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., et al. Presence and vitality of SARS-CoV-2 virus in wastewaters and rivers. medRxiv. 2020 doi: 10.1101/2020.05.01.20086009. this version posted May 5, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Manzano J., Hundesa A., Calgua B., et al. Adenovirus and Norovirus contaminants in commercially distributed shellfish. Food Environ Virol. 2014;6:31–41. doi: 10.1007/s12560-013-9133-1. [DOI] [PubMed] [Google Scholar]
- Rupprecht C.E., Smith J.S., Fekadu M., Childs J.E. The Ascension of wildlife rabies: a cause for public health concern or intervention? Emerg. Infect. Dis. 1995;1:107–114. doi: 10.3201/eid0104.950401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini J.M., Edward S.J.L. Host range of SARS-CoV-2 and implications for public health. Lancet Microbe. 2020;1:e141–e142. doi: 10.1016/S2666-5247(20)30069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar S.A, Treto J, Bidawid S, Farber J. Foodborne spread of hepatitis A: Recent studies on virus survival, transfer and inactivation. Can J Infect Dis. 2000;11(3):159–163. doi: 10.1155/2000/805156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlottau K., Rissmann M., Graaf A., Schön J., Sehl J., Wylezich C., et al. SARS-CoV-2 in fruit bats, ferrets, pigs, and chickens: an experimental transmission study. Lancet Microbe. 2020;1:e218–e225. doi: 10.1016/S2666-5247(20)30089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütze H. In: Aquaculture Virology. Kibenge F.S.B., Godoy M.M., editors. Elsevier; Amsterdam: 2016. Coronaviruses in aquatic organisms; pp. 327–335. Chapter 20, pages. [DOI] [Google Scholar]
- Sernapesca 2020. https://www.aqua.cl/2020/06/16/debido-a-situacion-en-china-sernapesca-detalla-acciones-de-control-sanitario-para-exportaciones-de-salmon-chileno/ Available at. (Accessed on July 08, 2020)
- Sharun K, Tiwari R, Natesan S, Dhama K. SARS-CoV-2 infection in farmed minks, associated zoonotic concerns, and importance of the One Health approach during the ongoing COVID-19 pandemic. Veterinary Quarterly. 2020;41(1):50–60. doi: 10.1080/01652176.2020.1867776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Wen Z., Zhong G., Yang H., Wang C., Huang B., Liu R., et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science. 2020;368:1016–1020. doi: 10.1126/science.abb7015. https://science.sciencemag.org/content/368/6494/1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai L., Zhong G., Yuan Q., Wen Z., Wang C., He X., Liu R., Wang J., Zhao Q., Liu Y., Huo N., Deng J., Bai J., Wu H., Guan Y., Shi J., Tian T., Xia N., Chen H., Bu Z. Replication, pathogenicity, and transmission of SARS-CoV-2 in minks. National Science Review. 2020:nwaa291. doi: 10.1093/nsr/nwaa291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia S.F., Yan L.-M., Chin A.W.H., Fung K., Choy K.-T., Wong A.Y.L., Kaewpreedee P., Perera R.A.P.M., et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020;583:834–838. doi: 10.1038/s41586-020-2342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman A.I., Boehm A.B. Systematic review and meta-analysis of the persistence and disinfection of human coronaviruses and their viral surrogates in water and wastewater. Environ. Sci. Technol. Lett. 2020;7:544–553. doi: 10.1021/acs.estlett.0c00313. [DOI] [PubMed] [Google Scholar]
- Singh M., Bansal V., Feschotte C. A single-cell RNA expression map of human coronavirus entry factors. Cell Reports. 2020;32(108175) doi: 10.1016/j.celrep.2020.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sit T.H.C., Brackman C.J., Ip S.M., Tam K.W.S., et al. Infection of dogs with SARS-CoV-2. Nature. 2020;586:776–778. doi: 10.1038/s41586-020-2334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.W., Berry E.S., Skilling D.E., et al. In vitro isolation and characterization of a calicivirus causing a vesicular disease of the hands and feet. Clin. Infect. Dis. 1998;26:434–439. doi: 10.1086/516311. https://www.jstor.org/stable/4481372 [DOI] [PubMed] [Google Scholar]
- Smith D.J., Lapedes A.S., de Jong J.C., Bestebroer T.M., Rimmelzwaan G.F., Osterhaus A.D.M.E., Fouchier R.A.M. Mapping the antigenic and genetic evolution of influenza virus. Science. 2004;305(5682):371–376. doi: 10.1126/science.1097211. [DOI] [PubMed] [Google Scholar]
- Tang X., Wu C., Li X., Song Y., et al. On the origin and continuing evolution of SARS-CoV-2. Natl. Sci. Rev. 2020;7:1012–1023. doi: 10.1093/nsr/nwaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D., Lindsay A.C., Halcox J.P. Aerosol and surface stability of SARSCoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. https://www.nejm.org/doi/10.1056/NEJMc2004973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tei S., Kitajima N., Takahashi K., Mishiro S. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet. 2003;362(9381):371–373. doi: 10.1016/S0140-6736(03)14025-1. [DOI] [PubMed] [Google Scholar]
- Thompson H. 2020. COVID-19 case clusters offer lessons and warnings for reopening.https://www.sciencenews.org/article/coronavirus-covid-19-case-clusters-lessons-warnings-reopening Available at. [Google Scholar]
- Tidey A. 2020. German COVID-19 infection rate jumps to 1.79 after meat plant outbreak.https://www.euronews.com/2020/06/21/german-covid-19-infection-rate-jumps-to-1-79-after-meat-plant-outbreak Available at. [Google Scholar]
- Tiwari R., Dhama K., Sharun K., Yatoo M., Malik Y.S., Singh R., Michalak I., Sah R., Bonilla-Aldana D.K., Rodriguez-Morales A.J. COVID-19: animals, veterinary and zoonotic links. Vet. Q. 2020;40(1):169–182. doi: 10.1080/01652176.2020.1766725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K.K-W., Tsang O., Yip C., Chan K.-H., Wu T.-C., Chan J., Leung W.-S., Chik T., Choi Chris Yau-Chung, Kandamby D., Lung D., Tam A., Poon R., Fung A., Hung I., Cheng V., Chan Jasper Fuk-Woo, Yuen K.-Y. Consistent detection of 2019 novel coronavirus in saliva. 2020. Clinical Infectious Diseases. 2020;71(15):841–843. doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd E.C.D., Greig J.D., Bartleson C.A., Michaels B.A. Outbreaks where food workers have been implicated in the spread of foodborne disease. Part 4. Infective doses and pathogen carriage. . Journal of Food Protection. 2008;71(11):2339–2373. doi: 10.4315/0362-028x-71.11.2339. [DOI] [PubMed] [Google Scholar]
- Tsang T.K., Cowling B.J., Fang V.J., et al. Influenza A virus shedding and infectivity in households. J. Infect. Dis. 2015;212(9):1420–1428. doi: 10.1093/infdis/jiv225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C., Crameri G., Kong X., Chen J., Sun Y., Yu M., Xiang H., Xia X., Liu S., Ren T., Yu Y., Eaton B.T., Xuan H., Wang L.F. Antibodies to SARS coronavirus in civets. Emerg. Infect. Dis. 2004;10(12):2244–2248. doi: 10.3201/eid1012.040520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA [United States Department of Agriculture] Confirmed cases of SARS-CoV-2 in animals in the United States. 2020. https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/sa_one_health/sars-cov-2-animals-us Available at. (Accessed July 21, 2020)
- van Boheemen S., de Graaf M., Lauber C., Bestebroer T.M., Raj V.S., Zaki A.M., Osterhaus A.D., Haagmans B.L., Gorbalenya A.E., Snijder E.J., Fouchier R.A. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. mBio. 2012;3(6) doi: 10.1128/mBio.00473-12. e00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Munster V.J. Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Euro Surveill. 2013;18(38) doi: 10.2807/1560-7917.es2013.18.38.20590. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20590 pii=20590. Available online: [DOI] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasickova P., Dvorska L., Lorencova A., Pavlik I. Viruses as a cause of foodborne diseases: a review of the literature. Vet. Med. –Czech. 2005;50:89–104. [Google Scholar]
- Velebit B., Radin D., Teodorovic V. Transmission of common foodborne viruses by meat products. Proc Food Sci. 2015;5:304–307. doi: 10.1016/j.profoo.2015.09.069. [DOI] [Google Scholar]
- Velebit B., Djordjevic V., Milojevic L., Babic M., Grkovic N., Jankovic V., et al. The common foodborne viruses: a review. Earth Env Sci. 2019;333(2019) doi: 10.1088/1755-1315/333/1/012110. [DOI] [Google Scholar]
- Vijgen L., Keyaerts E., Moes E., Thoelen I., Wollants E., Lemey P., et al. Complete genomic sequence of human coronavirus OC43: molecular clock analysis suggests a relatively recent zoonotic coronavirus transmission event. J. Virol. 2005;79:1595–1604. doi: 10.1128/JVI.79.3.1595–1604.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- V’kovski P., Gultom M., Kelly J., Steiner S., Russeil J., Mangeat B., Cora E., et al. Disparate temperature-dependent virus – host 1 dynamics for SARS-CoV-2 and 2 SARS-CoV in the human respiratory epithelium. bioRxiv preprint doi: https://doi.org/10.1101/2020.04.27.062315; this version posted December 18, 2020. 2020 doi: 10.1101/2020.04.27.062315. (Accessed February 4, 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahba L., Jain N., Fire A.Z., Shoura M.J., Artiles K.L., McCoy M.J., Jeong D.-E. An extensive meta-metagenomic search identifies SARS-CoV-2-homologous sequences in pangolin lung viromes. mSphere. 2020;5 doi: 10.1128/mSphere.00160-20. e00160-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P.J., Siddell S.G., Lefkowitz E.J., Mushegian A.R., et al. Changes to virus taxonomy and the international code of virus classification and nomenclature ratified by the international committee on taxonomy of viruses. Arch. Virol. 2019;164:2417–2429. doi: 10.1007/s00705-019-04306-w. [DOI] [PubMed] [Google Scholar]
- Waltenburg M.A., Victoroff T., Rose C.E., et al. Update: COVID-19 among workers in meat and poultry processing facilities — United States, April–may 2020. MMWR Morb Mortal Wkly Rep. 2020;69:887–892. doi: 10.15585/mmwr.mm6927e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94 doi: 10.1128/JVI.00127-20. e00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang V., Yu E. 2020. Beijing shuts down seafood market after dozens test positive for coronaviru.https://www.nytimes.com/2020/06/13/world/asia/beijing-market-coronavirus.html Available at: [Google Scholar]
- Wang X.-W., Li J.S., Guo T.K., et al. Concentration and detection of SARS coronavirus in sewage from Xiao Tang Shan hospital and the 309th hospital. J. Virol. Methods. 2005;128:156–161. doi: 10.1016/j.jviromet.2005.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.-W., Li J.-S., Jin M., Zhen B., et al. Study on the persistence of severe acute respiratory syndrome-associated coronavirus. J. Virol. Methods. 2005;126:171–177. doi: 10.1016/j.jviromet.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J. Am. Med. Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartecki A., Rzymski P. On the coronaviruses and their associations with the aquatic environment and wastewater. Water. 2020;12:1598. doi: 10.3390/w12061598. [DOI] [Google Scholar]
- Wei L., Lin J., Duan X., Huang W., Lu X., Zhou J., Zong Z. Asymptomatic COVID-19 patients can contaminate their surroundings: an environment sampling study. mSphere. 2020;5 doi: 10.1128/mSphere.00442-20. e00442-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO [World Health Organization] Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. Scientific Brief; March. 2020;29:2020. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations Available at. [Google Scholar]
- WHO [World Health Organization] Breastfeeding and COVID-19. Scientific Brief; June 23, 2020. 2020. https://www.who.int/news-room/commentaries/detail/breastfeeding-and-covid-19 Available at. (Accessed July 14, 2020)
- Wiersinga W.J., Rhosea A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- Wikipedia Fur Farming. 2021. https://en.wikipedia.org/wiki/Fur_farming
- Wölfel R., Corman V.M., Guggemos W., Sellmaler M., Zange S., Müller M.A., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Wong M.C., Javornik Cregeen S.J., Ajami N.J., Petrosino J.F. Evidence of recombination in coronaviruses implicating pangolin origins of nCoV-2019. bioRxiv. 2020 doi: 10.1101/2020.02.07.939207. [DOI] [Google Scholar]
- Woolhouse M., Scott F., Hudson Z., Howey R., Chase-Topping M. Human viruses: discovery and emergence. Philosophical Transactions of the Royal Society B: Biological Sciences. 2012;367:2864–2871. doi: 10.1098/rstb.2011.0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P., Duan F., Luo C., Liu Q., Qu X., Liang L., Wu K. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138:575–578. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Xiao A., Zhang J., Gu X., Lee W.L., Kauffman K., Hanage W., Matus M., Ghaeli N., Endo N., Duvallet C., Moniz K., Erickson T., Chai P., Thompson J., Alm E. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. medRxiv. 2020 doi: 10.1101/2020.04.05.20051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S., Marechal V., Mouchel J.M., Moulin L. Time course quantitative detection of SARS-CoV-2 in Parisian wastewaters correlates with COVID-19 confirmed cases. medRxiv. 2020:10–13. doi: 10.1101/2020.04.12.20062679. [DOI] [Google Scholar]
- Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K., Zhai J., Feng Y., Zhou N., et al. Isolation of SARS-CoV-2-related coronavirus from Malayan pangolins. Nature. 2020;583:286–289. doi: 10.1038/S41586-020-2313-X. [DOI] [PubMed] [Google Scholar]
- Xiao-Yu C., Yong Z., Xin C., Jian Z., Xian-Dong Z., Feng J., Xu L. Isolation and genetic analysis of a nidovirus from crucian carp (Carassius auratus) Arch. Virol. 2019;164:1651–1654. doi: 10.1007/s00705-019-04221-0. [DOI] [PubMed] [Google Scholar]
- Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y., et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., Li T., Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020;12:1–5. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of the SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z.-W., Yuan S., Yuen K.-S., Fung S.-Y., Chan C.-P., Jin D.-Y. Zoonotic origins of human coronaviruses. Int. J. Biol. Sci. 2020;16:1686–1697. doi: 10.7150/ijbs.45472. https://www.ijbs.com/v16p1686.htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekta R., Vahid-Dastjerdi L., Norouzbeigi S., Mortazavian A.M. Food products as potential carriers of SARS-CoV-2. Food Control. 2020:107754. doi: 10.1016/j.foodcont.2020.107754. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai X., Sun J., Yan Z., Zhang J., Zhao J., Zhao Z., Gao Q., He W.-T., Veit M., Su S. Comparison of SARS-CoV-2 spike protein binding to ACE2 receptors from human, pets, farm animals, and putative intermediate hosts. J. Virol. 2020;94 doi: 10.1128/JVI.00831-20. e00831-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Shen W., Xu C., Wang Y., Xu H., Liu X., Wei Y. Discovery of a novel Piscanivirus in yellow catfish (Pelteobagrus fulvidraco) in China. Infect. Genet. Evol. 2019;74:103924. doi: 10.1016/j.meegid.2019.103924. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Zhang H., Huang K., Yang Y., Hui X., et al. SARS-CoV-2 neutralizing serum antibodies in cats: a serological investigation. bioRxiv. 2020 (preprint doi: https://doi.org/10.1101/2020.04.01.021196. this version posted April 3, 2020. (Accessed February 4, 2021)) [Google Scholar]
- Zhang H., Kang Z., Gong H., et al. Digestive system is a potential route of COVID-19: an analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut. 2020;69:1010–1018. doi: 10.1136/gutjnl-2020-320953. [DOI] [Google Scholar]
- Zhang J., Wang S., Xue Y. Fecal specimen diagnosis 2019 novel coronavirus-infected pneumonia. J. Med. Virol. 2020;92:680–682. doi: 10.1002/jmv.25742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Chen D., Szabla R., Zheng M., Li G., Du P., Zheng S., Li X., Song C., Li R., Guo J.-T., Junop M., Zeng H., Lin H. Broad and differential animal angiotensin-converting enzyme 2 receptor usage by SARS-CoV-2. J. Virol. 2020;94 doi: 10.1128/JVI.00940-20. e00940-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Shi Z.-L. Sars-CoV-2 spillover events. Science. 2021;371(6525):120–122. doi: 10.1126/science.abf6097. [DOI] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]