Abstract
This study aims to examine whether maternal smoking, birth weight, birth month and breastfeeding are associated with COVID-19 infection and hospitalisation. Maternal smoking was positively associated with COVID-19 infection. Breastfeeding was negatively associated with COVID-19 infection. The odds of being hospitalised due to COVID-19 were higher among those who had lower birthweight and mothers who were smoking during pregnancy.
Keywords: UK Biobank, Early life factors, COVID-19
1. Introduction
Coronavirus disease 2019 (COVID-19) is a highly infectious condition caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). As of December 2020, more than 60 million laboratory confirmed cases and over 1 million deaths due to COVID-19 had been reported worldwide. The World Health Organization (WHO) declared COVID-19 a pandemic on March 11, 2020 [1]. While smoking [2] and the presence of chronic diseases [3] are susceptibility factors for COVID-19 infection, there is a wide variation in the risk of contracting the disease that might be explained by factors determined during early life.
The effect on environmental tobacco smoke in early life, especially maternal smoking on lower lung function later in life [4], lower respiratory infections [5] and obstructive pulmonary disease [6,7] is well established. A Norwegian study found that long-term breastfeeding was associated with a reduced risk of lower respiratory tract infections in later life [5]. Many diseases, including asthma, and acute upper respiratory infection, demonstrate birth month dependencies [8]. Prior studies have reported a link between birth weight and poorer adult lung function [9] and mortality associated with chronic obstructive airways disease [10]. Using a large UK population cohort (UK Biobank) this study aimed to identify associations between early life factors, including maternal smoking, breastfeeding, birth month and birthweight, and the risk of COVID-19 infection and hospitalisation.
2. Methods
2.1. Study population
We used the UK Biobank, a longitudinal population-based cohort of more than half million participants living in the UK aged between 37 and 73 years old when recruited in 2006–2010 [11]. UK Biobank collects information on self-reported demographic, lifestyle, health information and detailed biological measurements of the respondents assessed by touch-screen questionnaire and nurse-led interview at the baseline. COVID-19 test results from English participants were collected between 16th March 2020 and 21st December 2020, and were provided by Public Health England (PHE) [12]. Almost all of the respondents tested for COVID-19 infection are from combined nose/throat swabs, for PCR to be performed. Lower respiratory samples may also be analysed in intensive care settings. We excluded respondents who lived in Scotland, Wales, or Northern Ireland, were born outside UK and Ireland, and had died prior to March 2020. This resulted in a study population of 384,816 participants.
This study was conducted as part of UK Biobank project number 41877 and is covered by the generic ethics approval for UK Biobank studies from the NHS National Research Ethics Service (16/NW/0274).
2.2. Measurements
The outcomes were confirmed COVID-19 infection and hospitalisation due to COVID-19. We considered the respondents as positive if at least one of the laboratory tests performed were positive for SARS-CoV-2. We categorised the respondents as inpatients if they were confirmed COVID-19 infection, and one of their test results were assigned from an inpatient setting.
Early-life variables were self-reported by the respondents. Maternal smoking was defined based on the question ‘Did your mother smoke regularly around the time when you were born?’. We categorised respondents as breastfed if they confirmed that they were breastfed when they were babies. Information on birth month was retrieved from the birth date and was treated as the cosine of the values, which represents rhythmic seasonal length of night. Birthweight was collected as self-reported birthweight in kg [13].
We included sociodemographic and health behaviour as covariates. We calculated age at the time of the first COVID-19 test. Sex was included as a dummy variable representing the female gender. Average total household income before tax (%) was given as less than 18,000 (used as the reference), 18,000 to 30,999, 31,000 to 51,999, 52,000 to 100,000, greater than 100,000. Education was included as a dummy variable representing the college or university degree. Self-reported health status was given as very good (used as the reference), good, fair or poor. Body mass index (BMI) was calculated as body weight divided by the square of body height. We categorised respondents as non-smoker, past smoker or current smokers. Similarly, drinking behaviour was classified as never drinking alcohol, history of drinking alcohol in the past, and currently drinking alcohol.
2.3. Statistical analysis
We performed a multivariable logistic regression models to estimate the odds ratios of COVID-19 infection (7733 positive compared to 377,083 remaining participants) and inpatient due to COVID-19 (2494 inpatient compared to 382,322 remaining participants). The main predictors were maternal smoking around birth, breastfed as a baby, birth month and birthweight. All models were further adjusted for age, sex, higher education, average household income, health status, drinking behaviour, smoking status and BMI. In addition, we performed the same analyses to estimate the odds ratios of COVID-19 infection and hospitalisation in the first peak (March–July 2020) or the second peak of the pandemic (August–December 2020). Patients with missing data in any regressor were excluded from the logistic regression model. All statistical analyses were performed using STATA 16 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC.).
3. Results
Of 384,816 eligible respondents, 43,428 (11.29%) had been COVID-19 tested, 7733 (2.01%) had tested positive and 2494 (0.65%) were hospitalised due to COVID-19 (Table 1 ). 23.37% of the positive cases and 52.41% of the hospitalised individuals were detected during the first peak. The number of participants exposed to maternal smoking at birth was proportionally higher among COVID-19 infected participants and those who were hospitalised due to COVID-19 compared to those who did not have a positive COVID-19 test. The number of participants reported that they had breastfed were proportionally lower in those who tested positive for COVID-19 infection and who were hospitalised compared to those not tested. Volunteers who tested positive for COVID-19 were male, reported having less average household income, not have a higher education, current/former smokers, have a higher BMI and reported having poor health status compared to those who did not have a positive COVID-19 test (Table 1).
Table 1.
Descriptive statistics of the cohort.
| Variable | Total (N = 384,816) |
Not tested (N = 341,388) |
Tested for COVID-19 (N = 43,428) |
COVID-19 test positive (N = 7733) |
Inpatients due to COVID-19 (N = 2494) |
|---|---|---|---|---|---|
| Peak-1 (Mar–Jul 2020) (%) | 23.37 | 52.41 | |||
| Peak-2 (Aug–Dec 2020) (%) | 76.63 | 47.59 | |||
| Early-life experience | |||||
| Maternal smoking around birth (%) | 30.63 | 30.35 | 32.78 | 36.28 | 36.69 |
| Breastfed as a baby (%) | 71.74 | 71.77 | 71.58 | 65.48 | 70.35 |
| Birth month (%) | |||||
| January | 8.40 | 8.42 | 8.25 | 7.86 | 7.30 |
| February | 7.95 | 7.95 | 8.01 | 8.03 | 7.66 |
| March | 9.03 | 9.03 | 8.98 | 9.04 | 9.10 |
| April | 8.61 | 8.61 | 8.63 | 8.60 | 9.06 |
| May | 9.04 | 9.04 | 9.01 | 8.72 | 8.87 |
| June | 8.46 | 8.46 | 8.42 | 8.22 | 8.30 |
| July | 8.51 | 8.52 | 8.49 | 8.35 | 7.82 |
| August | 8.27 | 8.27 | 8.24 | 8.51 | 8.14 |
| September | 8.13 | 8.10 | 8.33 | 8.39 | 8.38 |
| October | 8.00 | 7.99 | 8.11 | 8.42 | 8.78 |
| November | 7.59 | 7.60 | 7.52 | 7.86 | 8.50 |
| December | 8.01 | 8.01 | 8.01 | 7.99 | 8.18 |
| Birthweight (kg) | 3.32 (SD = 0.66) | 3.32 (SD = 0.66) | 3.32 (SD = 0.69) | 3.33 (SD = 0.69) | 3.29 (SD = 0.73) |
| Sociodemographic | |||||
| Age (years) | 68.30 (SD = 8.06) | 68.16 (SD = 8.03) | 69.37 (SD = 8.23) | 66.17 (SD = 8.79) | 70.00 (SD = 8.65) |
| Male (%) | 45.13 | 44.86 | 47.26 | 49.19 | 55.13 |
| College or university degree (%) | 31.31 | 31.83 | 27.26 | 22.63 | 18.55 |
| Average total household income before tax (%) | |||||
| Less than 18,000 | 21.62 | 21.09 | 25.87 | 24.68 | 36.79 |
| 18,000 to 30,999 | 25.50 | 25.49 | 25.57 | 24.26 | 25.23 |
| 31,000 to 51,999 | 26.59 | 26.76 | 25.26 | 27.41 | 22.92 |
| 52,000 to 100,000 | 20.82 | 21.15 | 18.18 | 19.05 | 11.90 |
| Greater than 100,000 | 5.47 | 5.51 | 5.13 | 4.61 | 3.15 |
| Health status and health behaviour | |||||
| Self-reported health status (%) | |||||
| Excellent | 16.54 | 16.95 | 13.33 | 13.30 | 9.36 |
| Good | 59.20 | 59.73 | 55.06 | 55.10 | 49.35 |
| Fair | 20.42 | 19.85 | 24.96 | 25.32 | 30.47 |
| Poor | 3.83 | 3.47 | 6.66 | 6.28 | 10.82 |
| Drinking behaviour (%) | |||||
| Never | 3.27 | 3.23 | 3.58 | 3.06 | 3.95 |
| Drinking in the past | 3.30 | 3.18 | 4.23 | 3.98 | 6.20 |
| Drink currently | 93.43 | 93.59 | 92.18 | 92.96 | 89.85 |
| Smoking behaviour (%) | |||||
| Non-smoker | 55.00 | 55.60 | 50.29 | 50.44 | 42.52 |
| Past smoker | 35.19 | 34.74 | 38.70 | 38.35 | 44.94 |
| Current smoker | 9.81 | 9.66 | 11.02 | 11.21 | 12.54 |
| BMI (kg/m2) | 27.37 (SD = 4.74) | 27.29 (SD = 4.70) | 28.00 (SD = 4.99) | 28.33 (SD = 5.00) | 29.26 (SD = 5.42) |
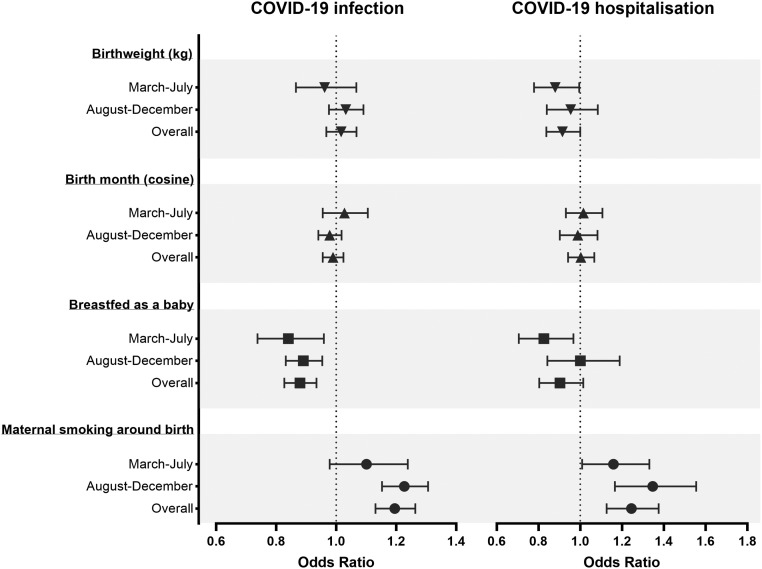
Table S1 reports the odds ratio of those who tested positive for COVID-19 and COVID-19 inpatients. In the sociodemographic, health status and health behaviour adjusted model (Fig. 1), odds ratio of COVID-19 infection shows that maternal smoking around birth was associated with a 20% higher risk of infection (95%CI 1.13 to 1.26). This risk was mostly observed in the positive cases of the second peak (adjusted OR 1.23, 95%CI 1.15 to 1.31). In addition, those who reported maternal smoking around birth had a 24% higher risk of hospitalisation due to COVID-19 (95%CI 1.13 to 1.38). This risk was present in the first and second peaks. Respondents who were breastfed had overall 12% lower odds of contracting COVID-19 (95%CI 0.83 to 0.93). This protective effect of having breastfed was present in both peaks. Breastfeeding was negatively associated with hospitalisation only in the first peak (adjusted OR 0.83, 95%CI 0.71 to 0.97). Birth month has no significant association with COVID-19 infection or inpatient due to COVID-19. Birthweight was not associated with an increased risk of contracting COVID-19, however lower birth weight was associated with inpatients due to the disease (adjusted OR 0.91, 95%CI 0.84 to 1.00). This association was mostly observed in the positive cases of the first peak (adjusted OR 0.88, 95%CI 0.78 to 0.99).
Fig. 1.
Associations between early-life factors and COVID-19 infection and hospitalisation. Logistic regression models were adjusted for age, sex, higher education, household income, health status, drinking behaviour, smoking behaviour and BMI. The graphs show odds ratio and 95% confidence intervals of maternal smoking around birth, breastfed as baby, cosine of birth month and birthweight (kg). 1st peak: March 2020–July 2020, 2nd peak August: 2020–December 2020.
4. Discussion
In this cohort study of 384,816 adults from England, a higher birth weight is associated with lower risk of COVID-19 hospitalisation. Low birth weight is commonly linked with poorer lung function later in life, which may lead to a higher risk of morbidity and mortality due to respiratory diseases [9,10]. Maternal smoking was found to be associated with higher risk of COVID-19 infection and hospitalisation. A considerable body of evidence has showed the link between maternal smoking and respiratory diseases [[5], [6], [7]]. For example, using information from the European Community Respiratory Health Survey, a study found that maternal smoking was associated with more respiratory symptoms, including asthma and chronic bronchitis symptoms [6]. A more recent study showed that the risk of COPD among adults in the UK was increased among those whose mothers smoked regularly around the time when they were born [7]. Smoking habits among mothers were used in prior studies as a measure of the child's exposure to environmental tobacco smoke [5]. In utero exposure to maternal smoking was related with lower lung function among young males, and this effect may persist to later stages of life [14]. Further research examining potential mechanisms that may explain the association between maternal smoking and respiratory infections, including COVID-19, is needed.
Breastfeeding had a significant relationship with lower risk of COVID-19 infection. This result supports a US study which showed that breastfeeding for at least 6 months provides greater protection against respiratory tract infections compared with shorter breastfeeding periods [15]. Breastfeeding has also been shown to have protective effects against lower respiratory tract infection in the first year of life [5], and our study suggests that this effect may last into adulthood.
The main limitation of the present study is the retrospective nature of the information about early life. Recall error and recall bias may lead to an underestimation of the association between early life conditions and COVID-19 infection. Examining detailed perinatal information covering the duration of breastfeeding or exposure to smoke, route of delivery and perinatal infections may provide a better understanding of relationship between early-life programming of vulnerability to COVID-19. Another limitation is that the observational nature of this study makes the findings susceptible to confounding effects and prevents us from inferring causality. This study includes COVID-19 data tested before 21st December 2020, therefore the overall effect size may change with the addition of recent cases.
We recommend further investigations into the pathways linking early life exposures with COVID-19 risk to better understand inequalities and differences in the severity of the disease. During the COVID-19 pandemic, protecting the vulnerable is a key policy driver that will require understanding risk factors both recent and from early life.
The following is the supplementary data related to this article.
Unadjusted and adjusted logistic regressions of positive COVID-19 test and inpatients due to COVID-19.
CRediT authorship contribution statement
A.D and A.M performed the data analysis. A.D and A.M drafted the manuscript. M.M.C., A.P. and N.P were involved in planning and supervised the work. All authors discussed the results and commented on the manuscript.
Declaration of competing interest
All authors declare no conflicts of interest.
Acknowledgements
Altug Didikoglu was supported by a grant from the Republic of Turkey Ministry of National Education. This research has been conducted using the UK Biobank Resource under Application Number 41877.
References
- 1.World Health Organization . World Health Organization; Geneva: 2020. Coronavirus Disease 2019 (COVID-19) Situation Report—91.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports URL: [Google Scholar]
- 2.Vardavas C.I., Nikitara K. COVID-19 and smoking: a systematic review of the evidence. Tob. Induc. Dis. 2020;18:20. doi: 10.18332/tid/119324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McQueenie R., Foster H., Jani B.D., Katikireddi S.V., Sattar N., Pell J.P., et al. Multimorbidity, polypharmacy, and COVID-19 infection within the UK Biobank cohort. PLoS One. 2020;15 doi: 10.1371/journal.pone.0238091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Upton M., Watt G., Smith G.D., McConnachie A., Hart C. Permanent effects of maternal smoking on offsprings’ lung function. Lancet. 1998;352:453. doi: 10.1016/s0140-6736(05)79187-x. [DOI] [PubMed] [Google Scholar]
- 5.Nafstad P., Jaakkola J., Hagen J., Botten G., Kongerud J. Breastfeeding, maternal smoking and lower respiratory tract infections. Eur. Respir. J. 1996;9:2623–2629. doi: 10.1183/09031936.96.09122623. [DOI] [PubMed] [Google Scholar]
- 6.Svanes C., Omenaas E., Jarvis D., Chinn S., Gulsvik A., Burney P. Parental smoking in childhood and adult obstructive lung disease: results from the European Community Respiratory Health Survey. Thorax. 2004;59:295–302. doi: 10.1136/thx.2003.009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magnus M.C., Henderson J., Tilling K., Howe L.D., Fraser A. Independent and combined associations of maternal and own smoking with adult lung function and COPD. Int. J. Epidemiol. 2018;47:1855–1864. doi: 10.1093/ije/dyy221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boland M.R., Shahn Z., Madigan D., Hripcsak G., Tatonetti N.P. Birth month affects lifetime disease risk: a phenome-wide method. J. Am. Med. Inform. Assoc. 2015;22:1042–1053. doi: 10.1093/jamia/ocv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawlor D.A., Ebrahim S., Smith G.D. Association of birth weight with adult lung function: findings from the British Women’s Heart and Health Study and a meta-analysis. Thorax. 2005;60:851–858. doi: 10.1136/thx.2005.042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barker D.J., Godfrey K., Fall C., Osmond C., Winter P., Shaheen S. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. Br. Med. J. 1991;303:671–675. doi: 10.1136/bmj.303.6804.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sudlow C., Gallacher J., Allen N., Beral V., Burton P., Danesh J., et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.UK Biobank COVID-19 Test Results Data 2020. 2020. https://www.ukbiobank.ac.uk/enable-your-research/about-our-data/covid-19-data URL.
- 13.De Kovel C.G., Carrión-Castillo A., Francks C. A large-scale population study of early life factors influencing left-handedness. Sci. Rep. 2019;9:1–11. doi: 10.1038/s41598-018-37423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayatbakhsh M.R., Sadasivam S., Mamun A.A., Najman J.M., Williams G., O’Callaghan M. Maternal smoking during and after pregnancy and lung function in early adulthood: a prospective study. Thorax. 2009;64:810–814. doi: 10.1136/thx.2009.116301. [DOI] [PubMed] [Google Scholar]
- 15.Chantry C.J., Howard C.R., Auinger P. Full breastfeeding duration and associated decrease in respiratory tract infection in US children. Pediatrics. 2006;117:425–432. doi: 10.1542/peds.2004-2283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Unadjusted and adjusted logistic regressions of positive COVID-19 test and inpatients due to COVID-19.