Abstract
Spontaneous intestinal perforation (SIP) of the newborn is a single intestinal perforation commonly found in the terminal ileum without distinct causes. These cases often associated with prematurity. The new COVID-19 in pregnancy increased the risk of premature rupture of membranes, preterm delivery, intrauterine fetal death (IUFD), and low birth weight (LBW). Here we report a premature twin with SIP that was born from Coronavirus-19 positive mother.
Keywords: COVID-19, Neonatology, Spontaneous intestinal perforation
Abbreviations
- COVID-19
Coronavirus Disease 2019
- CPAP
Continous positive airway pressure
- ELBW
Extremely low birth weight
- IUFD
Intrauterine fetal death
- LBW
Low birth weight
- MERS-CoV
Middle east respiratory syndrome coronavirus
- NICU
Neonatal intensive care unit
- PICC
Peripherally inserted central catheter (PICC)
- PPV
Positive pressure ventilation
- RDS
Respiratory distress syndrome
- RT-PCR
Reverse transcription-polymerase chain reaction
- SaO2
Saturation oxygen
- SARS-CoV
Severe acure respiratory syndrome coronavirus
- SIP
Spontaneous intestinal perforation
- TPN
Total parenteral nutrition
- USG
Ultrasonography
- VLBW
Very low birth weight
1. Introduction
Spontaneous intestinal perforation (SIP) is a single intestinal perforation typically involving the distal ileum's antimesenteric border and usually occurs in the extremely premature infant in the first 1–2 weeks of life [1]. SIP is the second common cause of intestinal perforation in neonates, especially in low-birth-weight newborn, the incidence rate is 1.1% in very low birth weight (VLBW, birth weight <1500 g) and 7.4% in extremely low birth weight (ELBW, birth weight <1000 g) [2]. SIP incidence was related to various perinatal factors, for example, intrauterine drug exposure, especially cocaine, intestinal anomalies (aganglionosis or atresia), congenital heart defects, sepsis, polycythemia, asphyxia, respiratory distress syndrome (RDS), and use of umbilical catheters, and exsanguine-transfusion. These factors cause mesenteric blood vessels disruption, resulting in hypoperfusion and hypoxia in the intestines [3].
The Novel Coronavirus disease-2019 (COVID-19) has rapidly spread globally with the number of confirmed cases increasing, threatening global public health. Allotey et al., 2020 reported that 10% of pregnant women who were admitted to the hospital were confirmed positive for COVID-19 [4]. Little is known about the correlation between Coronavirus-19 infection with pregnancy. Data from previous coronaviruses (SARS-CoV and MERS-CoV) suggests that pregnant women may be at higher risk of severe illness, morbidity, or mortality than the general population. However, there is no evidence that the infection affects infant outcomes [5]. In this case, we reported the premature twin with SIP that was born from Coronavirus-19 positive mother.
2. Case report
2.1. Case presentation
A 31-year-old gravida 3, para 1, severe preeclampsia, obesity grade III, with monochorionic diamniotic (MDCA) twins presented at 32 weeks’ gestation in the emergency room. We perform initial fetal monitoring, followed by administering a single dose of dexamethasone for fetal lung maturation and magnesium sulfate initial dose for tocolysis. Chest x-ray examination shows bacterial pneumonia and pleural effusion, meanwhile RT-PCR confirm the Coronavirus-19 infection. We decided to carry out an emergency caesarean section with COVID-19 health protocols.
An 1800 g male neonate was delivered with low general activities and minimal respiratory effort. His Apgar scores were 3, 5, and 7 at 1, 5, and 10 minutes. There were fine rales and wet sounding rhonchi with suspicious of Respiratory Distress Syndrome (RDS). After performed neonatal resuscitation, the baby starts breathing spontaneously. Respiratory rate was 40 breaths per minute, the temperature was 36.7°Celcius, pulse was 140 beats per minutes, and the saturation was 96% at 10 minutes. The patient transferred to the neonatal intensive care unit (NICU) with Continuous Positive Airway Pressure (CPAP) support. Meanwhile, the twins, with a birth weight of 1500 g, breathing spontaneously with room air after resuscitation. The laboratory examination of 1800 g infant shows blood glucose was 70, hematocrit was 51,7%, white blood cell count was 14.8 × 103/mcL, platelets were 255 × 103/mcL, sodium 133 mmol/L, potassium 5 mmol/L, calcium 7.2 mg/dL), and albumin 2.9 g/L. A peripheral blood smear was unremarkable. First line antibiotics were given.
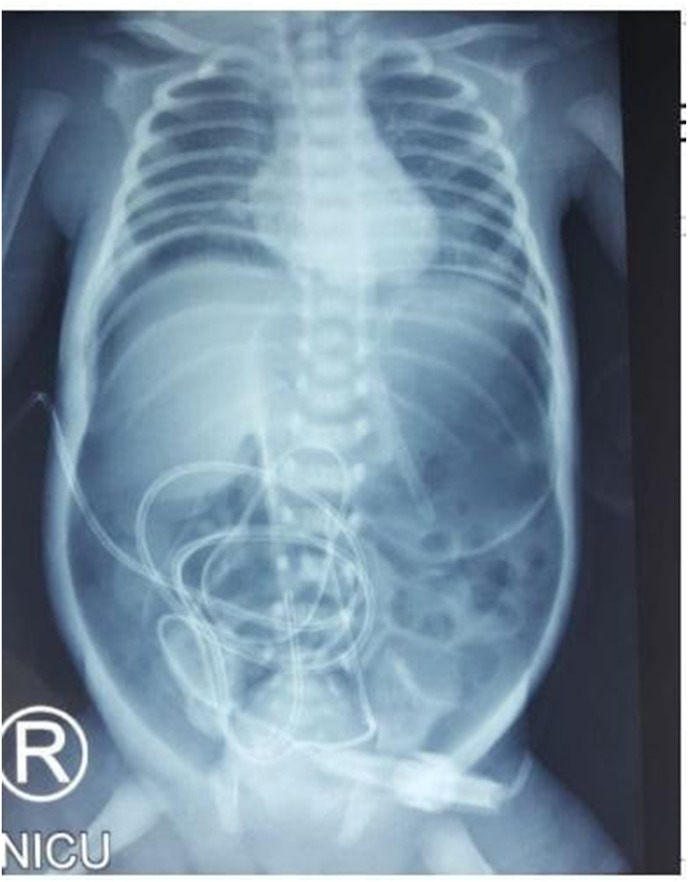
The patient was still weak, vomiting was present while feeding, and no defecation was noted 24 hours after born. The infant was immediately treated with fasting, total parenteral nutrition (TPN) and albumin administration. In the next day, abdominal distention and meconium defecation were noted. The abdominal x-ray confirmed pneumoperitoneum (Fig. 1 ). The patient RT-PCR swab examination was negative.
Fig. 1.
Pneumoperitoneum: shadow appearance in x-ray shown the presence of air or gas in the right and left sub-hemidiaphragm, forming a continuous diaphragm sign, and surround the entire abdominal wall, creating a football sign.
2.2. Treatment
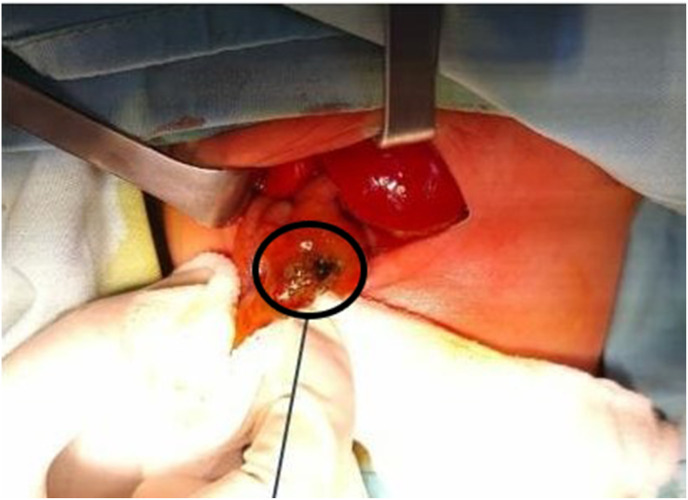
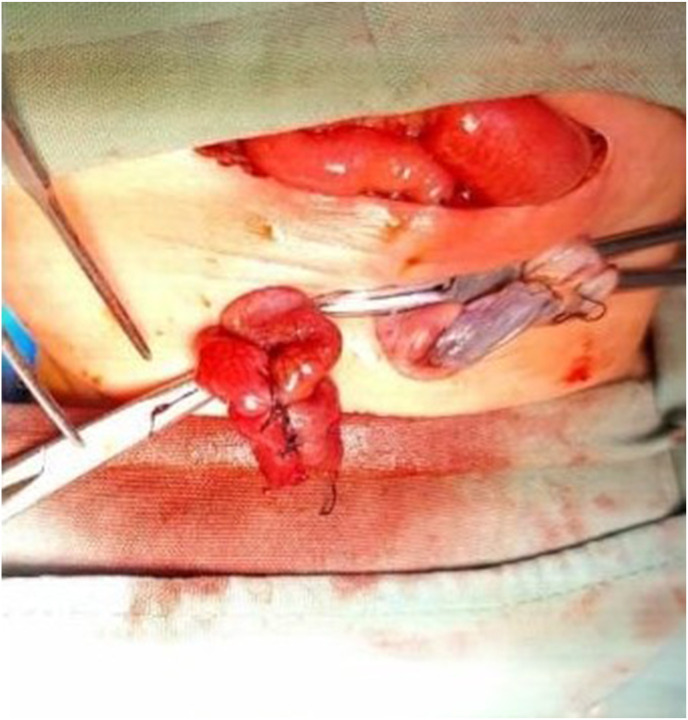
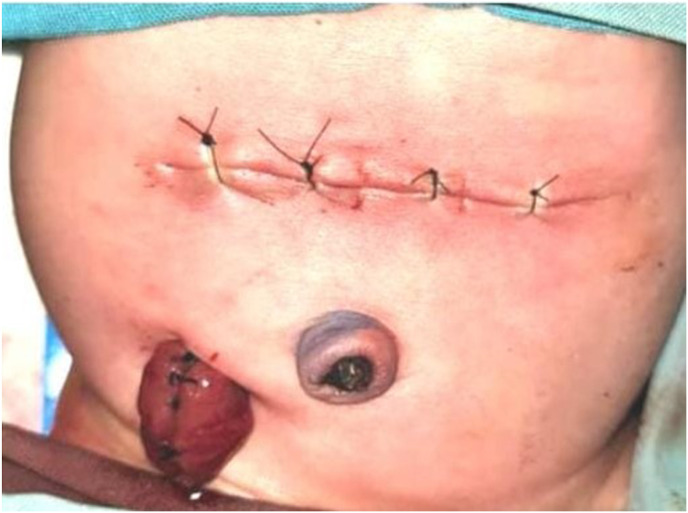
A pediatric surgeon consultant performed the surgery. The patient was placed in a supine position. 10% povidone-iodine was used to disinfect the operating area. A transverse incision was performed at supraumbilical and continued down to expose the peritoneum. Meconium mixed with cloudy peritoneum were found. One cm perforation in the ileum with a diameter of 1 cm was found, located 60 cm from the distal Treitz ligament and 40 cm proximal to the ileocecal junction (Fig. 2 A). Stomach, small intestine, and distal sigmoid were within normal limits, and microcolon was not found. The perforated segment of ileum was resected, and Mikulicz stoma ileostomy was performed. (Fig. 2B). Wash the abdominal cavity with warm 0.9% NaCl then the surgical wound was sutured (Fig. 2C). The postoperative course was uneventful and the patients was discharged home after 25 days.
Fig. 2.
SIP operation procedure. 2A. One cm of ileum perforation was found (black circle). 2B. Ileum resection and Mikulicz stoma ileostomy were performed. 2C. Post-operation, the surgical wound has been sutured.
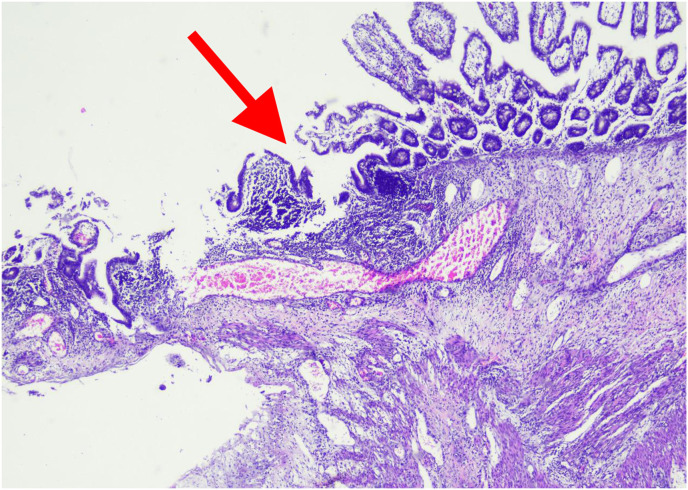
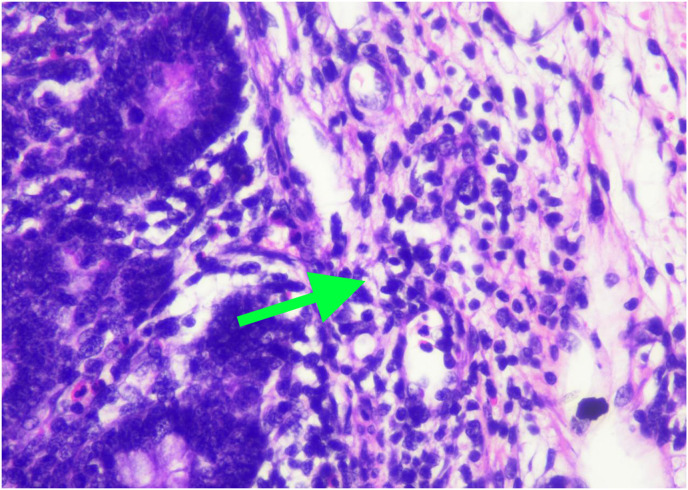
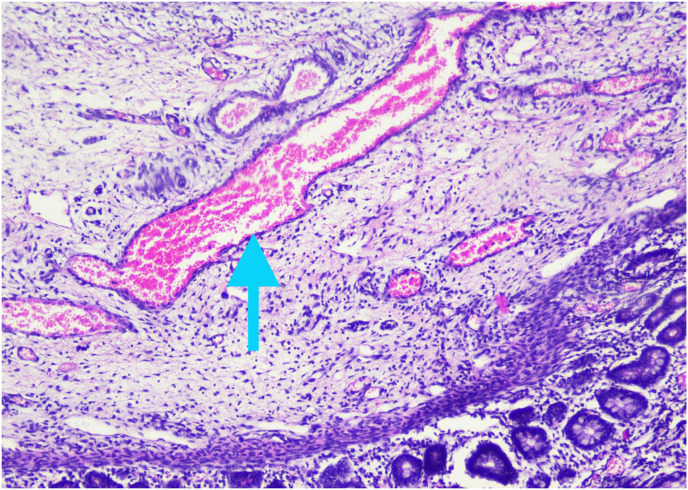
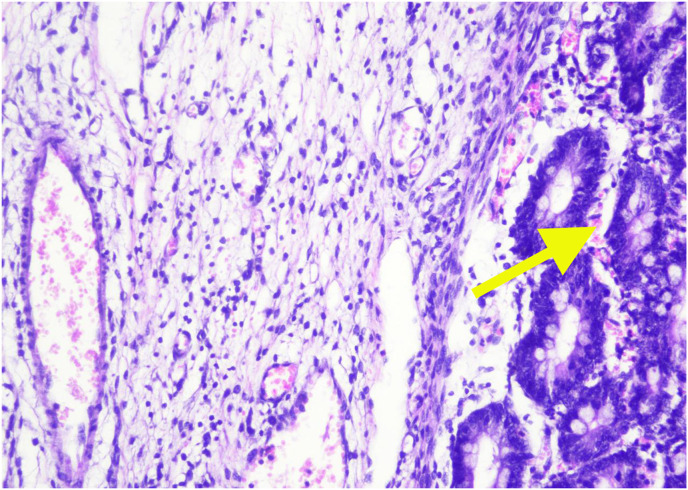
Histopathological examination showed pieces of ileal tissue with partially eroded mucosal surfaces (Fig. 3 A). Lamina propria and submucosal appear swollen, along with infiltration of inflammatory cells (lymphocytes, histiocytes, plasma cells, and eosinophils) (Fig. 3B). Dilatation of blood vessels (Fig. 3C) and extravasation of erythrocytes (Fig. 3D) were found. There was no evidence of malignancy found. The conclusion was nonspecific ileitis.
Fig. 3.
Histopathology examination of ileal tissue. 3A. Partial erosion of mucosal lining (red arrow). 3B. Inflammatory cells, lymphocytes, histiocytes, plasma cells, and eosinophils (green arrow). 3C. Dilatation of blood vessels (blue arrow). 3D. Extravasation of erythrocytes (yellow arrow). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3. Discussion
There are two major causes of intestinal perforations in the neonate. The most common type was secondary to necrotizing enterocolitis/NEC (43% of cases). The less common type occurs as focal or spontaneous/idiopathic perforation of the terminal ileum (22% of cases). SIP occurs in infants who are relatively younger and with LBW. It usually happens about seven days after birth, earlier than NEC [6]. In this case, perforation occurs two days after delivery with gestational age 32 weeks and birth weight 1800 g, thus belong to SIP.
The aetiology and pathogenesis of SIP were still unknown. Many theories have been proposed, but none has been proven to be the cause [2]. SIP is considered a secondary event to immaturity, postnatal exposure to dexamethasone or indomethacin, hypotension (reflected by the need for inotropes), leukocytosis, candidiasis, staphylococcus epidermidis infection, placement of an umbilical arterial catheter, or the presence of patent ductus arteriosus [1,7]. Stress, hypoxia, or shock may lead to regional hypoperfusion and transient intestinal ischemia resulting in SIP [2]. Premature rupture of membranes, low Apgar scores, perinatal asphyxia, feeding tubes, mechanical ventilation, and cardiovascular resuscitation in the perinatal period may be increasing risk of SIP [2,8]. Conditions associated with fetal or neonatal hypoxia are essential antecedents for this distinct emerging entity [9].
COVID-19 pandemic is still a worldwide health problem. Pregnant women and neonates were included in the age category that very vulnerable to Coronavirus-19 infection. There was no evidence of vertical transmission of COVID-19 in neonates, while the horizontal transmission is thought to be the source of infection in neonates. Viral pneumonia is one of the most important causes of morbidity and mortality in pregnant women [10]. Pneumonia in pregnant women is associated with various clinical outcomes of labour, such as premature rupture of membranes, preterm delivery, IUFD, LBW, and neonatal mortality [11]. Neonates may be at high risk for complications of COVID-19 infection due to low immune system and physiological changes in the respiratory and cardiovascular systems at birth [12]. Other studies reported that the manifestation of symptoms and clinical signs of COVID-19 infection in neonates could be less severe than adults. The main symptom is fever, vomit, cough, or shortness of breath. None causes neonatal death [13].
In this case, the patient was born from a mother who was Coronavirus-19 positive. Both of patient and his twins were Coronavirus-19 negative from RT-PCR swab examination. Placenta examination to identify Coronavirus-19 in these twins was not performed. Apart from prematurity itself as the main risk factor for SIP, the other risk factors were PPV treatment, low APGAR score, CPAP use, umbilical catheter, and tube feeding. Besides, adverse perinatal conditions like asphyxia may initiate intestinal ischemia, thus aggravate the risk of intestinal perforation. The associations between SIP incidence and Coronavirus-19 infection in mothers have not been proven in this case. Even though the other twin has lower birth weight (birth weight 1500g), SIP did not occur.
The SIP clinical manifestations were sudden abdominal distension, a bluish discolouration of the abdomen, hypotension, and metabolic acidosis. Complete blood count examination showed an increase or decrease in white blood cells, an increase in immature cells (left shift), and a decline in the number of platelets. Electrolyte examination may reveal hyponatremia and hyperkalemia. Radiological examination typically shows pneumoperitoneum (free air). However, a gasless abdomen may be found as well. Specific radiological findings associated with NEC were not observed. There were three clinical features to help distinguish SIP with perforation in NEC: (1) SIP occurs early (usually in the first 1–2 weeks of life); (2) abdominal distention; (3) free air with the absence of pneumatosis or portal venous gas in abdominal x-ray [1]. Our patient present with abdominal distension occurs in day three after born and pneumoperitoneum in the form of a football sign.
The management of SIP consists of medical management and surgical management. Medical management consists of resting the gastrointestinal tract (GIT) for 7–14 days, circulatory support, and antibiotics administration. Gastric decompression was performed by inserting an orogastric tube, providing TPN as needed, and fasting. Continue the patient's respiratory support. Give inotropic to keep normal blood pressure and observe urine production to 1–3 mL/kg/hour. Administer parenteral antibiotics for 7–10 days [1]. We immediately fasted the patient and administered the TPN when abdominal distension was found. Respiration support was maintain using CPAP, and an orogastric tube was inserted. Metronidazole was given when the diagnosis of ileum perforation established.
The optimal surgical management of SIP was still controversial. The traditional surgical approach was exploratory laparotomy by resecting the bowel; while another approach is primary peritoneal drainage (PPD). A prospective randomized trial study by Moss et al. and Rees et al. in the case of NEC or SIP perforation, both studies showed that there was no difference in outcome between the two methods [1]. In our case, the pediatric surgeon performed an exploratory laparotomy, resected the ilium, and Mikulicz stoma ileostomy. The histopathological examination of the tissue found swollen lamina propria and submucosa and inflammatory cells' infiltration (lymphocytes, histiocytes, plasma cells, and eosinophils). Dilatation of blood vessels and extravasation of erythrocytes were also found. These findings strongly support the diagnosis of SIP.
Mortality of neonatal intestinal perforation was still high, ranging from 40 to 70% [14]. Several recent studies have reported lower mortality. Prgomet et al. stated that the mortality rate for neonatal intestinal perforation was 31% [15]. A study conducted by Hyginus et al. said that NEC, prematurity, low birth weight, multiple perforations, and delay in identifying perforations were increasing the mortality in neonatal gastrointestinal perforations. However, unlike the NEC, SIP has a better prognosis with a survival rate of between 70% and 100% [16]. There were no NEC or multiple perforations found in our case. Early identification, immediate surgery, and optimal postsurgical care have the best outcomes in most cases. The postponement of hospital discharge for this patient was related to the mother's isolation period due to COVID-19, stoma care education to the family, breastfeeding optimization, and Kangaroo Mother Care education for her 1500 g twin.
4. Conclusion
The aetiology and pathogenesis of SIP were still unknown. Besides prematurity as a significant risk factor, perinatal risk factors such as PPV measures, low APGAR score, CPAP, umbilical catheter, and tube feeding were included as the SIP risk factos. However, the associations between SIP and Coronavirus-19 infection has not yet proven in this case. Early diagnosis, immediate surgery, and appropriate postoperative care were crucial in SIP management.
Patient consent
Patient's family gives permission for patient information to be published in scientific journal anonymously.
Funding
No funding or grant support.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
The following authors have no financial disclosures: Am H, Ag H, RE, MTU, DA, KDH, MTAS.
References
- 1.Gomella T.L., Eyal F.G., Bany-Mohammed F. 8th. McGraw Hill; New York: 2020. Spontaneous intestinal perforation. Gomella’s Neonatology: Management, Procedures, On-Call Problem, Diseases, and Drugs; pp. 1051–1056. [Google Scholar]
- 2.Tiwari C., Sandlas G., Jayaswal S., Shah H. Spontaneous intestinal perforation in neonates. J Neonatal Surg. 2015;4(2):14. [PMC free article] [PubMed] [Google Scholar]
- 3.Young C.M., Kingma S.D., Neu J. Ischemia-reperfusion and neonatal intestinal injury. J Pediatr. 2011;158:e25–e28. doi: 10.1016/j.jpeds.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Allotey J., Stallings E., Bonet M., Yap M., Chatterjee S., Kew T. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rasmussen S.A., Smulian J.C., Lednicky J.A., Wen T.S., Jamieson D.J. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrugia M.K., Morgan A.S., McHugh K., Kiely E.M. IMAGES IN NEONATAL MEDICINE neonatal gastrointestinal perforation. Arch Dis Child Fetal Neonatal Ed. 2003;88:F75. doi: 10.1136/fn.88.1.F75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah J., Singhal N., Silva O. Intestinal perforation in very preterm neonates: risk factors and outcomes. J Perinatol. 2015;35:595–600. doi: 10.1038/jp.2015.41. [DOI] [PubMed] [Google Scholar]
- 8.Basany L., Aepala R., Reddy M.M. Spontaneous jejunal perforation in a term neonate: case report. Indian J Neonatal Med Res. 2018, Oct;6(4):PC01–PC03. doi: 10.7860/IJNMR/2017/38419.2239. [DOI] [Google Scholar]
- 9.Korakaki E., Manoura A., Hatzidaki E., Arbiros J., Vlahakis J., Valari V. Spontaneous intestinal perforation in a full-term infant: association with infection. Minerva Pediatr. 2003;55:289–292. [PubMed] [Google Scholar]
- 10.Berkowitz K., LaSala A. Risk factors associated with the increasing prevalence of pneumonia during pregnancy. Am J Obstet Gynecol. 1990;163:981–985. doi: 10.1016/0002-9378(90)91109-. [DOI] [PubMed] [Google Scholar]
- 11.Karimi-Zarchi M., Neamatzadeh H., Dastgheib S.A., Abbasi H., Mirjalili S.R., Behforouz A. Vertical transmission of coronavirus disease 19 (COVID-19) from infected pregnant mothers to neonates: A review. Fetal Pediatr Pathol. 2020;39(3):246–250. doi: 10.1080/15513815.2020.1747120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicholson E.G., Avadhanula V., Ferlic-Stark L., Patel K., Gincoo K.E., Piedra P.A. The risk of serious bacterial infection in febrile infants 0-90 days of life with a respiratory viral infection. Pediatr Infect Dis J. 2019;38:355–361. doi: 10.1097/INF.0000000000002165. [DOI] [PubMed] [Google Scholar]
- 13.De Bernardo G., Giordano M., Zollo G., Chiatto F., Sordino D., De Santis R. The clinical course of SARS-CoV-2 positive neonates. J Perinatol. 2020;40:1462–1469. doi: 10.1038/s41372-020-0715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asabe K., Oka Y., Kai H.I., Shirakusa T. Neonatal gastrointestinal perforation. Tuk J Pediatr. 2009;51:264–270. [PubMed] [Google Scholar]
- 15.Prgomet S., Lukšić B., Pogorelić Z., Jurić I., Čapkun V., Arapović A. Perinatal risk factors in newborns with gastrointestinal perforation. World J Gastrointest Surg. 2017;9(2):46–52. doi: 10.4240/wjgs.v9.i2.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hyginus E.O., Jideoffor U., Victor M., N O.A. Gastrointestinal perforation in neonates: Aetiology and risk factors. J Neonatal Surg. 2013;2(3):30. [PMC free article] [PubMed] [Google Scholar]