Abstract
SARS-CoV-2 can spread by close contact through large droplet spray and indirect contact via contaminated objects. There is mounting evidence that it can also be transmitted by inhalation of infected saliva aerosol particles. These particles are generated when breathing, talking, laughing, coughing or sneezing. It can be assumed that aerosol particle concentrations should be kept low in order to minimize the potential risk of airborne virus transmission. This paper presents measurements of aerosol particle concentrations in a gym, where saliva aerosol production is pronounced. 35 test persons performed physical exercise and aerosol particle concentrations, CO2 concentrations, air temperature and relative humidity were obtained in the room of 886 m³. A separate test was used to discriminate between human endogenous and exogenous aerosol particles. Aerosol particle removal by mechanical ventilation and mobile air cleaning units was measured. The gym test showed that ventilation with air-change rate ACH = 2.2 h−1, i.e. 4.5 times the minimum of the Dutch Building Code, was insufficient to stop the significant aerosol concentration rise over 30 min. Air cleaning alone with ACH = 1.39 h−1 had a similar effect as ventilation alone. Simplified mathematical models were engaged to provide further insight into ventilation, air cleaning and deposition. It was shown that combining the above-mentioned ventilation and air cleaning can reduce aerosol particle concentrations with 80 to 90% , depending on aerosol size. This combination of existing ventilation supplemented with air cleaning is energy efficient and can also be applied for other indoor environments.
Keywords: COVID-19, Aerosol, Sports club, Fitness center, Building ventilation, Air purifier
1. Introduction
In the second week of 2021, the European Centre for Disease Prevention and Control reported 94,582,873 cases of SARS-CoV-2 including 2,036,713 deaths, world-wide [1]. It has been suggested that this virus can be transmitted by respiratory droplets and by contact routes [[2], [3], [4], [5], [6], [7]]. Direct transmission can occur when infective droplets produced by activities such as talking, laughing, coughing or sneezing reach the mucosae (mouth and nose) or conjunctiva (eyes) of another person. Indirect or contact route transmission can occur via handrails, keyboard buttons and other objects, where virus is deposited after contact with an infected person. There is mounting evidence that the virus can also be transmitted by inhalation of saliva aerosol particles because the virus has been found in small aerosol particles that can remain in the air for hours, and it has been shown to maintain viability in such aerosols [[8], [9], [10], [11], [12]]. Therefore, precautionary measures should not only be applied for the direct transmission route and the contact route, but also for the airborne route.
Respiratory droplets are generated from the fluid lining of the respiratory tract during expiratory activities such as breathing, talking, laughing, coughing and sneezing [[13], [14], [15], [16]]. A single sneeze can produce 10,000 droplets or more [17]. A cough can produce from 100 up to 1000 droplets or more. Talking can produce about 50 droplets per second [18]. On an hourly or daily basis however, normal mouth breathing is assumed to generate more aerosol particles than coughing or sneezing because the latter are intermittent events [13,[19], [20], [21], [22]].
Expired droplet sizes can range from about 0.1 μm to 1 mm [16]. Large droplets will generally settle rather quickly due to gravity and therefore can only contribute to virus transmission between individuals in close proximity. This is why “social distancing” has been introduced in countries around the world, although there is no strict consensus on the distance to be kept and the currently used 1.5 m, 1.8 m or 2 m distance is actually a compromise between avoiding large droplet spray and practical feasibility in keeping this distance in daily life. Small droplets however, and their residues or droplet nuclei after evaporation, can remain suspended in the air for a much longer time and could transfer SARS-CoV-2 over larger distances [16,[23], [24], [25]].
There is no clear consensus in the scientific literature on the diameter separating large droplets from small droplets or aerosol particles. Large droplets were initially defined as those with diameter larger than 100 μm by Wells [23]. In line with this definition, Hinds [26] defines aerosols as a suspension of solid or liquid particles in a gas with particle size from 0.001 to over 100 μm. Others however have labeled droplets larger than 5 μm [27,28] or 10 μm [29] as large droplets. Nicas et al. [30] suggested a particle with a diameter of a few tens of μm or larger to be a droplet. Xie et al. [25] revisited the Wells evaporation-falling curve and defined the critical droplet size as the diameter of a droplet that has completed evaporated at the time it hits the ground, falling from 2 m height. They found that a saline water droplet can have a critical diameter of about 30 μm to nearly 100 μm, depending on the drop ejection speed and the ambient temperature and relative humidity (RH). It is assumed that the consideration of different RH led to the different critical diameters from different studies [16,22,25].
Small droplets evaporate quickly to the size of droplet nuclei, which settle very slowly and can remain suspended in the air for a long time [30]. Nicas et al. [30] estimated that expired aerosol particles rapidly evaporate to a diameter slightly below half of the initial diameter if the concentration of non-volatile components is assumed to be 88 g/L. For a particle of 20 μm at RH = 30 and 70%, it would take only 0.17 s and 0.4 s, respectively, to evaporate to an equilibrium diameter of 10 μm [30]. Morawska et al. [31] stated that a 5 μm droplet of pure water evaporates in 0.8 s at 97% RH and a 3 μm droplet in less than 0.33 s. Holmgren et al. [32] found that the droplet diameter reduced by a factor 0.42 in 75% ambient RH and that evaporation is a very fast process, in line with Nicas et al. [30].
After expiration, the movement of the aerosol particles in the enclosure is initially influenced by the expiratory jet, which is a moist and turbulent buoyant gas cloud [25,33,34]. Evidently this jet is much less pronounced for breathing than for coughing and sneezing. After the influence of the expiratory jet, the indoor airflow patterns take over. Indoor airflow patterns can be very complex [[35], [36], [37], [38], [39], [40], [41], [42], [43]]. A person is also a source of heat and vapor and a – mainly thermal – convective plume is present around each person that yields a clear upward airflow near their body [22,44]. The movement of the aerosol particles is therefore determined by the interaction between the expiratory jet, the human thermal plume and other sources that affect the indoor airflow pattern, such as the ventilation system, thermal plumes from appliances and other heat sources, and the movement of people in the enclosure.
The droplet nuclei are submicrometer to approximately 10 μm in size and can remain suspended in the air for hours while each carrying multiple virions [16], and van Doremalen et al. [9] demonstrated an approximately 1-h viability half-life of the SARS-CoV-2 virus. Therefore, precautions against COVID-19 transmission should also address the potential aerosol or airborne transmission route. It can be assumed that aerosol particle concentrations in indoor environments should be kept low in order to minimize the potential risk of virus transmission. Respiratory aerosol particle concentration build-up in indoor environments can be pronounced, certainly when ventilation is insufficient.
The role of building ventilation in the airborne transmission of infectious agents was reviewed by Li et al. [45]. They concluded on the existence of an association between ventilation, air movement in buildings and the spread of infectious diseases such as influenza and SARS. However, they also indicated the lack of data to specify the minimum ventilation requirements in buildings such as hospitals, schools and offices to avoid the airborne spread of infectious diseases. Ai and Melikov [22] reviewed the airborne spread of expiratory droplet nuclei between the occupants of indoor environments. They highlighted the importance of indoor airflow patterns and stated the need for future research in three specific areas: the importance of the direction of indoor airflow patterns, the dynamics of airborne transmission and the application of computational fluid dynamics (CFD) simulations to obtain more detailed insights.
In the second half of the year 2020 during the COVID-19 pandemic, an increasingly large number of international organizations and national government authorities have stressed that “sufficient ventilation” should be ensured [[46], [47], [48], [49]]. However, to the best of our knowledge, there are three main questions for which, to date, no clear quantitative answer has been provided. First, it is not clear how much ventilation is required to keep aerosol concentrations limited. Clearly, this will depend on the number of persons per unit surface area, on the physical and respiratory activity of these persons, on their physiological characteristics in terms of aerosol particle emission and on the ventilation efficiency. Especially concerning human aerosol particle emission during various types of activities, the information available in the scientific literature is rather scarce. Second, in terms of exposure, it is not yet known which limit of aerosol concentrations can be considered safe. In other words, it is not yet known which level of potentially infected aerosol particle concentrations for which duration can be a risk for which people with which type of immune system. Third, in case ventilation is insufficient, it is not clear to what extent air cleaning can be engaged to bring the aerosol particle concentrations below a certain threshold value. The present study attempts to provide information that can help in answering the first and third question. The study does not explicitly focus on infection risk but on ventilation and air cleaning as measures to limit the build-up of aerosol concentrations in the indoor environment of a gym.
The paper is structured as follows. In section 2, some more information about the state-of-the-art in aerosol particle production during physical exercise and about the state-of-the-art in ventilation and in air cleaning is provided. Section 3 contains a short study to discriminate between human endogenous and exogenous aerosol particles. Section 4 presents the measurement set-up and associated measurement results in the gym under study. In sections 5, 6, simplified mathematical modeling is applied to provide insight into the effective ventilation, air cleaning and deposition fluxes, and to extrapolate the findings to scenarios with longer exercise sessions and more air cleaning units. Sections 7 (discussion) and 8 (conclusions) conclude the paper.
2. Aerosol production, ventilation and air cleaning in gyms
2.1. Gyms and aerosol particle production during physical exercise
A gym is an environment that houses equipment and services for the purpose of physical exercise. A gym was selected as a case study for several reasons. First, respiratory aerosol particle production and aerosol particle inhalation in gyms is expected to be more pronounced than in many other indoor environments. Although there are only a few studies that provide some indirect indication of how physical exercise influences the emission of respiratory droplets, these studies are consistent in indicating an overall substantial increase in aerosol expiration due to more intensive breathing compared to tidal breathing. Johnson and Morawska [13] found that deep exhalation resulted in a 4 to 6-fold increase in aerosol particle concentration. Rapid inhalation produced a further 2- to 3-fold increase in concentration, while rapid exhalation had little effect on the measured concentration. Almstrand et al. [14] studied the effect of airway opening on aerosol particle production. Test subjects performed different breathing maneuvers in which the initial lung volume preceding an inhalation to total lung capacity was varied between functional residual capacity (FRC; the volume of air in the lungs at the end of passive expiration) and residual volume (RV; the volume of air in the lungs after full exhalation). The number of expired aerosol particles showed a 2 to 18-fold increase after exhalations to RV compared with exhalations without airway closure. Concerning inhalation during physical exercise, at least three aggravating factors are discerned: (i) the quantity of inhaled pollutants increases proportionally with the minute ventilation; (ii) most of the air is inhaled through the mouth and therefore by-passes the normal nasal mechanisms for filtration of large particles; and (iii) the increased airflow velocity carries pollutants deeper into the respiratory tract [50]. A second reason for selecting a gym as case study is that gyms have been identified as key locations for possible infection transmission and even potential ‘superspreading’ events [[51], [52], [53]]. For example, COVID-19 outbreaks have been reported in 12 fitness dance classes in South Korea [53] and in a fitness center in Belgium [54] where aerosol transmission could have been a factor. Together with recent studies suggesting that asymptomatic carriers can transfer SARS-CoV-2 [55,56], these studies have fueled concerns on SARS-CoV-2 spreading in fitness centers. A third reason is public health and economy. Sports have an important role in society in view of the health and well-being of the population and reducing the burden on healthcare services. Certainly during the COVID-19 pandemic, sports have been and still are undoubtedly important [[57], [58], [59]]. However, due to the pandemic, authorities in many countries have ordered fitness centers and gyms to be closed and over the past months they have only gradually and partially reopened, and eventually in many countries closed again near the end of 2020. A long closure or a long reduced occupation density can negatively affect the health and well-being of the population. It can also have detrimental economic consequences, with bankruptcies and the associated negative consequences throughout the whole supply chain. As an example, in the Netherlands, fitness is the most practiced sport [60] with a total of 3,900 fitness centers that are registered at the main national branch organization and with an associated total revenue of 1.9 billion Euro in 2019 [61].
To the best of our knowledge, there is no study in the scientific literature that specifically focuses on respiratory aerosol production in fitness centers or gyms. There is even relatively little published research about air quality in fitness centers in general, as opposed to residential buildings and other types of public spaces such as schools and offices [[62], [63], [64]]. The few studies that are available in the scientific literature have measured particulate matter (PM) concentrations in fitness centers as part of indoor air quality studies, without focus on saliva aerosol particles. Indeed, PM concentrations in fitness centers can not only originate from respiratory activity (endogenous particles) but also from injection by the ventilation system, resuspension from room and equipment surfaces after earlier deposition, resuspension from clothing, skin and hair, friction between fitness machine components, friction between clothing, etc. The latter are termed exogenous particles. Salonen et al. [64] provided a review of studies on contaminants in indoor sports facilities including fitness centers. The PM concentration levels in fitness centers were found to be highly influenced by the occupancy level, the type or intensity of the indoor activity and the ventilation type. Ramos et al. [65] measured higher PM concentrations for aerobic than for holistic classes. Aerobic included all the classes that involved power, strength, vigorous and fast movements, however excluding cycling. Holistic included all classes that involved meditation, stability and flexibility movements. The higher concentrations during aerobic classes were attributed to the activity patterns that promoted resuspension of particles [[66], [67], [68]]. PM10 concentrations measured in the same classroom and on the same day were also higher during the aerobic class (average 45 μg/m³) than in the holistic class (average 33 μg/m³), which was again attributed to the greater resuspension caused by the aerobic activities. The relation between PM concentration and resuspension was also indicated by Ramos et al. [69] who found higher PM concentrations coinciding with the period of fitness classes. Concentrations were much lower in fitness centers with mechanical ventilation including filtration of outdoor air than in centers with natural ventilation with open windows [70]. Maximum PM concentrations were typically higher in rooms for group classes than in large workout areas such as those with cardiovascular equipment and free weights. The maxima occurred during high-intensity cardio group classes, with the highest PM10 concentration observed for a cycling class.
2.2. Ventilation in gyms
Ventilation can be defined as “the process of introducing and distributing outdoor and/or properly treated recycled air into a building or a room” [71] or “the process by which ‘clean’ air (normally outdoor air) is intentionally provided to a space and stale air is removed” [72]. Authoritative books and extensive reviews have been dedicated to this the topic over the past decades (e.g. Refs. [71,[73], [74], [75]]). A distinction is made between two main ventilation categories: displacement ventilation and mixing ventilation. To the best of our knowledge, the vast majority of gyms apply mixing ventilation. This can be either mechanical ventilation, natural ventilation or hybrid mechanical/natural ventilation. The indoor air flow patterns in mixing ventilation in general and around persons in particular can be very complex [[35], [36], [37], [38], [39], [40], [41], [42], [43], [44]]. These air flow patterns also govern the motion of the expired aerosol particles due to their low inertia. The intention of mixing ventilation is to dilute the concentrations of e.g. aerosol particles, after which part of this mixed air is expelled to the outside. In some cases ventilation includes recirculation of part of the heated or cooled exhausted air back to the inside, for the purpose of energy conservation. In case of infectious diseases, if recirculation is applied, which is not recommended (e.g. Refs. [48,49]), the recirculated air should be treated so that infectious aerosols are annihilated.
In the Netherlands, the minimum requirements for the ventilation of buildings are prescribed by the Building Code (“Bouwbesluit”) published in 2012 and last amended in 2020 [76]. The Building Code applies a person-based approach in which the minimum fresh air ventilation rates in dm³/s per person are stipulated. The minimum values for different types of buildings are given in Table 1 , where a distinction is made between new and existing buildings. Table 1 shows that the required flow rates for indoor sports centers are higher than for shops but – for new buildings – lower than for educational buildings and identical to those of industrial and office buildings. This does not seem to be aligned with the expected higher aerosol particle production during physical exercise [13,14].
Table 1.
Minimum required ventilation flow rates for different building usage types in the Dutch Building Code [76].
| Function | Requirement in dm³/s/person | |
|---|---|---|
| New buildings | Existing buildings | |
| Childcare | 6.5 | 3.44 |
| Meeting | 4 | 2.12 |
| Healthcare, bed area | 12 | 3.44 |
| Healthcare, other areas | 6.5 | 3.44 |
| Industrial | 6.5 | 3.44 |
| Office | 6.5 | 3.44 |
| Hotel, dormitory | 12 | 6.40 |
| Education | 8.5 | 3.44 |
| Sports | 6.5 | 3.44 |
| Shopping | 4 | 2.12 |
In 2008, the Dutch “Guidebook for Sports Accommodations” was published by the NOC*NSF [77]. The NOC*NSF (Dutch Olympic Committee * Dutch Sports Federation) is the overarching organization for all sports activities, professional and recreational, in the Netherlands. In 2014, specific guidelines for sports facilities for people with disabilities were published by a consortium of organizations including the NOC*NSF [78]. These guidelines stipulate a minimum ventilation flow rate of 11.1 dm³/s per exercising person for sports halls, which is 70% above the minimum required value in the Dutch Building Code for new buildings and even 3.2 times higher for existing buildings (see Table 1). The Guidebook [77] even suggests a total of 6 air change rates per hour (ACH) for fitness spaces, which implies that the volume of air in the room is replaced by fresh air 6 times per hour. For aerobics and martial arts spaces, it advices ACH = 8 h−1 and for indoor cycling ACH = 10 h−1. These higher values seem better aligned with the expected higher production of heat, vapor, CO2 and aerosol particles by people during physical exercise.
In view of the COVID-19 pandemic, ASHRAE, the American Society of Heating, Refrigerating and Airconditioning Engineers, has acknowledged the potential for aerosol transmission of SARS-CoV-2 and states that facilities of all types should follow, as a minimum, the latest published standards and guidelines and good engineering practice [79]. ASHRAE Standard 62.1 specifies ventilation rates for acceptable indoor air quality [80]. For gyms, health clubs, aerobics rooms and weight rooms, the minimum outdoor airflow rate is 10 dm3/s/person. This is higher than specified for most retail buildings (3.8 dm³/s/person, except for beauty and nail salons where 10 dm³/s/person is required) and educational buildings (3.8–5 dm³/s/person). Note that the ASRHAE value for gyms aligns well with the 11.1 dm³/s from the Dutch guidelines [77,78].
2.3. Air cleaning in gyms
Air cleaning can be defined as the removal of potentially harmful airborne contaminants, usually aerosol particles but sometimes also gases, from the air [81]. Air cleaners (ACs) can be installed in indoor environments as small stand-alone mobile units or inside HVAC (heating, ventilation, airconditioning) units or air handler units in buildings. A wide range of technologies for ACs exist, such as filtration, activated carbon, ultraviolet germicidal irradiation, electrostatic precipitators, photocatalytic oxidation and plasma. Large ACs are also referred to as professional ACs. ACs should have a sufficiently high aerosol particle removal efficiency and a sufficiently high volume flow rate, in comparison to the room volume to be treated. Fisk et al. [82] stated that filter efficiencies above 85% provide only modest gains in performance. Several authors mentioned that the air flow rates must be at least several ACH to obtain substantial particle reductions [[82], [83], [84], [85]]. The Association of Home Appliance Manufacturers (AHAM) Air Cleaner Council defines the steady state for air cleaning as at least an 80% continuous removal of smoke particles [86]. Asbach et al. [87] mention that ACs should yield 3 to 6 air changes per hour, with the higher value preferred in the context of the COVID-19 pandemic [88]. The US ANSI/AHAM AC-1:2015 standard [86] evaluates ACs based on their clean air delivery rate (CADR), which is defined as the measure of the delivery of contaminant free air, within the defined particle size range, by an AC, expressed in cubic feet per minute (cfm) or m³/h. The CADR is the rate of contaminant reduction in a test chamber when the AC is turned on, minus the rate of natural decay when the AC is not running, multiplied by the volume of the test chamber as measured in ft³ or m³ [86]. Assuming a room 8 ft high (= 2.44 m) and to achieve a 80% steady state removal, the floor area is related to the CADR by Ref. [86]:
| (1) |
with A in ft2 and CADR in cfm. In SI units with A in m2 and the CADR in m³/h, this is:
| (2) |
To the best of our knowledge, at the moment of writing this paper, the application of air cleaning in public spaces in the Netherlands and many other European countries is rather rare, even though AC technology is not new and the COVID-19 pandemic is already more than a year old. This is partly attributed to the sometimes less good reputation of commercially available ACs and the inferior performance of some of these ACs. First, while several high-quality ACs are available on the market, others have very low efficiencies and some even generate harmful by-products such as O3 and NOx [[89], [90], [91], [92]]. Asbach et al. [87] stated that evidence provided by manufacturers on the effectiveness of their ACs should always be critically reviewed. There is a lack of proper testing standards and certification. There is currently no European testing standard for ACs and an international IEC standard to replace the national standards is currently in preparation [87]. Second, mobile ACs are easy to install (plug and play) and it is tempting for uneducated individuals to perform the selection, purchase, installation and operation themselves. However, ACs will only provide good results if the efficiency is high enough, if the installed capacity is in line with the room air volume to be handled and if proper maintenance procedures and frequencies are applied.
3. Endogenously versus exogenously generated aerosol particles
3.1. Measurement set-up and protocol
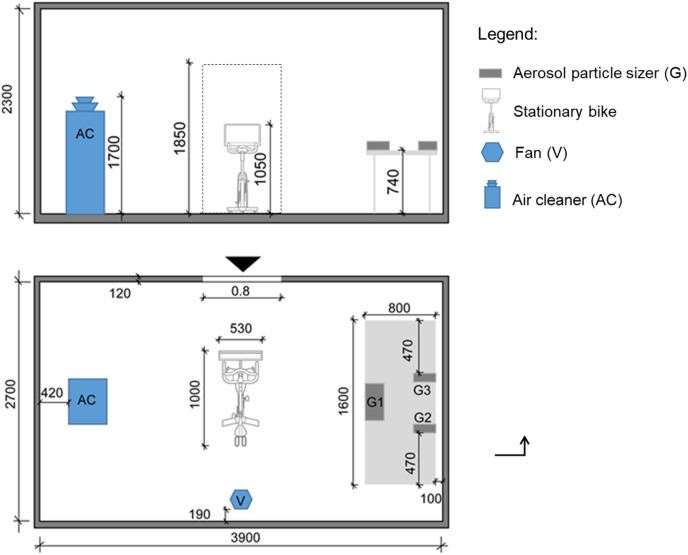
Measurements were conducted to provide a first indication on the amount of endogenously (i.e. saliva) versus exogenously generated aerosol particles during physical exercise. It is known that the amount of endogenously generated saliva aerosols is small compared to the particle concentrations typically found in outdoor and indoor environments [92,93]. So breathing only provides small additions to PM concentrations although it is these small amounts of saliva aerosol particles that are of concern in view of the spread of infectious diseases. Tests were performed in a 3.9 x 2.7 × 2.3 m³ = 24.2 m³ airtight stainless steel test room (Fig. 1 ). The room was equipped with a stationary bicycle in the center, an AC, a fan for generating well-mixed indoor conditions and three Grimm 11D aerosol particle sizers (APS) with a measurement range from about 0.25 to about 30 μm [94]. There was no supply or exhaust of air from the room. Three healthy human volunteers, aged 20–22 years and accustomed to regular physical exercise, participated in this study. Approval for use of human subjects was obtained from the Ethical Review Board of Eindhoven University of Technology with file number ERB2020BE58. The subjects signed an informed consent form prior to participating in the study. Every subject performed two times a session of 30 min exercise on the stationary bicycle in heart rate zones 3 and 4. In the first session, the subject released its breath freely into the room and the APS measured the aerosol particles from the different sources. In the second session, the subject released its breath via a mask into a tube that was connected to the outside environment. The mixing fan was operated during the two sessions. Prior to every session, the mask and tube were disinfected and air cleaning was performed while operating an additional fan inside during at least 30 min to reduce the aerosol particle concentration. Assuming that the amount of endogenously and exogenously generated particles was similar in both sets by the same individual, the difference between both sessions provided an indication of the amount of endogenous aerosol particles. The subjects were given at least 30 min rest between the two exercise sessions and were provided with a bottle of drinking water to be consumed in the rest period. The temperature was 21 °C and the RH ranged between 55 and 65%. Subject 1 had short hair, a short beard, wore a short-sleeved shirt and short trousers and applied a pedaling frequency of about 90 rpm. Subject 2 had medium long hair, no beard, wore a short-sleeved shirt and short trousers and applied a pedaling frequency of about 70 rpm. Subject 3 had short hair, a short beard, wore a short-sleeved shirt and long trousers and applied a pedaling frequency of about 80 rpm.
Fig. 1.
Measurement set-up in stainless steel test room. Dimensions in mm.
3.2. Results
Because only two measurements sessions were performed for only three persons, the results only provide a first indication on the proportion of exogenous versus endogenous particle concentrations. Table 2 lists the resulting aerosol particle concentrations in five size fractions: 10 to 2.5 μm, 2.5 to 1 μm, 1 to 0.5 μm, 0.5 to 0.25 μm and below 0.25 μm, averaged over the three measurement locations (Fig. 1). The following observations are made:
-
•
For every subject, rather similar concentrations of exogenously generated particles are found, except for the largest size range of 10-2.5 μm. This is attributed to the fact that only relatively few particles in this size range were present and therefore only few could be detected, giving rise to large uncertainties. Note that, assuming a density of 1004 kg/m³, 0.5 μg/m³ PM10 corresponds to about 951 particles of 10 μm diameter per m³. For 0.5 μg/m³ PM2.5 this is about 60,872 particles of 2.5 μm diameter per m³. For 0.1 μg/m³ PM1 this is about 190,225 particles of 1 μm diameter per m³. For 0.01 μg/m³ PM0.5 this is about 152,180 particles of 0.5 μm diameter per m³. Finally, for 0.01 μg/m³ PM0.25 this is about 1,217,440 particles of 0.25 μm diameter per m³. Additional variations in the exogenous particle emission among the subjects could be attributed to the different pedalling frequencies, clothing and hair style.
-
•
The results indicate a very high inter-subject variability for the endogenous particle emission. This is in line with previous studies that also showed very large variability [13,14,20,31]. The first subject yielded only very low concentrations of saliva aerosol particles, only significant in the size ranges below 1 μm. The second subject yielded higher concentrations of saliva aerosol particles, only detectable in the size range above 1 μm. For the largest particles (>2.5 μm), the concentration of endogenous particles was in the order of magnitude of that of the exogenous particles. Finally, the third subject emitted saliva aerosol particles in all size ranges, with the same order of magnitude as the exogenous particles, for all size ranges.
-
•
Apart from the largest size range, both the exogenously and endogenously generated particle concentrations showed an increasing trend over time in the 30-min sessions.
Table 2.
Aerosol particle concentrations (μg/m³) in five size fractions measured in test room during physical exercise on stationary bicycle in 5-min intervals during 30 min. Exo = exogeneous aerosol particles; Endo = endogenous (i.e. saliva) aerosol particles.
| Subject | t (min) | PM10-PM2.5 (μg/m³) |
PM2.5-PM1 (μg/m³) |
PM1-PM0.5 (μg/m³) |
PM0.5-PM0.25 (μg/m³) |
PM0.25 (μg/m³) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| exo | endo | Exo | endo | exo | endo | exo | endo | exo | endo | ||
| 1 | 5 | 0.63 | 0.00 | 0.12 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 10 | 0.08 | 0.00 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 15 | 0.00 | 0.00 | 0.11 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | |
| 20 | 0.06 | 0.00 | 0.21 | 0.00 | 0.06 | 0.00 | 0.02 | 0.01 | 0.00 | 0.00 | |
| 25 | 0.49 | 0.00 | 0.31 | 0.00 | 0.10 | 0.01 | 0.03 | 0.01 | 0.01 | 0.00 | |
| 30 | 0.20 | 0.00 | 0.45 | 0.00 | 0.18 | 0.03 | 0.06 | 0.02 | 0.01 | 0.01 | |
| 2 | 5 | 1.04 | 0.00 | 0.22 | 0.03 | 0.03 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 |
| 10 | 0.74 | 0.37 | 0.31 | 0.01 | 0.04 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | |
| 15 | 0.50 | 0.18 | 0.30 | 0.06 | 0.05 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | |
| 20 | 0.59 | 0.00 | 0.36 | 0.06 | 0.06 | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 | |
| 25 | 0.52 | 0.54 | 0.38 | 0.06 | 0.09 | 0.00 | 0.02 | 0.00 | 0.01 | 0.00 | |
| 30 | 0.40 | 0.67 | 0.39 | 0.07 | 0.11 | 0.00 | 0.03 | 0.00 | 0.01 | 0.00 | |
| 3 | 5 | 0.52 | 0.91 | 0.10 | 0.24 | 0.02 | 0.05 | 0.01 | 0.01 | 0.00 | 0.01 |
| 10 | 1.35 | 0.21 | 0.29 | 0.11 | 0.03 | 0.05 | 0.01 | 0.01 | 0.00 | 0.01 | |
| 15 | 1.04 | 1.72 | 0.36 | 0.24 | 0.05 | 0.05 | 0.01 | 0.01 | 0.00 | 0.00 | |
| 20 | 1.33 | 0.89 | 0.47 | 0.24 | 0.07 | 0.08 | 0.02 | 0.01 | 0.00 | 0.01 | |
| 25 | 1.16 | 1.15 | 0.43 | 0.34 | 0.09 | 0.06 | 0.02 | 0.01 | 0.01 | 0.01 | |
| 30 | 0.98 | 0.81 | 0.47 | 0.27 | 0.10 | 0.08 | 0.03 | 0.01 | 0.00 | 0.01 | |
Note that the APS itself did not allow to discriminate between solid and liquid particles and that all concentrations were obtained by assuming the particles had a density similar to that of saliva (1002–1006 kg/m³), as the density of the actual solid particles was unknown. Therefore, it could be assumed that solid (exogenous) particle concentrations as mentioned in Table 2 are underestimated due to their actual higher densities.
4. Measurements in the gym
4.1. Measurement set-up
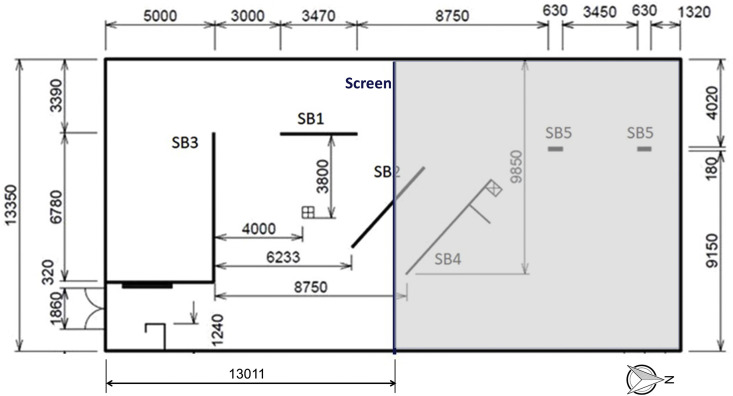
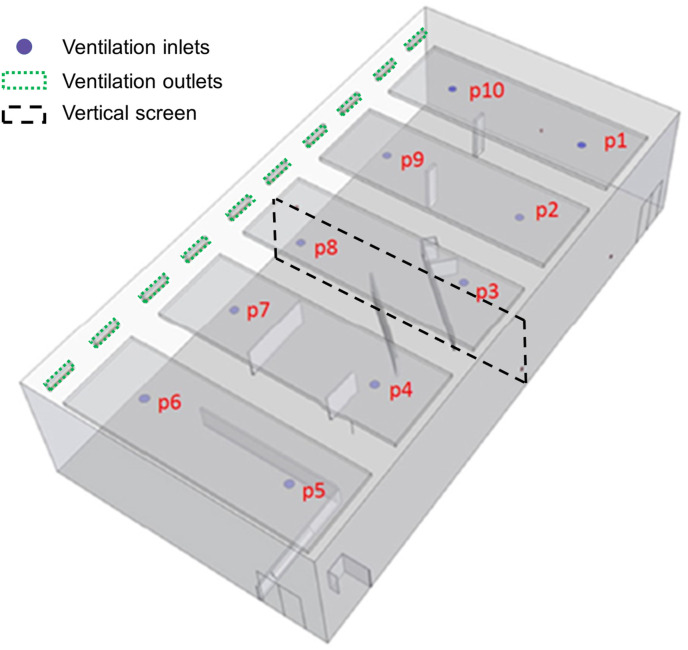
The measurements were performed in the fitness 3 room of the Student Sports Center at Eindhoven University of Technology in the Netherlands. Fig. 2 shows the plan view. The room was split in two parts by a vertical screen and only the south part was used for this study. The floor area of this part is 173.7 m2 and the height was about 5.1 m, yielding a room volume of about 886 m³. The ventilation system in the fitness room was a mechanical mixing ventilation system by which fresh air was supplied into the room by openings with swirl diffusors in the ceiling (indicated with p1-p10 in Fig. 3 ). The openings p4 to p8 were situated in the half of the room used in the present study. The exhaust openings were present on the west side of the room, near the ceiling (Fig. 3). The ventilation flow rate per opening was measured with a FlowFinder device to which a flow straightener was added to remove the swirl and allow an accurate measurement. Every measurement was performed five times and the resulting average measured volume flow rates were 377.6, 365.8, 375.3, 418.6 and 411.3 m³/h for positions p4, p5, p6, p7 and p8, respectively, yielding a total volume flow rate of 1948.6 m³/h. This implies ACH = 2.20 h−1, which is 4.5 times higher than the minimum requirement in the Dutch Building Code for existing buildings, assuming a near-full occupancy with 35 persons (see Table 1). However, note that this ACH is considerably lower than the recommended value of ACH = 6 h−1 in Ref. [77].
Fig. 2.
Plan view of fitness room 3 of Student Sports Center with indication of vertical screen that divides the room in two spaces of about equal volume. Left part is considered in this study. SB refers to vertical shield boards, also visible in Fig. 4, Fig. 5. Dimensions in mm.
Fig. 3.
Position of ventilation inlets and outlets near the ceiling. Openings p4 to p8 apply for the half of the room used in this study.
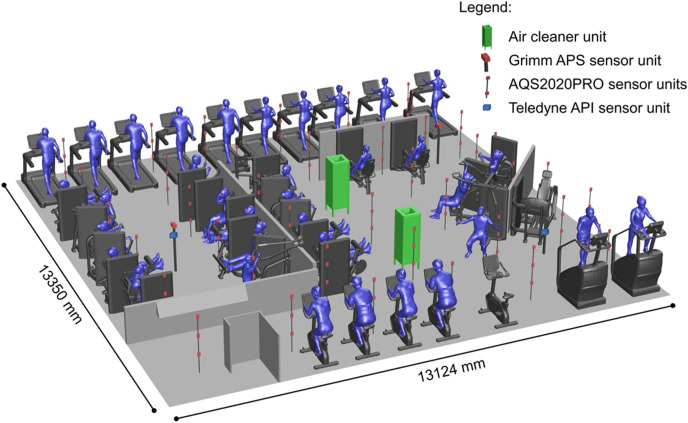
Fig. 4 shows a perspective view of the measurement set-up in the gym with the cardio and weight machines. We focused on a gym with cardio equipment consisting of stationary exercise bicycles and treadmills and with workout equipment consisting of weight-based exercise machines. When using this equipment, the people exercising are not moving throughout the room but instead remain confined at a rather fixed position in the room, which was aimed at limiting resuspension of particles. Two AC units [95], two Grimm APS [94] and 110 AQS2020PRO APS [96] were installed (Fig. 4). The two AC units each consisted of a combination of four cleaning components: a dielectric barrier discharge plasma component, an electrostatic exterior component, an electrostatic with glass fiber component and a carbon filter component. The units ingested the airflow at their bottom opening and exhausted the cleaned air at the top at 1.6 m height at an angle of 45° to the vertical. Every unit had an air flow rate of 617 m³/h, a required power of 104 W and a measured CADR value for artificial saliva aerosols based on a water-glycol mixture of 233, 261, 320, 412 and 645 m³/h for PM10, PM2.5, PM1, PM0.5 and PM0.25, respectively, based on standard 20-min tests [86] in a test room of 24.2 m³. In line with the findings in Ref. [97], the CADR is reduced as the particle size increases because larger particles fall under the influence of gravity and have a relatively higher deposition rate. Test room measurements indicated that the ACs did not produce substantial amounts of harmful byproducts NOx and O3. The Grimm sensors were mounted at measuring heights of 1.367 m and 1.247 m. The 110 APS were mounted on vertical poles at heights of 0.5, 1.0 and 1.5 m (Fig. 4).
Fig. 4.
Measurement set-up in gym.
4.2. Measurement protocol
40 healthy test subjects were recruited in the age range 18–60 years via a broadcast email invitation offering a modest cash incentive. The subjects signed an informed consent form prior to participating in the study. Both the subjects and the 15 members of the research team and support staff were subjected to a stringent protocol. The subject recruitment and safety protocol were approved by the Ethical Review Board of Eindhoven University of Technology with file number ERB2020BE29r, by the Safety Region of Brabant Southeast and the National Institute for Public Health and the Environment (RIVM) in the Netherlands. First, the subjects, research team and support staff were tested against COVID-19, quarantined for two days in single rooms in a hotel in Eindhoven city center and finally subjected to additional safety precautions on the measurement day of 11 July 2020. While all 55 persons tested negative for COVID-19, after stringent application of the safety protocol, 5 test subjects were excluded from the measurement campaign and 35 subjects remained. These 35 subjects performed cardio and/or weight machine exercises in sessions of 30 min (Fig. 4, Fig. 5 ).
Fig. 5.
Photo of (a) measurement set-up and (b) ongoing session with 35 test subjects.
Six of the experimental scenarios or 30-min sessions are listed in Table 3 . The scenarios can be grouped in three sets: Set 1: ventilation on and air cleaning off; Set 2: ventilation off and air cleaning off; Set 3: ventilation off and air cleaning on. Within every set, the parameter is the physical exercise: present or not. In those scenarios when physical exercise was not conducted, all subjects were removed from the room and directed to a large sports hall where they waited for the next exercise session. In between sessions, the subjects were provided with drinking water and sandwiches.
Table 3.
Six experimental sessions/scenarios in chronological order.
| Scenario | Set | Physical exercise and people present (Yes/No) | Ventilation On/Off | Air cleaning On/Off |
|---|---|---|---|---|
| 1 | 1 | Yes | On | Off |
| 2 | 1 | No | On | Off |
| 3 | 2 | Yes | Off | Off |
| 4 | 2 | No | Off | Off |
| 5 | 3 | Yes | Off | On |
| 6 | 3 | No | Off | On |
All exercise sessions were performed in the same way. The subjects were divided into two groups: cardio workout (CW) (16 subjects) and strength training (ST) (19 subjects). Within the strength training group, 10 subjects followed the protocol for muscle endurance (STME) and 9 subjects followed the protocol for muscle mass (STMM). There were three 30-min sessions in which the subjects had to follow a cardio workout (once or twice) and performed a strength training (one or twice). The CW performed their training for 30 min at an intensity between 60 and 75% of heart rate reserve. This was measured by a TICKR heart rate belt connected to the machine. An additional task was that subjects should be able to continue talking to each other not to end up with a too high exercise intensity. Both the STME and the STMM performed three sets of 20 or 10 repetitions on three different machines. Each repetition started with a start signal and lasted 3 min. After the execution of the exercise, the subjects were given rest until the next start signal. On the last machine they performed an extra set to complete the 30 min. In the CW the subjects had a choice of machine. The following CW machines were used in every session: 10 treadmills (LifeFitness, Elevation series), 2 Powermill climbers (LifeFitness, Elevation series) and 4 upright exercise bikes (LifeFitness, Elevation series). STME performed this protocol on the following machines (LifeFitness, Circuit Series): leg extension, seated row, chest press, seated leg curl, ab crunch, lat pulldown, triceps press, squat, shoulder press, biceps curl. STMM performed this protocol on the following machines (LifeFitness, Optima Series): leg extension, seated leg curl, chest press, seated row, hip abduction, hip adduction, biceps curl, shoulder press, machine fly. Fig. 5b shows an ongoing session.
4.3. Measurement results
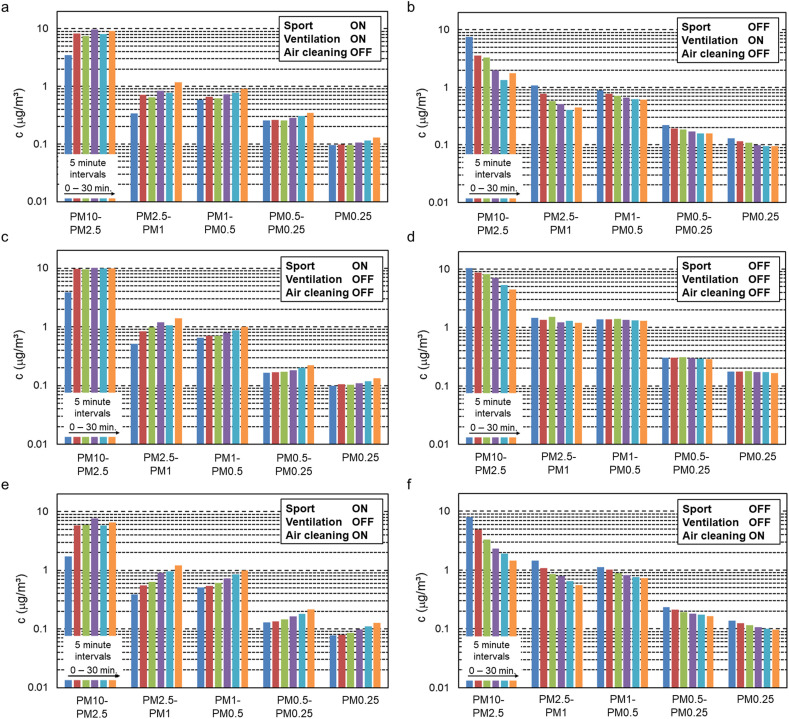
Fig. 6 displays the measured aerosol particle concentrations by the two Grimm APS (values from both sensors averaged) at the end of every 5-min interval in each of the six 30-min measurement sessions. Every row of two figures represents one set, as outlined above. The results are presented as concentrations in the size fractions 10–2.5 μm, 2.5–1 μm, 1–0.5 μm, 0.5–0.25 μm and the fraction below 0.25 μm. To aid in interpreting the semi-logarithmic graphs, Table 4 holds the differences between the concentration at the end and at the beginning of each session. For these six sessions, the ranges of the absolute values of the air temperature averaged over the 110 AQS2020PRO sensors, were: 18.6–19.6, 19.6-19.5, 19.6–20.7, 21.0-20.9, 21.3–22.2 and 22.3–22.3 °C for measurement sessions 1 to 6, respectively. Those of the relative humidity were: 44.0–46.3, 46.3-44.7, 44.8–49.5, 51.1-50.6, 45.5–49.8 and 50.0–50.0%, respectively. The CO2 measurement results are shown in Fig. 7 . The following observations are made:
-
•
As a general comment, evaporation is not considered a factor here because this process is very fast, therefore all measured concentrations are expected to be those of the droplet nuclei.
-
•
Fig. 6a shows that when physical exercise was performed and the ventilation system was engaged (with ACs off), the concentrations of aerosol particles in all size fractions increased almost monotonically. The ventilation system was clearly not effective in avoiding the rise in aerosol concentrations within the 30-min period. After 30 min, the subjects ceased their exercise.
-
•
Fig. 6b demonstrates that after the physical exercise had ceased and after everybody had left the room, the ventilation system was effective in reducing the aerosol concentrations – almost monotonically – in all size fractions during the period of 30 min.
-
•
Fig. 6c depicts the rise in aerosol concentrations when physical exercise was performed and neither ventilation nor ACs were engaged. The increase in the fraction 2.5–10 μm was most pronounced in the first 10 min, while afterwards the concentration in this fraction remained quite constant. In the other size fractions there was an almost monotonic increase.
-
•
Fig. 6d shows that when physical exercise had ceased, people had left the room and ventilation remained turned off, there was a substantial concentration decrease especially in the largest size fraction versus a much more limited decrease in the smaller fractions, both of which are attributed to natural deposition in the calm indoor environment.
-
•
Fig. 6e shows the increase when exercise was performed, ventilation was turned off but the ACs were engaged. It is clear that also air cleaning alone, at the flow rate provided, was not sufficient to limit the rise in aerosol concentrations within the 30-min time period.
-
•
Fig. 6f shows that the ACs were also effective in reducing the aerosol particle concentrations after the exercise had halted, people had left the room but the two ACs remained active.
-
•
Comparing Fig. 6b with 6f and rows 2 and 6 in Table 4, the aerosol particle concentration reductions by ventilation versus ACs were quite similar, with the ACs appearing to have been even more effective than ventilation in several size fractions. This in spite of the fact that the ventilation ACH was 2.20 h−1 while the air cleaning ACH was 1.39 h−1, which is a 58% difference. Note however that ventilation also injects a small portion of PM into the room (i.e. the concentration in the outdoor air after filtering in the mechanical ventilation system – see section 6).
-
•
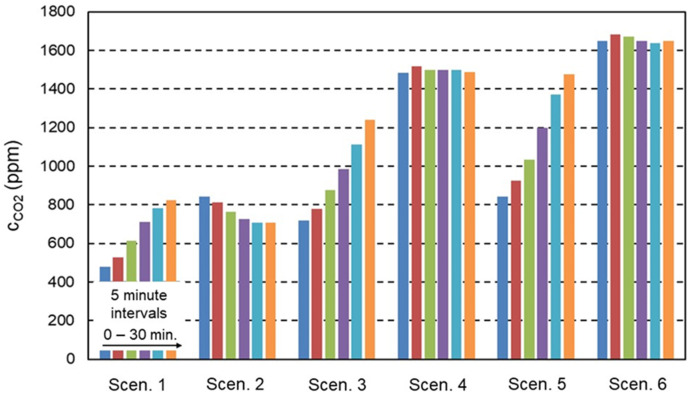
Fig. 7 depicts the 5-min CO2 concentrations throughout each of the six sessions as an average of the values measured by the 110 AQS2020PRO APS at 0.5, 1.0 and 1.5 m height. The first session shows an almost doubling of the concentration due to the physical exercise in spite of the ventilation system being active. The second session shows the concentration decay due to ventilation. In the third and fifth session, there are strong rises in concentration due to the absence of ventilation. Note that the ACs do not affect the CO2 concentration, as shown for sessions 4 and 6 where this concentration remains fairly constant.
Fig. 6.
Aerosol particle concentrations at the end of every 5-min interval in the six 30-min measurement sessions.
Table 4.
Change in aerosol particle concentrations (μg/m³) over 30-min sessions/scenarios.
| 10–2.5 μm | 2.5–1 μm | 1–0.5 μm | 0.5–0.25 μm | <0.25 μm | |
|---|---|---|---|---|---|
| Scenario 1: Sport ON/Vent ON/ACs OFF | 5.54 | 0.83 | 0.33 | 0.09 | 0.03 |
| Scenario 2: Sport OFF/Vent ON/ACs OFF | −5.68 | −0.63 | −0.29 | −0.06 | −0.03 |
| Scenario 3: Sport ON/Vent OFF/ACs OFF | 6.09 | 0.87 | 0.35 | 0.06 | 0.03 |
| Scenario 4: Sport OFF/Vent OFF/ACs OFF | −6.05 | −0.27 | −0.09 | −0.01 | −0.01 |
| Scenario 5: Sport ON/Vent OFF/ACs ON | 4.77 | 0.82 | 0.48 | 0.09 | 0.05 |
| Scenario 6: Sport OFF/Vent OFF/ACs ON | −6.49 | −0.63 | −0.38 | −0.07 | −0.04 |
Fig. 7.
CO2 concentrations at the end of every 5-min interval in the six 30-min measurement sessions.
5. Simplified mathematical model for CO2
A simplified mathematical model can be used to assess the effective ventilation rate. The model assumes a uniform CO2 concentration in the room with volume V, in other words: perfect mixing of the generated CO2 and of the supplied ventilation air with the CO2. It also assumes a steady release of CO2 by the subjects. With these assumptions, the mass balance for CO2 can be written as:
| (3) |
With c the CO2 concentration (ppm), G the CO2 emission rate (ppm.m³/h), QV the ventilation rate (m³/h) and c0 the CO2 concentration (ppm) in the supplied ventilation air. For scenario 1 (physical exercise and ventilation), the solution of this first-order ordinary differential equation is:
| (4) |
For scenario 2 (only ventilation), the solution is:
| (5) |
For scenarios 3 and 5 (physical exercise without ventilation), the solutions are:
| (6) |
| (7) |
Least squares fitting of Eq. (5) to the data of CO2 in Fig. 7 yields QV = 995 m³/h. The CO2 production by the subjects in scenarios 1, 3 and 5 is obtained by fitting Eqs. (4), (6), (7) to the data in Fig. 7, yielding G1 = 1.96 kg/h; G3 = 2.00 kg/h; G5 = 2.56 kg/h. The CO2 production for a person in rest is about 0.033 kg/h [98,99], implying a production rate of 1.16 kg/h for 35 persons. The values for G1, G3 and G5 suggest that the combined production of the 35 exercising subjects is about 70%–220% higher than for 35 persons in rest. A more accurate approach for estimation of CO2 generation rates by building occupants at different levels of physical activity was provided by Persily and de Jonge [100]. Assuming body mass of 65 kg and 70 kg for female and male subjects respectively, a “met” of 4 (moderate effort) yields 1.75 kg/h CO2 production for 35 persons, while a “met” of 8 (vigorous effort) yields 3.50 kg/h. The values of G1, G3 and G5 are indeed situated in between these two estimates. The value of QV implies that the effective ventilation rate is only 51% of the actual supply ventilation flow rate of 1948.6 m³/h. This is attributed to fact that the simplified mathematical model assumes a uniform CO2 source, a uniform concentration distribution and a uniform effect of the ventilation system. In reality, the CO2 generation occurred at test person height and the measurements were conducted close to the CO2 source, while both the ventilation supply openings and the exhaust openings were positioned near the ceiling. QV = 995 m³/h could therefore be considered as the “effective” or local ventilation flow rate for the zone in the lower part of the room, in which the test persons were present. This “effective” ventilation rate is more than twice that required by the Dutch Building Code (i.e. 433 m³/h), however, evaluation of ventilation systems with regard to building codes generally occurs based on the total supply ventilation flow rate. The variability in CO2 emission could be attributed to subjects having performed more or less intensive exercise from one session to another, subject fatigue, subjects switching from cardio to weight machines and some inter-subject variability in CO2 emission under similar physical exercise.
6. Simplified mathematical model for aerosol particle concentrations
6.1. Aerosol particle production, deposition, ventilation and air cleaning
We consider the five size fractions also used in Fig. 6: 10–2.5 μm, 2.5–1 μm, 1–0.5 μm, 0.5–0.25 μm and below 0.25 μm. Let G denote the aerosol particle production rate by physical exercise, which is the sum of the production rates by respiration, resuspension, machine component friction, clothing friction and the like. QV denotes the ventilation flow rate, QAC the total AC flow rate, ηAC the AC efficiency, KN the natural deposition loss rate under calm indoor airflow conditions (ventilation and ACs off), KV the deposition loss rate in the ventilation flow regime (ventilation on, ACs off), KAC the deposition loss rate in the AC flow regime (ventilation off, ACs on), V the room volume and c0 the concentration in the incoming ventilation air. Assuming well-mixed conditions and a steady emission of aerosol particles in all five size fractions by the 35 subjects, the mass balances for the six scenarios in Fig. 6 for a given size fraction are:
| (8) |
| (9) |
| (10) |
| (11) |
| (12) |
| (13) |
The corresponding solutions are:
| (14) |
| (15) |
| (16) |
| (17) |
| (18) |
| (19) |
Considering the left-hand sides of these equations as known by the data in Fig. 6, these six equations have seven unknowns: G1, G3, G5, KV, KN, ηACQAC and KAC. Note that ηACQAC cannot be considered known from the CADR tests in the small test room of 24.2 m³, as reported in subsection 4.1, because Noh and Oh [97] showed that for the same AC device, the experimental CADR decreased as the size of the test chamber increased. To solve the system of equations, the sum ηACQAC + KACV is taken as a single variable. Least squares fitting of Eqs. (15), (17), (19) to the data in Fig. 6 yields the values of QV + KVV, KNV and ηACQAC + KACV for every size fraction. Using these values into Eqs. (14), (16), (18) yields the values of G1, G3 and G5. KVV is calculated based on QV = 995 m³/h (see section 5). Table 5 holds the results. It also shows the deposition loss rates KN and KV based on V = 886 m³. The last row shows the measured c0 concentration values. The following observations are made:
-
•
The low measured c0 values are representative of a large degree of air filtering in the mechanical ventilation system.
-
•
Aerosol particle removal due to deposition in scenarios 2 (KV) and 4 (KN) rapidly decreases with decreasing size fraction. This is expected given the lower mass and associated smaller settling velocities of the smaller aerosol particles. The KN values found here are very similar to those by Mølgaard et al. [101]. Several studies indicated deposition rates of 0.02–0.55 h−1 for PM2.5 where the area and roughness of the deposition surfaces plays an important role [[102], [103], [104]]. Shaughnessy and Sextro [105] reported data from Thatcher et al. [106] and Xu et al. [107] where KN for the size fraction 10–2.5 μm ranged from 1 to 10 h−1, for 2.5–1 μm from 0.3 to 1 h−1, for 1–0.5 μm from 0.1 to 0.3 h−1, for 0.5–0.25 μm from 0.05 to 0.1 h−1 and finally for the size fraction below 0.25 μm from 0.035 to 0.05 h−1. Apart from the two smallest size fractions, the KN values in Table 5 are situated in these ranges. The larger values in the two smallest size fractions could be attributed to the large number of surfaces in the gym room.
-
•
Aerosol particle removal due to deposition in scenario 4 (KN; ventilation off, ACs off) is much less pronounced than in scenario 2 (KV; ventilation on, ACs off), which is attributed to the indoor airflow pattern in the latter scenario generated by the ventilation system. Indeed, Friedlander and Johnstone [108] demonstrated the strong increase in deposition from turbulent gas streams with increase in the flow Reynolds number, attributed to the larger eddies and larger inertial forces favoring deposition. For the size fraction 10–2.5 μm, the deposition rate is 2.2 times larger in scenario 2 than in 4, while for the size fraction 0.5–0.25 μm, it is 4.8 times larger.
-
•
Earlier, it was shown by comparing Fig. 6b with 6f and rows 2 and 6 in Table 4, that the aerosol particle concentration reductions by ventilation versus ACs were quite similar, with the ACs appearing a bit more effective than ventilation in several size fractions. This in spite the fact that the ventilation ACH was 2.20 h−1 while the AC ACH was 1.39 h−1, which is a 58% difference. This is confirmed by the fact that the sum QV + KVV is larger than the sum ηACQAC + KN,ACV. This is attributed to the lower effectiveness of the ventilation system which is attributed to two reasons: (1) the presence of the ventilation inlet and outlets near the ceiling and (2) the fact that the incoming ventilation air also contained – albeit fairly low – concentrations of aerosol particles. It is also attributed to the fact that the AC units were positioned in the region where the aerosol particles were generated, which can explain their relatively larger effectiveness.
-
•
The aerosol particle production rates are very different among scenarios 1, 3 and 5, with the differences also differing per size fraction. This could be attributed to inter-subject variability in aerosol particle emission under similar physical exercise regimes but also by subjects having performed more or less intensive exercise from one session to another, subject fatigue and subjects switching from cardio to weight machines. It could also be attributed, at least partly, due to the use of only two measurement points for aerosol particle concentrations.
-
•
In terms of the magnitude of aerosol particle production, You et al. [109] measured the short-term emission rates of particles by persons with different clothing and activity intensities in a sealed chamber. The activities did not involve gym machines but included walking, upper body and arm movements. Based on their data for a cotton suit and for slight to strong activity intensity and assuming a particle density of 1000 kg/m³, the following ranges can be derived for 35 persons: 5635–39238 μg/h for the size fraction 10–2.5 μm, 2004–2227 μg/h for 2.5–1 μm, ≈2738 μg/h for 1–0.5 μm and finally 760–844 μg/h for the size fraction below 0.5 μm. Taking into account that the 35 persons in the gym performed moderate rather than strong activity and that the numbers by You et al. [109] do not include particles generated by the friction between components of the cardio and weight machines, the values of G1, G3 and G5 in Table 5 can be considered in line with the findings by You et al. [109].
Table 5.
Flow rates associated with aerosol particle production, deposition, ventilation and air cleaning, for five size fractions. Deposition loss rates and the concentrations in the incoming ventilation air are also given.
| 10–2.5 μm | 2.5–1 μm | 1–0.5 μm | 0.5–0.25 μm | <0.25 μm | |
|---|---|---|---|---|---|
| QV + KV V (m³/h) | 4721 | 2656 | 1664 | 1629 | 1599 |
| KV V (m³/h) | 1704 | 394 | 156 | 127 | 126 |
| ηAC QAC + KAC V (m³/h) | 4306 | 2277 | 989 | 814 | 812 |
| G1 (μg/h) | 47361 | 3398 | 1362 | 488 | 143 |
| G3 (μg/h) | 23440 | 1951 | 796 | 134 | 73 |
| G5 (μg/h) | 32415 | 3871 | 2289 | 330 | 192 |
| QV (m³/h) | 995 | 995 | 995 | 995 | 995 |
| KV V (m³/h) | 3726 | 1661 | 669 | 634 | 604 |
| KV (h−1) | 4.21 | 1.87 | 0.76 | 0.72 | 0.68 |
| KN (h−1) | 1.92 | 0.45 | 0.18 | 0.14 | 0.14 |
| c0 (μg/m³) | 0.006 | 0.053 | 0.520 | 0.151 | 0.088 |
6.2. Combined effect of ventilation and air cleaning
A scenario that was not considered in the experimental campaign was the combination of ventilation and air cleaning. Therefore, the simplified model is applied to investigate this additional scenario with ventilation and air cleaning combined and using the aerosol particle production rate from the first scenario (G7 = G1):
| (20) |
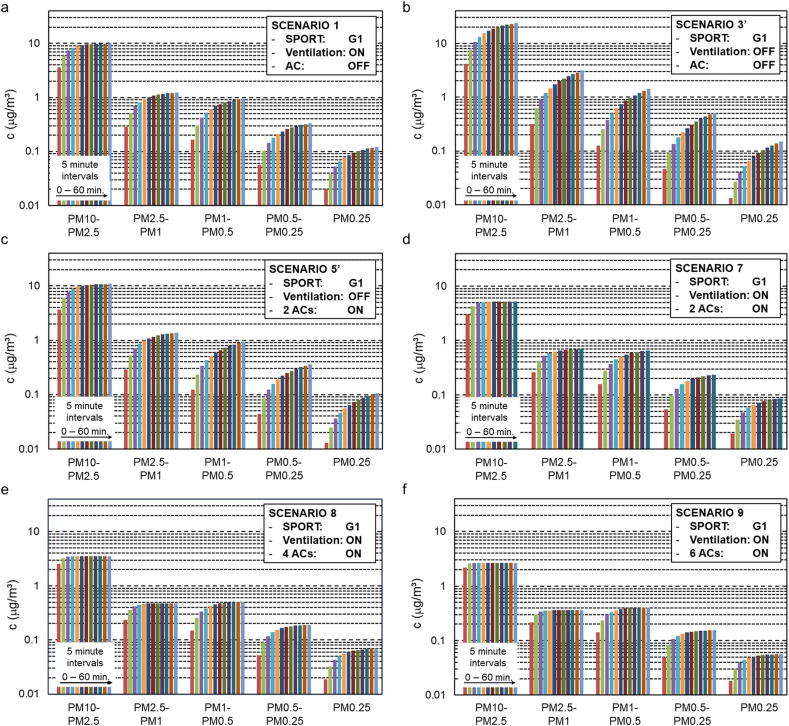
It is assumed that the combination of ventilation (with supply and exhaust near the ceiling) and air cleaning (near ground level) also combines the deposition rates by both technologies. The simplified model was also used for other scenarios, as shown in Fig. 8 that presents the results of six scenarios for a 60-min period, all with the same aerosol generation rate G1. Fig. 8a is the calculated result for scenario 1. Fig. 8b and c present scenarios 3′ and 5′ that are identical to scenarios 3 and 5 but now with G3’ = G5’ = G1. Fig. 8d presents scenario 7 (ventilation and air cleaning combined). Fig. 8e and f present two additional scenarios in which the number of ACs is raised from 2 to 4 and 6 units, respectively. All figures show that the concentrations tend towards an asymptote over time, as dictated by the exponential functions in the above-mentioned equations. Table 6 lists the asymptotic values as reached in every scenario at t = ∞, and Fig. 9 shows these asymptotic values in percentages of the values of scenario 3’ (only deposition). The following observations are made:
-
•
Fig. 8 shows that the duration during which concentrations keep rising significantly is largest for scenario 3’ (Fig. 8b; no ventilation, no ACs, only natural deposition in calm indoor airflow conditions). Evidently this is also the scenario in which the highest concentrations are obtained. Table 6 indicates that these concentrations go up to 27.80, 8.61, 8.72, 3.83 and 1.13 μg/m³ for the size fractions 10–2.5 μm, 2.5–1 μm, 1–0.5 μm, 0.5–0.25 μm and below 0.25 μm, respectively. The concentrations in the largest size fraction keep rising significantly for about 4.83 h, while those in the smallest size fraction keep rising beyond 15 h (not shown in figure).
-
•
Fig. 8 also shows that the duration at which near-equilibrium conditions are obtained is shortest for scenario 9 in which most intensive air cleaning is engaged. Evidently this is also the scenario in which the lowest concentrations are obtained. Table 6 indicates that these concentrations remain limited to 2.69, 0.36, 0.41, 0.16 and 0.06 μg/m³ for the size fractions 10–2.5 μm, 2.5–1 μm, 1–0.5 μm, 0.5–0.25 μm and below 0.25 μm, respectively. The concentrations in the largest size fraction keep rising significantly for only 0.63 h, while those in the smallest size fraction keep rising significantly for about 0.83 h.
-
•
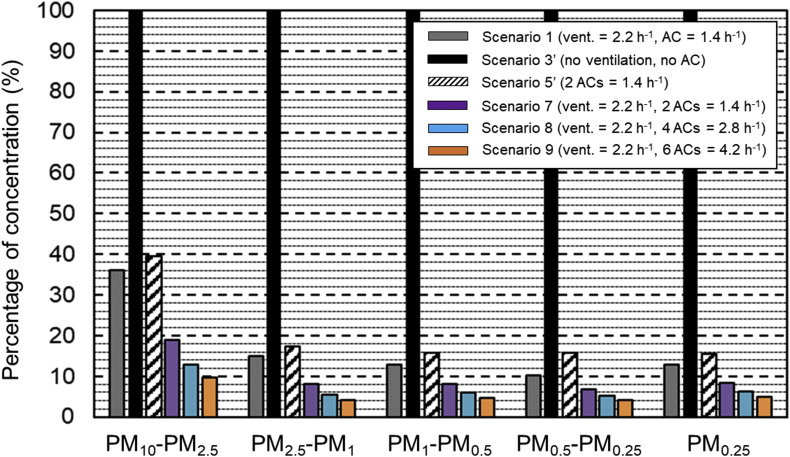
For all other scenarios, the duration towards near-equilibrium and the near-final concentrations are situated between those of scenarios 3′ and 9. In scenario 1, ventilation alone reduces the final concentrations of scenario 3’ (no ventilation, no ACs) by factors 2.8, 6.6, 7.7, 9.8 and 8.1 for the size fractions 10–2.5 μm, 2.5–1 μm, 1–0.5 μm, 0.5–0.25 μm and below 0.25 μm, respectively. In scenario 5′, air cleaning alone (with 2 ACs) yields slightly lower reduction factors: 2.5, 5.8, 6.3, 6.4 and 6.3, in relation to scenario 3’. Combining ventilation and air cleaning in scenario 7 reduces the final concentrations of scenario 3’ (no ventilation, no ACs) by factors of 5.3, 12.3, 12.3, 14.7 and 11.3 for the five consecutive size fractions. Adding more AC units increases these factors further. 4 AC units (scenario 8) correspond to ACH = 2.78 h−1, which is still below the recommendation by Ref. [87] that the air cleaner ACH should be between 3 and 6. In this case, the concentrations of scenario 3′ are reduced with factors of 7.8, 17.9, 16.8, 19.2 and 16.1, for each of the consecutive size fractions. Finally, 6 AC units (scenario 9) yields ACH = 4.17 h−1 which is above the lower limit of the recommendation in Ref. [87]. The resulting reduction factors are 10.3, 23.9, 21.3, 23.9 and 18.8. Note that [87] advises ACH = 6 h−1 which would lead to even lower asymptotic concentration values. Fig. 9 shows the percentages of the concentrations in the five size fractions, compared to scenario 3’ (only deposition). Ventilation alone or air cleaning alone reduces the concentrations in the largest size fraction with more than 60% and in the other size fractions with more than 80%. Combining ventilation and air cleaning (2 AC units) yields reductions of more than 80% and 90% in the largest and other size fractions, respectively. Ventilation combined with 6 AC units gives reductions of 90 an 95% for the largest and smaller size fractions, respectively. Note that 6 AC units yields ACH = 4.17 h-1 which is just above the lower limit of the recommendation in [87].
Fig. 8.
Calculated aerosol particle concentrations at the end of every 5-min interval in 60-min sessions for six scenarios: 1, 3′, 5′, 7, 8 and 9.
Table 6.
Calculated asymptotic values of aerosol particle concentrations (μg/m³) for six scenarios: 1, 3′, 5’, 7, 8 and 9.
| 10–2.5 μm | 2.5–1 μm | 1–0.5 μm | 0.5–0.25 μm | <0.25 μm | |
|---|---|---|---|---|---|
| Scenario 1 | 10.03 | 1.30 | 1.13 | 0.39 | 0.14 |
| Scenario 3′ | 27.80 | 8.61 | 8.72 | 3.83 | 1.13 |
| Scenario 5′ | 11.00 | 1.49 | 1.38 | 0.60 | 0.18 |
| Scenario 7 | 5.25 | 0.70 | 0.71 | 0.26 | 0.10 |
| Scenario 8 | 3.55 | 0.48 | 0.52 | 0.20 | 0.07 |
| Scenario 9 | 2.69 | 0.36 | 0.41 | 0.16 | 0.06 |
Fig. 9.
Calculated asymptotic aerosol particle concentrations for six scenarios: 1, 3′, 5′, 7, 8 and 9, expressed as percentages compared to scenario 3’.
7. Discussion
A major gap in the scientific literature is information about the aerosol particle emission by persons performing physical exercise. A previous study indicated that deep exhalation could yield a 4 to 6-fold increase in aerosol particle emission and rapid inhalation a further 2- to 3-fold increase in emission [13], yielding a maximum 18-fold increase. Another study revealed that the number of expired aerosol particles showed a 2 to 18-fold increase after exhalations to residual long volume compared with exhalations without airway closure [14]. Therefore, concerns about high aerosol particle concentrations in indoor sports centers, fitness centers and gyms are justified. Regardless, more research is needed to assess aerosol particle emissions by persons performing physical exercise at different levels of intensity and heart rate.
To the best of our knowledge, there was no study in the scientific literature that specifically focused on respiratory aerosol production in fitness centers. There was even relatively little published research about air quality in fitness centers in general, as opposed to residential buildings and other types of public spaces such as schools and offices [[62], [63], [64]]. The few studies that were available in the scientific literature had measured PM concentrations without a focus on saliva aerosol particles and without an attempt to discriminate between endogenous and exogenous particles. To provide some first preliminary insights in the proportions between endogenous versus exogenous particles, in the present study, a small test was performed. While some clear trends could be discerned, especially for the size fractions below 2.5 μm, especially the large inter-subject variability was noted. This however was in line with earlier studies that also indicated very large inter-subject variability [13,14,20,31]. Much more research is needed on endogenous versus exogenous aerosol particle emission as the lack of knowledge about their relative proportions will continue to complicate advice concerning saliva aerosol emission and reduction in view of limiting infection risk. Due to the inability to discriminate between endogenous and exogenous particles in the gym study in the present paper, the focus in the paper is on the combination of these two types.
The scenarios considered in the present study are neither a worst-case nor a most beneficial scenario. On the one hand, in actual gym settings, many persons performing physical exercise will apply long breaks between exercises, either to rest or to talk to other people. In that regard, the present study considered fairly vigorous and continuous exercise, in an attempt to obtain a steady release of both endogenous and exogenous aerosol particles. On the other hand, in intensive cycling sessions such as spinning, the intensity and the heart rates are higher than those in the present study that included a combination of cardio and weight machines. The measured CO2 emission rates confirm that the present scenarios are in between vigorous and moderate exercise.
A wide variety of gyms and ventilation systems exist. Nevertheless, most gyms are characterized by a large height and most gym have mixing ventilation systems with supply and exhaust openings near the ceiling. In order to generalize the results on ventilation effectiveness from the present study, a number of additional gyms will need to be investigated, after which potentially a common denominator could be defined and some general advices could be established in terms of required ventilation and/or air cleaning flow rates.
Similarly, a wide variety of ACs exist. As mentioned in this paper, ACs need to have both a sufficiently high efficiency and a proper capacity (flow rate) in order to be effective. ACs have a sometimes less good reputation because of the presence of some very deficient and even harmful types on the market. However, also high-quality ACs have been developed and are commercially available. High-quality certification and international standardization are imperative.
Aerosol particle deposition is an important factor. The present study suggests that the engagement of ventilation or air cleaning, by inducing an overall more turbulent airflow pattern in the room, substantially enhances the deposition, in line with a previous study [108].
In view of the COVID-19 pandemic and potential future pandemics, ventilation of indoor environments, gyms included, will need to be reconsidered. At the same time, energy efficiency should be upheld to the largest degree possible, in view of limiting climate change. Suggestions by politicians, scientists and opinion makers that ventilation has to be massively incremented to avoid potential aerosol SARS-CoV-2 infection, would unavoidably give rise to large investment costs to upgrade ventilation systems and large energy consumption and losses (if heat recovery is not massively deployed) and the associated costs. Therefore, we suggest to not engage in expensive upgrades of existing mechanical ventilation systems, on condition that they already enable – pandemics aside – a healthy and comfortable indoor environment, using ventilation rates that are above the minima required by building codes. Instead, these expensive and already available systems can be supplemented with lower-cost mobile professional AC units. The present study has shown that the effectiveness of high-quality AC units can be similar to that of a mechanical ventilation system (with aerosol filtering) with a 60% higher flow rate. AC units do not require the air to be heated, cooled or (de)humidified, as it is indoor air being handled and exhausted back into the room. Ventilation air coming from outside will often need extra energy for heating, cooling and (de)humidifying, even if heat recovery is applied. However, it should be stressed that ventilation at the minimum flow rates as required by building codes remains imperative, because many ACs do not remove gasses, such as CO2.
A gym is rather complex indoor environment in the sense that it has a large height, the sources are present near the floor while generally the ventilation supply and exhaust openings are present near the ceiling. Therefore, future work should consider measuring aerosol particle concentrations not only at two positions at similar height as in the present study, but also measuring concentration gradients along the height of the room. Given the large height, vertical concentration gradients could be present, irrespective of the type of mixing ventilation system or ACs that are present. Future work will include CFD simulations to provide more inside into the vertical gradients and the related effectiveness of ventilation and AC units.
The results from this study in terms of AC units supplementing ventilation can also be applied in other indoor environments. For rooms with lower height such as class rooms and offices, for example, the complexity could be smaller. For indoor environments with larger height however, such as football stadiums, basketball halls and concert halls, the complexity could be much larger. The authors are currently conducting a similar project for the Johan Cruijff Football stadium, home of the Amsterdam Ajax Football team and of the Dutch National Football Team [110].
The introduction mentioned three main questions for which, to date, no clear quantitative answer had been provided. The present study attempted to provide some information in terms of ventilation and air cleaning effectiveness in a realistic environment. It does not provide information about infection risk. Future work should develop strategies to allow various types of indoor activities to be safely maintained during pandemics. A first practical engineering strategy in this regard for indoor sports centers (including gyms) was presented by Blocken et al. [111]. This work should be supplemented with an infection risk analysis as well.
8. Summary and conclusions
SARS-CoV-2 can spread by close contact through large droplet spray and indirect contact via contaminated objects. There is mounting evidence that it can also be transmitted by inhalation of infected saliva aerosol particles. These particles are generated when breathing, talking, laughing, coughing or sneezing. It can be assumed that aerosol particle concentrations indoors should be kept low in order to minimize the potential risk of airborne virus transmission. This paper presents measurements of aerosol particle concentrations in a gym, where saliva aerosol production is pronounced. 35 test persons performed physical exercise and aerosol particle concentrations, CO2 concentrations, air temperature and relative humidity were obtained in the room of 886 m³. A separate test was used to provide some information on the amount of human endogenous versus exogenous aerosol particles. This test showed large inter-subject variability, with one person emitting much more exogenous than endogenous particles, while another emitted similar amounts of both types. Aerosol particle removal by mechanical ventilation and mobile air cleaning (AC) units was measured. The gym test showed that ventilation with ACH = 2.2 h−1, i.e. 4.5 times the minimum of the Dutch Building Code, was insufficient to stop the significant aerosol concentration rise over a 30-min measurement session. Air cleaning alone with ACH = 1.39 h−1 had a similar effect as ventilation alone. This difference can be attributed to the lower effectiveness of the ventilation system due to two reasons: (1) the presence of the ventilation inlet and outlets near the ceiling and (2) the fact that the incoming ventilation air also contained – albeit fairly low – concentrations of aerosol particles. It was also attributed to the fact that the AC units were positioned in the region where the aerosol particles were generated, which can explain the relatively larger effectiveness of ACs. Simplified mathematical models were engaged to provide further insight into ventilation, air cleaning and deposition. It was shown that combining ventilation and intensive air cleaning with up to six AC units with a total ACH of 4.17 h−1 – as recommended in the scientific literature – can reduce the concentrations by factors of 2.3 up to 3.7 depending on aerosol size, compared to ventilation alone, and by factors of 10.3 up to 23.9 depending on aerosol size, compared to a situation without ventilation and AC units. It is suggested that if aerosol particle concentrations need to be reduced in view of the COVID-19 pandemic, this should not necessarily be done by an expensive upgrade of the existing mechanical ventilation system. Instead, it could also be achieved by supplementing this system with mobile professional high-quality AC units. When the AC units are installed near ground-level in gyms with large height (e.g. 5 m), they can have a higher effectiveness than the ventilation system and together with the existing ventilation system, they can reduce the aerosol particle concentrations below a pre-defined threshold. This lowers investment and operational costs because AC units do not require the air to be heated, cooled or (de)humidified, as it is indoor air being handled and exhausted back into the room. Ventilation air coming from outside will often need extra energy for heating, cooling and (de)humidifying, even if heat recovery is applied. However, it should be stressed that ventilation at (at least) the minimum flow rates required by building codes remains imperative, because many ACs do not remove gasses, such as CO2.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge the many organizations that directly or indirectly enabled this research project. The Topteam Sports of the Netherlands is acknowledged for having funded part of this project. Martin Olde Weghuis (Board Member at Sportinnovator), Merit Cloquet (Sports Innovation Offer at Sportinnovator), Paul van der Kolk (Programme Manager at Sportinnovator) and Iris Nijland (Project Leader and Secretary at Sportinnnovator) are acknowledged for having brought the many partners in this project together, in record time, around the central goal of investigating options to allow gyms to stay open safely in times of COVID-19. Martin van der Sluis, Tom Koenderman, Hendrik Waanders and Kenny van der Sluis (PlasmaMade) are acknowledged for having provided the air cleaning units, the 110 AQS2020PRO sensors, their overall support during the set-up of the measurement campaign and many valuable discussions on the design, set-up and results of the campaign. Jan Hazelhof is acknowledged for his assistance with the regular medical disinfection of the measurement equipment on the measurement day. Wim Koch, Peter Geurts and their colleagues of the Student Sports Center of Eindhoven University of Technology are acknowledged for having allowed and facilitated the measurements in their building. Medical doctors Dr. Jelle Oosterhof and Dr. Wouter Bisseling are acknowledged for having attended the test day in case medical assistance would have been required. John Jorritsma, the Mayor of the city of Eindhoven and Stijn Steenbakkers, Alderman for Innovation, Sports, Economy, Brainport and Education are acknowledged for their support in the safe design of the protocol and final approval of the project. Prof.dr. Jaap van Dissel, Director of the Centre for Infectious Disease Control (Cib) of the Netherlands National Institute for Public Health and the Environment (RIVM) is acknowledged for his advice concerning the required COVID-19 testing procedure as part of the safety protocol. All test persons, most of them students of Eindhoven University of Technology, are acknowledged for their enthusiastic collaboration – one student even flew over on his own initiative from Milan, Italy, to be part of the test. Peter Jansen and Scarlett de Moor of YOMOYO are acknowledged for the professional recording of the test event (https://www.youtube.com/watch?v=PdBgr4U0BKg). The authors are also grateful to the anonymous peer reviewers whose valuable comments have improved this manuscript.
References
- 1.European Centre for Disease Prevention and Control COVID-19 Situation update 2nd week 2021. 2020. https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases Retrieved on 27 January 2021.
- 2.Chan J., Yuan S., Kok K., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 382: 1199-1207. [DOI] [PMC free article] [PubMed]
- 4.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burke R.M., Midgley C.M., Dratch A., et al. Active monitoring of persons exposed to patients with confirmed COVID-19 — United States, January–February 2020. MMWR Morbid. Mortal. Weekly Rep. 2020;69(9):245–246. doi: 10.15585/mmwr.mm6909e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . World Health Organization; Geneva: 2020. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) 16-24 February 2020.https://www.who.int/docs/default- source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf Available from: [Google Scholar]
- 7.World Health Organization . World Health Organization; Geneva: 2020. Modes of Transmission of Virus Causing COVID-19: Implications for IPC Precaution Recommendations. Scientific Brief.www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations Available from: [Google Scholar]
- 8.Liu Y., Ning Z., Chen Yu, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557–560. doi: 10.1038/s41586-020-2271-3. https://www.nature.com/articles/s41586-020-2271-3 [DOI] [PubMed] [Google Scholar]
- 9.van Doremalen N., Morris D.H., Holbrook M.G., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. New England J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morawska L., Cao J. Airborne transmission of SARS-CoV-2: the world should face the reality. Environ. Int. 2020;139:105730. doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadei M., Hopke P.K., Jonidi A., Shahsavani A. A letter about the airborne transmission of SARS-CoV-2 based on the current evidence. Aerosol Air Qual. Res. 2020;20:911–914. [Google Scholar]
- 12.Asadi S., Bouvier N., Wexler A.S., Ristenpart W.D. The coronavirus pandemic and aerosols: does COVID-19 transmit via expiratory particles? Aerosol. Sci. Technol. 2020;54(6):635–638. doi: 10.1080/02786826.2020.1749229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson G.R., Morawska L. The mechanism of breath aerosol formation. J. Aerosol Med. Pulm. Drug Deliv. 2009;22(3):229–237. doi: 10.1089/jamp.2008.0720. [DOI] [PubMed] [Google Scholar]
- 14.Almstrand A.C., Bake B., Ljungstrom E.L., et al. Effect of airway opening on production of exhaled particles. J. Appl. Physiol. 2010;108:584–588. doi: 10.1152/japplphysiol.00873.2009. [DOI] [PubMed] [Google Scholar]
- 15.Asadi S., Wexler A.S., Cappa C.D., et al. Effect of voicing and articulation manner on aerosol particle emission during human speech. PloS One. 2019;15(1) doi: 10.1371/journal.pone.0227699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitttal R., Ni R., Seo J.H. The flow physics of COVID-19. J. Fluid Mech. 2020;894 Article nr. F2. [Google Scholar]
- 17.Han Z.Y., Weng W.G., Huang Q.Y. Characterizations of particle size distribution of the droplets exhaled by sneeze. J. R. Soc. Interface. 2013;10(88):20130560. doi: 10.1098/rsif.2013.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asadi S., Wexler A.S., Cappa C.D., et al. Aerosol emission and superemission during human speech increase with voice loudness. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-38808-z. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fairchild C.I., Stampfer J.F. Paricle concentration in exhaled breath - summary Report. Am. Ind. Hyg. Assoc. J. 1987;48:948–949. doi: 10.1080/15298668791385868. [DOI] [PubMed] [Google Scholar]
- 20.Papineni R.S., Rosenthal F.S. The size distribution of droplets in the exhaled breath of healthy human subjects. J. Aerosol Med. 1997;10:105–116. doi: 10.1089/jam.1997.10.105. [DOI] [PubMed] [Google Scholar]
- 21.Edwards D.A., Man J.C., Brand P., et al. Inhaling to mitigate exhaled bioaerosols. Proc. Natl. Acad. Sci. Unit. States Am. 2004;101(50):17383–17388. doi: 10.1073/pnas.0408159101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ai Z.T., Melikov A.K. Airborne spread of expiratory droplet nuclei between the occupants of indoor environments: a review. Indoor Air. 2018;28:500–524. doi: 10.1111/ina.12465. [DOI] [PubMed] [Google Scholar]
- 23.Wells W.F. On air-borne infection. Study II. Droplets and droplet nuclei. Am. J. Hyg. 1934;20:611–618. [Google Scholar]
- 24.Wan M.P., Chao C.Y.H. Transport characteristics of expiratory droplets and droplet nuclei in indoor environments with different ventilation airflow patterns. J. Biomech. Eng. 2007;129:341–353. doi: 10.1115/1.2720911. [DOI] [PubMed] [Google Scholar]
- 25.Xie X., Li Y., Chwang A.T.Y., et al. How far droplets can move in indoor environments - revisiting the Wells evaporation-falling curve. Indoor Air. 2007;17:211–225. doi: 10.1111/j.1600-0668.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- 26.Hinds W.C. John Wiley & Sons; New York: 1982. Aerosol Technology. [Google Scholar]
- 27.CDC . Centers for Disease Control and Prevention; Atlanta, GA, USA: 1996. Guidelines for Isolation Precautions in Hospitals, Hospital Infection Control Advisory Committee.http://wonder.cdc.gov/wonder/prevguid/p0000419/p0000419.asp Available at: retrieved on 16 November 2020. [Google Scholar]
- 28.Mangili A., Gendreau M.A. Transmission of infectious diseases during commercial air travel. Lancet. 2005;365:989–996. doi: 10.1016/S0140-6736(05)71089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christian M.D., Loutfy M., McDonald L.C., et al. Possible SARS coronavirus transmission during cardiopulmonary resuscitation. Emerg. Infect. Dis. 2004;10:287–293. doi: 10.3201/eid1002.030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicas M., Nazaroff W., Hubbard A. Toward understanding the risk of secondary airborne infection: emission of respirable pathogens. J. Occup. Environ. Hyg. 2005;2(3):143–154. doi: 10.1080/15459620590918466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morawska L., Johnson G.R., Ristovski Z.D., et al. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J. Aerosol Sci. 2009;40(3):256–269. [Google Scholar]
- 32.Holmgren H., Bake B., Olin A.C., Ljungström E. Relation between humidity and size of exhaled particles. J. Aerosol Med. Pulm. Drug Deliv. 2011;24(5):253–260. doi: 10.1089/jamp.2011.0880. [DOI] [PubMed] [Google Scholar]
- 33.Bourouiba L., Dehandschoewercker E., Bush J.W.M. Violent expiratory events: on coughing and sneezing. J. Fluid Mech. 2014;745:537–563. [Google Scholar]
- 34.Bourouiba L. Clinical Review & Education; 2020. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. JAMA Insights. [DOI] [PubMed] [Google Scholar]
- 35.Nielsen P.V. Technical University of Denmark; Copenhagen: 1976. Flow in Air Conditioned Rooms. English Translation and Revision of PhD Thesis. [Google Scholar]
- 36.Kato S., Murakami S., Mochida A., Akabayashi S., Tominaga Y. Velocity-pressure field of cross-ventilation with open windows analyzed by wind tunnel and numerical simulation. J. Wind Eng. Ind. Aerod. 1992;44:2575–2586. [Google Scholar]
- 37.Chen Q. Prediction of room air motion by Reynolds-stress models. Build. Environ. 1996;31:233–244. [Google Scholar]
- 38.Chen Q., Chao N.T. Comparing turbulence models for buoyant plume and displacement ventilation simulation. Indoor Built Environ. 1997;6:140–149. [Google Scholar]
- 39.Nielsen P.V. Computational fluid dynamics and room air movement. Indoor Air. 2004;14(Suppl. 7):134–143. doi: 10.1111/j.1600-0668.2004.00282.x. [DOI] [PubMed] [Google Scholar]
- 40.van Hooff T., Blocken B. Coupled urban wind flow and indoor natural ventilation modelling on a high-resolution grid: a case study for the Amsterdam ArenA stadium. Environ. Model. Software. 2010;25(1):51–65. [Google Scholar]
- 41.Karava P., Stathopoulos T., Athienitis A.K. Airflow assessment in cross-ventilated buildings with operable facade elements. Build. Environ. 2011;46:266–279. [Google Scholar]
- 42.Ramponi R., Blocken B. CFD simulation of cross-ventilation for a generic isolated building: impact of computational parameters. Build. Environ. 2012;53:34–48. [Google Scholar]
- 43.Blocken B. Computational Fluid Dynamics for Urban Physics: importance, scales, possibilities, limitations and ten tips and tricks towards accurate and reliable simulations. Build. Environ. 2015;91:219–245. [Google Scholar]
- 44.Craven B.A., Settles G.S. A computational and experimental investigation of the human thermal plume. J. Fluids Eng.-Trans. ASME. 2006;128(6):1251–1258. [Google Scholar]
- 45.Li Y., Leung G.M., Tang J.W., et al. Role of ventilation in airborne transmission of infectious agents in the built environment - a multidisciplinary systematic review. Indoor Air. 2007;17(1):2–18. doi: 10.1111/j.1600-0668.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 46.CDC . Centers for Disease Control and Prevention; 2020. SARS-CoV-2 & Potential Airborne Transmission.https://www.cdc.gov/coronavirus/2019-ncov/more/scientific-brief-sars-cov-2.html Retrieved on 21 October 2020. [PubMed] [Google Scholar]
- 47.European Centre for Disease Prevention and Control . 2020. Heating, Ventilation and Air-Conditioning Systems in the Context of COVID-19. 22 June 2020.www.ecdc.europa.eu/en/publications-data/heating-ventilation-air-conditioning-systems-covid-19 Retrieved on 22 October 2020. [Google Scholar]
- 48.ASHRAE 2020. ASHRAE Issues Statements on Relationship between COVID-19 and HVAC in Buildings. 2020. https://www.ashrae.org/about/news/2020/ashrae-issues-statements-on-relationship-between-covid-19-and-hvac-in-buildings Retrieved on 22 October 2020. [Google Scholar]
- 49.REHVA 2020 How to operate HVAC and other building service systems to prevent the spread of the coronavirus (SARS-CoV-2) disease (COVID-19) in workplaces. REHVA COVID-19 guidance document. 2020 www.rehva.eu/fileadmin/user_upload/REHVA_COVID-19_guidance_document_V3_03082020.pdf Retrieved on 22 October 2020. [Google Scholar]
- 50.Carlisle A.J., Sharp N.C.C. Exercise and outdoor ambient air pollution. Br. J. Sports Med. 2001;35:214–222. doi: 10.1136/bjsm.35.4.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andrade A., Dominski F.H., Pereira M.L., et al. Infection risk in gyms during physical exercise. Environ. Sci. Pollut. Control Ser. 2018;25:19675–19686. doi: 10.1007/s11356-018-1822-8. [DOI] [PubMed] [Google Scholar]
- 52.Chang S., Pierson E., Koh P.W., et al. Mobility network models of COVID-19 explain inequities and inform reopening. Nature. 2020;589:82–107. doi: 10.1038/s41586-020-2923-3. [DOI] [PubMed] [Google Scholar]
- 53.Jang S., Han S.H., Rhee J.Y. vol. 268. Emerging Infectious Diseases; Research Letter: 2020. Cluster of Coronavirus Disease Associated with Fitness Dance Classes, South Korea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Serrure B., Verrycken R. Dutch: Hoe de Epidemie Ontspoorde in Antwerpen) Newspaper De Tijd; 2020. How the epidemic got derailed in Antwerp.https://www.tijd.be/dossiers/coronavirus/hoe-de-epidemie-ontspoorde-in-antwerpen/10242577.html 1 August 2020. Retrieved on 17 August 2020. [Google Scholar]
- 55.Ye F., Xu S., Rong Z., et al. Delivery of infection from asymptomatic carriers of COVID-19 in a familial cluster. Int. J. Infect. Dis. 2020;94:133–138. doi: 10.1016/j.ijid.2020.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rothe C., Schunk M., Sothmann P., et al. Transmission of 2019-ncov infection from an asymptomatic contact in Germany. N. Engl. J. Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nyenhuis S.M., Greiwe J., Zeiger J.S., et al. Exercise and fitness in the age of social distancing during the COVID-19 Pandemic. J. Allergy Clin. Immunol.: In Pract. 2020 doi: 10.1016/j.jaip.2020.04.039. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen P., Mao L., Nassis G.P., et al. Coronavirus disease (COVID-19): the need to maintain regular physical activity while taking precautions. J. Sport Health Sci. 2020;9(2):103–104. doi: 10.1016/j.jshs.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rubin G.J., Wessely S. The psychological effects of quarantining a city. Br. Med. J. 2020;368:m313. doi: 10.1136/bmj.m313. [DOI] [PubMed] [Google Scholar]
- 60.Hover P., van Eldert P. Mulier Instituut; Utrecht: 2019. Fitnessbranche in Nederland 2018: kerncijfers vraag- en aanbodzijde (in Dutch) factsheet 2019/1. [Google Scholar]
- 61.Platform ondernemende sportaanbieders. 2020. https://www.ondernemendesportaanbieders.nl/overzicht-statistieken Retrieved on 17 August 2020.
- 62.Alves C.A., Calvo A.I., Castro A., et al. Indoor air quality in two university sports facilities. Aerosol Air Qual. Res. 2013;13:1723–1730. [Google Scholar]
- 63.Andrade A., Dominski F.H. Indoor air quality of environments used for physical exercise and sports practice: systematic review. J. Environ. Manag. 2018:577–586. doi: 10.1016/j.jenvman.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 64.Salonen H., Salthammer T., Morawska L. Human exposure to air contaminants in sports environments. Indoor Air. 2020;30:1109–1129. doi: 10.1111/ina.12718. [DOI] [PubMed] [Google Scholar]
- 65.Ramos C.A., Reis J.F., Almeida T., Alves F., et al. Estimating the inhaled dose of pollutants during indoor physical activity. Sci. Total Environ. 2015;527–528:111–118. doi: 10.1016/j.scitotenv.2015.04.120. [DOI] [PubMed] [Google Scholar]
- 66.Canha N., Freitas M.C., Almeida S.M., et al. Indoor school environment: easy and low cost to assess inorganic pollutants. J. Radioanal. Nucl. Chem. 2010;286(2):495–500. [Google Scholar]
- 67.Canha N., Martinho M., Almeida-Silva M., et al. Indoor air quality in primary schools. Int. J. Environ. Pollut. 2012;(1/2/3/4):396–410. [Google Scholar]
- 68.Braniš M., Safranek J. Characterization of coarse particulate matter in school gym. Environ. Res. 2011;111(4):485–491. doi: 10.1016/j.envres.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 69.Ramos C.A., Wolterbeek H.T., Almeida S.M. Exposure to indoor air pollutants during physical activity in fitness centers. Build. Environ. 2014;82:349–360. [Google Scholar]
- 70.Slezakova K., Peixoto C., Oliveira M., et al. Indoor particulate pollution in fitness centres with emphasis on ultrafine particles. Environ. Pollut. 2018;233:180–193. doi: 10.1016/j.envpol.2017.10.050. [DOI] [PubMed] [Google Scholar]
- 71.Etheridge D., Sandberg M. Wiley; 1996. Building Ventilation: Theory and Measurements. -13: 978-0471960874. [Google Scholar]
- 72.AIVC . International Energy Agency; 2020. Air Infiltration and Ventilation Centre.https://www.aivc.org/resources/faqs/what-ventilation Retrieved on 11 May 2020. [Google Scholar]
- 73.Awbi H. Spon Press; 2003. Ventilation of Buildings. [Google Scholar]
- 74.Chen Q. Ventilation performance prediction for buildings: a method overview and recent applications. Build. Environ. 2009;44(4):848–858. [Google Scholar]
- 75.Blocken B. 50 years of computational wind engineering: past, present and future. J. Wind Eng. Ind. Aerod. 2014;129:69–102. [Google Scholar]
- 76.Ministerie van Binnenlandse Zaken en Koninkrijksrelaties Building code. Bouwbesluit online (in Dutch) 2012. https://rijksoverheid.bouwbesluit.com/Inhoud/docs/wet/bb2012/hfd3 Retrieved on 11 May 2020.
- 77.Ariëns J.P.E., Joosten T.A., Schriemer W., et al. Handboek Sportaccommodaties. ISA Sport. NOC*NSF, Arko Sports Media BV; 2008. Guidebook sports accommodations (in Dutch) [Google Scholar]
- 78.Stichting Onbeperkt Sportief Guidelines for accessibility of indoor sports accommodations (in Dutch) 2014. http://www.onbeperktsportief.nl/content/toegankelijke-indoor-sportaccommodaties Retrieved on 10 May 2020. 84.
- 79.ASHRAE Pandemic COVID-19 and airborne transmission. Environ. Health Committ.(EHC) Emerging Issue Brief. 2020 https://www.ashrae.org/file%20library/technical%20resources/covid-19/eiband-airbornetransmission.pdf Retrieved on 13 May 2020. [Google Scholar]
- 80.ASHRAE Ventilation for acceptable indoor air quality. ANSI/ASHRAE Standard. 2019;62:1–2019. [Google Scholar]
- 81.ASHRAE . American Society of Heating, Refrigerating and Air-Conditioning Engineers; 2015. ASHRAE Position Document on Filtration and Air Cleaning.http://www.ashrae.org/file%20library/about/position%20documents/filtration-and-air-cleaning-pd.pdfhttp://www.ashrae.org/file library/about/position documents/filtration-and-air-cleaning-pd.pdf"www.ashrae.org/file%20library/about/position%20documents/filtration-and-air-cleaning-pd.pdf [Google Scholar]
- 82.Fisk W.J., Faulkner D., Polonen J., et al. Performance and costs of particle air filtration technologies. Indoor Air. 2002;12:223–234. doi: 10.1034/j.1600-0668.2002.01136.x. [DOI] [PubMed] [Google Scholar]
- 83.Cheng Y.S., Lu J.C., Chen T.R. Efficiency of a portable indoor air cleaner in removing pollens and fungal spores. Aerosol Sci. Technol. 1998;29(2):92–101. [Google Scholar]
- 84.Green R., Simpson A., Cutovic A., et al. The effect of air filtration on airborne dog allergen. Allergy. 1999;54(5):484–488. doi: 10.1034/j.1398-9995.1999.00029.x. [DOI] [PubMed] [Google Scholar]
- 85.Emmerich S.J., Nabinger S.J. Measurement and simulation of the IAQ impact of particle air cleaners in a single-zone building. Int. J. Heating, Ventilating, Air-Condition. Refrig. Res. 2001;7(3):223–244. [Google Scholar]
- 86.AHAM . Approved American National Standard – Association of Home Appliance Manufacturers. ANSI/AHAM AC-1; 2015. Method for measuring performance of portable household electric room air cleaners. [Google Scholar]
- 87.Asbach C., Held A., Kiendler-Scharr A., et al. Position paper of the Gesellschaft für Aerosolforschung on understanding the role of aerosol particles in SARS-CoV-2 infection. Assoc. Aerosol Res. 2020;17 December 2020. [Google Scholar]
- 88.Kähler C., Fuchs T., Hain R. 2020. Können mobile Raumluftreiniger eine indirekte SARS-CoV-2 Infektionsgefahr durch Aerosole wirk-sam reduzieren? 5 August 2020.https://www.unibw.de/lrt7/raumluftreiniger.pdf [Google Scholar]
- 89.Waring M.S., Siegel J.A., Corsi R.L. Ultrafine particle removal and generation by portable air cleaners. Atmos. Environ. 2008;42:5003–5014. [Google Scholar]
- 90.Zhang Y., Mo J., Li Y., et al. Can commonly-used fan-driven air cleaning technologies improve indoor air quality? A literature review. Atmos. Environ. 2011;45:4329–4343. doi: 10.1016/j.atmosenv.2011.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim H.J., Han B., Kim Y.J., et al. Submicrometer particle removal indoors by a novel electrostatic precipitator with high clean air delivery rate, low ozone emissions, and carbon fiber ionizer. Indoor Air. 2013;23:369–378. doi: 10.1111/ina.12037. [DOI] [PubMed] [Google Scholar]
- 92.Gunschera J., Markewitz D., Bansen B., et al. Portable photocatalytic air cleaners: efficiencies and by-product generation. Environ. Sci. Pollut. Control Ser. 2016;23:7482–7493. doi: 10.1007/s11356-015-5992-3. [DOI] [PubMed] [Google Scholar]
- 93.Olofson K.F.G., Andersson P.U., Hallquist M.L., et al. Urban aerosol evolution and particle formation during winter time temperature inversions. Atmos. Environ. 2009;43:340–346. [Google Scholar]
- 94.Grimm Aerosol Technik . Grimm Aerosol Technik Ainring GmbH & Co.KG; Ainring, Germany: 2020. Portable Aerosol Spectrometer, Model 11-D. [Google Scholar]
- 95.PlasmaMade. 2020. https://www.plasmamade.nl Retrieved on 21 October 2020. [Google Scholar]
- 96.CloudGarden Product sheet Cloud Garden climate sensor CG.TRHCV.D. Cloud-Garden-Klimaatsensor-Nederlands-incl-technische-specs. 2020 pdf. Retrieved on 17 August 2020. [Google Scholar]
- 97.Noh K.C., Oh M.D. Variation of clean air delivery rate and effective air cleaning ratio of room air cleaning devices. Build. Environ. 2015;84:44–49. [Google Scholar]
- 98.ASHRAE . American Society of Heating, Refrigerating and Air-Conditioning Engineerings, Inc.; Atlanta: 2007. HVAC Application 2007. [Google Scholar]
- 99.Spengler J.D., Vallarino J., McNeely E., Estephan H. National Air Transportation Center of Excellence for Research in the Intermodal Transport Environment; Boston, MA: 2012. In-flight/onboard Monitoring: ACER's Component for ASHEAE 1262, Part 2. (RITE) [Google Scholar]
- 100.Persily A., de Jonge J. Carbon dioxide generation rates for building occupants. Indoor Air. 2017;27:868–879. doi: 10.1111/ina.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mølgaard B., Koivisto A.J., Hussein T., et al. A new clean air delivery rate test applied to five portable indoor air cleaners. Aerosol. Sci. Technol. 2014;48(4):409–417. [Google Scholar]
- 102.Long C.M., Suh H.H., Catalano P.J., et al. Using time-and size-resolved particulate data to quantify indoor penetration and deposition behavior. Environ. Sci. Technol. 2001;35(10):2089–2099. doi: 10.1021/es001477d. [DOI] [PubMed] [Google Scholar]
- 103.Chao C.Y.H., Wan M.P., Cheng E.C.K. Penetration coefficient and deposition rate as a function of particle size in non-smoking naturally ventilated residences. Atmos. Environ. 2003;37(30):4233–4241. [Google Scholar]
- 104.Wallace L. Indoor particles: a review. J. Air Waste Manag. Assoc. 1996;46(2):98–126. doi: 10.1080/10473289.1996.10467451. [DOI] [PubMed] [Google Scholar]
- 105.Shaughnessy R.J., Sextro R.G. What is an effective portable air cleaning device: a review. J. Occup. Environ. Hyg. 2006;3(4):169–181. doi: 10.1080/15459620600580129. [DOI] [PubMed] [Google Scholar]
- 106.Thatcher T.L., Lai A.C., Moreno-Jackson R., et al. Effects of room furnishings and air speed on particle deposition rates indoors. Atmos. Environ. 2002;36:1811–1819. [Google Scholar]
- 107.Xu M., Nematollahi M., Sextro R.G., et al. Deposition of tobacco smoke particles in a low ventilation room. Aerosol Sci. Technol. 1994;20:194–206. [Google Scholar]
- 108.Friedlander S.K., Johnstone H.F. Deposition of suspended particles from turbulent gas streams. Ind. Eng. Chem. 1957;49(7):1151–1156. [Google Scholar]
- 109.You R., Cui W., Chen C., Zhao B. Measuring the short-term emission rates of particles in the “Personal Cloud” with different clothes and activity intensities in a sealed chamber. Aerosol Air Qual. Res. 2013;13:911–921. [Google Scholar]
- 110.Eindhoven University of Technology You tube channel. https://www.youtube.com/watch?v=qS2MnmRLAAI
- 111.Blocken B., van Druenen T., van Hooff T., et al. Can indoor sports centers be allowed to re-open during the COVID-19 pandemic based on a certificate of equivalence? Build. Environ. 2020:107022. doi: 10.1016/j.buildenv.2020.107022. Article nr. [DOI] [PMC free article] [PubMed] [Google Scholar]