Abstract
Rationale
Delivery of continuous positive airway pressure (CPAP) is the standard treatment for obstructive sleep apnoea in children and adults. Treatment adherence is a major challenge, as many patients find the CPAP mask uncomfortable. The study aim was to demonstrate the feasibility of delivered CPAP through customised nasal masks by assessing mask leak and comfort of customised masks compared to commercially available CPAP masks.
Methods
Six healthy adult volunteers participated in a crossover study including commercial masks in three different sizes (petite, small/medium and large) from the same supplier and a customised mask fabricated for each subject using three-dimensional facial scanning and modern additive manufacturing processes. Mask leak and comfort were assessed with varying CPAP levels and mask tightness. Leak was measured in real time using an inline low-resistance Pitot tube flow sensor, and each mask was ranked for comfort by the subjects.
Results
Mask leak rates varied directly with CPAP level and inversely with mask tightness. When ranked for comfort, three subjects favoured the customised mask, while three favoured a commercial mask. The petite mask yielded the highest mask leaks and was ranked least comfortable by all subjects. Relative mask leaks and comfort rankings for the other commercial and customised masks varied between individuals. Mask leak was comparable when comparing the customised masks with the highest ranked commercial masks.
Conclusion
Customised masks successfully delivered target CPAP settings in all six subjects, demonstrating the feasibility of this approach.
Short abstract
This research details a methodology for fabrication of customised noninvasive ventilation masks based on 3D facial scans to use as an alternative to commercially available masks for the delivery of continuous positive airway pressure https://bit.ly/35WspAg
Introduction
Obstructive sleep apnoea (OSA) is a common sleep-related breathing disorder where individuals experience partial or complete interruptions in breathing due to obstruction of the upper airways. Moderate-to-severe OSA, defined in adults as an apnoea–hypopnoea index (AHI) ≥15 events·h−1 [1], has been linked to significant negative health consequences, ranging from daytime sleepiness in the short term to acute cardiovascular events and increased risk of a range of medical comorbidities in the long term [2]. Increased motor vehicle accidents have been linked to individuals experiencing excessive daytime sleepiness [1, 2]. It is estimated that 13% of males and 6% of females have moderate-to-severe OSA [3].
Currently, the standard treatment for OSA in adults is the delivery of continuous positive airway pressure (CPAP). CPAP acts as a pneumatic stent to prevent collapse of the upper airway during sleep, thus restoring breathing and sleep, and reducing OSA-related health consequences [1, 2, 4, 5]. Administration of CPAP requires a tight-fitting interface, normally a mask, which should fit the individual patient's facial structure [6]. Mask air leakage and discomfort have been linked to poor CPAP adherence [7, 8]. Mask leak can cause deleterious effects, such as eye irritation and high noise levels [2, 9]. Conversely, when mask leak is reduced through excessive tightening of the headgear, discomfort such as pain, rashes, skin breakdown and pressure sores may arise where the mask contacts the face [2, 9]. Improving the CPAP mask fit, such that mask leakage is minimised at a comfortable tightness of fit, may help improve access to CPAP therapy for a more diverse range of patients, and could potentially increase adherence to CPAP therapy.
To improve CPAP or noninvasive ventilation mask fit, several groups have recently investigated the manufacture of customised masks or mask cushions using a combination of three-dimensional (3D) facial scanning and modern additive manufacturing processes [10–13]. Such approaches may be particularly useful for individuals with OSA and craniofacial anomalies [13]. In one comparison between customised masks and a conventional commercial mask, subjects with severe OSA showed improved AHI compared to their baseline when using the customised mask versus subjects who used a commercial mask over a 14-day period [10].
Here, we report on the development of individualised, custom-built nasal CPAP masks using 3D facial scanning and 3D printing technologies. Mask leakage and comfort were evaluated in healthy, adult volunteers for customised masks and commercial masks of three different sizes in a crossover study.
Methods
This feasibility study of custom masks for noninvasive delivery of CPAP was conducted at the Respiratory Fluid Mechanics Lab at the University of Alberta (Edmonton, AB, Canada) between February 2019 and June 2019. The study was approved by the University of Alberta research ethics board (study ID: MS1_Pro00085707) and subjects provided informed written consent.
Subjects
Each subject enrolled in the study was aged ≥18 years and was a nonsmoker. Six subjects were enrolled in this feasibility study. Nonprobability, purposive sampling was used to select a useful group of subjects, based on their motivation, willingness to participate and capability to effectively communicate their experiences and opinions [14]. There were four male subjects and two female subjects, aged 24–62 years. No subjects had previously used nocturnal CPAP support.
Experimental apparatus
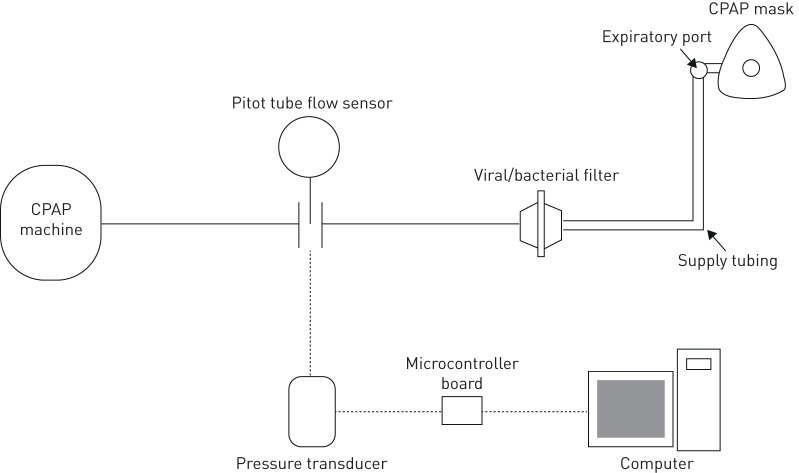
In this study, four different CPAP masks were tested. This included three different sizes of a commercial CPAP mask (Wisp Nasal CPAP Mask; Philips Respironics, Murrysville, PA, USA; petite, small/medium, and large) along with a customised CPAP mask designed and individually manufactured for each subject (Live Cell Imaging Laboratory, University of Calgary). The Wisp masks were selected because they fit a wide range of facial geometries, and three sizes were tested to provide comparative data ranging from a poor fit to a good fit in each subject. During the study, each subject wore standard headgear (Fabric Frame; Philips Respironics). The mask was connected to a supply tube which was connected to a CPAP machine (S8 Elite; ResMed, San Diego, CA, USA) with a viral/bacterial filter (VP7100; KEGO Corporation, London, ON, Canada) and an inline Pitot tube flow sensor (RespEQ, Baltimore, MD, USA) (figure 1). The Pitot tube flow sensor was used to obtain mask air leak values in real time by measuring the airflow, in standard litres per minute (SLPM; with standard conditions defined as 21.1°C and 101.3 kPa), between the CPAP machine and the mask. The Pitot tube flow sensor was selected over a conventional pneumotach due to its low flow resistance, limiting the effects it had on the delivery of CPAP [15]. A pressure transducer (Seleon, Heilbronn, Germany) was used to convert the differential pressure across the sensor into an electric signal which was transmitted using a microcontroller board (UNO Rev3; Arduino, Somerville, MA, USA) and recorded with LabView software (National Instruments, Austin, TX, USA). The flow sensor–pressure transducer combination was calibrated ahead of the study against a series of known airflow rates, ranging from −120 to 120 SLPM. Time-averaged airflow rate was obtained by analysing the airflow data with the programme MATLAB (MathWorks, Natick, MA, USA). Before each subject visit, the intentional air leak through the expiratory port of the supply tubing was measured at a CPAP setting of 4, 8 and 12 cmH2O using the Pitot tube flow sensor with the end of the tube sealed off. Mask air leak was then later calculated after completion of each subject's test by subtracting the intentional air leak from the time-averaged airflow.
FIGURE 1.
Schematic of experimental apparatus. CPAP: continuous positive airway pressure.
Fabrication of custom masks
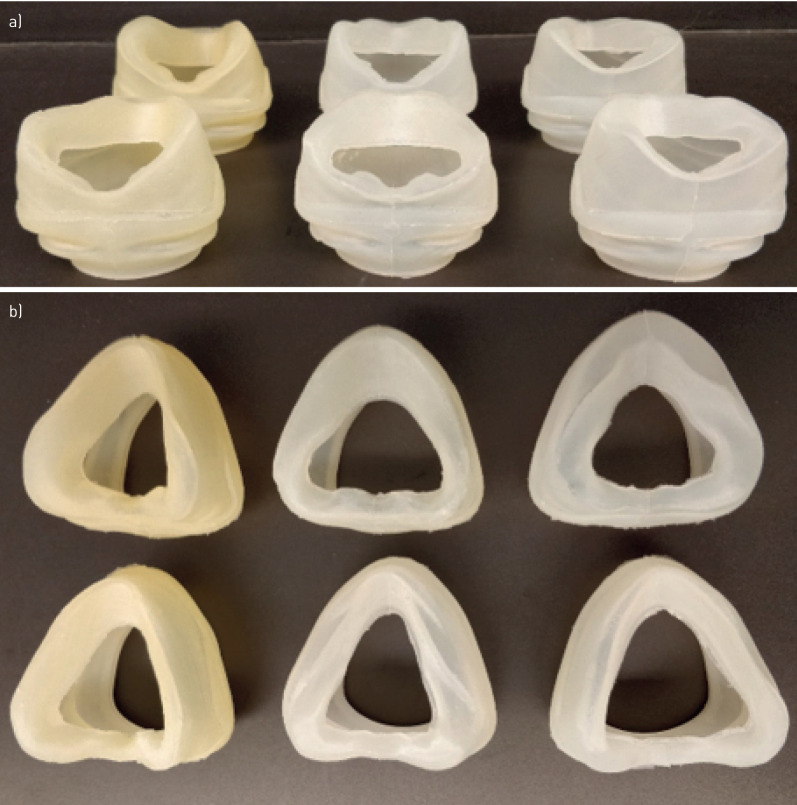
The CPAP mask design consists of two components: a standard coupler and a customised cushion fit to the individual face (figure 2). The coupler was designed to connect the cushion to the commercial headgear and supply tubing (Philips Respironics). The coupler is a hard-plastic component and was fabricated using a stereolithography apparatus 3D printer (Peopoly Moai, Los Angeles, CA, USA) using ultraviolet curable resin. The same coupler design was used for all custom masks that were tested. All six customised cushions are shown in figure 3.
FIGURE 2.
Final customised mask. a) Portion of mask labelled “coupler” (front) interfaces with the headgear and hosing that conducts positive pressure from the continuous positive airway pressure (CPAP) machine. The “cushion” (back) contacts the subject's face. b) Demonstration of the coupler attached to the headgear and hosing of the Wisp CPAP system. c) Customised mask worn in situ.
FIGURE 3.
a) Top-down view of all six customised cushions; b) front view of all six customised cushions.
Subjects' facial features were captured using a 3D facial scanning system (3dMDFace System, Atlanta, GA, USA). The facial scans were digitally edited to limit the regions examined (frontal, nasal, infraorbital, buccal and mental regions) using Meshmixer (Autodesk, San Rafael, CA, USA). The edited scans were imported into computer-aided design software to design custom cushions to match the facial features (Fusion 360; Autodesk).
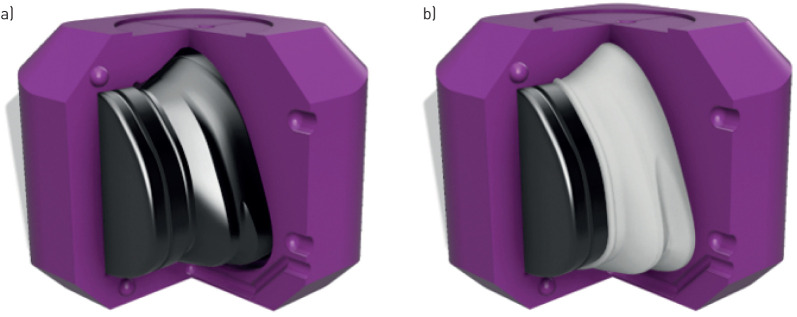
The cushion–skin interface was customised by point-wise matching the surface of the cushion, which contacts the skin, to the scanned mesh of corresponding facial features. To ensure skin safety and comfort, the cushions were fabricated from a skin safe silicone (Dragon Skin 30; Smooth-on, Macungie, PA, USA). As this material could not be printed directly using available 3D printers, it was cast into 3D-printed moulds. Using a constructive solid geometry approach, the desired cushion geometry was subtracted from the mould box interior, leaving a shell in the shape of the custom cushion between an inner and outer mould component (figure 4a). These components were manufactured using a fused filament fabrication 3D printer (Ultimaker 3; Ultimaker, Utrecht, the Netherlands) using 2.85-mm polylactic acid filament (6B14 FCAPLA2BK; Filaments.ca, Mississauga, ON, Canada). The 3D printer used has a manufacturer-reported accuracy of±12.5 µm XY (i.e. in the plane parallel to the print bed) and ±2.5 µm Z (normal to the print bed). The mould box was assembled and clamped, two-component silicone was prepared as per the manufacturer's instructions and injected into the mould box using a 60 mL Luer-lock syringe. After curing (16 h, 20˚C), the cushion was removed from the mould and visually inspected for tears and other sources of unintended leak prior to bench testing. In two instances, cushions failed visual inspection due to the presence of air pockets, which was attributed to inadequate material being injected. These cushions were rejected and remade for use in the present study.
FIGURE 4.
a) Assembled mould box components with one component removed for visualisation. Outer mould shown in magenta, inner mould shown in black. b) Demonstration of custom cushion created after injection of cavity with silicone (white).
Study design
This feasibility study aimed to investigate the use of customised 3D-printed CPAP masks through comparison of mask air leak rates and subject-reported comfort rankings against commercially available CPAP masks.
For each study subject, the headgear was adjusted to obtain three levels of mask tightness, each within a 50 g tolerance, that were measured by the force applied to the face: 100 g for a loose fit, 350 g for an appropriate fit and 600 g for a tight fit. These forces were measured using a force gauge (model DFG35-10; OMEGA, Norwalk, CT, USA). The mask tightness chosen to represent loose, appropriate and tight fits were determined in preliminary consultation and lab bench testing with a nurse practitioner highly experienced in fitting CPAP masks to patients. The appropriate fit was determined using the “two-finger rule” [16]. Three CPAP pressures were selected for testing: 4, 8 and 12 cmH2O. This coincides with the median CPAP of 8–10 cmH2O used in other OSA studies [17–22].
Each subject underwent testing of the four masks at each pressure level and mask tightness for a total of 36 individual tests. Each pressure was applied for 1 min with the flow sensor recording the airflow over the same period. Subjects were instructed to breathe normally while in a seated position. At the completion of the mask testing, each subject was asked to rank the four CPAP masks in order of comfort level.
Statistical analysis
Mask air leak was compared between all four CPAP masks using a three-factor repeated-measures ANOVA procedure along with Tukey post hoc analysis. Additionally, a three-factor repeated-measures ANOVA procedure was used to specifically compare the commercial CPAP nasal mask that each subject found most comfortable with their customised mask. The three compared factors were the CPAP mask, CPAP level and mask tightness. Results with two-sided p≤0.05 were considered significant. Statistical analysis was performed using MATLAB software.
Results
Six subjects were enrolled in this study. The mean±sd intentional air leak through the expiratory port for the three tested CPAP levels was found to be 14.0±0.4 SLPM for 4 cmH2O, 19±1 SLPM for 8 cmH2O and 24±1 SPLM for 12 cmH2O (n=6). When testing with a loose fit the CPAP machine would alarm “high leak”, indicating that target pressures were not maintained in the breathing circuit and mask. This occurred for two subjects using their customised masks at the 12 cmH2O CPAP setting; two and four subjects using the petite size commercial mask at 8 and 12 cmH2O, respectively; two subjects using the small/medium size commercial mask at both 8 and 12 cmH2O; and one subject using the large commercial mask at 8 cmH2O. When testing with the appropriate fit, the “high leak” alarm was given only for one subject using the petite size commercial mask at 12 cmH2O. For all other combinations of mask tightness and CPAP levels, the customised and commercial masks delivered and maintained target CPAP in all six adult subjects, with no alarms.
Average mask air leaks for each combination of mask tested, CPAP level and tightness are displayed in table 1. Each factor, CPAP mask, CPAP level and mask tightness had influence on mask air leak. Significant influences on leak were also observed in all interactions between the three factors apart from the interaction between CPAP mask and CPAP level. From post hoc analysis, the individual CPAP masks were compared against one another for each CPAP level and mask tightness combination. The pairs of masks observed to be significantly different in air leak have been displayed in table 2.
TABLE 1.
Average mask air leak for varying continuous positive airway pressure (CPAP) mask, CPAP level and mask tightness
| Mask air leak SLPM | |||
| CPAP level cmH2O | |||
| 4 | 8 | 12 | |
| Petite | |||
| 100 g | 27±13 | 48±15 | 60±22 |
| 350 g | 9±5 | 17±9 | 27±15 |
| 600 g | 2±2 | 5±2 | 8±4 |
| Small/medium | |||
| 100 g | 17±19 | 37±23 | 44±19 |
| 350 g | 3±1 | 7±4 | 13±9 |
| 600 g | 1.1±0.5 | 1.3±0.5 | 3±2 |
| Large | |||
| 100 g | 19±14 | 28±16 | 29±5 |
| 350 g | 5±6 | 16±16 | 18±10 |
| 600 g | 3±3 | 9±7 | 13±11 |
| Custom | |||
| 100 g | 9±2 | 28±11 | 45±20 |
| 350 g | 5±2 | 11±3 | 18±4 |
| 600 g | 5±3 | 8±4 | 11±2 |
Data are presented as mean±sd. n=6. SLPM: standard litres per minute.
TABLE 2.
Differences in air leak between masks for varying continuous positive airway pressure (CPAP) level and mask tightness
| CPAP level cmH2O | |||
| 4 | 8 | 12 | |
| 100 g | No differences | No differences | P>L |
| 350 g | No differences | No differences | P>SM |
| 600 g | C>P | P, C>SM | P, C>SM |
P: petite; L: large; SM: small/medium; C: custom. Mask pairings were observed to have significantly different mask air leaks when p<0.05 in post hoc analysis.
Mask comfort
Out of the four masks tested, three out of the six subjects found the customised mask to be the most comfortable; two subjects found it to be the second most comfortable; and one subject found it to be the third most comfortable. Excluding the customised mask, four subjects preferred the large-sized commercial mask and two subjects preferred the small/medium-sized commercial mask.
Preferred commercial CPAP mask versus customised CPAP mask
When comparing the mask air leak for each subjects' preferred commercial mask to their customised mask, the influence of both pressure level and mask tightness were statistically significant, but the influence of the mask itself was not. Significant influence on mask leak was only observed in the interaction between CPAP level and mask tightness.
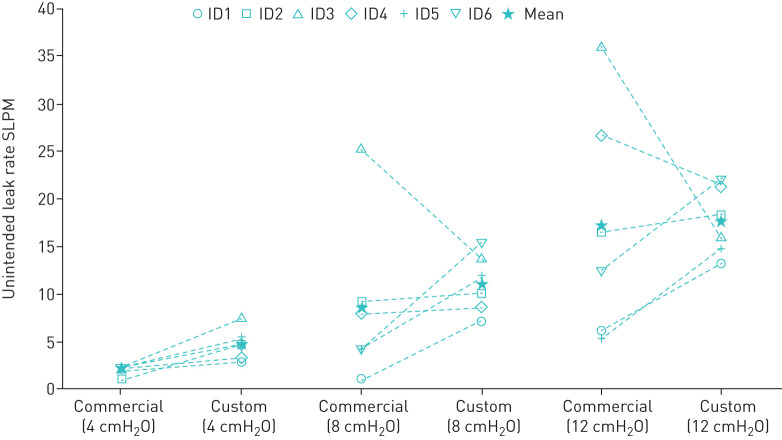
Relative mask air leak was variable between subjects when comparing their preferred commercial masks against their customised masks (figure 5). Some subjects showed a decrease in mask leak when using their customised masks, whereas other subjects showed an increase in mask leak.
FIGURE 5.
Mask air leak for each subject's preferred commercial continuous positive airway pressure mask and their customised counterparts at an appropriate fit (350 g). SLPM: standard litres per minute.
Discussion
Both mask leak and comfort results reported herein indicate that, on average, the customised nasal CPAP masks performed similarly to commercial masks (of appropriate size), for the small sample of healthy adults studied. For the tightness deemed appropriate, the customised masks did not produce any “high leak” alarm. These results suggest that customised masks could be used to deliver CPAP therapy safely and effectively. Conversely, customised masks did not consistently reduce mask leak nor improve comfort across study subjects. Rather, the relative leak and comfort between the customised masks and commercial masks were highly variable between subjects, presumably as a function of variable facial geometries. Methods of identifying individual patients most likely to benefit from provision of a customised CPAP mask have not been established.
Some limitations of the present feasibility study warrant comment. First, study subjects were in good health and had not previously required nocturnal CPAP support; therefore, they may not reflect patients who are prescribed CPAP. Results for commercial masks may represent a best-case scenario, as the subjects were all healthy without any craniofacial abnormalities. Additionally, the commercial masks used in the present study do not necessarily reflect the best available mask for each individual across the broad supply of all masks available from various manufacturers. Future trials conducted in patients who are prescribed CPAP should compare customised masks with each patient's currently used commercial mask. Second, mask leak testing was done while each subject was seated at rest in an upright position with their head facing forward. Whether or not mask customisation has an influence of variation in leak with body position, i.e. lying down or on one's side, or turning in bed, was not assessed in this study. Third, evaluation of all four masks was done in a single visit, which could have skewed comfort rankings due to potential testing fatigue of wearing masks for the entire visit. To mitigate this factor, the order of the masks was randomised for each subject, as well as by providing a 10-min recovery period between each mask tested. Fourth, although no significant difference was observed between customised and commercial masks in mask leak or in subject-reported comfort, these variables may not necessarily be similar between customised and commercial masks due the low power of the sample in this feasibility study. Finally, results presented here for customised masks are specific to the customised mask design and fabrication methods employed, including the silicone material used to form the portion of the mask which contacts the face. These results may not generalise to all methods of mask manufacturing.
Notwithstanding these limitations, two consistent trends were observed in the mask leakage data: mask leak varied directly with CPAP levels and inversely with mask tightness. In a comparison between all masks, the petite-sized commercial mask exhibited the highest mask leak and was ranked least comfortable by all subjects. Further comparisons between the customised masks and the commercial mask that each subject ranked most comfortable showed no statistically significant influence of mask selection on measured mask leak, which is consistent with results for leakage reported by Cheng et al. [10]. Cheng et al. [10] reported leakage monitored by a CPAP machine over 14 days of use by patients with severe OSA randomised into groups using customised or commercial masks. Mean±sd mask leak was 32±19 L·min−1 for customised masks versus 36±48 L·min−1 for commercial masks [10]. Although average mask leak was comparable between groups, variability in mask leak appeared notably lower for the group using customised masks. A similar reduction in variability was noted in the present work, particularly when masks were evaluated at the tightness of fit deemed appropriate.
Our study measured mask leakage in real time by using a low-resistance Pitot tube flow sensor. Other studies looking into customised CPAP masks obtained their leak data directly from the CPAP machine, which required overnight use to obtain an average leak rate [10, 13]. With mask leakage being the main comparative measurement made in the present study, measurements in real time allowed for rapid testing of varying masks, tightness and CPAP levels. This allowed for faster evaluation of mask leakage between the tested variables. However, unlike the other studies where AHI was an important comparative measurement, our study did not assess AHI since overnight mask usage was not conducted.
Based on the comfort rankings, half of the subjects ranked the customised mask most comfortable, with half ranking one of the commercial mask sizes as most comfortable. Comfort rankings were poorly correlated with measured mask leak, likely indicating that subjects' perception of comfort was more strongly influenced by other factors, such as the pressure of the mask contacting the face or localised leaks directing airflow towards the eyes. While measurement of airflow from the CPAP machine allows the total mask leak to be derived, it provides no information on the location of the leak around the interface between the mask cushion and face. When evaluating customised masks for CPAP, Cheng et al. [10] divided their comfort assessment into several components: headgear force, headgear comfort, cushion fit, cushion comfort, forehead comfort and usability. Their study found better performance in headgear force and cushion fit for their customised masks, but found no significant correlation between headgear tightness or cushion fit with cushion comfort [10]. Overall, no statistically significant difference in cushion comfort was found between customised and commercial masks [10].
Prevalence of OSA is higher in subjects with craniofacial abnormalities than in the general population [23], some of whom may not be able to maintain CPAP therapy using commercial masks. Morrison et al. [13] developed a custom-built oronasal mask for a child with craniofacial abnormalities and OSA which decreased mask leakage, improved AHI and increased adherence, which were all sustained after 3 months of use. Such benefits to patients present potential savings to healthcare systems, which may ultimately offset the fabrication costs of custom masks. Based on results from both our study and the study by Morrison et al. [13], custom-fabricated CPAP masks may be most suitable to a population with craniofacial abnormalities who are unable to use commercial masks. Further study of the custom-built nasal masks presented herein in subjects with craniofacial abnormalities is warranted.
Conclusion
Customised masks successfully delivered and maintained target CPAP settings in all six adult subjects, demonstrating the feasibility of the mask customisation methods employed. Customised masks should be explored for individuals with unique facial features who are unable to receive CPAP treatment using commercial masks.
Acknowledgments
We thank the Live Cell Imaging Laboratory team (University of Calgary, Calgary, AL, Canada) for the support of this project.
Footnotes
Author contributions: Conception and design of work: M. Ungrin, P. Colarusso, J.E. MacLean and A.R. Martin; acquisition and analysis of data: K. Duong, J. Glover, A.C. Perry and D. Olmstead; interpretation of data: K. Duong, D. Olmstead, J.E. MacLean and A.R. Martin; drafting manuscript: K. Duong, J. Glover, A.C. Perry and A.R. Martin; critically revising manuscript: D. Olmstead, M. Ungrin, P. Colarusso, J.E. MacLean and A.R. Martin.
Support statement: Part of this work was supported from a Research and Innovation Seed Grant from Respiratory Health Strategic Clinical Network (Alberta Health Services). A.C. Perry was funded by a studentship from the Respiratory Health Strategic Clinical Network for part of this work. Funding information for this article has been deposited with the Crossref Funder Registry.
Conflict of interest: K. Duong has nothing to disclose.
Conflict of interest: J. Glover has nothing to disclose.
Conflict of interest: A.C. Perry has nothing to disclose.
Conflict of interest: D. Olmstead has nothing to disclose.
Conflict of interest: M. Ungrin has nothing to disclose.
Conflict of interest: P. Colarusso is cofounder of Luxidea, Inc. Luxidea has no financial or related interest in the work described here.
Conflict of interest: J.E. MacLean reports grants from Alberta Economic Development and Trade during the conduct of the study.
Conflict of interest: A.R. Martin reports grants from Alberta Economic Development and Trade during the conduct of the study.
References
- 1.Donovan LM, Boeder S, Malhotra A, et al. New developments in the use of positive airway pressure for obstructive sleep apnea. J Thorac Dis 2015; 7: 1323–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shapiro GK, Shapiro CM. Factors that influence CPAP adherence: an overview. Sleep Breath 2010; 14: 323–335. doi: 10.1007/s11325-010-0391-y [DOI] [PubMed] [Google Scholar]
- 3.Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177: 1006–1014. doi: 10.1093/aje/kws342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weaver TE, Sawyer AM. Adherence to continuous positive airway pressure treatment for obstructive sleep apnoea: implications for future interventions. Indian J Med Res 2010; 131: 245–258. [PMC free article] [PubMed] [Google Scholar]
- 5.Rotenberg BW, Murariu D, Pang KP. Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg 2016; 45: 43. doi: 10.1186/s40463-016-0156-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strickland SL The patient experience during noninvasive respiratory support. Respir Care 2019; 64: 689–700. doi: 10.4187/respcare.06642 [DOI] [PubMed] [Google Scholar]
- 7.Valentin A, Subramanian S, Quan SF, et al. Air leak is associated with poor adherence to autoPAP therapy. Sleep 2011; 34: 801–806. doi: 10.5665/SLEEP.1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salepci B, Caglayan B, Kiral N, et al. CPAP adherence of patients with obstructive sleep apnea. Respir Care 2013; 58: 1467–1473. doi: 10.4187/respcare.02139 [DOI] [PubMed] [Google Scholar]
- 9.Massie CA, Hart RW. Clinical outcomes related to interface type in patients with obstructive sleep apnea/hypopnea syndrome who are using continuous positive airway pressure. Chest 2003; 123: 1112–1118. doi: 10.1378/chest.123.4.1112 [DOI] [PubMed] [Google Scholar]
- 10.Cheng YL, Hsu DY, Lee HC, et al. Clinical verification of patients with obstructive sleep apnea provided with a customized cushion for continuous positive airway pressure. J Prosthet Dent 2015; 113: 29–34. doi: 10.1016/j.prosdent.2014.01.030 [DOI] [PubMed] [Google Scholar]
- 11.Sela M, Toledo N, Honen Y, et al. Customized facial constant positive air pressure (CPAP) masks. ArXiv 2016: abs/1609.07049. [Google Scholar]
- 12.Shikama M, Nakagami G, Noguchi H, et al. Development of personalized fitting device with 3-dimensional solution for prevention of NIV oronasal mask-related pressure ulcers. Respir Care 2018; 63: 1024–1032. doi: 10.4187/respcare.05691 [DOI] [PubMed] [Google Scholar]
- 13.Morrison R, Vankoevering K, Nasser H, et al. Personalized 3D-printed CPAP masks improve CPAP effectiveness in children with OSA and craniofacial anomalies. Presented at the Combined Otolaryngology Spring Meetings, Boston, MA, USA, April 2015.
- 14.Daniel J Sampling Essentials: Practical Guidelines for Making Sampling Choices. Thousand Oaks, SAGE Publications, 2012. [Google Scholar]
- 15.Kirkness JP, Verma M, McGinley BM, et al. Pitot-tube flowmeter for quantification of airflow during sleep. Physiol Meas 2011; 32: 223–237. doi: 10.1088/0967-3334/32/2/006 [DOI] [PubMed] [Google Scholar]
- 16.Castro-Codesal ML, Olmstead DL, MacLean JE. Mask interfaces for home non-invasive ventilation in infants and children. Paediatr Respir Rev 2019; 32: 66–72. [DOI] [PubMed] [Google Scholar]
- 17.Rowland S, Aiyappan V, Hennessy C, et al. Comparing the efficacy, mask leak, patient adherence, and patient preference of three different CPAP interfaces to treat moderate-severe obstructive sleep apnea. J Clin Sleep Med 2018; 14: 101–108. doi: 10.5664/jcsm.6892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guralnick AS, Pant M, Minhaj M, et al. CPAP adherence in patients with newly diagnosed obstructive sleep apnea prior to elective surgery. J Clin Sleep Med 2012; 8: 501–506. doi: 10.5664/jcsm.2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McArdle N, Devereux G, Heidarnejad H, et al. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med 1999; 159: 1108–1114. doi: 10.1164/ajrccm.159.4.9807111 [DOI] [PubMed] [Google Scholar]
- 20.Bakker JP, Neill AM, Campbell AJ. Nasal versus oronasal continuous positive airway pressure masks for obstructive sleep apnea: a pilot investigation of pressure requirement, residual disease, and leak. Sleep Breath 2012; 16: 709–716. doi: 10.1007/s11325-011-0564-3 [DOI] [PubMed] [Google Scholar]
- 21.Shirlaw T, Duce B, Milosavljevic J, et al. A randomised crossover trial comparing nasal masks with oronasal masks: no differences in therapeutic pressures or residual apnea-hypopnea indices. J Sleep Res 2019; 28: e12760. doi: 10.1111/jsr.12760 [DOI] [PubMed] [Google Scholar]
- 22.Duarte RLM, Mendes BA, Oliveira-e-Sá TS, et al. Nasal versus oronasal mask in patients under auto-adjusting continuous positive airway pressure titration: a real-life study. Eur Arch Otorhinolaryngol 2020; 277: 3507–3512. doi: 10.1007/s00405-020-06242-x [DOI] [PubMed] [Google Scholar]
- 23.Tan HL, Kheirandish-Gozal L, Abel F, et al. Craniofacial syndromes and sleep-related breathing disorders. Sleep Med Rev 2016; 27: 74–88. doi: 10.1016/j.smrv.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]