Abstract
Aim:
The aim is to assess and compare the mineral gain and penetration depth of hydroxyapatite and silica nanoparticle infiltrates into artificially created erosive lesions of enamel and dentin.
Materials and Methods:
Sixty extracted human molars were sectioned to obtain enamel and dentin samples (n = 60 each). They were demineralized using citric and formic acid, respectively, to create erosive lesions on enamel and dentin surfaces. Samples were assigned into nanohydroxyapatite (nHA) or nanosilica groups (n = 30 each) according to the infiltrant used. Half of the enamel and dentin samples in each group (n = 15) were analyzed after erosive attack for mineral loss, after infiltrant application for mineral gain, using energy-dispersive X-ray spectroscopy. In another half of the enamel and dentin samples (n = 15), the penetration depth of the nanoinfiltrants was analyzed using confocal microscopy.
Statistical Analysis:
To compare the overall mineral gain between groups, a dependent t-test was applied. The intergroup comparisons were made using one-way ANOVA followed by Tukey post hoc test for pairwise comparisons for both penetration depth and mineral gain. The significance level was set to P ≤ 0.05.
Results:
The mineral gain in enamel was not statistically different between nHA and nanosilica infiltrants (P = 0.9950). nHA infiltrated dentin showed significantly more mineral gain (P = 0.0001) than nanosilica infiltrant. The depth of penetration of the nHA in enamel was statistically greater than that of nanosilica, but in dentin, the difference was not significant.
Conclusion:
nHA infiltrant performed better in mineral gain, and penetrated deeper into the demineralized erosive lesions, compared to nanosilica infiltrant in both enamel and dentin. The highest mineral precipitation and deeper penetration into both demineralized enamel and dentin was observed with nHA infiltrant compared to (as against) nanosilica infiltrant.
Keywords: Colloidal silica, confocal microscopy, energy dispersive X-rays, erosive lesions, nanohydroxyapatite, remineralization
INTRODUCTION
Tooth erosion or the chemical wear of tooth is a well-established dental pathology and has been reported among 20% of the world population. About 89% of documented patients with severe tooth wear were found to have an etiology of erosion.[1] The prevalence of erosion has increased in children and adults due to frequent consumption of acidic diet and due to the exposure of gastric acids during acid reflux or vomiting.[2] Prevalence data indicate that erosive lesions are most commonly seen on palatal surfaces of maxillary incisors, and occlusal surfaces of mandibular molars, followed by labial surfaces of maxillary anteriors. The least commonly involved teeth are mandibular anteriors.[3]
The demineralized erosive defects can be treated by minimally invasive procedures, i.e., through the application of remineralizing agents such as fluoride-containing agents, beta-tricalcium phosphate, and casein phosphopeptide-amorphous calcium phosphate complexes.[4,5] a Compared to enamel, dentin remineralization using fluoride is less effective, more complex, and challenging.[6] The inorganic content of dentin is mainly calcium deficient and carbonate-rich hydroxyapatite, and the organic content is collagen-type I and noncollagenous proteins (NCPs) such as dentin matrix protein (DMP1) and dentin phosphoprotein (DPP or DMP2). It was reported that NCPs act as inhibitors of dentin mineralization, and if removed, dentin remineralization can be enhanced.[7]Further, remineralization in dentin takes a longer time, and thus for ideal mineralization, the release of calcium and phosphate ions should be slow.[1]
Synthetic calcium hydroxyapatite (Ca10(PO4)6(OH2)) has similar mineral content like that of dental hard tissues and is highly bioactive and biocompatible. Nanohydroxyapatite (nHA) compared to HA has higher surface energy, solubility, and better biological activity.[8,9] It was recommended that 20 nm is the ideal dimension of the nHA particles that can firmly attach to the demineralized enamel surface and resist further damage due to erosive acidic attack.[10]Thus, synthetic nHA was considered a better substitute to increase the mineral gain of demineralized enamel and dentin.
Colloidal silica contains fine amorphous silica particles and due to their low mass, can infiltrate into the demineralized collagen matrix without surface precipitation.[11] Silica nanoparticles (nSiO2) with their affinity in the presence of hydroxyl ions on their surface can adhere to dentin surfaces easily. When these nanoparticles of HA or SiO2infiltrate the demineralized dentin, the particles act as a scaffold for the initiation of the remineralization process. When tested on demineralized dentin blocks, colloidal silica nanoparticles have shown better penetration and remineralization capacity compared to nHA. It was reported that up to 20% of phosphate levels and 16% of the mineral volume were recovered with nSiO2in comparison to nHA particles infiltration in demineralized dentin.[12,13]
Hence, the objective of this study was to assess and compare the efficacy of nHA and colloidal silica nanoparticles in remineralizing the artificially created enamel and dentin erosive lesions on molar teeth. The null hypothesis tested was that the penetration depth and ability to remineralize the eroded enamel and dentin were not different for hydroxyapatite and silica nanoparticle infiltrants.
MATERIALS AND METHODS
Sixty sound, freshly extracted mandibular molars were procured according to protocols approved by the State Health University and Institutional Review Board (D178601021). The collected teeth were examined at ×2.5 magnification, to rule out any fractures, cracks or craze lines, hypoplasia, or white spot lesions.
Sample preparation
Collected mandibular molar teeth with similar dimensions were disinfected and immersed in distilled water until their use. Each tooth was decoronated approximately 1 mm below the cementoenamel junction, and the crowns were sectioned mesiodistally along the central groove of the occlusal surface, to obtain buccal and lingual halves from each tooth sample. Then, both halves of each tooth sample were embedded in acrylic resin molds so that the coronal portion of buccal and lingual (8 mm × 4 mm) surfaces are exposed.
Teeth samples were placed in an artificial saliva solution throughout the experimental period as it was validated as a natural saliva substitute in remineralization in vitro studies.[12,14] To make 1 L of artificial saliva, 14.4 mM NaCl, 16.1 mM KCl, 0.3 mM CaCl2.2H2O, 2.9 mM K2HPO4, and 0.10 mM sodium carboxymethyl cellulose were mixed in 1000 ml water, and using 1M HCl, the pH (7.2) of the solution was adjusted.[15]
For creating erosive enamel lesions, the buccal surfaces (n = 60) of all the test specimens were immersed in the freshly prepared 1% W/V citric acid (pH 2.3) solution six times daily for 2 min at 37°C for 6 days. In between the demineralization periods, the enamel specimens were placed in artificial saliva.
For the formation of dentin erosive lesions (n = 60) on lingual surfaces, a straight fissure bur was used to remove the enamel, and the dentin underneath was exposed. Later, the samples were completely immersed in a 4N formic acid solution for 48 h.[14] The samples were then washed thoroughly with distilled water and stored in artificial saliva.
Infiltration of specimens
The demineralized enamel and dentin samples were assigned to two groups (n = 30 each) according to the infiltrant being used; nHA aqueous paste (Sigma Aldrich, St. Louis, Missouri USA) group and colloidal nanosilica infiltrate-Ludox HS-40 (Sigma Aldrich, St. Louis, Missouri USA) group.
nHA suspension was prepared by mixing the nano-HA paste with sodium hexametaphosphate (SHMP) (1:3 ratio) in distilled water and acetone. Nano silica (nSiO2) used was Ludox HS-40 (Sigma Aldrich, St. Louis, Missouri, USA), which is colloidal silica in an aqueous medium and thus needs no additional preparation. Infiltrants were applied at 3, 6, 8 10, 12 h Intervals and were immersed in artificial saliva during the interim phase.[4]
Scanning electron microscopy/energy-dispersive X-ray spectroscopy analysis
To calculate the baseline (demineralized) mineral content, only one half of enamel and dentin erosive surface (4 mm × 4 mm) was infiltrated with nanoinfiltrant. All the specimens were coated with carbon before performing energy-dispersive X-ray spectroscopy (Shimadzu EDX 7000, Tokyo, Japan) analysis. Two readings were taken on each half of the sample, and elemental values of silica, calcium, and phosphorus were obtained. The gain in mineral content after the infiltrants application was analyzed by peaks in weight % and volume % for calcium and phosphorus elements.
Penetration depth analysis
To assess the extent of penetration of the infiltrant, 0.1% ethanolic solution of tetramethyl rhodamine isothiocyanate dye (Macsen Labs Pvt. Ltd., Rajasthan, India) was added to the infiltrants before application.[13] Then, infiltrated enamel and dentin specimens were sectioned to a size of 150 mm thickness using a hard tissue microtome (Leica SP 1600, Nussloch, Germany) and were visualized under confocal laser scanning microscope (Neon 40-Carl Zeiss, Jena, Germany). To achieve reproducible measurements of lesion depth and infiltrant depth, three deepest measurements (in microns) were taken for each section, and their averages were calculated as mean maximum lesion depth and maximum penetration depth.
Statistical analysis
The analysis of data was done using the SPSS software version 2.0 (IBM, New York, NY, USA). The intergroup comparisons were made using one-way ANOVA followed by Tukey post hoc test for pairwise comparisons for both penetration depth and mineral gain. To compare the overall values of mineral gain between groups, a dependent t-test was applied. The level of significance was set to P ≤ 0.05.
RESULTS
The overall mineral content gain was not different in enamel samples between nHA and nSiO2 infiltrants (P = 0.9950) [Table 1]. The difference in mineral gain was highly significant in dentin samples between the two infiltrants tested (P = 0.0001), where nHA dentin samples revealed highest mineral gain. Compared to nHA enamel samples, the mineral gain was not significantly different for nSiO2dentin samples (P = 0.0920).
Table 1.
Comparison of mean mineral gain wt% from baseline erosive values between the two infiltrants by Tukey multiple post hoc procedures
| (I) Groups | (J) Groups | Mean difference (I-J) | SE | P |
|---|---|---|---|---|
| nHA enamel | nHA dentin | −37.46 | 4.34 | 0.0001* |
| nSiO2 enamel | 1.04 | 4.34 | 0.9950 | |
| nSiO2 dentin | −10.36 | 4.34 | 0.0920 | |
| nHA dentin | nSiO2 enamel | 38.50 | 4.34 | 0.0001* |
| nSiO2 dentin | 27.10 | 4.34 | 0.0001* | |
| nSiO2 enamel | nSiO2 dentin | −11.40 | 4.34 | 0.0500* |
*P≤0.05 is statistically significant, P>0.05 means statistically not significant. SE: Standard error, nHA: Nanohydroxyapatite, nSiO2: Silica nanoparticle
For the gain of Ca and P individually in enamel, there was no significant difference between the infiltrants (P > 0.05) [Figure 1]. Dentin samples gained more Ca and P than enamel samples for both the infiltrants (P < 0.05).
Figure 1.
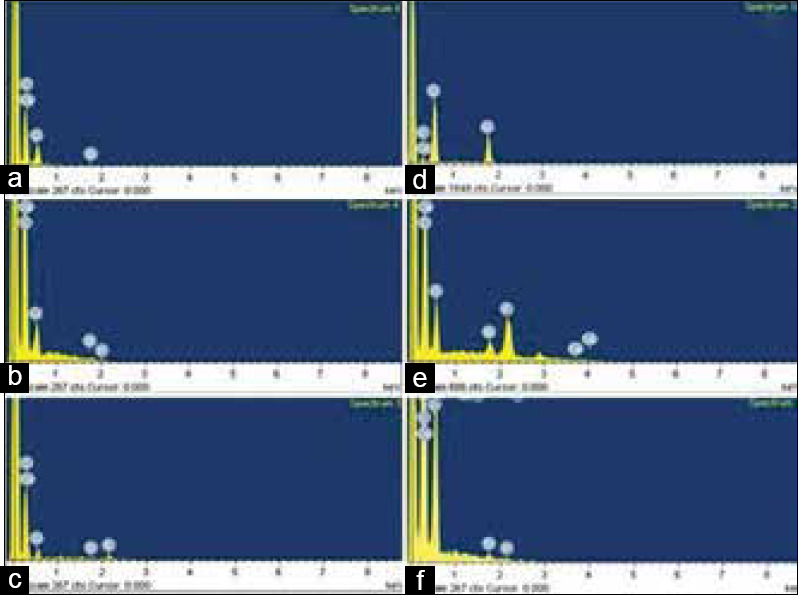
Energy-dispersive X-ray spectroscopy elemental spectrum (a) Energy-dispersive X-ray spectroscopy elemental spectrum of demineralized enamel sample (b) After nanohydroxyapatite infiltration in enamel sample (c) After nSiO2 infiltration in enamel sample (d) Spectrum of demineralized dentin sample (e) After nanohydroxyapatite infiltration in dentin sample (f) After nSiO2 infiltration in dentin sample
In dentin, both the infiltrants presented similar penetration depths without any difference statistically (P = 0.2370) [Table 2]. However, in enamel, the nHA infiltrant exhibited a statistically higher depth of penetration than the nSiO2infiltrant (P = 0.0150). The penetration depth of the infiltrants was significantly higher in dentin than in enamel for both the infiltrants [Figure 2].
Table 2.
Comparison of difference in mean penetration depth between the two infiltrants by Tukeys post hoc test
| (I) Groups | (J) Groups | Mean difference (I-J) | SE | P |
|---|---|---|---|---|
| nHA enamel | nHA Dentin | −375.92 | 4.30 | 0.0001* |
| nSiO2 Enamel | 13.38 | 4.30 | 0.0150* | |
| nSiO2 Dentin | −367.73 | 4.30 | 0.0001* | |
| nSiO2 enamel | nSiO2 Dentin | −381.10 | 4.30 | 0.0001* |
| nHA dentin | nSiO2 Enamel | 389.30 | 4.30 | 0.0001* |
| nSiO2 Dentin | 8.20 | 4.30 | 0.2370 |
*P≤0.05 is statistically significant, P>0.05 means statistically not significant. SE: Standard error, nHA: Nanohydroxyapatite, nSiO2: Silica nanoparticle
Figure 2.
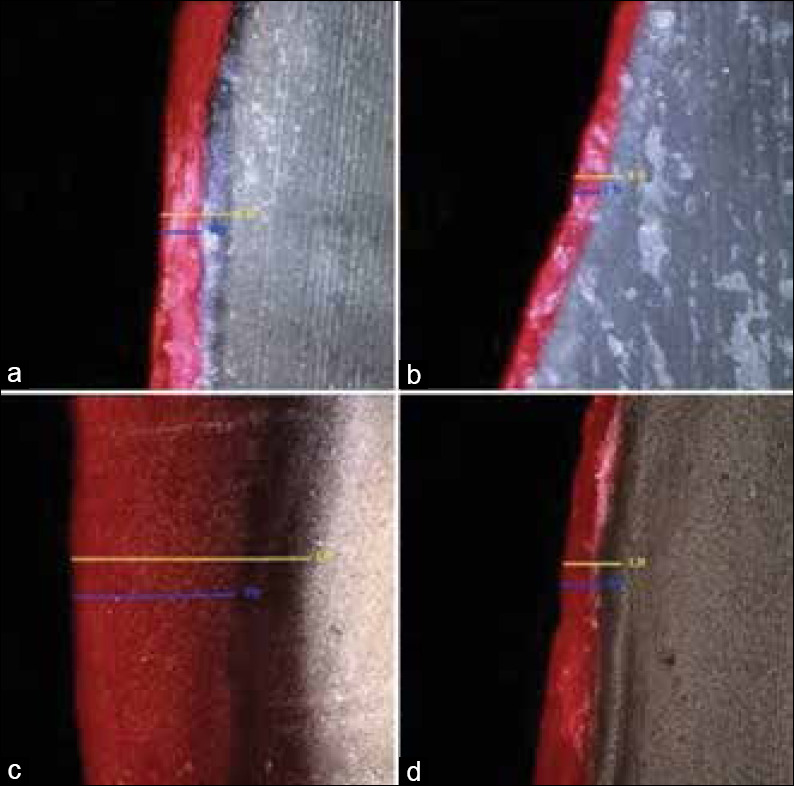
CLSM images showing lesion and penetration depths at ×10 magnification. (a) nanohydroxyapatite infiltration in enamel (b) nSiO2 infiltration in enamel (c) nanohydroxyapatite infiltration in dentin (d) nSiO2 infiltration in dentin. Yellow line indicates the depth of demineralized lesion (maximum lesion depth) and blue line depicts penetration depth (maximum penetration depth) of the infiltrants
DISCUSSION
The remineralization process involves diffusion of calcium and phosphate ions that are supplied from an external source through the demineralized enamel and dentin surfaces producing net mineral gain.[16]Nanoparticles have superior dispersion property than their counterpart bulk materials and thus can diffuse quickly through the dentinal tubules that have a diameter of 2–3 mm. These nanomaterials having a larger surface area provide high solubility and reactivity allowing faster deposition of particles on the rougher demineralized surfaces.[17]
Tay and Pashley were able to achieve 5 mm thick intra- and inter-fibrillar remineralization of demineralized dentin with guided tissue remineralization.[14] As dentin is devoid of inorganic hydroxyapatite content, the nanoparticles infiltrated can decrease the energy barrier essential for the inorganic ion cluster formation, that can be improved by placing the specimens in artificial saliva.[13,15] Hegde et al. highlighted the remineralization potential of saliva due to the presence of calcium and phosphate ions.[17] Thus, to simulate the oral environment, teeth samples were placed in artificial saliva throughout the experimental period.
In experimental in vitro studies, the depth of demineralization of enamel should be ideally between 500 and 900 mm to mimic the clinical situation.[18] Citric acid was selected as its enamel demineralization potential is well pronounced due to its chelating action and can bind calcium like minerals from the apatite.[19] Enamel eroded by citric acid produces a rougher surface with interprismatic mineral loss. For dentin demineralization, 4N formic acid was used in an attempt to achieve complete demineralization leaving behind only the collagen framework.[13] The demineralization process was carried out uniformly for all enamel and dentin samples, and thus no significant variations were observed in calcium and phosphorus depletion levels in the demineralized samples.
Hydroxyapatite used in the study was in the form of aqueous paste, and volatile acetone was added to make the nHA suspension for smooth and uniform application. A deflocculant agent SHMP was mixed with nHA at 1:3 ratio to prevent precipitation of particles on application. Earlier studies where lower concentration (0.03% W/V) of nHA was used, stated that this concentration was inadequate for visualization of particles using scanning electron microscope.[11,20,21] Thus, 15% W/V concentration of nHA was employed in this study. Colloidal silica used was available as a water-based suspension form, and thus, the infiltrant was applied directly onto the demineralized area.
The importance of depth of penetration of an infiltrant was highlighted in previous studies,[12,13] as these nanoparticles act as a scaffold, and have to reach the full extent of demineralized area for remineralization. Hence, the fluorescent dye was mixed with the infiltrants for better image analysis.
The results of the present study indicate that the application of nanoinfiltrants was able to raise the mineral content in demineralized enamel and dentin. The mineralization values for nHA and nSiO2infiltrants in enamel were similar without any statistical difference. In dentin, the mineralization values were significantly higher for nHA, similar to previous studies results.[22,23] This finding was in contrast to earlier results[12,13] where they reported significantly higher mineralization values for nSiO2infiltrant.
In the present study, for both the infiltrants used, calcium levels were restored up to 73% and also demonstrated a 21% recovery of overall mineral volume after remineralization. The phosphorus levels were higher in dentin after infiltrants application, especially for nHA. These findings were supported by the hypothesis stating that the remineralization in dentin is considerably slower than that in enamel. The reduced amount of nucleating minerals in the dentin and the presence of a minimal amount of phosphate, might have contributed to the slower mineralization of dentin.
In the study, between the infiltrants, the depth of penetration was not different, but a statistically significant difference was noticed in enamel, where nHA showed more penetration. The penetrability of a material into porous solids is dependent on its penetration coefficient that relies on the viscosity, surface tension, and contact angle of the infiltrant. nHA formulation was combined with SHMP and acetone in the study. The SHMP breaks down nHA particles to nanosize of <5 nm. Hydrophobic and volatile nature of acetone helps to penetrate the particles deeply into the demineralized area by displacing the water molecules that evaporates consequently. As the particle size decreases, the atomicity increases and therefore the depth of penetration.[24]Thus, the deeper infiltration of nHA can be attributed mainly to the smaller particle size. Inferior penetration and mineral gain concerning nSiO2infiltrant might be due to the fact that SHMP or acetone were not added, and the particle size was reported to be 10–20 mm in diameter. It was demonstrated in earlier studies[13,24] as well, that small particle size is an essential determinant of infiltrant penetrability.
The average lesion depth in enamel demineralized samples was 118 mm, and in dentin, it was 715 mm, in the present study. Improper demineralization might be one of the reasons for obtaining poor penetration in enamel samples in the study. Stronger acids such as phosphoric acid or hydrochloric acid are recommended for better penetration of infiltrant.[25] Furthermore, the infiltrant application was done gently without firm pressure and agitation, which could have resulted in less penetration.
CONCLUSION
The present study arrives at the conclusion that nHA infiltrant was better at regaining lost mineral content and have shown greater penetration into the enamel and dentin erosive lesions when compared to nanosilica infiltrant. Particle size and viscosity of the infiltrant play an essential role for deeper penetration and better mineral gain of the substrate.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hemmings K, Truman A, Shah S, Chauhan R. Tooth wear guidelines for the BSRD part 1; Aetiology, diagnosis and prevention. Dent update. 2018;45:483–95. [Google Scholar]
- 2.Sarode GS, Sarode SC. Abfraction: A review. J Oral Maxillofac Pathol. 2013;17:222–7. doi: 10.4103/0973-029X.119788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schlueter N, Luka B. Erosive tooth wear - A review on global prevalence and on its prevalence in risk groups. Br Dent J. 2018;224:364–70. doi: 10.1038/sj.bdj.2018.167. [DOI] [PubMed] [Google Scholar]
- 4.Thimmaiah C, Shetty P, Shetty SB, Natarajan S, Thomas NA. Comparative analysis of the remineralization potential of CPP-ACP with fluoride, tri-calcium phosphate and nano hydroxyapatite using SEM/EDX - An in vitro study. J Clin Exp Dent. 2019;11:e1120–6. doi: 10.4317/jced.55941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weir MD, Ruan J, Zhang N, Chow LC, Zhang K, Chang X, et al. Effect of calcium phosphate nanocomposite on in vitro remineralization of human dentin lesions. Dent Mater. 2017;33:1033–44. doi: 10.1016/j.dental.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 6.Featherstone JD. The science and practice of caries prevention. J Am Dent Assoc. 2000;131:887–99. doi: 10.14219/jada.archive.2000.0307. [DOI] [PubMed] [Google Scholar]
- 7.George A, Veis A. Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chem Rev. 2008;108:4670–93. doi: 10.1021/cr0782729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang SB, Gao SS, Yu HY. Effect of nano-hydroxyapatite concentration on remineralization of initial enamel lesion in vitro. Biomed Mater. 2009;4:34104. doi: 10.1088/1748-6041/4/3/034104. [DOI] [PubMed] [Google Scholar]
- 9.Scribante A, Dermenaki Farahani MR, Marino G, Matera C, Baena RR, Lanteri V, et al. Biomimetic effect of nano-hydroxyapatite in demineralized enamel before orthodontic bonding of brackets and attachments: Visual, adhesion strength, and hardness in in vitro tests. Biomed Res Int. 2020;2020:6747498. doi: 10.1155/2020/6747498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L, Pan J, Tao J, Xu X, Mao C, Gu X, et al. Repair of enamel using hydroxyapatite nanoparticles as the building blocks. J Mater Chem. 2008;18:4079–84. [Google Scholar]
- 11.Roveri N, Foresti E, Lelli M, Lesci IG. Recent advances in preventing teeth health hazard: The daily use of hydroxyapatite instead of fluoride. Recent Pat Biomed Eng. 2009;23:197–215. [Google Scholar]
- 12.Besinis A, van Noort R, Martin N. Remineralization potential of fully demineralized dentin infiltrated with silica and hydroxyapatite nanoparticles. Dent Mater. 2014;30:249–62. doi: 10.1016/j.dental.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Besinis A, van Noort R, Martin N. Infiltration of demineralized dentin with silica and hydroxyapatite nanoparticles. Dent Mater. 2012;28:1012–23. doi: 10.1016/j.dental.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Tay FR, Pashley DH. Guided tissue remineralisation of partially demineralised human dentine. Biomaterials. 2008;29:1127–37. doi: 10.1016/j.biomaterials.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Shaik ZA, Rambabu T, Sajjan G, Varma M, Satish K, Raju VB, et al. Quantitative analysis of remineralization of artificial carious lesions with commercially available newer remineralizing agents Using SEM-EDX - In vitro study. J Clin Diagn Res. 2017;11:ZC20–3. doi: 10.7860/JCDR/2017/22270.9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jawale KD, Kamat SB, Patil JA, Nanjannawar GS, Chopade RV. Grape seed extract: An innovation in remineralization. J Conserv Dent. 2017;20:415–8. doi: 10.4103/JCD.JCD_287_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hegde MN, Devadiga D, Jemsily PA. Comparative evaluation of effect of acidic beverage on enamel surface pre-treated with various remineralizing agents: An in vitro study. J Conserv Dent. 2012;15:351–6. doi: 10.4103/0972-0707.101902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer-Lueckel H, Paris S. Improved resin infiltration of natural caries lesions. J Dent Res. 2008;87:1112–6. doi: 10.1177/154405910808701201. [DOI] [PubMed] [Google Scholar]
- 19.Dündar A, Şengün A, Başlak C, Kuş M. Effects of citric acid modified with fluoride, nano-hydroxyapatite and casein on eroded enamel. Arch Oral Biol. 2018;93:177–86. doi: 10.1016/j.archoralbio.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Swarup JS, Rao A. Enamel surface remineralization: Using synthetic nanohydroxyapatite. Contemp Clin Dent. 2012;3:433–6. doi: 10.4103/0976-237X.107434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Memarpour M, Shafiei F, Rafiee A, Soltani M, Dashti MH. Effect of hydroxyapatite nanoparticles on enamel remineralization and estimation of fissure sealant bond strength to remineralized tooth surfaces: An in vitro study. BMC Oral Health. 2019;19:92. doi: 10.1186/s12903-019-0785-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tschoppe P, Zandim DL, Martus P, Kielbassa AM. Enamel and dentine remineralization by nano-hydroxyapatite toothpastes. J Dent. 2011;39:430–7. doi: 10.1016/j.jdent.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Huang S, Gao S, Cheng L, Yu H. Remineralization potential of nano-hydroxyapatite on initial enamel lesions: An in vitro study. Caries Res. 2011;45:460–8. doi: 10.1159/000331207. [DOI] [PubMed] [Google Scholar]
- 24.Kunam D, Manimaran S, Sampath V, Sekar M. Evaluation of dentinal tubule occlusion and depth of penetration of nano-hydroxyapatite derived from chicken eggshell powder with and without addition of sodium fluoride: An in vitro study. J Conserv Dent. 2016;19:239–44. doi: 10.4103/0972-0707.181940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paris S, Soviero VM, Chatzidakis AJ, Meyer-Lueckel H. Penetration of experimental infiltrants with different penetration coefficients and ethanol addition into natural caries lesions in primary molars. Caries Res. 2012;46:113–7. doi: 10.1159/000336961. [DOI] [PubMed] [Google Scholar]


