Abstract
Purpose:
To discuss the ocular manifestations provoked by novel coronavirus 2019 (COVID-19) disease in humans, the natural history of the disease in the eye, and its treatment.
Methods:
We designed a narrative review of the ocular manifestations of COVID-19 based on the literature published till July 30, 2020. The databases were PubMed, Scopus, Cochrane Library, Google Scholar, and ScienceDirect. The inclusion criteria were (1) all types of clinical studies and (2) the topic was COVID-19 and its association to the eye regarding the current guidelines.
Results:
From 168 abstracts screened, 61 papers fully filled the inclusion criteria after the full-text screening. The 61 records include 13 case reports, 17 prospective (case series or cross-sectional) studies, 8 retrospective studies, 12 literature reviews (one systematic review), and 11 letters to the editor. The majority of the papers agreed that ophthalmic manifestations due to COVID-19 were few and rarely encountered. The main ocular pathology seemed to be conjunctivitis, where the viral polymerase chain reaction also happened to be most detectable. Posterior segment or neuro-ophthalmic manifestations were scarce. Viral genome detection in the eye as well as viral portal of entry to the globe is still vague.
Conclusion:
The exact incidence of ocular manifestations in COVID-19 disease is uncertain. Conjunctivitis is the most prevalent ocular manifestation. It is still a debate whether the eye is a portal of entry for infection.
Keywords: Conjunctivitis, Contact lens, Novel coronavirus 2019, Ocular transmission, Retina, Severe acute respiratory syndrome coronavirus-2, Tear
INTRODUCTION
On December 31, 2019, Chinese authorities alerted the World Health Organization (WHO) about 41 of pneumonia-like cases of unknown origin and etiology detected in Wuhan City, Hubei Province, China. Symptoms of its infection are malaise, fever, dry cough, shortness of breath, and respiratory distress. They called the virus severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2).1
On January 30, 2020, the WHO was determined to declare a public health emergency of international concern,2 and on February 11, 2020, the WHO officially named the disease novel coronavirus 2019 (COVID-19).3
Severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus, and SARS-CoV-2 have also been known to cause other symptoms apart from the respiratory tract, including the ocular tissues and gastrointestinal tract.4 Although the majority of studies are focused on the respiratory tract because of its life-threatening nature, manifestations of COVID-19 in other systems should not be ignored as they may represent an alternative mode of transmission.5
A recent study stated that SARS-CoV-2 also uses the cell entry receptor, angiotensin-converting enzyme 2 (ACE2).6 Moreover, the activity and expression of ACE2 can be noticed in the ocular surface, including the cornea and conjunctiva, which provides transocular entry a possibility for COVID-19.7
Ocular manifestations of COVID-19 are reported in the form of increased conjunctival secretion, epiphora, and diminution of vision. Many infected cases were first presented by conjunctivitis before pneumonia occurred.8,9
Several concerns among the ophthalmologists have been raised about the potential ophthalmic manifestations associated with COVID-19. Hence, the focus has now been shifted toward evaluating the studies from China and other countries that highlight this association. Many imperative and indispensable queries need to be clarified, including the detailed ocular presentation, course, and its incidence in COVID-19 disease, the possibility of COVID-19 transmission through the tear, and the incidence of the disease among the contact lens (CL) users.
The available studies differ in their content and significance, causing further dilemmas among professionals. Hence, we designed this review to evaluate the currently available evidence for COVID-19 infection of ocular tissue in humans, as well as its ocular manifestations and treatment. Furthermore, we attempt to bridge the gap of knowledge in this field and put the base for further research from an ophthalmic point of view.
METHODS
We conducted a review of association between COVID-19 and the ocular manifestations, as well as the available therapeutic options and potential ocular toxicity in humans, based on the literature published till July 30, 2020, including the preprint and peer-reviewed studies. The following keywords used in various combinations were adapted for each search: “Coronaviruses” and “Ocular Implications” , “Ocular findings in coronavirus patients” , “Conjunctival infection in coronavirus patient” , “Coronavirus transmission by tears” , “Ocular manifestation for coronavirus” , “Coronavirus in tears” , “Coronavirus and eye” , “Coronaviruses and Ocular complications” , “COVID-19 and eye” , “Ophthalmic manifestation and COVID19” , “Contact lens and COVID-19” , and “Retina and COVID-19” . These search keywords were adapted for use in different databases: PubMed, Cochrane Library, ScienceDirect, Scopus, and Google scholar.
The search strategy was used to get the titles and the abstracts of the pertinent studies in any language as the first stage of search by two independent authors (S.S.E. and M.A.S.). Then, they retrieved the abstracts. In the second stage of the search, they reviewed the full text of articles to determine the suitability of implication. Dispute ruling was done by a third author (R.A.), and the final decision was taken by the first author (A.E.B.). The full-text articles were screened based on the following inclusion and exclusion criteria. The inclusion criteria were (1) clinical studies (case–control, case series, case report, or a cohort design). We also included reviews, viewpoints, commentaries, opinions, or letters to the editor. (2) The exposure of interest was COVID-19 and its association to the eye. We excluded the following: (1) papers studying in vitro ophthalmic manifestations and (2) studies with ocular manifestations but without the development of a complete clinical picture of COVID-19 or confirmation of coronavirus regarding the current guidelines. All authors collaborated in data extraction for further data analysis to convey the relevant data.
RESULTS
We started with a database of 168 papers in total. In Phase 1 screening (title and abstract screening), a total of 63 papers were excluded following the pre-established eligibility criteria. In Phase 2 screening (full-text screening), a total of 44 papers were excluded as they did not fulfill the criteria we were searching for. They did not report cases with ocular manifestations but mainly precautions and control measures for ophthalmologists to follow during this pandemic. Figure 1 represents the flowchart of the search steps and design. The final database included a total of 61 papers (43 original or primary studies and 18 secondary studies) on which we based our review. The 61 records include 13 case reports, 17 prospective (case series or cross-sectional) studies, 8 retrospective studies, 12 literature reviews (1 systematic review, and 11 letters to the editor).
Figure 1.
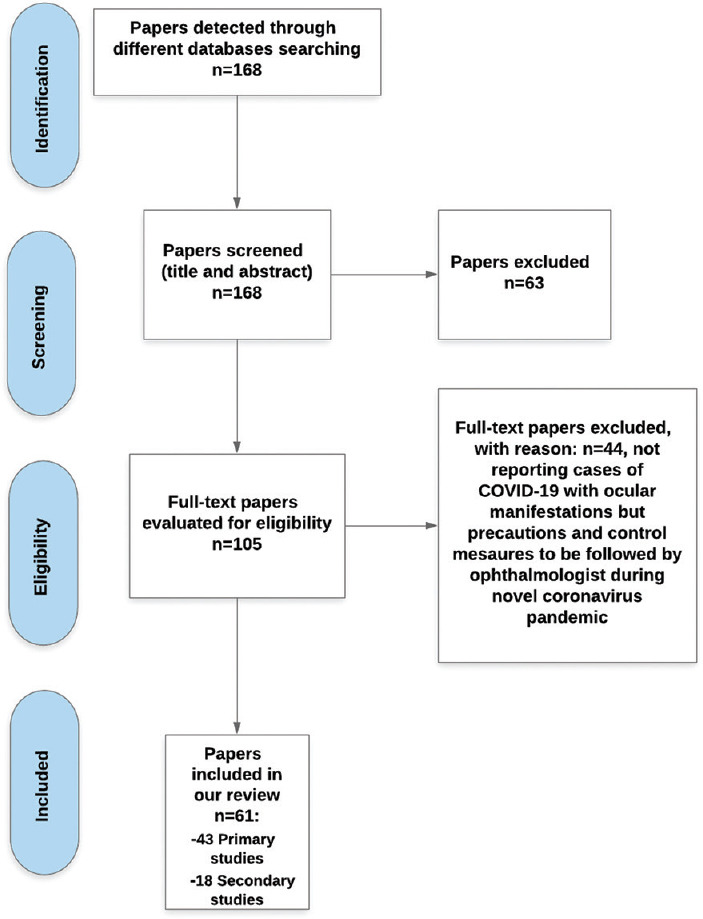
The flowchart of the search steps and design
Table 1 shows all included primary studies with the main findings of each study. Out of 18 secondary studies, we sufficed by just listing the important results of systematic reviews and meta-analysis studies in Table 2.
Table 1.
Primary studies included in the review*
| Author (s) | Country | Population/sample size | Inclusion criteria | Study design | Ocular symptoms |
|---|---|---|---|---|---|
| Xia et al. 9 | China | 30 patients Male: 70% Age: 54.50±14.17 years |
SARS-CoV-2-infected patients | Prospective interventional case series | Conjunctivitis in 1 patient (3%) with aqueous secretion |
| Cheema et al. 10 | Canada | A female aged 29 years | Not mentioned | Case report | Unilateral conjunctivitis, watery discharge then mucus discharge, and follicles Normal VA at the presentation and then declined owing to subepithelial infiltrates with overlying epithelial defects |
| Chen et al. 11 | China | A male aged 30 years | Not mentioned | Case report | Bilateral acute follicular conjunctivitis: moderate conjunctival injection, watery discharge, inferior palpebral follicles, tender palpable preauricular lymph nodes |
| Lan et al. 12 | China | 81 cases: Males: 40.7% Age: 41.69±18.6 years One of them was medical staff |
Confirmed COVID-19 patients | Prospective series of case studies | Conjunctivitis in 4% (3) patients who did not meet the typical features of viral conjunctivitis The time from the first symptom to the diagnosis was 3.33±1.15 days, and the ophthalmological consultation was carried out on the 25.67±1.53 days after diagnosis |
| Huang et al. 13 | China | 37 Chinese patients 12 severe cases, others were mild Age: 41-58 years |
Patients with laboratory-confirmed COVID-19 | Prospective cross-sectional study | Conjunctivitis in 3 cases (8%) only |
| Wu et al. 14 | China | 38 patients: Male: 65.8% Age: 65.8±16.6 years |
Patients with COVID-19 treated from February 9 to 15, 2020, at a hospital center | Retrospective case series | Ocular manifestations in 32% of the patients in the form of conjunctivitis |
| Zhang et al. 15 | China | 102 patients: Male: 47% Age: 57.63±14.90 years |
COVID-19 patients between December 30, 2019, and February 7, 2020 | Retrospective cross-sectional study | Bilateral conjunctivitis in 3% of 72 COVID-19 patients with watery discharges |
| Colavita et al. 16 | Italy | A 65-year-old female | Not mentioned | Case report | Bilateral conjunctivitis |
| Guan et al. 17 | China | 1099 patients from 552 hospitals Male: 58.1% Age: 47 years (35-58 years) Healthcare workers: 3.5% |
Confirmed COVID-19 patients, between December 11, 2019, and January 29, 2020 | Retrospective cohort | Conjunctivitis: in (0.8%): 4/1099 patients had severe COVID-19 and 5/1099 patients had non-severe disease |
| Marinho et al. 18 | Brazil | 12 adults Male: 50% 9 physicians 2 healthcare workers Age: 25-69 years |
SARS-CoV-2-infected patients | Case report | Retinal and OCT findings in the form of cotton wool spots and microhemorrhages along the retinal arcade in color fundus photograph (4 patients) Hyperreflective lesions at the level of ganglion cells and inner plexiform layers more prominently at the papillomacular bundle in both eyes Normal OCT-angiography |
| Leung et al. 19 | United States | 7 patients | Patients on hydroxychloroquine plus erlotinib therapy | Retrospective observational case series | 2 of 7 patients who received the high dose of HCQ developed macular abnormalities detected by retinal imaging and multifocal electroretinogram without visual symptoms |
| Chen et al.20 | China | 534 cases in China Male: 55.55% (conjunctivitis group) Age: 44 years (median) |
Patients were recruited from February 1 to March 1, 2020, not hospitalized, not severe cases, with smartphone and who accepted the questionnaire | Cross-sectional study | Conjunctivitis in 5% Hand-eye contact was independently correlated with conjunctivitis symptoms |
| Daruich et al.21 | Argentina | 27-year-old male | Not mentioned | Case report | Unilateral moderate conjunctivitis |
| Salducci and La Torre22 | Italy | An Italian patient: 72 years old with type 2 diabetes mellitus | Not mentioned | Case report | Bilateral conjunctivitis: serous secretions, conjunctival chemosis, and pseudomembranes on the tarsal conjunctiva |
| Karimi et al.23 | Iran | 43 patients: Male: 67.5% Age: 56±13 years (43-69 years) |
Clinically confirmed COVID-19 patients between March 28, 2020, and April 5, 2020 | Prospective case series | Bilateral conjunctivitis in 2.3% Mucus discharge, while another patient had bilateral foreign body sensation (2.3%) |
| Zhou et al.24 | China | 67 patients: majority were healthcare workers Male: 25 Age: 35.7±10.6 years |
Confirmed or suspected COVID-19 pneumonia during January 17-28, 2020 | Retrospective cohort study | Conjunctivitis |
| Sun et al.25 | China | 72 patients: Male: 50% Age: 58.68±14.81 years |
COVID-19 patients from December 30, 2019, to February 7, 2020, at Tongji Hospital | Single-center cross-sectional study | Bilateral conjunctivitis in 3% with watery discharges and without tenderness or enlargement of the preauricular lymph node |
| Navel et al.26 | France | A 63-year-old male | Not mentioned | Case report | Conjunctivitis was the first ocular manifestations Followed by follicles, tarsal hemorrhages, thin yellowish-white translucent pseudomembranes on the tarsal conjunctiva of lower lids, and superficial punctate keratitis |
| Deng et al.27 | China | 114 patients in China: Male: 54% Age: 61.4±16.7 years |
Patients with COVID-19 pneumonia recruited from February 3 to February 10, 2020, at Tongji Hospital | Observational study | No ocular complications or signs of ocular transmissible routes were reported |
| Zhang et al.28 | China | 14 confirmed, 16 suspected patients Age in the confirmed group: 48±13.4 years Age in the suspected group: 40±16.2 years |
All patients with diagnosed and suspected COVID-19 in Shenyang | Cross-sectional non-randomized study | Not mentioned |
| Xie et al.29 | China | 33 patients without ocular manifestation: Male: 66.7% Age: 57.6±14.0 years |
COVID-19 patients without any ocular manifestation from February 12 to 28, 2020 | Retrospective cohort study | SARS-CoV-2 might spread from normal conjunctiva of COVID-19 patients |
| Jun et al.30 | Singapore | 17 patients | SARS-COV-2-infected patients | Prospective study case series | Conjunctivitis in one patient |
| Dinkin et al. 31 | United States | 2 confirmed cases First case: a 36-year-old male Second case: a 71-year-old female |
Not mentioned | Case report | First case: a partial center oculomotor nerve palsy, bilateral abducens palsies, and bilateral distal leg paresthesias. The condition improved partially before discharge 3 days after admission Second case: right abducent (6th nerve) palsy, she presented with diplopia and defective abduction. The condition improved 2 weeks after discharge |
| Bostanci Ceran and Ozates32 | Turkey | 93 patients Male: 58.1% Age: 39.4±21.9 years |
Hospitalized and clinically confirmed COVID-19 patients between March 11 and April 30, 2020 | Cross-sectional study | 21.5% had at least one ocular manifestation: most common findings included hyperemia (20), epiphora (9), increased secretion (6), chemosis (3), follicular conjunctivitis (2), and episcleritis (2) The most common symptom was photophobia (15) |
| Abrishami et al. 33 | Iran | 142 patients Male: 54.2% Age: 62.6±15 years |
Consecutive patients with confirmed COVID-19 at the central referral center | Cross-sectional study | Ocular manifestations: in 65% patients Conjunctival hyperemia: 31%, conjunctival chemosis: 16%. Conjunctival chemosis was the most common ocular manifestation in ICU patients |
| Khavandi et al. 34 | Iran | A 65-year-old Caucasian diabetic male patient | Not mentioned | Letter to the editor | Follicular conjunctivitis with conjunctival chemosis and mucoid discharge |
| Nayak et al. 35 | India | A 65-year-old diabetic, hypertensive, and asthmatic patient on ventilator | Not mentioned | Case report | Unilateral severe follicular conjunctivitis Ocular signs without any complications |
| Ozturker36 | Turkey | A 32-year-old emergency healthcare worker | Not mentioned | Case report | Unilateral conjunctivitis and photophobia for 1 day |
| Scalinci and Trovato Battagliola37 | Italy | 5 confirmed cases 4 males: 41, 43, 65, 48 years One female: 37 years |
COVID-19-confirmed cases referred to their clinic | Case series | All showed only acute conjunctivitis: conjunctival hyperemia, epiphora, discharge, and photophobia |
| Méndez Mangana et al. 38 | Spain | A 31-year-old female | Not mentioned | Letter to the editor | Unilateral acute nodular episcleritis A slightly elevated nodule with hyperemia at the inferotemporal sector without or impaired VA |
| Gangaputra and Patel39 | United States | A total of 450 surveys 144 (32.0%) were positive COVID-19 |
Participants responded to the survey 1-4 weeks after receiving their COVID-19 test results (positive or negative) | Retrospective survey questionnaire | 47% of patients reported ocular symptoms Eye pain in 19%, photophobia in 14%, flashes or floaters in 12%, blurry vision in 11%, and red eyes in 10% 27% experiencing persistent eye symptoms despite recovery |
| Sindhuja et al. 40 | India | 127 mild COVID-19 patients Male: 88.98% Age: 38.8 years (5-73 years) |
Mild COVID-19-positive cases admitted between March 27 and April 19, 2020 | Retrospective cross-sectional study | 9% had ocular complaints (one case was excluded due to previous cataract surgery) 6% had conjunctivitis and conjunctival congestion |
| Guo et al. 41 | China | A 53-year-old male | Not mentioned | Case report | Unilateral viral conjunctivitis Relapsing bilateral keratoconjunctivitis 5 days after the complete relieve |
| Ying et al. 42 | Malaysia | A 54-year-old female | Not mentioned | Case report | Bilateral conjunctivitis lasting for 4 days |
| Pascual-Prieto et al. 43 | Spain | 8 cases of ophthalmoparesis | Not mentioned | Letter to the editor | D. 2 cases with unilateral 6th nerve palsy, one case of bilateral 6th nerve palsy, and one case of unilateral 4th nerve palsy E. One case of 4th nerve palsy: diagnosed only by clinical presentation C. 2 cases of unilateral 3rd nerve palsy and one case of unilateral 6th nerve palsy |
| Kumar et al. 44 | India | 45 patients Male: 78% Age: 31.26±12.8 years |
Confirmed COVID-19 cases with or without ocular symptoms | Case series | Not mentioned |
| Güemes-Villahoz et al. 45 | Spain | 36 patients (72 eyes) Male: 44% Age: 67.9 years |
Over the age of 18 years, positive RT?PCR test from nasopharyngeal swab, hospitalized because of COVID-19 and ability to give verbal consent | A cross-sectional study | 50% presented unilateral conjunctivitis and the other 50% were bilateral, 72% presented mild eye redness, 50% presented moderate secretions |
| Joob and Wiwanitkit46 | Thailand | 82 COVID-19 cases | Not mentioned | Letter to the editor | No patients had ocular manifestation The virus is a large virus and it is usually hard to secrete via exocrine gland |
| Atum et al. 47 | Turkey | 40 COVID-19 patients Male: 62.5% Age: 41.38±23.7 years |
Patients with positive PCR of nasopharyngeal and oropharyngeal swabs | Prospective interventional case series study | Conjunctivitis in 25% |
| Zhou et al. 48 | China | 121 patients Male: 44% Age: 8 years (22-89 years) Mild/moderate disease in 52% Severe disease in 48% |
COVID-19 patients from January 17 through February 16, 2020, in the Renmin Hospital | Case series study | Ocular symptoms in 6.6%, itching in 62.5%, redness in 37.5%, tearing in 37.5%, discharge in 25%, and foreign body sensation in 25%. 7 were severe cases and 1 was a mild/moderate case |
| Valente et al. 49 | Italy | 27 pediatric patients Male: 20 (74%) Age 84 months (8 days-210 months) |
Confirmed COVID-19 patients, hospitalized from March 16 to April 15, 2020 | Prospective observational case series study | Mild viral conjunctivitis in 15%. |
| Mungmungpuntipantip and Wiwanitkit50 | Thailand | 48 COVID-19 patients | SARS-COV-2-infected patients | Letter to the editor | No ocular manifestations in all patients examined in Thailand |
| Insausti-García et al. 51 | Spain | A 40-year-old White male patient | Not-mentioned | Case report | Unilateral papillophlebitis center persistent and painless decrease of the visual sensitivity, VA was 20/20 Severe inflammation of the ONH, retinal venous vasodilatation and tortuosity, cotton-wool spots, and moderate superficial hemorrhages in all four quadrants FA: venous staining, leakage, optic disc leakage, and late staining. No evidence of ischemia or peripheral vasculitis VF: a diffuse sensitivity decrease, a slight central scotoma, and a moderate increase in the blind spot OCT: papillary edema without macular edema. One week later: macular edema and VA decreased to 20/200 |
| Not mentioned | Not mentioned | Negative conjunctival swabs in patients without conjunctivitis | Antiviral drug | ||
| Started on the 3rd day of presentation while VA started to decline on the 6th day | Cervical lymphadenopathy | Not mentioned | Oral valacyclovir 500 mg and moxifloxacin eye drops | ||
| Started 13 days after illness onset, reduced on day 15, and resolved on day 19 | Not mentioned | Positive conjunctival swab 13 days after onset and lasted for 5 days Conjunctival specimens on days 13, 14, 17, and 19 showed decreasing levels of viral RNA |
Ribavirin eye drops | ||
| Ocular discomfort appeared on the 16.67±9.29 days after the diagnosis of COVID-19 | Not mentioned | Negative conjunctival PCR in both eyes of all 3 patients 78 patients with no ocular manifestations were not tested with PCR |
Not mentioned | ||
| Not mentioned | Not mentioned | Positive conjunctival swab in only one case of severe patients, but he had no conjunctivitis | Not mentioned | ||
| First symptom in 1 patient | Not mentioned | Positive nasopharyngeal PCR in 92% of ocular abnormality, positive conjunctival, and nasopharyngeal PCR in 17%, and positive conjunctival PCR in 5% | Not mentioned | ||
| Not mentioned | Not mentioned | Positive conjunctival swab of only one patient of the 2 patients with conjunctivitis | Ganciclovir eye drops | ||
| Started before fever and other symptoms, improved on day 15, and resolved on day 20 | Not mentioned | Ocular swabs: PCR was positive from the 3rd day up to day 21, with declining virus concentration The virus was still detected in ocular swabs days after it was no longer detectable in nasal ones |
Not mentioned | ||
| Not mentioned | Enlargement of lymph nodes in 0.2% patients | Not mentioned | Not mentioned | ||
| 11-33 days from the onset of COVID-19 symptoms | Not mentioned | Not mentioned | Not mentioned | ||
| Not mentioned | No risk factors | Not mentioned | Not mentioned | ||
| Conjunctivitis as initial symptom in 4 patients with a duration of 5.9±4.5 days/range 2-24 days | Not mentioned | Not mentioned | Ofloxacin, tobramycin, and ganciclovir eye drops | ||
| Conjunctivitis as the first presentation | Followed by severe respiratory symptoms | Not mentioned | A topical association of antibiotic and corticoids | ||
| Not-mentioned | Preauricular and submaxillary lymph nodes enlargement | Not mentioned | Cold compress, artificial tears, and topical ganciclovir 5 times/day for 7 days | ||
| Started 3 days before the onset of systemic manifestations | 95.3% had fever at the time of sampling | 7% of tear samples and 70% of nasopharyngeal samples were positive Positive tear RT-PCR in the patient with conjunctivitis but negative in the patient with ocular foreign body sensation All patients with positive tear RT-PCR had positive nasopharyngeal RT-PCR results |
Not mentioned | ||
| First symptom in one patient with a negative conjunctival swab | Not mentioned | 3 had positive conjunctival swab without ocular symptoms | Not mentioned | ||
| Not mentioned | Not mentioned | Positive conjunctival swab in one patient The time for sampling: 18.15±7.57 days |
Ganciclovir eye drops | ||
| Started on day 17 of illness and decreased from day 21 to day 26 | Not mentioned | On day 20, negative PCR of conjunctival scrapings and swabs for COVID-19 | Azithromycin eye drops twice/day for 3 days and dexamethasone with daily debridement of pseudomembranous | ||
| Not mentioned | Not mentioned | Negative PCR in conjunctival swabs of all patients | Not mentioned | ||
| Not mentioned | Not mentioned | All the conjunctival results of PCR-test were negative | Not mentioned | ||
| Not mentioned | Not mentioned | Strong positive results in both eyes of 2 out of the 33 (90-year-old female and 48-year-old male) | Not mentioned | ||
| Not mentioned | Not mentioned | Negative PCR in all tear samples (64 samples) collected over 3 weeks, suggesting that transmission through tears is low | Not mentioned | ||
| Not mentioned | Not mentioned | Not mentioned | Not mentioned | ||
| Not mentioned | Not mentioned | Not mentioned | Not mentioned | ||
| Ocular manifestations appeared after the systemic symptoms | Not mentioned | Not mentioned | Not mentioned | ||
| Not mentioned | Fever, dry cough, shortness of breath, and bilateral pneumonia appeared 2 days later | Not mentioned | Not mentioned | ||
| Symptoms had a late onset and resolved over 2 weeks | No data | Not mentioned | Not mentioned | ||
| Not mentioned | No other extraocular manifestations | Negative conjunctival swab but positive nasopharyngeal swab | Not mentioned | ||
| Not mentioned | No reported extraocular manifestations | Not mentioned | Moxifloxacin eye drops | ||
| Appeared 7 days after the systemic manifestations | Not mentioned | Not mentioned | Artificial tears and fluorometholone for 3 days, tapered over weeks | ||
| 20.6% showed ocular symptoms before systemic ones | Not mentioned | Not mentioned | Not mentioned | ||
| 45%: before systemic symptoms, 4 patients during the 1st week and one patient during the 3rd week | 55% developed systemic symptoms along with ocular manifestations | Not mentioned | Not mentioned | ||
| Started 10 days after the onset of disease and relieved 6 days after treatment | Not mentioned | Not mentioned | Glucocorticoids for 5 days | ||
| Not mentioned | No other systemic manifestation | Positive PCR | Not mentioned | ||
| C. Respiratory symptoms preceded diplopia by 2 weeks Respiratory symptoms preceded diplopia by 30 days |
Not mentioned | A. All had positive PCR B. No PCR was tested |
Not mentioned | ||
| Not mentioned | Not mentioned | 2% was positive for RT-PCR in the conjunctival swab | Not mentioned | ||
| The mean days since the disease onset until conjunctivitis manifestation: 8 days The mean duration of the conjunctivitis: 3 days |
Not mentioned | Positive conjunctival swab in 5.5%, only one of the 18 patients with conjunctivitis, and one patient without conjunctivitis | Not mentioned | ||
| Not mentioned | Not mentioned | Not mentioned | Not mentioned | ||
| Not mentioned | Not mentioned | Positive conjunctival swab in 7.5%, one case only had conjunctivitis | Not mentioned | ||
| Not mentioned | Not mentioned | Positive conjunctival swab in only one patient with ocular manifestations and two without manifestations, showed The positivity was not correlated significantly with the duration of disease |
Not mentioned | ||
| Resolution of conjunctivitis was after 3-5 days from onset | No mentioned | Positive conjunctival swab in one patient 2 patients were also positive but without conjunctivitis Swab became negative for all patients in 4 days |
Not mentioned | ||
| Not mentioned | Not mentioned | Not mentioned | Not mentioned | ||
| Appeared 6 weeks after the onset of the disease with gradual recovery of vision to 20/40 2 weeks later | Not mentioned | Not mentioned | Acetylsalicylic acid orally and bromfenac 0.9 mg/mL eye drops. Then sustained-release dexamethasone implant (Ozurdex, Allergan) |
*Primary studies: Means studies recording the primary study data. This is intended to answer scientific questions and to gain new knowledge. SARS-CoV-2: Severe acute respiratory syndrome coronavirus-2, VA: Visual acuity, COVID-19: Novel coronavirus 2019, RNA: Ribonucleic acid, PCR: Polymerase chain reaction, OCT: Optical coherence tomography, HCQ: Hydroxychloroquine, ICU: Intensive care unit, RT-PCR: Reverse transcription-PCR, ONH: Optic nerve head, FA: Fluorescein angiography, VF: Visual field
Table 2.
Selected secondary studies* “systematic reviews and meta-analysis” discussing novel coronavirus 2019 and eye
| Author (s) | Study design | Results |
|---|---|---|
| Ulhaq and Soraya52 | Letter to the editor | A meta-analysis was conducted The prevalence of ocular manifestations among COVID-19-infected patients ranged from 2% to 32% |
| Liu et al.53 | Letter to the editor | A meta-analysis of 62 studies Significant increased probability of conjunctivitis in non-severe stages of COVID-19 compared to severe subjects |
| Lawrenson and Buckley54 | Letter to the editor | A meta-analysis of selected 9 studies Conjunctivitis is a rare complication of COVID-19, with an estimated pooled prevalence of 4% or less. Virus presents in approximately 3% of tear/conjunctival swab samples |
| Aiello et al.55 | Systematic review of 11 studies | Conjunctivitis was demonstrated to be as high as 32% in one study, three patients had conjunctivitis with a positive tear-PCR, 8 patients had positive tear-PCR in the absence of conjunctivitis, and 14 had conjunctivitis with negative tear-PCR The majority of the available data regarding SARS-CoV-2 colonization of ocular tissues and secretions have to be considered controversial |
*Secondary studies mean studies on secondary results and involve the analysis of research which have already been performed and published. COVID-19: Novel coronavirus 2019, SARS-CoV: Severe acute respiratory syndrome coronavirus, SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus-2, PCR: Polymerase chain reaction
Among the 43 original studies we came upon on the final search, we found 29 about conjunctivitis, which seems to be the most prevalent ocular manifestation. Most of these studies also examined the positivity of polymerase chain reaction (PCR) of conjunctival swab/tear samples. Five studies reported mainly the viral PCR on tear film swabs, ocular transmission of SARS-CoV-2, and ACE2 expression in the conjunctiva. Other ocular manifestations were rarely encountered and reported in only 8 studies (2 studies reported keratoconjunctivitis/keratitis, 1 study reported acute nodular scleritis, 2 studies reported neuro-ophthalmic manifestations, and 2 studies reported the retinal and optic nerve findings). Besides ophthalmic medications, trial and side effects showed up in one study, while two studies confirmed the absence of ocular manifestations in COVID-19 patients.
Contact lens practice is one that can be greatly impacted by an infectious and transmissible disease. Although we did not find any original articles mentioning this dilemma, we addressed this issue based on the data in two review articles. The other secondary research (reviews and letters to the editor) dealt with ocular manifestations and ocular transmissions of SARS-CoV-2 comprehensively, and it was hard to classify them.
DISCUSSION
Consistent with previous studies, most patients with COVID-19 present with various respiratory symptoms, such as fever, dry cough, and chest tightness, that may rapidly progress to pneumonia.56,57 However, COVID-19 disease could present with distinguished extrapulmonary manifestations including diarrhea, headache, myalgia, or arthralgia. Some patients even present with asymptomatic infection.58
Yet, there is a large controversy about the relationship between SARS-CoV-2 virus and ocular pathology – conjunctivitis, in particular – as well as the infectivity and transmissibility of the virus from ocular surfaces. The authors categorized the virus impacts and the possible ocular manifestations in essential points based on the current literature.
Anterior segment impacts
Conjunctivitis has been convened to be the most prevalent ocular manifestation in COVID-19–infected patients. Authors depended on clinical examination and conjunctival swabs to detect the viral ribonucleic acid (RNA) by quantitative reverse transcription-polymerase chain reaction (RT-PCR).10,14,15
The exact pathogenesis of conjunctivitis is unclear. It may be purely a synchronous disease unrelated to the infection, or it may be correlated to SARS-CoV-2 infection.59 It could be through an exogenous mechanism. At the early phase of the disease, the local inflammatory invasion occurs and does not exceed 1 week. At the late stages, keratoconjunctivitis exacerbates due to cytokine surge.41
To date, the precise incidence of ocular manifestations in COVID-19 disease is uncertain. It ranges from 0.8%17 to 64%33 in different published studies. However, it could be stated that the majority recorded a percentage close to 3%,9,15,25 while other studies listed relatively far results (5%20 and 8%13 ). In contrast, some authors have emphasized that the ocular manifestation is an infrequent finding in COVID-19.46,50 The overall pooled prevalence of ocular manifestations among COVID-19 infected patients ranged from 4%54 to 5.5%,52 using the random-effects model, whereas the incidence of conjunctivitis varies in the pediatric population, reaching 15%.49 Pediatric ocular manifestations seem to be milder than that in adults. However, there is a possible existence of viral load in pediatric conjunctival secretions and therefore a risk of transmission. The different prevalence may be due to variations in population characteristics, sample size, study designs, and severity of COVID-19 cases.
Conjunctivitis could be unilateral10,21,35,36,38,45 or bilateral.11,15,16,22,23,25,42,45 With an overview of the published literature, no significant sex9,14,18,60 or age predilection9,18,61 could be assumed. Moreover, no evident correlation between the severity of the systemic viral infection and the seriousness of ocular disease might be highlighted. In spite of this, Wu et al. reported a frequency of about one-third more ophthalmic manifestations in patients with severe COVID-19.14 Chen et al. reported compatible results as well.20 Contrary to these studies, Liu et al. stated that the non-severe COVID-19 patients seem to be more likely to develop conjunctivitis. The probability of conjunctivitis between severe COVID-19 patients and not severe patients was statistically significant.53 This controversial issue may require further investigation.
The spectrum of acute conjunctivitis' signs and symptoms fluctuated reportedly from mild, such as foreign body sensation, conjunctival hyperemia, and tearing,11,22 to more severe photophobia, swollen eyelid, mucus secretions, chemosis, dry eye, and follicular reaction in forniceal conjunctiva.10,11,20,32,34,35 In addition, pseudomembranes of fibrin and inflammatory cells on the tarsal conjunctiva were noted by Salducci and La Torre22 and Navel et al.,26 who described follicles, petechiae, tarsal hemorrhages, chemosis, mucus filaments, and tarsal pseudomembranes. Systemic correlations were mostly preauricular, submaxillary, or cervical lymphadenopathy,10,11,17,22 in addition to the now well-known constitutional and respiratory symptoms of COVID-19 infection.
Laboratory findings were generally normal in COVID-19 patients with ocular signs. Whereas, Wu et al reported higher white blood cell (WBC) counts, neutrophils counts, and higher levels of procalcitonin, C-reactive protein, and lactate dehydrogenase in patients with ocular symptoms than in those without symptoms.14
The timing of conjunctivitis appearance throughout the COVID-19 course is still uncertain. Some authors reported late-onset conjunctivitis (10–13 days).11,35,41 Others reported synchronous conjunctivitis along with the systemic manifestations.12,14,40 Some authors described that acute conjunctivitis was the sole presenting sign without any systemic manifestations as an atypical presentation of COVID-19 disease,36,37,40,42 while it was the initial symptom in other reports.14,20,21,24,39
Usually, conjunctivitis resolves within 1–2 weeks without any complications.35,42,45,54 However, Guo et al.41 published a case report of relapsing bilateral keratoconjunctivitis 5 days after full recovery.
Keratitis
Corneal affection was seldom noticed. Mainly, superficial punctate keratitis was observed.26 Small pseudodendrites and small localized subepithelial infiltrates with overlying epithelial defects were remarked as well. They look like herpetic keratitis and evolved later into over 50 discrete areas of subepithelial infiltrates with overlying diffusely, spreading epithelial defects throughout the whole cornea.10
Episcleritis
Episcleritis was reported as a possible ocular complication of COVID-19.32,38 It may be due to immune-vascular inflammation and coagulation/thrombotic complications recorded in COVID-19 patients.38
Retinal and optic nerve impacts
There are almost no rigorous and satisfactory data on how the disease affects the retina or the optic nerve except for a recent study by Marinho et al.18 Retinal and optical coherence tomography (OCT) findings have been perceived in 12 cases; 9 confirmed by PCR (using nasal and oral swabs), and 2 tested positive in antibody tests for COVID-19. All patients appeared to have hyperreflective lesions at the level of ganglion cell and inner plexiform layers, especially at the papillomacular bundle zone in both eyes. OCT-angiography and ganglion cells complex analysis seemed ordinary. Besides, four patients had subtle cotton wool spots and microhemorrhages along the arterial arcades. The author suggested a potential association between ganglion cell and plexiform layer findings and central nervous system (CNS) manifestations.18
Another study illustrated unilateral papillophlebitis as a possible ocular complication of COVID-19. The condition started 6 weeks after the disease onset without macular edema. One week later, the macular edema developed and visual acuity (VA) decreased. There are three essential agents implicated the coagulopathy and venous occlusions in patients with COVID-19: endotheliitis, which leads to mechanical vasoconstriction, cytokine storm which activates clotting factors, and finally, stasis and hypoxia that stimulate coagulation mechanisms as well.51
The presence of SARS-CoV-2 in the retinal cells has been demonstrated in three human retinal biopsies out of 14 deceased COVID-19 patients. Retinal biopsies were prepared from the enucleated eyes. It is still unknown whether the virus replicates in the retina and in which retinal structures it is exactly located, as there is no evidence if SARS-CoV-2 is present in the choroid or vitreous. Blood is another probable source of the virus that cannot be fully excluded.62 Further studies are warranted to detect the existence of the SARS-CoV-2 virus in different ocular tissues to clarify this query.
Neuro-ophthalmological impacts
Cranial nerves
Interestingly, Dinkin et al.31 reported two COVID-19 patients with ophthalmoplegia within a few days of respiratory symptoms and the disease onset. Hence, they concluded worthy theories related to SARS-CoV-2 infection. They described a case of unilateral third nerve palsy that began partially before it was fully paralyzed. The condition was accompanied by leg paresthesia and bilateral sixth nerve palsy. They attributed this to an acute direct infection rather than demyelinating inflammatory neuropathy secondary to a virus-mediated immune response, based on the time of occurrence of neurological symptoms within a few days of disease onset. The second case was unilateral sixth nerve palsy with lacking radiological evidence of abducens nerve involvement and the presence of optic nerve sheath enhancement. They hypothesized that the viral leptomeningeal invasion or ischemic process was the cause. Both cases showed lymphopenia that seemed to be prevalent in patients with CNS manifestations.31
Another report declared eight cases of ophthalmoplegia in COVID-19 patients: three cases with unilateral sixth nerve palsy, one case of bilateral sixth nerve palsy, and two cases of unilateral fourth nerve palsy, and two cases of unilateral third nerve palsy.43
Visual acuity and pupillary reaction
The majority of the reviewed literature established that VA was within normal. In fact, few studies denoted a decline in VA that could be justified by viral keratitis.10 Other reasonable associated etiologies such as senile lens opacity or dry eye disease might vindicate.12 So far, neither pupillary light reflex anomalies nor relative afferent pupillary defect has been designated. Intraocular inflammation also appears implausible.10,18,26,61
Contact lens wear during novel coronavirus 2019
Notably, COVID-19 is not correlated with CLs; however, it has been suggested that silicone lenses are more prone to bind the pathogen than hydrogels.63 Nevertheless, in the pandemic of coronavirus, CL practice has become more sophisticated to reduce the transmission rate of COVID-19 in CL users.64
Therapeutic options of ophthalmic manifestations and potential ocular toxicity
A variety of treatments have been applied to treat ophthalmic manifestations, mainly antiviral drugs such as ganciclovir eye drop15,20,25 or gel combined with artificial tears,22,38 ribavirin eye drop,11 oral acyclovir 500 mg in conjunction with moxifloxacin eye drop based on a presumed diagnosis of herpetic keratoconjunctivitis.10,37 Topical antibiotics and corticosteroids have been used,21,38 azithromycin eye drops with low-dose dexamethasone and pseudomembranes debridement have been used as well.26 However, the majority of cases resolved spontaneously.
Chloroquine (CQ) and hydroxychloroquine (HCQ) have been recommended in the treatment of COVID-19 infection in many protocols. Suggested doses for the treatment of COVID-19 (1000 mg/day for 10 days, CQ; 800 mg 1st day and then 400 mg/day for 5 days, HCQ) in many guidelines worldwide are considerably higher than the maximum recommended daily safe doses of both drugs (2.3 mg/kg/day, CQ; 5.0 mg/kg/day, HCQ). Otherwise, the development of retinal toxicity is potential. Irreversible retinal damage can occur if the exposure to the safe doses is for more than 5 years, and visual loss despite ceasing prescription is inevitable. Nevertheless, Leung et al. found that two of seven cases of COVID-19 who were treated with the high dose of HCQ showed abnormalities of the macula on the retinal imaging and multifocal electroretinogram without visual symptoms.19 It is worth mentioning that the newest investigations fight against the use of those two agents in the treatment protocols due to their ineffectiveness and impending harm.65,66
Detection of severe acute respiratory syndrome coronavirus-2 in ocular secretions and ocular transmission
Another important structure that seemingly holds the virus is the tear film. The ocular surface may represent a portal of entry for the SARS-CoV-2 in the human body.50 The isolation of SARS-CoV-2 in the tear samples in patients with confirmed COVID-19 has prompted concerns that the virus could be transmitted through ocular secretions as well as by fomite transmission to the eyes via contaminated hand.67
Conjunctival swabs for RT-PCR showed positivity in concurrence with conjunctivitis61 or in isolation.9 In a study on 67 patients, conjunctival swab samples from one patient showed positive RT-PCR results, and two other patients had probable positive results lacking ocular symptoms.24 Another study of 72 COVID-19 patients concluded that SARS-CoV-2 was spotted in the conjunctival secretions' samples of only one patient who was an emergency department nurse.25 On the contrary, a case series in Singapore disclosed the absence of SARS-CoV-2 RNA in conjunctival secretions through RT-PCR, where multiple tear samples were collected from 17 cases.30 In agreement, others have concluded the absence of COVID-19 virus in tears samples.12,16,23,27,28,63 Evidence of viral particles in tears/conjunctiva is low. It presents in approximately 3% of tear/conjunctival swab samples54 and ranges from 0% to 7.14%.68
Studies have been conducted to isolate the virus from conjunctival and tear swabs,13,14,15,25 the above studies confirmed positive SARS-CoV-2 RT-PCR results from very few patients' tears, but the isolation of the virus was unsuccessful. This could be due to the hardness of collecting tears and further analysis. Besides, the virus has a large size and usually difficult to secrete via exocrine gland.46
Some patients showed ocular symptoms during the disease course without evidence of SARS-CoV-2 in the tear samples, thus hypothesizing that SARS-CoV-2 can exist in tears or the conjunctival sac.55 Otherwise, viral shedding in the ocular secretions has been demonstrated in asymptomatic patients without ocular disease.55,69
Although the ocular transmission of SARS-CoV-2 is theoretically possible, it remains uncertain, and there is a debate over its spread via tears.20,52 Some authors have emphasized that the eye could be an important window for infection via respiratory droplets, as well as the possibility of ocular transmission by infected tears or secretions.67,70,71 On the other hand, others have asserted that SARS-CoV-2 is unlikely to be transmitted via the conjunctiva or ocular secretions.69,72 Moreover, the high positivity rate of nasopharyngeal swabs by RT-PCR in contrary to its conjunctiva/tear counterpart makes the estimation that the nasolacrimal duct is an important entry gate for the virus doubtful.67 The pooled sensitivity of the ocular tissue/fluid in identifying SARS-CoV-2 is still very low (0.6%) compared with standard sample collection from nasopharyngeal swab/sputum.52
On the other hand, some critical points should be kept in mind. First, although PCR is a widely accepted means to spot viral genome, the shedding loads of the SARS-CoV-2 are perhaps below the sensitivity of the test, even with repeated sampling,9 or are actually not shedding at the time of sampling.30 Second, most studies sampled ocular tissue/secretions after the onset of respiratory and systemic disease. However, ocular manifestations have been reported to begin few hours8 or days before respiratory symptoms instigate.21,23,25 Furthermore, conjunctival swabs have occurred to regain positivity after nasopharyngeal swabs were negative and conjunctivitis regressed,16 suggesting that nasopharyngeal and conjunctival swabs should be obtained in concurrence.30 Third, since COVID-19 patients have viremia during the acute phase, the positivity of SARS-CoV-2 RNA is probably the result of viral exudation in the conjunctiva rather than actual replication.60 Fourth, SARS-CoV-2 is anticipated to bind its spikes to ACE2 as a cellular receptor to gain entry into cell's insides as other SARS-CoV strains.28 Current evidence on whether conjunctival epithelium can express ACE2 is still scarce. ACE2 is expressed in the conjunctival samples at a low level (range, 0.26–1% of elongation factor 1α [Elα1a]) and at a lower expression level in the cornea (0.1% of El1α).73 However, upper respiratory tract epithelia, such as the oral, nasal, and nasopharynx mucosa did not express ACE2 receptors on the epithelial surface, proposing that these tissues are not entrance gateways for SARS-CoV-2.5,74 The same debate is applied for the contribution of sialylated ocular mucins to host defense against viral pathogens as well as the role of the interdependence of the ocular mucosal immune system with nasal cavity-associated lymphoid tissue in the nasolacrimal ducts. These topics merit further investigations.61 Fifth, the conjunctiva and the cornea can adopt antiviral countermeasures.73 Lactoferrin in tears can inhibit the virus binding as may decoy receptors such as 9-O-acetylated sialic acid on tear glycoproteins, which may explain the low prevalence of eye involvement.75
In conclusion, conjunctivitis or keratoconjunctivitis is the most customary ocular manifestation in COVID-19. Nonetheless, in the pandemic of COVID-19, the occurrence of other ocular manifestations as a retinal impact or ophthalmoplegia should pay concerns to SARS-CoV-2 infection even in patients with mild symptoms or signs. In addition, the deficient and imprecise ophthalmic data call attention to the necessity of ophthalmologists in evaluating and screening COVID-19 patients with neuro-ophthalmic manifestation. Further studies are required urgently to understand the natural course of ocular hazers during COVID-19 pandemics as well as prognostic significance of used treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol. 2020;92:401–2. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Novel Coronavirus (2019-nCoV) Situation Report-11 (31 January 2020) Geneva, Switzerland: World Health Organization; 2020. [Last accessed on 2020 Oct 11]. Available from: https://www.who.int/docs/defaultsource/coronaviruse/situation-reports/20200131-sitrep-11-ncov.pdf?sfvrsn=de7c0f7_4 . [Google Scholar]
- 3.World Health Organization. Novel Coronavirus (2019-nCoV) Situation Report-22 (11 February 2020) Geneva, Switzerland: World Health Organization; 2020. [Last accessed on 2020 Oct 11]. Available from: https://apps.who.int/iris/handle/10665/330991 . [Google Scholar]
- 4.Gralinski LE, Baric RS. Molecular pathology of emerging coronavirus infections. J Pathol. 2015;235:185–95. doi: 10.1002/path.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seah I, Agrawal R. Can the coronavirus disease 2019 (COVID-19) affect the eyes.A review of coronaviruses and ocular implications in humans and animals. Ocul Immunol Inflamm. 2020;28:391–5. doi: 10.1080/09273948.2020.1738501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94:1–9. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma D, Chen CB, Jhanji V, Xu C, Yuan XL, Liang JJ, et al. Expression of SARS-CoV-2 receptor ACE2 and TMPRSS2 in human primary conjunctival and pterygium cell lines and in mouse cornea. Eye (Lond) 2020;34:1212–9. doi: 10.1038/s41433-020-0939-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu CW, Liu XF, Jia ZF. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020;395:E39. doi: 10.1016/S0140-6736(20)30313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia J, Tong J, Liu M, Shen Y, Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020;92:589–94. doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheema M, Aghazadeh H, Nazarali S, Ting A, Hodges J, McFarlane A, et al. Keratoconjunctivitis as the initial medical presentation of the novel coronavirus disease 2019 (COVID-19) Can J Ophthalmol. 2020;55:e125–9. doi: 10.1016/j.jcjo.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Liu M, Zhang Z, Qiao K, Huang T, Chen M, et al. Ocular manifestations of a hospitalised patient with confirmed 2019 novel coronavirus disease. Br J Ophthalmol. 2020;104:748–51. doi: 10.1136/bjophthalmol-2020-316304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lan QQ, Zeng SM, Liao X, Xu F, Qi H, Li M. Screening for novel coronavirus related conjunctivitis among the patients with corona virus disease-19. Zhonghua Yan Ke Za Zhi. 2020;56:E009. doi: 10.3760/cma.j.cn112142-20200322-00213. [DOI] [PubMed] [Google Scholar]
- 13.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu P, Duan F, Luo C, Liu Q, Qu X, Liang L, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in hubei province, China. JAMA Ophthalmol. 2020;138:575–8. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Chen X, Chen L, Deng C, Zou X, Liu W, et al. The evidence of SARS-CoV-2 infection on ocular surface. Ocul Surf. 2020;18:360–2. doi: 10.1016/j.jtos.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colavita F, Lapa D, Carletti F, Lalle E, Bordi L, Marsella P, et al. SARS-CoV-2 isolation from ocular secretions of a patient with COVID-19 in Italy with prolonged viral RNA detection. Ann Intern Med. 2020;173:242–3. doi: 10.7326/M20-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marinho PM, Marcos AA, Romano AC, Nascimento H, Belfort R., Jr Retinal findings in patients with COVID-19. Lancet. 2020;395:1610. doi: 10.1016/S0140-6736(20)31014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung LS, Neal JW, Wakelee HA, Sequist LV, Marmor MF. Rapid onset of retinal toxicity from high-dose hydroxychloroquine given for cancer therapy. Am J Ophthalmol. 2015;160:799–8050. doi: 10.1016/j.ajo.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Chen L, Deng C, Chen X, Zhang X, Chen B, Yu H, et al. Ocular manifestations and clinical characteristics of 535 cases of COVID-19 in Wuhan, China: A cross-sectional study. Acta Ophthalmol. 2020 doi: 10.1111/aos.14472. Published online ahead of print/ DOI: 101111/aos14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daruich A, Martin D, Bremond-Gignac D. Unilateral conjunctivitis as first presentation of Coronavirus Disease 2019 (COVID-19): A telemedicine diagnosis. J Fr Ophtalmol. 2020;43:e167–8. doi: 10.1016/j.jfo.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salducci M, La Torre G. COVID-19 emergency in the cruise's ship: A case report of conjunctivitis. Clin Ter. 2020;171:e189–91. doi: 10.7417/CT.2020.2212. [DOI] [PubMed] [Google Scholar]
- 23.Karimi S, Arabi A, Shahraki T, Safi S. Detection of severe acute respiratory syndrome coronavirus.2 in the tears of patients with coronavirus disease 2019. Eye (Lond) 2020;34:1220–3. doi: 10.1038/s41433-020-0965-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou YY, Zeng YY, Tong YQ, Chen CZ. Ophthalmologic evidence against the interpersonal transmission of 2019 novel coronavirus through conjunctiva. Unpublished (PREPRINT) from medRxiv. 2020 posted online: Feb.12.2020. [Google Scholar]
- 25.Sun X, Zhang X, Chen X, Chen L, Deng C, Zou X, et al. The infection evidence of SARS-COV-2 in ocular surface: A single-center cross-sectional study. Unpublished (PREPRINT) from medRxiv. 2020 Posted online: Feb.26.2020. [Google Scholar]
- 26.Navel V, Chiambaretta F, Dutheil F. Haemorrhagic conjunctivitis with pseudomembranous related to SARS-CoV-2. Am J Ophthalmol Case Rep. 2020;19:100735. doi: 10.1016/j.ajoc.2020.100735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng C, Yang Y, Chen H, Chen W, Chen Z, Ma K, et al. Ocular Detection of SARS-CoV-2 in 114 Cases of COVID-19 Pneumonia in Wuhan, China: An Observational Study (2/19/2020) [Last accessed on 2020 Oct 11]. Available at SSRN: https://ssrn.com/abstract=3543587 .
- 28.Zhang X, Song W, Sun B, Mu J, Dong X, Wang B. Conjunctival polymerase chain reaction-tests of 2019 novel coronavirus in patients in Shenyang, China. Unpublished (PREPRINT) from medRxiv. 2020 Posted online: Feb.25.2020. [Google Scholar]
- 29.Xie HT, Jiang SY, Xu KK, Liu X, Xu B, Wang L, et al. SARS-CoV-2 in the ocular surface of COVID-19 patients. Eye Vis (Lond) 2020;7:23. doi: 10.1186/s40662-020-00189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jun IS, Anderson DE, Kang AE, Wang LF, Rao P, Young BE, et al. Assessing viral shedding and infectivity of tears in coronavirus disease 2019 (COVID-19) patients. Ophthalmology. 2020;127:977–979. doi: 10.1016/j.ophtha.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dinkin M, Gao V, Kahan J, Bobker S, Simonetto M, Wechsler P, et al. COVID-19 presenting with ophthalmoparesis from cranial nerve palsy. Neurology. 2020;95:221–3. doi: 10.1212/WNL.0000000000009700. [DOI] [PubMed] [Google Scholar]
- 32.Bostanci Ceran B, Ozates S. Ocular manifestations of coronavirus disease 2019. Graefes Arch Clin Exp Ophthalmol. 2020;258:1959–63. doi: 10.1007/s00417-020-04777-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abrishami M, Tohidinezhad F, Daneshvar R, Omidtabrizi A, Amini M, Sedaghat A, et al. Ocular manifestations of hospitalized patients with COVID-19 in Northeast of Iran. Ocul Immunol Inflamm. 2020;28:739–44. doi: 10.1080/09273948.2020.1773868. [DOI] [PubMed] [Google Scholar]
- 34.Khavandi S, Tabibzadeh E, Naderan M, Shoar S. Corona virus disease-19 (COVID-19) presenting as conjunctivitis: Atypically high-risk during a pandemic. Cont Lens Anterior Eye. 2020;43:211–2. doi: 10.1016/j.clae.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nayak B, Poddar C, Panigrahi MK, Tripathy S, Mishra B. Late manifestation of follicular conjunctivitis in ventilated patient following COVID-19 positive severe pneumonia. Indian J Ophthalmol. 2020;68:1675–7. doi: 10.4103/ijo.IJO_1682_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ozturker ZK. Conjunctivitis as sole symptom of COVID.19: A case report and review of literature. Eur J Ophthalmol. 2020 doi: 10.1177/1120672120946287. DOI: “https://doi.org/10.1177/1120672120946287”10.1177/1120672120946287; Posted online: Jul.24.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scalinci SZ, Trovato Battagliola E. Conjunctivitis can be the only presenting sign and symptom of COVID-19. IDCases. 2020;20:e00774. doi: 10.1016/j.idcr.2020.e00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Méndez Mangana C, Barraquer Kargacin A, Barraquer RI. Episcleritis as an ocular manifestation in a patient with COVID.19. Acta Ophthalmol. 2020 doi: 10.1111/aos.14484. Published online ahead of print/ DOI: 10.1111/aos.14484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gangaputra SS, Patel SN. Ocular symptoms among nonhospitalized patients who underwent COVID-19 testing. Ophthalmology. 2020;127:1425–7. doi: 10.1016/j.ophtha.2020.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sindhuja K, Lomi N, Asif MI, Tandon R. Clinical profile and prevalence of conjunctivitis in mild COVID-19 patients in a tertiary care COVID-19 hospital: A retrospective cross-sectional study. Indian J Ophthalmol. 2020;68:1546–50. doi: 10.4103/ijo.IJO_1319_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo D, Xia J, Wang Y, Zhang X, Shen Y, Tong JP. Relapsing viral keratoconjunctivitis in COVID-19: A case report. Virol J. 2020;17:97. doi: 10.1186/s12985-020-01370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ying NY, Idris NS, Muhamad R, Ahmad I. Coronavirus Disease 2019 Presenting as Conjunctivitis. Korean J Fam Med. 2020 doi: 10.4082/kjfm.20.0090. Epubahead of print/ published online: June 1, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pascual-Prieto J, Narváez-Palazón C, Porta-Etessam J, Gómez-de-Liaño R. COVID-19 epidemic: Should ophthalmologists be aware of oculomotor paresis? Arch Soc Esp Oftalmol. 2020;95:361–2. doi: 10.1016/j.oftal.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar K, Prakash AA, Gangasagara SB, Rathod SB, Ravi K, Rangaiah A, et al. Presence of viral RNA of SARS-CoV-2 in conjunctival swab specimens of COVID-19 patients. Indian J Ophthalmol. 2020;68:1015–7. doi: 10.4103/ijo.IJO_1287_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Güemes-Villahoz N, Burgos-Blasco B, Arribi-Vilela A, Arriola-Villalobos P, Rico-Luna CM, Cuiña-Sardiña R, et al. Detecting SARS-CoV-2 RNA in conjunctival secretions: Is it a valuable diagnostic method of COVID-19? J Med Virol. 2020:1–6. doi: 10.1002/jmv.26219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joob B, Wiwanitkit V. COVID-19, ocular manifestation and ophthalmic risks. J Fr Ophtalmol. 2020;43:e231. doi: 10.1016/j.jfo.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atum M, Boz AA, Çakir B, Karabay O, Köroglu M, Ögütlü A, et al. Evaluation of conjunctival swab PCR results in patients with SARS-CoV-2 infection. Ocul Immunol Inflamm. 2020;28:745–8. doi: 10.1080/09273948.2020.1775261. [DOI] [PubMed] [Google Scholar]
- 48.Zhou Y, Duan C, Zeng Y, Tong Y, Nie Y, Yang Y, et al. Ocular findings and proportion with conjunctival SARS-COV-2 in COVID-19 patients. Ophthalmology. 2020;127:982–3. doi: 10.1016/j.ophtha.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valente P, Iarossi G, Federici M, Petroni S, Palma P, Cotugno N, et al. Ocular manifestations and viral shedding in tears of pediatric patients with coronavirus disease 2019: a preliminary report. J AAPOS. 2020:1–4. doi: 10.1016/j.jaapos.2020.05.002. Article in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mungmungpuntipantip R, Wiwanitkit V. Ocular manifestation, eye protection, and COVID-19. Graefes Arch Clin Exp Ophthalmol. 2020;258:1339. doi: 10.1007/s00417-020-04662-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Insausti-García A, Reche-Sainz JA, Ruiz-Arranz C, López Vázquez Á, Ferro-Osuna M. Papillophlebitis in a COVID-19 patient: Inflammation and hypercoagulable state. Eur J Ophthalmol. doi: 10.1177/1120672120947591. Epub ahead of print / Published Online: July 30 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ulhaq ZS, Soraya GV. The prevalence of ophthalmic manifestations in COVID-19 and the diagnostic value of ocular tissue/fluid. Graefes Arch Clin Exp Ophthalmol. 2020;258:1351–2. doi: 10.1007/s00417-020-04695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu M, Dai C, Lv X, Li B. Letter to the Editor: Are severe COVID-19 patients more susceptible to conjunctivitis? J Med Virol. 2020;92:2394–5. doi: 10.1002/jmv.26084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lawrenson JG, Buckley RJ. COVID-19 and the eye. Ophthalmic Physiol Opt. 2020;40:383–8. doi: 10.1111/opo.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aiello F, Gallo Afflitto G, Mancino R, Li JO, Cesareo M, Giannini C, et al. Coronavirus disease 2019 (SARS-CoV-2) and colonization of ocular tissues and secretions: A systematic review. Eye (Lond) 2020;34:1206–11. doi: 10.1038/s41433-020-0926-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–33. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in germany. N Engl J Med. 2020;382:970–1. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo D, Xia J, Shen Y, Tong J. SARS-CoV-2 may be related to conjunctivitis but not necessarily spread through the conjunctiva SARS-CoV-2 and conjunctiva. J Med Virol. 2020;92:1757–58. doi: 10.1002/jmv.25856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peng Y, Zhou YH. Is novel coronavirus disease (COVID-19) transmitted through conjunctiva? J Med Virol. 2020;92:1408–9. doi: 10.1002/jmv.25753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Belser JA, Rota PA, Tumpey TM. Ocular tropism of respiratory viruses. Microbiol Mol Biol Rev. 2013;77:144–56. doi: 10.1128/MMBR.00058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Casagrande M, Fitzek A, Püschel K, Aleshcheva G, Schultheiss HP, Berneking L, et al. Detection of SARS-CoV-2 in human retinal biopsies of deceased COVID-19 patients. Ocul Immunol Inflamm. 2020;28:721–5. doi: 10.1080/09273948.2020.1770301. [DOI] [PubMed] [Google Scholar]
- 63.Jones L, Walsh K, Willcox M, Morgan P, Nichols J. The COVID-19 pandemic: Important considerations for contact lens practitioners. Cont Lens Anterior Eye. 2020;43:196–203. doi: 10.1016/j.clae.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zeri F, Naroo SA. Contact lens practice in the time of COVID-19. Cont Lens Anterior Eye. 2020;43:193–5. doi: 10.1016/j.clae.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guastalegname M, Vallone A. Could chloroquine /hydroxychloroquine be harmful in Coronavirus Disease 2019 (COVID-19) treatment? Clin Infect Dis. 2020;71:888–9. doi: 10.1093/cid/ciaa321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saleh M, Gabriels J, Chang D, Soo Kim B, Mansoor A, Mahmood E, et al. Effect of chloroquine, hydroxychloroquine, and azithromycin on the corrected QT interval in patients with SARS-CoV-2 infection. Circ Arrhythm Electrophysiol. 2020;13:e008662. doi: 10.1161/CIRCEP.120.008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun CB, Wang YY, Liu GH, Liu Z. Role of the eye in transmitting human coronavirus: What we know and what we do not know. Front Public Health. 2020;8:155. doi: 10.3389/fpubh.2020.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Emparan JP, Sardi-Correa C, López-Ulloa JA, Viteri-Soria J, Penniecook JA, Jimenez-Román J, et al. COVID-19 and the eye: How much do we really know.A best evidence reviews? Arq Bras Oftalmol. 2020;83:250–61. doi: 10.5935/0004-2749.20200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ho D, Low R, Tong L, Gupta V, Veeraraghavan A, Agrawal R. COVID-19 and the ocular surface: A review of transmission and manifestations. Ocul Immunol Inflamm. 2020;28:726–34. doi: 10.1080/09273948.2020.1772313. [DOI] [PubMed] [Google Scholar]
- 70.Dockery DM, Rowe SG, Murphy MA, Krzystolik MG. The ocular manifestations and transmission of COVID-19: Recommendations for prevention. J Emerg Med. 2020;59:137–40. doi: 10.1016/j.jemermed.2020.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Amesty MA, Alió Del Barrio JL, Alió JL. COVID-19 disease and ophthalmology: An update. Ophthalmol Ther. 2020;9:1–2. doi: 10.1007/s40123-020-00260-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu Z, Sun CB. Conjunctiva is not a preferred gateway of entry for SARS-CoV-2 to infect respiratory tract. J Med Virol. 2020;92:1410–2. doi: 10.1002/jmv.25859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leonardi A, Rosani U, Brun P. Ocular surface expression of SARS-CoV-2 receptors. Ocul Immunol Inflamm. 2020;28:735–8. doi: 10.1080/09273948.2020.1772314. [DOI] [PubMed] [Google Scholar]
- 74.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–7. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Willcox MD, Walsh K, Nichols JJ, Morgan PB, Jones LW. The ocular surface, coronaviruses and COVID-19. Clin Exp Optom. 2020;103:418–24. doi: 10.1111/cxo.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]


