Abstract
Background:
There remains a paucity of real-world observational evidence comparing percutaneous coronary intervention (PCI) to coronary artery bypass grafting (CABG) in patients with diabetes and multivessel coronary artery disease (CAD).
Objectives:
To compare early and long-term outcomes of PCI versus CABG in patients with diabetes.
Methods:
Clinical and administrative databases in Ontario, Canada, were linked to obtain records of all diabetic patients with angiographic evidence of 2-vessel or 3-vessel CAD who were treated with either PCI or isolated CABG from 2008–2017. 1:1 propensity score matching was performed to account for baseline differences. All-cause mortality and the composite of myocardial infarction, repeat revascularization, stroke, or death (termed major cardiovascular and cerebrovascular events [MACCE]) were compared between the matched groups using a stratified log rank test and Cox-proportional hazards model.
Results:
A total of 4,519 and 9,716 patients underwent PCI and CABG respectively. Prior to matching, CABG patients were significantly younger (65.7 vs 68.3 years), more likely male (78% vs 73%) and had more severe CAD. Propensity score matching based on 23 baseline covariates yielded 4,301 well-balanced pairs. There was no difference in early mortality between PCI and CABG (2.4% vs 2.3%, p=0.721) after matching. The median and maximum follow-up were 5.5 and 11.5 years respectively. All-cause mortality (hazard ratio (HR) 1.39, 95%CI; 1.28–1.51) and overall MACCE (HR 1.99, 95%CI; 1.86–2.12) were significantly higher with PCI compared to CABG.
Conclusions:
In patients with multivessel CAD and diabetes, compared to PCI, CABG was associated with improved long-term mortality and freedom from MACCE.
Keywords: diabetes, percutaneous coronary intervention, coronary artery bypass grafting, propensity score
CONDENSED ABSTRACT
A propensity score matched analysis comparing percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) in 4,301 patient-pairs with diabetes and multivessel coronary artery disease (CAD) was undertaken utilizing clinical and administrative databases housed in Ontario, Canada. While there was no difference in early mortality between PCI and CABG (2.4% vs 2.3%, p=0.721), the rate of all-cause mortality over the entire follow-up was significantly higher with PCI (hazard ratio (HR): 1.39, 95%CI; 1.28–1.51) compared to CABG. Overall composite of major adverse cardiac and cerebrovascular events was higher with PCI compared to CABG (HR: 1.99, 95%CI; 1.86–2.12).
INTRODUCTION
Clinical trials in the late 1990s to early 2000s comparing percutaneous coronary interventions (PCI) to coronary artery bypass grafting (CABG) demonstrated improved outcomes with CABG in diabetic patients in post-hoc subgroup analyses. The Bypass Angioplasty Revascularization (BARI) trial showed a mortality benefit with CABG compared to balloon angioplasty while the Arterial Revascularization Therapy Study (ARTS-I) demonstrated a higher incidence of repeated revascularization with PCI over CABG.(1, 2) Subgroup analyses of the BARI-2D trial showed that patients with diabetes who underwent surgical revascularization had fewer major adverse cardiac events than medical therapy.(3) Similarly, a subgroup analysis of diabetic patients in the Synergy Between Percutaneous Coronary Intervention With TAXUS and Cardiac Surgery (SYNTAX) trial showed both a reduction in repeat revascularization and major adverse cardiac and cerebrovascular events (MACCE) with CABG over PCI.(4)
The Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Diseases (FREEDOM) trial was designed to compare PCI performed using drug eluting stents (DES) to CABG in diabetic patients with multi-vessel disease.(5) The trial randomized 1900 diabetic patients to intervention with PCI-DES or CABG, and demonstrated a mortality benefit and reduced risk of non-fatal MI favoring CABG. The FREEDOM trial led to a change in the 2014 AHA/ACC guideline recommendations for the revascularization of stable ischemic heart disease, indicating that patients with diabetes and complex 3-vessel disease or 2-vessel disease with proximal left anterior descending (LAD) artery involvement, who are good surgical candidates, should be treated with CABG (2012: Class IIa level of evidence (LOE): B to 2014: Class I LOE: B).(6) Similar recommendations were also made in 2014 in the ESC/EACTS European guidelines, with a higher level of evidence in their recommendation for CABG over PCI in diabetic patients with multivessel disease (Class I, LOE: A).(7) More recently, the FREEDOM follow-on study demonstrated improved survival with CABG at mean 7.5 years follow-up in the original FREEDOM cohort.(8)
Despite strong RCT evidence supporting the use of CABG over PCI in diabetic patients with multivessel disease, it remains unclear if the efficacy observed in randomized clinical trials translates to comparative effectiveness in the more heterogeneous population in real world practice. Our objectives were to compare the primary outcome of long-term mortality and the secondary outcome of a composite of long-term MACCE at the population level between PCI and CABG in diabetic patients with multivessel coronary artery disease.
METHODS
Study design
We conducted a retrospective analysis of records for all patients in the province of Ontario, Canada, who had invasive coronary angiography from October 1, 2008 to December 31, 2018 and subsequently underwent revascularization with CABG or PCI within 90 days of the index angiography. Clinical registries of all patients undergoing angiography, CABG and PCI, were linked to multiple population-based health databases using patient-level encrypted health card numbers at ICES. As a prescribed entity under Ontario’s Personal Health Information Protection Act, ICES is able to collect, construct and store registries, link, and analyze individual health data without the need to obtain individual patient consent (see link to Data and Privacy at www.ices.on.ca). Therefore, we were able to study all patients undergoing these revascularization procedures without participation bias. The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a Research Ethics Board. These datasets were linked using unique encoded identifiers and analyzed at ICES.
Data Sources
Similar to previously published studies, coronary angiography records were obtained from the CorHealth Ontario Registry.(9, 10) Baseline demographics were obtained from the CorHealth Registry, Canadian Institute for Health Information Discharge Abstract Database (CIHI-DAD), or previously validated algorithms where applicable.(11) Hospitalizations and the primary admission diagnoses were determined via the CIHI-DAD, using the International Classification of Diseases 10th edition coding system, as previously described.(12) Deaths were determined using the Registered Persons Database (RPDB), which contains vital status information for all Ontarians. In-hospital deaths were determined using a combination of the RPDB and CIHI-DAD. Physicians’ claims for procedures were determined using the Ontario Health Insurance Plan fee billing database. The Ontario Diabetes Database was used to identify patients with diabetes, as employed previously.(12) We used Statistics Canada’s census data to determine sociodemographic information based on median neighbourhood income of individuals, to serve as a proxy for socioeconomic status.
Study population and Data Linkages
Significant coronary artery narrowing in the CorHealth angiographic database was defined as ≥70% for all coronary arteries, with the exception of the left main where ≥50% was considered a significant narrowing. (9) In our primary analysis, we included only patients with pre-existing diabetes and significant multivessel disease, defined as: two vessel disease consisting of proximal left anterior descending (LAD) artery and either circumflex disease or right coronary artery disease, or three vessel disease involving the LAD, circumflex, and right coronary artery. Patients with significant left main disease were excluded in the primary analysis to be consistent with the coronary anatomic inclusion criteria in the FREEDOM trial but were included in a secondary analysis to examine the robustness of our findings. In patients with more than one coronary angiogram, we used the first angiogram that demonstrated one of the disease patterns described above as the index angiogram.
Since patients could undergo revascularization after some time has passed after angiography, for example, due to the need for surgical or interventional consultation after an elective angiogram, we assigned patients who underwent CABG within 90 days after angiography to the CABG cohort and those who underwent PCI within 90 days to the PCI cohort, whichever procedure occurred first was considered the index procedure. Those who did not undergo CABG or PCI within 90 days were considered medically managed and were excluded from the analysis. Patients who underwent another concomitant cardiac operation (such as valve procedures and aortic surgery) and those who underwent TAVR following the index CABG or PCI were excluded to ensure that patients were comparable in terms of procedural risk. For all patients, we excluded those with a history of previous cardiac surgical procedures by using a “look back” period of 20 years prior to the index procedure date to identify patients that had undergone previous cardiac surgery in the CIHI-DAD and the CorHealth cardiac surgery registry and patients who underwent PCI procedure within the six months prior to the index coronary angiogram using the CIHI-DAD and the CorHealth PCI registry data. Finally, we excluded patients with cardiogenic shock, STEMI, or those undergoing emergent or salvage procedures as these patients are primarily treated by PCI and are at high risk for mortality.
Early outcomes
First, we examined 30-day mortality, defined as death that occurred in hospital or within 30 days of the index revascularization procedure (PCI/CABG). Non-fatal early events including in-hospital complications of new clinically-diagnosed acute myocardial infarction and stroke were obtained from the CIHI-DAD.
Long-term outcomes
Long-term outcomes included all events from the index procedure (defined as the date of either PCI or CABG) to date of last follow-up on March 31st, 2020. All patients were followed for at least 1-year and the maximum follow-up was 11.5 years. Our primary study outcome was long-term all-cause mortality. The secondary outcomes included long-term MACCE and the individual components of MACCE: new hospital admission for myocardial infarction, stroke and repeat revascularization. Since revascularization procedures may be staged, PCI of a new vessel (ie. not previously stented) within 90 days of the index procedure PCI were not counted as repeat revascularization procedures. Repeat PCI was subcategorized based on the target of intervention (vessel with prior PCI, PCI after CABG, or PCI of a new vessel). However, if PCI was performed on the same vessel or CABG was performed after index PCI, this was considered a repeat revascularization procedure.
Statistical analyses
Baseline characteristics were first compared in the overall sample between those undergoing CABG and those undergoing PCI. Student’s t-test was used for normally distributed continuous variables, Wilcoxon rank-sum test for non-normally distributed continuous variables, while the Chi-square test was used for categorical variables. Propensity score (PS) matching was performed to account for baseline differences in patient characteristics between PCI and CABG in order to minimize confounding. The PS for each patient was estimated using a multivariable logistic regression model in which the intervention performed, (PCI versus CABG), was regressed on 23 important baseline characteristics that may influence the choice of intervention or that were prognostically important for the outcome. These baseline characteristics included: age, sex, frailty, cardiac comorbidities, lung disease, peripheral vascular disease, cerebrovascular disease, cancer, dementia, renal function, CCS and NYHA classification, left ventricular function, the extent of coronary artery disease, and surrogates for socioeconomic status (neighbourhood income quintile and rural status). Subjects were matched on the logit of the propensity score using 1:1 greedy nearest-neighbour matching with a caliper distance of 0.2 times the standard deviation of the logit of the PS.(13) Success of matching was assessed by computing the standardized difference of each covariate with a cut-off of 0.1 to denote acceptable balance.(14) Early events were compared between the two cohorts using the McNemar test for binary outcomes and paired t-test and the Wilcoxon signed rank test for normally and non-normally distributed continuous variables, respectively. All tests were two-sided and p-values<0.05 were considered significant.
For long-term mortality and MACCE, a time to event analysis using Kaplan-Meier survival curves was conducted in the matched sample, using a log-rank P test stratified on the matched pairs to test the equality of the estimated survival curves.(15) In addition, cause-specific hazard ratios were estimated using a Cox-proportional hazards model, which incorporated a robust sandwich-type variance estimator to account for the matched nature of the data, which has been shown to result in more accurate estimates of standard errors compared to the conventional maximum-likelihood estimate of the standard error.(16) For the individual components of MACCE (late MI, stroke, and repeat revascularization), we estimated cumulative incidence functions (CIFs) to estimate the incidence of these events after accounting for death as a competing risk. In the matched sample, a Fine-Gray subdistribution hazard model were used to regress the outcome on a single variable denoting treatment status.(17) For both models, robust variance estimators were used to estimate the standard errors.
For outcomes with <6 events per group, privacy legislation prevents the reporting of the actual number of events; as such, we instead report absolute risk differences between the PCI and CABG which is compliant with Ontario privacy legislation. All curves were truncated to 8-years given the small numbers at risk after that time point.
Secondary analysis
Our primary analysis excluded patients with LM disease as these patients were excluded in the FREEDOM trial. We performed a secondary analysis that compared outcomes in patients inclusive of those with LM disease. Therefore, the secondary analysis cohort included: isolated LM, LM with one or two vessel disease, two or three vessel disease, and three vessel disease with LM involvement for the primary outcome of long-term mortality.
Sensitivity analyses
In a series of sensitivity analyses, we excluded patients of higher risk for either PCI or CABG to ascertain the robustness of our primary outcome of long-term mortality. Specifically, we excluded patients that underwent PCI after a cardiac surgery consult, since these patients might be considered possible surgical turndowns, those with an initial diagnosis of acute coronary syndrome (i.e. non-STEMI or unstable angina), and those with severe renal disease requiring dialysis. Baseline characteristics were compared, and the primary outcome of long-term survival was compared as described above. Two additional sensitivity analyses were conducted to examine the temporal robustness of the primary outcomes. In the first sensitivity analysis, patients that underwent PCI and received a bare metal stent were excluded. In the second sensitivity analysis, we performed an exact match on year of procedure such that pairs were only matched within the same year to account for potential era differences.
All analyses were conducted with SAS (version 9.4; SAS Institute, Inc, Cary, NC).
RESULTS
Primary matched analysis
In total, there were 4,519 patients in the PCI group and 9,716 patients in the CABG group (Supplemental Figure 1). There were significant differences in important baseline characteristics in the PCI and CABG cohort before propensity matching (Table 1, Supplemental Figure 2). Those who underwent PCI were older, more often female, and had a higher burden of comorbidities, but had fewer diseased coronary vessels. Propensity score matching on 23 baseline covariates yielded 4,301 pairs of patients (i.e., 95% of PCI patients were matched to a CABG patient), who were well-matched with standardized mean differences <0.10 for all covariates. Importantly, the extent of CAD was similar between the groups after matching. Early deaths did not differ between the PCI and CABG groups (2.4% vs 2.3%, absolute risk difference [ARD]; 0.12, 95%CI: −0.52% to 0.76%, p=0.721, Table 2). There was no difference in the rate of new in-hospital MI between PCI and CABG (0.88% vs. 1.2%, ARD; −0.35%, 95%CI; −0.78% to 0.08%, p=0.112). The rate of new in-hospital stroke was higher with CABG compared to PCI (ARD; −0.58% 95%CI: −0.84% to −0.32%, p<0.001). Early outcomes before and after PS matching are provided in Table 2.
Table 1.
Baseline characteristics and procedural description
| Before propensity score matching | After propensity score matching | |||||||
|---|---|---|---|---|---|---|---|---|
| VARIABLE | VALUE | PCI (N=4,519) | CABG (N=9,716) | SMD | P-VALUE | PCI (N=4,301) | CABG (N=4,301) | SMD |
| Age | Mean ± SD | 68.3 ± 11.2 | 65.7 ± 9.4 | 0.26 | <.001 | 67.9 ± 11.1 | 67.5 ± 9.2 | 0.04 |
| Charlson Index | Mean ± SD | 3.2 ± 1.9 | 3.1 ± 1.6 | 0.04 | 0.015 | 3.1 ± 1.9 | 3.1 ± 1.7 | 0 |
| Hospital Frailty Risk Score | Mean ± SD | 2.4 ± 4.0 | 2.2 ± 3.4 | 0.08 | <.001 | 2.4 ± 4.0 | 2.3 ± 3.7 | 0.01 |
| BMI | 0–25 | 695 (15.4%) | 1,352 (13.9%) | 0.04 | <.001 | 653 (15.2%) | 642 (14.9%) | 0.01 |
| 26–30 | 1,132 (25.0%) | 2,180 (22.4%) | 0.06 | 1,065 (24.8%) | 1,035 (24.1%) | 0.02 | ||
| ≥ 31 | 1,060 (23.5%) | 2,226 (22.9%) | 0.01 | 1,011 (23.5%) | 1,011 (23.5%) | 0 | ||
| Unknown | 1,632 (36.1%) | 3,958 (40.7%) | 0.1 | 1,572 (36.5%) | 1,613 (37.5%) | 0.02 | ||
| Creatinine level (mg/dl) | 0–120 | 3,339 (73.9%) | 7,566 (77.9%) | 0.09 | <.001 | 3,207 (74.6%) | 3,183 (74.0%) | 0.01 |
| 121–180 | 525 (11.6%) | 887 (9.1%) | 0.08 | 478 (11.1%) | 491 (11.4%) | 0.01 | ||
| ≥ 181 | 243 (5.4%) | 456 (4.7%) | 0.03 | 229 (5.3%) | 221 (5.1%) | 0.01 | ||
| Unknown | 412 (9.1%) | 807 (8.3%) | 0.03 | 387 (9.0%) | 406 (9.4%) | 0.02 | ||
| Male Sex | M | 3,276 (72.5%) | 7,573 (77.9%) | 0.13 | 3,161 (73.5%) | 3,174 (73.8%) | 0.01 | |
| Income Quintile | 1 | 1,018 (22.6%) | 2,012 (20.8%) | 0.04 | 0.005 | 954 (22.2%) | 959 (22.4%) | 0 |
| 2 | 1,004 (22.3%) | 2,088 (21.6%) | 0.02 | 950 (22.2%) | 948 (22.1%) | 0 | ||
| 3 | 968 (21.5%) | 2,049 (21.2%) | 0.01 | 934 (21.8%) | 935 (21.8%) | 0 | ||
| 4 | 788 (17.5%) | 1,916 (19.8%) | 0.06 | 758 (17.7%) | 757 (17.7%) | 0 | ||
| 5 | 727 (16.1%) | 1,615 (16.7%) | 0.01 | 692 (16.1%) | 688 (16.0%) | 0 | ||
| Rural Status | 572 (12.7%) | 1,314 (13.5%) | 0.03 | 0.156 | 554 (12.9%) | 569 (13.2%) | 0.01 | |
| History of smoking | Current | 737 (16.3%) | 1,871 (19.3%) | 0.08 | <.001 | 721 (16.8%) | 737 (17.1%) | 0.01 |
| Former | 1,349 (29.9%) | 2,932 (30.2%) | 0.01 | 1,297 (30.2%) | 1,297 (30.2%) | 0 | ||
| Never | 2,264 (50.1%) | 4,532 (46.6%) | 0.07 | 2,126 (49.4%) | 2,105 (48.9%) | 0.01 | ||
| Unknown | 169 (3.7%) | 381 (3.9%) | 0.01 | 157 (3.7%) | 162 (3.8%) | 0.01 | ||
| Recent MI within 30 days | 1,105 (24.5%) | 2,472 (25.4%) | 0.02 | 0.205 | 1,039 (24.2%) | 1,065 (24.8%) | 0.01 | |
| History of MI | 1,237 (27.4%) | 2,281 (23.5%) | 0.09 | <.001 | 1,152 (26.8%) | 1,159 (26.9%) | 0 | |
| Hypertension | 3,763 (83.3%) | 7,826 (80.5%) | 0.07 | <.001 | 3,558 (82.7%) | 3,570 (83.0%) | 0.01 | |
| Cerebrovascular disease | 364 (8.1%) | 541 (5.6%) | 0.1 | <.001 | 322 (7.5%) | 319 (7.4%) | 0 | |
| COPD | 1,012 (22.4%) | 1,730 (17.8%) | 0.11 | <.001 | 930 (21.6%) | 919 (21.4%) | 0.01 | |
| Hyperlipidemia | 2,271 (50.3%) | 4,434 (45.6%) | 0.09 | <.001 | 2,145 (49.9%) | 2,142 (49.8%) | 0 | |
| Peripheral Vasc Disease | 251 (5.6%) | 410 (4.2%) | 0.06 | <.001 | 230 (5.3%) | 229 (5.3%) | 0 | |
| Dementia | 114 (2.5%) | 111 (1.1%) | 0.1 | <.001 | 90 (2.1%) | 85 (2.0%) | 0.01 | |
| History of cancer | 513 (11.4%) | 790 (8.1%) | 0.11 | <.001 | 453 (10.5%) | 459 (10.7%) | 0 | |
| Dialysis | 180 (4.0%) | 287 (3.0%) | 0.06 | 0.001 | 164 (3.8%) | 169 (3.9%) | 0.01 | |
| CCS Class | 0 | 582 (12.9%) | 1,255 (13.0%) | 0 | <.001 | 556 (13.0%) | 563 (13.2%) | 0.01 |
| 1 | 435 (9.7%) | 945 (9.8%) | 0 | 416 (9.7%) | 424 (9.9%) | 0.01 | ||
| 2 | 1,100 (24.4%) | 2,239 (23.2%) | 0.03 | 1,056 (24.7%) | 980 (22.9%) | 0.04 | ||
| 3 | 761 (16.9%) | 1,457 (15.1%) | 0.05 | 713 (16.6%) | 742 (17.3%) | 0.02 | ||
| 4 | 133 (3.0%) | 268 (2.8%) | 0.01 | 125 (2.9%) | 124 (2.9%) | 0 | ||
| 4 - High risk features | 355 (7.9%) | 652 (6.8%) | 0.04 | 328 (7.7%) | 332 (7.8%) | 0 | ||
| 4 - Intermediate risk features | 692 (15.4%) | 1,805 (18.7%) | 0.09 | 667 (15.6%) | 687 (16.1%) | 0.01 | ||
| 4- Low risk features | 442 (9.8%) | 1,033 (10.7%) | 0.03 | 422 (9.9%) | 426 (10.0%) | 0 | ||
| NYHA Class | 1 | 2,125 (47.0%) | 4,868 (50.1%) | 0.06 | 0.014 | 2,048 (47.6%) | 2,066 (48.0%) | 0.01 |
| 2 | 504 (11.2%) | 1,030 (10.6%) | 0.02 | 472 (11.0%) | 468 (10.9%) | 0 | ||
| 3 | 261 (5.8%) | 539 (5.5%) | 0.01 | 244 (5.7%) | 233 (5.4%) | 0.01 | ||
| 4 | 118 (2.6%) | 218 (2.2%) | 0.02 | 105 (2.4%) | 102 (2.4%) | 0 | ||
| Unknown | 1,511 (33.4%) | 3,061 (31.5%) | 0.04 | 1,432 (33.3%) | 1,432 (33.3%) | 0 | ||
| Extent of CAD | 2 vessel disease | 1,566 (34.7%) | 1,442 (14.8%) | 0.47 | <.001 | 1,362 (31.7%) | 1,349 (31.4%) | 0.01 |
| 3 vessel disease | 2,953 (65.3%) | 8,274 (85.2%) | 0.47 | 2,939 (68.3%) | 2,952 (68.6%) | 0.01 | ||
| Left ventricular ejection fraction | ≥ 50% | 1,822 (40.3%) | 3,794 (39.0%) | 0.03 | 0.305 | 1,735 (40.3%) | 1,725 (40.1%) | 0 |
| 35% – 49% | 537 (11.9%) | 1,224 (12.6%) | 0.02 | 517 (12.0%) | 499 (11.6%) | 0.01 | ||
| 20% – 34% | 223 (4.9%) | 474 (4.9%) | 0 | 206 (4.8%) | 208 (4.8%) | 0 | ||
| < 20% | 49 (1.1%) | 83 (0.9%) | 0.02 | 44 (1.0%) | 39 (0.9%) | 0.01 | ||
| Unknown | 1,888 (41.8%) | 4,141 (42.6%) | 0.02 | 1,799 (41.8%) | 1,830 (42.5%) | 0.01 | ||
| Stent type used | BMS | 1,009 (23.3%) | 0 (.%) | . | 945 (22.9%) | 0 (.%) | . | |
| DES | 3,545 (81.8%) | 0 (.%) | . | 3,395 (82.2%) | 0 (.%) | . | ||
| # of grafted vessels (CABG) | 1 | 0 (.%) | 120 (1.2%) | . | 0 (.%) | 76 (1.8%) | . | |
| 2 | 0 (.%) | 1,076 (11.1%) | . | 0 (.%) | 627 (14.6%) | . | ||
| ≥ 3 | 0 (.%) | 8,403 (86.5%) | . | 0 (.%) | 3,551 (82.6%) | . | ||
| Unknown | 0 (.%) | 117 (1.2%) | . | 0 (.%) | 47 (1.1%) | . | ||
| # of stented vessels (PCI) | 1 | 2,538 (56.2%) | 0 (.%) | . | 2,408 (56.0%) | 0 (.%) | . | |
| 2 | 1,464 (32.4%) | 0 (.%) | . | 1,393 (32.4%) | 0 (.%) | . | ||
| ≥ 3 | 209 (4.6%) | 0 (.%) | . | 205 (4.8%) | 0 (.%) | . | ||
| Unknown | 308 (6.8%) | 0 (.%) | . | 295 (6.9%) | 0 (.%) | . | ||
CAD, coronary artery disease, CABG, coronary artery bypass grafting, COPD, chronic obstructive pulmonary disease, PCI, percutaneous coronary intervention
Note, matching was not performed on the last three variables (stent type used, number of stented vessels, number of grafted vessels)
Table 2.
Early outcomes for unmatched and matched cohorts
| Before propensity score matching | After propensity score matching | |||||
|---|---|---|---|---|---|---|
| PCI (n=9,716) |
CABG (n=4,519) |
P-value | PCI (n=4,301) |
CABG (n=4,301) |
P-value | |
| Early death | 117 (2.6%) | 180 (1.9%) | <0.001 | 104 (2.4%) | 99 (2.3%) | 0.721 |
| ARD: 0.74% (95%CI: 0.22% to 1.29%) | ARD: 0.12% (95%CI: −0.52% to 0.76%) | |||||
| In-hospital stroke | ARD: −0.52% (95%CI; −0.34 to −0.71%) | ARD: −0.58% (95%CI; −0.84% to −0.32%) | ||||
| In-hospital MI | 44 (1.0%) | 108 (1.1%) | 0.427 | 38 (0.88%) | 53 (1.2%) | 0.112 |
| ARD: −0.14%, (95%CI: −0.48% to 0.24%) | ARD: −0.35% (95%CI: −0.78% to 0.008%) | |||||
95%CI, 95% confidence interval, ARD, absolute risk difference, CABG, coronary artery bypass grafting, MI, myocardial infarction, PCI, percutaneous coronary intervention. For outcomes with <6 events per group, privacy legislation prevents the reporting of the actual number of events; as such, we instead report absolute risk differences between the PCI and CABG which is compliant with Ontario privacy legislation.
Risk difference < 0 means that risk of the event was higher for CABG than PCI
At 8-year follow-up (maximum: 11.5 years, IQR: 2.7–7.5 years), all-cause mortality in the propensity score matched cohorts was 27.0% for patients who underwent PCI and 19.4% for patients undergoing CABG surgery and the HR was 1.39 (95%CI; 1.28–1.51, p<0.0001) over the entire follow-up period. Survival curves are shown in Figure 1. Additionally, in long-term follow-up, 51.1% of patients undergoing PCI and 30.4% undergoing CABG surgery experienced MACCE at 8-years (see Figure 2). The HR was 1.99 (95%CI; 1.86–2.12) over the entire follow-up period, again indicating higher risk of adverse outcomes among those undergoing PCI (p<0.001). Supplemental Table 1 shows the hazard ratios and 95%CI in the samples before and after propensity score matching.
Figure 1.
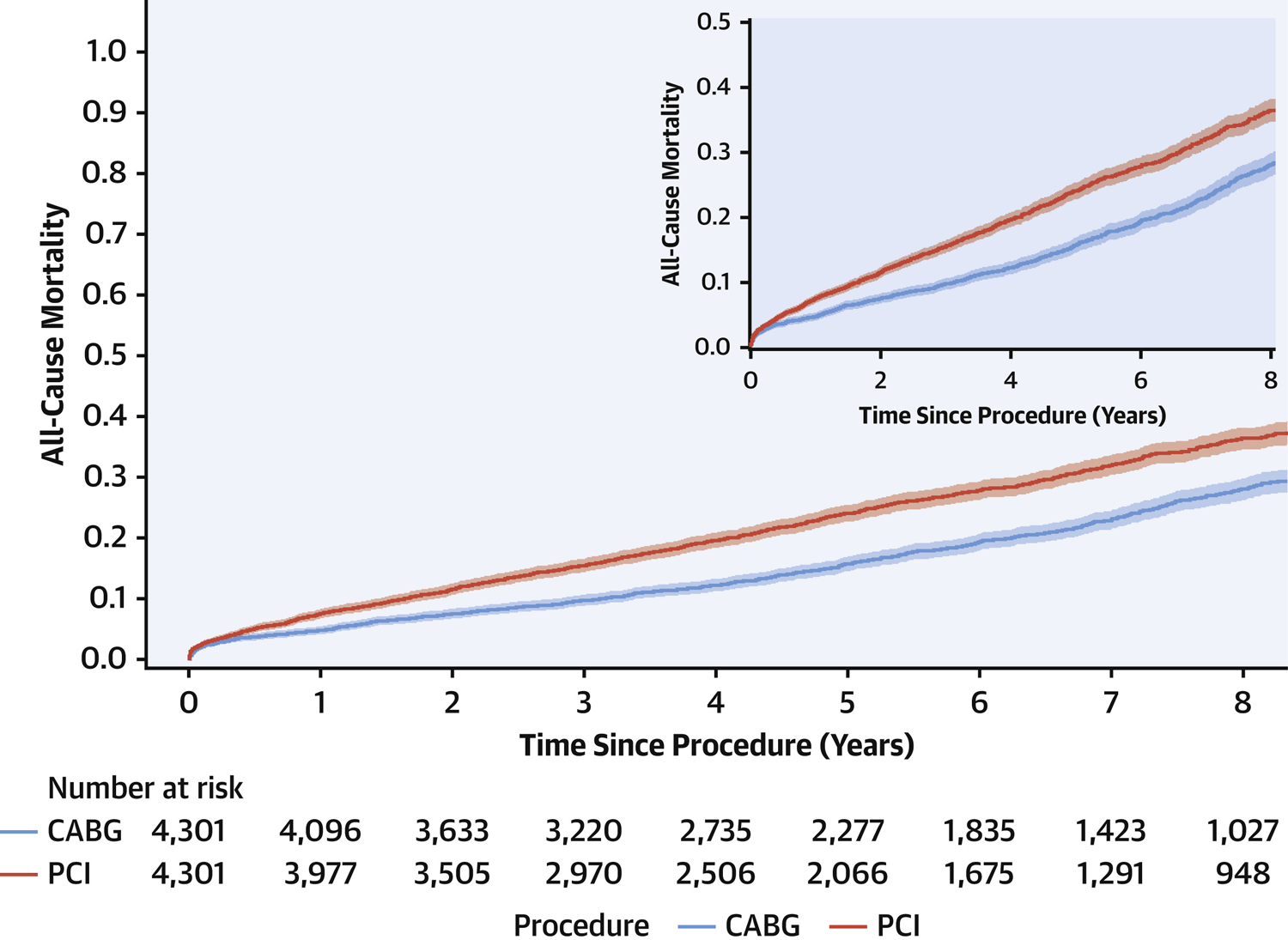
Cumulative incidence curves for 8-year survival. All cause mortality was compared between percutaneous coronary intervention (PCI) versus coronary artery bypass grafting (CABG) after propensity score matching in patients with diabetes and multivessel coronary artery disease (Hazard ratio: 1.39, 95%CI: 1.28–1.51, p<0.001). The shaded region around the curve represents the 95% confidence interval.
Figure 2.
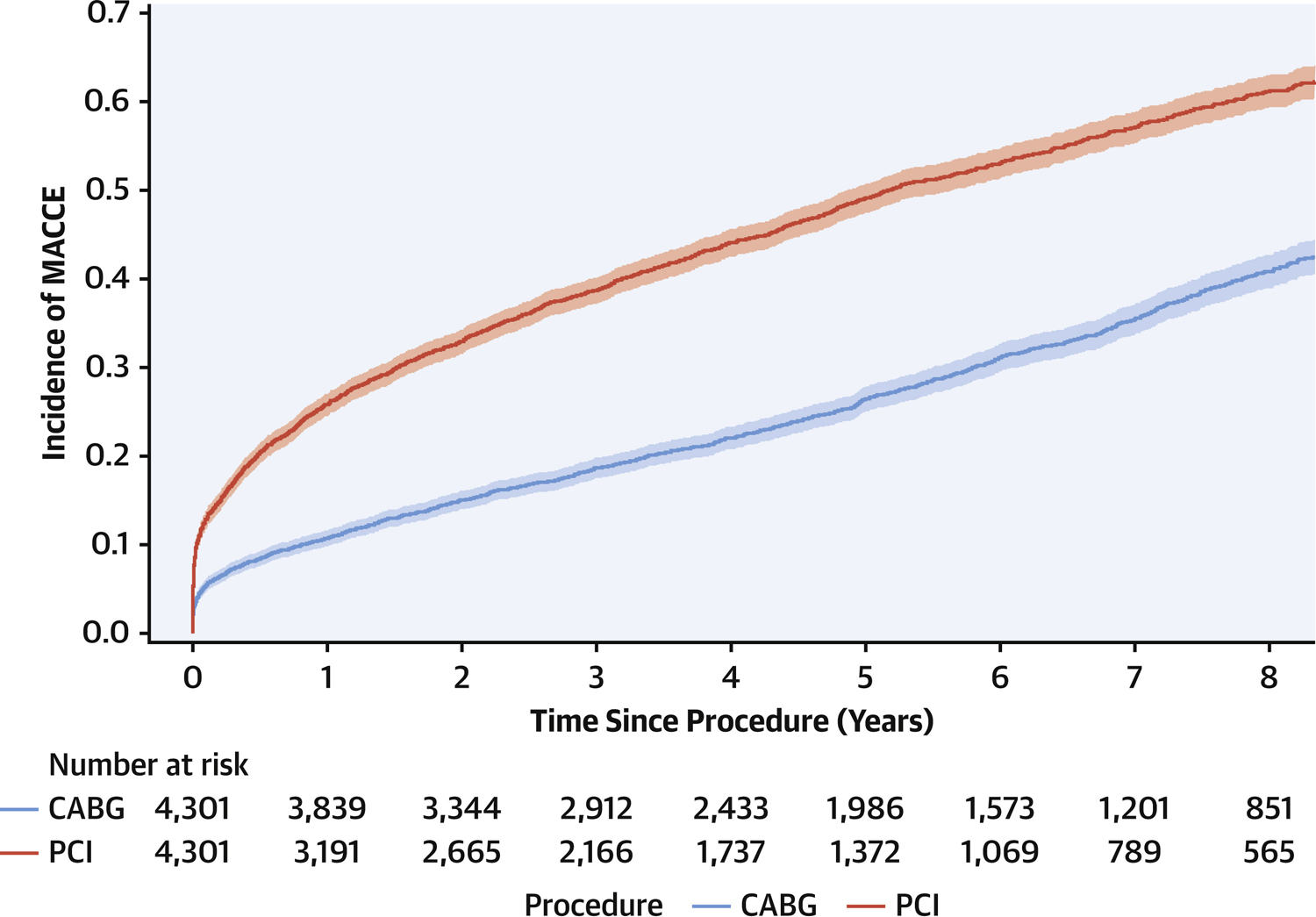
Cumulative incidence curves for 8-year freedom from major adverse cardiac and cerebrovascular events (MACCE). MACCE was a composite of death, myocardial infarction, stroke, repeat revascularization and compared between percutaneous coronary intervention (PCI) versus coronary artery bypass grafting (CABG) after propensity score matching in patients with diabetes and multivessel coronary artery disease (HR: 1.99, 95%CI: 1.86–2.12, p<0.001). The shaded region around the curve represents the 95% confidence interval.
When the components of MACCE were analyzed separately, we found that the cumulative incidence of MI was 16.4% among those undergoing PCI compared with 7.2% after CABG (see Supplemental Figure 3). The subdistribution HR was 2.32 (95%CI; 2.04–2.64) indicating an increased risk of MI in the PCI group (p<0.001). Repeat revascularization occurred more frequently in the PCI group compared to CABG (see Supplemental Figure 4 – 25.9% vs 7.8%). The risk of repeat revascularization was more than three-fold higher in the PCI group (subdistribution HR: 3.65, 95%CI; 3.24–4.34, p<0.001). The details of the repeat revascularization procedures can be found in Supplemental Table 2. The cumulative incidence of stroke at 8-years was similar between PCI and CABG (3.5% vs 4.1%, Supplemental Figure 5) with a subdistribution HR of 0.87 (95%CI: 0.71–1.08, p=0.203) over the entire study period. Overall key findings from the primary analysis are presented in the Central Illustration.
Central Illustration:
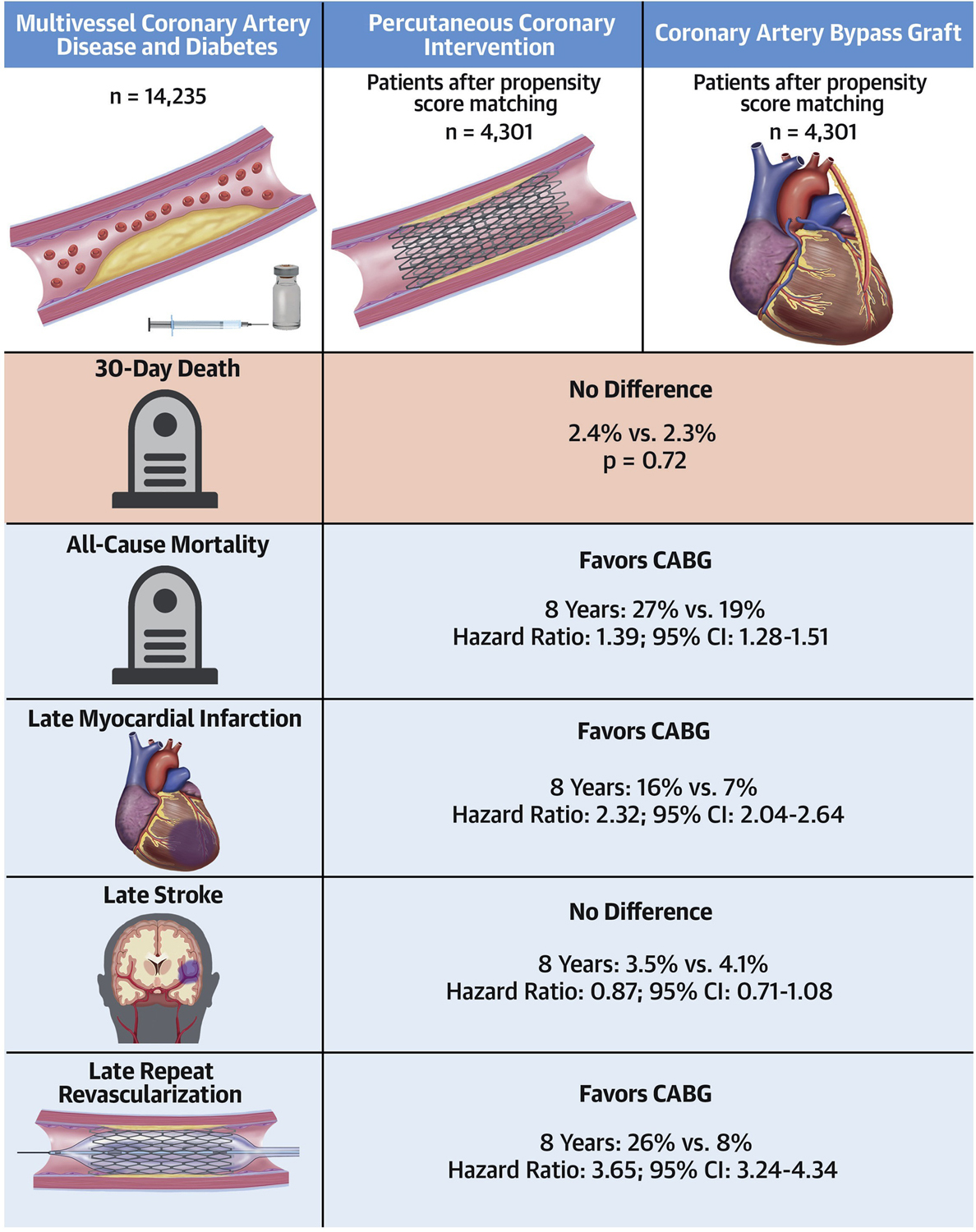
A propensity score cohort matched analysis of coronary artery bypass grafting versus percutaneous coronary intervention for patients with multivessel coronary artery disease and diabetes
Secondary analysis
In the secondary analysis, patients with left main disease were included in the sample. Propensity score matching on the same covariates as the primary analysis was performed, yielding 5,139 pairs of patients that were well-matched (SMD<0.1 for all covariates). Findings were consistent with the primary analysis; the risk of late mortality was significantly higher with PCI compared to CABG as shown in Supplemental Figure 6 (HR: 1.47, 95%CI: 1.36–1.58, p<0.001).
Sensitivity analysis
In the cohort of 4519 diabetic patients with two and three vessel disease undergoing PCI, only 375 patients (8.3%) were seen by a cardiac surgeon for consultation. These 375 patients were older and had more comorbidities compared to patients that underwent PCI without a cardiac surgical consult. After exclusion of patients who had pre-PCI cardiac surgical consultation and repeating the propensity score match, there were 3968 pairs of patients. Again, late mortality was higher in the PCI group (HR: 1.43, 95%CI; 1.31–1.57, see Supplemental Figure 7). When patients with a diagnosis of acute coronary syndrome (i.e. non-STEMI and unstable angina) were excluded, late mortality was higher in the PCI group (HR: 1.36, 95%CI: 1.19–1.56, see supplemental Figure 8) in 2,337 pairs of well-matched patients. The full details of the sensitivity analysis that excluded those with a history of dialysis, those that received bare-metal stents, and exact match by year can be found in the Supplemental Appendix. Findings supported the robustness of the primary analysis.
DISCUSSION
There were several key findings from this analysis. First, long-term survival was significantly higher in patients with multivessel CAD and diabetes, undergoing CABG as compared to PCI. Freedom from MACCE was also higher with CABG compared to PCI, and these findings were driven by lower mortality, new MI, and reduced need for repeat revascularization. There was no excess early mortality with CABG compared with PCI. While the rate of early stroke was higher with CABG compared to PCI, the incidence of stroke was similar between the two treatments at 8 years follow-up. Finally, less than 10% of diabetic patients with multivessel CAD that underwent PCI had a consultation with a cardiac surgeon.
Overall, these findings suggest that in diabetic patients with multivessel CAD, revascularization with CABG may be the preferred approach. While our primary analysis excluded left main patients to be consistent with the FREEDOM trial, the findings were robust even when these patients were added into the study. While the FREEDOM trial was the largest RCT that compared PCI and CABG in diabetic patients with multivessel disease, nearly 33,000 patients were screened, 3309 were eligible, and ultimately only 1900 were randomized.(8) In the extended follow-up study of FREEDOM, mortality was higher in the PCI group (24.3%) compared to the CABG group (18.3%) at 8-years, numbers that were strikingly similar to the findings of our observational study (26.1% vs 18.5% at 8 years). The findings of our observational study derived from registry data are consistent with that of the FREEDOM trial. Similar to the FREEDOM trial, we did not find any difference in early mortality, but we did detect an increase peri-operative stroke in the CABG group, although there was no difference at 8-years. Importantly, we note that our patient cohort was similar to that of FREEDOM. Although our patients were, on average, 5 years older, the proportion of men (~80%) and proportion of patients with three-vessel disease (~70%) was similar between the trial and our study.
Ramanathan and colleagues examined outcomes following PCI and CABG in a cohort of diabetic patients with multivessel coronary artery disease. They concluded that CABG was associated with lower risk of MACCE at 5 years, particularly in patients admitted with acute coronary syndrome rather than those with stable ischemic heart disease.(18) While our analysis shared similar findings, there were several important differences between the two studies. Our primary analysis included over 8000 patients and our secondary analysis included patients with left main disease in addition to clinically significant two and three vessel disease. Furthermore, we used propensity score matching to create two well-balanced groups rather than multivariable analysis to compare long-term outcomes. Nonetheless, the similar conclusions of these two studies continue to support the use of CABG in patients with advanced multivessel CAD for long term benefit.
Our analysis also found significant reductions in myocardial infarction and need for repeat revascularization events in the CABG cohort, consistent with findings from FREEDOM and the diabetic subgroup of the SYNTAX trial.(19) Our findings may be explained by the differing mechanisms of PCI and CABG in the revascularization of diseased vessels. In PCI, stents are placed to treat focal flow limiting lesions while any distal non-flow limiting lesions are left untreated. In contrast, in CABG, the bypass graft is often placed distal to both the flow limiting and non-flow limiting lesion.(20) Future events after revascularization may occur when an acute thrombosis of the non-flow limiting lesion occurs, resulting in an acute myocardial infarction and potential need for subsequent revascularization – in PCI, the distal coronary bed is not protected whilst in CABG, there is protection of the distal coronary bed if the bypass graft remains patent. In patients with diabetes, the coronary arteries are often diffusely diseased and thus CABG may be more effective for protection against late thrombotic events compared to PCI.(21) In addition, numerous studies have shown that the risk of stent restenosis is doubled in diabetic patients compared to non-diabetic patients and thus diabetic patients are at especially higher risk for myocardial infarction and need for repeat revascularization.(22, 23) Finally, there is some concern for higher rates of incomplete revascularization with PCI compared to CABG; incomplete revascularization is associated with worse late survival and higher late MACCE events.(24, 25)
A concerning finding in our study was that only 10% of patients undergoing PCI had a consultation with a cardiac surgeon. According to both American and European guidelines, there is a class I recommendation that CABG is preferred to PCI in patients with diabetes and multivessel disease (both three vessel and complex two vessel disease involving the proximal LAD).(26, 27) These recommendations are further supported by the ACC/AHA appropriate use criteria for patients with diabetes and two- and three-vessel coronary disease which suggests that CABG is more often appropriate compared to PCI in patients with stable ischemic heart disease.(28) Furthermore, the heart team approach is recommended in patients with diabetes and complex multivessel CAD (Class I recommendation). Thus, while the majority of patients in our analysis underwent CABG, it remains concerning that only 10% of those patients that underwent PCI had ever seen a cardiac surgeon despite guidelines recommending that a heart team approach be undertaken. Our study is the largest to-date to examine the real world clinical practice of revascularization in patients with diabetes, and supports the generalizability of the FREEDOM trial results. However, we note that both PCI and CABG techniques continue to improve and evolve and that the use of intravascular ultrasound, optical coherence tomography, and fractional flow reserve may improve outcomes as shown in the SYNTAX II study that examined state of the art PCI. (29)
Limitations
This study must be interpreted in the context of some significant limitations. This was a retrospective observational study and thus may be confounded by treatment allocation bias. While we attempted to mitigate any potential bias by performing propensity score matching on 23 variables, we recognize that unmeasured or unknown confounders may exist. Another concern was that patients with a history of acute coronary syndrome or patients that underwent PCI after a cardiac surgery consult may be considered higher risk patients (i.e. surgical turndown patients) and thus may bias against PCI. However, we performed several sensitivity analyses which excluded these patients and findings were robust and still favoured CABG. There is a paucity of detailed surgical information in the CABG patients such as the use of sequential grafts, target vessel bypassed, vessel size and completeness of revascularization as this is not captured in our dataset. Finally, we note that the definition of early MI used in our analysis depended on a clinical diagnosis and differed from those used in clinical trials that relied on mostly biochemical markers. This may have underestimated the number of peri-procedural myocardial infarctions in both groups. There remains controversy surrounding the definition of clinically important myocardial infarction and in particular the use of only biochemical markers.(30)
Conclusion
In summary, CABG should be considered the preferred approach in patients with diabetes and multivessel CAD that are good surgical candidates. Further work is warranted to ensure that diabetic patients with extensive CAD are treated within a heart team framework.
Supplementary Material
PERSPECTIVES.
Competency in Medical Knowledge:
In suitable candidates, CABG surgery is the preferred over PCI for revascularization in patients with diabetes and multivessel coronary artery disease.
Competency in Patient Care:
Surgical consultation should be strongly considered in patients with diabetes and multivessel coronary artery disease on angiography.
Translational Outlook:
Health policy recommendations should be developed to ensure that patients with diabetes and multivessel coronary artery disease are treated utilizing a Heart-Team approach.
FUNDING SOURCES
ICES is supported in part by a grant from the Ontario Ministry of Health and Long-Term Care. The opinions, results and conclusions are those of the authors and no endorsement by the Ministry of Health and Long-Term Care or by ICES is intended or should be inferred. Parts of this material are based on data and information compiled and provided by CIHI. However, the analyses, conclusions, opinions and statements expressed herein are those of the author, and not necessarily those of CIHI. The authors acknowledge that the clinical registry data used in this publication is from participating hospitals through the CorHealth Ontario Cardiac Registry, which serves as an advisory body to the Minister of Health and Long-Term Care (MOHLTC), is funded by the MOHLTC, and is dedicated to improving the quality, efficiency, access and equity in the delivery of the continuum of adult cardiac services in Ontario, Canada. This study was supported by a Foundation Grant from the Canadian Institutes of Health Research (grant # FDN 148446). DYT is supported by a CIHR Fellowship. RVR is supported by the Black Family Foundation Fellowship Award. SEF is supported by the Bernard S. Goldman Chair in Cardiovascular Surgery (Toronto, Ontario). PCA is supported by a Mid-Career Investigator Award from the Heart and Stroke Foundation. DSL is supported by a Mid-Career Investigator Award from the Heart and Stroke Foundation and is the Ted Rogers Chair in Heart Function Outcomes, a joint Hospital-University Chair of the University Health Network and the University of Toronto.
DISCLOSURES
Dr. Michael Farkouh has received research grant support from Amgen, Novo Nordisk, and Novartis. Dr. Husam Abdel-Qadir served on the endpoint adjudication committee for the THEMIS trial funded by Astra Zeneca. The remaining authors have nothing to disclose.
ABBREVIATIONS
- CABG
coronary artery bypass grafting
- CAD
coronary artery disease
- CIHI-DAD
Canadian Institute for Health Information Discharge Abstract Database
- FREEDOM
Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Diseases
- HR
hazard ratio
- LAD
left anterior descending
- MACCE
major adverse cardiac and cerebrovascular event
- PCI
percutaneous coronary intervention
- RPDB
Registered Persons Database
Contributor Information
Derrick Y Tam, Division of Cardiac Surgery, Department of Surgery, Schulich Heart Centre, Sunnybrook Health Sciences Centre, University of Toronto, Toronto, Canada; Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada.
Christoffer Dharma, ICES, Toronto, Canada.
Rodolfo Rocha, Division of Cardiac Surgery, Department of Surgery, Peter Munk Cardiac Centre, University Health Network, University of Toronto, Toronto, Canada.
Michael E. Farkouh, Division of Cardiology, Department of Medicine, Peter Munk Cardiac Centre, University Health Network, University of Toronto, Toronto, Canada.
Husam Abdel-Qadir, ICES, Toronto, Canada; Division of Cardiology, Department of Medicine, Women’s College Hospital, University of Toronto, Toronto, Canada.
Louise Y. Sun, University of Ottawa Heart Institute, University of Ottawa, Ottawa, Canada.
Harindra C. Wijeysundera, Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Division of Cardiology, Department of Medicine, Schulich Heart Centre, University of Toronto, Toronto, Canada.
Peter C. Austin, ICES, Toronto, Canada.
Jay Udell, Division of Cardiology, Department of Medicine, Women’s College Hospital, University of Toronto, Toronto, Canada.
Mario Gaudino, Department of Cardiothoracic Surgery, Weill Cornell Medical College, New York, New York.
Stephen E. Fremes, Division of Cardiac Surgery, Department of Surgery, Schulich Heart Centre, Sunnybrook Health Sciences Centre, University of Toronto, Toronto, Canada.
Douglas S. Lee, Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; ICES, Toronto, Canada; Division of Cardiology, Department of Medicine, Peter Munk Cardiac Centre, University Health Network, University of Toronto, Toronto, Canada.
References
- 1.Bypass Angioplasty Revascularization Investigation (BARI) Investigators. Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. N. Engl. J. Med 1996;335:217–225. [DOI] [PubMed] [Google Scholar]
- 2.Abizaid A, Costa MA, Centemero M, et al. Clinical and economic impact of diabetes mellitus on percutaneous and surgical treatment of multivessel coronary disease patients: insights from the Arterial Revascularization Therapy Study (ARTS) trial. Circulation 2001;104:533–538. [DOI] [PubMed] [Google Scholar]
- 3.BARI 2D Study Group, Frye RL, August P, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N. Engl. J. Med 2009;360:2503–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kappetein AP, Head SJ, Morice M-C, et al. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardiothorac Surg 2013;43:1006–1013. [DOI] [PubMed] [Google Scholar]
- 5.Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N. Engl. J. Med 2012;367:2375–2384. [DOI] [PubMed] [Google Scholar]
- 6.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130:e344–426. [DOI] [PubMed] [Google Scholar]
- 7.Kolh P, Alfonso F, Collet J-P, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg 2014;46:517–592. [DOI] [PubMed] [Google Scholar]
- 8.Farkouh ME, Domanski M, Dangas GD, et al. Long-term Survival following Multivessel Revascularization in Patients with Diabetes (FREEDOM Follow-On Study). J Am Coll Cardiol 2018;73:629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braga JR, Austin PC, Ross HJ, Tu JV, Lee DS. Importance of Nonobstructive Coronary Artery Disease in the Prognosis of Patients With Heart Failure. J Am Coll Cardiol HF 2019;7:493–501. [DOI] [PubMed] [Google Scholar]
- 10.Johnston A, Mesana TG, Lee DS, Eddeen AB, Sun LY. Sex Differences in Long-Term Survival After Major Cardiac Surgery: A Population-Based Cohort Study. J Am Heart Assoc 2019;8:e013260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tu JV, Chu A, Donovan LR, et al. The Cardiovascular Health in Ambulatory Care Research Team (CANHEART): using big data to measure and improve cardiovascular health and healthcare services. Circ Cardiovasc Qual Outcomes 2015;8:204–212. [DOI] [PubMed] [Google Scholar]
- 12.Lipscombe LL, Hux JE. Trends in diabetes prevalence, incidence, and mortality in Ontario, Canada 1995–2005: a population-based study. Lancet 2007;369:750–756. [DOI] [PubMed] [Google Scholar]
- 13.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011;10:150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Austin PC, Schuster T. The performance of different propensity score methods for estimating absolute effects of treatments on survival outcomes: A simulation study. Stat Methods Med Res 2016;25:2214–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med 2013;32:2837–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austin PC, Fine JP. Propensity-score matching with competing risks in survival analysis. Stat Med 2019;38:751–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramanathan K, Abel JG, Park JE, et al. Surgical Versus Percutaneous Coronary Revascularization in Patients With Diabetes and Acute Coronary Syndromes. J Am Coll Cardiol 2017;70:2995–3006. [DOI] [PubMed] [Google Scholar]
- 19.Verma S, Farkouh ME, Yanagawa B, et al. Comparison of coronary artery bypass surgery and percutaneous coronary intervention in patients with diabetes: a meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol 2013;1:317–328. [DOI] [PubMed] [Google Scholar]
- 20.Doenst T, Haverich A, Serruys P, et al. PCI and CABG for Treating Stable Coronary Artery Disease: JACC Review Topic of the Week. J Am Coll Cardiol 2019;73:964–976. [DOI] [PubMed] [Google Scholar]
- 21.Scognamiglio R, Negut C, Ramondo A, Tiengo A, Avogaro A. Detection of coronary artery disease in asymptomatic patients with type 2 diabetes mellitus. J Am Coll Cardiol 2006;47:65–71. [DOI] [PubMed] [Google Scholar]
- 22.Daemen J, Wenaweser P, Tsuchida K, et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet 2007;369:667–678. [DOI] [PubMed] [Google Scholar]
- 23.Machecourt J, Danchin N, Lablanche JM, et al. Risk factors for stent thrombosis after implantation of sirolimus-eluting stents in diabetic and nondiabetic patients: the EVASTENT Matched-Cohort Registry. J Am Coll Cardiol 2007;50:501–508. [DOI] [PubMed] [Google Scholar]
- 24.Takagi H, Watanabe T, Mizuno Y, Kawai N, Umemoto T, ALICE (All-Literature Investigation of Cardiovascular Evidence) Group. A meta-analysis of adjusted risk estimates for survival from observational studies of complete versus incomplete revascularization in patients with multivessel disease undergoing coronary artery bypass grafting. Interact Cardiovasc Thorac Surg 2014;18:679–682. [DOI] [PubMed] [Google Scholar]
- 25.Bangalore S, Guo Y, Samadashvili Z, Blecker S, Hannan EL. Revascularization in Patients With Multivessel Coronary Artery Disease and Severe Left Ventricular Systolic Dysfunction: Everolimus-Eluting Stents Versus Coronary Artery Bypass Graft Surgery. Circulation 2016;133:2132–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2014;130:1749–1767. [DOI] [PubMed] [Google Scholar]
- 27.Neumann F-J, Sousa Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J 2018;34:2949. [Google Scholar]
- 28.Patel MR, Calhoon JH, Dehmer GJ, et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 Appropriate Use Criteria for Coronary Revascularization in Patients With Stable Ischemic Heart Disease: A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Am Coll Cardiol 2017;69:2212–2241. [DOI] [PubMed] [Google Scholar]
- 29.Tam DY, Bakaeen F, Feldman DN, et al. Modality Selection for the Revascularization of Left Main Disease. Can J Cardiol 2019;35:983–992. [DOI] [PubMed] [Google Scholar]
- 30.Ruel M, Farkouh ME. Why NOBLE and EXCEL Are Consistent With Each Other and With Previous Trials. Circulation 2017;135:822–824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


