Abstract
Objective:
A study was conducted to identify metabolic-related effects of benzo(ghi)perylene (BghiP) and 1,2,3,4,6,7,8-heptachlorodibenzo-p-dioxin (HpCDD), on primary human fibroblasts to verify biological associations previously found in occupational health research.
Methods:
Human lung fibroblasts were exposed to BghiP or HpCDD and extracts were analyzed with a metabolome-wide association study to test for pathways and metabolites altered relative to controls. Gene expression was measured by quantitative-real time PCR.
Results:
Metabolic perturbations in amino-acid, oxidative stress, and fatty-acid pathways were observed for BghiP and HpCDD. HpCDD but not BghiP exposure increased gene expression of the amino acid transporters SLC7A5 and SLC7A11.
Conclusions:
Exposure to PAH or dioxin perturbs amino acid pathways at physiologically relevant concentrations with different mechanisms. These findings imply an effect on central homeostatic systems by environmental exposures which could have implications on disease susceptibility.
Keywords: high-resolution metabolomics, exposure bio-monitoring, environmental toxicology, exposome
INTRODUCTION
Open burn bits represent a major source of airborne pollutants including polycyclic aromatic hydrocarbons (PAH), polychlorinated dibenzo-p-dioxins (PCDD), and polychlorinated dibenzofurans (PCDF) (1, 2). Additional occupational exposures on active military bases include airborne particulate PAH from aircraft and diesel fuel combustion (3). Recently, our group studied samples from the Department of Defense Serum Repository (DoDSR) for personnel stationed in areas with these types of environmental hazards to test for evidence of exposure. PAHs and PCDDs/PCDFs were analyzed in serum samples collected pre and post-deployment from 200 persons deployed to areas with open burn pits (Balad, Iraq, and Bagram, Afghanistan) and 200 persons not deployed (control) by gas chromatography-mass spectrometry (4). These studies revealed numerous PAH, PCDD, and PCDF present in many serum samples from both control and case samples.
Among these biomarkers of exposure, naphthalene was the most commonly detected PAH (83% of serum samples). Other compounds of interest include benzo(ghi)perylene (BghiP) which that was found in 21% of serum samples, and a dioxin, 1,2,3,4,6,7,8-heptachlorodibenzo-p-dioxin (HpCDD), which was found in 33% of samples. Metabolome-wide association studies (MWAS) of pre- and post-deployment samples showed that metabolic associations of deployment to Balad included amino sugar metabolism, detoxification pathways (pentose and glucuronide; methionine and cysteine), and several metabolic pathways associated with amino acid metabolism and the urea cycle (Go et al. JOEM 2019 Current Supplement).
To determine whether the metabolic associations with deployment could be caused by PAH or dioxin exposure, we used primary human lung fibroblasts (HLFs) as a surrogate model to study pathway effects of BghiP and HpCDD. HLFs were chosen for three important reasons: 1) they are primary cells isolated from lung tissue explants from human beings, 2) fibroblasts are most abundant cells in the lung (5) and 3) fibroblasts are sentinel cells that play a major role in airway inflammation and remodeling and are often the first responders to external stimuli such as environmental insults like airborne PAHs, PCDDs, and PCDFs (6-8).
We selected BghiP as a priority for study because there was significantly more BghiP in case samples after deployment compared to pre-deployment; BghiP exposure is associated with combustion of diesel fuel and also has a biological half-life of several days (3). In contrast, naphthalene was more commonly detected, but we excluded naphthalene because naphthalene is associated with cigarette smoke, has a short biological half-life (4-12 h), and did not differ in the deployment study (4). We selected HpCDD because it remains in the body for years after exposure and was higher in serum from post-deployment samples than for controls (4). In contrast, other PCDD and PCDF did not differ between cases and controls.
The objectives of the present study were to 1) identify metabolic changes in human lung fibroblasts exposed to BghiP or HpCDD at concentrations found in post-deployment serum samples, 2) determine whether those HLF changes were similar to MWAS results associated with deployment to Balad or Bagram, and 3) determine whether specific changes in gene expression can account for metabolic disruptions in exposed HLFs. Results show a number of key changes in amino acid metabolites in both BghiP- and HpCDD-exposed HLFs and identified gene expression changes in amino acid transporters that may account for some of the metabolic changes associated with HpCDD exposure.
METHODS
Source of Samples
Eight-hundred de-identified DoDSR samples collected from Armed Forces personnel during active duty as previously described along with exclusion criteria (Go et al. JOEM 2019 Current Supplement). In the current analyses, the Case group excluded 2 more individuals due to undetected levels of dioxins or furans (4). Therefore, the overall numbers in the current study consisted of paired pre- and post-deployment data for 370 individuals, with 185 in the Case group.
Measurement of BghiP, Dioxins, and Furans
Data for quantification of benzo(ghi)perylene and polychlorinated dibenzo-p-dioxins/dibenzofurans was previously reported (4). Briefly, chemicals were extracted from serum samples using Triton X-100 and NaCl solutions and then spiked with the EPA Method 1613 internal standard, which contains a mixture of deuterated surrogates (Wellington EPA-1613LCS). Hexane was added to chemical extracts and samples of chemical extracts were dried down using dry nitrogen. The samples were reconstituted in 20 μL of nonane. Detection and quantification of dioxin/furan measurements were performed using high-resolution GC-MS (HRGC) utilizing an Agilent 7890A GC with a Micromass Autospec Premier high resolution mass spectrometry. The mean and maximal values for post-deployment samples were 87 nM and 2.8 μM for BghiP and 60 pM and 0.84 nM for HpCDD.
Chemicals and HPLC Columns
Acetonitrile (HPLC grade), formic acid (HPLC grade), and water (HPLC grade) were obtained from Sigma–Aldrich (St. Louis). A mixture of internal standard stable isotopic chemicals was obtained from Cambridge Isotope Laboratories, Inc. (Andover, Pennsylvania). Analytical columns consisted of Accucore HILIC (100 × 2.1 mm) with a short, end-capped HILIC pre-column (Thermo, Defender guard). Benzo(ghi)perylene (CAS # 191-24-2) was purchased from Sigma-Aldrich (St. Louis, MO), and 1,2,3,4,6,7,8-heptachlorodibenzo-p-dioxin (CAS # 35822-46-9) was purchased from Wellington Labs (Guelph, ON, Canada). Stock solutions were prepared in DMSO and stored per manufacturer instructions. Reagents were used in cell culture at the final concentrations indicated in the figure legends.
Cell Culture and Treatments
Primary human lung fibroblasts (HLFs) derived from control tissue explants as previously described in (see also Woeller et al. JOEM 2019 Current Supplement) were used for the current study. Control or “normal” tissues represented surgical waste tissue obtained from patients undergoing diagnostic biopsy for hamartoma, a benign tumor, and cell strains were established by explant technique from tissue distal to the lesion. All patient tissue explants were obtained with written informed consent under the approval of Research Subject Review Board of the University of Rochester Medical Center. Briefly, fresh obtained human lung tissue was washed with sterile PBS and minced in 100 mm dishes with sterile scissors. Explanted HLFs were cultured in Minimal Eagle’s Medium (MEM) supplemented with 10% fetal bovine serum (FBS) and antibiotics. All media and supplements were purchased from Gibco (Carlsbad, CA). FBS was from Hyclone (Logan, UT). These cells are morphologically consistent with fibroblasts and express collagen and vimentin, but do not express CD45, factor VIII, or cytokeratin. HLFs were used between passages 4 and 10. HLFs were placed in 0.5% FBS-MEM for 48 hours before addition of vehicle (DMSO), benzo(ghi)perylene or 1,2,3,4,6,7,8-heptachlorodibenzo-p-dioxin for 24 hours at the concentrations indicated (n = 7-8 replicates for each treatment per each HLF strain). After treatments, for high resolution metabolomics (HRM) using mass spectrometry, culture media were collected, and cells were washed 3 times in ice cold PBS before being lysed directly on culture plates in 2:1 acetonitrile: water (HPLC Grade; Sigma-Aldrich ACN: 34998-4L; H2O:270733-4L). For RNA analysis, cells were treated as described above and lysed directly on plates in Qiazol lysis reagent (Qiagen, Valencia, CA).
Ultra-High-Resolution Mass Spectrometry and Data Pre-processing
For HRM, cells were lysed and extracted by addition of 300 μL of lysis solution [2:1, acetonitrile (ACN): water] containing a mixture of isotopic standards (9). Samples were allowed to stand on ice for 30 min and centrifuged at 13,000 x g for 15 min to remove protein. Supernatants were analyzed by liquid chromatography coupled to ultra-high-resolution mass spectrometry (LC-HRMS) as described below. A quality control pooled reference sample (QStd3) was included at the beginning and end of each analytical batch of 20 samples for quality control and quality assurance (10). Samples were analyzed in triplicate by liquid chromatography with Fourier transform mass spectrometry (Dionex Ultimate 3000, Velos, Thermo Fisher) with HILIC chromatography/positive electrospray ionization (ESI) mode and resolution of 70,000 (11). Spectral m/z features were acquired in scan range 85-2,000 mass-to-charge ratio (m/z). Raw data files were extracted using apLCMSv6.3.3 (12) with xMSanalyzer v2.0.7 (13), followed by batch correction with ComBat (14). Resulting mass spectrometry data, referred to as m/z features, included accurate mass m/z, retention time (s) and ion abundance.
Metabolic Feature Selection
Data preprocessing included averaging intensities for triplicate injections and retaining only m/z features with at least 80% non-missing values in either of the groups and more than 50% non-missing values across all samples. Data were then log2 transformed and quantile normalized (15). Selection of differentially expressed m/z features was performed based on one-way ANOVA using the limma package in R with a p <0.05 cutoff (16). Unsupervised two-way hierarchal clustering analysis (HCA) of discriminating m/z features utilizing the hclust() function in R was used to determine the clustering pattern of selected m/z features and samples. Principal component analysis (PCA) was performed using the pca() function implemented in R package pcaMethods. Discriminatory metabolites (p < 0.05) were used with Mummichog 1.0.9 (12) for pathway enrichment analysis. For pathway enrichment analysis, features differing at p < 0.05 were selected to protect against type 2 error, and permutation testing (p < 0.05) in pathway enrichment analysis was used to protect against type 1 error (17, 18). Annotation of the m/z features was obtained with xMSannotator using the KEGG and HMDB databases (19). Identities of hundreds of m/z metabolic features (called metabolites hereafter) corresponding to m/z features obtained with this protocol have been established by coelution with authentic standards and ion dissociation mass spectrometry (MS/MS) and are provided as appropriate.
Mapping the interactions of the metabolome with environmental chemicals
Dioxin/furan measurements and high-resolution metabolomic data from the same set of samples (Go et al. JOEM 2019 Current Supplement) were integrated by using xMWAS (19). We used sparse partial least-squares regression, a variable selection and dimensionality reduction method, to conduct pairwise correlation analysis between the dioxin and furan concentrations (185 samples × 24 chemicals) and the metabolome (185 samples × 925 m/z features previously selected by LIMMA analysis). Thresholds for determining significant associations must have met the correlation threshold criteria (∣r∣ > 0.4) and p < 0.05 as determined by Student's t-test.
RNA isolation and Real-Time PCR analysis
Total cell RNA was extracted using Qiazol and isolated by subsequent phenol:chloroform extraction. RNA was precipitated and pellets washed with 80% ethanol two times before resuspension in nuclease free water. RNA concentrations were determined with a DS-11 FX spectrophotometer (Denovix, Wilmington, DE). cDNA was generated using the iScript reverse transcription kit (Bio-Rad) and gene expression quantified via real time PCR with gene specific primers and the SsoFast Evergreen 2X PCR master mix (Bio-Rad, Hercules, CA). Reactions were run on an iCycler iQ5 PCR thermal cycler. Gene specific primers are as follows: SLC7A5 (5’-CTATCACCTGGGCGTCATG-3’ and 5’-TGGATCATGGAGAGGATGGAG-3’); SLC7A11 (5-TTTTGTACGAGTCTGGGTGG-3’ and 5’-CGCAAGTTCAGGGATTTCAC-3’); TBP (5’- CGAAACGCCGAATATAATCCC-3’ and 5’-CCCAACTTCTGTACAACTCTAGC-3’) and HPRT1 (5’-ATGACCAGTCAACAGGGGAC-3’ and 5’-TGCCTGACCAAGGAAAGCAA-3’). Gene expression was normalized using the ΔΔCt method (20) using the average Ct value for the reference genes TBP and HPRT1.
Statistical analysis
Student’s t-test and one-way analysis of variance (ANOVA) were used for statistical analysis, with p < 0.05 used for selection of metabolites for pathway enrichment analysis and p < 0.01 for targeted real-time PCR analysis.
RESULTS
Correlations of dioxins and furans with the serum metabolome
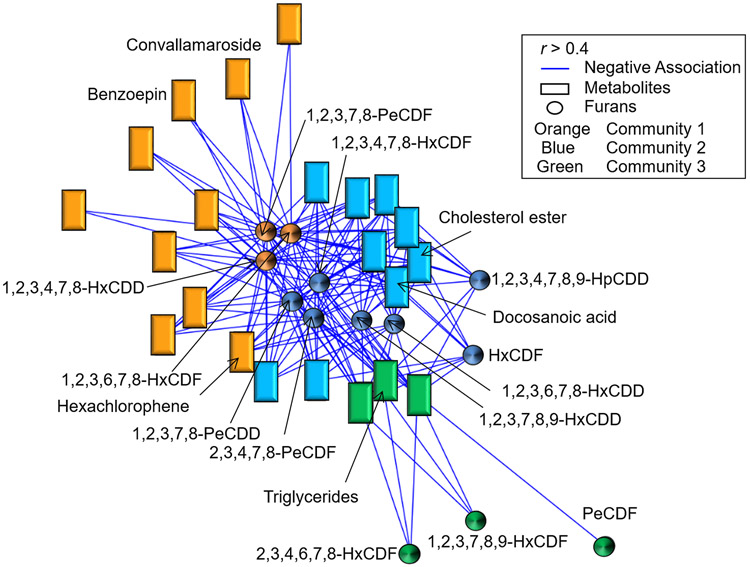
Based on our previous findings of associations between serum BghiP and flouroanthrene which was also associated with detoxification, and sphingolipid metabolism in soldiers deployed to high risk areas (Go et al. JOEM 2019 Current Supplement), we used xMWAS to test for interactions of dioxins and furans with the serum metabolome (19). Thirteen of the 24 dioxins and furans correlated with 22 metabolites in 3 chemical-metabolite communities (Figure 1, Supplemental Table 1). These included dioxins/furans which were clustered into 5 groups: the pentachlorodibenzofurans (PeCDF), pentachlorodibenzo-p-dioxins (PeCDD), hexachlorodibenzofurans (HxCDF), hexachlorodibenzo-p-dioxins (HxCDD), and heptachlorodibenzo-p-dioxin (HpCDD). The largest hub, community one (Orange in Figure 1, Supplemental Table 1), had associations between 1-2-3-6-7-8-HxCDF, 1-2-3-4-7-8-HxCDD, and 1-2-3-7-8-PeCDF and 10 metabolites, including the organopesticide, benzoepin, the steroid, convallamaroside, and the antiseptic, hexaclorophene. Community two (blue in Figure 1, Supplemental Table 1), identified associations of 1-2-3-4-7-8-9-HpCDD, 1-2-3-4-7-8-HxCDF, 2-3-4-7-8-PeCDF, 1-2-3-6-7-8-HxCDD, 1-2-3-7-8-9-HxCDD, HxCDF, and 1-2-3-7-8-PeCDD with 9 metabolites, which were annotated as cholesterol esters and the long-chain fatty acid, docosanoic acid. Community three (Green in Figure 1, Supplemental Table 1) identified associations of 2-3-4-6-7-8-HxCDF, 1-2-3-7-8-9-HxCDF, and PeCDF with 3 metabolites, one of which was annotated as a triglyceride. These results show that in addition to the metabolite associations found for BghiP (Go et al. JOEM 2019 Current Supplement), serum lipid metabolites correlate with serum HpCDD and other dioxins and furans.
FIGURE 1.
Association of the metabolome with environmental chemicals. The 925 metabolites associated with deployment in the Case group were examined for their association with 24 detected furans and dioxins using xMWAS (19). Three major metabolic communities were detected; orange (community 1), 1-2-3-6-7-8-HxCDF, 1-2-3-4-7-8-HxCDD, and 1-2-3-7-8-PeCDF were found to be associated with 10 metabolites including benzoepin, convallamaroside and hexachlorophene; blue (community 2); 1-2-3-4-7-8-9-HpCDD, 1-2-3-4-7-8-HxCDF, 2-3-4-7-8-PeCDF, 1-2-3-6-7-8-HxCDD, 1-2-3-7-8-9-HxCDD, HxCDF, and 1-2-3-7-8-PeCDD were found to be associated with 9 metabolites including docosanoic acid and cholesterol esters; green (community 3), 2-3-4-6-7-8-HxCDF, 1-2-3-7-8-9-HxCDF, and PeCDF were found to be associated with 3 metabolites which includes triglycerides. (∣r∣ > 0.4 at p < 0.05). Blue lines, rectangles and circles indicate negative associations, metabolites, and furans/dioxins, respectively.
Dose Response of BghiP and HpCDD on lung fibroblasts
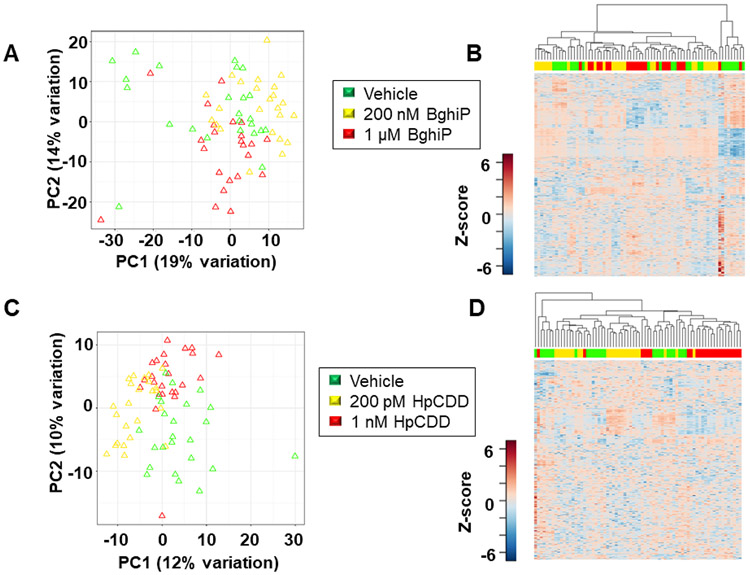
Because the correlations found in the human serum analyses do not establish cause-effect relationships between the serum PAH or dioxin, and metabolites, we performed cell studies to identify metabolic changes that occur in response to BghiP or HpCDD. We used normal lung fibroblasts from 3 different donors strains, exposed to them to BghiP (200 nM or 1 μM), HpCDD (200 pM or 1 nM) or vehicle control for 24 h, and extracted samples for HRM. We performed one-way ANOVA to test whether metabolic differences existed at doses for BghiP and HpCDD within ranges observed for service personnel deployed to Balad, Iraq, and Bagram, Afghanistan (4). Results showed 720 metabolites differed for the BghiP-exposed cells and 388 features differed for the HpCDD-exposed cells (Supplemental Table 2). Hierarchical cluster analysis (HCA) of the differentiating metabolites for BghiP-treated cells showed that most of the 1 μM treated cells clustered together while the lower dose and vehicle-treated cells mostly clustered together (Fig 2A). Principal component analysis (PCA) showed that PC1 and PC2 accounted for 34% of the variance, and about one-third of the vehicle-treated samples (green) separated from the others in PC1 (Fig 2B). In PC2, the lower-dose (yellow) and vehicle-treated samples did not separate, but the higher dose (red) mostly separated. HCA-heat map for the HpCDD-treated cells similarly showed clustering of most of the higher-dose samples (red) from lower dose (yellow) and controls (green) (Fig 2C). PCA results for HpCDD showed a relatively clear trajectory in PC1 and PC2 from vehicle control to lower dose to higher dose (Fig 2D). Because the results showed relatively little effect of the lower dose of BghiP or HpCDD on metabolism, we focused analyses on the higher dose treatments for pathway enrichment analysis.
FIGURE 2.
Metabolic dose responses associated with exposure to BghiP and HpCDD. A, Unsupervised HCA-heatmap indicates that intensity of 720 metabolites drive the separation between the vehicle, 200 nM BghiP and 1 μM BghiP exposure. B, PCA plot showing separation of the vehicle (green) 200 nM BghiP (yellow) and 1 μM BghiP (red), through the 1st (19% variation) and 2nd (14% variation) principal components. C, Unsupervised HCA-heatmap indicates that intensity of 388 metabolites drive the separation between the vehicle, 200 pM HpCDD and 1 nM HpCDD exposure. D, PCA plot showing separation of the vehicle (green) 200 pM HpCDD (yellow) and 1 nM HpCDD (red), through the 1st (12% variation) and 2nd (10% variation) principal components. n=23 (BghiP 1 μM); n=24 (BghiP 200nM, vehicle, 200 pM HpCDD, and 1 nM HpCDD).
Pathway effects of BghiP in human lung fibroblasts
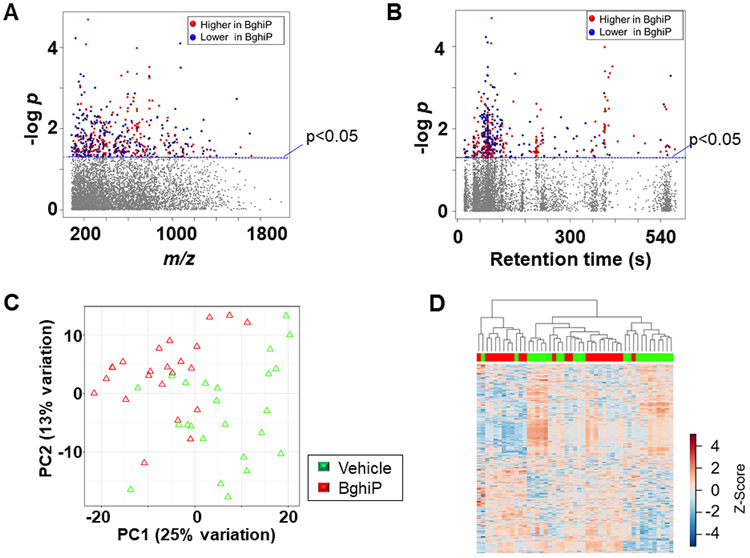
Of the 498 metabolites that differed after 1 μM BghiP treatment, 275 were increased (red) and 223 decreased (blue) (Fig 3A, Supplemental Table 3). A Type I Manhattan plot of -log p as a function of m/z showed that most had m/z between 200 and 800. Plotting the data as a function of retention time in a Type II Manhattan plot (Fig 3B) showed that many of the metabolites that decreased eluted early, indicating a relatively non-polar character. PCA showed that 38% of variance was in PC1 and PC2 with incomplete separation according to treatment. HCA similarly showed some clustering of BghiP (red) and vehicle (green), but this was incomplete. Pathway enrichment analysis showed fifteen pathways were altered by BghiP treatment, including N-glycan, amino acid and urea cycle pathways (Fig 4). Other pathways included porphyrin and multiple lipid metabolism pathways. In a separate analysis of data for 200 nM BghiP treatment, many of these pathways were also found for this lower dose (pathways identified by asterisks in Fig 4).
Figure 3.
Metabolic responses associated with exposure to 1 μM benzo(ghi)perylene. A, Type I Manhattan plot of metabolites plotted against the –log p value indicates that features are altered after exposure to BghiP at p < 0.05; (275 metabolites increased after BghiP exposure (red) while 223 were decreased (blue); 5775 features were not affected by BghiP exposure (gray). B, Type II Manhattan plot using retention time (RT, s) plotted against –log p value. n=23 (BghiP) n=24 (Veh). C, PCA plot showing separation of the vehicle treated (green) and 1 μM BghiP exposed (red), through the 1st (25% variation) and 2nd (13% variation) principal components. D, Unsupervised HCA-heatmap indicates that intensity of 498 metabolites drive the separation between the vehicle treated and BghiP treated cells.
Figure 4.
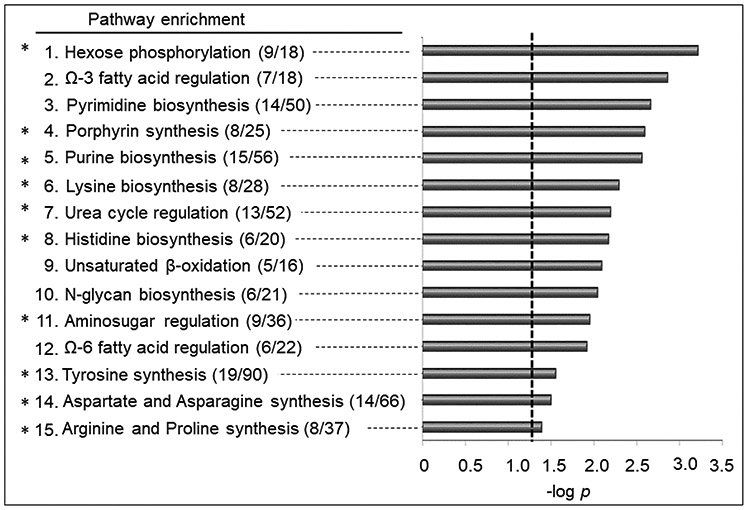
Pathway enrichment analysis associated with exposure to BghiP. A total of (15/119) enriched pathways were determined (Filled gray bars indicate significance less than p < 0.05, and the cutoff is indicated by the dotted line). Numbers in the parentheses indicate the overlap of the metabolites as matched through KEGG and the Edinburgh human metabolic network databases. *Indicates overlapped enriched pathways with 200 nM BghiP exposure.
Pathway effects of HpCDD in human lung fibroblasts
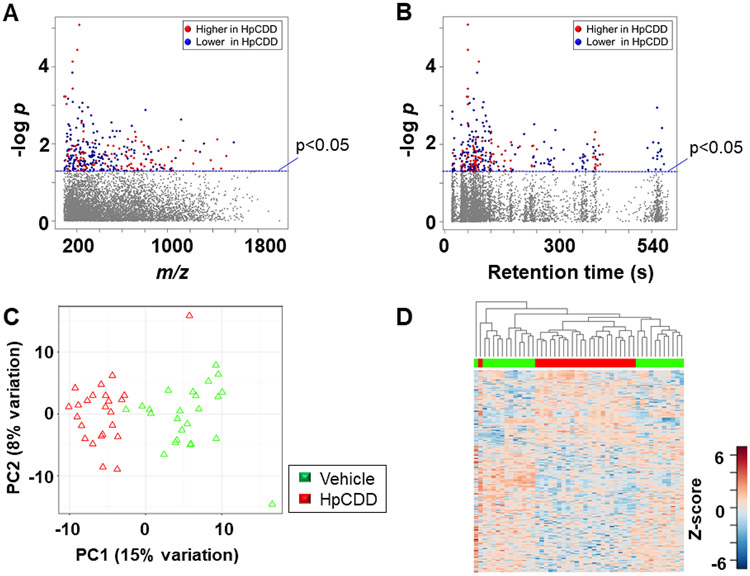
Of the 299 metabolites that differed after 1 nM HpCDD treatment compared with vehicle group, 108 were increased (red) and 191 were decreased (blue) (Fig 5A, Supplemental Table 4). Plotting the data in a Type II Manhattan plot (Fig 5B) showed that many of the metabolites that decreased in abundance eluted early, indicating a relatively non-polar character. PCA showed that PC1 and PC2 included only 23% of the variance, but separation was almost complete in PC1 (Fig 5C). HCA showed that almost all of the HpCDD treated samples clustered together (Fig 5D). Relative intensities of selected metabolites are provided in Figure 6. The amino acids tyrosine (Fig 6A) and methionine (Fig 6B) both decreased after BghiP exposure. In contrast, 4-methyl-glutamate (Fig 6C), an important metabolite in C5-basic acid metabolism, increased.
Figure 5.
Metabolic responses associated with exposure to 1 nM HpCDD. A, Type I Manhattan plot of metabolites plotted against the –log p value indicates that features are altered after exposure to HpCDD at p < 0.05; 108 metabolites increased after HpCDD exposure (red) while 191 were decreased (blue); 6003 features were not affected by HpCDD exposure (gray) (6030 m/z features) were not affected by exposure. B, Type II Manhattan plot using retention time (RT, s) plotted against –log p value. n=24 for both conditions. C, PCA plot showing separation of the vehicle treated (green) and 1 μM HpCDD exposed (red), through the 1st (15% variation) and 2nd (8% variation) principal components. D, Unsupervised HCA-heatmap indicates that intensity of 299 metabolites drive the separation between the vehicle treated and HpCDD treated cells.
Figure 6.
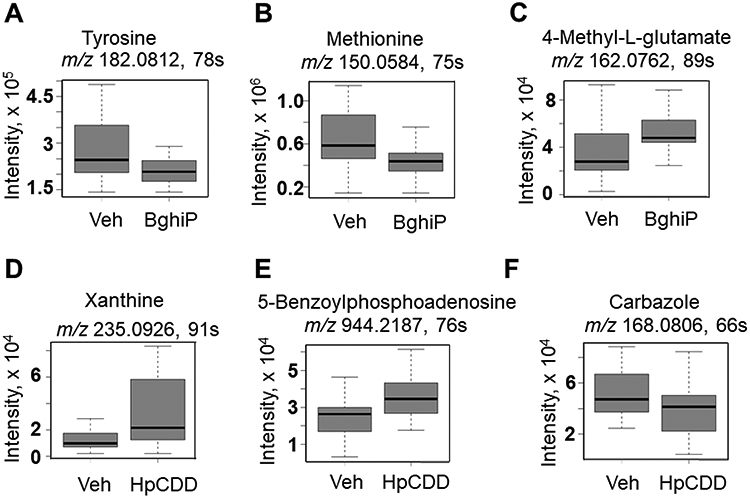
Comparison of selected metabolites between the BghiP and HpCDD groups. Features identified as significant by pathway were annotated and key amino acids and purine metabolites were found altered either by BghiP or HpCDD exposure. A. Tyrosine (m/z 182.0812, 78s), B. methionine (m/z 150.05 75 s), and C. 4-methyl-glutamate (162.0762, 89 s). D. Xanthine (m/z 235.0926, 91 s), E. 5-benzoylphosphoadenosine (m/z 944.2187, 76 s) and lastly, F. carbazole (m/z 168.0806, 66 s). n=23 (BghiP 1 μM); n=24 (vehicle and 1 nM HpCDD).
Pathway enrichment analysis showed that eight pathways were affected by HpCDD, three of which (lysine, urea, purine) were also significant at the lower dose of HpCDD (pathways identified by asterisks in Fig 7). Five of the pathways substantially overlapped with the BghiP pathways, including amino-sugar, lysine, tyrosine, urea cycle and purine metabolism. Additional pathways included biopterin, butanoate, and xenobiotic metabolism. Relative intensities of selected metabolites are provided in Figure 6. After HpCDD exposure, the purine metabolites xanthine (Fig 6D) and 5-benzoylphospho-adenosine (Fig 6E) were increased while the xenobiotic carbazole, an aromatic hydrocarbon, was found to have decreased (Fig 6F). Notably, associations between HpCDD and cholesterol, triglyceride or fatty acid metabolism was not observed.
Figure 7.
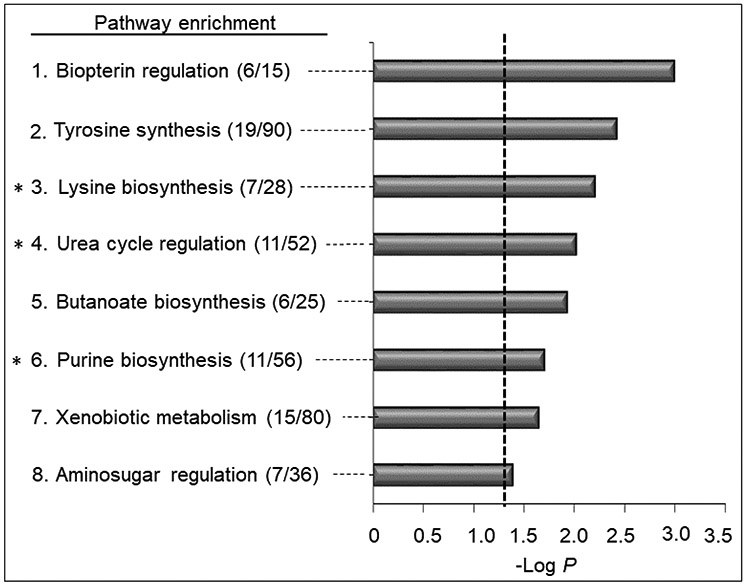
Pathway enrichment analysis associated with exposure to HpCDD. A total of (8/119) enriched pathways were determined (filled gray bars indicate significance less than p < 0.05, and the cutoff is indicated by the dotted line). Numbers in the parentheses indicate the overlap of the metabolites as matched through KEGG and the Edinburgh human metabolic network databases matching using m/z and RT. *Indicates overlapped enriched pathways with 200 pM HpCDD exposure.
Overall, these results show that exposure to the PAH, BghiP, or the dioxin, HpCDD, each elicits changes in amino acid, amino-sugar and urea cycle metabolism. BghiP impacts a broader range of amino acid pathways, however, and also has effects on lipid pathways that were not observed for HpCDD. In contrast, HpCDD had a greater impact on xenobiotic metabolism, with additional effects on biopterin and butanoate metabolism.
Effect of BghiP and HpCDD on gene expression
Because of the common and widespread effects on amino acid metabolism in BghiP- and HpCDD-treated cells, we hypothesized that BghiP and HpCDD affected expression of key amino acid transporters. To test this, we focused on glutamate metabolism because of its central role in amino acid homeostasis and examined several genes including amino acid transporters SLC7A5, also called LAT1 (L-type amino acid transporter 1) and SLC7A11 by quantitative real-time PCR. SLC7A5 is a dominant neutral amino acid transporter for tyrosine, phenylalanine, tryptophan, leucine and arginine (21). SLC7A11 is part of a heterodimeric Na+-independent anionic amino acid transport system for glutamate and cystine (22). Real-time PCR did not reveal any differences in glutamate metabolic mRNA levels but showed increases in abundance of transcripts for two amino acid transporters only in response to HpCDD, namely SLC7A5 and SLC7A11 (Figure 8). Because BghiP impacted amino acid pathways without affecting these transporters, these results indicate that BghiP and HpcDD disrupted amino acid metabolism through different mechanisms.
Figure 8.
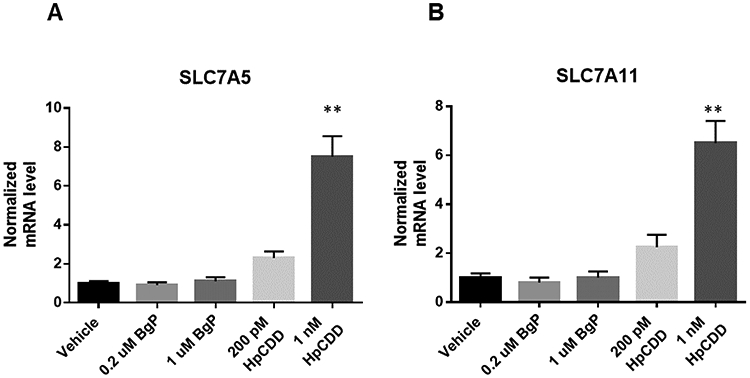
Alterations in amino acid transporters gene expression by BghiP or HpCDD exposure. Lung fibroblasts were exposed to BghiP (0.2 and 1 μM) and HpCDD (0.2 and 1 nM) for 24 h and changes to SLC7A5 (A); and SLC7A11 (B) were monitored. Results were normalized using the ΔΔCt method with the reference genes TBP and HPRT1. HpCDD exposure at 1 nM increased both transporters compared to vehicle. p < 0.01, n=4 per group.
DISCUSSION
Previous studies showed lung pathology associations with PAH and dioxin exposure (23-27). Go et al (Go et al. JOEM 2019 Current Supplement) found that Service Personnel deployed to Balad showed altered amino acid metabolism in the post-deployment serum samples. The present research extends these observations by providing a complementary MWAS of dioxins in post-deployment serum of service personnel. Results showed associations of dioxins and furans with cholesterol, triglyceride, and fatty acid metabolism (Fig 1), which are consistent with previous observations for benzo(a)pyrene (BaP) in both human serum studies and cell culture models (Smith et al. JOEM 2019 Current Supplement). The present research builds upon the human serum correlation studies by identifying changes in amino acid pathways and expression of amino acid transporters in human fibroblast cell strains in response to the dioxin HpCDD at concentrations found in service personnel. Together with accompanying in vitro studies (Smith et al. JOEM 2019 Current Supplement; Woeller et al. JOEM 2019 Current Supplement), this research provides strong evidence for cause-effect relationships of PAHs, dioxins, and furans with metabolic changes observed in DoDSR. These results provide a critical link to understand the relationships between external exposures, internal body burden, biologic responses, and health outcomes.
Concentrations selected for both BghiP and HpCDD were close to those detected in serum samples from the original cohort. For example, the mean detection value of BghiP in case post samples was around 0.087 μM with a max detection of 2.8 μM (4). We used doses of 0.2 and 1 μM BghiP and found several significant changes in the HLF metabolome. The mean concentration of HpCDD in post-deployment samples was 0.06 nM with a maximum detection of 0.84 nM (4). We used a similar range from 0.2 to 1 nM HpCDD, and the results showed a continuum of effects over this range. The results of the cell studies showed that metabolic characteristics were overlapping for the different exposure levels used, with indistinct separation of metabolic profiles for different exposure levels. Thus, even though the concentrations used were realistic, the extent of perturbations for the lower doses of BghiP and HpCDD (Fig 2) was small. This result highlights the difficulty in evaluation of adverse impacts of exposure, especially when there are multiple agents that affect common systems (e.g., see Fig 1).
BghiP exposure most often occurs due to incomplete combustion of diesel fuels (3, 4, 28, 29). We observed that in the high-dose exposed cells, 498 features were altered in response to BghiP with approximately half being increased, and the remaining decreased (Fig 3). Many of these features were mapped to central metabolic pathways, such as pyrimidine and purine regulation for nucleic acid metabolism, β-oxidation for energy production and ω-3 and ω-6 fatty acid metabolism for cell signaling (Fig 4). Several amino acid pathways, lysine, histidine, tyrosine, aspartate and asparagine, as well as the arginine and proline pathways were also found to vary with BghiP. Tyrosine and methionine, which are used in many cellular processes such neurotransmitter biosynthesis, phosphorylation signaling, and ubiquinone biosynthesis , were decreased in response to BghiP (Fig 6A, B). Decreased methionine is of particular interest because methionine is a precursor for cysteine which is required for maintenance of the antioxidant, glutathione. 4-methyl-glutamate, a metabolite within the C5-branched dibasic acid metabolism, was increased, implying that BghiP could induce changes in carbohydrate metabolism.
The dose response characteristics to BghiP showed that the exposure levels were in a range causing increased impact with higher dose. One third of the pathways were detected only at the higher dose. These effects could reflect increased adaptive responses through signaling by products of ω-3 and ω-6 fatty acids, increased pyrimidine turnover for cell repair, and/or increased N-glycan from turnover and repair of basement membrane and interstitial elements. Changes in β-oxidation could similarly represent perturbation of energy metabolism to maintain cellular homeostasis or respond to cellular damage. Many of these pathway effects were apparent in the MWAS of deployment to either Balad or Bagram (Go et al. JOEM 2019 Current Supplement). For instance, N-glycan and pyrimidine metabolism changed with deployment to Balad while ω-3 fatty acid metabolism and β-oxidation were altered with deployment to Bagram.
With HpCDD treatment of cells, we found alterations in five of the same pathways as observed for BghiP, with amino-sugar and urea cycle changes being common to changes seen with deployment to Balad. We did not observe alterations in specific metabolic pathways associated with deployment to Bagram, however the lipid associations with HpCDD seen in xMWAS analysis of post-deployment samples (Fig 1) were observed with deployment to Bagram (Go et al. JOEM 2019 Current Supplement). These results indicate that studies of HpCDD in cell types that are more active in lipid metabolism may be needed to test whether changes in lipid metabolism are mechanistically linked to HpCDD exposure. In the human lung fibroblasts, purine, lysine, and the urea cycle pathways were the most sensitive pathways to HpCDD exposure. Metabolites found to be increased by HpCDD included xanthine, which is a purine that leads to oxidative stress through generation of the toxic superoxide anion radical by xanthine oxidase (30). Other pathways altered by HpCDD included biopterin regulation, butanoate, and xenobiotic metabolism (Fig 7). Biopterin is needed for nitric oxide production and tyrosine metabolism.
Altogether, the most common pathways altered due to deployment and in vitro due to treatment with BghiP and HpCDD were the pathways of amino acid metabolism. Prior research has shown that abundance of key amino acid transporters is affected by dioxin exposure (31, 32). Results confirmed that 1 μM HpCDD increased expression of genes for SLC7A5 and SLC7A11 amino acid transporters (Fig 8). Because SLC7A5 encodes a transporter for tyrosine, and SLC7A11 encodes a protein subunit for cysteine and glutamate transport, both of which are linked to lysine transport, these results implicate amino acid transport in the biologic responses to HpCDD and possibly other dioxins. SLC7A11 is also recognized as a key response to oxidative stress, thereby implicating oxidative stress as an important contributing mechanism.
CONCLUSIONS
The present research shows that fatty acid and steroid metabolism varied with dioxin and furan contents in human serum as reflected in measurements of DoDSR samples for Service Personnel deployed to Balad, Iraq and Bagram, Afghanistan. In vitro studies with 3 strains of human lung fibroblasts showed metabolic perturbation to fatty acids by exposure to BghiP and additional amino effects by BghiP or HpCDD treatment. HpCDD treatment increased abundance of transcripts for key amino acid transporters, which could contribute to underlying mechanisms. The results further indicated that the effects of BghiP and HpCDD occurred through different mechanisms. In combination with other metabolomics and gene expression studies, these results begin to clarify the complex pathway responses contributing to diseases associated with deployment to high-risk areas.
Supplementary Material
Acknowledgements
The authors would like to acknowledge ViLinh Tran and Ken Liu for their technical expertise with the mass spectrometer. This public health surveillance project was supported by funding from the Department of Defense award (306889-1.00-64239), and National Institute of Health (award R01 ES023485, P30 ES019776 and S10 OD 018006).
Footnotes
Disclaimer
The opinions expressed are those of the authors and do not necessarily reflect the official positions of the Uniformed Services University, the U.S. Departments of Defense, the Army and the Air Force, the U.S. Army Public Health Center (Provisional) or Emory University.
Conflicts of interest: None to declare
REFERENCES
- 1.Abraham JH, Eick-Cost A, Clark LL, et al. A retrospective cohort study of military deployment and postdeployment medical encounters for respiratory conditions. Military medicine. 2014;179:540–546. [DOI] [PubMed] [Google Scholar]
- 2.Smith B, Wong CA, Boyko EJ, et al. The effects of exposure to documented open-air burn pits on respiratory health among deployers of the Millennium Cohort Study. J Occup Environ Med. 2012;54:708–716. [DOI] [PubMed] [Google Scholar]
- 3.Masiol M, Mallon CT, Haines KM Jr., Utell MJ, Hopke PK. Source Apportionment of Airborne Dioxins, Furans, and Polycyclic Aromatic Hydrocarbons at a United States Forward Operating Air Base During the Iraq War. J Occup Environ Med. 2016;58:S31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia X, Carroll-Haddad A, Brown N, Utell MJ, Mallon CT, Hopke PK. Polycyclic Aromatic Hydrocarbons and Polychlorinated Dibenzo-p-Dioxins/Dibenzofurans in Microliter Samples of Human Serum as Exposure Indicators. J Occup Environ Med. 2016;58:S72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crapo JD, Barry BE, Gehr P, Bachofen M, Weibel ER. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis. 1982;126:332–337. [DOI] [PubMed] [Google Scholar]
- 6.Lacy SH, Woeller CF, Thatcher TH, et al. Human lung fibroblasts produce proresolving peroxisome proliferator-activated receptor-gamma ligands in a cyclooxygenase-2-dependent manner. Am J Physiol Lung Cell Mol Physiol. 2016;311:L855–L867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baglole CJ, Ray DM, Bernstein SH, et al. More than structural cells, fibroblasts create and orchestrate the tumor microenvironment. Immunol Invest. 2006;35:297–325. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman J, Graf BA, Leung EC, et al. Fibroblasts as sentinel cells: role of the CDcd40-CDcd40 ligand system in fibroblast activation and lung inflammation and fibrosis. Chest. 2001;120:53S–55S. [DOI] [PubMed] [Google Scholar]
- 9.Go YM, Kim CW, Walker DI, et al. Disturbed flow induces systemic changes in metabolites in mouse plasma: a metabolomics study using ApoE(−)/(−) mice with partial carotid ligation. Am J Physiol Regul Integr Comp Physiol. 2015;308:R62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Go YM, Kim CW, Walker DI, et al. Disturbed flow induces systemic changes in metabolites in mouse plasma: a metabolomics study using ApoE(−)/(−) mice with partial carotid ligation. Am J Physiol Regul Integr Comp Physiol. 2015;308:R62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones DP, Walker DI, Uppal K, Rohrbeck P, Mallon CT, Go YM. Metabolic Pathways and Networks Associated With Tobacco Use in Military Personnel. Journal of occupational and environmental medicine 2016;58:S111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu T, Park Y, Li S, Jones DP. Hybrid feature detection and information accumulation using high-resolution LC-MS metabolomics data. J Proteome Res. 2013;12:1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uppal K, Soltow QA, Strobel FH, et al. xMSanalyzer: automated pipeline for improved feature detection and downstream analysis of large-scale, non-targeted metabolomics data. BMC Bioinformatics. 2013;14:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8:118–127. [DOI] [PubMed] [Google Scholar]
- 15.Patel RM, Roback JD, Uppal K, Yu T, Jones DP, Josephson CD. Metabolomics profile comparisons of irradiated and nonirradiated stored donor red blood cells. Transfusion. 2015;55:544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smyth GK, Michaud J, Scott HS. Use of within-array replicate spots for assessing differential expression in microarray experiments. Bioinformatics. 2005;21:2067–2075. [DOI] [PubMed] [Google Scholar]
- 17.Uppal K, Walker DI, Liu K, Li S, Go YM, Jones DP. Computational Metabolomics: A Framework for the Million Metabolome. Chem Res Toxicol. 2016;29:1956–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khatri P, Sirota M, Butte AJ. Ten years of pathway analysis: current approaches and outstanding challenges. PLoS Comput Biol. 2012;8:e1002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uppal K, Ma C, Go YM, Jones DP, Wren J. xMWAS: a data-driven integration and differential network analysis tool. Bioinformatics. 2018;34:701–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. [DOI] [PubMed] [Google Scholar]
- 21.Scalise M, Galluccio M, Console L, Pochini L, Indiveri C. The Human SLC7A5 (LAT1): The Intriguing Histidine/Large Neutral Amino Acid Transporter and Its Relevance to Human Health. Front Chem. 2018;6:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koppula P, Zhang Y, Shi J, Li W, Gan B. The glutamate/cystine antiporter SLC7A11/xCT enhances cancer cell dependency on glucose by exporting glutamate. J Biol Chem. 2017;292:14240–14249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker DI, Pennell KD, Uppal K, et al. Pilot Metabolome-Wide Association Study of Benzo(a)pyrene in Serum From Military Personnel. J Occup Environ Med. 2016;58:S44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker DI, Go Y-M, Liu K, Pennell KD, Jones DP. Chapter 7 - Population Screening for Biological and Environmental Properties of the Human Metabolic Phenotype: Implications for Personalized Medicine In: Holmes E, Nicholson JK, Darzi AW, Lindon JC, eds. Metabolic Phenotyping in Personalized and Public Healthcare. Boston: Academic Press; 2016:167–211. [Google Scholar]
- 25.Kampa M, Castanas E. Human health effects of air pollution. Environ Pollut. 2008;151:362–367. [DOI] [PubMed] [Google Scholar]
- 26.Woeller CF, Thatcher TH, Van Twisk D, et al. MicroRNAs as Novel Biomarkers of Deployment Status and Exposure to Polychlorinated Dibenzo-p-Dioxins/Dibenzofurans. J Occup Environ Med. 2016;58:S89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woeller CF, Thatcher TH, Van Twisk D, et al. Detection of Serum microRNAs From Department of Defense Serum Repository: Correlation With Cotinine, Cytokine, and Polycyclic Aromatic Hydrocarbon Levels. J Occup Environ Med. 2016;58:S62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simoneit BRT. Biomass burning - A review of organic tracers for smoke from incomplete combustion. Appl Geochem. 2002;17:129–162. [Google Scholar]
- 29.Baek SO, Field RA, Goldstone ME, Kirk PW, Lester JN, Perry R. A Review of Atmospheric Polycyclic Aromatic-Hydrocarbons - Sources, Fate and Behavior. Water Air Soil Poll. 1991;60:279–300. [Google Scholar]
- 30.Kuppusamy P, Zweier JL. Characterization of free radical generation by xanthine oxidase. Evidence for hydroxyl radical generation. J Biol Chem. 1989;264:9880–9884. [PubMed] [Google Scholar]
- 31.Le Vee M, Jouan E, Fardel O. Involvement of aryl hydrocarbon receptor in basal and 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced expression of target genes in primary human hepatocytes. Toxicol In Vitro. 2010;24:1775–1781. [DOI] [PubMed] [Google Scholar]
- 32.Goode G, Pratap S, Eltom SE. Depletion of the aryl hydrocarbon receptor in MDA-MB-231 human breast cancer cells altered the expression of genes in key regulatory pathways of cancer. PLoS One. 2014;9:e100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.