Abstract
Osteoporosis is an age-related deterioration in bone health that is, at least in part, a stem cell disease. The different mechanisms and signaling pathways that change with age and contribute to the development of osteoporosis are being identified. One key upstream mechanism that appears to target a number of osteogenic pathways with age is kynurenine, a tryptophan metabolite and an endogenous Aryl hydrocarbon receptor (AhR) agonist. The AhR signaling pathway has been reported to promote aging phenotypes across species and in different tissues. We previously found that kynurenine accumulates with age in the plasma and various tissues including bone and induces bone loss and osteoporosis in mice. Bone marrow mesenchymal stem cells (BMSCs) are responsible for osteogenesis, adipogenesis, and overall bone regeneration. In the present study, we investigated the effect of kynurenine on BMSCs, with a focus on autophagy and senescence as two cellular processes that control BMSCs proliferation and differentiation capacity. We found that physiological levels of kynurenine (10 and 100 μM) disrupted autophagic flux as evidenced by the reduction of LC3B-II, and autophagolysosomal production, as well as a significant increase of p62 protein level. Additionally, Kynurenine also induced a senescent phenotype in BMSCs as shown by the increased expression of several senescence markers including senescence associated β-galactosidase in BMSCs. Additionally, western blotting reveals that levels of p21, another marker of senescence, also increased in kynurenine-treated BMSCs, while senescent-associated aggregation of nuclear H3K9me3 also showed a significant increase in response to kynurenine treatment. To validate that these effects are in fact due to AhR signaling pathway, we utilized two known AhR antagonists: CH-223191, and 3’,4’-Dimethoxyflavone to try to block AhR signaling and rescue kynurenine/AhR mediated effects. Indeed, AhR inhibition restored kynurenine-suppressed autophagy levels as shown by levels of LC3B-II, p62 and autophagolysosomal formation demonstrating a rescuing of autophagic flux. Furthermore, inhibition of AhR signaling prevented the kynurenine-induced increase in senescence associated β-galactosidase and p21 levels, as well as blocking aggregation of nuclear H3K9me3. Taken together, our results suggest that kynurenine inhibits autophagy and induces senescence in BMSCs via AhR signaling, and that this may be a novel target to prevent or reduce age-associated bone loss and osteoporosis.
Keywords: Osteoporosis, Kynurenine, Aging, Autophagy, Senescence, Human Bone Marrow Stromal Cells
1. Introduction
Bone loss is one of the most deleterious outcomes to aging. As humans age, the chance for bone structure deterioration and loss increases. As a result, bone fracture rates become higher. Recent studies recognized that in age-related osteoporosis, pathological changes in bone marrow mesenchymal stem cells (BMSCs) play an important role in their ability to differentiate into various bone cell types as well as to secrete a number of regulatory cytokines [1, 2]. In aged bone, osteoblasts demonstrate functional decline while osteoclasts show excessive proliferation and activity. This disrupts the balance between bone resorption and remodeling contributing to the bone loss and increased fragility. Therefore, interference with normal homeostatic functions of BMSCs is deleterious for maintaining bone integrity [3]. A critical gap in our understanding of aging bone, age-associated bone loss and osteoporosis is related to the factors that drive changes in osteogenic homeostasis and the mechanisms that are affected leading to those changes.
Tryptophan metabolites may be a key factor in bone aging. Tryptophan is an essential amino acid that is a main substrate for de novo production of nicotinamide adenine dinucleotide (NAD), a key component in sirtuin activation. Sirtuin is implicated in a wide variety of biological functions including control of cellular metabolism, energy homeostasis, aging and longevity [4] [5] [6]. Importantly a number of metabolites of the tryptophan degradation pathways demonstrate significant bioactivities and are involved in several physiological and pathological processes. Kynurenine (KYN), the first major stable tryptophan metabolite, together with quinolinic acid, another tryptophan metabolite, both have been shown to accumulate in plasma and tissues with age [7, 8]. We have concentrated on kynurenine since our group and others have demonstrated that increased levels of kynurenine in aged mice and humans contribute to age-induced bone loss and correlate with increased bone frailty, affecting the balance between bone resorption and remodeling by targeting BMSCs and osteoclasts [9, 10] [11]. Previously, our group demonstrated the protective role of cytokine SDF-1 (CXCL12) on bone marrow stem cell survival and osteogenic differentiation [12]. CXCL12 is involved in bone formation through regulation of recruitment, engraftment, proliferation, and differentiation of stem/progenitor cells. We also have shown that the protective effect of the CXCR4/CXCL12 pathway during oxidative stress in BMSCs is associated with enhancement of autophagy [13]. Our group and others have demonstrated that the increased level of kynurenine in aged mice and humans contribute to age-induced bone loss and correlates with increased bone loss by affecting both bone resorption and formation [9, 10] [11]. Recently, our group demonstrated that kynurenine acts at least in part through AhR activation of the microRNA miR29b-1–5p to decrease the expression and activity of cytokine CXCL12 [14]. Previous studies have shown that the increase of kynurenine levels in aged bones can be attributed to both the direct oxidation of tryptophan as a result of elevated free radicals, and more importantly as a result of increased activity of the tryptophan degrading enzyme indoleamine-2,3-dioxygenase (IDO) [15, 16]. kynurenine’s actions are thought to be principally mediated by the aryl hydrocarbon receptor (AhR) [17, 18]. Upon binding of kynurenine to the AhR in the cytoplasm the ligand-receptor complex is transported into the nucleus where it competes with HIF-1a to bind to the aryl hydrocarbon receptor nuclear translocator (ARNT) and then acts as a transcription factor binding to the xenobiotic response element, rather than the HIF-1a associated hypoxia response element, for a number of genes [19, 20]. In addition to kynurenine targeting CXCL12 regulating epigenetic miRNA and Hdac pathways [14], there is evidence that kynurenine targets metabolic pathways critical for osteogenic homeostasis, in particular autophagy and senescence.
Autophagy, a process of recycling of non-essential or damaged organelles and proteins plays an important role in maintaining cell homeostasis and survival under stressful conditions. It helps to maintain a delicate balance between various bone marrow populations and promoting damage control. Beclin-1 is required for RANKL-induced osteoclast differentiation [21]. Activation of autophagy through HIF-1 alpha pathway is needed for hypoxia-induced osteoclastogenesis as well as MSC differentiation and protection from ROS, which increase with age [22–24]. An increase in autophagy can reduce bone loss, while a decrease in autophagy or its disregulation is associated with a decline in bone growth and increased bone loss with aged mice,. [25, 26].
Premature cellular senescence is considered as one of the hallmarks of aging in addition to genomic instability, epigenetic alterations and stem cell exhaustion [27]. Although senescent cells and their senescence-associated secreted phenotype (SASP) may play a beneficial role in tissue regeneration and the prevention of cell loss at low levels, they contribute to various pathological processes when they accumulate excessively in aged tissues [28], For example, it has been shown that SASP accumulated in aged bones and correlated with bone growth decline [29]. Age-related senescence and increased SASP expression has been shown to be responsible for osteoblasts and BMSC dysfunction leading to decreased proliferation, and the accumulation of DNA damage [30, 31].
In the current paper we explored the effect of the AhR-kynurenine pathway on BMSC homeostasis in relations with aged bone remodeling, with a focus on kynurenine-mediated changes in BMSC autophagy and senescence.
2. Materials and Methods:
2.1. Reagents and antibodies:
Kynurenine (K8625), anti-beta-actin antibody (A5441), Aryl hydrocarbon (AhR) inhibitors, CH-223191 (C8124) and 3’4’-dimetylflavone (3’4’DMF) (D6571) and all other reagents were purchased from Sigma (St. Louis, MO) unless otherwise specified. Anti-LC3B antibody (2775S) and anti p62 antibodies (23214) were from Cell Signaling (Danvers, MA). Anti-Histone H3K9me3 (ab176916), anti-p21 (ab109199), anti-LAMP2 (ab13524) antibodies were from Abcam (Cambridge, MA). Anti-p16 antibody (sc-166760) were from Santa Cruz (Dallas TX). Anti-AhR antibody (AF6697) were from R&D System (Minneapolis, MN).
2.2. BMSCs isolation and culture:
BMSCs were isolated from 18 months old mice as described previously [9, 10]. Briefly, BMSCs were isolated from the long bones of 18-month-old C57BL/6 mice. The mice were euthanized and the femora and humeri were removed. The marrow was then flushed with PBS and the cellular material harvested. The cellular material was then centrifuged, and the pellet washed with PBS. The cells were then plated in 100 cm2 culture plates with DMEM medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 50 U/mL penicillin/streptomycin, and 2mM L-glutamine. After 24 hours, the supernatants were removed, and the adherent stromal cells were trypsinized. A negative selection process was then used to deplete the cells of hematopoietic lineages (T-lymphocytic, B-lymphocytic, myeloid, and erythroid cells) using a commercially available kit (BD Biosciences, San Jose, CA, USA), thus retaining the progenitor (stem) cell population. The positive fractions were collected using the following parameters: negative for CD3e (CD3 e chain); CD11b (integrin aMchain); CD45R/B220; Ly-6G and Ly-6C (Gr-1); and TER-119/Erythroid Cells (Ly-76). Next, positive selections were performed using the anti-Stem cell antigen-1 (Sca-1) column magnetic bead sorting kit (Miltenyi Biotec, Bergisch Gladbach, Germany).
2.3. Kynurenine treatment and proteins expression detection:
Cells were plated and cultured in DMEM supplemented with 10% FBS and antibiotics. Serum starvation was performed by incubation in DMEM supplemented with 1% FBS overnight. After that, cells were treated with kynurenine in doses indicated. Some cells were co-treated with 25μM of autophagy inhibitor, chloroquine (CQ) or with AhR inhibitors as described below. After the treatment, cells were lysed in RIPA buffer supplemented with protease and phosphatase inhibitors. Proteins expression was determinate by Western blotting using appropriate antibodies. Changes in protein expression were quantified using Image Lab software (Bio-Rad). Alternatively, kynurenine-treated cells were subject to immunofluorescent staining as described below.
2.4. Autophagy detection and immunofluorescent staining:
Several methods were used to detect autophagic processes in kynurenine-treated BMSC consistent with guidelines for monitoring autophagy [32]. First, LC3B-II, which is required during autophagosome membrane formation and elongation, together with changes in expression of p62, a receptor for cargo to be degraded by autophagy, which includes ubiquitinated protein aggregates destined for clearance, were analyzed by measuring the protein’s expression using Western blotting with LC3B-II or p62 antibodies. Additionally, the formation of mature autophagosomes was detected by staining them with monodansylcadaverine (MDC), a selective fluorescent marker for autophagic vacuoles under in vivo conditions [33] using an autophagy detection kit (ab139484, Abcam, Cambridge, MA). Briefly, BMSCs were plated in 8-well chamber slides and serum-starved overnight with DMEM medium supplemented with 1% FBS to drive autophagy, and then were treated with 10 or 100 μM of kynurenine, with or without, 25μM of chloroquine (CQ) to assess autophagic flux. Alternatively, some cells were additionally treated with AhR inhibitors, CH-223191 or 3’4’DMF. After the treatment, lysosomal/autophagic vacuoles were visualized with MDC according to manufacturer’s protocol. Cells were fixed with 4% paraformaldehyde (PFA) solution and immunofluorescent images were taken using ImageXpress Micro High Content Imager (Molecular Device, San Jose, CA). The green fluorescence intensity was quantified using ImageJ software. Finally, colocalization of the autophagy marker, LC3B-II and lysosomal marker, LAMP2 which occurs with autophagolysosomal formation were detected by immunofluorescent staining. BMSCs were plated on 8-well chambers and treated as described above. Cells were fixed with 4% PFA solution, permeabilized with 0.1% Triton X-100 solution in PBS. Cells were then blocked with 1% normal donkey serum and LC3B and LAMP2 proteins were detected using appropriate antibodies. Alexa Fluor-488 and Alexa Fluor-596-conjugated secondary antibodies were used to visualize the LC3B-II and LAMP2, respectively. Nuclei were stained with DAPI. Images were taken with a Nikon i90 fluorescent microscope.
2.5. Senescence detection:
To detect senescence in kynurenine-treated BMSC, several methods were used. First, the increase of SA-beta-galactosidase (β-gal), which is a accumulating in senescent cells [34] [35], was detected using a SA-β-gal senescence assay kit (Cell Signaling, cat# 9860, Denver, MA). Briefly, after treatment with kynurenine with or without AhR inhibitors, BMSCs were washed with PBS and fixed with fixative solution and stained with β-gal reaction solution at 37°C for 48h according to manufacturer’s protocol. Images were taken using a Nikon i90 inverted microscope with bright field and the intensity of SA-β-gal staining was analyzed using ImageJ software. Additionally, the changes of expression of senescence markers, p21 and p16 were detected using Western blotting analysis using appropriate antibodies. Age-related DNA damage accumulation leads to specific heterochromatin reorganization with formation of distinct nuclear structures known as Senescence-Associated Chromatin Foci (SAHF). SAHF contribute to senescence-associated cell growth arrest by sequestering and silencing proliferation-promoting genes such as the E2F target gene cyclin A [36]. SAHF were detected by staining BMSCs with Anti-Histone H3 (tri methyl K9) antibody and visualized using anti-rabbit Alexa Fluor-488 conjugated antibody. Nuclei were stained with DAPI. Immunofluorescent images were taken using Nikon i90 inverted fluorescent microscope using appropriate settings.
2.6. Statistical analysis:
In each experiment, control and experimental cells were matched for cell line, age, seeding density, number of passages, and number of days post-confluence to avoid variation in tissue culture factors that can influence measurements. Results are shown as means ± SE for n experiments. One-way ANOVA and post t test analyses were used to determine the significance of differences between the means of different groups. P<0.05 was considered statistically significant.
3. Results:
3.1. Kynurenine disrupts autophagy inhibiting serum-starvation induced macroautophagy in BMSCs:
To assess the effect of KYN on serum-starvation induced autophagy, we treated murine BMSCs isolated from bone marrow of 18 months old mice with different doses of KYN. To assess autophagic flux, or the overall level of autophagy, we performed the experiments in the presence or absence of the lysosomal inhibitor chloroquine, which blocks the final stages of autolysosomal turnover allowing them to accumulate and report total flux. Following autophagosome and lysosome fusion, chloroquine prevents lysosomal enzyme activation and the degradation of autolysosome contents by blocking acidification. This also blocks the overall turnover of the autolysomes and their cargos allowing their accumulation and measurement of LC3B-II as a marker of the degree of autophagic flux. As shown in Figs. 1A–B, control treatment of BMSCs in the presence of chloroquine resulted in accumulation of LC3B-II, demonstrating normal flux. In contrast, samples treated with kynurenine in the presence of chloroquine showed a reduction in the accumulation of LC3B-II, suggesting that the overall autophagic process was impaired. Additionally, we analyzed the level of mature autophagosomes by detecting the incorporation of monodansylcadaverien (MDC) into the BMSCs. Again, while control treatment of BMSCs showed robust accumulation of MDC-labeled puncta representing the accumulation of autophagosomes and autophagolysosomes. Kynurenine treatment at both 10 and 100 μM inhibited autophagic flux in a dose dependent manner (Figure 1C.). We also measured an expression level of p62, a third autophagy marker, which can be used to assess flux without inhibiting the final autophagolysosomal degradation step (e.g. with chloroquine). P62 is selectively incorporated into autophagosomes by binding to membrane LC3 and is degraded during normal autophagy. The total cellular expression levels of p62 are inversely correlated with the degree of autophagic activity or flux. Accumulation of p62 protein suggests that cells are autophagy deficient or autophagic flux is disrupted [37]. Consistently with other autophagy markers, the level of p62 protein in kynurenine-treated cells was elevated in dose-dependent fashion after 48hr of treatment indicating an impairment of autophagy (Fig. 1D). Finally, we detected changes in co-localization of LC3B with the lysosomal marker, LAMP2 that occurs in the late stage of autophagic flux during autophagolysosome formation. In control cells, consistently with effect of CQ on LC3B-II level accumulation and autophagosome formation, treatment with 25μM of CQ resulted in increased colocalization of LC3B and LAMP2 suggesting the last stages of autophagy with autophagosome-lysosome fusion were achieved demonstrating normal robust autophagic flux. (Fig. 1E). On the contrary, BMSCs treated with different doses of kynurenine in the presence of CQ, showed a significant reduction in the colocalization of LC3B and LAMP with more individual lysosome staining, supporting the idea that the late stage fusion of autophagosomes and lysosomes was inhibited. Together, these data indicated that in BMSCs kynurenine inhibits autophagy in a dose dependent fashion. Further, that this appears to be occurring at the later stages of autophagy affecting autophagosome maturation and autophagolysosome formation.
Figure 1. Kynurenine treatment disrupts autophagic flux.
A-B. LC3B-II expression in BMSCs treated with (10 μM or 100 μM) kynurenine (KYN) for 12h with, or without, 25 μM of chloroquine (CQ). Data are expressed as changes of LC3B-II/β-actin ratio adjusted to Vehicle-control. C. Monodansylcadaverine fluorescence intensity staining in BMSCs treated with indicated doses of KYN with or without 25 μM of CQ for 12h. D. A representative immunoblotting of p62 protein expression changes under KYN treatment. Data are expressed as changes of LC3B-II/β-actin ratio adjusted to Vehicle-control. E. LC3B/LAMP2 colocalization under KYN treatment detected by immunostaining with LC3B (green) and LAMP2 (red) antibodies, respectively. Nuclei were stained with DAPI (blue). All data are representative of at least three independent experiments with two replicates for each group. * - p< 0.05 vs. vehicle-control; # - p< 0.05 vs. vehicle+CQ
3.2. Kynurenine increases senescence in serum-starved BMSCs:
Our previous studies have shown that kynurenine elevation correlates with inhibition of osteogenesis in BMSCs and may be related to the decreased expression of the cytokine stromal cell-derived factor 1 (SDF-1/CXCL12) which is essential for BMSCs proliferation and homing [11, 38]. In the present study, we tested the idea that kynurenine induces the cell-function inhibitor and antiproliferation process of senescence in BMSCs. As shown in Figs. 2 A–B, treatment of BMSCs with KYN for 24hr significantly increased SA-beta-galactosidase activity, a hallmark of senescence. Additionally, the expression levels of the Cyclin D kinase (CDK) inhibitor, p21 was significantly elevated under kynurenine treatment while the level of another CDK inhibitor, p16 remained unchanged (Fig. 2C, D). These data suggest that treatment with KYN promotes senescence in BMSCs. Interestingly, the increase of p21 but not p16 indicate that p53/p21 pathway rather than pRb/p16 pathway is responsible for cell cycle arrest resulting in senescence in kynurenine-treated BMSC. Using a third independent assessment of senescence induction we also analyzed the formation of senescence-associated chromatin foci (SAHF) by detecting the aggregation of methylated Histone3 condensation in nuclei. Treating BMSCs with different doses of kynurenine for 48 hrs. resulted in increased numbers of condensed Histone-3 methylation foci in the nuclei (Fig. 2E). Together, these data indicate that kynurenine increases senescence in BMSCs.
Figure 2.
A-B. Representative images of β-galactosidase staining in BMSCs treated with indicated doses of KYN for 3 and 24h. Data are expressed as β-gal-positive staining area percentage and are representative of at least 3 independent experiments. * p< 0.05 vs. 0μM KYN; ** - p< 0.05 vs. KYN 100μM at 3 hr. treatment. C., D. – Expression of p21 and p16 in BMSCs treated with indicated doses of KYN for 24 hr. Data are expressed as protein/β-actin ratio adjusted to 0μM KYN. * p< 0.05 vs. 0 μM KYN. E. SAHP detection by Histone 3-methyl-9K nuclear staining in BMSCs treated with indicated doses of KYN for 24h. Nuclei were stained with DAPI (blue). All data are representative of at least 3 independent experiments.
3.3. Kynurenine suppresses autophagy and increases senescence via the Aryl hydrocarbon receptor pathway:
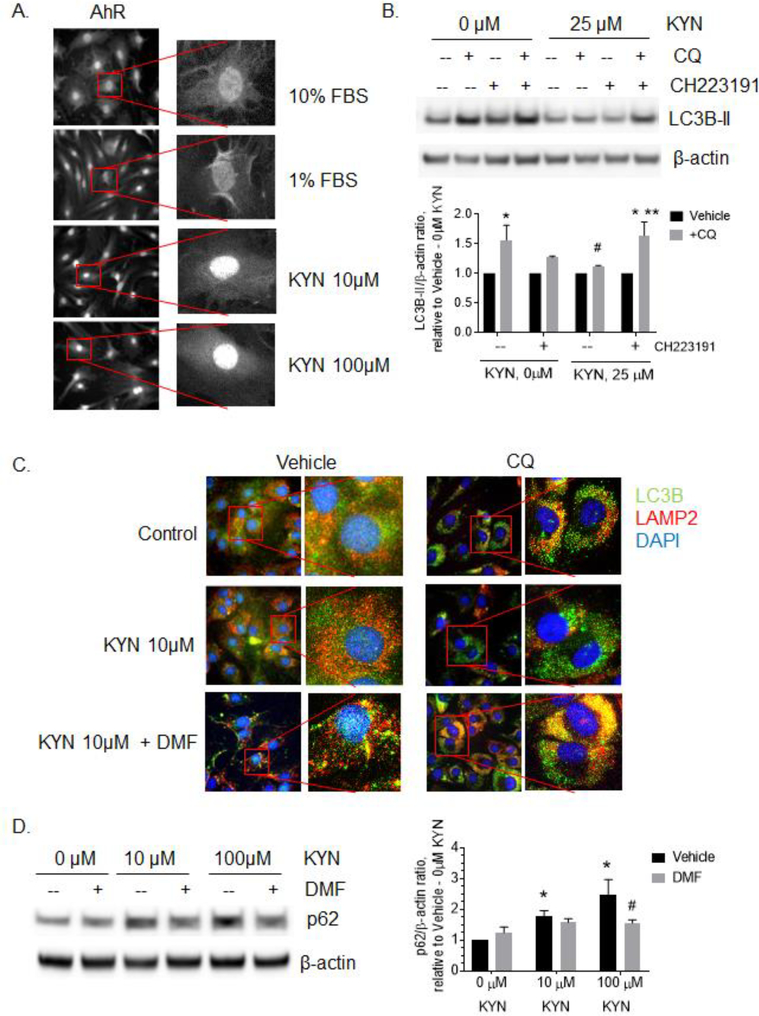
Kynurenine is a well-known ligand of the Aryl hydrocarbon receptor (AhR), which is emerging to play a significant role in bone metabolism by suppressing osteogenesis [39, 40]. We tested the potential role of kynurenine mediated AhR activation in BMSCs. Upon activation, AhR translocates to the nucleus where it binds to the transcription factor, ARNT, and activates the transcription of target genes. As shown in Fig. 3A, treatment of BMSCs with different doses of kynurenine resulted in elevated immunofluorescent AhR nuclear staining. Interestingly, serum starvation alone, induced by incubating BMSCs in medium supplemented with 1% FBS for 24 hours rather than 10% FBS, resulted in only minimal AhR nuclear translocation (Fig. 3A). To test whether AhR activation is important to mediate kynurenine action on autophagy and senescence in serum-depleted BMSCs, we treated BMSCs with 10 or 100 μM of kynurenine in the presence of two AhR competitive inhibitors, CH-223191 or 3’4’-dimethyl flavone (DMF). Inhibition of AhR by CH-223191 partially restored kynurenine suppressed autophagic flux as assessed by LC3B-II accumulation (Fig. 3B). Additionally, DMF treatment also demonstrated a restoration of autophagic flux following kynurenine mediated inhibition showing increased LC3/LAMP2 colocalization in BMSCs in the presence of chloroquine (Fig. 3C). Additionally, inhibition of AhR by DMF abolished the kynurenine-induced elevation of autophagy marker, p62 (Fig. 3D). These independent approaches support both that the AhR pathway is involved in the kynurenine-mediated disruption of autophagy during nutrient depletion, and that autophagy can be rescued by inhibiting AhR regulated kynurenine signaling.
Figure 3.
Inhibition of AhR restores kynurenine-suppressed autophagy in BMSCs. A. AhR nuclear translocation is demonstrated with AhR immunofluorescent localization following treatment with 10 or 100 μM KYN for 12h. B. LC3B-II expression levels in BMSCs treated with 25μM of KYN and 25 μM of CQ with or without 2 μg/ml of AhR inhibitor, CH223191 for 12h. Data expressed as LC3B-II/ β-actin ratio adjusted to 0μM KYN + vehicle. * - p< 0.05 vs. vehicle, # - p< 0.05 vs. 0μM KYN + CQ, ** - p< 0.05 vs no CH223191. All data are representative of at least 3 independent experiments C. LC3B (green) and LAMP (red) immunostaining in BMSCs treated with indicated doses of KYN and CQ with or without 2 μg/ml of AhR inhibitor, 3’4’DMF (DMF). Nuclei were stained with DAPI (blue). Images are representative of 3 independent experiments. D. Expression of p62 in BMSCs treated with indicated doses of KYN for 48h with or without CQ in the presence or absence of 10μM of AhR inhibitor, 3’4’DMF (DMF). Images are representative of at least three independent experiments. Data are expressed as p62/β-actin ratio, adjusted to vehicle-0μM KYN. * p< 0.05 vs. 0μM KYN + vehicle, # - p< 0.05 vs. 100μM KYN + vehicle. All data are representative of at least 3 independent experiments.
At the same time, inhibition of AhR by CH-223191 and 3’4’-DMF, also affected senescence. CH-223191 treatment prevented the increase of kynurenine-induced SA-beta-galactosidase activity (Fig. 4A). Treatment of BMSCs with 3’4’-DMF inhibited kynurenine-induced overexpression of senescence marker, p21 (Fig. 4B). Additionally, AhR inhibition by DMF prevented the formation of senescence-associated chromatin foci (Fig. 4C). Together, these data support that KYN upregulates senescence and suppresses autophagy in BMSC through the AhR pathway.
Figure 4.
Inhibition of AhR prevents kynurenine-induced senescence in BMSCs. A. beta-galactosidase assay in BMSCs treated with indicated doses of KYN for 24h in the presence or absence of 2μg/ml of AhR inhibitor, CH223191. Data are expressed as β-gal-positive staining area percentage and are representative of at least 3 independent experiments with three replicates for each group. * - p< 0.05 vs. 0μM KYN + vehicle, # - p< 0.05 vs. 100μM KYN + vehicle. B. Expression of p21 in BMSCs treated with indicated doses of KYN 48 hr. with or without 10μM of AhR inhibitor, 3’4’-DMF (DMF). Data are expressed as p21/β-actin ratio adjusted to vehicle-0μM KYN. * - p< 0.05 vs. 0μM KYN, # - p< 0.05 vs. KYN + vehicle. C. Histone 3-methyl-9K nuclear staining (green) in BMSCs treated with indicated doses of KYN for 24h with or without 10μM of 3’4’DMF. Nuclei were stained with DAPI (blue). Images are representative of 3 independent experiments with three replicates for each group.
4. Discussion.
Aging is associated with bone loss. Our previous work in murine models has shown that the ratio of the plasma and bone marrow levels of tryptophan, an essential amino acid, relative to its primary metabolite, kynurenine declines with age, while its metabolite increases [9, 11]. Previously, our group have shown that supplementing mice with a kynurenine rich diet results in bone loss, decreased osteogenic activity and an elevated numbers of osteoclasts [9 5]. In this study, we investigate the role of KYN and AhR in disrupting autophagy and increasing senescence in BMSCs linking mechanisms tied to the downregulation of osteogenesis to kynurenine.
Autophagy is a complex dynamic process that has been shown to be essential for the maintenance of long-lived cells, such as neurons, cardiomyocytes, osteocytes, and bone homeostasis [41]. During stressful conditions such as food deprivation, under oxidative stress or other stressors including bio-mechanical stress, autophagy helps in protecting cells by recycling damaged and non-essential organelles and proteins by lysosomes generating new molecular building blocks and energy. Recently, several studies have shown that autophagy is essential for maintaining healthy bone homeostasis [42, 43]. For example, induction of autophagy helps to maintain the function of BMSCs playing the protective role during estrogen-deficiency-induced osteoporosis [26]. Autophagy activation is considered a promising therapeutic approach in glucocorticoid-induced osteoporosis [44]. Autophagy appears to be one of the key factors for efficient MSC differentiation and function, which are in turn critical for the early stages of osteogenesis and inhibition of adipogenesis [22]. Our data indicate that kynurenine suppresses starvation-induced autophagy in BMSC and this may contribute to age-related osteogenesic impairment. Several studies have demonstrated that efficiency of autophagic processes are suppressed with age [45]. However, overactive autophagy itself may result in bone-related pathology. For example, the autophagic/lysosomal degradation of TNF receptor–associated receptor 3 (TRAF3) is an important step in RANKL-induced NF-kB activation leading to stimulation of osteoclastogenesis, which in turn disrupts of balance between osteoclastic bone resorption and osteoblastic bone formation [46]. The cytokine CXCL12 is critical in mediating BMSC osteogenesis. Of interest our group and others recently have shown that activation of the CXCL12/CXCR4 axis under oxidative stress results in increased autophagy in BMSC [13] and that autophagy is essential for bone homeostasis [47, 48] as well as playing a critical role for osteogenic cell survival. We recently have shown that kynurenine treatment decreases CXCL12 protein levels and osteogenesis via the AhR signaling pathway [14]. Together, this may provide a novel mechanism in age-associated autophagy-mediated regulation of BMSC homeostasis.
In addition, cellular senescence, a premature termination of cell division and cell function, is recognized to play both beneficial and detrimental roles in cell physiology and various pathological conditions. Senescence is one of the hallmarks of aging [34]. As we age, several cytokines and ROS accumulate in the cells which lead to induction of DNA damage and disruption of cell cycle [49]. Therefore, senescence – in addition to autophagy - is an important mechanism by which our body attempts to preserve damaged cells. It has been shown that premature senescence affects mesenchymal stem cell function [50]. Senescence-induced growth arrest targets cell cycle regulators including depression of cyclin-dependent kinases (CDKs) and their target proteins, Cyclin A and E through either p53 – p21/Waf1 pathway or through the retinoblastoma protein (pRb) – p16/INK4a pathway. The most prominent CDK inhibitor, p16, acts primarily by binding to CDK4 and CDK6 preventing cells from entering the G1-phase. Another CDK Inhibitor, p21, is activated through the tumor suppressor transcription factor p53 which is a critical regulator of cell protection under various stressful conditions such as low dose radiation, hypoxia, oxidative stress and viral infections. The p21/CDKN1A pathway induces senescence mainly at G1/S1 phase of cell cycle [51, 52]. Lately, a number of studies have been exploring targeting senescence as anti-cancer therapeutic approach suggesting the same approaches may have value in treating osteoporosis [28, 53].
Our results showed that treatment of BMSCs with kynurenine for 48hr significantly increased the level of p21 while the level of p16 remained unchanged. Although both markers are senescent biomarkers elevated during senescence processes, they can be expressed independently and as part of distinct senescent mechanisms. Although both p21 and p16 protein are elevated in response to stress-induced and replicative senescence, the dynamics of changes in protein expression for both are different. The rapid increase of p21 occurs in cells approaching replicative senescence [54] while elevation of p16 occurs after senescence has been already established in culture [55, 56]. This dynamic may explain the differences in p21 and p16 expression observed in our cell culture model of serum starvation-induced senescence. Our findings are consistent with a recent study by Kim et al, in which it has been shown that in different populations of osteoprogenitor cells the level of p21/Cip1, but not p16/Ink4a, was elevated with aging, and that this osteoprogenitor population isolated from old mice also displayed characteristics of DNA damage and secreted SASPs [57]. Since p21 and p16 are regulated by slightly different mechanisms (p53-p21 and p16-pRb, respectively), our results may indicate that kynurenine works through activating p53 tumor suppressor gene or protein. It would be of particular interest to explore the activation of p53 oncogene in aged BMSCs.
Aryl hydrocarbon receptor (AhR) plays an important role in regulation of various pathological and physiological processes. AhR is a main receptor for dioxin compounds and mediates dioxin toxic effects [58]. Upon ligand binding, AhR translocates from cytoplasm into nucleus where it binds to AhR nuclear translocator (ARNT) forming a transcription complex that in turn initiates ligand-specific transcription activity. A number of studies have shown AhR involvement in bone metabolism. Tong et al have shown that in a collagen-induced arthritis mouse model, AhR expression and nuclear translocation were significantly increased. This increase correlated with suppression of osteoblastic markers Runx2 and Alp which reduced MSC differentiation into osteoblasts [39]. Another study by Yu et al showed that a systemic AhR knockout mouse model resulted in an in increase in bone mass together with a decrease in bone resorption and osteoclast formation. The authors showed that osteoclast-specific AhR knockout mice were more resistant to sex hormone deficiency-induced bone loss [40]. In this study, we demonstrated kynurenine treatment induces AhR nuclear translocation in BMSCs and disrupted autophagy where autophagy was previously induced by serum-starvation. Inhibition of the AhR pathway prevented the kynurenine-induced increase of senescence and preserved autophagy in BMSCs, suggesting that AhR pathway is important for mediating kynurenine’s effect in bone remodeling. Interestingly, although a lack of nutrients is a well-known factor triggering senescence and autophagy, our data shown that serum starvation alone did not significantly induce AhR nuclear translocation. This indicates that ligand-specific activation of AhR by kynurenine is essential for AhR-mediated effect on autophagy and senescence under stress conditions. These results are in agreement with findings by Wan et al, which demonstrated that TCDD exposure triggered premature senescence in rat pheochromocytoma (PC12) and human neuroblastoma SH-SY5Y cell lines [59]. Interestingly, a recent study by Yamamoto et al showed that kynurenine expressed by undifferentiated human embryonic stem cells activates AhR in a paracrine signaling fashion and that activation of the IDO-AhR pathway prevented hESCs from differentiating [60]. At the same time the homeostatic imbalance in bone maybe further tipped through an increase in osteoclastogenesis, an area targeted for future studies. Altogether, activation of AhR by kynurenine in aged BMSCs may contribute via targeting autophagic and senescent mechanisms to impair BMSC survival and differentiation disrupting the balance between bone resorption and formation.
In conclusion, our study revealed a potential mechanism contributing to age-related bone homeostasis disruption. Activation of AhR by kynurenine in aged BMSCs leads to increased cell senescence, with a concomitant disruption of autophagy. Increased kynurenine levels in aged bone marrow may contribute to impairment of osteogenic differentiation of MSCs, loss of osteoprogenitor cell populations through a decline of cell survival pathways and increased dysfunction due to senescence. Those processes may shift the balance between bone resorption and new bone formation with kynurenine playing a crucial role in multiple mechanisms driving age-associated bone loss and osteoporosis. As such the kynurenine pathway is an attractive therapeutic target to explore to restore normal bone homeostasis and reverse age-related bone loss.
Highlights.
Kynurenine disrupts autophagy in bone marrow mesenchymal stem cells (BMSCs)
Kynurenine increases BMSC cellular senescence in a dose dependent fashion
Inhibiting Kynurenine binding to AhR blocks KYN-induced senescence and restores autophagy
Funding:
This publication is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Science Research and Development Program (VA Merit Award 1I01CX000930-01, WDH) and the National Institutes of Health (NIA-AG036675 SF, MWH, CMI, MM-L, and WDH). The contents of this publication do not represent the views of the Department of Veterans Affairs, or the United States Government.
Abbreviations:
- 3’4’DMF
3’,4’-Dimethoxyflavone
- AhR
Aryl hydrocarbon receptor
- ARNT
Aryl hydrocarbon receptor nuclear translocator
- BMSC
bone marrow stem cells
- CXCL12
C-X-C motif chemokine 12
- CXCR4
C-X-C Motif Chemokine Receptor 4
- Hdac
Histone deacetylase
- HIF-1
Hypoxia induced factor one
- IDO
indoleamine-2,3-dioxygenase
- KYN
Kynurenine
- LC3B
Microtubule-associated proteins 1A/1B light chain 3B
- MDC
Monodansylcadaverine
- MSC
Mesenchymal stromal cells
- OP
Osteoporosis
- P62
ubiquitin-binding protein p62
- RANKL
Receptor activator of nuclear factor kappa-B ligand
- SASP
Senescence-associated secretory phenotype
- SDF-1
Stromal derived factor 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors also declare that there is no other conflict of interest regarding the publication of this manuscript.
Author Statement
All authors have participated in the research and revision and declare that there is no other conflict of interest regarding the publication of this manuscript. This manuscript has not been submitted for review to any other journal.
5. References:
- 1.Teitelbaum SL, Stem cells and osteoporosis therapy. Cell Stem Cell, 2010. 7(5): p. 553–4. [DOI] [PubMed] [Google Scholar]
- 2.Li X, et al. , IL-6 Contributes to the Defective Osteogenesis of Bone Marrow Stromal Cells from the Vertebral Body of the Glucocorticoid-Induced Osteoporotic Mouse. PLoS One, 2016. 11(4): p. e0154677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Q, et al. , Decreased proliferation ability and differentiation potential of mesenchymal stem cells of osteoporosis rat. Asian Pac J Trop Med, 2014. 7(5): p. 358–63. [DOI] [PubMed] [Google Scholar]
- 4.Anderson KA, et al. , SnapShot: Mammalian Sirtuins. Cell, 2014. 159(4): p. 956–956.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verdin E, et al. , Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem Sci, 2010. 35(12): p. 669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srivastava S, Emerging therapeutic roles for NAD(+) metabolism in mitochondrial and age-related disorders. Clin Transl Med, 2016. 5(1): p. 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braidy N, et al. , Changes in kynurenine pathway metabolism in the brain, liver and kidney of aged female Wistar rats. Febs j, 2011. 278(22): p. 4425–34. [DOI] [PubMed] [Google Scholar]
- 8.de Bie J, et al. , Central kynurenine pathway shift with age in women. J Neurochem, 2016. 136(5): p. 995–1003. [DOI] [PubMed] [Google Scholar]
- 9.Refaey ME, et al. , Kynurenine, a Tryptophan Metabolite That Accumulates With Age, Induces Bone Loss. J Bone Miner Res, 2017. 32(11): p. 2182–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Refaey M, et al. , Oxidation of the aromatic amino acids tryptophan and tyrosine disrupts their anabolic effects on bone marrow mesenchymal stem cells. Mol Cell Endocrinol, 2015. 410: p. 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim BJ, et al. , The Detrimental Effects of Kynurenine, a Tryptophan Metabolite, on Human Bone Metabolism. J Clin Endocrinol Metab, 2019. 104(6): p. 2334–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herberg S, et al. , Stromal cell-derived factor-1beta potentiates bone morphogenetic protein-2-stimulated osteoinduction of genetically engineered bone marrow-derived mesenchymal stem cells in vitro. Tissue Eng Part A, 2013. 19(1–2): p. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herberg S, et al. , Stromal cell-derived factor-1beta mediates cell survival through enhancing autophagy in bone marrow-derived mesenchymal stem cells. PLoS One, 2013. 8(3): p. e58207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elmansi Ahmed M., H. KA, Sudharsan Periyasamy-Thandavan, Xue Jiang, Alexandra Aguilar-Pérez, Galina Kondrikova, Dmitry Kondrikov, Pierce Jessica L., Kaiser Helen, Ding Ke-Hong, Walker Aisha L., Fulzele Sadanand, Bollag Wendy B., Mohammed Elsalanty, Xing-ming Shi Qing Zhong, Johnson Maribeth, Chen Jie, Hunter Monte, Volkman Brian F., Hamrick Mark W., Isales Carlos M., McGee-Lawrence Meghan E., Hill William D.., Age-related increase of kynurenine enhances miR29b-1–5p to decrease both CXCL12 signaling and the epigenetic enzyme Hdac3 in bone marrow stromal cells. The Journal of Bone and Mineral Research 2019. under review. [Google Scholar]
- 15.Brooks AK, et al. , Interactions between inflammatory mediators and corticosteroids regulate transcription of genes within the Kynurenine Pathway in the mouse hippocampus. J Neuroinflammation, 2016. 13(1): p. 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pertovaara M, et al. , Indoleamine 2,3-dioxygenase activity in nonagenarians is markedly increased and predicts mortality. Mech Ageing Dev, 2006. 127(5): p. 497–9. [DOI] [PubMed] [Google Scholar]
- 17.Mezrich JD, et al. , An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory Tcells. J Immunol, 2010. 185(6): p. 3190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurz K, et al. , Effects of adalimumab therapy on disease activity and interferon-gamma-mediated biochemical pathways in patients with rheumatoid arthritis. Autoimmunity, 2011. 44(3): p. 235–42. [DOI] [PubMed] [Google Scholar]
- 19.Murray IA, Patterson AD, and Perdew GH, Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nature reviews. Cancer, 2014. 14(12): p. 801–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beischlag TV, et al. , The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr, 2008. 18(3): p. 207–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung YH, et al. , Beclin-1 is required for RANKL-induced osteoclast differentiation. J Cell Physiol, 2014. 229(12): p. 1963–71. [DOI] [PubMed] [Google Scholar]
- 22.Nuschke A, et al. , Human mesenchymal stem cells/multipotent stromal cells consume accumulated autophagosomes early in differentiation. Stem Cell Res Ther, 2014. 5(6): p. 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou J, et al. , Autophagy prevents irradiation injury and maintains stemness through decreasing ROS generation in mesenchymal stem cells. Cell Death Dis, 2013. 4: p. e844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y, et al. , Autophagy regulates hypoxia-induced osteoclastogenesis through the HIF-1alpha/BNIP3 signaling pathway. J Cell Physiol, 2012. 227(2): p. 639–48. [DOI] [PubMed] [Google Scholar]
- 25.Carames B, et al. , Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum, 2010. 62(3): p. 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qi M, et al. , Autophagy Maintains the Function of Bone Marrow Mesenchymal Stem Cells to Prevent Estrogen Deficiency-Induced Osteoporosis. Theranostics, 2017. 7(18): p. 4498–4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.López-Otín C, et al. , The Hallmarks of Aging. Cell, 2013. 153(6): p. 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thangavel C, et al. , Therapeutically activating RB: reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocr Relat Cancer, 2011. 18(3): p. 333–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farr JN, et al. , Identification of Senescent Cells in the Bone Microenvironment. J Bone Miner Res, 2016. 31(11): p. 1920–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Q, et al. , DNA damage drives accelerated bone aging via an NF-kappaB-dependent mechanism. J Bone Miner Res, 2013. 28(5): p. 1214–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kassem M and Marie PJ, Senescence-associated intrinsic mechanisms of osteoblast dysfunctions. Aging Cell, 2011. 10(2): p. 191–7. [DOI] [PubMed] [Google Scholar]
- 32.Klionsky DJ, et al. , Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy, 2016. 12(1): p. 1–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biederbick A, Kern HF, and Elsasser HP, Monodansylcadaverine (MDC) is a specific in vivo marker for autophagic vacuoles. Eur J Cell Biol, 1995. 66(1): p. 3–14. [PubMed] [Google Scholar]
- 34.Dimri GP, et al. , A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A, 1995. 92(20): p. 9363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farr JN and Khosla S, Cellular senescence in bone. Bone, 2019. 121: p. 121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narita M, et al. , Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell, 2003. 113(6): p. 703–16. [DOI] [PubMed] [Google Scholar]
- 37.Mizushima N, Yoshimori T, and Levine B, Methods in mammalian autophagy research. Cell, 2010. 140(3): p. 313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilbert W, et al. , Stromal cell-derived factor-1 (CXCL12) and its role in bone and muscle biology. Cytokine, 2019. 123: p. 154783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tong Y, et al. , Aryl hydrocarbon receptor suppresses the osteogenesis of mesenchymal stem cells in collagen-induced arthritic mice through the inhibition of beta-catenin. Exp Cell Res, 2017. 350(2): p. 349357. [DOI] [PubMed] [Google Scholar]
- 40.Yu TY, et al. , Aryl hydrocarbon receptor catabolic activity in bone metabolism is osteoclast dependent in vivo. Biochem Biophys Res Commun, 2014. 450(1): p. 416–22. [DOI] [PubMed] [Google Scholar]
- 41.Guan J-L, et al. , Autophagy in stem cells. Autophagy, 2013. 9(6): p. 830–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang B, et al. , Mechanically induced autophagy is associated with ATP metabolism and cellular viability in osteocytes in vitro. Redox Biol, 2018. 14: p. 492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Z, et al. , Autophagy activation facilitates mechanical stimulation-promoted osteoblast differentiation and ameliorates hindlimb unloading-induced bone loss. Biochem Biophys Res Commun, 2018. 498(3): p. 667–673. [DOI] [PubMed] [Google Scholar]
- 44.Shen G, et al. , Autophagy as a target for glucocorticoid-induced osteoporosis therapy. Cell Mol Life Sci, 2018. 75(15): p. 2683–2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu JJ, et al. , Mitochondrial dysfunction and oxidative stress mediate the physiological impairment induced by the disruption of autophagy. Aging; (Albany NY: ), 2009. 1(4): p. 425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiu Y, et al. , Chloroquine reduces osteoclastogenesis in murine osteoporosis by preventing TRAF3 degradation. J Clin Invest, 2014. 124(1): p. 297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jaber FA, et al. , Autophagy plays an essential role in bone homeostasis. J Cell Physiol, 2019. 234(8): p. 12105–12115. [DOI] [PubMed] [Google Scholar]
- 48.Thomas N, et al. , Autophagy Regulates Craniofacial Bone Acquisition. Calcif Tissue Int, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pignolo RJ, et al. , Targeting Cell Senescence for the Treatment of Age-Related Bone Loss. Curr Osteoporos Rep, 2019. 17(2): p. 70–85. [DOI] [PubMed] [Google Scholar]
- 50.Chilosi M, et al. , Premature lung aging and cellular senescence in the pathogenesis of idiopathic pulmonary fibrosis and COPD/emphysema. Transl Res, 2013. 162(3): p. 156–73. [DOI] [PubMed] [Google Scholar]
- 51.Brugarolas J, et al. , Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature, 1995. 377(6549): p. 552–7. [DOI] [PubMed] [Google Scholar]
- 52.Kim YY, et al. , Cooperation between p21 and Akt is required for p53-dependent cellular senescence. Aging Cell, 2017. 16(5): p. 1094–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michaud K, et al. , Pharmacologic inhibition of cyclin-dependent kinases 4 and 6 arrests the growth of glioblastoma multiforme intracranial xenografts. Cancer Res, 2010. 70(8): p. 3228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herbig U, et al. , Real-time imaging of transcriptional activation in live cells reveals rapid up-regulation of the cyclin-dependent kinase inhibitor gene CDKN1A in replicative cellular senescence. Aging Cell, 2003. 2(6): p. 295–304. [DOI] [PubMed] [Google Scholar]
- 55.Stein GH, et al. , Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol Cell Biol, 1999. 19(3): p. 2109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alcorta DA, et al. , Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc Natl Acad Sci U S A, 1996. 93(24): p. 13742–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim H-N, et al. , DNA damage and senescence in osteoprogenitors expressing Osx1 may cause their decrease with age. Aging Cell, 2017. 16(4): p. 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bock KW, Human and rodent aryl hydrocarbon receptor (AHR): from mediator of dioxin toxicity to physiologic AHR functions and therapeutic options. Biol Chem, 2017. 398(4): p. 455–464. [DOI] [PubMed] [Google Scholar]
- 59.Wan C, et al. , 2, 3, 7, 8-Tetrachlorodibenzo-P-dioxin (TCDD) induces premature senescence in human and rodent neuronal cells via ROS-dependent mechanisms. PLoS One, 2014. 9(2): p. e89811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamamoto T, et al. , Kynurenine signaling through the aryl hydrocarbon receptor maintains the undifferentiated state of human embryonic stem cells. Sci Signal, 2019. 12(587). [DOI] [PubMed] [Google Scholar]