Abstract
Bone fractures in older adults are often preceded by a loss of muscle mass and strength. Likewise, bone loss with prolonged bed rest, spinal cord injury, or with exposure to microgravity is also preceded by a rapid loss of muscle mass. Recent studies using animal models in the setting of hindlimb unloading or botulinum toxin (Botox) injection also reveal that muscle loss can induce bone loss. Moreover, muscle-derived factors such as irisin and leptin can inhibit bone loss with unloading, and knockout of catabolic factors in muscle such as the ubiquitin ligase Murf1 or the myokine myostatin can reduce osteoclastogenesis. These findings suggest that therapies targeting muscle in the setting of disuse atrophy may potentially attenuate bone loss, primarily by reducing bone resorption. These potential therapies not only include pharmacological approaches but also interventions such as whole-body vibration coupled with resistance exercise and functional electric stimulation of muscle.
Keywords: osteoclasts, myostatin, microgravity, resistance training, irisin
Introduction
The mechanical forces applied to bone, which are critical to skeletal health, come from two primary sources: external gravitational loading via ground reaction forces and internal loading via muscle contractions [1, 2]. These forces can be reduced significantly with a more sedentary lifestyle that often accompanies advanced age, prolonged bedrest, spinal cord injury or exposure to microgravity with spaceflight. Reduced activity and less frequent muscle contraction induce muscle atrophy as well as bone loss [3, 4]. In many cases the association between muscle atrophy and bone loss may be correlative emanating from systemic factors such as elevated glucocorticoids [5]; however, there also appear to be local factors related to altered muscle-bone crosstalk that underlie the coordinated loss of muscle and bone in these settings. For example, muscle is a source of myokines that can stimulate bone formation and also contribute to bone loss [6, 7], whereas bone secretes factors such as osteocalcin and connexin 43 that have direct effects on muscle [8, 9]. Defining the cellular and molecular mechanisms linking muscle and bone within the setting of disuse is critical for developing therapeutic approaches to inhibit muscle and bone loss and ultimately prevent bone fractures. This review examines patterns of muscle atrophy and bone loss in human subjects, reviews evidence from animal models that shed light on the cellular and molecular mechanisms underlying muscle and bone loss with disuse, and highlights various therapeutic strategies for preventing the loss of muscle and bone that occurs with a sedentary lifestyle and unloading.
Clinical studies
Muscle and bone loss with aging
Osteoporosis is defined as a skeletal disease characterized by low bone mass and micro-architectural deterioration of bone tissue [10]. Sarcopenia corresponds to a progressive and general loss of muscle mass with either low muscle strength or physical performance [11]. Importantly, both osteoporosis and sarcopenia are well-known contributors to low trauma fractures through increased bone fragility, poor balance, reduced walking speed, or falls [12]. Moreover, sarcopenia with aging is also associated with a more sedentary lifestyle, and thus represents a form of disuse atrophy [13]. Therefore, the combination of these two diseases may pose additive risks for fragility fractures, including hip fractures, which are associated with extensive disability and morbidity in the elderly population [14–16]. In fact, several epidemiological studies have supported the close association between low muscle mass and poor bone health in older adults. Verschueren et al. [17] reported that men with sarcopenia had significantly lower BMD and were more likely to have osteoporosis compared with those without sarcopenia in a study on middle-aged and elderly community-dwelling Europeans. Another study including 1,308 men and 1,171 women over 65 years showed that low muscle mass was significantly associated with higher risk of osteoporosis, even after adjusting for potential risk factors [18]. Lean mass affects not only overall BMD but also key cross-sectional bone parameters related to bone strength. Men in the lowest quartile of relative lean appendicular mass have significantly lower section modulus of both the femoral neck and distal radius compared to men in higher quartiles of relative lean mass [19], and men in the lowest quartile of grip strength have significantly lower cortical bone area and thickness of the distal radius compared with men having higher measures of grip strength [20] (Fig. 1). The cellular and molecular mechanisms contributing to the concomitant loss of muscle and bone with aging in these studies is still unclear; however, the highly integrated changes in skeletal muscle and bone observed from these clinical studies suggests that therapeutic approaches independently targeting either sarcopenia or osteoporosis alone may not be enough for effective fracture prevention, and that new strategies for improving both tissues simultaneously are necessary. Due to the clinical importance of concurrent sarcopenia and osteoporosis, the term “osteosarcopenia” has been proposed [21] and extensively discussed in a recent review paper [22].
Figure 1.
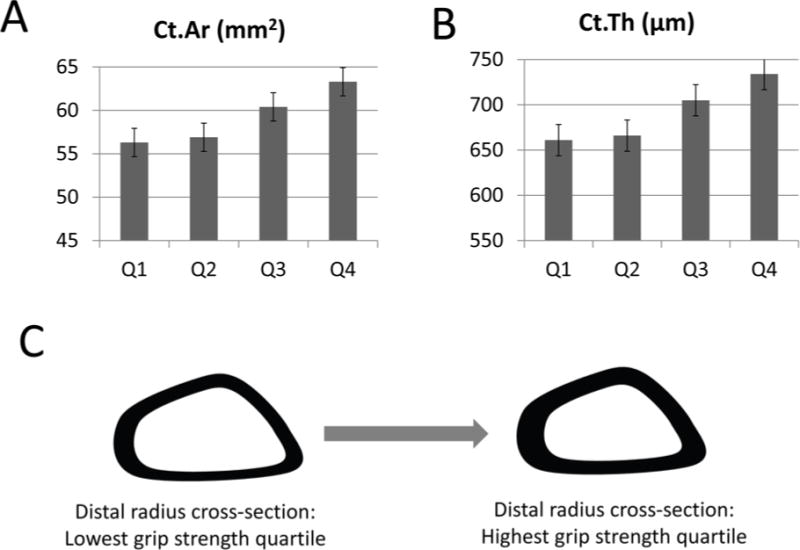
Graphs showing measures of cortical area (A; p<0.001 ANOVA) and cortical thickness (B; p<0.05 ANOVA) in men with grip strength values in the lowest (Q1) and highest (Q4) quartiles. Data from ref. [20]. C. Schematic showing the significant differences in bone cross-sectional shape of the radius among men in the lowest and highest quartiles of grip strength.
Muscle and bone loss with spaceflight and bed rest
Spaceflight and prolonged bed rest are useful human models to understand the effects of unloading on muscle and bone, despite their limited clinical relevance in terms of falls or fractures. After 6 months aboard the International Space Station, 9 crewmembers with an exercise prescription had a 10 to 15% reduction in calf muscle mass and a 32% decreased peak power along with a slow-to-fast fiber type transition in the gastrocnemius and soleus muscles [23]. Astronauts on even short duration spaceflight, such as 8- and 17-day mission, experienced a marked decrease in muscle volume and strength [24, 25]. Besides muscle atrophy, there is the well-documented development of bone loss during spaceflight. Lang et al. [26, 27] showed that bone mass was lost at a rate of 0.5% to 1.5% per month during 4- to 6-month spaceflight, and that proximal femur BMD was only partially recovered at 1 year after re-exposure to Earth’s gravity. Bone loss in space is mainly attributable to the uncoupling of bone remodeling, reflecting increased bone resorption and decreased bone formation [28]. Long-term bed rest is also used as a microgravity model to simulate the physiological changes that occur in humans since bed rest can significantly decrease both muscle volume and force [29, 30] as well as bone mass [31, 32]. Interestingly, the changes of muscle and bone under these conditions occur in such a way that muscle atrophy precedes the decline in bone mass [2, 33]. Furthermore, muscle loss can be recovered about six times faster than bone loss in astronauts after returning to normal gravity [34]. These data suggest that decreased muscle derived forces may primarily drive bone loss with unloading. There is, however, accumulating evidence that muscle can secrete cytokines in response to contraction and exercise [35, 36]. Changes in these muscle-derived factors with unloading may also contribute to bone loss, and these are referenced below under animal studies of disuse.
Loss of muscle and bone with paralysis and spinal cord injury
Spinal cord injury (SCI) is a traumatic event that is equivalent to neurectomy using surgical interventions to induce sarcopenia and osteopenia. As a result of paralysis, individuals with SCI experience severe muscle atrophy of about 30–60%, depending on muscle type and completeness of the lesion, leading to marked reductions in maximal force generation and endurance [37–39]. In addition, SCI-related bone loss proceeds at a rate of 1% per week for the first 6–12 months, a rate that is 5 to 20 times greater than that observed in microgravity, long-term bed rest, and aging post-menopause [37, 40–42]. The primary cause of SCI-related bone loss is the removal of muscle-induced mechanical stimuli on the bone. For example, a comparative study including 10 male pairs of monozygotic twins discordant for SCI showed that leg lean mass loss was significantly correlated with leg BMD loss after SCI [43]. Muscle contraction elicited by electrical stimulation slowed bone loss and even partially reversed it after SCI [44–46]. The interesting point is that this protective effect of muscle contraction on bone seems to be independent of the central nervous system [47]. Neuronal, endocrine, and lifestyle factors caused by paralysis, such as dysfunction of the pituitary-hypothalamic axis, increased adiposity, insulin resistance, and autonomic dysfunctions [48–50] may additionally worsen the rapidity of SCI-related bone loss [37].
Evidence from animal models
Inducing muscle and bone loss with hindlimb unloading (HLU) and botulinum toxin (BTX)
Hindlimb unloading- (HLU) and botulinum toxin- (BTX) induced muscle paralysis represent two of the most commonly explored rodent disuse models that alter the force applied to bone in subtle yet significant ways. HLU removes or reduces external ground reaction forces, but spares muscle contraction, while botulinum toxin-induced paralysis eliminates muscle contractions but allows for the application of external forces [51]. In the HLU model, a device is attached to the animal’s tail to prevent hindlimb weight bearing, but unlike botulinum toxin induced paralysis the hindlimbs are not fully immobilized [33]. Thus, passive muscle contractions are allowed to continue but muscle forces are theoretically reduced since they do not oppose the torque of ground reaction forces [51, 33]. In the botulinum toxin model, intramuscular injection of BTX in the hindlimbs elicits temporary muscle paralysis, which secondarily alters gait and impacts external forces such that peak ground reaction forces are reduced by 11% four days after BTX injection and are recovered by 14 days post-injection [51]. Both models induce muscle atrophy and bone loss; however, the time course and interdependence of these effects have yet to be fully characterized [33, 52–53]. Here we focus our attention on recent animal studies utilizing HLU and BTX, describing the relative effects of each on muscle atrophy and bone metabolism.
The effects of BTX and HLU on bone are cumulative
Forces produced during muscle contraction can account for 70% of the bending moments applied to the lower limb, thus the forces applied to bone from muscle far exceed the loads applied to bone from ground reaction forces [2, 54, 55]. Since BTX injection removes all muscle contraction, it is not necessarily surprising that total bone loss following muscle paralysis via BTX injection often occurs more quickly and drastically than with removal of ground reaction forces via HLU [51, 56, 57]. Importantly, Warden et al. observed that the combination of HLU and BTX injection together has a more significant impact on bone than either HLU or BTX alone, suggesting that muscle can indeed have positive effects on bone even in the setting of unloading [58] (Table 1). This observation is further supported by the fact that muscle-specific ectopic expression if IGF-1 can suppress osteopenia in the setting of hindlimb unloading [59]. Ellman et al. also examined the combined effects of BTX and HLU, but added groups of both cage dwelling and HLU groups without botox injection to serve as additional controls [51]. Their results were consistent with Warden et al. in many regards, including combined HLU+BTX intervention showing more deleterious effects on bone than that of either intervention alone for structural parameters such as proximal tibia trabecular bone volume fraction and thickness as well as hindlimb BMD [51]. Ellman et al. also revealed significant, indirect effects of BTX such as lower BMD and poor bone architecture in the uninjected legs of both BTX-treated groups compared to their respective control groups with no exposure to BTX [51]. The indirect effects of BTX on hindlimb BMD and tibia microarchitecture were almost as drastic as those seen in direct BTX treatment of the injected leg, and even exceeded the effects of hindlimb unloading alone in some measures [51]. These findings, in addition to the studies noted below, suggest that BTX hindlimb injections can affect multiple bone sites in addition to local regions adjacent to the injection area.
Table 1.
Comparison of the effects of Hindlimb Unloading (HLU), Botulin Toxin Injection (BTX), and combination of Hindlimb Unloading and Botulin Toxin Injection (HLU + BTX) in mouse studies [refs. 48–65]. External ground reaction forces are preserved with injection of BTX alone, internal muscle derived forces are preserved with HLU alone, while both forces are removed with the combination of HLU + BTX. The degree of bone resorption is greatest with the combination of Hindlimb Unloading and Botulin Toxin Injection, followed by BTX injection, and least with HLU.
| HLU | BTX | HLU + BTX | |
|---|---|---|---|
| External Ground Reaction Forces | − | + | − |
| Internal Muscle Derived Forces | + | − | − |
| Relative Degree of Bone Resorption | + | ++ | +++ |
HLU and BTX can produce contrasting site-specific patterns of bone resorption
The two studies compared above and numerous others have demonstrated that decreased mechanical loading, regardless of the loading source, results in bone catabolism and muscle atrophy (Table 1). The time course of muscle and bone loss with HLU and BTX indicates that muscle atrophy generally precedes bone loss. Lloyd et al. performed HLU in mice for three weeks and found significant muscle atrophy of HLU subjects beginning at day 7, whereas decreases in tibial and femoral cortical thickness were not demonstrated until day 14 [33]. Thus, in this experiment, changes in muscle preceded those in bone, suggesting that sarcopenia may contribute to the subsequent osteopenia that develops during HLU [33]. Muscle mass, cortical bone structure, and bone strength in control mice did not decline over the experimental period, suggesting the possibility that muscle may have a greater influence on cortical than trabecular bone [33]. When similar parameters were monitored in the tibia following BTX-induced muscle paralysis it was found that initial bone resorption occurs in metaphyseal trabeculae, and then later occurs homogeneously along the long axis of the diaphyseal cortex [60]. The timing of bone resorption at these specific locations (3 days and 9 days post injection respectively) suggests that initial bone resorption in the metaphysis occurs via upregulation of basal osteoclastic activity that may then drive a secondary wave of bone resorption in the diaphysis [60].
It is known that acute trabecular bone loss following muscle paralysis arises via RANKL-mediated osteoclastogenesis, but the initial signaling cascade responsible for the rapid activation of this bone catabolic pathway does not appear to be RANKL-dependent [61]. In HLU mice, increases in osteocyte apoptosis and RANKL production preceded increases in bone resorption at both endocortical and trabecular surfaces [62]. Osteocytes are a primary source of RANKL, and it is thought that apoptotic osteocytes trigger RANKL expression by neighboring osteocytes [62]. Additionally, apoptosis inhibition with QVD completely prevents HLU-triggered increase in RANKL expression and bone resorption at both sites, supporting the idea that osteocyte apoptosis is a common final pathway for activating and targeting bone resorption in response to many diverse stimuli [62]. In addition to apoptosis, many cytokines may play a role in the initiation of bone resorption. TNF and IL-1 have been upregulated prior to the onset of bone resorption at 7 and 14 days post BTX induced paralysis, and in separate experiments cytokines have been shown to upregulate osteoclast activity [58, 63–67]. Ausk et al. found that within 24 hours of BTX injection there are alterations of the bone marrow microenvironment marked by inflammatory cell infiltrate, followed by an increase in TNF and IL-4 at day 3 [68]. These alterations coincided with bone marrow becoming permissive to the formation of large osteoclasts with more nuclei, but not total number of osteoclasts [68]. This experiment demonstrated for the first time that BTX injection induces inflammation within the bone marrow cavity adjacent to the muscle and explains, at least in part, the spatiotemporal pattern of initial bone resorption in the metaphyseal trabecula.
Muscle-derived factors can modulate bone loss in the setting of disuse atrophy
A number of different factors have been identified that play key roles in bone mechanotransduction, and altering the expression of certain mechano-responsive genes during unloading can significantly impact the sensitivity of bone to disuse-induced bone loss. For example, bone-specific deletion of connexin 43, Nod2, Fas, beta catenin, and periostin each attenuate cortical bone loss with unloading [69–72]. More recently it has been discovered that muscle-derived factors can also impact bone loss with unloading, consistent with the studies referenced above indicating that muscle loss precedes bone loss with disuse resulting from spaceflight, bed rest, and hindlimb unloading. The myokine irisin is released with muscle contraction and stimulates bone formation by osteoblasts [73], and irisin treatment prevents muscle and bone loss with hindlimb unloading in mice [74]. Irisin can suppress osteoclast differentiation in vitro and inhibit RANKL expression by osteoblasts [75], providing evidence for a direct effect of this myokine on bone resorption (Fig. 2).
Figure 2.
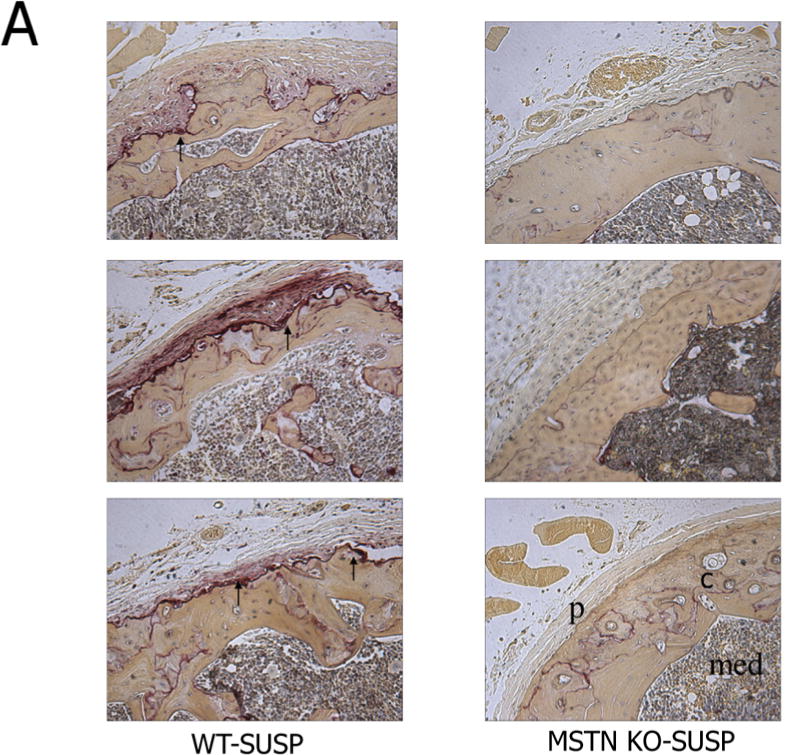
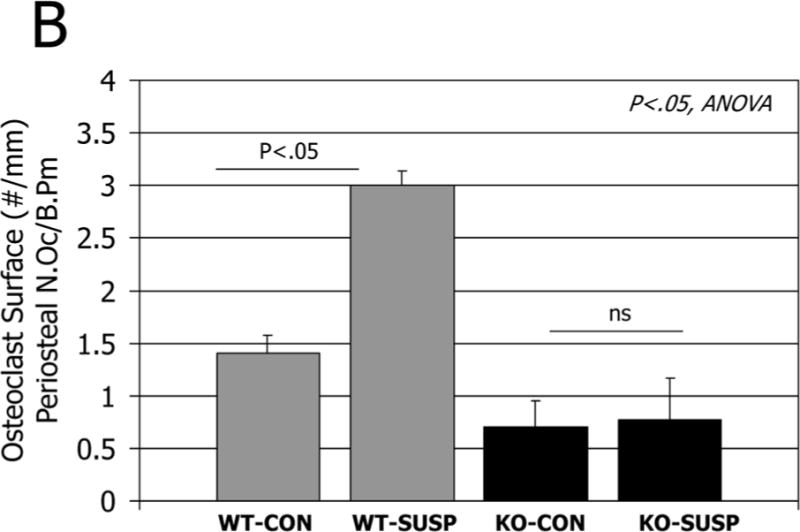
A. Histological sections of the distal femur stained for tartrate resistant acid phosphatase in normal mice (WT, left column) and mice lacking myostatin (MSTN KO, right column) after 7 days hindlimb unloading (SUSP). Arrows point to numerous osteoclasts on the subperiosteal surface in wild-type mice but not in myostatin-deficient mice after unloading. C=cortical bone, p=periosteum, med=medullary cavity. B. Quantification of osteoclast number per bone surface on the periosteum of ground control (CON) and tail-suspended (SUSP) normal (WT) and myostatin-deficient (MSTN KO) mice.
The myokine myostatin is also known to be elevated in catabolic conditions [7], and myostatin also has direct effects on osteoclastogenesis [76]. We previously found that osteoclast number on trabecular bone surfaces increased with unloading in both wild-type and myostatin-deficient mice [77]. However, myostatin deficiency suppresses subperiosteal resorption with unloading (Fig. 3), suggesting that some of myostatin’s effects on osteoclasts may be localized to the muscle-bone interface. Follistatin is a potent antagonist of myostatin, and follistatin is elevated with hypergravity, decreased with microgravity, and follistatin can attenuate myostatin-induced osteoclastogenesis [78]. These findings point to a key role for myostatin signaling in loss of both muscle and bone during unloading (Fig. 2). Muscle catabolism involves not only myostatin activity but also degradation by the ubiquitin ligases Murf1 and Mafbx. Importantly, congenital absence of Murf1 is also protective against cortical and trabecular bone loss with hindlimb unloading [79]. Finally, the adipokine leptin is now recognized to also be expressed in muscle and secreted by muscle cells as well as by adipocytes [80]. Hindlimb unloading in rats induces a reduction in circulating leptin, and leptin replacement therapy inhibits trabecular bone loss due to increased bone resorption [81]. The extent to which fat- or muscle-derived leptin contributes to serum leptin levels during unloading is not known, but it is certainly possible that muscle-derived leptin may be involved in the overall reduction in circulating leptin that occurs with unloading.
Figure 3.
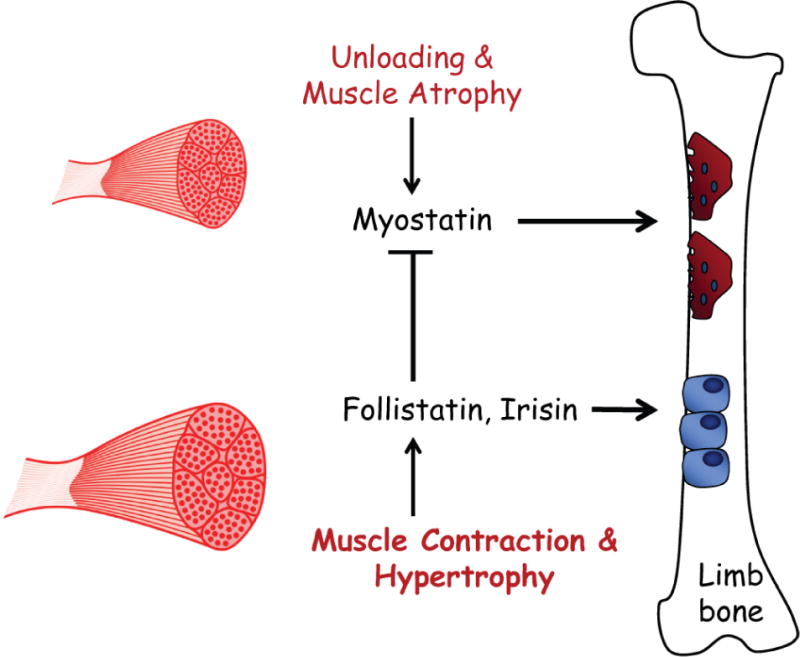
Relationship of muscle atrophy, and muscle hypertrophy, to myokines impacting bone resorption by osteoclasts (red) and bone formation by osteoblasts (blue). Myostatin expression and secretion is increased with unloading, increasing bone resorption. Follistatin is increased with muscle contraction and hypertrophy, suppressing myostatin-induced bone resorption. Follistatin and the myokine irisin, which is also increased with muscle contraction, can both directly inhibit osteoclastogenesis and irisin stimulates osteogenesis.
Discussion
Studies in humans and animal models indicate that muscle loss can occur rapidly with disuse or immobilization. Furthermore, muscle atrophy appears to contribute directly to bone loss primarily via increased bone resorption by osteoclasts, although decreases in irisin levels with disuse may also suppress osteogenesis. These observations suggest that therapeutic approaches targeting muscle to prevent atrophy could also reduce bone loss in the settings of decreased ambulatory activity with aging, bed rest, or spinal cord injury. Certain interventions utilizing this muscle-centric approach have shown some promising results. Functional electric stimulation of the soleus muscle in spinal cord injury patients attenuates bone loss in the tibia, particularly in the posterior tibia adjacent to the soleus muscle itself [37, 44]. Whole-body vibration has also been explored as a treatment modality for disuse atrophy. Low-magnitude, high-frequency vibration improved retention of postural stability and flexor (but not extensor) muscle strength following 90 days of bedrest [82]. Bone parameters were not examined in this study, but the positive impact of vibration on gait has important implications for reducing falls in sedentary individuals with muscle weakness. This is further indicated by a meta-analysis showing that while vibration had no significant effect on hip or lumbar spine bone mineral density in older women it did significantly improve functional measures of leg muscle strength [83]. Vibration has also been used in conjunction with resistance exercise in bedrest patients, and the combination of both vibration and exercise appears to be more effective than either intervention alone for preventing muscle atrophy [84], reducing bone marrow fat accumulation in the spine [85], and preserving bone mass in the distal tibia [86]. The resistance exercise data are consistent with results from the Advanced Resistance Exercise Device (ARED) showing that resistance exercise during spaceflight can prevent significant loss of muscle and bone with prolonged exposure to microgravity [87]. Together these data underscore the importance of frequent and forceful muscle contractions for preserving bone health.
Pharmacological approaches are also being explored to reduce loss of muscle and bone with aging and disuse. Myostatin inhibitors referenced earlier represent one approach, and treatment of older patients with a myostatin antibody can improve appendicular lean mass and functional measures of muscle power, though changes in bone are not known [88]. Other pharmacological strategies include selective androgen receptor modulators (SARMs) [89, 90]. Testosterone treatment itself has been found to reduce bone loss with Botox injection [91] and sciatic nerve injury [92] in rodents, but surprisingly the effects on muscle atrophy using these models are negligible and not significant. On the other hand, the SARM MK-0773 increased lean mass in older women but had no significant impact on bone mineral content [90]. These pharmacological studies suggest, in light of the data from resistance exercise presented above, that mechanical stimuli related to muscle contraction may have the greatest potential for preserving bone health when bone is at risk from unloading. Myokines such as irisin are released with muscle contraction but also with whole-body vibration [93], underscoring the importance of muscle contraction and physical stimuli for muscle-bone crosstalk. As Girgis and colleagues recently noted [90], “the simplest of all therapies to treat, or in this case prevent, musculoskeletal disease is also the most difficult to implement”. Strength training can increase muscle power, improve balance, and in many cases prevent bone loss even in settings of disuse. Novel strategies that can mimic the multiple physiological signals emanating from frequent, forceful muscle contraction are most likely to provide the greatest benefit to the skeleton in cases of immobilization, disuse, and microgravity.
Acknowledgments
Funding for this research was provided by the National Institute on Aging, US National Institutes of Health (AG 036675).
Footnotes
Conflict of Interest Statement: Tucker Bettis, Beom-Jun Kim, and Mark W. Hamrick declare that they have no conflicts of interest.
References
- 1.Schoenau E, Frost HM. The “muscle-bone unit” in children and adolescents. Calcif Tissue Int. 2002;70:405–407. doi: 10.1007/s00223-001-0048-8. [DOI] [PubMed] [Google Scholar]
- 2.Burr DB. Muscle strength, bone mass, and age-related bone loss. J Bone Miner Res. 1997;12:1547–1551. doi: 10.1359/jbmr.1997.12.10.1547. [DOI] [PubMed] [Google Scholar]
- 3.Bloomfield SA. Changes in musculoskeletal structure and function with prolonged bed rest. Med Sci Sports Exerc. 1997;29:197–206. doi: 10.1097/00005768-199702000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Reilly B, Franklin C. Prevention of muscle wasting and osteoporosis: the value of examining novel animal models. J Exp Biol. 2016;219:2582–2595. doi: 10.1242/jeb.128348. [DOI] [PubMed] [Google Scholar]
- 5.Sato A, et al. Glucocorticoids induce muscle and bone atrophy by tissue-specific mechanisms upstream of E3 ubiquitin ligases. Endocrinology. 2017;158:664–677. doi: 10.1210/en.2016-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamrick MW, McNeil PL, Patterson SL. Role of muscle-derived growth factors in bone formation. J Musculoskelet Neuronal Interact. 2010;10(1):64–70. [PMC free article] [PubMed] [Google Scholar]
- 7.Hamrick MW. A role for myokines in muscle-bone interactions. Exerc Sport Sci Rev. 2011;39:43–7. doi: 10.1097/JES.0b013e318201f601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karsenty G, Mera P. Molecular bases of the crosstalk between bone and muscle. Bone. 2017 doi: 10.1016/j.bone.2017.04.006. doi: 10.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen H, Grimston S, Civitelli R, Thomopoulos S. Deletion of connexin43 in osteoblasts/osteocytes leads to impaired muscle formation in mice. J Bone Miner Res. 2015;30:596–605. doi: 10.1002/jbmr.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NIH consensus development panel. Osteoporosis prevention, diagnosis, and therapy 2001. JAMA. 2001;285:785–795. [Google Scholar]
- 11.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age and ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu R, Leung J, Woo J. Incremental predictive value of sarcopenia for incident fracture in an elderly Chinese cohort: results from the Osteoporotic Fractures in Men (MrOs) Study. Journal of the American Medical Directors Association. 2014;15:551–558. doi: 10.1016/j.jamda.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Steffl M, et al. Relationship between sarcopenia and physical activity in older people: a systematic review and meta-analysis. Clin Interv Aging. 2017;12:835–845. doi: 10.2147/CIA.S132940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frisoli A, Jr, Chaves PH, Ingham SJ, Fried LP. Severe osteopenia and osteoporosis, sarcopenia, and frailty status in community-dwelling older women: results from the Women’s Health and Aging Study (WHAS) II. Bone. 2011;48:952–957. doi: 10.1016/j.bone.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 15.Yu R, Leung J, Woo J. Sarcopenia combined with FRAX probabilities improves fracture risk prediction in older Chinese men. Journal of the American Medical Directors Association. 2014;15:918–923. doi: 10.1016/j.jamda.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Huo YR, Suriyaarachchi P, Gomez F, Curcio CL, Boersma D, Gunawardene P, Demontiero O, Duque G. Comprehensive nutritional status in sarco-osteoporotic older fallers. J Nutr, Health, Aging. 2015;19:474–480. doi: 10.1007/s12603-014-0543-z. [DOI] [PubMed] [Google Scholar]
- 17.Verschueren S, Gielen E, O’Neill TW, et al. Sarcopenia and its relationship with bone mineral density in middle-aged and elderly European men. Osteoporosis Intl. 2013;24:87–98. doi: 10.1007/s00198-012-2057-z. [DOI] [PubMed] [Google Scholar]
- 18.Kim S, Won CW, Kim BS, Choi HR, Moon MY. The association between the low muscle mass and osteoporosis in elderly Korean people. J Korean Med Sci. 2014;29:995–1000. doi: 10.3346/jkms.2014.29.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szulc P, Beck TJ, Marchand F, Delmas PD. Low skeletal muscle mass is associated with poor structural parameters of bone and impaired balance in elderly men—the MINOS study. J Bone Miner Res. 2005;20:721–29. doi: 10.1359/JBMR.041230. [DOI] [PubMed] [Google Scholar]
- 20.Szulc P, Blaizot S, Boutroy S, et al. Impaired bone microarchitecture at the distal radius in older men with low muscle mass and grip strength: The STRAMBO study. J Bone Miner Res. 2013;28:169–178. doi: 10.1002/jbmr.1726. [DOI] [PubMed] [Google Scholar]
- 21.Binkley N, Buehring B. Beyond FRAX: it’s time to consider “sarco-osteopenia”. J Clin Densitometry. 2009;12:413–416. doi: 10.1016/j.jocd.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Hirschfeld HP, Kinsella R, Duque G. Osteosarcopenia: where bone, muscle, and fat collide. Osteoporosis Intl. 2017;28:2781–2790. doi: 10.1007/s00198-017-4151-8. [DOI] [PubMed] [Google Scholar]
- 23.Trappe S, Costill D, Gallagher P, Creer A, Peters JR, Evans H, Riley DA, Fitts RH. Exercise in space: human skeletal muscle after 6 months aboard the International Space Station. J Appl Physiol. 2009;106:1159–1168. doi: 10.1152/japplphysiol.91578.2008. [DOI] [PubMed] [Google Scholar]
- 24.Tesch PA, Berg HE, Bring D, Evans HJ, LeBlanc AD. Effects of 17-day spaceflight on knee extensor muscle function and size. Eur J Appl Physiol. 2005;93:463–468. doi: 10.1007/s00421-004-1236-9. [DOI] [PubMed] [Google Scholar]
- 25.LeBlanc A, Rowe R, Schneider V, Evans H, Hedrick T. Regional muscle loss after short duration spaceflight. Aviation, Space, Envmtl Med. 1995;66:1151–1154. [PubMed] [Google Scholar]
- 26.Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19:1006–1012. doi: 10.1359/JBMR.040307. [DOI] [PubMed] [Google Scholar]
- 27.Lang TF, Leblanc AD, Evans HJ, Lu Y. Adaptation of the proximal femur to skeletal reloading after long-duration spaceflight. J Bone Miner Res. 2006;21:1224–1230. doi: 10.1359/jbmr.060509. [DOI] [PubMed] [Google Scholar]
- 28.McCarthy EF. Perspective: skeletal complications of space flight. Skeletal Radiology. 2011;40:661–663. doi: 10.1007/s00256-011-1100-z. [DOI] [PubMed] [Google Scholar]
- 29.Ferrando AA, Stuart CA, Brunder DG, Hillman GR. Magnetic resonance imaging quantitation of changes in muscle volume during 7 days of strict bed rest. Aviation, Space, Envmtl Med. 1995;66:976–981. [PubMed] [Google Scholar]
- 30.Bloomfield SA. Changes in musculoskeletal structure and function with prolonged bed rest. Med Sci Sports Exerc. 1997;29:197–206. doi: 10.1097/00005768-199702000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Krasnoff J, Painter P. The physiological consequences of bed rest and inactivity. Advances in renal replacement therapy. 1999;6:124–132. doi: 10.1016/s1073-4449(99)70030-0. [DOI] [PubMed] [Google Scholar]
- 32.Morgan JL, Zwart SR, Heer M, Ploutz-Snyder R, Ericson K, Smith SM. Bone metabolism and nutritional status during 30-day head-down-tilt bed rest. J Appl Physiol. 2012;113:1519–1529. doi: 10.1152/japplphysiol.01064.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lloyd SA, Lang CH, Zhang Y, Paul EM, Laufenberg LJ, Lewis GS, Donahue HJ. Interdependence of muscle atrophy and bone loss induced by mechanical unloading. J Bone Miner Res. 2014;29:1118–1130. doi: 10.1002/jbmr.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keyak JH, Koyama AK, LeBlanc A, Lu Y, Lang TF. Reduction in proximal femoral strength due to long-duration spaceflight. Bone. 2009;44:449–453. doi: 10.1016/j.bone.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 35.Pedersen BK. Muscles and their myokines. J Exp Biol. 2011;214:337–346. doi: 10.1242/jeb.048074. [DOI] [PubMed] [Google Scholar]
- 36.Pedersen BK, Fischer CP. Beneficial health effects of exercise–the role of IL-6 as a myokine. Trends Pharmacol Sci. 2007;28:152–156. doi: 10.1016/j.tips.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Qin W, Bauman WA, Cardozo C. Bone and muscle loss after spinal cord injury: organ interactions. Annals NY Acad Sci. 2010;1211:66–84. doi: 10.1111/j.1749-6632.2010.05806.x. [DOI] [PubMed] [Google Scholar]
- 38.Dudley-Javoroski S, Shields RK. Muscle and bone plasticity after spinal cord injury: review of adaptations to disuse and to electrical muscle stimulation. J Rehab Resch Dev. 2008;45:283–296. doi: 10.1682/jrrd.2007.02.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biering-Sorensen B, Kristensen IB, Kjaer M, Biering-Sorensen F. Muscle after spinal cord injury. Muscle & Nerve. 2009;40:499–519. doi: 10.1002/mus.21391. [DOI] [PubMed] [Google Scholar]
- 40.Jiang SD, Dai LY, Jiang LS. Osteoporosis after spinal cord injury. Osteoporosis Intl. 2006;17:180–192. doi: 10.1007/s00198-005-2028-8. [DOI] [PubMed] [Google Scholar]
- 41.Warden SJ, Bennell KL, Matthews B, Brown DJ, McMeeken JM, Wark JD. Quantitative ultrasound assessment of acute bone loss following spinal cord injury: a longitudinal pilot study. Osteoporosis Intl. 2002;13:586–592. doi: 10.1007/s001980200077. [DOI] [PubMed] [Google Scholar]
- 42.Garland DE, Adkins RH, Kushwaha V, Stewart C. Risk factors for osteoporosis at the knee in the spinal cord injury population. J Spinal Cord Med. 2004;27:202–206. doi: 10.1080/10790268.2004.11753748. [DOI] [PubMed] [Google Scholar]
- 43.Bauman WA, Spungen AM, Wang J, Pierson RN, Jr, Schwartz E. Relationship of fat mass and serum estradiol with lower extremity bone in persons with chronic spinal cord injury. Am J Physiol Endocrinol Metab. 2006;290:E1098–1103. doi: 10.1152/ajpendo.00250.2005. [DOI] [PubMed] [Google Scholar]
- 44.Shields RK, Dudley-Javoroski S. Musculoskeletal plasticity after acute spinal cord injury: effects of long-term neuromuscular electrical stimulation training. J Neurophysiology. 2006;95:2380–2390. doi: 10.1152/jn.01181.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shields RK, Dudley-Javoroski S, Law LA. Electrically induced muscle contractions influence bone density decline after spinal cord injury. Spine. 2006;31:548–553. doi: 10.1097/01.brs.0000201303.49308.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dudley-Javoroski S, Shields RK. Asymmetric bone adaptations to soleus mechanical loading after spinal cord injury. J Musculoskelet Neuronal Interact. 2008;8:227–238. [PMC free article] [PubMed] [Google Scholar]
- 47.Qin W, Sun L, Cao J, et al. The central nervous system (CNS)-independent anti-bone-resorptive activity of muscle contraction and the underlying molecular and cellular signatures. J Biol Chem. 2013;288:13511–13521. doi: 10.1074/jbc.M113.454892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bauman WA, Adkins RH, Spungen AM, Waters RL. The effect of residual neurological deficit on oral glucose tolerance in persons with chronic spinal cord injury. Spinal Cord. 1999;37:765–71. doi: 10.1038/sj.sc.3100893. [DOI] [PubMed] [Google Scholar]
- 49.Bauman WA, Spungen AM. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism. 1994;43:749–756. doi: 10.1016/0026-0495(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 50.Tsitouras PD, Zhong YG, Spungen AM, Bauman WA. Serum testosterone and growth hormone/insulin like growth factor-1 in adults with spinal cord injury. Horm Metab Res. 1995;27:287–292. doi: 10.1055/s-2007-979961. [DOI] [PubMed] [Google Scholar]
- 51.Ellman R, Grasso DJ, van Vliet M, et al. Combined effects of botulinum toxin injection and hindlimb unloading on bone and muscle. Calc Tissue Intl. 2014;94:327–337. doi: 10.1007/s00223-013-9814-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hanson A, Harrison B, Young M, Stodieck L, Ferguson V. Longitudinal characterization of functional, morphologic, and biochemical adaptations in mouse skeletal muscle with hindlimb suspension. Muscle & Nerve. 2013;498:393–402. doi: 10.1002/mus.23753. [DOI] [PubMed] [Google Scholar]
- 53.Lloyd SA, Lewis GS, Zhang Y, Paul EM, Donahue HJ. Connexin 43 deficiency attenuates loss of trabecular bone and prevents suppression of cortical bone formation during unloading. J Bone Miner Res. 2012;27:2359–72. doi: 10.1002/jbmr.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu TW, Taylor SJ, O’Connor JJ, Walker PS. Influence of muscle activity on the forces in the femur: An in vivo study. J Biomech. 1997;30:1101–1106. doi: 10.1016/s0021-9290(97)00090-0. [DOI] [PubMed] [Google Scholar]
- 55.Novotny SA, Warren GL, Hamrick MW. Aging and the Muscle-Bone Relationship. Physiology. 2015;30:8–16. doi: 10.1152/physiol.00033.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Warner SE, Sanford DA, Becker BA, Bain SD, Srinivasan S, Gross TS. Botox induced muscle paralysis rapidly degrades bone. Bone. 2006;38:257–264. doi: 10.1016/j.bone.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gross TS, Poliachik SL, Prasad J, Bain SD. The effect of muscle dysfunction on bone mass and morphology. J Musculoskelet Neuronal Interact. 2010;10:25–34. [PubMed] [Google Scholar]
- 58.Warden SJ, Galley MR, Richard JS, George LA, Dirks RC, Guildenbecher EA, Judd AM, Robling AG, Fuchs RK. Reduced gravitational loading does not account for the skeletal effect of botulinum toxin-induced muscle inhibition suggesting a direct effect of muscle on bone. Bone. 2013;54:98–105. doi: 10.1016/j.bone.2013.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alzghoul M, Gerrard D, Watkins B, Hannon K. Ectopic expression of IGF-1 and Shh by skeletal muscle inhibits disuse-mediated skeletal muscle atrophy and bone osteopenia in vivo. FASEB J. 2004;18:221–3. doi: 10.1096/fj.03-0293fje. [DOI] [PubMed] [Google Scholar]
- 60.Ausk BJ, Huber P, Srinivasan S, Bain SD, Kwon RY, McNamara EA, Poliachik SL, Sybrowsky CL, Gross TS. Metaphyseal and diaphyseal bone loss in the tibia following transient muscle paralysis are spatiotemporally distinct resorption events. Bone. 2013;57:413–22. doi: 10.1016/j.bone.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aliprantis AO, Stolina M, Kostenuik PJ, Poliachik SL, Warner SE, Bain SD, Gross TS. Transient muscle paralysis degrades bone via rapid osteoclastogenesis. FASEB J. 2012;26:1110–1118. doi: 10.1096/fj.11-196642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cabahug-Zuckerman P, Frikha-Benayed D, Majeska RJ, Tuthill A, Yakar S, Judex S, Schaffler MB. Osteocyte apoptosis caused by hindlimb unloading is required to trigger osteocyte RANKL production and subsequent resorption of cortical and trabecular bone in mice femurs. J Bone Miner Res. 2016;31:1356–65. doi: 10.1002/jbmr.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adamopoulos IE, Bowman EP. Immune regulation of bone loss by Th17 cells. Arthritis Res Ther. 2008;10:225. doi: 10.1186/ar2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Colburn NT, Zaal KJ, Wang F, Tuan RS. A role for gamma/delta T cells in a mouse model of fracture healing. Arthritis Rheum. 2009;60:1694–1703. doi: 10.1002/art.24520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kong YY, Feige U, Sarosi I, Bolon B, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 66.Marchand-Libouban H, Le Drévo MA, Chappard D. Disuse induced by botulinum toxin affects the bone marrow expression profile of bone genes leading to a rapid bone loss. J Musculoskelet Neuronal Interact. 2013;13:27–36. [PubMed] [Google Scholar]
- 67.Worton LE, Gardiner EM, Bain SD, Gross TS. Transient muscle paralysis increases the osteoclastogenic differentiation potential of marrow. Orthopaedic Research Abstract. 2011:231766. [Google Scholar]
- 68.Ausk BJ, Worton LE, Smigiel KS, Kwon RY, Bain SD, Srinivasan S, Gardiner EM, Gross TS. Muscle paralysis induces bone marrow inflammation and predisposition to formation of giant osteoclasts. Am J Physiol Cell Physiol. 2017;313:C533–C540. doi: 10.1152/ajpcell.00363.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grimston S, Goldberg D, Watkins M, et al. Connexin43 deficiency reduces the sensitivity of cortical bone to the effects of muscle paralysis. J Bone Miner Res. 2011;26:2151–60. doi: 10.1002/jbmr.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maurel D, Duan P, Farr J, et al. Beta-catenin haplo insufficient male mice do not lose bone in response to hindlimb unloading. PLoS One. 2016;11:e0158381. doi: 10.1371/journal.pone.0158381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sankaran J, Li B, Donahue LR, Judex S. Modulation of unloading-induced bone loss in mice with altered ERK signaling. Mamm Genome. 2016;27:47–61. doi: 10.1007/s00335-015-9611-x. [DOI] [PubMed] [Google Scholar]
- 72.Gerbaix M, Vico L, Ferrari SL, Bonnet S. Periostin expression contributes to cortical bone loss during unloading. Bone. 2015;71:94–100. doi: 10.1016/j.bone.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 73.Colaianni G, Cuscito C, Mongelli T, Pignataro P, Buccoliero C, Liu P, Lu P, Sartini L, Di Comite M, Mori G, Di Benedetto A, Brunetti G, Yuen T, Sun L, Reseland JE, Colucci S, New MI, Zaidi M, Cinti S, Grano M. The myokine irisin increases cortical bone mass. Proc Natl Acad Sci U S A. 2015;112:12157–62. doi: 10.1073/pnas.1516622112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Colaianni G, Mongelli T, Cuscito C, et al. Irisin prevents and restores bone loss and muscle atrophy in hindlimb suspended mice. Sci Rep. 2017;7:2811. doi: 10.1038/s41598-017-02557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kawao N, Moritake A, Tatsumi K, Kaji H. Roles of irisin in the linkage from muscle to bone during mechanical unloading in mice. Calcif Tissue Int. 2018 doi: 10.1007/s00223-018-0387-3. doi: 10.1007. [ePub ahead of print] [DOI] [PubMed] [Google Scholar]
- 76.Dankbar B, Fennen M, Brunert D, et al. Myostatin is a direct regulator of osteoclast differentiation and its inhibition reduces inflammatory joint destruction in mice. Nature Medicine. 2015;21:1085–90. doi: 10.1038/nm.3917. [DOI] [PubMed] [Google Scholar]
- 77.Hamrick MW, Shi X, Zhang W, Pennington C, et al. Loss of myostatin (GDF8) function increases osteogenic differentiation of bone marrow-derived mesenchymal stem cells but the osteogenic effect is ablated with unloading. Bone. 2007;40:1544–53. doi: 10.1016/j.bone.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kawao N, Morita H, Obata K, Tatsumi K, Kaji H, et al. Role of follistatin in muscle and bone alterations induced by gravity change in mice. J Cell Physiol. 2018;233:1191–1201. doi: 10.1002/jcp.25986. [DOI] [PubMed] [Google Scholar]
- 79.Kondo H, Ezura Y, Nakamoto T, et al. Murf1 deficiency suppresses unloading-induced effects on osteoblasts and osteoclasts to lead to bone loss. J Cell Biochem. 2011;112:3525–30. doi: 10.1002/jcb.23327. [DOI] [PubMed] [Google Scholar]
- 80.Hamrick MW. Role of the Cytokine-like Hormone Leptin in Muscle-bone Crosstalk with Aging. J Bone Metab. 2017;24:1–8. doi: 10.11005/jbm.2017.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baek K, Bloomfield SA. Beta-adrenergic blockade and leptin replacement effectively mitigate disuse bone loss. J Bone Miner Res. 2009;24:792–9. doi: 10.1359/jbmr.081241. [DOI] [PubMed] [Google Scholar]
- 82.Muir J, Judex S, Qin Y, Rubin C. Postural instability caused by extended bed rest is alleviated by brief daily exposure to low magnitude mechanical signals. Gait Posture. 2011;33:429–35. doi: 10.1016/j.gaitpost.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lau RW, Liao LR, Yu F, Teo T, Chung RC, Pang M. The effects of whole body vibration therapy on bone mineral density and leg muscle strength in older adults: a systematic review and meta-analysis. Clin Rehabil. 2011;25:975–88. doi: 10.1177/0269215511405078. [DOI] [PubMed] [Google Scholar]
- 84.Salanova M, Gelfi, Miriggi M, et al. Disuse deterioration of human skeletal muscle challenged by resistive exercise superimposed with vibration: evidence from structural and proteomic analysis. FASAB J. 2014;28:4748–63. doi: 10.1096/fj.14-252825. [DOI] [PubMed] [Google Scholar]
- 85.Trudel G, Coletta E, Cameron I, et al. Resistive exercises, with or without whole body vibration, prevent vertebral marrow fat accumulation during 60 days of head-down tilt bed rest in men. J Appl Physiol. 2012;112:1824–31. doi: 10.1152/japplphysiol.00029.2012. [DOI] [PubMed] [Google Scholar]
- 86.Belavy D, Beller G, Ritter Z, Felsenberg D. Bone structure and density via HR-pQCT in 60 d bed rest, 2 years recovery with and without countermeasures. J Musculoskelet Neuronal Interact. 2011;11:215–26. [PubMed] [Google Scholar]
- 87.Smith SM, Heer MA, Shackelford LC, et al. Benefits for bone from resistance exercise and nutrition in long-duration spaceflight: Evidence from biochemistry and densitometry. J Bone Miner Res. 2012;27:1896–906. doi: 10.1002/jbmr.1647. [DOI] [PubMed] [Google Scholar]
- 88.Becker C, Lord SR, Studenski SA, Warden SJ, et al. Myostatin antibody (LY2495655) in older weak fallers: a proof-of-concept, randomised, phase 2 trial. Lancet Diabetes Endocrinol. 2015;3:948–57. doi: 10.1016/S2213-8587(15)00298-3. [DOI] [PubMed] [Google Scholar]
- 89.Cohen S, Nathan J, Goldberg A. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discovery. 2015;14:58–74. doi: 10.1038/nrd4467. [DOI] [PubMed] [Google Scholar]
- 90.Girgis C, Mokbel N, DiGirolamo Therapies for musculoskeletal disease: can we treat two birds with one stone? Curr Osteop Rep. 2014;12:142–153. doi: 10.1007/s11914-014-0204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Laurent M, Jardi F, Dubois V, et al. Androgens have antiresorptive effects on trabecular disuse osteopenia independent from muscle atrophy. Bone. 2016;93:33–42. doi: 10.1016/j.bone.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 92.Yarrow J, Conover C, Beggs L, et al. Testosterone dose dependently prevents bone and muscle loss in rodents after spinal cord injury. J Neurotrauma. 2014;31:834–45. doi: 10.1089/neu.2013.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huh JY, Mougios V, Skraparlis A, Kabasakalis A, Mantzoros CS. Irisin in response to acute and chronic whole-body vibration exercise in humans. Metabolism. 2014;63:918–21. doi: 10.1016/j.metabol.2014.04.001. [DOI] [PubMed] [Google Scholar]


