Abstract
Background & Aims:
Gamma chain (γc) cytokines (interleukin 2 [IL2], IL4, IL7, IL9, IL15 and IL21) signal via a common γc receptor. IL2 regulates the immune response, whereas IL21 and IL15 contribute to development of autoimmune disorders, including celiac disease. We investigated whether BNZ-2, a peptide designed to inhibit IL15 and IL21, blocks these cytokines selectively and its effects on intraepithelial cytotoxic T cells.
Methods:
We obtained duodenal biopsies from 9 patients with potential celiac disease (positive results from tests for anti-TG2 but no villous atrophy), 30 patients with untreated celiac disease (with villous atrophy), and 5 patients with treated celiac disease (on a gluten-free diet), as well as 43 individuals without celiac disease (controls). We stimulated primary intestinal intraepithelial CD8+ T cell lines, or CD8+ T cells directly isolated from intestinal biopsies, with γc cytokines in presence or absence of BNZ-2. Cells were analyzed by immunoblots, flow cytometry, or RNA-seq analysis for phosphorylation of signaling molecules, gene expression profiles, proliferation and levels of granzyme B.
Results:
Duodenal tissues from patients with untreated celiac disease had increased levels of mRNAs encoding IL15 receptor subunit alpha (IL15RA) and IL21 compared with tissues from patients with potential celiac disease and controls. Activation of intraepithelial cytotoxic T cells with IL15 or IL21 induced separate signaling pathways; incubation of the cells with IL15 and IL21 cooperatively increased their transcriptional activity, proliferation, and cytolytic properties. BNZ-2 specifically inhibited the effects of IL15 and IL21, but not of other γc cytokines.
Conclusions:
We found increased expression of IL15RA and IL21 in duodenal tissues from patients with untreated celiac disease compared with controls. IL15 and IL21 cooperatively activated intestinal intraepithelial cytotoxic T cells. In particular, they increased their transcriptional activity, proliferation and cytolytic activity. The peptide BNZ-2 blocked these effects, but not those of other γc cytokines, including IL2. BNZ-2 might be used to prevent cytotoxic T-cell mediated tissue damage in complex immune disorders exhibiting upregulation of IL15 and IL21.
Keywords: autoimmunity, immune response, treatment, signal transduction
Lay Summary:
Increased levels of cytokines (IL15 and IL21) in intestinal tissues of patients with celiac disease promote proliferation and cytolytic activity of immune cells, which can be prevented with an agent that specifically blocks these 2 cytokines.
Graphical Abstract

Introduction
The common gamma chain (γc) cytokine family includes six members, interleukin(IL)-2, 4, 7, 9, 15 and 21, displaying a similar four-helical bundle structure and sharing the common γc receptor signaling subunit1. These cytokines are critically implicated both in immune homeostasis and immunopathology. IL15 and IL21 play a key role in the pathogenesis of organ-specific autoimmune and autoimmune-like disorders such as type-1 diabetes (T1D)2–3, graft-versus-host disease (GVHD)4–5 and celiac disease6–7. IL2 is a pivotal cytokine for T cell differentiation and activation8. Despite signaling through the same receptor (IL2/IL15Rβγ)9, IL2 and IL15 have distinct roles in vivo, with IL15 overexpression being associated with autoimmunity, while IL2 playing a critical immunoregulatory role by ensuring survival and expansion of regulatory T cells10.
Celiac disease is an immune-mediated disorder triggered by dietary gluten in genetically susceptible individuals. Small intestinal enteropathy in Celiac disease is promoted by a crosstalk between epithelial stress signals associated with IL15 upregulation and an anti-gluten CD4+ T cell response11. These two events are required for the licensing of intraepithelial cytotoxic T cells (IE-CTL), responsible for tissue destruction6. Importantly, IL21, produced by gluten-specific T cells12, enhances the adaptive immune response and contributes to interferon-γ (IFN-γ) production in active celiac disease7. On a milder side of disease spectrum, potential celiac disease is characterized by the development of anti-gluten immunity in the absence of tissue damage. Importantly neither IL1511 nor IL2113, were upregulated in potential celiac disease, suggesting they could play a role in villous atrophy. Whether both cytokines are co-expressed in active celiac disease and cooperate to activate human IE-CTL, remains to be established. In line with this hypothesis, IL15 and IL21 synergistically promoted proliferation and effector function of murine CTL in vitro14.
A life-long gluten-free diet (GFD) is currently the only effective treatment for Celiac disease, however poor adherence and decreased quality of life have been reported. Furthermore, up to 30% of adult patients do not fully respond to gluten withdrawal15 and 1–2% develop refractory Celiac disease (RCD), a pre-cancerous condition16. Thus, the need for alternative treatments for patients who fail to heal on a GFD.
In complex inflammatory disorders, like Celiac disease, more than a single cytokine is often implicated in the immune response, thus the lack of full therapeutic efficacy of monoclonal antibodies. To address this concern, a novel multi-cytokine pharmacological inhibition approach has been recently proposed. Taking advantage of the structural information on the cytokine binding site on the γc, multi γc-cytokines inhibitors have been designed17. The leading peptide BNZ-1 safely and selectively blocked IL2, 9 and 15 signaling18 in the context of T-cell malignancies19 where both IL2 and −15 have been implicated in driving the expansion and survival of malignant CTL. A second peptide, BNZ-2, designed to selectively block IL15 and IL21, has been synthetized, but its ability to inhibit IL15 and IL21 without hampering signaling of other γc-cytokines in human CTL remains to be determined.
In this study, we investigated the combined impact of IL15 and IL21 on signaling, transcription and function of human tissue-resident IE-CTL both in vitro and ex vivo and tested in a pre-clinical setting the ability of BNZ-2 to selectively and concomitantly block IL15 and IL21.
Materials and Methods
Patients
Eighty-seven individuals undergoing esophago-gastro-duodenal endoscopy (EGDS) during diagnostic work-up were enrolled from three centers: University of Chicago Medicine, Celiac Disease Center at Columbia University and Department of Pediatrics, University of Naples Federico II. Forty-three controls, nine potential, thirty untreated and five treated celiac patients have been included (Table S1). For each subject, 4–6 biopsies were obtained from the duodenum. Control subjects underwent EGDS during diagnostic work-up about abdominal discomfort, failure to thrive or other intestinal disorders unrelated to Celiac disease. They all had normal duodenal histology, no family history of Celiac disease, normal serum levels of anti-TG2 IgA. Potential patients had positive anti-TG2 and no villous atrophy (histological Marsh score of 0–1). Untreated (or active) Celiac disease patients had positive anti-TG2 IgA and an enteropathy with increased intraepithelial lymphocytes (IEL), crypts hyperplasia and villous atrophy, according to current diagnostic guidelines15, 20. Treated celiac patients were on a gluten-free-diet (GFD) for at least 6 months and had normal duodenal histology and serum anti-TG2 IgA levels. Analysis of patient samples was approved by the Institutional Review Board of the University of Chicago (IRB-12623B), Columbia University (IRB-AAAB2472) and University of Naples Federico II (CE 230/05).
Cytokine and BNZ-2 stimulation
CD8+ T cell lines or ex vivo single cell suspensions from the epithelial compartment (obtained as described in Supplemental Methods) were stimulated with human recombinant IL15 (Biolegend), IL21 (Biolegend) or IL2 (NIH AIDS Reagent Program) at the indicated concentrations for 20 minutes for signaling experiments, western blot (WB) and flow cytometry, or 2 hours for RNA-sequencing (RNA-seq). BNZ-2, or a control peptide (CP), scrambled sequence of BNZ-2, was added at the indicated concentrations ten minutes prior to cytokine stimulation.
Additional details are in the Supplementary materials and methods section.
Results
Concomitant expression of IL15 and IL21 is associated with intestinal damage in Celiac disease
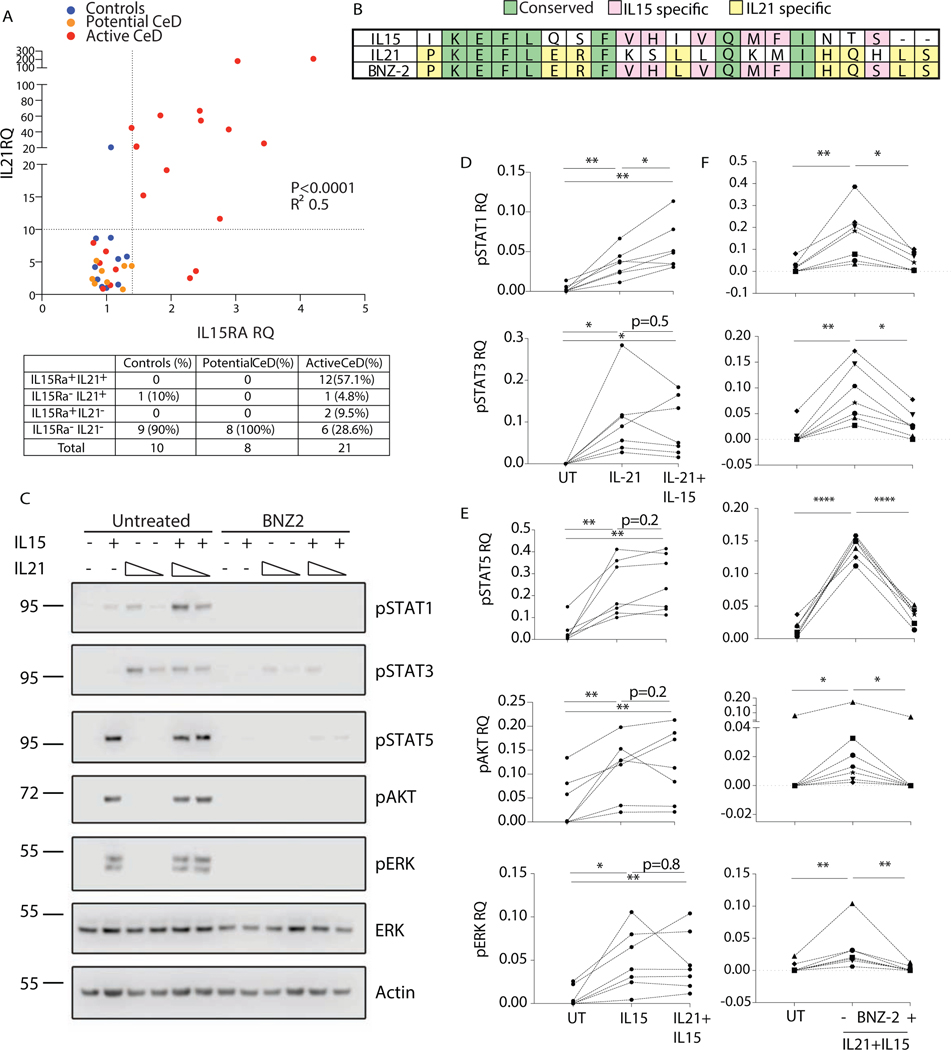
IL15 and IL21 were both proven to promote cytolytic activity in CTLs14. Their expression in the intestinal mucosa of celiac patients was individually investigated10,13,21–23. However, whether the two cytokines are co-expressed or define two distinct subsets of celiac patients is unknown. To address this question, we analyzed transcript levels of IL21 and IL15 private receptor alpha (IL15Rα), which is required for IL15 trans-presentation and signaling24, in the duodenum of celiac subjects and controls. IL15RA and IL21 transcripts were concomitantly overexpressed in 57.1% of active, but absent in potential celiac patients (Fig. 1A). Among active celiac patients, 4.8% overexpressed only IL21, 9.5% only IL15RA, while one third (28.6%) displayed neither IL15 nor IL21 upregulation.
Figure 1. IL15 and IL21 are concomitantly up-regulated in full-blown celiac disease and BNZ-2 impairs their cooperative signaling in human tissue-resident IE-CTL.
(A) Relative quantification (RQ) of IL15 receptor alpha (IL15RA) and IL21 transcripts levels in duodenal biopsies of 10 controls (blue), 8 Potential (orange) and 21 Active (red) celiac patients, as assessed by Taqman PCR. Two dotted lines indicate the cut-off used to define positive vs negative subjects. Number and percentages of patients in each quadrant are displayed in the table. IL15RA and IL21 correlation was assessed by Pearson’s test, p<0.0001, R2=0.5. (B) Alignment of the amino acid sequences of the D-helices of IL15 (NP_000576.1, 144–162) and IL21 (NP_068575.1, 133–153) and BNZ-2. Conserved positions are in green, IL15 specific in pink, IL21 specific in yellow, unique residues in white. (C) One representative blot displaying pSTAT1, pSTAT3 and pSTAT5, pAKT and pERK in human IE-CTL lines in response to IL15 (140pM) and IL21 (4 and 14pM) in absence (left) or presence (right) of BNZ-2 (3uM). (D-E) Quantification by densitometry of seven WB for pSTAT1 and pSTAT3 (D) upon stimulation with IL21 (14pM) alone or in combination with IL15 and pSTAT5, pAKT and pERK (E) upon stimulation with IL15 alone or in combination with IL21 (14pM). (F) Quantification of seven WB displaying levels of pSTAT1, pSTAT3, pSTAT5, pAKT, pERK in response to IL15 (140pM) + IL21 (14pM) in absence or presence of 3uM BNZ-2. (D-F) Phospho-protein levels are normalized to averaged ERK and actin total protein levels for each condition. Paired one-way ANOVA was performed, *p<0.05.
In conclusion, selective upregulation of IL15 takes place in less than 10% of active celiac patients, with IL15 and/or IL21 expression being dysregulated in over 71% and a large majority of active celiac patients overexpressing both cytokines. Upregulation of IL15 and IL21 occurring only in active but in none of potential celiac patients, suggests that these two cytokines could cooperatively contribute to tissue damage in Celiac disease.
BNZ-2 blocks the signaling pathways enhanced by IL15 and IL21 in tissue-resident IE-CTL
BNZ-2 is a novel γc-binding peptide designed to specifically antagonize IL15 and IL21, but not the other members of the γc cytokine family17. Its 21 amino-acid sequence, disclosed here for the first time, includes IL15- and IL21-specific, as well as shared residues (Fig. 1B).
IE-CTL are the critical effector cells mediating epithelial destruction in Celiac disease25–26. To test the hypothesis that IL15 and IL21 cooperatively contribute to IE-CTL activation and that this could be blocked by BNZ-2, we treated human IE-CTL short-term lines with IL15 and IL21, in presence and absence of BNZ-2. These cell lines were generated from small intestinal CD45+CD103+CD3+CD8+TcRαβ+ IEL (Fig. S1). Phosphorylation at key residues was assessed by WB (Fig. S2) for signal transducer and activator of transcription (STAT) molecules, including STAT1 (pY701), STAT3 (pY705) and STAT5 (pY694), as well as protein kinase B (AKT, pS473), and extracellular signal-regulated kinase (ERK, pT202/Y204) in response to increasing concentrations of IL15 and IL21. In line with studies in human and mouse lymphocytes27–31, we found that IL15 induced pSTAT5 and pERK in human IE-CTL in a dose-dependent manner starting at concentrations as low as 5pM, while phosphorylation of STAT1 and AKT required at least 140pM of IL15 (Fig. S2). In contrast to previous reports in peripheral blood lymphocytes27 and NK cells30, but in agreement with what demonstrated in CD4+ T cells31, IL15 was overall ineffective at inducing pSTAT3 in IE-CTL: concentrations above 700pM were required to observe STAT3 phosphorylation. On another hand, in accordance with previous studies32, IL21 promoted pSTAT1 and pSTAT3 starting at concentrations as low as 5pM (Fig. S2). However, it failed to induce phosphorylation of STAT5, AKT and ERK even at high concentrations. Based on these results in human IE-CTL, we used concentrations of IL15 (140 pM) and IL21 (5 and 14 pM) that induced sub-maximal levels of pSTAT molecules to investigate whether they could have additive and/or synergistic effects (Fig. 1C-E). A clear additive effect was observed for pSTAT1 upon stimulation with IL21+IL15 compared to IL21 alone (p<0.05, Fig. 1D). The increase in pSTAT3 remained overall solely driven by IL21 (Fig. 1D), while phosphorylation of STAT5, AKT and ERK were induced selectively by IL15 (Fig. 1E).
We next tested the ability of BNZ-2 to impair IL15 and IL21 signaling alone (Fig. S3) and in combination in human IE-CTL (Fig. S4 and 1F). BNZ-2 efficiently blocked IL21-mediated phosphorylation of STAT1 (p=0.01) and STAT3 (p=0.03) (Fig. S3A), and IL15-induced phosphorylation of STAT5 (p=0.01), AKT (p=0.002) and ERK (p=0.003) (Fig. S3B). More importantly, BNZ-2, but not its scrambled sequence used as a control, inhibited the combined activation of IE-CTL by IL15 and IL21 (Fig. 1F and S4).
Altogether, these results indicate that in human tissue-resident IE-CTL stimulation with IL15 and IL21 results in the merger of their distinct individual signaling pathways, suggesting that they do not substitute for each other. Furthermore, we identify a new molecule, BNZ-2, that effectively blocks the signaling promoted by each (Fig. S3) and both (Fig. 1F and S4) cytokines.
IL21 further modulates IL15 driven transcriptional program in tissue-resident IE-CTL
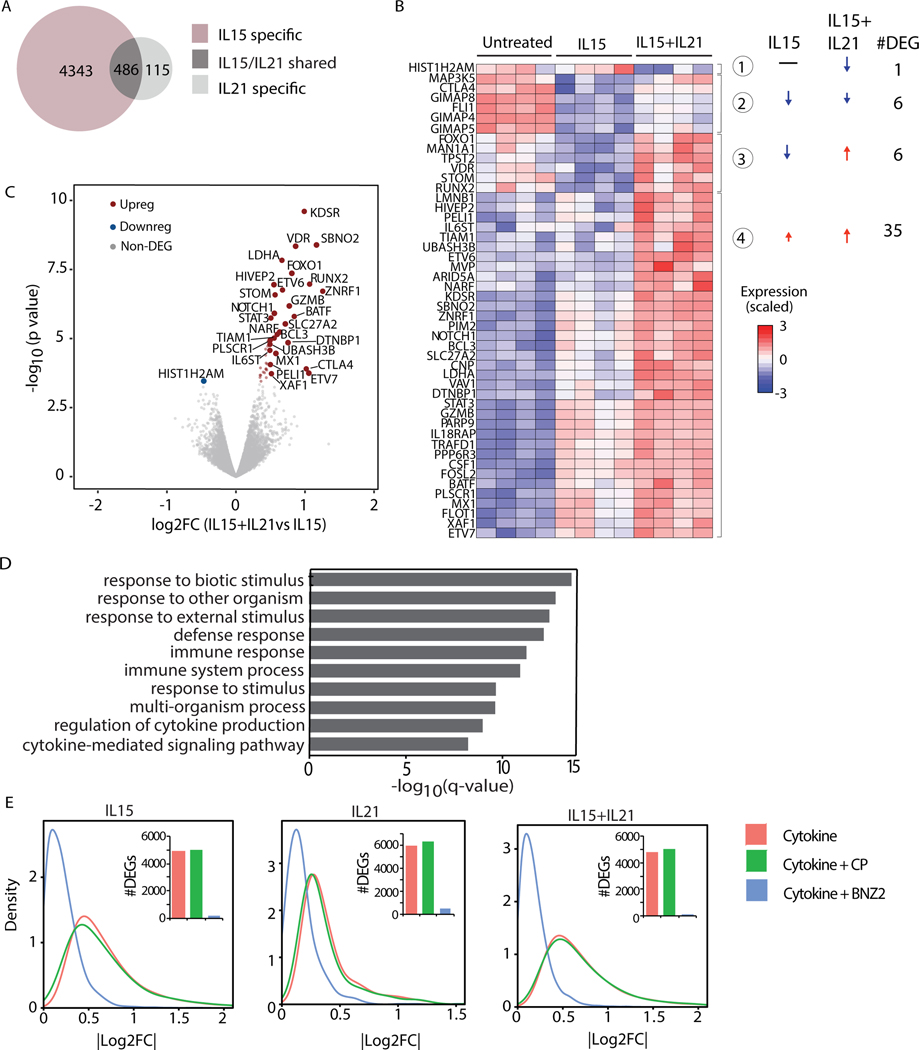
Prompted by the evidence that IL15 and IL21 induce a combined signaling profile in human IE-CTL that could not be triggered by them individually, we next investigated the impact of these cytokines on the transcriptional program of IE-CTL by RNA-seq. IE-CTL were stimulated with increasing doses of IL15 and IL21 to assess by qPCR the half maximal effective concentration (EC50) for the induction of Granzyme B (GzmB) (Fig. S5A-B), a key mediator of cytolysis in CTL that is transcriptionally upregulated by both cytokines33. The EC50 for IL15 (700pM) and IL21 (20pM) was further confirmed by analyzing STAT3 and TNFS10 transcripts (Fig. S5A-B). RNA-seq analysis of seven IE-CTL lines (52 profiles, ~20M reads/sample; Table S2) stimulated with IL15 (700pM) and/or IL21 (20pM) revealed that 4829 genes were differentially expressed (DEGs) compared to unstimulated cells in response to IL15, while IL21 only induced 601 DEGs (Fig. 2A and Table S3). 486 were shared genes, 115 and 4343 were differentially expressed selectively in response to IL21 (IL21 specific), and IL15 (IL15 specific), respectively. The evidence that IL15 can induce more massive transcriptional changes than IL21 prompted us to investigate whether and how IL21 altered IL15-driven transcriptional response in IE-CTL. The significance of the inquiry was further supported by the observation that IL15 is overexpressed in the intestinal epithelium of a subset of family members of celiac patients, lacking any signs of adaptive anti-gluten immunity11, while IL21 is produced by gluten-specific T cells13 in the gut of active patients with villous atrophy. To address this question, we interrogated the RNA-seq data and identified 48 genes (FDR<0.05) for which the intensity of the response to IL15 changed when IL21 was added (Fig. 2B). Interestingly, most of these genes (35/48, 73%) were further upregulated upon IL15+IL21 stimulation (group 4). The genes more strongly upregulated by IL15+IL21 vs. IL15 alone (red in Fig. 2C) contribute to immune pathways such as defense response and regulation of cytokine production and cytokine-mediated signaling pathways, as highlighted by the gene ontology (GO) enrichment analysis (Fig. 2D). Among these genes were GZMB, STAT3, FOXO3, BATF, BCL3, MX1 and NOTCH1 (Fig. 2B). Furthermore, 6 genes, including RUNX2 and metabolic genes such as VDR were upregulated in response to IL15+IL21, despite being down-regulated in response to IL15 alone (Fig. 2B, group 3), suggesting that in few cases IL21 could reverse the effect of IL15.
Figure 2. BNZ-2 blocks the transcriptional program individually and cooperatively induced by IL15 and IL21 in tissue-resident IE-CTL.
(A) Venn Diagram displaying the numbers of differentially expressed genes (DEGs) in response to 700pM IL15 or 20pM IL21. The expression of 4343 genes changed in response to IL15 (FDRIL15<0.05 and FDRIL21≥0.05, IL15 specific, pink), while 115 in response to IL21 (FDRIL15≥0.05 and a FDRIL21<0.05, IL21 specific, light grey). 486 genes are changed by both cytokines (FDRIL15<0.05 and FDRIL21<0.05, IL15/IL21 shared, dark grey). (B) Heat-map displaying changes in expression levels upon stimulation with IL15 alone (FDRIL15<0.05) or IL15+IL21 (FDRIL15<0.05 and FDRIL21<0.05) combination compared to untreated IE-CTL lines. Only DEGs in response to IL21+IL15 vs IL15 alone are dispayed: 4 distinct groups were identified based on their change in expression levels. Arrows indicate up- vs down-regulation. Numbers of DEGs in each group are indicated. (C) Volcano plot displaying DEGs in response to the combination of IL15+IL21 vs IL15. Expression levels upon stimulation with IL15 were used as a baseline. p-values are on the y-axis, log2 fold changes (log2FC) in expression upon IL21 addition (IL15+IL21 vs IL15) are on the x axis. The up-regulated genes are in red on the right side, the only downregulated gene is in blue on the left. (D) Gene Ontology (GO) enrichment analysis performed including all up-regulated genes (N=41) when IL21 is added to IL15 (red dots in B), ranked based on p-values. Corrected p-value (q-value) for enrichment of DEGs in each GO term is dispayed. (E) Density plots representing the impact of 1uM BNZ-2 (blue) on IL15 and/or IL21-induced (red) differential gene expression in IE-CTL lines. A scrambled sequence of BNZ-2 served as control peptide (CP, green). Absolute log2FC are on the x axis. Number of DEGs is in the histograms. All data (A-E) are based on gene expression levels in human IE-CTL lines as assessed by RNA-seq. FDR<0.05 was used as a cut-off to evaluate DEGs.
Taken together, these observations suggest that, despite IL15 having a major impact on IE-CTL, IL21 has the capacity to further enhance IL15-driven transcriptional signature.
BNZ-2 prevents IL15 and IL21 mediated transcriptional changes in IE-CTL lines and in the epithelial compartment ex vivo.
Next, we looked at the impact of BNZ-2 treatment on IL15 and/or IL21 driven transcriptional profile of IE-CTL. Importantly, BNZ-2, but not its scrambled sequence, reverted the transcriptional changes induced by IL15 and IL21 individually as well as combined (Fig. 2E), attesting its ability to prevent cytokine-mediated transcriptional re-programing of IE-CTL lines.
Furthermore, we investigated whether BNZ-2 was effective under ex vivo conditions involving both epithelial cells and IE-CTL. Previous studies demonstrated that IL15 was not only expressed but also able to signal in intestinal epithelial cells34. The single cell suspension obtained from the epithelial compartment of duodenal biopsies included ~80% of CD45-EpCam+ intestinal epithelial cells and 1–5% of CD45+EpCam-CD3+CD8+ IE-CTL as assessed by flow cytometry (Fig. S6A-B). Accordingly, when comparing transcriptional profiles of untreated IE-CTL lines and cells isolated ex vivo from the epithelial compartment to publicly available cell-type-specific data, ex vivo samples aligned with epithelial cell transcriptional profiles, while IE-CTL lines aligned with those from lymphocytes (Fig. S6C). Next, we assessed by RNA-seq the gene expression profile of the epithelial compartment upon stimulation with IL15 and/or IL21 for 2 hours. In addition, pSTAT3 and pSTAT5 were assessed by flow cytometry after 20 minutes (Fig. S6A and S7A-B). Ex vivo transcriptional data revealed 175 and 36 DEGs in response to IL15 and IL21, respectively, including 24 shared DEGs (Fig. S6D and Table S3). GO analysis revealed a much stronger impact of IL15 on both immune and non-immune related genes as compared to IL21 (Fig. S6E and Table S4). Importantly, looking at the baseline expression levels in vitro vs. ex vivo of the genes significantly changed in response to IL15 and/or IL21 ex vivo (Fig. 3A), we identified a subset only expressed ex vivo (on the left side of the plot, Fig. 3A), including mainly epithelial genes. In particular, genes coding for apolipoproteins (APOA1 and 4), junctional proteins (CLDN3, 4, 23 and PTPRF) and epithelial transporters (SLC6A19, PIGR, SLC39A5) were selectively modulated by IL15, while the gene coding for the enzyme gastrin (GAST) was selectively changed by IL21 (Fig. 3A). Nevertheless, despite the presence of genes selectively changed ex vivo, a concordant response was observed between cell lines and ex vivo samples for IL15 (r=0.451; p-value=2.254e-11) and IL21 (r=0.58; p-value=0.000361) responsive genes (Fig. S5F).
Figure 3. Cooperative and synergistic effects of IL15 and IL21 in the small intestinal epithelial compartment are efficiently blocked by BNZ-2 ex vivo.
(A) Scattered plot displaying DEGs in response to IL15 only (FDRIL15<0.05 and FDRIL21≥0.05, purple dots), IL21 only (FDRIL21<0.05 and FDRIL15≥0.05, yellow dots) or their combination (FDRIL15<0.05 and FDRIL21<0.05, green dots) ex-vivo. Baseline expression levels ex vivo (y) and in vitro in cell lines (x) are plotted for each gene. Correlation between ex vivo and in vitro expression: r=−0.33, Pearson’s test. (B) Volcano-plot displaying genes significantly changed in response to IL15+IL21 vs IL15 only (baseline), p-values are plotted on the y-axis, log2FC in expression when adding IL21 (IL15+IL21 vs IL15) on the x axis. Up-regulated genes are in red, the down-regulated are in blue. (C) Density plots indicating the impact of BNZ-2 (blue) on IL15 and IL21-induced (red) gene expression changes. Number of DEGs is indicated in the histograms in each plot. Data in A-C were assessed by RNA-seq in a single cell suspension of the epithelial compartment stimulated ex vivo with 700pM of IL15 and/or 20pM of IL21, in presence or absence of 1uM BNZ-2. FDR<0.05 was used to evaluate DEGs. (D-E) On the left, representative FACS plot displaying GzmB intracellular expression in live CD3+CD8+ cells in response to IL15 (700pM), IL21 (20pM) and their combination (D), and the impact of 1uM of BNZ-2 or a control peptide (CP, E). On the right, quantification of 6 experiments (D), including 4 GFD patients (red) and 2 Controls (CTR, blue) and of N=9 experiments (E, left plot), including 4 GFD patients (red) and 5 CTR, (blue) with BNZ-2 and N=3 CTR with the CP (E, right plot). A single cell suspension of the duodenal epithelial compartment was stained ex vivo and analyzed by flow cytometry. Each distinct symbol shape refers to an individual. Paired one-way ANOVA was performed, **p<0.01, ***p<0.001, ****p<0.0001.
In accordance with our observations in IE-CTL lines (Fig. 3B), a distinct ex vivo transcriptional profile emerged in response to combined IL15 and IL21 as compared to IL15 stimulation alone (Fig. 3B). Of note, GZMB was the highest upregulated gene in the ex vivo analysis. In addition, there was a significant upregulation of perforin (PRF1), suggesting that the two cytokines cooperatively enhance IE-CTL cytolytic program. Importantly, BNZ-2 efficiently prevented IL15/IL21-mediated transcriptional alterations not only in IE-CTL lines (Fig. 2E), but also in the small intestinal epithelial compartment as a whole, including both IE-CTL and epithelial cells (Fig. 3C). Finally, BNZ-2 blocked STAT5 and STAT3 phosphorylation upon IL15 (Fig. S7A and S8A) and IL21 (Fig. S7B) stimulation, respectively.
All together, these results indicate that IL15 and IL21 can reprogram IE-CTL and epithelial cells ex vivo. Furthermore, they demonstrate the ability of BNZ-2 to block the cytokine-induced reprogramming in a setting that reproduces, at least to a degree, the complexity of the small intestinal epithelial compartment where both lymphocytes and epithelial cells can respond to the presence of IL15 and IL21.
BNZ-2 blocks IL15 and IL21-mediated synergistic activation of IE-CTL ex-vivo.
Having proven that IL21 could further modulate IL15-induced gene transcriptional profile in IE-CTL (Fig. 1D and 2C), we analyzed the impact of the two cytokines on GzmB production (Fig. 3D) and IE-CTL proliferation (Fig. S7C) by flow cytometry in freshly isolated IE-CTL from celiac patients and controls. As anticipated, IL15 and IL21 synergistically increased the frequency of GzmB+ IE-CTL (Fig. 3D). Moreover, their combination led to increased IE-CTL proliferation, as assessed by Ki67 staining (Fig. S7C). Importantly, BNZ-2, but not its scrambled sequence, reverted the functional synergistic effects of IL15 and IL21 on GzmB expression (Fig. 3E and Fig. S8B) and IE-CTL proliferation (Fig. S7C).
Altogether, these results indicate that IL15 and IL21 may synergistically promote the expansion of IE-CTL and their ability to kill epithelial cells in active celiac patients. Furthermore, the combined effect of IL15 and IL21 on IE-CTL can be blocked by BNZ-2.
BNZ-2 prevents cytokine and gliadin-induced release of IFN-γ.
Gliadin, the dietary protein responsible for Celiac disease development, was reported to increase the production of IL1521, IL217,25 and IFN-γ35 in small intestinal biopsies of celiac patients. Furthermore, IL15 in synergy with IL21 was proven to up-regulate IFN-γ30. Importantly, BNZ-2 prevented the increase in IFN-γ transcript levels in IE-CTL in response to IL15 and IL21 stimulation alone or in combination (Fig. S9A).
Next, we determined whether BNZ-2 could prevent the release of IFN-γ in intestinal organ cultures in response to gliadin via its ability to block IL15 and IL21 mediated signaling. Duodenal organ culture generated from biopsies of active celiac patients were stimulated with 1mg/ml of peptic-tryptic digest of gliadin (PTG) in presence or absence of BNZ-2. As displayed in Fig. S9B, IFN-γ produced upon PTG stimulation in the supernatants of organ cultures (p<0.01) was significantly reduced in presence of BNZ-2 (p<0.05). These results further suggest that BNZ-2 may prevent the pathogenic effects of gluten challenge in celiac patients.
BNZ-2 specifically blocks IL15, without impacting IL2 mediated activation of IE-CTL
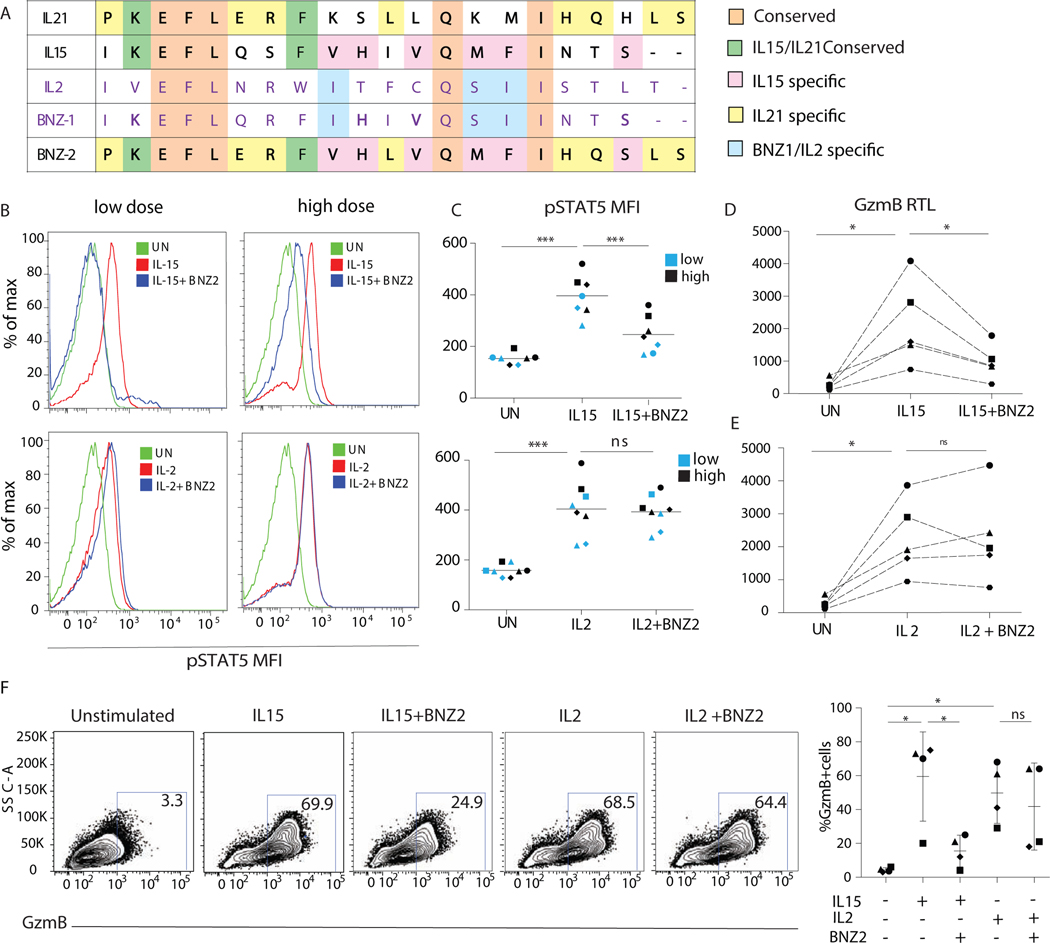
IL2, IL4, IL7, IL9, IL15, and IL21, all interact with the common γc (IL2Rγ) via the γc box present in their D-helices17. Each γc box is composed of a unique sequence of amino acids. BNZ-2, similarly to BNZ-1, that selectively blocks IL2, IL9, and IL15, was designed based on the structural information of the γc cytokine binding site. More specifically, the sequence of BNZ-1 is a composite of the IL2 and IL15 D-helices, while BNZ-2 is a composite of IL15 and IL21. Importantly, BNZ-2 shares 13 and 15 residues with IL15 and IL21 respectively, and only 6 amino acids with IL2 (Fig. 4A).
Figure 4. BNZ-2 specifically inhibits IL15 but not IL2-mediated changes in signaling and function in tissue-resident IE-CTL.
(A) Alignment of BNZ-1 and 2 with IL2, IL21 and IL15. The aminoacidic sequences of the D-helices for the three γc cytokines: IL2 (purple font), IL15 and IL21 (black font) were aligned to those of BNZ-1 (purple) and BNZ-2 (black). Shared residues are highlighted in a color-coded fashion: in common to all cytokines and both BNZ-1 and BNZ-2 (orange), conserved between IL21, IL15 and BNZ-2 (green), shared by BNZ-2 only with IL15 (pink) and IL21 (yellow), in common to BNZ-1 and IL2 (light blue). (B) Representative density plot displaying the MFI of pSTAT5 in response to 100pM (left) and 700pM (right) of IL15 (upper panel) or IL2 (lower panel) with or without 3uM BNZ-2 (blue) in IE-CTL lines, as compared to unstimulated (UN) cells (green). MFI quantification in response to low (blue) and high (black) concentrations of IL15 and IL2. (E-F) GZMB relative transcripts levels (RTL) in response to IL15 (D) or IL2 (E) with or without 1uM BNZ-2. (F) Intracellular expression of GzmB in response to IL15 or IL2 with or without 1uM BNZ-2. One representative plot (left) and frequency of GzmB+ cells in 4 independent experiments (right). A-F, paired one-way ANOVA, *p<0.05, ***p<0.001. C-F Each symbol refers to a distinct cell line.
IL2 was indicated, in contrast to IL15, to prevent organ-specific autoimmunity9. In addition to the γc, it also shares the IL2Rβ signaling subunit with IL1536. To determine whether BNZ-2 would block IL15 while preserving IL2 signaling in IE-CTL lines, we analyzed induction of pSTAT5 (Fig. 4B-C). Remarkably, BNZ-2 blocked IL15 (upper panels) but not IL2- signaling (lower panels), even when IL2 was used at low concentration (100pM).
In line with the ability of BNZ-2 to block IL15 but not IL2 signaling, 1uM BNZ-2 impaired IL15 (Fig. 4D), but not IL2 (Fig. 4E), mediated upregulation of GZMB transcripts (p<0.05) (Fig. S5) and protein (Fig. 4F) in IE-CTL lines, assessed by qPCR and flow cytometry, respectively. The EC50, determined by qPCR for each cytokine, was used for these experiments.
The extent to which BNZ-2 was able to block IL15 signaling depended also on the concentration of IL15 used to stimulate IE-CTL. More specifically, 3uM of BNZ-2 reduced pSTAT5 mean fluorescence intensity (MFI) from 500 to 300, and from 400 to around 200 when 700pM (high dose) and 100pM (low dose) of IL15 were used, respectively (Fig. 4C). In addition, the impact of higher doses of IL15 on the upregulation of GZMB transcript levels was dose-dependent (Fig. S10), indicating that it may be possible to calibrate the in vivo impact of BNZ-2 by adjusting its dose. In particular, to preserve the physiological functions of IL15 at low concentration, while preventing its pathogenic effects at high concentrations.
Because IL7, another member of the γc cytokine family, plays an important role in survival and proliferation of human T cells37, and in intestinal homeostasis38, we determined the impact of BNZ-2 on IL7 mediated signaling in IE-CTL. Ex vivo analysis of pSTAT5 by flow cytometry proved that IL7 mediated increase in pSTAT5 was not impaired by BNZ-2 (Fig. S11A-B). Preservation of IL7 and IL2 signaling in presence of BNZ-2 was further confirmed by WB in IE-CTL lines (Fig. S11C-E). Finally, because the γc receptor is also shared with other γc cytokines, we confirmed that BNZ-2 selectively prevented IL21 and IL15, but not IL2, IL9 or IL4, mediated proliferation in NK92 cells (Fig. S12).
Together, these results indicate that BNZ-2 is an effective yet modulable compound, that selectively inhibits IL15 and IL21 signaling. In particular it preserves IL2 signaling (Fig. 4 and S11), despite IL2 sharing the same signaling receptor as IL15.
Discussion
Our study reveals that Celiac disease with villous atrophy, in contrast to potential celiac disease, is characterized by the concomitant over-expression of IL15 and IL21 in the intestinal mucosa. Furthermore, our data indicate that IL15 and IL21 synergize in tissue-resident IE-CTL activation, and promote non-redundant signaling pathways. In particular IL15 induced pSTAT5 and IL21 pSTAT3, while they cooperatively enhanced pSTAT1 and synergistically increased cytolytic properties and proliferation in IE-CTL. Altogether, these findings support a cooperative role for IL15 and IL21 in promoting tissue damage in celiac disease. Notably, we identify BNZ-2 as a novel γc binding peptide that concurrently inhibit IL15 and IL21 mediated activation of tissue resident IE-CTL, while preserving IL2 signaling, suggesting that BNZ-2 represents a unique novel therapeutic candidate for active celiac patients.
In addition to indicating that IL15 and IL21 induce distinct and non-overlapping signaling pathways, our study also provides support to the concept that IL21 may cooperate with IL15 to promote active Celiac disease by enhancing IL15-driven transcriptional signature in human IE-CTL. Even though IL15 induces massive transcriptional changes in IE-CTL, IL21 enhances the impact of IL15 by upregulating genes associated with important immune functions (RUNX2, FOXO1, BATF, STAT3, BCL3 and NOTCH1). Ex vivo studies further confirmed that IL21 enhanced IL15-mediated transcriptional reprogramming of IE-CTL. In particular, IL15 in combination with IL21 led to a more significant upregulation of GZMB, PRF1, VAV1 and NOTCH1 and downregulation of RGS1 and IRF4, vs. IL15 alone. Moreover, non-saturating concentrations of the two cytokines synergistically promoted GzmB (Fig. 3D) and IFN-γ (Fig. S9A) production and enhanced IE-CTL proliferation (Fig. S7C). All together our ex vivo observations provide evidence that IL15 and IL21 cooperatively and non-redundantly activate human IE-CTL, key effector tissue-resident cell type mediating tissue destruction in Celiac disease. Importantly, our finding that only active, but not potential celiac patients had increased expression of both cytokines, further supports the concept that the two cytokines have a role in overt inflammation. In accordance, a recently published clinical trial39 reports that anti-IL-15 monoclonal antibody failed to prevent mucosal injury in response to gluten challenge, despite reducing CD3+ epithelial infiltration in celiac patients. This indicates that blocking IL-15 alone is not sufficient and that targeting in addition IL-21 may contribute to preventing villous atrophy.
In recent years, a strategy based on the usage of peptides that share specific amino acids with particular γc cytokines was designed to target the binding interface on the γc receptor and selectively block their signaling, without impairing other γc cytokines17. This is of particular interest for diseases in which multiple cytokines of the same family are involved. The first multi-γc-cytokines inhibitor, BNZ-1, was designed to block IL2, IL9 and IL1517-18 and proven to decrease regulatory T cells, NK cells and CD8+ Central Memory T-Cells in T-Cell malignancies19. Thanks to its safety profile it is currently tested in phase Ia clinical trials for T-cell large granular lymphocyte leukemia, and refractory cutaneous T cell lymphoma (ClinicalTrials.gov Identifier: NCT03239392). BNZ-2, a novel peptide with a distinct amino acidic sequence, inhibits individual and cooperative effects of IL15 and IL21 in human IE-CTLs without interfering with other γc cytokines, in particular IL2. This is remarkable given that IL2 and IL15 share the same heterodimeric signaling receptor (IL2/IL15Rβγ). The rationale behind wanting to preserve IL2 signaling lies in its key function in immune tolerance through its role in regulatory Foxp3+ T cell homeostasis10.
In addition to being a potential treatment for active CeD patients, BNZ-2 may also represent an approach to prevent development of RCD. While several studies pointed to a critical role for IL15 in the pathogenesis of RCD, three distinct sets of evidences indicate that blocking IL21 in addition to IL15 may contribute to prevent future RCD development in CeD patients: (i) RCD onset is linked to the intensity of the anti-gluten CD4+ T cell response associated with IFN-γ and IL21 production40, indeed HLA-DQ2 homozygous subjects are at higher risk for RCD41; (ii) IL21 promotes pSTAT3 and IELs cytolytic function (Fig. 2D); (iii) mutations facilitating IL15 mediated STAT3 activation are positively selected in refractory IELs16,42. Despite potentially useful to prevent its development, a strategy blocking also IL21 would have no additional advantage over anti-IL15 treatment for established RCD on a gluten-free diet because STAT3 activation can be induced by IL15 alone and doesn’t depend on IL21 in IELs43. Moreover, IE-CTL cytolytic properties and consequently villous atrophy, persists even on a GFD, indicating that IFN-γ and IL21 produced by gluten-specific CD4+ T cells12 are not critical in RCD. Taken together, these observations indicate that BNZ-2 might be effective in preventing, but not treating RCD (Fig. S13).
In summary, our study suggests that BNZ-2 is a unique therapeutic candidate for active Celiac disease where IL15 and IL21 play a joint role in the activation of tissue-resident IE-CTL that mediate tissue destruction and can undergo STAT3-dependent lymphomagenesis40,42. Importantly, BNZ-2 will be effective in over 70% of celiac patients, targeting not only those overexpressing IL15 or IL21, but also 57% of patients upregulating both. Furthermore, suggesting that PEGylated BNZ-2 will be effective in patients, BNZ-1 has been proven to have a good pharmacodynamic profile in vivo in humans in its PEGylated form19. This novel approach has the dual advantage of being more wide-ranging than targeting a single cytokine (i.e. anti-IL15 blockers43–44) and more specific than blocking a wide range of cytokines, (i.e. JAK inhibitors45).
More generally, BNZ-2 may also have a therapeutic potential in other autoimmune disorders such as T1D2–3 and GVHD4–5 where IL15 and IL21 driven activation of CTL plays a critical role in tissue destruction. Finally, whether BNZ-2 might exert wider effects including blocking IL21-mediated B-cell activation and antibody production remain to be demonstrated.
Supplementary Material
What you need to know:
BACKGROUND AND CONTEXT:
Interleukin 2 (IL2) regulates the immune response, whereas IL21 and IL15 contribute to development of autoimmune disorders, including celiac disease. We investigated whether BNZ-2, a peptide designed to inhibit IL15 and IL21, blocks these cytokines selectively and its effects on intraepithelial cytotoxic T cells.
NEW FINDINGS:
We found increased expression of IL15RA and IL21 in duodenal tissues from patients with untreated celiac disease compared with controls. Incubation of intraepithelial cytotoxic T cells with IL15 or IL21 increased their proliferation and cytolytic activity, and the peptide BNZ-2 specifically blocked these effects, but not effects of IL2.
LIMITATIONS:
This study was performed using cell lines and intraepithelial cytotoxic T cells isolated from duodenal biopsies. Further studies are needed in patients.
IMPACT: BNZ-2
might be used to prevent cytotoxic T-cell mediated damage to the intestinal mucosa in patients with immune regulatory disorders associated with overexpression of IL15 and IL21.
Acknowledgments.
The authors thank all patients and their family members as well as the University of Chicago Celiac Disease Center for supporting our research. The authors thank Valerie Abadie, University of Chicago, for critical reading of the manuscript and fruitful discussion. The authors thank Vania Yotova and Anne Dumaine (CHU Sainte-Justine Research Center, Montreal, QC, Canada), Maria Vittoria Barone and Giuliana Lania (University of Naples Federico II) for technical support. The authors thank Nicholas Di Nardi for helping consenting patients. Human recombinant IL2 used for stimulation of cell lines and ex vivo samples was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH.
Grant Support. This work was supported by the following grant from the NIH: R01DK067180 to B.J. Additional support includes: Cancer Center Support Grant P30CA014599, Digestive Diseases Research Core Center P30 DK42086 at the University of Chicago to B.J. and the “Carlino fellowship for celiac disease research” from the University of Chicago Celiac Disease Center to V.D. This work was also supported in part by research funding from BIONIZ therapeutics.
Disclosures. N.D., A.B., L.A.M., and N.A. are employees of BIONIZ therapeutics, the developer of the peptide used in this study. B. J. is an advisor to and shareholder of BIONIZ therapeutics. BIONIZ therapeutics holds the United States patent for the peptide. We also declare that the financial involvements of BIONIZ therapeutics did not undermine the scientific objectivity and integrity of the presented work.
Abbreviations:
- AKT
protein kinase B
- CP
control peptide
- CTL
cytotoxic T lymphocytes
- DEGs
differentially expressed genes
- EGDS
esophago-gastro-duodeno-scopy
- ERK
extracellular signal-regulated kinase
- Foxp3
regulatory forkhead box P3
- GZMB
granzyme B
- GFD
gluten free diet
- GVHD
graft-versus-host disease
- GWAS
genome wide association studies
- IE-CTL
intra-epithelial cytotoxic T lymphocytes
- IELs
intraepithelial lymphocytes
- IL
interleukin
- IL15Rα
Interleukin 15 receptor alpha
- IFN-γ
interferon gamma
- Log2FC
log2 fold changes
- NK
natural killer
- PTG
pepto-triptic digest of gliadin
- RNA-seq
RNA sequencing
- RCD
refractory celiac disease
- STAT
signal transducer and activator of transcription
- T1D
type-1 diabetes
- TG2
tissue transglutaminase
- WB
western blot
- γc
gamma chain
Footnotes
Data availability: RNA-sequencing was performed by the University of Chicago Genomics Facility (http://genomics.bsd.uchicago.edu). The RNA-seq data reported in this paper were deposited into the National Center for Biotechnology Information Gene Expression Omnibus database under the SuperSeries record GSE120904.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All other authors disclose no conflicts of interest.
References
Author names in bold designate shared co-first authorship
- 1.Rochman Y, Spolski R, Leonard WJ, New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol 9, 480–490 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spolski R, Kashyap M, Robinson C, Yu Z, Leonard WJ, IL-21 signaling is critical for the development of type I diabetes in the NOD mouse. Proc Natl Acad Sci U S A 105, 14028–14033 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J, Feigenbaum L, Awasthi P, et al. Insulin-dependent diabetes induced by pancreatic beta cell expression of IL-15 and IL-15Ralpha. Proc Natl Acad Sci U S A 110, 13534–13539 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaser BW, Roychowdhury S, Kim DJ, et al. Donor-derived IL-15 is critical for acute allogeneic graft-versus-host disease. Blood 105, 894–901 (2005) [DOI] [PubMed] [Google Scholar]
- 5.Hippen KL, Bucher C, Schirm DK, et al. Blocking IL-21 signaling ameliorates xenogeneic GVHD induced by human lymphocytes. Blood 119, 619–628 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jabri B, Abadie V, IL-15 functions as a danger signal to regulate tissue-resident T cells and tissue destruction. Nat Rev Immunol 15, 771–783 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fina D, Sarra M, Caruso R, et al. Interleukin 21 contributes to the mucosal T helper cell type 1 response in coeliac disease. Gut 57, 887–892 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Ross Sarah H. and Doreen A. Cantrell Signaling and Function of Interleukin-2 in T Lymphocytes. Ann Rev Immunol 26; 36: 411–433 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waldmann TA, The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol 6, 595–601 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Josefowicz SZ, Lu LF, Rudensky AY, Regulatory T cells: mechanisms of differentiation andfunction. Annu Rev Immunol 30, 531–564 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Setty M, Discepolo V, Abadie V, et al. Distinct and Synergistic Contributions of Epithelial Stress and Adaptive Immunity to Functions of Intraepithelial Killer Cells and Active Celiac Disease. Gastroenterology 149, 681–691 e610 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bodd M, Ráki M, Tollefsen S, et al. HLA-DQ2-restricted gluten-reactive T cells produce IL-21 but not IL-17 or IL-22. Mucosal Immunol 3, 594–601 (2010). [DOI] [PubMed] [Google Scholar]
- 13.Borrelli M, Gianfrani C, Lania G, et al R. In the Intestinal Mucosa of Children with Potential Celiac Disease IL-21 and IL-17A are Less Expressed than in the Active Disease. Am J Gastroenterol 111, 134–144 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Zeng R, Spolski R, Finkelstein SE, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med 201, 139–148 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubio-Tapia A, Hill ID, Kelly CP, et al. American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol 108, 656–676 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malamut G, Cellier C. Refractory Celiac Disease Gastroenterol Clin North Am. 2019;48(1):137–144. [DOI] [PubMed] [Google Scholar]
- 17.Nata T, Basheer A, Cocchi F, et al. Targeting the binding interface on a shared receptor subunit of a cytokine family enables the inhibition of multiple member cytokines with selectable target spectrum. J Biol Chem 290, 22338–22351 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Massoud R, Enose-Akahata Y, Tagaya Y, et al. Common gamma-chain blocking peptide reduces in vitro immune activation markers in HTLV-1-associated myelopathy/tropical spastic paraparesis. Proc Natl Acad Sci U S A 112, 11030–11035 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frohna P, Tagaya Y, Ratnayake A, et al. Results from a First-in-Human Study with Bnz-1: A Novel Peptide Inhibitor of IL-2, IL-9 and IL-15 for the Treatment of T-Cell Malignancies that Safely and Selectively Decreases Regulatory T-Cells, Natural Killer Cells, and CD8+ Central Memory T-Cells. Blood 130, 695 (2017).28798057 [Google Scholar]
- 20.Husby S, Koletzko S, Korponay-Szabó IR, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 54,136–60 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Maiuri L, Ciacci C, Auricchio S, et al. Interleukin 15 mediates epithelial changes in celiac disease. Gastroenterology 119, 996–1006 (2000). [DOI] [PubMed] [Google Scholar]
- 22.Mention JJ, Ben Ahmed M, Bègue B, et al. Interleukin 15: a key to disrupted intraepithelial lymphocyte homeostasis and lymphomagenesis in celiac disease. Gastroenterology 125, 730–745 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Sarra M, Cupi ML, Monteleone I, et al. IL-15 positively regulates IL-21 production in celiac disease mucosa. Mucosal Immunol 6, 244–255 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Fehniger TA, Caligiuri MA, Interleukin 15: biology and relevance to human disease. Blood 97, 14–32 (2001). [DOI] [PubMed] [Google Scholar]
- 25.Jabri B, Sollid LM. Tissue-mediated control of immunopathology in coeliac disease. Nat Rev Immunol; 9(12):858–70. (2009). [DOI] [PubMed] [Google Scholar]
- 26.Abadie V, Discepolo V, Jabri B, Intraepithelial lymphocytes in celiac disease immunopathology. Semin Immunopathol 34, 551–566 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Johnston JA, Bacon CM, Finbloom DS, et al. Tyrosine phosphorylation and activation of STAT5, STAT3, and Janus kinases by interleukins 2 and 15. Proc Natl Acad Sci U S A 92, 8705–8709 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin JX, Migone TS, Tseng M, et al. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity 2, 331–339 (1995). [DOI] [PubMed] [Google Scholar]
- 29.Miyazaki T, Kawahara A, Fujii H, et al. Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science 266, 1045–1047 (1994). [DOI] [PubMed] [Google Scholar]
- 30.Strengell M, Matikainen S, Sirén J, et al. IL-21 in synergy with IL-15 or IL-18 enhances IFN-gamma production in human NK and T cells. J Immunol 170, 5464–5469 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Marzec M, Halasa K, Kasprzycka M, et al. Differential effects of interleukin-2 and interleukin-15 versusinterleukin-21 on CD4+ cutaneous T-cell lymphoma cells. Cancer Res 68(4):1083–91 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Spolski R. and Leonard WJ, Interleukin-21: Basic Biology and Implications for Cancer and Autoimmunity. Annu. Rev. Immunol 26:57–79 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Pouw N, Treffers-Westerlaken E, et al. Combination of IL-21 and IL-15 enhances tumour-specific cytotoxicity and cytokine production of TCR-transduced primary T cells. Cancer Immunol Immunother 59, 921–931 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reinecker HC, MacDermott RP, Mirau S, et al A. Intestinal epithelial cells both express and respond to interleukin 15. Gastroenterology 111, 1706–1713 (1996). [DOI] [PubMed] [Google Scholar]
- 35.Nilsen EM, Jahnsen FL, Lundin KE, et al. , Gluten induces an intestinal cytokine response strongly dominated by interferon gamma in patients with celiac disease. Gastroenterology 115, 551–563 (1998). [DOI] [PubMed] [Google Scholar]
- 36.Ring AM, Lin JX, Feng D, et al. Mechanistic and structural insight into the functional dichotomy between IL-2 and IL-15. Nat Immunol 13, 1187–1195 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niu N. and Qin X. New insights into IL-7 signaling pathways during early and late T cell development. Cellular & Molecular Immunology 10, 187–189 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belarif L, Danger R, Kermarrec L. et al. IL-7 receptor influences anti-TNF responsiveness and T cell gut homing in inflammatory bowel disease. J Clin Invest 129(5):1910–1925 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lähdeaho ML, Scheinin M, Vuotikka P et al. Safety and efficacy of AMG 714 in adults with coeliac disease exposed to gluten challenge: a phase 2a, randomised, double-blind, placebo-controlled study. Lancet Gastroenterol Hepatol. doi: 10.1016/S2468-1253(19)30264-X (2019) [DOI] [PubMed] [Google Scholar]
- 40.Kooy-Winkelaar YM, Bouwer D, Janssen GM, et al. CD4 T-cell cytokines synergize to induce proliferation of malignant and nonmalignant innate intraepithelial lymphocytes. Proc Natl Acad Sci U S A 114, E980–E989 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Toma A, Goerres MS, Meijer JW, et al. Human leukocyte antigen-DQ2 homozygosity and the development of refractory celiac disease and enteropathy associated T-cell lymphoma. Clin Gastroenterol Hepatol 4:315–9 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Ettersperger J, Montcuquet N, Malamut G, et al. Interleukin-15-Dependent T-Cell-like Innate Intraepithelial Lymphocytes Develop in the Intestine and Transform into Lymphomas in Celiac Disease. Immunity 45, 610–625 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Morris JC, Janik JE, White JD, et al. Preclinical and phase I clinical trial of blockade of IL-15 using Mikbeta1 monoclonal antibody in T cell large granular lymphocyte leukemia. Proc Natl Acad Sci U S A 103, 401–406 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yokoyama S, Watanabe N, Sato N, et al. Antibody-mediated blockade of IL-15 reverses the autoimmune intestinal damage in transgenic mice that overexpress IL-15 in enterocytes. Proc Natl Acad Sci U S A 106, 15849–15854 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yokoyama S, Perera PY, Waldmann TA, et al. Tofacitinib, a janus kinase inhibitor demonstrates efficacy in an IL-15 transgenic mouse model that recapitulates pathologic manifestations of celiac disease. J Clin Immunol 33, 586–594 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.