Abstract
Objective:
Clinical and biological correlates of resilience in major depressive disorder are scarce. We aimed to investigate the effect of the Val66Met polymorphism in the BDNF gene on resilience scores in major depressive disorder patients and evaluate the polymorphism’s moderation effect on resilience scores in response to cognitive therapy.
Method:
A total of 106 major depressive disorder patients were enrolled in this clinical randomized study. The Resilience Scale and the Hamilton Rating Scale for Depression were applied at baseline, post-treatment, and at six months of follow-up. Blood samples were obtained at baseline for molecular analysis.
Results:
The baseline resilience scores were higher in patients with the Met allele (114.6±17.6) than in those with the Val/Val genotype (104.04±21.05; p = 0.037). Cognitive therapy treatment increased resilience scores (p ≤ 0.001) and decreased depressive symptoms (p ≤ 0.001). In the mixed-effect model, the Val/Val genotype represented a decrease in resilience scores (t 218 = -1.98; p = 0.048), and the Val66Met polymorphism interacted with sex to predict an increase in total resilience scores during cognitive treatment (t 218 = 2.69; p = 0.008).
Conclusion:
Our results indicate that cognitive therapy intervention could improve resilience in follow-up, considering that gender and genetic susceptibility are predicted by the Val66Met polymorphism.
Keywords: Resilience, Val66Met polymorphism, psychotherapy
Introduction
Major depressive disorder (MDD) is a severe and recurrent disorder linked to functional impairment, poor quality of life, medical morbidity, and mortality.1 The etiology of MDD is complex and may involve the interplay of multiple environmental and genetic factors.2 The way individuals react and respond to life adversities (resilience) corresponds to increased vulnerability to psychiatric disorders such as MDD.
Resilience refers to a person’s ability to adapt successfully to acute stress, trauma, or chronic forms of adversity. Studies suggest that, as an adaptive process, stress resilience may change over time due to developmental and environmental factors.3 A resilient individual, tested by adverse events, demonstrates adaptive psychobiological stress responses, or psychobiological allostasis. Although the psychosocial and social determinants of resilience are well defined,4 the biological underpinnings of resilience have just begun to be characterized. These efforts have focused on peripheral neuroendocrine changes predictive of resilience or on genetic variations that are linked with resilient outcomes.
Brain-derived neurotrophic factor (BDNF) is a member of the nerve growth factor family and is one of the most abundant neurotrophic factors in the brain. Although it plays an essential role during development in the guidance, functioning, and survival of neurons, it is also highly expressed in the adult brain, playing a role in brain plasticity and neuronal survival.5 BDNF is regulated at multiple levels, including transcriptional regulation of messenger RNA (mRNA), as well as activity-dependent release from nerve terminals. Decreased BDNF levels and expression could reduce neuroplasticity and neurogenesis in the hippocampus after acute and chronic stress, and they have been associated with depressive symptoms in pre-clinical and clinical studies.6 Typical antidepressant treatment increases BDNF mRNA expression in the hippocampus and cortical regions,7 as well as significantly increases blood levels of BDNF in MDD patients.8 Importantly, results from a genetic rat model support the hypothesis that reduced BDNF/trkB signaling in the hippocampus could contribute to greater vulnerability to stress-induced depression.9 Moreover, stressful early life experiences may induce adaptive plasticity, including increased BDNF expression after acute stress, which, in adolescent rats, was found to influence coping strategies and the response to an acute stress challenge.10
A functional BDNF polymorphism, Val66Met is found in 25-30 percent of humans,11,12 and results in decreased processing and trafficking of BDNF transcripts to dendrites.9,13 There is evidence that Met carriers exposed to early life stress or trauma are at increased risk for depression and present hippocampal volume alterations when exposed to childhood adversity.14 However, a meta-analysis reported no association between Val66Met polymorphism and reduced hippocampal volume in neuropsychiatric patients.15 Paradoxically, clinical studies indicate a higher incidence of depression in Val carriers and greater antidepressant response rates in Met carriers.16 Only one study has thus far evaluated the association between Val66Met polymorphism and resilience, finding higher scores in participants with the Met allele.17 These contradictory findings suggest the importance of genetic and environmental studies in MDD patients. Thus, the present study aims to assess the effect of the BDNF Val66Met polymorphism on resilience scores, as well as to evaluate the impact of this polymorphism on resilience scores in response to cognitive therapy.
Methods
Study design and participants
This randomized clinical trial included young adults (aged 18-29 years) diagnosed with MDD according to the Structured Clinical Interview (SCID) for DSM-IV.18 The participants were recruited through advertisements in local community health centers and psychosocial assistance centers and were enrolled between June 2010 and June 2012. The patients in current psychiatric or psychological treatment, at risk of suicide, or who met the criteria for psychoactive substance abuse were excluded from the study and referred to other treatment facilities.
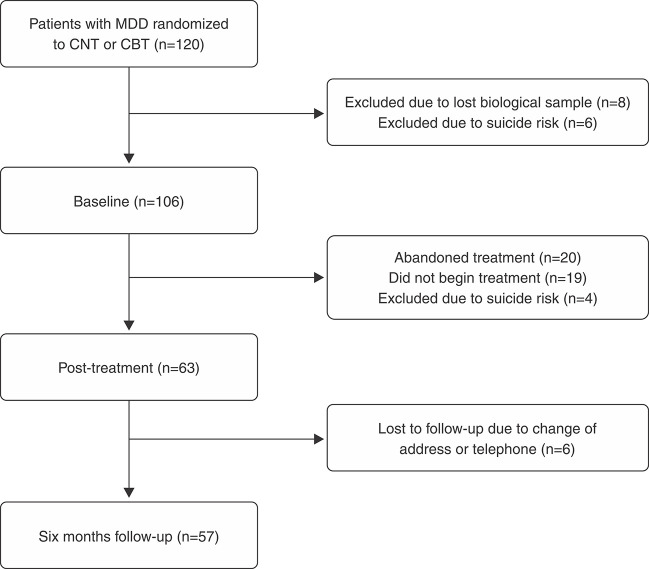
The study included 120 patients, who were randomized between two models of psychotherapy: cognitive behavioral therapy (CBT) or cognitive narrative therapy. Six individuals were excluded due to suicide risk and eight due to amplification failure during molecular analysis. Thus, 106 individuals were included in the study, 63 concluded the psychotherapy, and 57 were followed-up at six months after treatment, as shown in the flow chart in Figure 1.
Figure 1. Patient flow chart. CBT = cognitive behavioral therapy; CNT = cognitive narrative therapy; MDD = major depressive disorder.
The participants were interviewed to collect sociodemographic data (sex, age, education). Economic status was assessed using a Brazilian national economic index based on principal component analysis that included the Brazilian census of 2000 as a parameter. The instrument assesses 12 assets and the education level of the head of household.19
Interventions
Young adults who met the inclusion criteria were randomized in a clinical study of two models of cognitive therapy. A researcher not involved in the evaluation process or the psychotherapeutic interventions performed the randomization, raffling papers in a manila envelope to determine which psychotherapy model each participant would be allocated to.
The CBT handbook was structured according to the theory of Aaron Beck.20 This model of psychotherapy proposes that distorted or dysfunctional thinking, which influences mood and behavior, is common to all psychological disorders. Thus, accurate assessment and modification of thinking should lead to improvements in mood and behavior. Lasting improvements result from modifying basic dysfunctional beliefs.
The cognitive narrative therapy handbook was structured following the work of Oscar Gonçalves.21 This model aims to reframe personal narratives by writing a new biography based on life stories that lost meaning and became incoherent. The new narratives and interpretations are integrated into the patient’s personal story in a coherent and meaningful way.
Both interventions included seven 1-hour weekly sessions at the Hospital Universitário São Francisco de Paula, Pelotas, Brazil. Final year undergraduate psychology students conducted the sessions. All of the students received two months of training in 2-hour weekly meetings. Psychologists with training and clinical practice in the proposed interventions closely supervised the students. Supervision sessions discussing all of the cases were held weekly.
Outcomes
The depressive symptoms and resilience scores were assessed three times, at baseline, post-treatment, and at six months of follow-up. The Hamilton Rating Scale for Depression was used to assess the severity of depressive symptoms. This instrument includes 17 items that are classified quantitatively according to symptom intensity. The total score is the sum of all the items, with higher total scores indicating greater symptom severity.22 The scale’s internal consistency coefficients are considered adequate, ranging from 0.83 to 0.94. Its inter-rater reliability has been found consistent across several studies.23
The Resilience Scale was used to assess the degree of individual resilience.24 The scale considers positive personality traits that increase adaptability and also measures psychosocial adaption levels from important life events. It includes 25 items, with responses ranging from 1 (strongly disagree) to 7 (strongly agree). Total scores range from 25 to 175 points, with higher values indicating greater resilience.
Molecular analysis
DNA was extracted from peripheral blood leucocytes by a standardized salting-out procedure.25 Genotyping of the Val66Met polymorphism (rs6265) of the BDNF gene was determined using the forward (GGCTTGACATCATTGGCTGAC) and reverse (GGTCCTCATCCAACAGCTCTT) primers and probes in the Human Custom TaqMan Genotyping Assay 40x (Applied Biosystems, Foster City, CA, USA). One allele probe was labeled with VIC dye and the other was labeled with FAM dye. The reactions were conducted in a 96-well plate with a total reaction volume of 20 µl, using 2 ng of genomic DNA, TaqMan Genotyping Master Mix 1x (Applied Biosystems), and a custom TaqMan genotyping assay 1x. The plates were then positioned in a real-time PCR thermal cycler (7500 Fast Real PCR System; Applied Biosystems) and heated for 10 min at 95 °C, followed by 45 cycles of 95 °C for 15 s and 60 °C for 1 min. Fluorescence data files from each plate were analyzed using automated allele-calling software (SDS 2.1; Applied Biosystems).
Statistical analyses
Allelic frequencies were verified by gene counting, and departures from the Hardy-Weinberg equilibrium were assessed with the chi-square test. Comparisons of allelic and genotypic frequencies among groups of patients were performed with the chi-square test. Sociodemographic characteristics according to genotype were compared with an unpaired Student’s t-test or the chi-square test, as appropriate. Paired one-way analysis of variance (ANOVA) was used to evaluate the resilience and depressive symptom scores at baseline, post-treatment, and at six months of follow-up. A linear mixed-effect model was used to assess the effect of the Val66Met polymorphism on resilience scores during cognitive therapy intervention. Variables with normal distribution are presented as mean and standard deviation or absolute and relative frequencies. SPSS version 21.0 was used to perform the statistical analysis, and p-values < 0.05 were considered statistically significant.
Ethics statement
All ethical procedures set forth in National Health Council Resolution 196 (October 1996) were followed. The project was approved by the ethics committee of Universidade Católica de Pelotas (protocol 2009/24) and all participants signed the informed consent.
Results
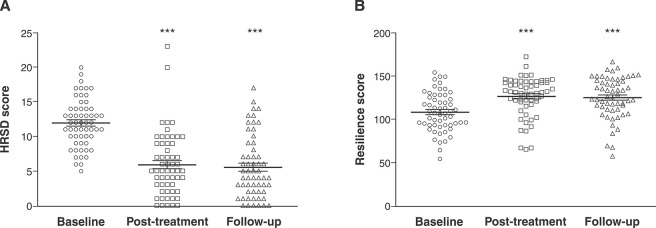
A total of 106 individuals were included in the analysis at baseline. Of these, 83 (78.3%) were female, with a mean age of 23.75 (±3.4) years. The genotype distribution was 77.4% (n=82) Val/Val and 22.6% (n=24) Val/Met or Met/Met. Figure 2A shows the efficacy of cognitive therapy for reducing depressive symptoms at baseline (11.9±3.6), post-treatment (5.8±4.5), and at six months of follow-up (8.2±5.3; p ≤ 0.001). Resilience scores increased significantly after the cognitive therapy intervention: from baseline (106.7±21.0) to post-treatment (125.2±23.2) to six months of follow-up (125.0±23.9; p ≤ 0.001; Figure 2B).
Figure 2. A) HRSD scores and B) resilience scores at baseline, post-treatment with cognitive psychotherapy, and at six-months of follow-up. Intergroup comparison of means by repeated measures ANOVA (n=57 in each group; p < 0.01). HRSD = Hamilton Rating Scale for Depression.
The data showed no association between sociodemographic characteristics, genotypes, and the Val66Met polymorphism (Table 1). Depressive symptom scores did not differ according to genotype distribution at baseline (p = 0.781), post-treatment (p = 0.976), or at six months of follow-up (p = 0.622) (Table 1). The baseline resilience scores were higher in Met allele carriers (114.6±17.6) than in patients with the Val/Val genotype (104.04±21.05; p = 0.037). No differences were observed in resilience scores according to genotype distribution at post-treatment (p = 0.427) or after six months of follow-up (p = 0.874) (Table 1). The genotype distribution of the Val66Met polymorphism agreed with that predicted by the Hardy-Weinberg equilibrium (χ2 = 0.45; p = 0.50).
Table 1. Sociodemographic and clinical characteristics of depressive subjects at baseline according to Val66Met genotype distribution.
| Variables | Val66Met Genotype | p-value | |
|---|---|---|---|
| Val/Val | Val/Met and Met/Met | ||
| Age (years) | 23.9±3.3 | 23.3±3.7 | 0.491 |
| Sex (%) | |||
| Male | 20 (87%) | 3 (13%) | 0.214 |
| Female | 62 (75%) | 21 (25%) | |
| Therapy abandonment | |||
| Yes | 36 (84%) | 7 (16%) | 0.291 |
| No | 46 (73%) | 17 (27%) | |
| HRSD scores baseline | 11.9±3.6 | 12.1±3.5 | 0.781 |
| HRSD scores post treatment | 5.8±4.9 | 5.7±3.3 | 0.976 |
| HRSD scores follow-up | 8.3±5.4 | 7.7±5.2 | 0.622 |
| Resilience scores baseline | 104.4±21.5 | 114.6±17.6 | 0.037 |
| Resilience scores post treatment | 123.8±25.0 | 129.1±17.6 | 0.427 |
| Resilience scores follow-up | 125.3±23.4 | 124.2±25.7 | 0.874 |
| Total | 82 | 24 | |
Data presented as mean ± standard deviation or n (%) considering the 106 patients evaluated at baseline.
HRSD = Hamilton Rating Scale for Depression.
Bold type denotes statistically significant findings (p < 0.05). The differences were evaluated by Student’s t-test or χ2, as appropriate; p-values ≤ 0.05 were considered significant for differences in sociodemographic and clinical characteristics between Val66Met genotypes.
Table 2 shows the mixed-effect model regression analysis regarding the effects of Val66Met polymorphism and sex on total resilience score changes during cognitive therapy and at six months of follow-up. The Val66Met polymorphism had a significant effect on total resilience scores during cognitive treatment, whereas Val/Val was associated with decreased resilience scores (t 218 = -1.98; p = 0.048). Moreover, Val66Met and sex interacted to predict an increase in total resilience scores during cognitive treatment (t 218 = 2.69; p = 0.008).
Table 2. Mixed-effects model: the results of change in total resilience scores (n=106) during cognitive therapy; Val66Met genotypes x sex interaction effect.
| Sample characteristics | Estimate | SE | df | t-value/z-value | p-value |
|---|---|---|---|---|---|
| Intercept | 137.78 | 29. 50 | 218 | 4.67 | < 0.001 |
| Age (centered), years | -0.98 | 1.22 | 218 | -0.80 | 0.421 |
| Val66Met | |||||
| Val/Val | -126.83 | 63.84 | 218 | -1.98 | 0.048 |
| Met (Ref) | |||||
| Sex | |||||
| Male | -52.16 | 33.03 | 218 | -1.58 | 0.116 |
| Female (Ref) | |||||
| Val66Met* sex | 185.62 | 69.00 | 218 | 2.69 | 0.008 |
df = degrees of freedom; SE = standard error of the parameter estimate.
Bold type denotes statistically significant findings (p < 0.05).
Discussion
Overall, this study provides evidence that the BDNF Val66Met polymorphism could be related to resilience. The results indicate that cognitive therapy could improve resilience depending on the patient’s Val66Met genotype, which shows the relevance of genetic and environmental studies of MDD. Besides genetic, neurobiological, and epigenetics factors that can interact and affect neurochemical regulation, environmental factors can induce epigenetic modifications, influencing stress resilience and risk of psychiatric disorders.26
The conceptual framework for the present study was resilience (i.e., the ability to cope with stressful situations and develop adequate behavioral and psychological adaptations to chronic stress), which minimizes negative thoughts in the face of difficult circumstances.27 Therefore, the development of a psychiatric condition may depend on both the individual’s resilience and the severity of the stress.28 Indeed, resilience has been shown to mediate the relationship between traumatic experience and depression, since some resilient individuals can function exceptionally well despite traumatic experiences.29 Furthermore, personality, temperament, physical aptitude, and social support have an important role in individual resilience.30 In this context, resilience has been considered an unstable system that arises from intrinsic and environmental factors, showing new possibilities for therapeutic intervention.28
Several studies have found an association between positive emotion and resilience,31 as well as that resilience has moderating effect on depressive and anxiety symptoms and successful adaptation to stress.31,32 In addition, studies support the idea that resilience can protect against mental health conditions such as depression and anxiety.33 CBT, which is effective in helping individuals face adversity and develop emotional health, has also been described as promoting resilience.34 Accordingly, our results showed the efficacy of cognitive psychotherapy for both reducing depressive symptoms and increasing resilience scores in depressive patients. This shows the importance of therapy for modifying automatic thoughts, behaviors, and dysfunctional cognitive schemas, as well as for promoting positive coping responses.
The brain’s plasticity under stress depends on genetic and environmental factors that may be involved in the development of resilience or psychiatric disorders.35 Resilience appears to be a neural adaptation to stress that is probably facilitated by a person’s genetic constitution. In this context, BDNF, an essential brain factor involved in the mechanisms of brain plasticity, appears to be related to resilience.36 For example, persistent BDNF alterations after social stress allow neural adaptation in the amygdala and ventral tegmental area.37 The modulatory effects of the BDNF Val66Met polymorphism on distinct sub-regions of the prefrontal cortex support the idea that the Met allele has a reduced surface area in the anterior cingulate and subregions of the middle frontal cortices in MDD.38 Moreover, allelic variation in the BDNF Val66Met polymorphism suggests specific neural correlates in MDD according to allelic groups, given that depressive patients with the Met allele showed more activation in areas associated with the cognitive appraisal of emotional information than did Val homozygotes.39 In contrast, a recent meta-analysis suggests that there is no association between BDNF Val66Met polymorphism and hippocampal volumes in neuropsychiatric patients.15
Our result that the Val66Met polymorphism had no main genetic effect on depressive symptoms is in agreement with previous similar studies, which found mixed or negative results.40 However, based on experimental observations brain anatomy and functioning differences in depressive patients according to BDNF Val66Met polymorphism genotype, we found that Met allele carriers had higher resilience scores. One recent study showed that individuals with the Val/Val genotype of this polymorphism had lower resilience scores in association with higher adversity and depressive symptoms, while another found that male carriers of a genetic variation in COMT gene had lower resilience.17,40 Moreover, our data show an important interaction between sex, the Val66Met polymorphism, and resilience, i.e., female Met allele carriers had higher resilience scores in response to cognitive therapy. These results could be explained by an interplay of environmental and genetic factors, which differently modulate epigenetic mechanisms during psychological treatment, given that women are more susceptible to stress and MDD. Moreover, previous meta-analysis showed that significantly more males with MDD had the Met allele than male controls, although no differences between cases and controls were observed for women.41
However, although the previous study suggested that MDD patients with the Val homozygote have lower resilience, our study found that cognitive psychotherapy treatment is effective in raising total resilience scores according to genotype. This shows the importance of environmental and genetic data for a better understanding of the neurobiology of resilience. According to the literature, BDNF genotypes can influence the response to antidepressant treatment, e.g., Met allele carriers have a better response rate than Val homozygotes.16 It should be pointed out that the results of these studies were inconsistent, and distinct responses were found according to race and time of treatment. Moreover, our study found an association between the Met allele and enhanced response to cognitive psychotherapy with respect to resilience. These data reveal the potential importance of genetic screening in depressive patients to determine the most appropriate treatment type.
At this point, it is clear that genetic variations in combination with external non-genetic factors affect the regulation and expression of genes through epigenetic mechanisms that influence protein functions. There is now evidence that environmental events can directly modify the epigenetic state of the genome during sensitive developmental periods and possibly in adulthood, leading to changes in gene expression and neural function.42 Thus, BDNF Val66Met genotypes can be predisposed to epigenetic alterations, causing susceptibility to (or protection from) environmental adversity. Based on the results of the present study, we suggest that the Met allele could be used as a possible predictive biomarker of resilience and that carriers in at-risk populations should be considered for personalized treatment. In conclusion, we found that the BDNF Val66Met polymorphism could be associated with resilience, as well as that cognitive psychotherapy is independently effective in improving resilience scores and depressive symptoms. Although our findings reveal the importance of genetic and environmental studies, they should be considered with caution due to insufficient power from our relatively small sample, which could affect the generalizability of our conclusions. Studying the effects of genetic therapy in association with psychotherapy could be a promising area for psychiatric research and contribute to personalized treatment. Finally, further studies are required to corroborate our findings, and the interaction between gender and BDNF should also be considered in future antidepressant pharmacogenetic studies.
Disclosure
The authors report no conflicts of interest.
Acknowledgements
The authors would like to thank the Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPEGRS), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), as well as the Brazilian Government for supporting the present research. We gratefully acknowledge the participation of all of the subjects in this study.
Footnotes
How to cite this article: Peters RB, Xavier J, Mondin TC, Cardoso TA, Ferreira FB, Teixeira L, et al. BDNF Val66Met polymorphism and resilience in major depressive disorder: the impact of cognitive psychotherapy. Braz J Psychiatry. 2021;43:22-28. http://dx.doi.org/10.1590/1516-4446-2019-0726
References
- 1.Saveanu RV, Nemeroff CB. Etiology of depression: genetic and environmental factors. Psychiatr Clin North Am. 2012;35:51–71. doi: 10.1016/j.psc.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Kendler KS, Aggen SH, Neale MC. Evidence for multiple genetic factors underlying DSM-IV criteria for major depression. JAMA Psychiatry. 2013;70:599–607. doi: 10.1001/jamapsychiatry.2013.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim-Cohen J, Turkewitz R. Resilience and measured gene-environment interactions. Dev. Psychopathol. 2012;24:1297–306. doi: 10.1017/S0954579412000715. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong MI, Birnie-Lefcovitch S, Ungar MT. Pathways between social support, family well being, quality of parenting, and child resilience: what we know. J Child Fam Stud. 2005;14:269–81. [Google Scholar]
- 5.Numakawa T, Odaka H, Adachi N. Actions of brain-derived neurotrophin factor in the neurogenesis and neuronal function, and its involvement in the pathophysiology of brain diseases. Int J Mol Sci. 2018;19:3650. doi: 10.3390/ijms19113650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–27. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–47. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee HY, Kim YK. Plasma brain-derived neurotrophic factor as a peripheral marker for the action mechanism of antidepressants. Neuropsychobiology. 2008;57:194–9. doi: 10.1159/000149817. [DOI] [PubMed] [Google Scholar]
- 9.Serra MP, Poddighe L, Boi M, Sanna F, Piludu MA, Corda MG, et al. Expression of BDNF and trkB in the hippocampus of a rat genetic model of vulnerability (Roman low‐avoidance) and resistance (Roman high‐avoidance) to stress‐induced depression. Brain Behav. 2017;7:e00861. doi: 10.1002/brb3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majcher-Maślanka I, Solarz A, Wędzony K, Chocyk A. Previous early-life stress modifies acute corticosterone-induced synaptic plasticity in the medial prefrontal cortex of adolescent rats. Neuroscience. 2018;379:316–33. doi: 10.1016/j.neuroscience.2018.03.038. [DOI] [PubMed] [Google Scholar]
- 11.Casey BJ, Glatt CE, Tottenham N, Soliman F, Bath K, Amso D, et al. Brain-derived neurotrophic factor as a model system for examining gene by environment interactions across development. Neuroscience. 2009;164:108–20. doi: 10.1016/j.neuroscience.2009.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF Val66Met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–69. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 13.Chiaruttini C, Vicario A, Li Z, Baj G, Braiuca P, Wu Y, et al. Dendritic trafficking of BDNF mRNA is mediated by translin and blocked by the G196A (Val66Met) mutation. Proc Natl Acad Sci U S A. 2009;106:16481–6. doi: 10.1073/pnas.0902833106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frodl T, Skokauskas N, Frey EM, Morris D, Gill M, Carballedo A. BDNF Val66 Met genotype interacts with childhood adversity and influences the formation of hippocampal subfields. Hum Brain Mapp. 2014;35:5776–83. doi: 10.1002/hbm.22584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrisberger F, Smieskova R, Schmidt A, Lenz C, Walter A, Wittfeld K, et al. BDNF Val66Met polymorphism and hippocampal volume in neuropsychiatric disorders: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2015;55:107–18. doi: 10.1016/j.neubiorev.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Tsai SJ, Hong CJ, Liou YJ. Effects of BDNF polymorphisms on antidepressant action. Psychiatry Investig. 2010;7:236–42. doi: 10.4306/pi.2010.7.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cruz‐Fuentes CS, Benjet C, Martínez‐Levy GA, Pérez‐Molina A, Briones‐Velasco M, Suárez‐González J. BDNF Met66 modulates the cumulative effect of psychosocial childhood adversities on major depression in adolescents. Brain Behav. 2014;4:290–7. doi: 10.1002/brb3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del-Ben CM, Vilela JAA, Crippa JAdS, Hallak JEC, Labate CM, Zuardi AW. Confiabilidade de "Entrevista Clínica Estruturada para o DSM-IV-Versão Clínica" traduzida para o português. Braz J Psychiatry. 2001;23:156–9. [Google Scholar]
- 19.Barros AJ, Victora CG. [A nationwide wealth score based on the 2000 Brazilian Demographic Census] Rev Saude Publica. 2005;39:523–9. doi: 10.1590/s0034-89102005000400002. [DOI] [PubMed] [Google Scholar]
- 20.Beck AT, Rush AJ, Emery G. Terapia cognitiva da depressão. Porto Alegre: Artmed; 1997. [Google Scholar]
- 21.Gonçalves OF, Machado PP. Cognitive narrative psychotherapy: research foundations. J Clin Psychol. 1999;55:1179–91. doi: 10.1002/(SICI)1097-4679(199910)55:10<1179::AID-JCLP2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 23.Moreno RA, Moreno DH. Escalas de avaliação clínica em psiquiatria e psicofarmacologia: escalas de avaliação para depressão de Hamilton (HAM-D) e Montgomery-Asberg (MADRS) Rev Psiquiatr Clin (São Paulo) 1998;25:1–17. [Google Scholar]
- 24.Pesce RP, Assis SG, Avanci JQ, Santos NC, Malaquias JV, Carvalhaes R. [Cross-cultural adaptation, reliability and validity of the Resilience Scale] Cad Saude Publica. 2005;21:436–48. doi: 10.1590/s0102-311x2005000200010. [DOI] [PubMed] [Google Scholar]
- 25.Lahiri DK, Nurnberger JI., Jr A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res. 1991;19:5444. doi: 10.1093/nar/19.19.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15:1475–84. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10:446–57. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- 29.Robinson JS, Larson CL, Cahill SP. Relations between resilience, positive and negative emotionality, and symptoms of anxiety and depression. Psychol Trauma. 2014;6(Suppl 1):S92–8. doi: 10.1037/a0033733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osório C, Probert T, Jones E, Young AH, Robbins I. Adapting to stress: understanding the neurobiology of resilience. Behav Med. 2017;43:307–22. doi: 10.1080/08964289.2016.1170661. [DOI] [PubMed] [Google Scholar]
- 31.Ong AD, Bergeman CS, Bisconti TL, Wallace KA. Psychological resilience, positive emotions, and successful adaptation to stress in later life. J Pers Soc Psychol. 2006;91:730–49. doi: 10.1037/0022-3514.91.4.730. [DOI] [PubMed] [Google Scholar]
- 32.Millear PM, Liossis P, Shochet IM, Biggs HC, Donald M. Being on PAR: outcomes of a pilot trial to improve mental health and wellbeing in the workplace with the Promoting Adult Resilience (PAR) program. Behav Change. 2008;25:215–28. [Google Scholar]
- 33.Shrivastava A, Desousa A. Resilience: a psychobiological construct for psychiatric disorders. Indian J Psychiatry. 2016;58:38–43. doi: 10.4103/0019-5545.174365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reivich K, Gillham JE, Chaplin TM, Seligman MEP. From helplessness to optimism: The role of resilience in treating and preventing depression in youth. In: Goldstein S, Brooks RB, editors. Handbook of resilience in children. New York: Springer; 2005. pp. 223–38. [Google Scholar]
- 35.Vialou V, Maze I, Renthal W, LaPlant QC, Watts EL, Mouzon E, et al. Serum response factor promotes resilience to chronic social stress through the induction of DeltaFosB. J Neurosci. 2010;30:14585–92. doi: 10.1523/JNEUROSCI.2496-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mallei A, Ieraci A, Popoli M. Chronic social defeat stress differentially regulates the expression of BDNF transcripts and epigenetic modifying enzymes in susceptible and resilient mice. World J Biol Psychiatry. 2019;20:555–66. doi: 10.1080/15622975.2018.1500029. [DOI] [PubMed] [Google Scholar]
- 37.Fanous S, Hammer RP, Jr, Nikulina EM. Short-and long-term effects of intermittent social defeat stress on brain-derived neurotrophic factor expression in mesocorticolimbic brain regions. Neuroscience. 2010;167:598–607. doi: 10.1016/j.neuroscience.2010.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Legge RM, Sendi S, Cole JH, Cohen-Woods S, Costafreda SG, Simmons A, et al. Modulatory effects of brain-derived neurotrophic factor Val66Met polymorphism on prefrontal regions in major depressive disorder. Br J Psychiatry. 2015;206:379–84. doi: 10.1192/bjp.bp.113.143529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lisiecka DM, O’Hanlon E, Fagan AJ, Carballedo A, Morris D, Suckling J, et al. BDNF Val66Met polymorphism in patterns of neural activation in individuals with MDD and healthy controls. J Affect Disord. 2015;184:239–44. doi: 10.1016/j.jad.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Kang JI, Kim SJ, Song YY, Namkoong K, An SK. Genetic influence of COMT and BDNF gene polymorphisms on resilience in healthy college students. Neuropsychobiology. 2013;68:174–80. doi: 10.1159/000353257. [DOI] [PubMed] [Google Scholar]
- 41.Verhagen M, van der Meij A, van Deurzen PA, Janzing JG, Arias-Vasquez A, Buitelaar JK, et al. Meta-analysis of the BDNF Val66Met polymorphism in major depressive disorder: effects of gender and ethnicity. Mol Psychiatry. 2010;15:260–71. doi: 10.1038/mp.2008.109. [DOI] [PubMed] [Google Scholar]
- 42.Fagiolini M, Jensen CL, Champagne FA. Epigenetic influences on brain development and plasticity. Curr Opin Neurobiol. 2009;19:207–12. doi: 10.1016/j.conb.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]