Abstract
This study aimed to analyze the proportion, characteristics and prognosis of untreated hepatocellular carcinoma (HCC) patients in a large representative nationwide study. A cohort study was conducted using the National Health Insurance Service (NHIS) database in Korea. A total of 63,668 newly-diagnosed HCC patients between January 2008 and December 2013 were analyzed. Patients were categorized into treatment group and no treatment group using claim codes after HCC diagnosis. The proportion of untreated HCC patients was 27.6%, decreasing from 33.4% in 2008 to 24.8% in 2013. Compared to treated patients, untreated patients were more likely to be older (P < 0.001), female (P < 0.01), to have a distant SEER stage (P < 0.001), severe liver disease (P < 0.001), and lower income (P < 0.001). The fully-adjusted hazard ratio for all-cause mortality comparing untreated to treated patients was 3.11 (95% CI, 3.04–3.18). The risk of mortality was higher for untreated patients in all pre-defined subgroups, including those with distant SEER stage and those with severe liver disease. About one fourth of newly diagnosed HCC patients did not receive any HCC-specific treatment. Untreated patients showed higher risk of mortality compared to treated patients in all subgroups. Further studies are needed to identify obstacles for HCC treatment and to improve treatment rates.
Introduction
Primary liver cancer, mostly hepatocellular carcinoma (HCC), is the sixth most common cancer and the second most common cause of cancer mortality in the world [1–3]. While the prognosis of HCC is dismal, due in large part to late detection and to compromised liver function by the underlying chronic liver disease [4–6], recent advances in HCC management include several potentially efficacious treatments for HCC [5, 7]. In addition, modern antiviral agents induce sustained suppression of viral replication or viral eradication in most patients with chronic hepatitis B virus or hepatitis C virus infection [8, 9], improving or maintaining liver function [10] and allowing for the application of HCC treatments. In spite of these advances, a substantial number of HCC patients still do not receive HCC-specific treatment. In an analysis of 128 hospitals from the US Veterans Administration, 24% of HCC patients did not receive HCC-specific treatment [11], and over 60% of HCC patients in the US Surveillance, Epidemiology, and End Results (SEER)-Medicare database did not receive any HCC-specific treatment [12]. Similarly, in the Italian Liver Cancer (ITA.LI.CA) database from 21 medical institutions, 11.7% of patients did not receive HCC-specific treatment [13].
Advanced stage at diagnosis, end-stage liver disease, old age and comorbidities have been suggested as possible reasons for not receiving treatment [12–15]. However, even patients with early-stage HCC and no co-morbidities may receive no treatment due to uncertain reasons [13, 14]. So far, the characteristics and clinical implications of untreated HCC have not been assessed in a comprehensive national analysis. In this study, we used a national cohort database to evaluate the proportion, characteristics, and clinical outcome of untreated HCC.
Materials and methods
Study population and design
We conducted a retrospective nationwide cohort analysis of newly diagnosed HCC patients 18 to 80 years of age between January 1st, 2008 and December 31st, 2013 (N = 68,558). We then excluded patients who had a history of any type of cancer (N = 3,973) and those who were diagnosed at the time of death (N = 1,022). The final sample size was 63,668 (50,963 men and 12,705 women). The Institutional Review Board of the Korean National Cancer Center approved this study and waived the requirement for informed consent because of the retrospective nature of our study using de-identified data.
Data sources
In this study, we used three sources of data. Cancer-related information was collected using the Korea National Cancer Incidence (KNCI) database, a nationwide hospital-based cancer registry that collects information from all cancer cases in Korea since 1999 [16]. The KNCI database contains information on age, sex, residential area, date of diagnosis, primary cancer site, and SEER stage of the primary tumor.
Information on treatments and diagnostic procedures, including details of diseases and prescriptions, was obtained from claims data from the Korean National Health Insurance Service (NHIS) database [17]. The NHIS is the public single payer for Korea’s mandatory universal medical insurance system. To claim reimbursement for patient care, all clinics and hospitals in Korea submit data to NHIS on inpatient hospitalizations and outpatient visits, allowing follow-up of the entire healthcare service utilization of any given patient over the course of treatment [18]. This information includes diagnoses, prescriptions, and medical procedures, which are coded using ICD-10, International Classification of Diseases, Tenth Revision (ICD-10) codes and Korean Drug and Anatomical Therapeutic Chemical (ATC) Codes. NHIS routinely audits the claims, and the data are considered reliable and have been used in numerous peer-reviewed publications [17, 19]. The NHIS database comprises four databases on insurance eligibility, medical treatments, medical care institutions, and general health exams. The medical treatment database contains information from treatment bills, including details of diseases and prescriptions [20]. Finally, vital status through December 31st, 2014 was ascertained from mortality and population registration data from the Ministry of the Interior.
Study variables
Our analysis compared patients who received vs. those who did not receive any HCC-specific anticancer treatment. HCC anticancer treatments included radiofrequency ablation (RFA), surgical resection, liver transplantation, transarterial chemoembolization (TACE), target drugs, radiation therapy, and other HCC-specific treatments. Treatments were identified by procedure codes and Korean ATC codes. If a patient did not receive any HCC-specific treatment after HCC diagnosis, we considered the patient untreated.
Potential confounders included age, sex, year of diagnosis, SEER tumor stage, severity of liver disease, and income percentile. HCC tumor stage was classified using SEER staging as localized (limited to the liver), regional (tumor extension beyond the limits of the liver), distant (away from the primary tumor), and unknown (lacking sufficient information to assign a stage). For each HCC patient, we assigned the likely cause of chronic liver disease based on diagnostic codes in claims during the overall study period. Hepatitis C was defined as the presence of a code for chronic hepatitis C only (B18.2). Hepatitis B was defined as the presence of a code for chronic hepatitis B (B18.0, B18.1, B18.10, B18.18, or Z22.5). The etiology of chronic liver disease in patients not meeting any of the above criteria was defined as other.
Severity of liver disease was defined based on diagnostic codes in claims during the year prior to HCC diagnosis. Severe liver disease was defined as the presence of a code for alcoholic hepatic failure (k704), hepatic failure (K720, K721, K729), hepatorenal syndrome (k729), esophageal varices with bleeding (I850), jaundice (R17), ascites (R18), hematemesis (K920), melena, hematochezia (K921), hepatopulmonary syndrome (K768), or spontaneous bacterial peritonitis (K658).
We also considered the following co-morbidities that could severely reduce life expectancy diagnosed during the year prior to HCC diagnosis: myocardial infarction (I21, I22), congestive heart failure (I43, I50, I09.9, I11.0, I13.0, I13.2, I25.5, I42.0, I42.5–I42.9, P29.0), cerebrovascular disease (G45, G46, I60–I69, H34.0), dementia (F00–F03, F05.1, G30, G31.1), renal disease (N18, N19, N05.2 –N05.7, N25.0, I12.0, I13.1, N03.2 –N03.7, Z49.0 –Z49.2, Z94.0, Z99.2), paraplegia and hemiplegia (G81, G82, G04.1, G11.4, G80.0, G83.0, G83.1–G83.4,G83.9), diabetes mellitus with complication (E10.2–E10.5, E10.7, E11.2–E11.5, E11.7, E12.2–E12.5, E12.7, E13.2–E13.5, E13.7, E14.2–E14.5, E14.7), and AIDS/HIV (B20–B22, B24).
Statistical analysis
We used logistic regression to identify factors associated with no treatment. The primary outcome of this study was all-cause mortality and the secondary outcome was liver-specific mortality (ICD-10 code C22). Person-time was calculated from the date of HCC diagnosis to death or the end of the study period on December 31st, 2014. Survival curves were generated by the Kaplan-Meier product-limit method and compared by log-rank tests. We used Cox proportional hazards regression models to estimate hazard ratios (HRs) with 95% confidence intervals (CIs) for all-cause mortality comparing treated versus untreated HCC patients. Since treatment decisions and patient survival could be clustered by hospital, we used hospital as a stratification factor in Cox models. We adjusted for sex, age, year of diagnosis, SEER stage, severe liver disease, residential area (urban vs rural), and income percentiles. We examined the proportional hazards assumption using plots of the log(-log) survival function and Schoenfeld residuals.
We performed subgroup analyses to evaluate if the association of treatment status with mortality varied across pre-specified subgroups defined by age (< 50, 50–60, 60–70, and ≥70 years), sex, SEER stage, comorbidities, etiology (hepatitis B only or combined with hepatitis C virus, hepatitis C virus only, and other), severe liver disease, income percentile (Medical aid beneficiary, ≤ 30th, > 30th– ≤ 70th, > 70th) and residential area (urban vs rural). We considered a p-value of < 0.05 as statistically significant. All analyses were performed using STATA version 14 (StataCorp LP, College Station, TX, USA) and SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
The median (interquartile range) age at HCC diagnosis of study participants (N = 63,668) was 59 (51–68) years and 80.0% of participants were men. The proportion of untreated patients was 27.6%, and this proportion decreased progressively from 33.4% in 2008 to 24.8% in 2013. Untreated patients were more likely to be older, female, have a distant SEER stage, have comorbidities that could severely reduce life expectancy, have severe liver disease, have lower income and live in a rural area compared to treated patients (Table 1). In multivariable analysis, older age, female, distant SEER stage, comorbidity, severe liver disease, being a Medical aid beneficiary, and living in a rural area were significantly associated with an increased probability of not getting treatment (Table 2).
Table 1. Characteristics of study participants.
| Untreated | Treated | P-value | |
|---|---|---|---|
| (N = 17,569) | (N = 46,099) | ||
| N (%) | N (%) | ||
| Age (years), median (IQR) | 61 (52–71) | 58 (51–66) | <0.001 |
| Age categories | |||
| <50 | 3,161 (18.0) | 9,214 (20.0) | |
| 50≤-<60 | 4,875 (27.8) | 16,430 (35.6) | |
| 60≤-<70 | 4,379 (24.9) | 13,085 (28.4) | |
| >70 | 5,154 (29.3) | 7,370 (16.0) | |
| Sex | < 0.01 | ||
| Male | 13,908 (79.2) | 37,055 (80.4) | |
| Female | 3,661 (20.8) | 9,044 (19.6) | |
| Year of Diagnosis | <0.001 | ||
| 2008 | 3,467 (19.7) | 6,915 (15.0) | |
| 2009 | 3,151 (17.9) | 7,607 (16.5) | |
| 2010 | 3,126 (17.8) | 7,774 (16.9) | |
| 2011 | 2,693 (15.3) | 8,093 (17.6) | |
| 2012 | 2,560 (14.6) | 7,921 (17.2) | |
| 2013 | 2,572 (14.6) | 7,789 (16.9) | |
| Seer Stage | <0.001 | ||
| Localized | 6,169 (35.1) | 26,999 (58.6) | |
| Regional | 4,669 (26.6) | 10,285 (22.3) | |
| Distant | 3,409 (19.4) | 4,014 (8.7) | |
| Unknown | 3,322 (18.9) | 4,801 (10.4) | |
| Comorbidity | |||
| Myocardial infarction | 131 (0.8) | 292 (0.6) | 0.12 |
| Congestive heart failure | 693 (3.9) | 1,167 (2.5) | <0.001 |
| Cerebrovascular disease | 1,341 (7.6) | 2,289 (5.0) | <0.001 |
| Dementia | 487 (2.8) | 428 (0.9) | <0.001 |
| Renal disease | 328 (1.9) | 625 (1.4) | <0.001 |
| Paraplegia and hemiplegia | 188 (1.1) | 225 (0.5) | <0.001 |
| Diabetes mellitus with complication | 2,059 (11.7) | 4,674 (10.1) | <0.001 |
| AIDS/HIV. | 7 (0.0) | 14 (0.0) | 0.56 |
| Severe liver disease, yes | 2,572 (14.6) | 4,624 (10.0) | <0.001 |
| Etiology | <0.001 | ||
| Hepatitis B virus* | 8,288 (47.2) | 33,465 (72.6) | |
| Hepatitis C virus | 1,195 (6.8) | 3,683 (8.0) | |
| Other† | 8,086 (46.0) | 8,951 (19.4) | |
| Income percentile | <0.001 | ||
| Medical aid beneficiary | 2,991 (17.0) | 5,861 (12.7) | |
| ≤30 | 4,328 (24.6) | 11,032 (23.9) | |
| 31–70 | 5,387 (30.7) | 14,323 (31.1) | |
| >70 | 4,863 (27.7) | 14,883 (32.3) | |
| Residency area | <0.001 | ||
| Urban | 10,627 (60.5) | 29,308 (63.6) | |
| Rural | 6,675 (38.0) | 16,116 (35.0) | |
| Unknown | 267 (1.5) | 675 (1.5) |
Values were presented n (%) or median (IQR)
* Including patients co-infected with HBV and HCV
† Including patients who had other liver disease except HBV and HCV (cirrhosis, steatohepatitis, and fatty liver etc.) or unknown.
Table 2. Odds ratios (95% confidence interval) for factors associated with no treatment in patients with newly diagnosed hepatocellular carcinoma.
| Univariable | Multivariable | |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Age | 1.03 (1.02, 1.03) | 1.01 (1.01, 1.02) |
| Sex | ||
| Male | Reference | Reference |
| Female | 1.08 (1.03, 1.13) | 1.14 (1.09, 1.20) |
| Year of Diagnosis | ||
| 2008 | 1.52 (1.43, 1.61) | 1.58 (1.48, 1.68) |
| 2009 | 1.25 (1.18, 1.33) | 1.27 (1.19, 1.36) |
| 2010 | 1.22 (1.15, 1.29) | 1.24 (1.16, 1.36) |
| 2011 | 1.01 (0.95, 1.07) | 1.06 (0.99, 1.13) |
| 2012 | 0.98 (0.92, 1.04) | 0.99 (0.93, 1.06) |
| 2013 | Reference | Reference |
| Seer Stage | ||
| Localized | Reference | Reference |
| Regional | 1.99 (1.90, 2.08) | 2.06 (1.97, 2.16) |
| Distant | 3.72 (3.52, 3.92) | 3.67 (3.46, 3.88) |
| Unknown | 3.03 (2.87, 3.19) | 2.59 (2.46, 2.74) |
| Comorbidity (reference: no comorbidity) | ||
| Myocardial infarction | 1.18 (0.96, 1.45) | 0.88 (0.70, 1.10) |
| Congestive heart failure | 1.58 (1.44, 1.74) | 1.20 (1.08, 1.33) |
| Cerebrovascular disease | 1.58 (1.48, 1.70) | 1.12 (1.04, 1.22) |
| Dementia | 3.04 (2.67, 3.47) | 2.09 (1.80, 2.41) |
| Renal disease | 1.38 (1.21, 1.58) | 1.33 (1.15, 1.54) |
| Paraplegia and hemiplegia | 2.21 (1.81, 2.68) | 1.46 (1.18, 1.82) |
| Diabetes mellitus with complication | 1.18 (1.11, 1.24) | 0.92 (0.86, 0.98) |
| AIDS/HIV. | 1.31 (0.53, 3.25) | 1.89 (0.73, 4.93) |
| Etiology | ||
| Hepatitis B virus* | Reference | Reference |
| Hepatitis C virus | 1.31 (1.22, 1.40) | 1.15 (1.07, 1.24) |
| Other | 3.64 (3.51, 3.79) | 2.98 (2.86, 3.11) |
| Severe liver disease | ||
| No | Reference | Reference |
| Yes | 1.54 (1.46, 1.62) | 1.56 (1.48, 1.65) |
| Income percentile | ||
| Medical aid beneficiary | 1.56 (1.48, 1.65) | 1.51 (1.42, 1.60) |
| ≤30 | 1.20 (1.14, 1.26) | 1.20 (1.14, 1.26) |
| 31–70 | 1.15 (1.10, 1.20) | 1.16 (1.11, 1.22) |
| >70 | Reference | Reference |
| Residency area | ||
| Metropolitan | Reference | Reference |
| Rural | 1.14 (1.10, 1.18) | 1.06 (1.02, 1.10) |
* Including patients co-infected with HBV and HCV
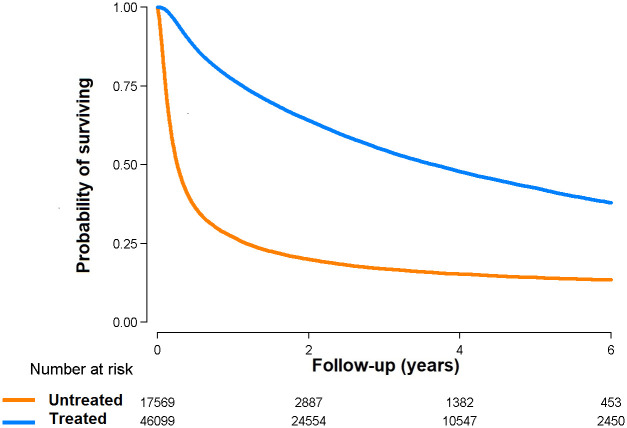
During 135,123 person-years of follow-up (median follow-up 2.1 years), we observed 37,407 deaths. All-cause mortality rates in the treated and untreated groups were 19.3 and 83.7 per 100 person-years, respectively (Table 3 and Fig 1). Untreated patients had a significantly higher risk of all-cause mortality compared to treated patients even after adjusting for age, sex, year of diagnosis, SEER stage, comorbidity, etiology, severe liver disease, income percentile, and urban vs rural location (fully-adjusted HR comparing untreated vs. treated patients 3.11, 95% CI 3.04–3.18).
Table 3. Hazard ratios (95% confidence intervals) for all-cause mortality comparing untreated vs. treated patients with newly diagnosed hepatocellular carcinoma, overall and by SEER stage.
| Mortality rate (per 100 pys) | Crude | Model 1 | Model 2 | |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
| Overall | ||||
| Untreated | 83.75 | 3.72 (3.64, 3.80) | 3.74 (3.56. 3.72) | 3.11 (3.04, 3.18) |
| Treated | 19.33 | Reference | Reference | Reference |
| Localized | ||||
| Untreated | 40.03 | 3.15 (3.04, 3.27) | 3.10 (2.99. 3.21) | 2.94 (2.83, 3.05) |
| Treated | 11.91 | Reference | Reference | Reference |
| Regional | ||||
| Untreated | 168.20 | 4.04 (3.88, 4.19) | 3.90 (3.75. 4.05) | 3.71 (3.57, 3.86) |
| Treated | 34.56 | Reference | Reference | Reference |
| Distant | ||||
| Untreated | 289.09 | 2.78 (2.65, 2.91) | 2.65 (2.53, 2.78) | 2.49 (2.38, 2.61) |
| Treated | 93.20 | Reference | Reference | Reference |
| Unknown | ||||
| Untreated | 82.78 | 3.73 (3.54. 3.94) | 3.61 (3.42, 3.81) | 3.36 (3.19, 3.55) |
| Treated | 19.71 | Reference | Reference | Reference |
Abbreviations: HR, hazard ratio; CI, confidence interval.
Model 1: Adjusted for sex, age, year of HCC diagnosis, SEER stage, income percentile (Medical aid, ≤ 30th, > 30th– 70th, and > 70th), residency area (urban vs rural) and comorbidities; Model 2: Further adjusted for etiology, and severe liver disease.
Fig 1. Overall survival from diagnosis of hepatocellular carcinoma by treatment status (N = 63,668).
Among participants who died, 91.7% and 88.9% died due to liver cancer specific cause in the treated and untreated group, respectively. Fully-adjusted HR for liver cancer specific mortality comparing untreated vs. treated patients was 3.05 (95% CI 2.98–3.12) (S1 Table).
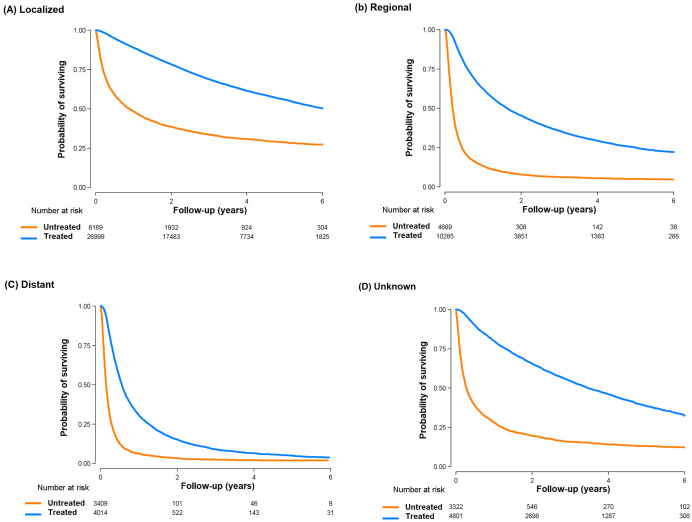
In subgroup analysis, the excess mortality in untreated patients was present in all pre-defined subgroups (Fig 2). The association was stronger in men, in patients ≥50 –<70 years of age, and in those with regional disease, without comorbidities and in the highest income category. When the association between treatment and mortality was evaluated by SEER stage (Table 3 and Fig 3), the higher risk of mortality in untreated vs. treated patients was evident in all stages, even in patients with distant disease (HR 2.49, 95% CI 2.38–2.61).
Fig 2. Hazard ratios (95% confidence intervals) for mortality comparing untreated with treated patients with hepatocellular carcinoma in selected subgroups.
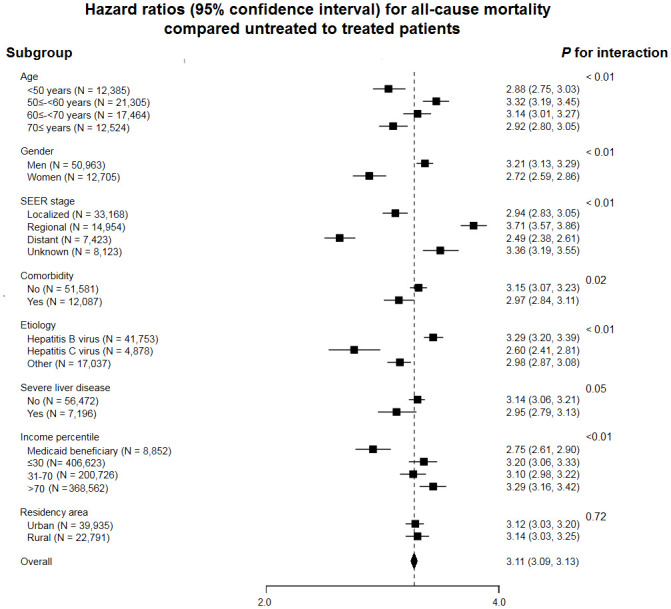
Adjusted for sex, age, year of HCC diagnosis, SEER stage, income percentile (Medical aid, ≤ 30th, > 30th– 70th, and > 70th), residency area (urban vs rural), comorbidities, etiology, and severity of liver disease.
Fig 3. Overall survival from diagnosis of hepatocellular carcinoma by treatment status in subgroups of Surveillance, Epidemiology, and End Results (SEER) stage.
(a) Localized disease; (b) Regional disease; (c) Distant disease; (d) Unknown.
Discussion
In this large nationwide study, the proportion of untreated HCC patients declined from 2008 to 2013, but still one fourth (24.8%) of newly-diagnosed HCC patients did not receive any HCC-specific treatment in 2013. Older age, advanced SEER stage, severe liver disease, and lower income were associated with being untreated. Untreated patients had a 3-fold higher risk of mortality compared to treated patients. The increased mortality in untreated vs. treated patients was evident in all subgroups, including older age, advanced stage, severe liver disease, and lower income.
The proportion of HCC patients who do not receive specific anticancer treatment varies from 10% to more than 60% across studies [11–13, 21]. Several reasons may explain these differences, including differences in study period, health insurance system, and medical costs. South Korea has a single payer system and all population is covered by the NHIS, explaining the relatively high treatment rates. However, even with a single payer system, we still found differences in treatment status by income category. In the US, patients who were elderly, non-Caucasian, and who had low socioeconomic status, poor liver function, and poor performance status were less likely to receive HCC specific treatment [11, 12, 21]. Consistent with these findings, we also found that elderly patients, those with severe liver disease, and those with lower income less likely to be treated. In addition, female patients had higher risk of not having HCC treatment after controlling confounding factors. It was similar with previous literature that gender inequities manifests itself in both lower health investments as well as worse health status of women relative to men [22].
Stage at diagnosis was also significantly associated with treatment status [11]. Advanced stage HCC was associated both with lower likelihood of receiving treatment and with poor survival [4]. Diagnosing HCC at early stages using surveillance may thus lead to increased likelihood of receiving treatment and of improving HCC survival. Indeed, HCC surveillance has been linked to earlier stage diagnoses and improved survival [23–25], and implementation of a national cancer surveillance program in South Korea was proposed as an explanation of HCC prognosis improvement over the last 20 years [26]. Nevertheless, even with a national surveillance program in Korea, a country with relatively high prevalence of hepatitis B virus infection and high risk of HCC, adherence to regular HCC surveillance remains poor [27, 28]. Intensifying population HCC surveillance efforts in high-risk populations could increase treatment rates and ultimately improve survival of HCC patients.
A relatively high proportion of patients with early stage disease at diagnosis also do not receive HCC-specific treatment (in our study, 18.6% of patients with localized SEER stage at diagnosis did not receive treatment) [21]. Since we used an administrative claims database, we did not have detailed information on the reasons why these patients did not receive treatment. Hence, future studies need to identify reasons for withholding treatment as well as barriers to HCC treatment in these patients.
In our study, untreated patients showed a 3-fold higher risk of mortality compared to treated patients, and the association between treatment and mortality was observed in all predefined subgroups including older patients, distant SEER stage, and severe liver disease. Active HCC therapy is associated with better outcomes [11], including in old patients, in patients with extrahepatic metastasis, and in patients with decreased liver function [29–32]. As a consequence, increasing treatment rates may benefit these subgroups as well. A multidisciplinary approach may help guide treatment in these difficult-to-treat populations [33].
Our data has several limitations. Although HCC-treatment improves outcomes, the difference in mortality between treated and untreated patients in our study cannot be only attributed to treatment effects. Since we used an administrative claims data, important prognostic information that might be associated with treatment and survival, such as functional status, and reason for no treatment was not available. However, when we conducted subgroup analysis in all predefined subgroups including older patients, distant SEER stage, and severe liver disease, the results were similar in all subgroup. Thus, non-treatment affects to the clinical outcomes in HCC in any reason. Thus, non-treatment affects to the clinical outcomes in HCC in any reason. Also, as this study was performed in South Korea, which is one of the most ethnically homogeneous countries that has a unique single payer system, generalizability of the study findings to other population at different healthcare system requires further evaluation.
Conclusions
In summary, untreated HCC is still common, even among patients diagnosed at early stage. Untreated HCC patients had a higher risk of mortality compared to treated patients. Older age, advanced stage, severe liver disease and lower income was associated with a lower likelihood of receiving HCC treatment. The proportion of untreated HCC patients declined progressively from 2008 to 2013 in South Korea, but almost one fourth newly-diagnosed patients did not receive HCC-specific treatment in 2013. Efforts to identify HCC cases at an early stage, to identify and remove barriers for HCC treatment, and to improve treatment rates could continue the trend towards improved HCC survival.
Supporting information
(DOCX)
Data Availability
The data underlying the results presented in the study are available from the National Health Insurance Sharing Service (https://nhiss.nhis.or.kr/). Data cannot be shared publicly because of the policy of the National Health Insurance Sharing Service. We confirm that authors had no special access to the data, and that qualified researchers can obtain access to the data in the same way the authors obtained it (contact +82-33-736-3425 or moonhj@nhis.or.kr).
Funding Statement
This work was supported by National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (1520240) and National Cancer Center Grant (NCC-1911275). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–86. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 2015; 19:223–38. 10.1016/j.cld.2015.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim BH, Park JW. Epidemiology of liver cancer in South Korea. Clin Mol Hepatol. 2018; 24:1–9. 10.3350/cmh.2017.0112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim KM, Sinn DH, Jung SH, Gwak GY, Paik YH, Choi MS, et al. The recommended treatment algorithms of the BCLC and HKLC staging systems: does following these always improve survival rates for HCC patients? Liver Int. 2016; 36:1490–7. 10.1111/liv.13107 [DOI] [PubMed] [Google Scholar]
- 5.2014 KLCSG-NCC Korea Practice Guideline for the Management of Hepatocellular Carcinoma. Gut Liver. 2015; 9:267–317. 10.5009/gnl14460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018; 69:182–236. 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 7.Yu SJ. A concise review of updated guidelines regarding the management of hepatocellular carcinoma around the world: 2010–2016. Clin Mol Hepatol. 2016; 22:7–17. 10.3350/cmh.2016.22.1.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.2017 KASL clinical practice guidelines management of hepatitis C: Treatment of chronic hepatitis C. Clin Mol Hepatol. 2018; 24:169–229. 10.3350/cmh.2018.1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinn DH, Cho SJ, Gu S, Seong D, Kang D, Kim H, et al. Persistent Nonalcoholic Fatty Liver Disease Increases Risk for Carotid Atherosclerosis. Gastroenterology. 2016; 151:481–8 e1. 10.1053/j.gastro.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 10.Kim JH, Sinn DH, Kim K, Kim H, Gwak GY, Paik YH, et al. Lamivudine versus Entecavir for Newly Diagnosed Hepatitis B Virus-Related Hepatocellular Carcinoma. Gut Liver. 2016; 10:939–47. 10.5009/gnl15527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serper M, Taddei TH, Mehta R, D’Addeo K, Dai F, Aytaman A, et al. Association of Provider Specialty and Multidisciplinary Care With Hepatocellular Carcinoma Treatment and Mortality. Gastroenterology. 2017; 152:1954–64. 10.1053/j.gastro.2017.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaya FT, Breunig IM, Seal B, Mullins CD, Chirikov VV, Hanna N. Comparative and cost effectiveness of treatment modalities for hepatocellular carcinoma in SEER-Medicare. Pharmacoeconomics. 2014; 32:63–74. 10.1007/s40273-013-0109-7 [DOI] [PubMed] [Google Scholar]
- 13.Giannini EG, Farinati F, Ciccarese F, Pecorelli A, Rapaccini GL, Di Marco M, et al. Prognosis of untreated hepatocellular carcinoma. Hepatology. 2015; 61:184–90. 10.1002/hep.27443 [DOI] [PubMed] [Google Scholar]
- 14.Devaki P, Wong RJ, Marupakula V, Nangia S, Nguyen L, Ditah IC, et al. Approximately one-half of patients with early-stage hepatocellular carcinoma meeting Milan criteria did not receive local tumor destructive or curative surgery in the post-MELD exception era. Cancer. 2014; 120:1725–32. 10.1002/cncr.28639 [DOI] [PubMed] [Google Scholar]
- 15.Khalaf N, Ying J, Mittal S, Temple S, Kanwal F, Davila J, et al. Natural History of Untreated Hepatocellular Carcinoma in a US Cohort and the Role of Cancer Surveillance. Clin Gastroenterol Hepatol. 2017; 15:273–81.e1. 10.1016/j.cgh.2016.07.033 [DOI] [PubMed] [Google Scholar]
- 16.Shin HR, Won YJ, Jung KW, Kong HJ, Yim SH, Lee JK, et al. Nationwide cancer incidence in Korea, 1999~2001; first result using the national cancer incidence database. Cancer Res Treat. 2005; 37:325–31. 10.4143/crt.2005.37.6.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song SO, Jung CH, Song YD, Park CY, Kwon HS, Cha BS, et al. Background and data configuration process of a nationwide population-based study using the korean national health insurance system. Diabetes Metab J. 2014; 38:395–403. 10.4093/dmj.2014.38.5.395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JA, Yoon S, Kim LY, Kim DS. Towards Actualizing the Value Potential of Korea Health Insurance Review and Assessment (HIRA) Data as a Resource for Health Research: Strengths, Limitations, Applications, and Strategies for Optimal Use of HIRA Data. J Korean Med Sci. 2017; 32:718–28. 10.3346/jkms.2017.32.5.718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin DW, Cho B, Guallar E. Korean National Health Insurance Database. JAMA Intern Med. 2016; 176:138 10.1001/jamainternmed.2015.7110 [DOI] [PubMed] [Google Scholar]
- 20.Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017; 46:e15 10.1093/ije/dyv319 [DOI] [PubMed] [Google Scholar]
- 21.Tan D, Yopp A, Beg MS, Gopal P, Singal AG. Meta-analysis: underutilisation and disparities of treatment among patients with hepatocellular carcinoma in the United States. Aliment Pharmacol Ther. 2013; 38:703–12. 10.1111/apt.12450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denton M, Prus S, Walters V. Gender differences in health: a Canadian study of the psychosocial, structural and behavioural determinants of health. Soc Sci Med. 2004; 58:2585–600. 10.1016/j.socscimed.2003.09.008 [DOI] [PubMed] [Google Scholar]
- 23.Kim KM, Kim J, Sinn DH, Kim HS, Kim K, Kang W, et al. Treatment for occult hepatocellular carcinoma: does it offer survival advantages over symptom-driven treatment? Scand J Gastroenterol. 2018; 53:727–33. 10.1080/00365521.2018.1458895 [DOI] [PubMed] [Google Scholar]
- 24.Costentin CE, Layese R, Bourcier V, Cagnot C, Marcellin P, Guyader D, et al. Compliance With Hepatocellular Carcinoma Surveillance Guidelines Associated With Increased Lead-Time Adjusted Survival of Patients With Compensated Viral Cirrhosis: A Multi-Center Cohort Study. Gastroenterology. 2018; 155:431–42.e10. 10.1053/j.gastro.2018.04.027 [DOI] [PubMed] [Google Scholar]
- 25.Hanouneh IA, Alkhouri N, Singal AG. Hepatocellular carcinoma surveillance in the 21st century: Saving lives or causing harm? Clin Mol Hepatol. 2019. 10.3350/cmh.2019.1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yim SY, Seo YS, Jung CH, Kim TH, Lee JM, Kim ES, et al. The management and prognosis of patients with hepatocellular carcinoma: what has changed in 20 years? Liver Int. 2016; 36:445–53. 10.1111/liv.12960 [DOI] [PubMed] [Google Scholar]
- 27.Tran SA, Le A, Zhao C, Hoang J, Yasukawa LA, Weber S, et al. Rate of hepatocellular carcinoma surveillance remains low for a large, real-life cohort of patients with hepatitis C cirrhosis. BMJ Open Gastroenterol. 2018; 5:e000192 10.1136/bmjgast-2017-000192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao C, Jin M, Le RH, Le MH, Chen VL, Jin M, et al. Poor adherence to hepatocellular carcinoma surveillance: A systematic review and meta-analysis of a complex issue. Liver Int. 2018; 38:503–14. 10.1111/liv.13555 [DOI] [PubMed] [Google Scholar]
- 29.Kim YJ, Jang BK, Kim ES, Chung WJ, Park KS, Cho KB, et al. Hepatocellular carcinoma in the elderly: clinical characteristics, treatment, survival analysis in Korean patients older than 70 years. J Korean Med Sci. 2012; 27:1147–54. 10.3346/jkms.2012.27.10.1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang H, Woo HY, Lee SK, Han JW, Jang B, Nam HC, et al. A comparative study of sorafenib and metronomic chemotherapy for Barcelona Clinic Liver Cancer-stage C hepatocellular carcinoma with poor liver function. Clin Mol Hepatol. 2017; 23:128–37. 10.3350/cmh.2016.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nouso K, Kokudo N, Tanaka M, Kuromatsu R, Nishikawa H, Toyoda H, et al. Treatment of hepatocellular carcinoma with Child-Pugh C cirrhosis. Oncology. 2014; 87 Suppl 1:99–103. 10.1159/000368152 [DOI] [PubMed] [Google Scholar]
- 32.Lee HS. Management of patients with hepatocellular carcinoma and extrahepatic metastasis. Dig Dis. 2011; 29:333–8. 10.1159/000327572 [DOI] [PubMed] [Google Scholar]
- 33.Sinn DH, Choi GS, Park HC, Kim JM, Kim H, Song KD, et al. Multidisciplinary approach is associated with improved survival of hepatocellular carcinoma patients. PLoS One. 2019; 14:e0210730 10.1371/journal.pone.0210730 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
The data underlying the results presented in the study are available from the National Health Insurance Sharing Service (https://nhiss.nhis.or.kr/). Data cannot be shared publicly because of the policy of the National Health Insurance Sharing Service. We confirm that authors had no special access to the data, and that qualified researchers can obtain access to the data in the same way the authors obtained it (contact +82-33-736-3425 or moonhj@nhis.or.kr).