Abstract
The goal of this study was to test for long-term benefits three years after the completion of a cognitive training intervention (Project: EVO™) in a subset of children with Sensory Processing Dysfunction (SPD). Our initial findings revealed that children with SPD who also met research criteria for Attention Deficit Hyperactivity Disorder (SPD+IA) showed a significant decrease in parent-observed inattentive behaviors, which remained stable in a nine-month follow-up assessment. Forty nine caregivers of participants who completed the Project: EVO™ training were contacted to be included in this follow up study. Each was emailed an invitation to complete the Vanderbilt ADHD Diagnostic Parent Rating Scale, which yielded a completion rate of 39/49 (80%). A Generalized Estimating Equations analysis was used to assess changes in symptoms over time, specifically to determine whether the initial improvements were retained. The SPD+IA cohort continued to show sustained benefits on their parent-reported scores of inattention, with 54% of SPD+IA individuals no longer meeting criteria for ADHD three years following intervention. These findings provide initial insights into the potential long-term benefits of a digital health intervention for children with attention-based issues.
Introduction
Sensory Processing Dysfunction (SPD), expressed as exaggerated aversive, withdrawal, or seeking behaviors associated with sensory inputs [1], affects almost five percent of all children [2]. Even though SPD is categorized as atypical responses to sensory input, many of those affected also exhibit attentional challenges [1, 3]. These findings suggested that a robust assessment and subsequent intervention of these deficient cognitive abilities may be warranted in this particular population. Research along these lines from our group has indeed demonstrated that compared to their typically developing peers, approximately 40% children with SPD demonstrate diminished cognitive control (selective attention in particular) as well as visuomotor control—abilities crucial to academic achievement and social development [4, 5]. In addition, these individuals also show decreased white matter microstructural integrity that correlated with their issues of inattention [6], providing a structural suggestion as to why such inattention effects may be present.
The use of digital technologies, especially those with adaptive mechanics, to assessing cognitive control have been especially powerful to help contend with inherently elevated testing variability in different clinical populations [5, 7]. Such approaches also underlie the basis for many digital therapeutic interventions: our work with the NeuroRacer intervention demonstrated that a ‘video game’ with adaptive mechanics can lead to improved attention abilities both behaviorally and neurally in older adults, with such effects persisting 6 months beyond the training period [8]. Out of the NeuroRacer platform came Project: EVO™, a digital intervention designed to enhance cognitive control abilities, specifically attention and goal management [5, 9]. Project: EVO was modified into an iOS compatible platform that deploys modern videogame interface with engaging visual and auditory feedback and adaptive algorithms designed to constantly challenge each player’s abilities. The effectiveness of Project: EVO was supported by improvements in cognitive control in different populations, including children with Autism Spectrum Disorder (ASD) and Attention Deficit Hyperactivity Disorder (ADHD) as well as older adults with geriatric depression [7, 9–11]. Most recently, a randomized, controlled trial found Project:EVO, currently known as EndeavorRx, to significantly improve attention in a large sample of children with ADHD [12]. Based on those results, The U.S Food and Drug Administration granted EndeavorRx™ (AKL-T01) clearance to be prescribed as a treatment for children with ADHD.
Previous study data published in 2017 provided promising evidence for cognitive benefits of Project: EVO in a subset of children with SPD who experience greater cognitive control deficiencies (SPD+IA) compared to typically developing children (TDC) evidenced by poorer neural and behavioral performance on measures of attention [5]. These SPD+IA individuals showed improvements in parent-reported attention following EVO training, with the improvements persisting at a 9-month follow-up. In contrast, SPD children without attention difficulties and TDC did not demonstrate training related benefits. In addition to the decrease in ADHD symptoms, participants in the SPD+IA group showed increased midline frontal theta power, a neural marker of attention [13–16]. These improvements correlated with the parental reports of inattention improvement. The 2017 study demonstrated the potential of Project: EVO to significantly improve attention after 4 weeks of training in children with SPD who experience attentional challenges. While findings from the original study are promising, it was not clear whether these benefits would persist over a longer time period than the 9-month follow-up period. Indeed, a number of longitudinal studies have shown persistence of benefits for several years [17, 18], but such effects and their duration following a behavioral intervention in the SPD population are unknown. The purpose of the current follow-up study is to assess whether the parent-reported benefits observed after EVO training in the SPD+IA group were sustained at a 3-year follow-up.
Materials and methods
Participants
Participant recruitment for the pilot study took place between February 2014 and January 2015 from the Sensory Neurodevelopment and Autism Program at University of California, San Francisco (UCSF). This follow-up study was conducted between March and May of 2018 involving 49 participants from the original cohort who successfully completed EVO training. Out of those, parents of 39 participants (80%) responded to this three-year follow-up, which involved the Vanderbilt ADHD Diagnostic Parent Rating Scale. Participants’ caregivers were contacted via email and completed the study through REDCap, a secure online platform for consenting and data collection. UCSF’s IRB committee approved this study through IRB #10–01940. An electronic written consent was obtained from all participants.
Measures
Vanderbilt ADHD Diagnostic Parent Rating Scale (Vanderbilt)
Participants’ caregivers were administered the Vanderbilt scale [19, 20] prior to the intervention as well as at each follow-up session to assess observed changes in participants’ attention and behavior. The Vanderbilt scale was shown to have an excellent internal consistency reliability (0.90 ≤ α ≤ 0.95) as well as high concurrent validity (r = 0.79; [21]) when related to the Computerized Diagnostic Interview Schedule for Children (C-DISC-IV; National Institute of Mental Health, 1997) [22]. The Vanderbilt scale assesses the 18 symptoms of ADHD as outlined in the DMS-IV [23]. The Vanderbilt scale was also utilized to distinguish two subgroups within the SPD cohort: those that reached the standardized threshold for inattention or hyperactivity (SPD+IA) and those that did not (SPD). The first follow-up assessment took place shortly after a participant completed 20 days of training with a second follow-up 9 months later [5]. The present study assesses the retainment of improvements three years post intervention. Parents were administered the first 18 questions of the Vanderbilt scale, where the first 9 questions assess symptoms of Inattention and the other 9 questions assess Hyperactivity/Impulsivity on a scale from 0 (“Never”) to 3 (“Very often”). To meet the criteria for either Inattention or Hyperactivity/Impulsivity, scores of 2 or 3 must be selected for at least 6 out of the 9 items. For quantitative analyses, sum from both scales were calculated as well as a total score combining both scales.
Project: EVO training
The Project: EVO™ (EVO) is proprietary software developed by Akili Interactive Labs, specifically designed as an investigational medical device to assess and adaptively train cognitive control for populations with cognitive disorders or decline and executive function deficits. EVO is a self-guided treatment designed for at-home use that involves a combination of visuomotor and perceptual discrimination tasks played on an iPad. Each session of the EVO training lasts approximately 4 minutes and consists of a multitasking condition, during which participants perform the visuomotor and perceptual discrimination tasks simultaneously [5]. The EVO intervention program involved participants playing 7 sessions (~30 minutes), 5 times a week for the duration of 1 month (20 days total). Research assistants remotely monitored EVO play and provided support and feedback to the parents and children during training. If a research assistant noticed a participant had more than two incomplete days of training, a reminder phone call would be made to the parents.
Statistical analyses
Age and gender comparability of the current groups (SPD, SPD+IA and TDC) were assessed with one-way ANOVA and chi-square test, respectively. Considering that the sample of individuals participating in this follow-up was 20% smaller than the original sample, we also checked for significant differences between our subsample and those who did not fill out the follow-up questionnaire. Specifically, age and baseline inattention contrasts were assessed using independent samples t-tests and gender representation with a chi-square test.
Improvements in Inattention were analyzed using Generalized Estimating Equations (GEE) with robust standard error estimators. GEE is a regression-based method used for analyzing longitudinal data where observations across time are not independent [24]. Since Inattention is a continuous variable, we selected Gaussian distribution with identity link function. The main goal of this study was to determine whether children in the SPD+IA group sustained the improvements observed after EVO. Thus, the first GEE model assessed changes over time within the SPD+IA group with baseline as the reference. Additionally, the interaction of Group and Time was assessed in a separate model with SPD+IA group as the reference for the Group variable and baseline scores as the reference of time.
All statistical analyses were performed using the SPSS 20.0 (IBM SPSS Statistics).
Results
Out of the 49 contacted participants, 39 parents (80%) completed the Vanderbilt 3-year follow-up assessment. The SPD, SPD+IA and TDC groups did not differ significantly in terms of age (p = .326) or gender (p = .283). Additionally, the subsample involved in the current follow-up was comparable to the original sample analyzed in the pilot study in terms of age, gender and baseline Vanderbilt Inattention scores with one exception in the TDC group—those who participated in this follow-up (M = 6.37, SD = 3.06) had a significantly higher or more impaired (p = .042) baseline inattention scores compared to those who did not (M = 2.33, SD = 2.31; Table 1).
Table 1. Sample characteristics.
| TDC | SPD | SPD+IA | Total | |||||
|---|---|---|---|---|---|---|---|---|
| Pilot | FU | Pilot | FU | Pilot | FU | Pilot | FU | |
| N | 22 | 19 | 10 | 7 | 17 | 13 | 49 | 39 |
| Males | 12 (55%) | 10 (53%) | 8 (80%) | 6 (86%) | 10 (59%) | 7 (54%) | 30 (61%) | 23 (59%) |
| Mean Age (SD) | 14.32 (1.1) | 14.47 (1.1) | 14.8 (1.6) | 14.57 (1.5) | 13.65 (1.5) | 13.77 (1.7) | 14.18 (1.4) | 14.26 (1.4) |
| Baseline Inattention (SD) | 5.82 (3.25) | 6.37 (3.06)* | 13.5 (2.07) | 14.14 (2.12) | 20.94 (3.11) | 20.69 (3.15) | 12.63 (7.38) | 12.54 (7.11) |
Sample characteristics of the original cohort (Pilot) and the subsample involved in the current follow-up study (FU).
*statistically significant at p≤0.05
To assess the long-term effects of the Project: EVO training on parental observations of inattention, we compared the Vanderbilt scores at 4 points in time (pre-assessment, post-assessment, 9-month and 3-year follow-up) for each of the 3 groups (SPD, SPD+IA, TDC). Mean total scores as well as mean scores from each subscale (Inattention, Hyperactivity) at all points in time are presented in Table 2. When setting up the GEE model, several choices need to be made. First, a working correlation matrix that best represents the relationships between measurements at different time points needs to be identified. Initially, autoregressive correlation matrix was considered based on a trend toward diminishing correlations over time. However, this type of correlation matrix assumes equal time intervals between any two observations [25], therefore exchangeable correlation matrix was chosen in the end. One of the advantages of GEE is that it is considered robust against misspecified correlation matrices [26]. The results of the GEE analysis (Table 3) revealed that the Inattention scores of the SPD+IA group at each follow-up assessment (post intervention, 9 months, and 3 years later) were significantly lower relative to baseline. This supports our hypothesis that the improvements of Inattention observed in children with SPD+IA immediately after intervention were sustained 3 years later. Notably, 7 (54%) out of the 13 SPD+IA individuals no longer met clinical criteria (scores 2 or 3 on at least 6 out of 9 items) for Inattention at 3-year follow-up.
Table 2. Changes in Vanderbilt mean scores.
| Vanderbilt TOTAL Mean (SD) | ||||
| PRE | POST | 9M | 3Y | |
| TDC | 9.82 (5.16) | 9.00 (3.99) | 7.62 (5.31) | 6.16 (7.18) |
| SPD | 23.80 (5.20) | 23.20 (8.89) | 19.00 (6.02) | 19.00 (12.32) |
| SPD+IA | 37.65 (5.31) | 31.06 (6.77) | 29.50 (9.11) | 26.23 (10.88) |
| Vanderbilt INATTENTION Mean (SD) | ||||
| PRE | POST | 9M | 3Y | |
| TDC | 5.82 (3.25) | 5.75 (2.69) | 4.76 (3.60) | 4.16 (4.40) |
| SPD | 13.50 (2.07) | 12.70 (4.27) | 10.13 (2.85) | 12.86 (8.51) |
| SPD+IA | 20.94 (3.11) | 16.63 (3.50) | 16.00 (4.50) | 14.38 (5.64) |
| Vanderbilt HYPERACTIVITY/IMPULSIVITY Mean (SD) | ||||
| PRE | POST | 9M | 3Y | |
| TDC | 4.00 (2.80) | 3.25 (2.88) | 2.86 (2.61) | 2.00 (3.22) |
| SPD | 10.30 (3.97) | 10.50 (5.19) | 8.88 (3.72) | 6.14 (4.78) |
| SPD+IA | 17.71 (5.17) | 14.44 (4.59) | 13.50 (6.74) | 11.85 (6.43) |
| N | 49 | 46 | 45 | 39 |
Vanderbilt mean scores at 4 time points: pre-assessment (PRE), post-assessment (POST), 9-month follow-up (9M), and 3-year follow-up (3Y).
Table 3. GEE analysis of Inattention scores.
| Estimated change | SE | 95% CI | ||
| SPD+IA change from baseline | ||||
| POST | -4.44*** | 1.12 | -6.64 | -2.25 |
| 9M | -5.16*** | 1.1 | -7.3 | -2.98 |
| 3Y | -6.59*** | 1.57 | -9.66 | -3.52 |
| Group comparisons | ||||
| Estimated difference | SE | 95% CI | ||
| SPD vs. SPD+IA | ||||
| POST | 3.64* | 1.5 | 0.7 | 6.59 |
| 9M | 1.64 | 1.41 | -1.12 | 4.39 |
| 3Y | 5.38 | 2.94 | -0.37 | 11.13 |
| TDC vs. SPD+IA | ||||
| POST | 4.29** | 1.22 | 1.9 | 6.69 |
| 9M | 3.94** | 1.44 | 1.12 | 6.77 |
| 3Y | 4.84*** | 1.81 | 1.29 | 8.38 |
Results of the GEE analysis performed on the Vanderbilt Inattention scores collected at 4 time points: baseline, post intervention (POST), 9-month follow-up (9M), and 3-year follow-up (3Y). First third of the table shows changes in scores within the group of children with Sensory Processing Dysfunction and Inattention (SPD+IA) relative to baseline. The rest of the table shows estimated differences between those changes and changes observed in the other two groups: typically developing children (TDC) and children with Sensory Processing Dysfunction only (SPD).
SE, Standard error; CI, Confidence interval.
*p<0.05,
**p<0.01,
***p<0.001
As can be seen in Fig 1, the scores of all three groups decreased over time to some degree. Thus, we also examined the interaction of Group and Time, in order to determine whether the changes in symptoms in SPD+IA were different from the changes observed in typically developing children and SPD children without attentional challenges. The interaction of Group and Time was statistically significant (p = .017). Inattention scores of the TDC group at the 3-year follow-up changed from baseline by approximately -6.59 + 4.84 = -1.75 points, which was a significantly smaller decrease in symptoms compared to the one seen in children in the SPD+IA group (p = .000445). The scores of the SPD-only group changed from baseline by approximately -6.59 + 5.38 = -1.21 over the 3-year period. While the difference in changes between the SPD and SPD+IA group was not statistically significant (p = .067), possibly because of a smaller sample size of the SPD-only group at the 3-year follow-up (N = 7), the actual difference in changes was greater than the difference between the TDC and SPD+IA group (4.84<5.38).
Fig 1. Changes in Vanderbilt Inattention scores.
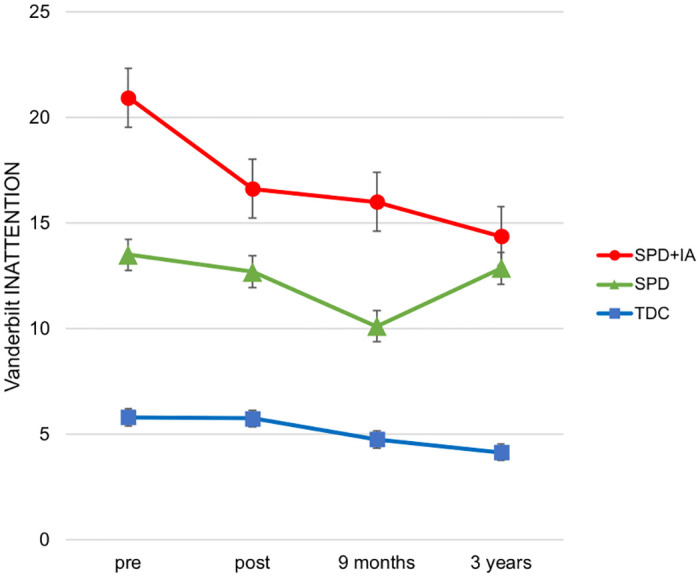
Vanderbilt Inattention mean scores displayed separately for typically developing children (TDC), children with Sensory Processing Dysfunction only (SPD), and children with SPD combined with inattention (SPD+IA) groups at 4 time points: pre-assessment [N = 49, N(TDC) = 22, N(SPD) = 10, N(SPD+IA) = 17], post-assessment [N = 46, N(TDC) = 20, N(SPD) = 10, N(SPD+IA) = 16], 9-month follow-up [N = 45, N(TDC) = 21, N(SPD) = 8, N(SPD+IA) = 16], and 3-year follow-up [N = 39, N(TDC) = 19, N(SPD) = 7, N(SPD+IA) = 13]. Error bars indicate standard error of the mean.
In addition to the Inattention scale, we conducted GEE analyses on the Hyperactivity/Impulsivity and Total scores. The interaction of Group and Time was not statistically significant in the analysis of Hyperactivity/Impulsivity scores (p = .114) and any changes within the SPD+IA group did not differ significantly from those observed in the other two groups. Outcomes of the Vanderbilt Total scale, which is a combination of the two subscales, were comparable to those of Inattention. These results are presented in S1 Table.
Discussion
The purpose of this study was to assess the long-term retention of parent reported inattention-based improvements following digital intervention with Project: EVO in children with Sensory Processing Dysfunction (SPD). Using a parent report, we found that children with SPD who also experience attentional challenges (SPD+IA) sustained their improvements three years post intervention and the majority of these children no longer meet clinical criteria for inattention. Below we discuss the implications of these findings with respect to targeted cognitive remediation in this cohort, as well as limitations that need to be considered.
Numerous studies have found beneficial effects of cognitive training in substitution or in addition to pharmacological treatments in children with attentional challenges [27–42] as well as in healthy children [43]. A handful of those studies followed-up with their participants to see whether any observed changes persisted over time, ranging from 6 weeks to 1 year after training [34–37, 39, 41, 43]. To our best knowledge, this is the longest follow-up study assessing long-term effects of cognitive training in children as well as the first study of this kind in children with SPD.
Our findings of long-lasting improvements in attention among SPD children with ADHD symptoms are consistent with previous reports of children diagnosed with ADHD showing sustained cognitive and behavioral benefits months after interventions [34–37, 39]. For example, Rabiner et al. [41] found that only children with six or more symptoms of inattention based on DSM-IV criteria demonstrated persistent benefits of computer-based attention training a year later. Taken together, these findings provide support for potential long-term benefits of cognitive training in children with attentional difficulties.
While it is difficult to determine the primary reason(s) for the sustained benefits in our SPD+IA cohort, we can draw from previous work to propose plausible explanation. The premise of cognitive training programs is that controlled engagement in cognitive tasks with adaptive algorithms provides constant challenge thus leading to strengthening of brain networks and enhancing the corresponding cognitive functions [44]. This hypothesis was recently corroborated by an fMRI study of children with ADHD [42] that demonstrated that cognitive training led to increased task-related activation in right dorsolateral prefrontal cortex (DLPFC) as well as inferior and superior parietal regions. The DLPFC along with parieto-temporal regions, cerebellum, thalamus and basal ganglia, are all brain areas considered to play an important role in mediating sustained attention, and that have been previously found to be underactivated in children with ADHD [45].
Taking into account the considerable symptom overlap and possible common neuroanatomical factors in ADHD and SPD [46, 47], the hypothesis of mechanism of change induced by cognitive training in ADHD may also be relevant in our SPD+IA cohort. Electroencephalography (EEG) data from our pilot study [5] showed significantly lower midline frontal theta (MFT) power, a known neuromarker of attention [8, 13, 14], in the SPD+IA group when compared to typically developing children (TDC). Following EVO training, MFT increased significantly in SPD+IA and reached levels comparable to those observed in the TDC group at baseline. Moreover, the MFT power gain correlated positively with improvements on the Vanderbilt Inattention scale. This regional theta activity is thought to reflect the communication between basal ganglia and frontal regions [48], brain areas involved in the sustained attention network.
These findings suggest that cognitive training might stimulate neural circuitry under-activated in children with attentional challenges, thus helping them “get on track” and resulting in sustained improvements years after intervention. Normalization effects have previously been observed for other modes of intervention, specifically pharmacological treatment. Structural and functional neuroimaging studies found that brain regions involved in modulating attention, including right DLPFC, basal ganglia, and cerebellar vermis, reached normal levels in long-term stimulant medicated ADHD patients [49–52]. This points to the possibility that cognitive training acts on these neural regions in a similar way as psychostimulant medication. To our best knowledge, neuroimaging data supporting long-term effects of cognitive training are not yet available, which stresses the importance of including neural assessment in future studies.
The clearest limitation of the present study is its sole reliance on parent report questionnaires. In the future, we hope to assess each participant not just on a parent report basis, but obtain neural data for the same assessments that were completed in the initial study. These data would aid in establishing whether the changes in neural activity observed in SPD+IA at post-assessment were also preserved three years later and whether they correlated with behavioral data reported by parents.
The absence of a control group is another notable limitation of this study. Looking at data from all 4 time points, we observed a pattern of symptom reduction, to varying degrees, among all 3 groups, which could be accounted for by the process of maturation. Even though the changes observed within the SPD+IA group were significantly greater than changes measured within the other 2 groups, having a sex- and age-matched control group of SPD+IA children without exposure to Project EVO, would shed more light into the specific contribution of this cognitive intervention program on the reported behavioral changes.
Conclusions
Despite the limitations, this follow-up study provides promising evidence for long-term benefits of Project: EVO in a subset of children with SPD. These children experience attention deficits that often impact their academic and social development. Our results demonstrate that just four weeks of videogame-like cognitive training have the potential to improve symptoms of inattention in this group of SPD children and that these improvements are sustained years after intervention with the majority of these children no longer meeting clinical criteria for Inattentive subtype of ADHD. Further studies are needed, however, to verify these results with the inclusion of control groups as well as multifaceted assessments.
Supporting information
(DOCX)
Individual Vanderbilt scores at all measurement times.
(XLSX)
Acknowledgments
We would like to thank all the children and families for their continued participation and support in our research. We would also like to thank Akili Interactive Labs, Inc., a digital therapeutics company, for providing the digital training platform used for this study as well as their assistance with data analysis.
Data Availability
All relevant data are within the manuscript and its Supporting information files.
Funding Statement
Funding for this project came from the Mickelson-Brody Family Foundation, the Wallace Research Foundation, the James Gates Family Foundation, the Kawaja-Holcombe Family Foundation, and crowdfunding support to the UCSF Sensory Neurodevelopment and Autism Program. Additionally, two of the authors (EM, MG] are employed by Cortica Healthcare, a provider of advanced neurological therapies for children. The funder provided support in the form of salaries for authors [EM, MG], but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Miller LJ, Anzalone ME, Lane SJ, Cermak SA, Osten ET. Concept evolution in sensory integration: A proposed nosology for diagnosis. Am J Occup Ther 2007. 10.5014/ajot.61.2.135 [DOI] [PubMed] [Google Scholar]
- 2.Ahn RR, Miller LJ, Milberger S, McIntosh DN. Prevalence of parents’ perceptions of sensory processing disorders among kindergarten children. Am J Occup Ther 2004. 10.5014/ajot.58.3.287 [DOI] [PubMed] [Google Scholar]
- 3.Bundy AC, Murray EA. Sensory integration: A. Jean Ayres’ theory revisited In: Bundy A. C., Murray E. A. & L SJ (Eds., editor. Sens. Integr. Theory Pract. 2nd ed, Philadelphia: F. A. Davis; 2002, p. 3–33. [Google Scholar]
- 4.Brandes-Aitken A, Anguera JA, Rolle CE, Desai SS, Demopoulos C, Skinner SN, et al. Characterizing cognitive and visuomotor control in children with sensory processing dysfunction and autism spectrum disorders. Neuropsychology 2018;32:148–60. 10.1037/neu0000404 [DOI] [PubMed] [Google Scholar]
- 5.Anguera JA, Brandes-Aitken AN, Antovich AD, Rolle CE, Desai SS, Marco EJ. A pilot study to determine the feasibility of enhancing cognitive abilities in children with sensory processing dysfunction. PLoS One 2017;12:1–19. 10.1371/journal.pone.0172616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owen JP, Marco EJ, Desai S, Fourie E, Harris J, Hill SS, et al. Abnormal white matter microstructure in children with sensory processing disorders. NeuroImage Clin 2013;2:844–53. 10.1016/j.nicl.2013.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anguera JA, Gunning FM, Areán PA. Improving late life depression and cognitive control through the use of therapeutic video game technology: A proof-of-concept randomized trial. Depress Anxiety 2017;34:508–17. 10.1002/da.22588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anguera JA, Boccanfuso J, Rintoul JL, Al-Hashimi O, Faraji F, Janowich J, et al. Video game training enhances cognitive control in older adults. Nature 2013. 10.1038/nature12486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis NO, Bower J, Kollins SH. Proof-of-concept study of an at-home, engaging, digital intervention for pediatric ADHD. PLoS One 2018;13:e0189749 10.1371/journal.pone.0189749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arean PA, Hallgren KA, Jordan JT, Gazzaley A, Atkins DC, Heagerty PJ, et al. The Use and Effectiveness of Mobile Apps for Depression: Results From a Fully Remote Clinical Trial. J Med Internet Res 2016;18:e330 10.2196/jmir.6482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yerys BE, Bertollo JR, Kenworthy L, Dawson G, Marco EJ, Schultz RT, et al. Brief Report: Pilot Study of a Novel Interactive Digital Treatment to Improve Cognitive Control in Children with Autism Spectrum Disorder and Co-occurring ADHD Symptoms. J Autism Dev Disord 2019;49:1727–37. 10.1007/s10803-018-3856-7 [DOI] [PubMed] [Google Scholar]
- 12.Kollins SH, DeLoss DJ, Cañadas E, Lutz J, Findling RL, Keefe RSE, et al. A novel digital intervention for actively reducing severity of paediatric ADHD (STARS-ADHD): a randomised controlled trial. Lancet Digit Heal 2020;2:e168–78. 10.1016/S2589-7500(20)30017-0 [DOI] [PubMed] [Google Scholar]
- 13.Nigbur R, Ivanova G, Stürmer B. Theta power as a marker for cognitive interference. Clin Neurophysiol 2011. 10.1016/j.clinph.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 14.Sauseng P, Hoppe J, Klimesch W, Gerloff C, Hummel FC. Dissociation of sustained attention from central executive functions: Local activity and interregional connectivity in the theta range. Eur J Neurosci 2007. 10.1111/j.1460-9568.2006.05286.x [DOI] [PubMed] [Google Scholar]
- 15.Ishii R, Shinosaki K, Ukai S, Inouye T, Ishihara T, Yoshimine T, et al. Medial prefrontal cortex generates frontal midline theta rhythm. Neuroreport 1999;10:675–9. 10.1097/00001756-199903170-00003. [DOI] [PubMed] [Google Scholar]
- 16.Ishihara T., Yoshii N. Multivariate analytic study of EEG and mental activity in juvenile delinquents. Electroencephalogr Clin Neurophysiol 1972;33:71–80. 10.1016/0013-4694(72)90026-0 [DOI] [PubMed] [Google Scholar]
- 17.Rebok GW, Ball K, Guey LT, Jones RN, Kim H-Y, King JW, et al. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc 2014;62:16–24. 10.1111/jgs.12607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valenzuela M, Sachdev P. Can cognitive exercise prevent the onset of dementia? systematic review of randomized clinical trials with longitudinal follow-up. Am J Geriatr Psychiatry 2009;17:179–87. 10.1097/JGP.0b013e3181953b57 [DOI] [PubMed] [Google Scholar]
- 19.Wolraich ML, Lambert W, Doffing MA, Bickman L, Simmons T, Worley K. Psychometric Properties of the Vanderbilt ADHD Diagnostic Parent Rating Scale in a Referred Population. J Pediatr Psychol 2003;28:559–67. 10.1093/jpepsy/jsg046 [DOI] [PubMed] [Google Scholar]
- 20.Bard DE, Wolraich ML, Neas B, Doffing M, Beck L. The psychometric properties of the Vanderbilt attention-deficit hyperactivity disorder diagnostic parent rating scale in a community population. J Dev Behav Pediatr 2013;34:72–82. 10.1097/DBP.0b013e31827a3a22 [DOI] [PubMed] [Google Scholar]
- 21.Wolraich ML, Lambert W, Doffing MA, Bickman L, Simmons T, Worley K. Psychometric Properties of the Vanderbilt ADHD Diagnostic Parent Rating Scale in a Referred Population. J Pediatr Psychol 2003. 10.1093/jpepsy/jsg046. [DOI] [PubMed] [Google Scholar]
- 22.National Institute of Mental Health. Computerized diagnostic interview schedule for children, IV. New York Author, Columbia Univ 1997.
- 23.Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR). 2000. 10.1176/appi.books.9780890423349. [DOI] [PubMed]
- 24.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13–22. 10.1093/biomet/73.1.13. [DOI] [Google Scholar]
- 25.Ahmad S, Schwarz M, Flick RJ, Rees CA, Harawa M, Simon K, et al. Analyzing Correlated Data in SAS® Niloofar Ramezani, University of Northern Colorado. Trop Med Int Heal 2016;21:170–485. [Google Scholar]
- 26.Zeger L, Liang S. Longitudinal Data Analysis for Discrete and Continuous Outcomes Author (s): Scott L. Zeger and Kung-Yee Liang Published by: International Biometric Society Stable URL: http://www.jstor.org/stable/2531248. Biometrics 1986;42:121–30. [PubMed] [Google Scholar]
- 27.Gray SA, Chaban P, Martinussen R, Goldberg R, Gotlieb H, Kronitz R, et al. Effects of a computerized working memory training program on working memory, attention, and academics in adolescents with severe LD and comorbid ADHD: A randomized controlled trial. J Child Psychol Psychiatry Allied Discip 2012;53:1277–84. 10.1111/j.1469-7610.2012.02592.x. [DOI] [PubMed] [Google Scholar]
- 28.Green CT, Long DL, Green D, Iosif AM, Dixon JF, Miller MR, et al. Will Working Memory Training Generalize to Improve Off-Task Behavior in Children with Attention-Deficit/Hyperactivity Disorder? Neurotherapeutics 2012;9:639–48. 10.1007/s13311-012-0124-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Semrud-Clikeman M, Nielsen KH, Clinton A, Sylvester L, Parle N, Connor RT. An intervention approach for children with teacher-and parent-identified attentional difficulties. J Learn Disabil 1999. 10.1177/002221949903200609 [DOI] [PubMed] [Google Scholar]
- 30.Shalev L, Tsal Y, Mevorach C. Computerized progressive attentional training (CPAT) program: Effective direct intervention for children with ADHD. Child Neuropsychol 2007. 10.1080/09297040600770787 [DOI] [PubMed] [Google Scholar]
- 31.Steiner NJ, Sheldrick RC, Gotthelf D, Perrin EC. Computer-based attention training in the schools for children with attention deficit/hyperactivity disorder: A preliminary trial. Clin Pediatr (Phila) 2011;50:615–22. 10.1177/0009922810397887 [DOI] [PubMed] [Google Scholar]
- 32.Tamm L, Epstein JN, Peugh JL, Nakonezny PA, Hughes CW. Preliminary data suggesting the efficacy of attention training for school-aged children with ADHD. Dev Cogn Neurosci 2013;4:16–28. 10.1016/j.dcn.2012.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tucha O, Tucha L, Kaumann G, König S, Lange KM, Stasik D, et al. Training of attention functions in children with attention deficit hyperactivity disorder. ADHD Atten Deficit Hyperact Disord 2011;3:271–83. 10.1007/s12402-011-0059-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Oord S, Ponsioen AJGB, Geurts HM, Brink ELT, Prins PJM. A Pilot Study of the Efficacy of a Computerized Executive Functioning Remediation Training With Game Elements for Children With ADHD in an Outpatient Setting: Outcome on Parent- and Teacher-Rated Executive Functioning and ADHD Behavior. J Atten Disord 2014. 10.1177/1087054712453167. [DOI] [PubMed] [Google Scholar]
- 35.Holmes J, Gathercole SE, Place M, Dunning DL, Hilton KA, Elliott JG. Working memory deficits can be overcome: Impacts of training and medication on working memory in children with ADHD. Appl Cogn Psychol 2010. 10.1002/acp.1589 20174591 [DOI] [Google Scholar]
- 36.Hovik KT, Saunes BK, Aarlien AK, Egeland J. RCT of working memory training in ADHD: Long-term near-transfer effects. PLoS One 2013. 10.1371/journal.pone.0080561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnstone SJ, Roodenrys S, Blackman R, Johnston E, Loveday K, Mantz S, et al. Neurocognitive training for children with and without AD/HD. ADHD Atten Deficit Hyperact Disord 2012;4:11–23. 10.1007/s12402-011-0069-8 [DOI] [PubMed] [Google Scholar]
- 38.Kerns KA, Karen E, Thomson J, Kerns KA. Investigation of a direct intervention for improving attention in young children with ADHD. Dev Neuropsychol 1999. 10.1207/S15326942DN1602_9. [DOI] [Google Scholar]
- 39.Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlström K, et al. Computerized training of working memory in children with ADHD—A randomized, controlled trial. J Am Acad Child Adolesc Psychiatry 2005. 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Lange KW, Tucha L, Hauser A, Hauser J, Lange KM, Stasik D, et al. Attention training in Attention Deficit Hyperactivity Disorder. Aula Abierta, ISSN 0210-2773, Vol 40, No 3, 2012, Págs 55–60 2012;40:55–60.
- 41.Rabiner DL, Murray DW, Skinner AT, Malone PS. A randomized trial of two promising computer-based interventions for students with attention difficulties. J Abnorm Child Psychol 2010. 10.1007/s10802-009-9353-x [DOI] [PubMed] [Google Scholar]
- 42.de Oliveira Rosa V, Rosa Franco A, Abrahão Salum Júnior G, Moreira-Maia CR, Wagner F, Simioni A, et al. Effects of computerized cognitive training as add-on treatment to stimulants in ADHD: a pilot fMRI study. Brain Imaging Behav 2019. 10.1007/s11682-019-00137-0. [DOI] [PubMed] [Google Scholar]
- 43.Jaeggi SM, Buschkuehl M, Jonides J, Shah P. Short- and long-term benefits of cognitive training. Proc Natl Acad Sci U S A 2011;108:10081–6. 10.1073/pnas.1103228108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cortese S, Ferrin M, Brandeis D, Buitelaar J, Daley D, Dittmann RW, et al. Cognitive Training for Attention-Deficit/Hyperactivity Disorder: Meta-Analysis of Clinical and Neuropsychological Outcomes From Randomized Controlled Trials. J Am Acad Child Adolesc Psychiatry 2015;54:164–74. 10.1016/j.jaac.2014.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christakou A, Murphy CM, Chantiluke K, Cubillo AI, Smith AB, Giampietro V, et al. Disorder-specific functional abnormalities during sustained attention in youth with Attention Deficit Hyperactivity Disorder (ADHD) and with Autism. Mol Psychiatry 2013;18:236–44. 10.1038/mp.2011.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koziol LF, Budding D. ADHD and sensory processing disorders: Placing the diagnostic issues in context. Appl Neuropsychol Child 2012. 10.1080/21622965.2012.709422 [DOI] [PubMed] [Google Scholar]
- 47.Pfeiffer B, Daly BP, Nicholls EG, Gullo DF. Assessing sensory processing problems in children with and without attention deficit hyperactivity disorder. Phys Occup Ther Pediatr 2015. 10.3109/01942638.2014.904471 [DOI] [PubMed] [Google Scholar]
- 48.Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends Cogn Sci 2014. 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakao T, Radua J, Rubia K, Mataix-Cols D. Gray Matter Volume Abnormalities in ADHD: Voxel-Based Meta-Analysis Exploring the Effects of Age and Stimulant Medication. Am J Psychiatry 2011;168:1154–63. 10.1176/appi.ajp.2011.11020281 [DOI] [PubMed] [Google Scholar]
- 50.Hart H, Radua J, Mataix-Cols D, Rubia K. Meta-analysis of fMRI studies of timing in attention-deficit hyperactivity disorder (ADHD). Neurosci Biobehav Rev 2012;36:2248–56. 10.1016/j.neubiorev.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 51.Bledsoe J, Semrud-Clikeman M, Pliszka SR. An MRI Study of the Cerebellar Vermis in Chronically-Treated and Treatment-Naïve Children with ADHD-Combined Type. Biol Psychiatry 2009;65:620 10.1016/j.biopsych.2008.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pretus C, Ramos-Quiroga JA, Richarte V, Corrales M, Picado M, Carmona S, et al. Time and psychostimulants: Opposing long-term structural effects in the adult ADHD brain. A longitudinal MR study. Eur Neuropsychopharmacol 2017;27:1238–47. 10.1016/j.euroneuro.2017.10.035 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Individual Vanderbilt scores at all measurement times.
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting information files.


