Abstract
Background
The association between personal hair dye use and breast cancer risk is currently debated. The aim of this work is to investigate the association between the use of hair care products and breast cancer risk in women.
Methods
Based on the PRISMA-IPD statement, the PubMed, Embase, Cochrane Library, Web of Science, OVID and Scopus databases were used to identify eligible studies published from inception to 22 April 2020. A pooled odds ratio (OR) with a 95% confidential interval (CI) was calculated to assess this correlation via fixed- or random-effect Mantel-Haenszel models using a heterogeneity Chi2 test with a significance level of p<0.1. All statistical tests were performed using StataSE software (version 12.0).
Results
The analyzed data comprised 14 eligible studies with 210319 unique subjects. The pooled results suggested that there was a significant association between the use of hair dyes and breast cancer occurrence (pooled OR = 1.07; 95% CI, 1.01–1.13). Regarding the individual analysis regarding the different types of hair chemicals, permanent hair dye users (pooled OR = 1.08; 95% CI, 1.03–1.14) and rinse users (pooled OR = 1.17; 95% CI, 1.02–1.35) were both found to have a significantly elevated breast cancer risk compared to natural hair subjects, whereas there was an insignificant relationship between the use of semipermanent hair dyes (pooled OR = 1.09; 95% CI, 0.92–1.28) and straighteners (pooled OR = 1.04; 95% CI, 0.96–1.14) and breast cancer risk. No impact on the overall correlation between hair dyes and breast cancer risk due to race (White vs non-White) (pooled OR = 1.05; 95% CI, 0.86–1.29), timing of use (<10 years vs ≥10 years) (pooled OR = 0.96; 95% CI, 0.85–1.08) or dye color (Darker than natural hair vs Lighter than natural hair) (pooled OR = 0.91; 95% CI, 0.62–1.32) was found.
Conclusions
Chemicals in hair dyes may play a role in breast carcinogenesis and increase breast cancer risk.
Introduction
Worldwide hair dye sales are extraordinarily remarkable, valued at approximately $12 billion per year [1]. Statistically, nearly 1/3 of females in the USA, Europe and East Asia, as well as 5–10% of males in Europe, use some type of hair dye every year, some people use them more than once [2–4]. The excessive application of hair colorants possibly increases users’ risk of cancer and has become a public health concern, as some hair products are potential mutagens and demonstrate endocrine-disrupting characteristics, both of which may be associated with the occurrence of several human carcinomas [5,6]. Hair care products contain more than 5,000 chemicals, of which aromatic amines are mutagenic in vitro and carcinogenic in animals and humans [7,8].
The major categories of hair products are temporary (rinse), semipermanent and permanent hair dyes, as well as straightening/relaxing hair chemicals (hereinafter collectively referred to as straighteners); almost all of them are compounded by para-phenylenediamine (PPD: C6H8N2) [9], a powerful skin sensitizer that can induce breast tumors in rats [6]. The potential carcinogenic effect of PPD is presumably attributable to its contamination with 4-aminobiphenyl (4-ABP) during production. In 2010, the International Agency for Research on Cancer (IARC) officially labeled 4-ABP as a human carcinogen because of its mutagenic effect on human DNA [10,11]. Moreover, 4-ABP can permeate the mammary gland to activate estrogen, which is a fundamental etiology of breast cancer [12,13]. Examination of breast ductal epithelial cells shows that 4-ABP-DNA adducts in women who used hair dyes in the past year were 8 times higher than those who never used [14].
A prospective cohort study suggested that hair dyes and straighteners containing endocrine-disrupting chemicals and other carcinogenic compounds might increase the risk of breast cancer, specifically in black women using permanent hair colorants [15]. Breast cancer is still the most common cause of cancer-related death in women, although its overall mortality is gradually decreasing in developed and developing countries [16]. Due to widespread use of hair products, even if they only slightly increase breast cancer risk, they pose tremendous negative consequences for public health. However, previous studies have reached discordant conclusions regarding the impact of personal use of hair chemicals on breast cancer risk by virtue of differences in study design, characteristics of the study population and changes in the chemical formulations of hair products [4,17–24]; the results of three relevant meta-analyses reinforced this contradiction due to differences in included trials and methodology [8,25,26]. To provide instructional evidence-based medical data to inform public health, we undertook a meta-analysis to summarize all the scientific data on the association between hair chemicals and new-onset breast cancer.
Methods
Search strategy and study design
In light of the PRISMA-IPD Statement [27], electronic searches were performed using six databases, including PubMed, Embase, Cochrane Library, Web of Science, OVID and Scopus, using the following retrieval strategy: ("Breast Neoplasms"[Mesh] OR (breast cancer) OR (breast tumor) OR (breast tumor)) AND ("Hair Dyes"[Mesh] OR (hair dye) OR straightener). No restrictions were required during the retrieval. Potential citations after 22 April 2020 were not included.
In addition to evaluating the relationship between hair coloring and breast cancer risk, the associations between different types of hair products and breast cancer risk were investigated. Furthermore, the impacts of race (White vs non-White), usage duration (<10 years vs ≥10 years) and hair dye color (lighter than natural hair vs darker than natural hair) on the overall correlation between the use of hair dyes and breast cancer risk were explored.
Inclusion criteria
Retrospective or prospective clinical studies published in English;
Female subjects;
Studies provided the original odds ratio (OR) and its 95% confidence interval (CI) or the raw data to calculate them;
The exposure element was the use of any type of hair care product;
The outcomes were clearly classified into cancer events (cases) and nonevents (controls). Hair care products included semipermanent, permanent hair dyes, rinses and straighteners. Cases referred to women who were healthy initially and developed breast cancer during follow-up.
Exclusion criteria
Non-English published articles;
Article type: review, case report, study protocol or conference paper;
Other elements that did not meet the inclusion criteria.
Two coauthors (HW and YL) separately screened the titles and abstracts of all citations and removed the unmatched citations. The full texts of the remaining potential studies were further scrutinized, and only satisfactory publications were retained. If there were inconsistencies, they were resolved by the third coauthor (CZ).
Data extraction
The following information was extracted from the qualified studies by two coauthors (HW and YL) using Excel software, version 2016 (Microsoft Corporation, Redmond, Washington, USA): first author, publication year, study duration, study type, nation of origin, median follow-up, mean age, sample size, categories of hair products, race, dye color, and timing of use. If any variabilities were identified, they were addressed by discussion. We additionally evaluated whether these studies assessed menopausal status, age at first birth, parity, family history of breast cancer, education, history of oral contraceptive use, body mass index, smoking history, number of alcoholic drinks per week and marital status.
Quality assessment
The Cochrane Collaboration's tool was used to assess the methodological quality of all included studies [28]. Bias was evaluated by two coauthors (HW and YL) independently. Any disagreements were resolved by discussion.
Statistical analysis
The association between the use of personal hair chemicals and breast cancer risk, and the influence of race, use duration and dye color on this overall association, were presented in the form of a pooled OR that was calculated using the crude ORs with their 95% CIs from all the included studies. The heterogeneity among different trials was evaluated via the heterogeneity Chi2 test with a significance level of p<0.1 [29]. When the heterogeneity test was not statistically significant (p≥0.1), a fixed-effect Mantel-Haenszel (MH) model was applied to pool the data; if not, a random-effect MH model was used [29]. Publication bias was investigated using Egger’s test with a significance level of p<0.05 and a Begg’s funnel plot. All statistical tests were analyzed via StataSE software, version 12.0 (StataCorp LP, College Station, TX, USA).
Results
Search results
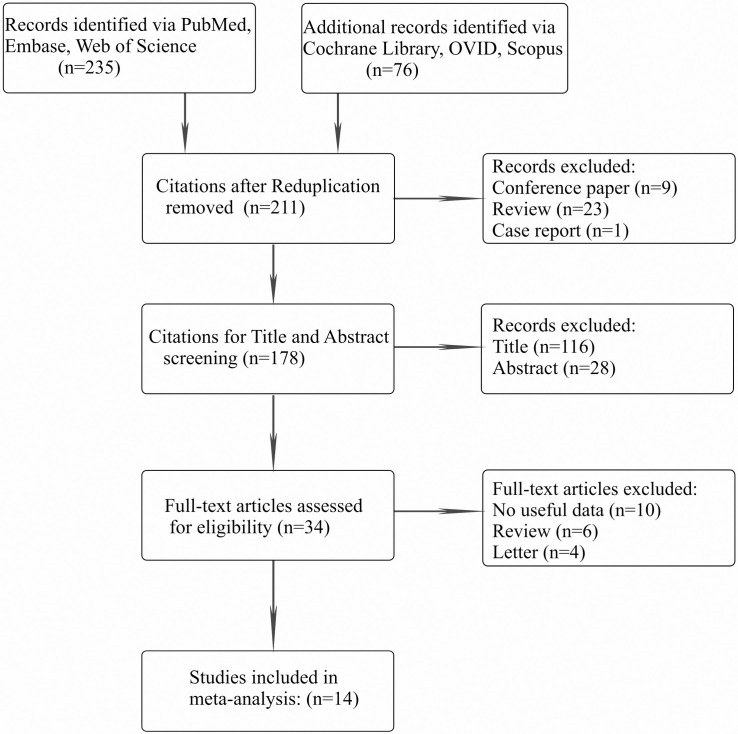
We identified a total of 311 potential citations after systematic retrieval, with duplicated studies (n = 100), reviews (n = 23), case reports (n = 1) and conference papers (n = 9) excluded. Of the 178 remaining citations, 144 were removed during title and abstract screening. Therefore, 34 studies were left for full-text scrutiny, and 20 of them were deleted because they lacked useful data (n = 10), were reviews (n = 6) or were letters (n = 4)). Ultimately, 14 eligible publications [4,15,18–24,30–34] with 210319 unique subjects met the inclusion criteria. The PRISMA flow diagram of the inclusion process is shown in Fig 1.
Fig 1. The PRISMA flow diagram of the inclusion process.
Characteristics of eligible studies
With respect to the included articles, publication years ranged from 1978 to 2019 (median: 1993), median follow-up ranged from 6 to 8.3 years (median: 7.25), and the mean subject age ranged from 48 to 57.5 years old (median: 54.7). The most common article type was a case-control study (78.6%); the most common nation of origin was the USA (64.5%). The most common interview method was a questionnaire survey alone (35.7%) (Table 1). As outlined in Table 2, seven studies [4,15,18,19,23,31,33] examined semipermanent or permanent hair dyes and rinses, and two [15,24] examined straighteners, while the Green et al. article [20] only examined permanent hair coloring. Several studies specifically considered the impact of race (n = 5), hair dye color (n = 4) and usage duration (n = 8) on the association between hair products and breast cancer risk. Table 2 also shows other detailed characteristics of the included studies.
Table 1. Summary of the subject-number distribution in terms of baseline characteristics.
| Characteristic | Studies, No. (%) (N = 14) | Analyzed subjects, No. (%) (N = 210319) |
|---|---|---|
| Study type | ||
| Case-control | 11 (78.6) | 44614 (21.2) |
| Prospective cohort | 3 (21.4) | 165785 (78.8) |
| Publication date, median (range), y | 1993 (1978–2019) | |
| Follow-up, median (range), y* | 7.25 (6–8.3) | |
| Mean age, median (range), y* | 54.7 (48–57.5) | |
| Original nation | ||
| USA | 9 (64.5) | 62221 (29.6) |
| China | 1 (7.1) | 592 (0.3) |
| Finland | 1 (7.1) | 27795 (13.2) |
| Canada | 1 (7.1) | 255 (0.1) |
| Iran | 1 (7.1) | 1052 (0.5) |
| Australia | 1 (7.1) | 118404 (56.3) |
| Interview method | ||
| questionnaire only | 5 (35.7) | 32581 (15.5) |
| telephone only | 1 (7.1) | 1336 (0.6) |
| Questionnaire and telephone | 4 (28.6) | 168669 (80.2) |
| In-person and telephone | 1 (7.1) | 1804 (0.9) |
| In-person and questionnaire | 2 (14.3) | 4877 (2.3) |
| Hospital files and questionnaire | 1 (7.1) | 1052 (0.5) |
| Assessment of menopause | ||
| Yes | 7 (50.0) | 173549 (82.5) |
| No | 7 (50.0) | 36850 (17.5) |
| Assessment of age at first birth | ||
| Yes | 7 (50.0) | 174681 (83.1) |
| No | 7 (50.0) | 35638 (16.9) |
| Assessment of parity | ||
| Yes | 9 (64.3) | 88949 (42.3) |
| No | 5 (35.7) | 121370 (57.7) |
| Assessment of family history of breast cancer | ||
| Yes | 6 (42.9) | 156574 (74.4) |
| No | 8 (57.1) | 53745 (25.6) |
| Assessment of education | ||
| Yes | 11 (78.6) | 90634 (43.1) |
| No | 3 (21.4) | 119685 (56.9) |
| Assessment of history of oral contraceptive use | ||
| Yes | 8 (57.1) | 85715 (40.8) |
| No | 6 (42.9) | 124604 (59.2) |
| Assessment of body mass index | ||
| Yes | 8 (57.1) | 88627 (42.1) |
| No | 6 (42.9) | 121692 (57.9) |
| Assessment of smoking history | ||
| Yes | 10 (71.4) | 204094 (97.0) |
| No | 4 (28.6) | 6225 (3.0) |
| Assessment of alcohol drinks per week | ||
| Yes | 5 (35.7) | 36581 (17.4) |
| No | 9 (64.3) | 173738 (82.6) |
| Assessment of marital status | ||
| Yes | 6 (42.9) | 34799 (16.5) |
| No | 8 (57.1) | 175520 (83.5) |
*The median value is calculated using the available data.
Table 2. The details of the 14 included studies.
| Study (Year) | Study duration | Original nation | Study type | Sample size | Interview method | Hair colorants | Race | Dye color | Dye duration (y) | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| Boice (1995) | 1926–1982 | USA | Case-control | 3156 | Questionnaire | NA | NA | NA | NA | [34] |
| Cook (1999) | 1983–1990 | USA | Case-control | 1804 | In-person; Telephone | Rinse; Semipermanent HD; Permanent HD; Bleach then dye; Frosting/tipping | White | Lighter; Darker | ≤5; 6–15; 16–25; >25 | [33] |
| Dianatinasab (2017) | 2014–2016 | Iran | Case-control | 1052 | Hospital files; Questionnaire | NA | NA | NA | NA | [32] |
| Eberle (2019) | 2003–2009 | USA | Prospective cohort | 46709 | Questionnaire; Telephone | Rinse; Semipermanent HD; Permanent HD; Straighteners | Non-Hispanic White; Black; Hispanic White; Other | Lighter; Darker; Combination | <5; ≥5 | [15] |
| Heikkinen (2015) | 2000–2007 | Finland | Case-control | 27795 | Questionnaire | Rinse; Semipermanent HD; Permanent HD; Bleach; Partial dye | NA | NA | NA | [4] |
| Koenig (1991) | 1977–1981 | USA | Case-control | 1336 | Telephone | Rinse; Semipermanent HD; Permanent HD | White; non-White | Black; Brown; Red; Blond; Silver/gray | NA | [23] |
| Mendelsohn (2009) | 1996–2000 | China | Prospective cohort | 592 | Questionnaire; In-person | NA | NA | NA | 1–2; 3–4; 5–9; ≥10† | [21] |
| Nasca (1979) | 1975–1976 | USA | Case-control | 349 | Questionnaire | Rinse; Semipermanent HD; Permanent HD | NA | NA | NA | [31] |
| Nasca (1992) | 1982–1984 | USA | Case-control | 3234 | Questionnaire; Telephone | Rinse; Semipermanent HD; Permanent HD | White; non-White | NA | <10; 10–19; 19–29; ≥30 | [18] |
| Shore (1978) | 1964–1976 | USA | Case-control | 322 | Questionnaire; Telephone | Permanent oxidative HD; Total HD* | NA | NA | 1–4; 5–10; ≥11 | [30] |
| Stavraky (1979) | 1964–1978 | Canada | Case-control | 255 | Questionnaire | Rinse; Semipermanent HD; Permanent HD | NA | NA | NA | [19] |
| Wynder (1983) | 1979–1981 | USA | Case-control | 1026 | Questionnaire | Permanent HD; Other | Jewish; non-Jewish | NA | <10; 10–19; 19–29; ≥30 | [22] |
| Llanos (2017) | 2002–2008 | USA | Case-control | 4285 | Questionnaire; In-person | Total HD; Straighteners | White; Black | Lighter; Darker; Medium | 1–10; ≥10 | [24] |
| Green (1987) | 1976–1982 | Australia | Prospective cohort | 118404 | Questionnaire; Telephone | Permanent HD | NA | NA | ≤5; 6–10; 11–15;16–20;>20† | [20] |
Abbreviations: HD, hair dye; NA, not assessed in study.
*Total HD refers to permanent or semipermanent hair dye or rinse.
†Dye duration in these studies is only for cases.
Hair dyes significantly increased breast cancer risk
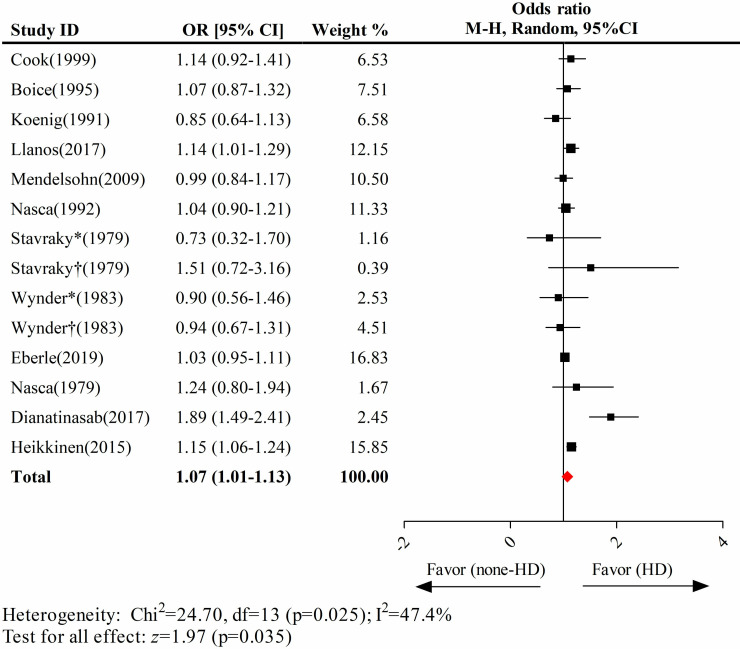
For the analysis of the association between hair dyes and breast cancer risk, 12 eligible studies were included. Of these, the study by Stavraky et al. [19] contained subjects in London and Toronto, and the Wynder et al. study [22] reported on Jewish and non-Jewish women, thereby bringing the number of usable studies to 14. The pooled results indicated that the use of hair dyes was significantly associated with breast cancer occurrence (pooled OR = 1.07; 95% CI, 1.01–1.13) (Fig 2).
Fig 2. The association between the use of hair dyes and breast cancer risk.
The study by Stavraky (1979) contains a Toronto group and a London group; Stavraky* refers to the Toronto group and Stavraky† refers to the London group. The study by Wynder (1983) involves a Jewish group and a non-Jewish group; Wynder* refers to the Jewish group and Wynder† refers to the non-Jewish group. Abbreviations: HD, hair dyes.
Permanent hair dyes and rinses were associated with elevated breast cancer risk
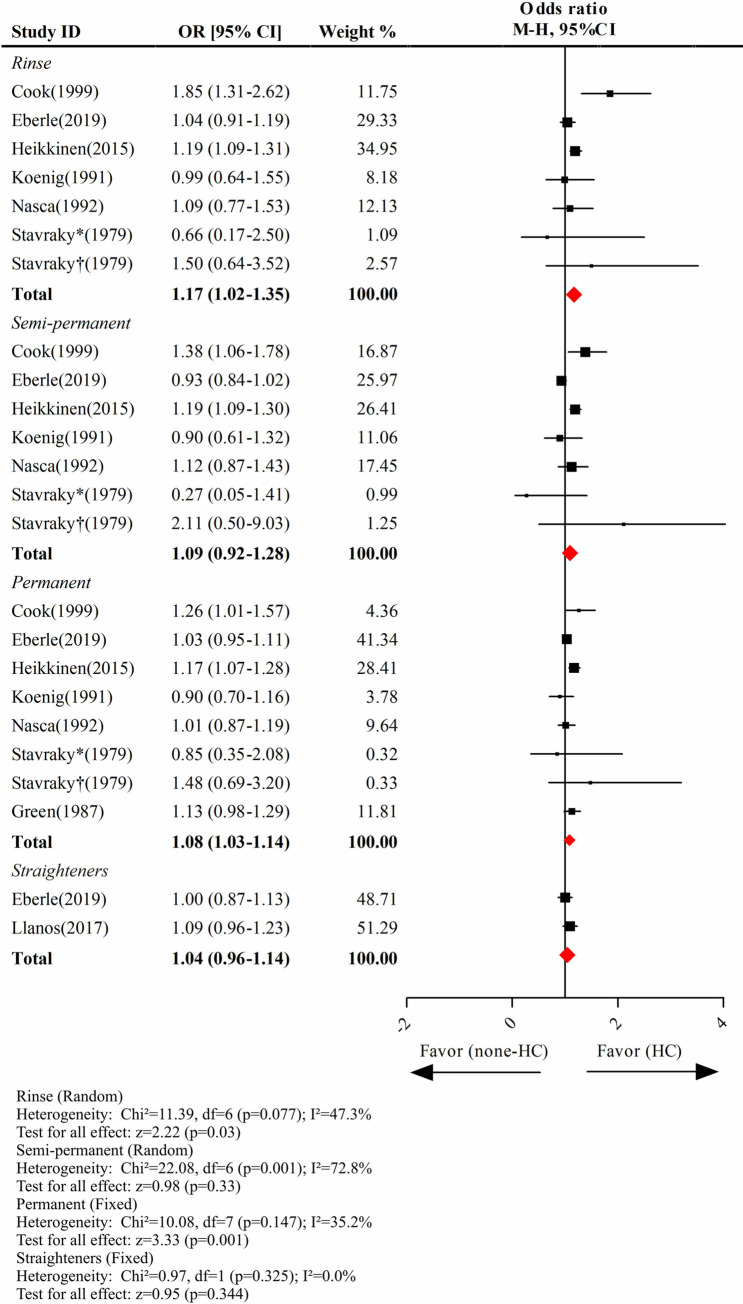
In the investigation of the relationship between different types of hair products and breast cancer risk, the final group of included studies used for the analysis of semipermanent and permanent hair dyes and rinses, as well as straighteners, was not entirely identical. Again, Stavraky et al. [19] classified their subjects into London and Toronto cohorts. The pooled data revealed that breast cancer risk was significantly increased by permanent hair dyes (pooled OR = 1.08; 95% CI, 1.03–1.14) and rinses (pooled OR = 1.17; 95% CI, 1.02–1.35) but was not significantly associated with semipermanent hair dyes (pooled OR = 1.09; 95% CI, 0.92–1.28) or straighteners (pooled OR = 1.04; 95% CI, 0.96–1.14) (Fig 3).
Fig 3. The association between the use of different types of hair chemicals and breast cancer risk.
The study by Stavraky (1979) contains a Toronto group and a London group; Stavraky* refers to the Toronto group and Stavraky† refers to the London group. Abbreviations: HC, hair chemicals.
Race, dye color and use duration did not impact the overall relationship between hair dyes and breast cancer risk
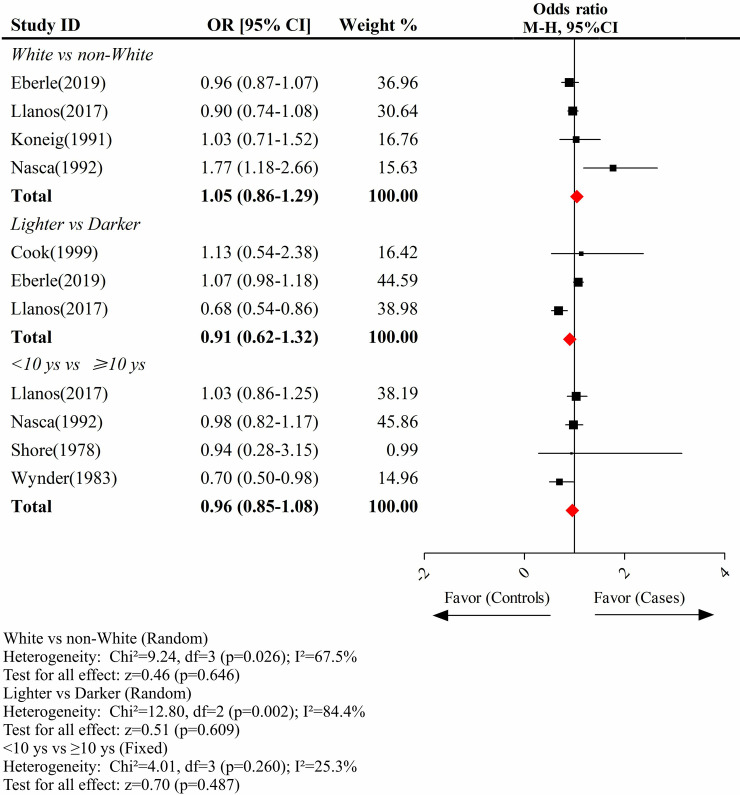
Although we previously mentioned several articles containing information for this analysis, fewer were usable. As demonstrated in Fig 4, the overall difference in breast cancer risk between White females and non-White females after using hair dyes was not significant (pooled OR = 1.05; 95% CI, 0.86–1.29); the results of this analysis mirrored those seen in the comparisons between lighter than natural hair and darker than natural hair (pooled OR = 0.91; 95% CI, 0.62–1.32) and <10 years and ≥10 years (pooled OR = 0.96; 95% CI, 0.85–1.08).
Fig 4. The impact of race, dyeing color and timing of use on the overall association between hair dyes and breast cancer risk.
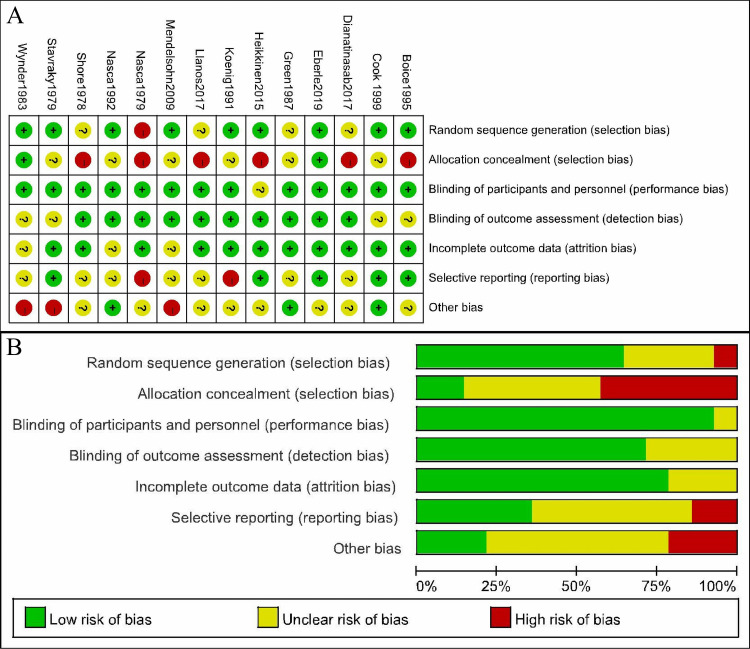
Risk of bias in included studies
Fig 5A and 5B present the judgement of the risk of bias summary and the risk of bias graph of the analyzed studies for each “risk of bias” domain, respectively. Nine studies adequately described the method of random sequence generation, while 6 studies did not perform allocation concealment. Thirteen studies had employed some strategies to blind participants and investigators, and 10 studies provided their methods for blinding outcome assessments. There were 11 studies that reported complete outcomes data and 2 studies that showed a high risk of selective reporting. Additionally, 3 studies had an imbalance in baseline characteristics.
Fig 5. The judgments of the risk of bias summary and the risk of bias graph.
A. The risk of bias summary; B. The risk of bias graph.
Heterogeneity and publication bias
The following meta-analyses appeared to contain heterogeneity: hair dyes and breast cancer risk, rinse and breast cancer risk, semipermanent hair dye and breast cancer risk, White vs non-White and lighter vs darker; thus, the random-effect MH model was adopted to calculate the value of the pooled OR. The remaining meta-analyses did not manifest any heterogeneity, and their pooled ORs were calculated using the fixed-effect MH model. All publication bias was not significant in terms of the results of Egger’s test (S1 Table) or the individual Begg’s funnel plot (S1–S8 Figs).
Discussion
The present large meta-analysis provides robust evidence supporting the conclusion that hair chemicals, particularly in permanent hair colorants and rinses, overtly increase the risk of breast carcinogenesis. Irrespective of our findings demonstrating no impacts of semipermanent hair dyes and straighteners on breast cancer risk, from a public health concern perspective, the results of this study are a meaningful addition to the pool of evidence on the potential association between hair dye use and breast cancer risk, and it further justifies additional research on the topic.
Over the past several decades, many case-control studies and prospective cohort studies have ascribed incredible importance to investigating the relationship between hair care products and new-onset breast cancer [15,17–19,21–23,30,31,33–38]; nevertheless, their conclusions are somewhat controversial. Using scientific methodology, our work concludes that a higher breast cancer risk is found in hair care product users, which is similar to the results of some other research groups [31,33,37]. As previously reviewed, one reason for this positive association is that these hair dyes contain PPD, which is a likely contributor to the carcinogenicity of hair dyes/straighteners. PPD activates the reactive oxygen species (ROS)-mediated mitochondrial pathway and inhibits the NF-κB, mTOR and Wnt pathways that promote healthy cell apoptosis [39]. Furthermore, it damages the lysosomal membrane in fibroblast cells by increasing ROS generation and lipid peroxidation and resulting in the collapse of the mitochondrial membrane potential and cytochrome c release [40].
Due to their different chemical compositions, hair colorants are classified as temporary, semipermanent and permanent dyes. A highly sensitive perovskite oxide sensor was used to determine that more than 80% of permanent hair dyes contain PPD [41]. Previous studies have suggested that the use of permanent dyes is closely associated with the occurrence of bladder cancer, lung cancer and Hodgkin's lymphoma [42]. Our study further confirms the positive correlation between the use of permanent dyes and breast cancer risk. By contrast, in 1987, a prospective cohort study found that permanent dyes do not increase the risk of breast cancer, likely due to the particular age of analyzed women (30–55 years) and the limited follow-up duration (only 6 years) [20]. Reaching a conclusion similar to ours, the Sister Study examined a broader age range of subjects and used a longer follow-up period; it observed that personal use of permanent hair dye was associated with a higher breast cancer risk [15].
Rinses, such as fuchsin basic, basic red 2 and Victoria blue B, have gained popularity in the last decade due to their inexpensive formulations, low prices, expedient applications and practical utilization at home. Additionally, study of the association between rinses and cancer risk has advanced recently, and some epidemiological studies have demonstrated insufficient evidence to support the existence of this association [43]. However, one study by Lizier et al. [44] showed that the molecules in hair dyes could be cleaved into noxious PPD. Orange 1 and basic red 51 are commonly applied in rinse formulations; cell experiments demonstrate that they can cause cell necrosis and drive tumorigenesis in healthy cells, respectively [45,46]. One of the predominant targets for the toxicity of most hair dyes is DNA, and many types of rinses can strongly interact with DNA and a result in disturbances in DNA replication, suggesting that rinses pose a potential risk to human health [42]. The findings of our meta-analysis confirm the increased breast cancer risk in rinse users compared with females who have never used them. To date, no previous case-control or prospective cohort studies can provide a direct comparison for ours.
Historically, semipermanent hair dyes have utilized preoxidizing agents deposited on or in the cuticle of the hair shaft as the essential element of their formulations [33]; recently, some oxidants (usually hydrogen peroxide) have been used to manufacture modern semipermanent hair products, similar to permanent hair colorants, that deposit dyes on the cuticle of the hair shaft that then penetrate into the inner portion of the hair shaft [33], resulting in an increased risk to human health. Similarly, sodium hydroxide and thioglycolate, which lack carcinogenic effects in humans, were used as the main active ingredients in traditional straighteners, but the chemical compositions in newer straighteners have changed [47]. Although our work does not uncover positive links between semipermanent hair dyes or straighteners and breast cancer risk, we still recommend avoiding their use, as the publications included in our analysis span a long period, meaning that a combination of traditional and popular semipermanent hair dyes and straighteners were analyzed. Additionally, many current semipermanent hair dyes and straighteners may contain azo bonds that can release aromatic amines after being cleaved [46]. Therefore, future studies focusing on the relationship between modern semipermanent hair dyes and straighteners and breast cancer risk will need to be conducted.
The findings from our study indicate that the overall relationship between hair dyes and breast cancer risk does not vary with race, timing of use and dye color, which is consistent with the results of the Women’s Circle of Health Study (WCHS) [24]. Additionally, of note, the WCHS concludes that breast cancer risk linked to the use of dark hair dye shades in Blacks is higher than in Whites. One possible explanation for this is that patterns of hair dye use vary between Black and White females (the WCHS observed that Black women had a less frequent use of hair dyes but a more frequent use of dark hair dyes than White women). Moreover, the chemical composition of the dark hair dyes used by Whites may be different from those used by Blacks; for instance, the toxicological assessment of some dark hair dyes uncovered higher concentrations of estrogen and endocrine-disrupting compounds in those that were specifically sold to Black women [15], which might correlate with their increased breast cancer risk. Furthermore, dark shades of hair dye contain a large concentration of commercial oxidative agents that pose a greater risk for breast tumorigenesis in Black females [12].
Admittedly, there were some limitations to our work. First, only English publications were included, which might contribute to selection bias and publication bias; indeed, according to the judgment of the risk of bias graph, nearly 50% of included studies were at high risk of selection bias. If we changed the inclusion criteria to decrease the selection bias, it might impact our observed results. Second, since few studies assessed the correlation between the use of straighteners and breast cancer risk, only two qualified articles were included, which might lead to result bias. Third, some studies revealed that, in Black females, straightener users had a greater risk of developing estrogen receptor-negative (ER-) breast cancer than those who never used straighteners [15,24]; because this association was underdocumented and understudied, there was insufficient data for us to analyze. Finally, differences in the chemical formulations of hair care products, dyeing frequency and the breast cancer family history of analyzed subjects across all included studies might cause clinical heterogeneity.
Conclusion
The use of hair beauty products such as hair dyes, straighteners and rinses may be associated with an increased risk of developing breast cancer in women; therefore, natural hair is preferable to dyed hair with respect to public health. Further studies need to evaluate the relationships between popular semipermanent hair dyes and breast cancer risk and between straighteners and ER- breast cancer risk.
Supporting information
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
*Significant level: p<0.05. Abbreviations: ys, years.
(DOCX)
Abbreviations
- PPD
para-phenylenediamine
- 4-ABP
4-aminobiphenyl
- IARC
International Agency for Research on Cancer
- OR
odds ratio
- CI
confidence interval
- MH
Mantel-Haenszel
- ROS
reactive oxygen species
- WCHS
Women’s Circle of Health Study
- ER-
estrogen receptor-negative
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Zhang Y, Kim C, Zheng T : Hair dye use and risk of human cancer. Front Biosci (Elite Ed) 2012, 4:516–528. 10.2741/397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.La Vecchia C, Tavani A: Hair dyes and lymphoid neoplasms: an update. Eur J Cancer Prev 2002, 11:409–412. 10.1097/00008469-200210000-00001 [DOI] [PubMed] [Google Scholar]
- 3.IARC working group on the evaluation of carcinogenic risks to humans: occupational exposures of hairdressers and barbers and personal use of hair colourants; some hair dyes, cosmetic colourants, industrial dyestuffs and aromatic amines. Proceedings. Lyon, France, 6–13 October 1992. IARC Monogr Eval Carcinog Risks Hum 1993, 57:7–398. [PMC free article] [PubMed]
- 4.Heikkinen S, Pitkäniemi J, Sarkeala T, Malila N, Koskenvuo M: Does hair dye use increase the risk of breast cancer? A population-based case-control study of finnish women. PLoS ONE 2015, 10 10.1371/journal.pone.0135190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnett CM, Goldenthal EI: Multigeneration reproduction and carcinogenicity studies in Sprague-Dawley rats exposed topically to oxidative hair-colouring formulations containing p-phenylenediamine and other aromatic amines. Food Chem Toxicol 1988, 26:467–474. 10.1016/0278-6915(88)90059-2 [DOI] [PubMed] [Google Scholar]
- 6.Rojanapo W, Kupradinun P, Tepsuwan A, Chutimataewin S, Tanyakaset M: Carcinogenicity of an oxidation product of p-phenylenediamine. Carcinogenesis 1986, 7:1997–2002. 10.1093/carcin/7.12.1997 [DOI] [PubMed] [Google Scholar]
- 7.Ames BN, Kammen HO, Yamasaki E : Hair dyes are mutagenic: identification of a variety of mutagenic ingredients. Proc Natl Acad Sci U S A 1975, 72:2423–2427. 10.1073/pnas.72.6.2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takkouche B, Etminan M, Montes-Martinez A: Personal use of hair dyes and risk of cancer: a meta-analysis. Jama 2005, 293:2516–2525. 10.1001/jama.293.20.2516 [DOI] [PubMed] [Google Scholar]
- 9.Clausen T S-JA, Lang G, Schuh W, Liebscher KD, Springob C et al. : Hair preparations. In: Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim: 2006, 10.1002/14356007.a12_571pub2,:1–46. [DOI] [Google Scholar]
- 10.IARC working group on the evaluation of carcinogenic risks to humans, Organisation mondiale de la santé, Centre international de recherche sur le cancer, editors. Some aromatic amines, organic dyes, and related exposures. 5–12 february 2008. Lyon: IARC Press 2010. [PMC free article] [PubMed]
- 11.IARC Working Group Members4-Aminobiphenyl—IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 2010, Vol. 99. Available from: http://monographsiarcfr/ENG/Monographs/vol99/mono99-8pdf.
- 12.Turesky RJ, Freeman JP, Holland RD, Nestorick DM, Miller DW, Ratnasinghe DL, et al. : Identification of aminobiphenyl derivatives in commercial hair dyes. Chem Res Toxicol 2003, 16:1162–1173. 10.1021/tx030029r [DOI] [PubMed] [Google Scholar]
- 13.Stiel L, Adkins-Jackson PB, Clark P, Mitchell E, Montgomery S: A review of hair product use on breast cancer risk in African American women. Cancer Med 2016, 5:597–604. 10.1002/cam4.613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambrosone CB, Abrams SM, Gorlewska-Roberts K, Kadlubar FF: Hair dye use, meat intake, and tobacco exposure and presence of carcinogen-DNA adducts in exfoliated breast ductal epithelial cells. Arch Biochem Biophys 2007, 464:169–175. 10.1016/j.abb.2007.05.018 [DOI] [PubMed] [Google Scholar]
- 15.Eberle CE, Sandler DP, Taylor KW, White AJ: Hair dye and chemical straightener use and breast cancer risk in a large US population of black and white women. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. : Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015, 136:E359–386. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 17.Kinlen LJ, Harris R, Garrod A, Rodriguez K: Use of hair dyes by patients with breast cancer: a case-control study. Br Med J 1977, 2:366–368. 10.1136/bmj.2.6083.366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nasca PC, Baptiste MS, Field NA, Metzger BB, DeMartino R: An epidemiologic case-control study of breast cancer and exposure to hair dyes. Ann Epidemiol 1992, 2:577–586. 10.1016/1047-2797(92)90002-8 [DOI] [PubMed] [Google Scholar]
- 19.Stavraky KM, Clarke EA, Donner A: Case-control study of hair dye use by patients with breast cancer and endometrial cancer. J Natl Cancer Inst 1979, 63:941–945. [PubMed] [Google Scholar]
- 20.Green A, Willett WC, Colditz GA, Stampfer MJ, Bain C, Rosner B, et al. : Use of permanent hair dyes and risk of breast cancer. J Natl Cancer Inst 1987, 79:253–257. [PubMed] [Google Scholar]
- 21.Mendelsohn JB, Li QZ, Ji BT, Shu XO, Yang G, Li HL, et al. : Personal use of hair dye and cancer risk in a prospective cohort of Chinese women. Cancer Science 2009, 100:1088–1091. 10.1111/j.1349-7006.2009.01149.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wynder EL, Goodman M: Epidemiology of breast cancer and hair dyes. J Natl Cancer Inst 1983, 71:481–488. [PubMed] [Google Scholar]
- 23.Koenig KL, Pasternack BS, Shore RE, Strax P: Hair dye use and breast cancer: a case-control study among screening participants. Am J Epidemiol 1991, 133:985–995. 10.1093/oxfordjournals.aje.a115818 [DOI] [PubMed] [Google Scholar]
- 24.Llanos AAM, Rabkin A, Bandera EV, Zirpoli G, Gonzalez BD, Xing CY, et al. : Hair product use and breast cancer risk among African American and White women. Carcinogenesis 2017, 38:883–892. 10.1093/carcin/bgx060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gera R, Mokbel R, Igor I, Mokbel K: Does the Use of Hair Dyes Increase the Risk of Developing Breast Cancer? A Meta-analysis and Review of the Literature. Anticancer Res 2018, 38:707–716. 10.21873/anticanres.12276 [DOI] [PubMed] [Google Scholar]
- 26.Takkouche B, Regueira-Mendez C, Montes-Martinez A: Risk of cancer among hairdressers and related workers: a meta-analysis. Int J Epidemiol 2009, 38:1512–1531. 10.1093/ije/dyp283 [DOI] [PubMed] [Google Scholar]
- 27.Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, et al. : Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: the PRISMA-IPD Statement. Jama 2015, 313:1657–1665. 10.1001/jama.2015.3656 [DOI] [PubMed] [Google Scholar]
- 28.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. : The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj 2011, 343:d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutton AJ AK, Jones DR Methods for metaanalysis in medical research. Wiley series in probability and statistics-applied probability and statistics section. Wiley, Hoboken: 2000. [Google Scholar]
- 30.Shore RE, Pasternack BS, Thiessen EU, Sadow M, Forbes R, Albert RE: A case-control study of hair dye use and breast cancer. J Natl Cancer Inst 1979, 62:277–283. [PubMed] [Google Scholar]
- 31.Nasca PC, Lawrence CE, Greenwald P, Chorost S, Arbuckle JT, Paulson A: Relationship of hair dye use, benign breast disease, and breast cancer. J Natl Cancer Inst 1980, 64:23–28. [PubMed] [Google Scholar]
- 32.Dianatinasab M, Fararouei M, Mohammadianpanah M, Zare-bandamiri M, Rezaianzadeh A: Hair Coloring, Stress, and Smoking Increase the Risk of Breast Cancer: A Case-Control Study. Clinical Breast Cancer 2017, 17:650–659. 10.1016/j.clbc.2017.04.012 [DOI] [PubMed] [Google Scholar]
- 33.Cook LS, Malone KE, Daling JR, Voigt LF, Weiss NS: Hair product use and the risk of breast cancer in young women. Cancer Causes Control 1999, 10:551–559. 10.1023/a:1008903126798 [DOI] [PubMed] [Google Scholar]
- 34.Boice JD Jr., Mandel JS, Doody MM: Breast cancer among radiologic technologists. Jama 1995, 274:394–401. [PubMed] [Google Scholar]
- 35.Stavraky KM, Clarke EA, Donner A: A case-control study of hair-dye use and cancers of various sites. Br J Cancer 1981, 43:236–239. 10.1038/bjc.1981.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng T, Holford TR, Mayne ST, Owens PH, Boyle P, Zhang B, et al. : Use of hair colouring products and breast cancer risk: a case-control study in Connecticut. Eur J Cancer 2002, 38:1647–1652. 10.1016/s0959-8049(02)00138-7 [DOI] [PubMed] [Google Scholar]
- 37.Petro-Nustas W, Norton ME, Al-Masarweh I: Risk factors for breast cancer in Jordanian women. Journal of Nursing Scholarship 2002, 34:19–25. 10.1111/j.1547-5069.2002.00019.x [DOI] [PubMed] [Google Scholar]
- 38.Hennekens CH, Speizer FE, Rosner B, Bain CJ, Belanger C, Peto R: Use of permanent hair dyes and cancer among registered nurses. Lancet 1979, 1:1390–1393. 10.1016/s0140-6736(79)92021-x [DOI] [PubMed] [Google Scholar]
- 39.Seydi E, Fatahi M, Naserzadeh P, Pourahmad J: The effects of para-phenylenediamine (PPD) on the skin fibroblast cells. Xenobiotica 2019, 49:1143–1148. 10.1080/00498254.2018.1541264 [DOI] [PubMed] [Google Scholar]
- 40.Reena K, Ng KY, Koh RY, Gnanajothy P, Chye SM: para-Phenylenediamine induces apoptosis through activation of reactive oxygen species-mediated mitochondrial pathway, and inhibition of the NF-kappaB, mTOR, and Wnt pathways in human urothelial cells. Environ Toxicol 2017, 32:265–277. 10.1002/tox.22233 [DOI] [PubMed] [Google Scholar]
- 41.He J, Sunarso J, Miao J, Sun H, Dai J, Zhang C, et al. : A highly sensitive perovskite oxide sensor for detection of p-phenylenediamine in hair dyes. J Hazard Mater 2019, 369:699–706. 10.1016/j.jhazmat.2019.02.070 [DOI] [PubMed] [Google Scholar]
- 42.Liu B, Jin SF, Li HC, Sun XY, Yan SQ, Deng SJ, et al. : The Bio-Safety Concerns of Three Domestic Temporary Hair Dye Molecules: Fuchsin Basic, Victoria Blue B and Basic Red 2. Molecules 2019, 24 10.3390/molecules24091744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Preston RJ, Skare JA, Aardema MJ: A review of biomonitoring studies measuring genotoxicity in humans exposed to hair dyes. Mutagenesis 2010, 25:17–23. 10.1093/mutage/gep044 [DOI] [PubMed] [Google Scholar]
- 44.Lizier TMZ T.B.; de Oliveria D.P.; Zanoni M.V.B: Electrochemicalreduction as a powerful tool to highlight the possible formation of by-productsmore toxic than Sudan III dye. Int J Electrochem Sci 2012, 7:7784–7796. [Google Scholar]
- 45.Liu H, Chen J, Jiang J, Giesy JP, Yu H, Wang X: Cytotoxicity of HC Orange NO. 1 to L929 fibroblast cells. Environ Toxicol Pharmacol 2008, 26:309–314. 10.1016/j.etap.2008.06.002 [DOI] [PubMed] [Google Scholar]
- 46.Zanoni TB, Tiago M, Faiao-Flores F, de Moraes Barros SB, Bast A, Hageman G, et al. : Basic Red 51, a permitted semi-permanent hair dye, is cytotoxic to human skin cells: Studies in monolayer and 3D skin model using human keratinocytes (HaCaT). Toxicol Lett 2014, 227:139–149. 10.1016/j.toxlet.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 47.Rosenberg L, Boggs DA, Adams-Campbell LL, Palmer JR: Hair relaxers not associated with breast cancer risk: evidence from the black women's health study. Cancer Epidemiol Biomarkers Prev 2007, 16:1035–1037. 10.1158/1055-9965.EPI-06-0946 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
*Significant level: p<0.05. Abbreviations: ys, years.
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.