Abstract
The taxonomic relationship of Lentzea atacamensis and Lentzea deserti were re-evaluated using comparative genome analysis. The 16S rRNA gene sequence analysis indicated that the type strains of L. atacamensis and L. deserti shared 99.7% sequence similarity. The digital DNA-DNA hybridization (dDDH) and average nucleotide identity (ANI) values between the genomes of two type strains were 88.6% and 98.8%, respectively, greater than the two recognized thresholds values of 70% dDDH and 95–96% ANI for bacterial species delineation. These results suggested that L. atacamensis and L. deserti should share the same taxonomic position. And this conclusion was further supported by similar phenotypic and chemotaxonomic features between them. Therefore, we propose that L. deserti is a later heterotypic synonym of L. atacamensis.
Introduction
The genus Lentzea was proposed by Yassin et al. in 1995 [1] and was subsequently emended by Lee et al. [2] and Fang et al. [3]. The strains of this genus form abundant aerial hyphae that fragment into rod-shaped elements. Whole-cell hydrolysates contain meso-diaminopimelic acid as diagnostic diamino acid and MK-9 (H4) was the predominant menaquinone; The G+C content of genomic DNA ranges from 64.1 to 71.0 mol%. According to the List of LPSN (https://lpsn.dsmz.de/genus/lentzea), there are currently over 20 species of the genus Lentzea with validly published names. Lentzea species are distributed in different habitats, such as human pathological tissue [1], an equine placenta [4], a limestone [5] and different soils [6–8]. As separate genomic species, Lechevalieria atacamensis and Lechevalieria deserti were isolated from hyperarid soils of the Atacama Desert and proposed in 2010 as two validly named species based on a polyphasic taxonomic approach [9]. In 2018, Lechevalieria atacamensis and Lechevalieria deserti were transferred to the genus Lentzea from the genus Lechevalieria based on comparative genome analysis [10]. However, our research showed that the two species belong to the same genome species. The aim of the present study was to clarify the relationship between the type strains of L. atacamensis and L. deserti based on genomic and associated phenotypic data.
Materials and methods
Phenotypic characterization
L. atacamensis CGMCC 4.5536T (= C61T = DSM 45479T) and L. deserti CGMCC 4.5535T (= C68T = DSM 45480T), were purchased from the CGMCC (China General Microbiological Culture Collection Center). The cultural properties of L. atacamensis CGMCC 4.5536T and L. deserti CGMCC 4.5535T were observed on various media including Gause’s synthetic No. 1 medium [11] and ISP 2–7 media as described by Shirling and Gottlieb [12]. The color of aerial hyphae, substrate mycelia and soluble pigments were determined by the Color Standards and Color Nomenclature [13]. Growth at different temperature, pH range and NaCl tolerance was tested on ISP2 agar medium for 14 days according to the method described in the literature [12]. Carbon source utilization tests and enzyme activities were performed using the Biolog GEN III MicroPlates (Biolog, USA) and API ZYM system (bioMérieux, France) according to the manufacturer’s instructions. Other biochemical tests such as hydrolysis of aesculin, starch and urea, hydrogen sulfide production and nitrate reduction, etc. were carried out according to the methods described by Xu et al. [14]. The isomer of diaminopimelic acid and sugar analysis of whole-cell hydrolysates were performed according to the procedures described by Hasegawa et al. [15] and Lechevalier and Lechevalier [16]. Menaquinones were extracted according to the method of Collins et al. [17] and analyzed by HPLC [18]. The experiments were carried out in triplicate. Whole genome sequences of the type strains of L. atacamensis and L. deserti are available from the GenBank database. Their genomic data such as GenBank assembly accessions, genomic lengths, number of contigs, contig N50 and DNA G+C contents, etc., are presented in Table 1.
Table 1. Genomic data of L. atacamensis DSM 45479T (= CGMCC 4.5536T = C61T) and L. deserti DSM 45480T (= CGMCC 4.5535T = C68T).
| Strain | L. deserti DSM 45480T | L. atacamensis DSM 45479T |
|---|---|---|
| Assembly accession | GCA_003148865.1 | GCA_003269295.1 |
| Genome size (bp) | 9,529,573 | 9,306,230 |
| No. of contigs | 50 | 38 |
| Contig N50 (bp) | 442,485 | 785,641 |
| G+C content (mol%) | 68.8 | 68.9 |
| Protein coding genes | 9221 | 9075 |
| RNA genes | 86 | 90 |
| rRNA genes | 8 | 12 |
| 5S rRNA | 6 | 6 |
| 16S rRNA | 1 | 5 |
| 23S rRNA | 1 | 1 |
| tRNA genes | 70 | 70 |
| Other RNA genes | 8 | 8 |
| Protein coding genes with function prediction | 6780 | 6635 |
| without function prediction | 2441 | 2440 |
| Protein coding genes with enzymes | 1589 | 1569 |
Phylogenetic analysis and genomic DNA-DNA correlation analysis
The phylogenetic analysis of L. atacamensis C61T and L. deserti C68T was carried out using the Type (Strain) Genome Server (https://tygs.dsmz.de/) [19]. The average nucleotide identity (ANI) analysis was used for evaluating the genetic relationship between L. atacamensis C61T and L. deserti C68T by using the orthoANIu algorithm and an online ANI calculator (www.ezbiocloud.net/tools/ani) [20, 21]. At the same time, the digital DNA-DNA hybridization (dDDH) value between two genome sequences was calculated by the online Genome-to-Genome Distance Calculator (http://ggdc.dsmz.de/distcalc2.php) [22].
Results and discussion
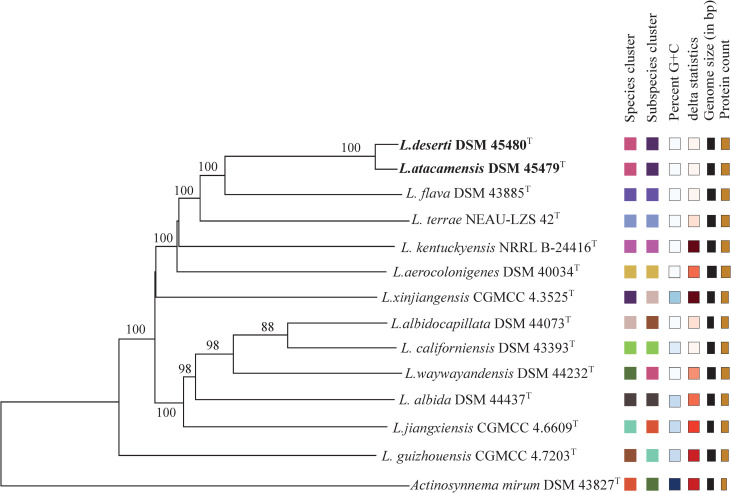
The phylogenomic analysis showed that L. deserti C68T was most closely related to L. atacamensis C61T (Fig 1), confirming that they should belong to the same genomic species. This result further suggested that the phylogenetic analysis based on whole genome sequences exhibited better resolution than the phylogenetic analysis based on 16S rRNA gene sequence [23]. ANI analysis indicated that L. atacamensis C61T and L. deserti C68T exhibited 98.8% ANI value, greater than the 95–96% threshold for species demarcation [24], confirming that they have the same taxonomic position. Meanwhile, L. atacamensis C61T and L. deserti C68T had an 88.6% dDDH value, greater than the 70% threshold for species demarcation, confirming that they belong to the same species [25]. In addition, comparative phenotypic characteristics of L. atacamensis CGMCC 4.5536T and L. deserti CGMCC 4.5535T are presented in Table 2 and S1 Fig. As shown in Table 1 and S1 Fig, most features between them were almost identical. For example, they were produced white aerial mycelium on ISP 3, ISP 4 and ISP 5. In Biolog GENIII test, positive for growth on acetoacetic acid, acetic acid, acid Methyl Ester, aztreonam, etc.; And negative for growth on d-aspartic acid, d-fructose-6-PO4, d-fucose etc. They both contained meso-DAP in the cell-wall, and galactose, mannose and rhamnose as the whole-cell sugar. The major menaquinones of strains consisted of MK-9 (H4) (Table 1). At the same time, the genomes size of strain L. atacamensis DSM 45479T is 9,306,230 bp in 38 of contigs. 9075 protein coding genes, 90 rRNA genes, 6635 protein coding genes, 2440 without function prediction and 1569 protein coding genes with enzymes were predicted. While the genomes size of strain L. deserti DSM 45480T is 9,529,573 bp in 50 of contigs. 9221 protein coding genes, 86 rRNA genes, 6780 protein coding genes, 2442 without function prediction and 1589 protein coding genes with enzymes were predicted (Table 2). Consequently, we propose that L. deserti C68T is a later heterotypic synonym of L. atacamensis C61T based on the results above and rule 42 of the Bacteriological Code [26].
Fig 1. Phylogenetic tree based on whole genome sequences of L. atacamensis DSM 45479T, L. deserti DSM 45480T and related reference strains.
Tree inferred with FastME 2.1.6.1 [27] from GBDP distances calculated from genome sequences. The branch lengths are scaled in terms of GBDP distance formula d5. The numbers above branches are GBDP pseudo-bootstrap support values >60% from 100 replications, with an average branch support of 96.0%. The tree was rooted at the midpoint [28].
Table 2. Phenotypic characteristics between L. atacamensis CGMCC 4.5536T and L. deserti CGMCC 4.5535T.
| Characteristic | 1 | 2 |
|---|---|---|
| Aerial mycelium on Gause’s synthetic No. 1 | TB | TB |
| Aerial mycelium on ISP 2 | AY | WAAY |
| Aerial mycelium on ISP 3, 4 and 5 | WH | WH |
| Substrate mycelium on ISP 2, 3, 4 and 5 | YO | YO |
| Melanin or diffusible pigments on ISP 6 and 7 | – | – |
| aAcid production from: | ||
| Adonitol, erythritol, melezitose, methyl, α-d-glucoside | – | + |
| Inulin, lactose, mannitol, mannose, rhamnose, salicin, trehalose | + | + |
| API ZYM test | ||
| Acid phosphatase, alkaline phosphatase, cystine arylamidase, esterase (C4) | + | + |
| Esterase lipase (C8), trypsin, leucine arylamidase, lipase (C14) | + | + |
| N-acetyl-β-glucosaminidase, valine arylamidase, α-chymotrypsin, α-mannosidase | + | + |
| α-galactosidase, α-glucosidase, β-galactosidase, β-glucosidase | + | + |
| naphtol-AS-BI-phosphohydrolase, β-glucuronidase | W | W |
| α-fucosidase | – | – |
| BiologGEN III test | ||
| Acetoacetic acid, acetic acid, aztreonam, bromo-succinic acid, d-arabitol, d-cellobiose, dextrin, d-fructose, d-galactose, d-gluconic acid, d-glucuronic acid, d-lactic acid, d-maltose, d-mannitol, d-mannose, d-melibiose, d-malic acid, d-raffinose, d-salicin, d-serine, d-trehalose, d-turanose, gentiobiose, gelatin, glycerol, glycyl-l-proline, inosine, lactate, l-fucose, myo-inositol, l-alanine, l-arginine, l-glutamic acid, l-malic acid, l-serine, l-histidine, lithium chloride, l-pyroglutamic acid, l-rhamnose, l-aspartic acid, methyl pyruvate, N-acetyl-d-glucosamine, nalidixic acid, propionic acid, sodium bromate, sucrose, stachyose, α-d-glucose, α-d-lactose, 1% sodium citric acid, Tween 40, α-keto-glutaric acid, γ-amino-butryric acid, β-hydroxy-d, l-butyric acid. | + | + |
| d-aspartic acid, d-fructose-6-PO4, d-fucose, d-serine, d-sorbitol, formic acid, l-galactonic acid lactone, l-lactic acid, minocycline, N-acetyl-β-d-mannosamine, N-acetyl-d-galactosamine, N-acetyl neuraminic acid, 3-methyl glucose, fusidic acid d-galacturonic acid, d-sorbitol, d-glucose-6-PO4, guanidine HCl, niaproof 4, quinic acid, d-saccharic acid, sodium butyrate, tetrazolium violet, tetrazolium blue, troleandomycin, p-hydroxy-phenylacetic acid, α-hydroxy-butyric acid, β-methyl-d-glucoside | – | – |
| Lincomycin | + | – |
| Acid Methyl Ester, pectin | W | W |
| Glucuronamide, α-keto-butyric acid | + | W |
| Mucic acid, vancomycin | W | – |
| Potassium tellurite | W | + |
| Hydrolysis of starch, aesculin and allantoin | + | + |
| H2S production, nitrate reduction and urea hydrolysis | – | – |
| a Decomposition of: arbutin, uric acid | – | + |
| a Decomposition of: casein, elastin, hypoxanthine, tyrosine, xylan | + | + |
| Growth at/in: 4% NaCl (w/v), 20–40°C and pH 5.0–11.0 | + | + |
| Growth at/in: 0.1% Methyl violet (w/v) | – | – |
| a Major fatty acids (%) | ||
| iso-C16:0 | 23.1 | 24.5 |
| C16:0 | 9.9 | 10.8 |
| anteiso-C15:0 | 9.3 | 9.7 |
| anteiso-C17:0 | 8.5 | 8.4 |
| iso-C15:0 | 6.6 | 7.5 |
| C16:1 | 6.5 | 5.8 |
| iso-C14:0 | 3.5 | 3.5 |
| iso-C17:0 | 1.8 | 2.8 |
| C17:0 | 1.5 | ND |
| Predominant menaquinones | MK-9(H4) | MK-9(H4) |
| Cell-wall diamino acid | mes-DAP | mes-DAP |
| Whole-cell sugars | G, M, R | G, M, R |
Note:
a, data are from Okoro et al. [9].
Note: 1, L. atacamensis CGMCC 4.5536T; 2, L. deserti CGMCC 4.5535T. All data are from this study except where specified; +, Positive reaction;–, negative reaction; W, weak reaction; ND, Not detected; TB, Tilleul-buff; AY, Antimony yellow; YO, Yellow ocher; WAAY, White and Antimony yellow; WH, White; G, galactose; M, mannose; R, rhamnose.
Emended description of Lentzea atacamensis (Okoro et al. 2010) Nouioui et al. 2018
The description is as before (Okoro et al. 2010 and Nouioui et al. 2018) with the following additions. Tilleul-buff aerial mycelia and olive-buff massicot yellow substrate mycelia are produced on Gause’s synthetic agar medium at 28°C for 21 days. Positive for acid phosphatase, alkaline phosphatase, cystine arylamidase, esterase (C4), esterase lipase (C8), trypsin, leucine arylamidase, lipase (C14), N-acetyl-β-glucosaminidase, naphtol-AS-BI-phosphohydrolase, valine arylamidase, α-chymotrypsin, α-mannosidase, α-galactosidase, α-glucosidase, β-galactosidase, β-glucosidaseβ-glucuronidase; but negative for α-fucosidase. The cell wall contains meso-DAP. Whole-cell sugars are galactose, mannose and rhamnose. The predomiant menaquinones is MK-9 (H4). The DNA G+C content of the genome sequence, consisting of 19306230 bp, is 68.9 mol%.
The type strain is CGMCC 4.5536 (= C61 = NRRL B-24706 = JCM 17492 = DSM 45479).
Supporting information
(DOCX)
Acknowledgments
We sincerely thank MCCC (Marine Culture Collection of China) for providing excellent technical assistance.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by the State Forestry and Grassland Bureau (2018-01 to ZYL), Major Science and Technology Program of Hunan Province (2017NK1014 to ZYL), Forestry Science and Technology Project of Hunan Province (XLK201825 to ZYL), Key Technology R&D Program of Changsha (kq190114 to ZYL) and Natural Science Foundation of Hunan Province (2019JJ50027 to XZG). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Yassin AF, Rainey FA, Brzezinka H, Jahnke KD, Weissbrodt H, Budzikiewicz H, et al. Lentzea gen. nov., a New Genus of the Order Actinomycetales. Int J Syst Evol Microbiol. 1995; 45(2): 357–363. 10.1099/00207713-45-2-357 [DOI] [PubMed] [Google Scholar]
- 2.Lee SD, Kim ES, Roe JH, Kim J, Kang SO, Hah YC. Saccharothrix violacea sp. nov., isolated from a gold mine cave, and Saccharothrix albidocapillata comb. nov. Int J Syst Evol Microbiol. 2000; 50(Pt3): 1315–1323. 10.1099/00207713-50-3-1315 [DOI] [PubMed] [Google Scholar]
- 3.Fang B-Z, Han M-X, Liu L, Zhang Z-T, Liu W-L et al. Lentzea cavernae sp. nov., an actinobacterium isolated from a karst cave sample, and emended description of the genus Lentzea. Int J Syst Evol Microbiol. 2017; 67(7): 2357–2362. 10.1099/ijsem.0.001958 [DOI] [PubMed] [Google Scholar]
- 4.Labeda DP, Hatano K, Kroppenstedt RM, Tamura T. Lentzea kentuckyensis sp. nov., of equine origin. Int J Syst Evol Microbiol. 2007; 57(Pt 8): 1780–1783. 10.1099/ijs.0.64245-0 [DOI] [PubMed] [Google Scholar]
- 5.Cao C, Yuan B, Qin S, Jiang J, Lian B. Lentzea pudingi sp. nov., isolated from a weathered limestone sample in a karst area. Int J Syst Evol Microbiol. 2017; 67(11): 4873–4878. 10.1099/ijsem.0.002400 [DOI] [PubMed] [Google Scholar]
- 6.Li X, Zhang L, Ding Y, Gao Y, Ruan J, Huang Y. Lentzea jiangxiensis sp. nov., isolated from acidic soil. Int J Syst Evol Microbiol. 2012; 62(10): 2342–2346. 10.1099/ijs.0.033795-0 [DOI] [PubMed] [Google Scholar]
- 7.Li Dongmei, Zheng Weiwei, Zhao Junwei, Han Liyuan, Zhao Xueli, Jiang Hao, et al. Lentzea soli sp. nov., an actinomycete isolated from soil. Int J Syst Evol Microbiol. 2018; 68: 1496–1501. 10.1099/ijsem.0.002698 [DOI] [PubMed] [Google Scholar]
- 8.Li Dongmei, Jiang Hao, Han Liyuan, Li Yuanyuan, Zhao Junwei, Jiang Shanwen et al. Lentzea terrae sp. nov., isolated from soil and an emended description of lentzea soli. Int J Syst Evol Microbiol. 2018; 68(11): 3528–3533. 10.1099/ijsem.0.003024 [DOI] [PubMed] [Google Scholar]
- 9.Okoro CK, Bull AT, Mutreja A, Rong X, Huang Y, Goodfellow M. Lechevalieria atacamensis sp. nov., Lechevalieria deserti sp. nov., and Lechevalieria roselyniae sp. nov., isolated from hyperarid soils. Int J Syst Evol Microbiol. 2010; 60(2): 296–300. [DOI] [PubMed] [Google Scholar]
- 10.Nouioui I, Carro L, García-López M, Meier-Kolthoff JP, Woyke T, Kyrpides NC, et al. Genome-Based Taxonomic Classification of the Phylum Actinobacteria. Front Microbiol. 2018; 9: 1–119. 10.3389/fmicb.2018.02007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atlas R.M., Parks L.C(editor). Handbook of microbiological media: CRC Press; 1993. [Google Scholar]
- 12.Shirling EB, Gottlieb D. Methods for characterization of Streptomyces species. Int J Syst Bacteriol. 1966; 16(3): 313–340. [Google Scholar]
- 13.Ridgway, R. Color standards and color nomenclature. Published by the author. Washington, DC. 1912; 1–43.
- 14.Xu LH, Li WJ, Liu ZH, Jiang CL. Actinomycete Systematics: Principle, Methods and Practice: Beijing: Science press; 2007. [Google Scholar]
- 15.Hasegawa T, Takizawa M, Tanida S. A rapid analysis for chemical grouping of aerobic actinomycetes. J Gen Appl Microbiol. 1983; 29(4): 319–322. [Google Scholar]
- 16.Lechevalier MP, Lechevalier H. Chemical composition as a criterion in the classification of aerobic actinomycetes. Int J Syst Bacteriol.1970; 20(4): 435–443. 10.1016/s0065-2164(08)70539-2 [DOI] [PubMed] [Google Scholar]
- 17.Collins MD, Pirouz T, Goodfellow M, Minnikin DE. Distribution of Menaquinones in Actinomycetes and Corynebacteria [J]. J Gen Microbiol. 1977, 100(2): 221–230. 10.1099/00221287-100-2-221 [DOI] [PubMed] [Google Scholar]
- 18.Kroppenstedt RM. Fatty acid and menaquinone analysis of actinomycetes and related organisms In: Goodfellow M and London Minnikin DE (editors). Chemical Methods in Bacterial Systematics. England: Academic Press; 1985; pp. 173–199. [Google Scholar]
- 19.Meier-Kolthoff JP, Göker M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat Commun. 2019; 10(1): 2182 10.1038/s41467-019-10210-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee I, Ouk Kim Y, Park SC, Chun J. OrthoANI: An improved algorithm and software for calculating average nucleotide identity. Int J Syst Evol Microbiol. 2016; 66(2): 1100–1103. 10.1099/ijsem.0.000760 [DOI] [PubMed] [Google Scholar]
- 21.Yoon SH, Ha SM, Lim J, Kwon S, Chun J. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie van Leeuwenhoek. 2017; 110(10): 1281–1286. 10.1007/s10482-017-0844-4 [DOI] [PubMed] [Google Scholar]
- 22.Meier-Kolthof J.P., Auch A.F., Klenk H. P., Goker M., (2013). Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics. 2013, 14(1): 60–60. 10.1186/1471-2105-14-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez RL, Gunturu S, Harvey WT, Rossello-Mora R, Tiedje JM, Cole JR, et al. The Microbial Genomes Atlas (MiGA) webserver: taxonomic and gene diversity analysis of Archaea and Bacteria at the whole genome level. Nucleic Acids Res. 2018; 46(W1): W282–W288. 10.1093/nar/gky467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richter M, Rosselló-Móra R. Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci U S A. 2009; 106(45): 19126–31. 10.1073/pnas.0906412106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wayne LG, Brenner DJ, Colwell RR, Grimont PAD, Kandler O, Krichevsky MI, et al. Report of the Ad Hoc Committee on Reconciliation of Approaches to Bacterial Systematics. Int J Syst Bacteriol.1987; 37(4): 463–464. [Google Scholar]
- 26.Garrity GM, Parker CT, Tindall BJ. International Code of Nomenclature of Prokaryotes. Int J Syst Evol Microbiol. 2019; 69(1A): S1–S111. 10.1099/ijsem.0.000778 [DOI] [PubMed] [Google Scholar]
- 27.Vincent L, Richard D, Olivier G. FastME 2.0. FastME 2.0 a comprehensive, accurate, and fast distance-based phylogeny inference program. Mol Biol Evol. 2015; 32(10): 2798–2800. 10.1093/molbev/msv150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farris JS. Estimating Phylogenetic Trees from Distance Matrices. Am Nat. 1972; 106(951): 645–668. [Google Scholar]