Abstract
Background:
Diagnosis of engraftment syndrome (ES) following allogeneic hematopoietic stem cell transplantation (HSCT) can be a challenge due to the systemic presentation and alternative etiologies. With a goal of establishing biomarkers to more accurately distinguish ES, we prospectively analyzed levels of cytokines during HSCT.
Procedures:
We performed a prospective study of children ≤21 years who underwent allogeneic HSCT. Blood samples for interleukin (IL)-6, IL-8, IL-10, IL-1b, IL-12p70, interferon-γ, tumor necrosis factor alpha (TNF-α) and procalcitonin were obtained from each subject prior to conditioning, at day 0, and then biweekly through engraftment and at days 30, 60 and 100. Patients were evaluated for ES, infection and acute graft-versus-host disease. Cytokines were analyzed by values at engraftment, and also compared to pre-conditioning and day 0 values to evaluate for change from baseline.
Results:
A total of 30 subjects (median age: 7 years,min.-max.: 1-21 years) were enrolled of whom 5 had ES. Characterization of the cytokine profile revealed differences between day 0 from pre-HSCT, with a trend towards differences in IL-10, IL-12p70, interferon-γ and TNF-α at the time of ES. For IL8 and procalcitonin, there was evidence that the absolute difference (or fold change) between engraftment and pre-conditioning or day 0 differed according to ES. In particular, procalcitonin increased from baseline (15.1 median fold increase in ES+ versus 2.31 median fold increase in ES−, P = 0.0006, median difference: 13.8, 95% confidence interval: 6.33, 65.6).
Conclusions:
Our data provide one of the first prospective studies evaluating cytokines in pediatric allogeneic HSCT and suggest that elevated procalcitonin may serve as a biomarker for ES. Further studies to evaluate this finding are warranted.
Keywords: engraftment syndrome, pediatric, procalcitonin, stem cell transplantation
1 ∣. INTRODUCTION
Engraftment syndrome (ES) following allogeneic hematopoietic stem cell transplantation (HSCT) can be a diagnostic challenge given the systemic presentation that can mimic a host of other etiologies, with symptoms overlapping with infection or hyperacute graft-versus-host disease (GVHD).1,2 With a reported incidence ranging from 7% to 90%,1,2 the diagnosis of ES is based on a set of clinical findings, separated into major and minor criteria (Table 1),1 that include non-infectious fever, erythematous skin rash, capillary leak, as well as impairment of pulmonary, hepatic and renal function.1,2 It can remain as a mild, self-limited illness or progress to a more serious complication, leading to multi-organ failure and death.3 The difficulty in distinguishing ES from other etiologies limits the ability to tailor and may potentially delay necessary therapy, while early recognition of ES and prompt initiation of therapy with steroids may limit symptoms and severity, leading to improved outcomes post-transplant.
TABLE 1.
Diagnostic criteria for engraftment syndrome
| Major criteria | Temperature ≥ 38.3 °C with no identifiable infectious etiology |
| Erythrodermatous rash involving > 25% of body surface area and not attributable to a medication | |
| Noncardiogenic pulmonary edema, manifested by diffuse pulmonary infiltrates consistent with this diagnosis, and hypoxia | |
| Minor criteria | Hepatic dysfunction with either bilirubin ≥ 2 mg/dL or transaminase levels ≥ 2 times normal |
| Renal insufficiency (serum creatinine) ≥ 2 times baseline | |
| Weight gain ≥ 2.5% of baseline body weight | |
| Transient encephalopathy unexplainable by other causes |
Diagnosis: all three major criteria or two major criteria and one or more minor criterion, within 96 hours of engraftment.
Although the pathophysiology of ES is poorly understood, it has been hypothesized that proinflammatory cytokines such as interleukin-1 (IL-1), tumor necrosis factor alpha (TNF-α) and interferon gamma (IFN-γ), amongst others, are released during neutrophil recovery, which may play a role in the development of ES.1 The production and release of these cytokines can lead to local and systemic tissue injury causing the signs and symptoms mentioned above. Characterization of the cytokine profile in transplantation, particularly in ES, is limited.4 Based on the cytokine profiles in patients with ES, if biomarkers for ES could be established, then these markers could potentially be used to facilitate an early diagnosis and shorten the time to treatment initiation with steroids, the typical agent used in severe ES. Importantly, it would be optimal to identify these cytokine elevations early, prior to the development of severe ES. In addition, given the number of anti-cytokine targeted therapies in clinical use (e.g., etanercept to target TNF-α or tocilizumab to target IL-6) if specific cytokines are known to be elevated in ES, targeted agents may more selectively be used to treat ES, limiting toxicity of systemic steroids.
In addition to standard cytokines, procalcitonin is another inflammatory biomarker that has been used to help identify patients with systemic infection, and may be particularly useful in discriminating fever due to infection versus fever from non-infectious etiologies.5-7 As a 116-amino-acid peptide typically produced in the thyroid, systemic production is substantially increased in the setting of microbial infection8,9 and higher levels may be associated with more severe disease or sepsis.10 Evaluation of procalcitonin in the setting of allogeneic HSCT is limited,11-13 with no data regarding its role in the setting of ES. A clinical utility of using procalcitonin in oncology in general is demonstrated.14-17
On the basis of the potential clinical utility in being able to accurately diagnose ES and given the limited analysis of biomarkers for ES, particularly in children, we sought to systematically and prospectively evaluate for biomarkers predictive for ES in children undergoing transplant. With the recent advances in laboratory techniques, it is now possible to measure multiple cytokines from small samples of blood making a study in the pediatric population more feasible. With a goal of evaluating the correlation of specific cytokine levels with the diagnosis of ES to potentially decrease time to treatment and therefore decrease morbidity and mortality associated with ES, we systematically evaluated levels of inflammatory cytokines (including IL-6, IL-8, IL-10, IL-1b; IL-12p70; IFN-γ, TNF-α and procalcitonin) in the early post-transplant period leading up to engraftment and followed through day 100 post-transplant in a cohort of pediatric transplant recipients. Our data, based on a limited and heterogeneous pediatric transplant population, characterize the cytokine profiles during transplant and demonstrate a potential utility of using procalcitonin as predictive of ES.
2 ∣. METHODS
2.1 ∣. Patients and inclusion criteria
This was a prospective study of children ≤21 years old who under-went allogeneic HSCT at Children’s National Medical Center (CNMC) between September 2009 and August 2012 and provided informed consent or parental permission for enrollment in the study. The CNMC Institutional Review Board approved this protocol. Subjects eligible for the study included any pediatric subject who proceeded to allogeneic transplantation,with only exclusion of children who weighed ≤5 kg due to the limitations on the ability to obtain sufficient serial blood quantities due to restrictions on pediatric blood volume draws for research. Clinical data collected on all study participants included conditioning regimens, GVHD prophylaxis, day of engraftment and development of transplant-related complications such as infection, ES or acute GVHD. Information was stored in a secure database.
2.2 ∣. Definitions
ES was defined by the first day of the presence of all three major criteria, or two major criteria and at least one minor criterion within 96 hours of neutrophil engraftment (absolute neutrophil count ≥ 500/μL for 2 days), as defined by Spitzer (Table 1).1 All subjects were also monitored for the development of GVHD and infection. Acute GVHD was graded on a modified Glucksburg Scale.18 Serial blood cultures were obtained as needed in febrile patients based on standard clinical guidelines.
2.3 ∣. Laboratory assessments
Blood samples were obtained from each enrolled subject through a central venous device at the following time points: prior to initiation of conditioning regimen (Pre or −7 days); day 0 (day of stem cell infusion); biweekly through engraftment and at days 30, 60 and 100. Approximately 2–3 mm (0.4–0.6 teaspoons) of blood was withdrawn from the patient and placed in a red top tube for each scheduled blood draw. Serum was collected after centrifugation and samples were stored in 300–500 μL aliquots. Cytokines evaluated included IL-6, IL-8, IL-10, IL-1b; IL-12p70; IFN-γ, TNF-α; and procalcitonin. Serum cytokines were measured using a multiplex assay using a commercially available Th1/Th2 platform as per the manufacturer’s instructions (Mesoscale, Rockville, MD). Procalcitonin levels were analyzed from frozen serum using a Brahms Kryptor machine (Thermo Scientific, Berlin, Germany), which was granted FDA approval for this analysis in 2008.
2.4 ∣. Statistical analysis
All cytokine and procalcitonin data were tabulated longitudinally per subject and time point. Time periods included the following: Pre, day (D) 0, D3-4, D7-8, D11-12, D15-16, D19-20, engraftment, D30, D60 and D100. For each of the cytokine and procalcitonin variables, two types of analyses were performed.
First, a variable was created that included the maximum value for each patient following day 0 through day 30, or until the day of established ES, whichever came first. Subjects were subsequently stratified by the absence or presence of GVHD, ES and infection, and these groups were used to create dichotomous categorical outcome variables. Some variables were missing as many as 12 values. For two group comparisons of continuous data (e.g., for those who developed ES versus those who did not), the Wilcoxon rank sum test was used. Exact tests were used as appropriate. For the procalcitonin variable, two dichotomous variables were created based on the cut-off point of 0.5 or 2.0, which have been established in the critical care setting as reliable cut-off points for diagnosis of infection or sepsis.19-21 The two dichotomous procalcitonin variables were crossed with the three dichotomous outcome variables mentioned above and Fisher’s exact test was used to test for a general association.
Second, an analysis to examine the differences between engraftment and both prior conditioning and the day of stem cell infusion, as well as between prior conditioning and day of stem cell infusion, was performed. Both absolute differences (later period – earlier period) and fold changes (later period/earlier period) were analyzed for each cytokine and procalcitonin. Thus, six new variables (three absolute differences and three fold changes) were created for each of the cytokines and procalcitonin. On these newly created out-come variables, two types of analyses were performed. First, a one-sample test using the Wilcoxon signed rank test was performed with the null hypothesis that the median absolute difference and log fold change (log(later/earlier) = log(1)) between the time points was 0 in each case. Second, a comparison of the distributions of absolute difference or fold changes according to one of the three classification variables (ES, infection and GVHD) was performed using the Wilcoxon rank sum test. Prior to analysis, values of zero were replaced with 0.015, based on the reported lower limit of detection of 0.020 for the assay, in order to avoid division by zero and better estimate absolute differences.
Logistic modeling was used to estimate the sensitivity and specificity of procalcitonin fold change from pre-treatment to engraftment relative to a diagnosis of ES.
All P-values were two-tailed, and in view of the exploratory nature of the analysis and the large number of tests performed, statistical significance was set at P < 0.005, while those for which 0.005 < P < 0.05 were considered trends. Confidence intervals (CI, 95%) are provided to aid interpretation of the data. Statistical analysis was performed using SAS® software.
3 ∣. RESULTS
3.1 ∣. Patient characteristics
A total of 30 subjects, with a median age of 7 years (minimum–maximum, 1–21 years), were enrolled in the study. Patient characteristics are shown in Table 2. There were 17 males and 13 females. Patients had the following diagnosis: leukemia (n = 6), relapsed leukemia (n = 8), bone marrow failure (n = 2), thalassemia (n = 3), sickle cell disease (n = 3), Shwachman–Diamond syndrome n = 3), Hurler syndrome (n = 2), severe aplastic anemia (1), myelodysplastic syndrome (1), Wiskott-Aldrich syndrome (n = 1). Stem cell sources included human leukocyte antigen (HLA) matched related donor (n = 10), matched unrelated donor (n = 13) and cord blood (n = 7). The conditioning regimen was ablative in 17 patients and reduced intensity in 13. Of note, two patients were enrolled in the study twice because they underwent second transplants due to engraftment failure. GVHD prophylaxis consisted of calcineurin inhibitor (primarily cyclosporine) in all patients (n = 27) with concomitant methotrexate in the majority of the subjects (n = 23). Data on GVHD prophylaxis were missing in three subjects. Five subjects, all with non-malignant disease, received methylprednisolone as part of GVHD prophylaxis.
TABLE 2.
Subject characteristics
| n | ||
|---|---|---|
| Subjects enrolled | 30 | |
| Median age of subjects (minimum–maximum), years | 7(1–21) | |
| Male gender | 17 | |
| Diagnosis | Leukemia | 6 |
| Relapsed leukemia | 8 | |
| Bone marrow failure | 2 | |
| Thalassemia | 3 | |
| Sickle cell disease | 3 | |
| Shwachman–Diamond | 3 | |
| Hurler syndrome | 2 | |
| Severe aplastic anemia | 1 | |
| Myelodysplastic syndrome | 1 | |
| Wiskott–Aldrich syndrome | 1 | |
| Conditioning | Myeloablative | 17 |
| Non-myeloablative | 13 | |
| Donor Source | Matched related | 10 |
| Matched unrelated | 13 | |
| Cord blood | 7 | |
| Median time to neutrophil engraftment (minimum–maximum), days | 21(11–50) | |
| Engraftment syndrome | 5 | |
| Acute GVHD | 15 | |
| Culture + Infection | 6 | |
GVHD, graft-versus-host disease.
3.2 ∣. Transplant outcomes
Amongst these 30 subjects, 5 met the criteria for ES, 15 developed acute GVHD (inclusive of 3 subjects who also had ES which was followed by the development of GVHD at a median of 10 days after the diagnosis of ES) and 6 developed systemic infection (confirmed by positive blood culture). One subject with culture-positive infection (which developed at day +50) had both ES and subsequently developed GVHD. In addition, two subjects with ES, who did not have blood culture-positive infections, had documented respiratory viral illnesses with influenza A and parainfluenza, respectively. The median time to neutrophil engraftment was 21 days (minimum–maximum, 11–50 days). All subjects who developed ES did so within 30 days post-HSCT. All of the patients who developed ES received steroids at a starting dose ranging from 1 to 2 mg/kg methylprednisolone. Two of these subjects subsequently went on to develop acute GVHD and had a protracted course of steroids. The others received steroids for 7 days for ES which were then rapidly tapered; one of these patients died early from infection complications. None of the patients who received methylprednisolone for GVHD prophylaxis developed ES.
3.3 ∣. Cytokine and procalcitonin profiles
Each subject had a longitudinal assessment of cytokines and procalcitonin (Supplemental Figures S1-S8, Supplemental Tables S1-S8). There were no appreciable difference in the cytokine profiles or procalcitonin at engraftment between those who had a matched related donor (n = 10) and those who had a matched unrelated donor (n = 13) and a cord transplant (n = 7), or between those who had in vivo T-cell depletion (n = 17) and those who did not (n = 13). However, IL-8 values decreased more between day 0 and engraftment in patients who received reduced intensity conditioning (RIC) (n = 13) than in patients who received myeloablative (MA) conditioning (n = 17); and IL-12p70 values deceased between day 0 and engraftment in RIC patients but slightly increased in MA patients. Moreover, TNF values increased more between day 0 and engraftment in MA patients than in RIC patients. Using the Wilcoxon rank sum test, none of the cytokines or procalcitonin were predictors for ES, GVHD or infection (Table 3). IL-10 was the only variable found to be elevated (P = 0.015; median difference: 63.3, 95% CI: 6.80, 109) in the setting of culture-positive infection. Although procalcitonin has been associated with infection in other patient populations, elevated procalcitonin in this cohort was not associated with positive blood culture. Based on established parameters for elevated procalcitonin, measures were subsequently dichotomized with a cut-off point of >2.0 ng/mL (previously shown to be suggestive of systemic bacterial infection/sepsis, localized bacterial infection or in the setting of severe non-infectious inflammatory settings).21 Based on the dichotomized values for procalcitonin, only 8.7% (2/23) were ES+ for procalcitonin ≤ 2.0, whereas 43% (3/7) were ES+ for procalcitonin > 2.0 (Fisher’s exact test, P = 0.068; odds ratio: 7.88, 95% CI: 0.62, 113).
TABLE 3.
Maximum values of cytokines and procalcitonin (PCT) between day 0 (exclusive) and day 30 (inclusive), or day of engraftment, whichever came first. Medians and upper and lower quartiles are reported
| Engraftment syndrome |
Culture positive infection |
Acute GVHD |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Present | Absent | P | Present | Absent | P | Present | Absent | P | |
| n | 5 | 25 | 6 | 24 | 15 | 15 | |||
| IFN-γ | 18.4 (9.20, 31.5) | 12.1 (3.50, 25.7) | 0.70 | 25.7 (17.5, 40.1) | 10.3 (3.05, 25.3) | 0.12 | 5.80 (2.70, 24.6) | 18.9 (8.90, 32.5) | 0.10 |
| IL-1b | 0.90 (0.60, 1.10) | 0.60 (0.30, 0.60) | 0.11 | 0.60 (0.10, 0.90) | 0.60 (0.55, 0.90) | 0.65 | 0.60 (0.30, 1.10) | 0.60 (0.50, 0.60) | 0.23 |
| IL-6 | 22.4 (7.70, 47.2) | 10.9(6.20, 38.2) | 0.45 | 24.7 (7.90, 47.2) | 10.2 (5.90, 38.3) | 0.35 | 9.5 (5.6, 93.0) | 12.4 (6.70, 36.9) | 0.87 |
| IL-8 | 398.9 (70.6, 1463.1) | 106.7 (37.8, 161.6) | 0.21 | 158.9(126.5, 398.9) | 83.9 (38.1, 189.6) | 0.25 | 84.0 (57.5, 292.9) | 126.5 (37.8, 215.4) | 0.80 |
| IL-10 | 48.5 (18.0, 48.6) | 13.0 (5.60, 26.6) | 0.25 | 91.3 (27.2, 116.7) | 12.1 (5.45,21.2) | 0.015 | 13.9 (7.00, 61.7) | 17.8 (5.30, 27.2) | 0.66 |
| IL-12p70 | 1.50 (1.10, 3.00) | 1.20 (0.70, 2.40) | 0.62 | 6.70 (1.00, 41.6) | 1.20 (0.70, 2.35) | 0.23 | 1.50 (0.70, 2.40) | 1.20 (0.80, 3.00) | 0.97 |
| TNF-α | 10.6 (4.80, 18.4) | 8.70 (5.10, 13.2) | 0.66 | 9.30 (7.00, 17.5) | 7.50 (5.10, 13.5) | 0.53 | 8.70 (5.10, 17.5) | 8.90 (5.10, 13.2) | 0.62 |
| PCTa | 2.05 (0.95, 6.80) | 0.54 (0.20, 0.95) | 0.068 | 1.03 (0.38, 5.10) | 0.54 (0.21, 1.05) | 0.60 | 0.79 (0.31, 5.10) | 0.50 (0.17, 1.50) | 1.0 |
GVHD, graft-versus-host disease; IFN, interferon; IL, interleukin; TNF, tumor necrosis factor.
All values shown as the median (lower quartile, upper quartile).
Procalcitonin was dichotomized at 2.0 and was analyzed using Fisher’s exact test. All other cytokines were analyzed using the Wilcoxon rank sum test.
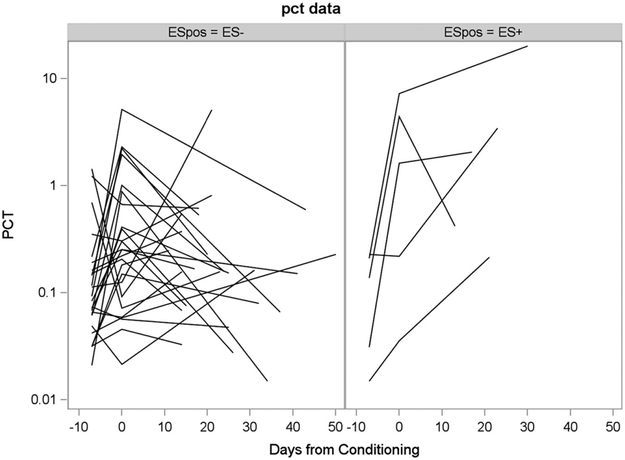
Given the variability in baseline levels for cytokines (both prior to initiation of the conditioning time point and at day 0) and the changes that occur in patients during the early transplant period, we were also interested in evaluating changes in cytokines over time as indicative of changes in the inflammatory milieu. Therefore, we also evaluated the absolute difference and log fold change from baseline to engraftment. Based on this analysis, for most of the cytokines, the one-sample tests indicated that the pre-conditioning and day 0 values differed. For four of the cytokines (IFN, IL-10, IL-12 and TNF-α), there was weak evidence that distributions of absolute differences (or fold changes) between engraftment and pre-conditioning and day 0 differed according to infection status (i.e., at engraftment, cytokine levels increased more in the culture-positive patients than in the culture-negative patients). For procalcitonin, there was strong evidence that distributions of absolute differences (or fold changes) between engraftment and Pre differed according to ES (Fig. 1, Table 4). Based on this analysis, the only biomarker elevated at the time of engraftment compared to pre-treatment in those with subsequent development of ES was procalcitonin (median-fold increase from baseline in those with ES was 15.1 versus 2.31 in those without ES, P = 0.0006; median difference: 13.8, 95% CI: 6.33, 65.6) (Table 4).
FIGURE 1.
Change in procalcitonin from pre-transplant conditioning to D0 and from pre-transplant to engraftment for patients with engraftment syndrome vs. those without engraftment syndrome
TABLE 4.
Fold increase from pre-treatment to the time of engraftment for patients with and without ES. Medians and upper and lower quartiles are reported
| Engraftment syndrome |
|||
|---|---|---|---|
| Present | Absent | P | |
| N | 5 | 25 | |
| IFN-γ | 1.00 (1.00, 2.97)a | 1.81 (1.14, 7.67) | 0.54 |
| IL-1b | 1.00 (1.00, 1.50) | 1.00 (1.00, 1.00) | 0.39 |
| IL-6 | 2.79 (1.00, 5.63) | 1.10 (0.35, 3.68) | 0.36 |
| IL-8 | 2.97 (1.18, 4.99) | 0.85 (0.45, 1.89) | 0.23 |
| IL-10 | 4.21 (2.05, 4.60) | 1.57 (0.87, 3.25) | 0.17 |
| IL-12p70 | 1.00 (0.44, 2.57) | 1.00 (0.37, 1.00) | 0.58 |
| TNF-α | 0.74 (0.60, 2.63) | 0.83 (0.45, 1.20) | 0.67 |
| PCT | 15.1 (14.2, 66.0) | 2.31 (0.64, 2.47) | 0.0006 |
ES, engraftment syndrome; IFN, interferon; IL, interleukin; PCT, procalcitonin; TNF, tumor necrosis factor.
Median (lower and upper quartile), all values represent the fold increase from pre-treatment (prior to the start of conditioning) to the time of engraftment.
Wilcoxon rank sum test was used to compare ES groups.
We estimated the sensitivity and specificity of procalcitonin fold change from pre-treatment to engraftment relative to a diagnosis of ES. While there were only five patients with ES, when the procalcitonin fold change from pre-treatment to engraftment was >11.41, ES could be identified with 80% sensitivity (4 of 5 with ES correctly identified) and 96% specificity (24 of 25 without ES correctly identified). Alternatively, when the fold change was >2.67, ES could be identified with 100% sensitivity (5 of 5 with ES correctly identified) and 80% specificity (20 of 25 without ES correctly identified). Thus, with the limitation of very few patients with ES, procalcitonin fold change may be potentially useful to assist in diagnosing ES if the changes are great enough.
4.∣. DISCUSSION
Recent studies have shown that the development of ES following allogeneic HSCT can be associated with worse overall outcomes— including higher non-relapse mortality, despite prompt initiation of therapy with steroids.2,22 An enhanced understanding of the pathophysiology of ES and the implications for subsequent development of GVHD is needed to improve treatment options and potentially out-comes. If specific biomarkers could be found to predict the development of ES, it may be possible to start treatment early before more severe complications develop and therefore decrease the associated morbidity and mortality. Our results provide one of the first prospective studies serially and systematically evaluating the cytokine profile in children undergoing allogeneic HSCT with a focus primarily on ES. Based on the preliminary nature of our findings in a heterogeneous pediatric transplant population, further studies are needed to establish the significance of this observation.
Results from this study help to establish a baseline for the cytokine profile in the early pre-transplant period, which incorporates an evaluation period that extends out to day 100 post-transplant. As illustrated in the supplemental figures, there is a general increase in cytokines and procalcitonin from baseline to engraftment that generally resolves by day 100. ES developed in 16.7% of patients, consistent with the previously published incidence in a diverse group of HSCT recipients. Although there was no clear association of any cytokine with the development of ES (Table 3), there was a trend towards an association with elevated procalcitonin, which was further supported by the fold change seen in this value from baseline in subjects with ES and not supported by the increase seen with other cytokines despite the small number of patients developing ES in this cohort (Table 4). Given the dynamic nature of transplant, the multiple pre-existing variables and confounders and disease subtypes, understanding biomarkers in the context of an individual’s pre-existing baseline needs to be further studied as changes from baseline may be equally or even more important in predicting clinically significant outcomes.
Despite the strength inherent in the prospective nature of our study, there are several limitations. Most notably is the baseline inherent complexity of the patient population under study that leads to multiple confounders, especially given the small sample size and the various underlying diseases and transplantation regimens, although the diversity is representative of typical pediatric HSCT population. Other confounders include infection, GVHD, and the impact of the conditioning regimen where some difference in certain cytokines was appreciated, the significance of which is unclear. In our study, amongst the five subjects with a diagnosis of ES, three subjects subsequently developed acute GVHD within a short interval (median time: 10 days), of whom one also had concurrent influenza and another developed a culture positive bacteremia. Another subject had concurrent parainfluenza, leaving only one subject with ES who did not have infection or GVHD. In addition, serial prospective evaluations of C-reactive protein or erythrocyte sedimentation rate were not performed, and these readily available laboratory markers could have served as an adjunct in evaluation of ES, though correlations between IL-6 and C-reactive protein have been seen.23,24 Future studies of cytokines should incorporate these evaluations as they provide a more readily available assessment of inflammatory status.
In conclusion, our data provide a preliminary foundation for future studies of biomarkers associated with ES and a suggestion for the role of procalcitonin in distinguishing ES from other potential etiologies. Ultimately, identifying biomarkers for ES may allow for pre-emptive treatment leading to improved outcomes. Further study in a larger population is warranted.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank our patients and their families as well as the clinical care teams.
Abbreviations
- CI
confidence interval
- CNMC
Children’s National Medical Center
- ES
engraftment syndrome
- GVHD
graft-versus-host disease
- HSCT
hematopoietic stem cell transplantation
- IFN
interferon
- IL
interleukin
- MA
myeloablative
- RIC
reduced intensity conditioning
- TNF
tumor necrosis factor
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- 1.Spitzer TR. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27:893–898. [DOI] [PubMed] [Google Scholar]
- 2.Spitzer TR. Engraftment syndrome: double-edged sword of hematopoietic cell transplants. Bone Marrow Transplant. 2015;50:469–475. [DOI] [PubMed] [Google Scholar]
- 3.Foncillas MA, Diaz MA, Sevilla J,et al. Engraftment syndrome emerges as the main cause of transplant-related mortality in pediatric patients receiving autologous peripheral blood progenitor cell transplantation. J. Pediatr. Hematol. Oncol. 2004;26:492–496. [DOI] [PubMed] [Google Scholar]
- 4.Khandelwal P, Mellor-Heineke S, Rahman N, et al. Cytokine Profile of Engraftment Syndrome in Pediatric Hematopoietic Stem Cell Transplant Recipients. Biol. Blood Marrow Transplant. 2016;22:690–697. [DOI] [PubMed] [Google Scholar]
- 5.Assicot M,Gendrel D,Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993;341:515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakr Y, Sponholz C, Tuche F, Brunkhorst F, Reinhart K. The role of procalcitonin in febrile neutropenic patients: review of the literature. Infection 2008;36:396–407. [DOI] [PubMed] [Google Scholar]
- 7.Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect. Dis. 2013;13:426–435. [DOI] [PubMed] [Google Scholar]
- 8.Simon L, Saint-Louis P, Amre DK, Lacroix J,Gauvin F. Procalcitonin and C-reactive protein as markers of bacterial infection in critically ill children at onset of systemic inflammatory response syndrome. Pediatr. Crit. Care Med. 2008;9:407–413. [DOI] [PubMed] [Google Scholar]
- 9.Linscheid P, Seboek D, Nylen ES, et al. In vitro and in vivo calcitonin I gene expression in parenchymal cells: a novel product of human adipose tissue. Endocrinology 2003;144:5578–5584. [DOI] [PubMed] [Google Scholar]
- 10.de Jager CP, de Wit NC, Weers-Pothoff G, van der Poll T, Wever PC. Procalcitonin kinetics in Legionella pneumophila pneumonia. Clin. Microbiol. Infect. 2009;15:1020–1025. [DOI] [PubMed] [Google Scholar]
- 11.Massaro KS, Macedo R, de Castro BS, et al. Risk factor for death in hematopoietic stem cell transplantation: are biomarkers useful to foresee the prognosis in this population of patients? Infection 2014;42:1023–1032. [DOI] [PubMed] [Google Scholar]
- 12.Sato M, Nakasone H, Terasako-Saito K, et al. Prediction of infectious complications by the combination of plasma procalcitonin level and localized infection before allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2014;49:553–560. [DOI] [PubMed] [Google Scholar]
- 13.Lyu YX, Yu XC, Zhu MY. Comparison of the diagnostic value of procalcitonin and C-reactive protein after hematopoietic stem cell transplantation: a systematic review and meta-analysis. Transpl. Infect. Dis. 2013;15:290–299. [DOI] [PubMed] [Google Scholar]
- 14.Sedef AM, Kose F, Mertsoylu H, Ozyilkan O. Procalcitonin as a biomarker for infection-related mortality in cancer patients. Curr. Opin. Support. Palliat. Care 2015;9:168–173. [DOI] [PubMed] [Google Scholar]
- 15.Shomali W, Hachem R, Chaftari AM, et al. Can procalcitonin distinguish infectious fever from tumor-related fever in non-neutropenic cancer patients? Cancer 2012;118:5823–5829. [DOI] [PubMed] [Google Scholar]
- 16.Robinson JO, Lamoth F, Bally F, Knaup M, Calandra T, Marchetti O. Monitoring procalcitonin in febrile neutropenia: what is its utility for initial diagnosis of infection and reassessment in persistent fever? PLoS One 2011;6:e18886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Secmeer G, Devrim I, Kara A, et al. Role of procalcitonin and CRP in differentiating a stable from a deteriorating clinical course in pediatric febrile neutropenia. J. Pediatr. Hematol. Oncol. 2007;29:107–111. [DOI] [PubMed] [Google Scholar]
- 18.Vogelsang GB, Wagner JE. Graft-versus-host disease. Hematol. Oncol. Clin. North Am. 1990;4:625–639. [PubMed] [Google Scholar]
- 19.Pacifico L, Osborn JF, Natale F, Ferraro F, De Curtis M, Chiesa C. Procalcitonin in pediatrics. Adv. Clin. Chem. 2013;59:203–263. [DOI] [PubMed] [Google Scholar]
- 20.Chiesa C, Panero A, Rossi N, et al. Reliability of procalcitonin concentrations for the diagnosis of sepsis in critically ill neonates. Clin. Infect. Dis. 1998;26:664–672. [DOI] [PubMed] [Google Scholar]
- 21.Uzzan B, Cohen R, Nicolas P, Cucherat M, Perret GY. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: a systematic review and meta-analysis. Crit. Care Med. 2006;34:1996–2003. [DOI] [PubMed] [Google Scholar]
- 22.Chang L, Frame D, Braun T, et al. Engraftment syndrome after allogeneic hematopoietic cell transplantation predicts poor outcomes. Biol. Blood Marrow Transplant. 2014;20:1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015;385:517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J. Clin. Invest. 2003;111:1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.