Abstract
Coronavirus disease 2019 (COVID-19) is emerging as the greatest public health crisis in the early 21st century. Its causative agent, Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2), is an enveloped single-stranded positive-sense ribonucleic acid virus that enters cells via the angiotensin converting enzyme 2 receptor or several other receptors. While COVID-19 primarily affects the respiratory system, other organs including the brain can be involved. In Western clinical studies, relatively mild neurological dysfunction such as anosmia and dysgeusia is frequent (~70-84%) while severe neurologic disorders such as stroke (~1-6%) and meningoencephalitis are less common. It is unclear how much SARS-CoV-2 infection contributes to the incidence of stroke given co-morbidities in the affected patient population. Rarely, clinically-defined cases of acute disseminated encephalomyelitis, Guillain-Barré syndrome and acute necrotizing encephalopathy have been reported in COVID-19 patients. Common neuropathological findings in the 184 patients reviewed include microglial activation (42.9%) with microglial nodules in a subset (33.3%), lymphoid inflammation (37.5%), acute hypoxic-ischemic changes (29.9%), astrogliosis (27.7%), acute/subacute brain infarcts (21.2%), spontaneous hemorrhage (15.8%), and microthrombi (15.2%). In our institutional cases, we also note occasional anterior pituitary infarcts. COVID-19 coagulopathy, sepsis, and acute respiratory distress likely contribute to a number of these findings. When present, central nervous system lymphoid inflammation is often minimal to mild, is detected best by immunohistochemistry and, in one study, indistinguishable from control sepsis cases. Some cases evince microglial nodules or neuronophagy, strongly supporting viral meningoencephalitis, with a proclivity for involvement of the medulla oblongata. The virus is detectable by reverse transcriptase polymerase chain reaction, immunohistochemistry, or electron microscopy in human cerebrum, cerebellum, cranial nerves, olfactory bulb, as well as in the olfactory epithelium; neurons and endothelium can also be infected. Review of the extant cases has limitations including selection bias and limited clinical information in some cases. Much remains to be learned about the effects of direct viral infection of brain cells and whether SARS-CoV-2 persists long-term contributing to chronic symptomatology.
Keywords: CNS, COVID-19, SARS-CoV-2, Brain, Pituitary
1. Introduction
In late 2019, a novel infectious disease associated with pneumonia and acute respiratory distress emerged in Wuhan, China. The implicated human coronavirus was genetically related to but distinct from the underlying viral agent behind the 2003 Severe Acute Respiratory Syndrome (SARS) outbreak, SARS coronavirus (SARS-CoV). The International Committee of Taxonomy of Viruses designated this novel coronavirus as Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2)1 and the World Health Organization termed the associated disease as coronavirus disease 2019 (COVID-19).1 As unsuspecting asymptomatic patients readily spread the disease, the COVID-19 pandemic has swept rapidly across the world resulting, by the end of December 2020, in over 79 million confirmed human infections and over 1.7 million deaths.2 These numbers are believed to be substantial underestimates of the true toll. COVID-19 is best known for its pulmonary involvement, but other organs are often affected including heart, kidney and nervous system. This review will provide an overview of tissue-based COVID-19 neuropathological analyses, almost entirely autopsy derived, as well as a brief discussion of SARS-CoV-2 virology to provide context for understanding its pathogenesis, and of diagnostic testing limitations that may bias the examined cases. The nervous system is affected both secondarily from systemic complications such as hypoxia and coagulopathy, and also likely from primary infection though much remains to be elucidated regarding viral invasion of the brain, spinal cord and other components of the nervous system.
2. SARS-CoV-2 virology
SARS-CoV-2 is the latest coronavirus to emerge as a human pathogen. Coronaviruses are single-stranded, positive-sensed ribonucleic acid (RNA) viruses subdivided into four genera: alpha-coronavirus (α-CoV), beta-coronavirus (β-CoV), gamma-coronavirus (γ-CoV), and delta-coronavirus (δ-CoV).3 SARS-CoV-2 is a beta-coronavirus, typically 60 to 140 nm4–6 in size, that shares genetic similarities to SARS-CoV (~79% homology) and Middle East Respiratory Syndrome coronavirus (MERS-CoV) (~50% homology).7 The SARS-CoV-2 genome encodes 16 non-structural proteins involved in viral replication and four structural proteins consisting of the envelope, membrane, nucleocapsid, and spike glycoprotein (Figure 1).3,8,9 The virus is constantly evolving with numerous strains of SARS-CoV-2 identified, some preferentially localized (at least temporarily) to geographic regions such as Europe or North America.10 The mutation rate of SARS-CoV-2 is estimated to be 0.84-1.12 x 10-3 substitutions per site per year,7,11 which is lower than that of human immunodeficiency virus (HIV)12 or influenza A.13 The genetic spectrum of disparate SARS-CoV-2 strains undergoing continued mutation could contribute to the variability of neuropathological findings discussed later. Antibodies against nucleocapsid and spike proteins have been used for immunohistochemical studies.
Figure 1:
Severe Acute Respiratory Coronavirus 2 (SARS-CoV-2) structure. There are four structural proteins: spike (S) protein (red), envelope (E) protein (violet), membrane (M) protein (blue), and nucleocapsid (N) protein (orange).
3. ACE2 and other receptors mediate SARS-CoV-2 entry
The entry of SARS-CoV-2 into human cells is commonly thought to be mediated by the interaction of the spike protein with the angiotensin converting enzyme 2 (ACE2) receptor, an important regulator of the renin-angiotensin system (RAS).14 Gender, age, lifestyle, smoking, and other patient co-morbidities are implicated in the modulation of ACE2 receptor expression in various tissues.15–21 ACE2 receptor expression has been reported in the cerebrum, cerebellum, brainstem, retina, and olfactory mucosa.22–24 Neurons, vascular pericytes and smooth muscle, and glia express the ACE2 receptor.22,23,25 In addition to the ACE2 receptor, in vitro studies show that SARS-CoV-2 may gain entry using other cell receptors, such as basigin (BSG; CD147),26 neuropilin-1 (NRP1),27 transmembrane serine protease 2 and 4 (TMPRSS2/4),28,29 and cathepsin L (CTSL).9 Expression of ACE2 receptor is highest in oligodendrocytes, TMPRSS2/4 in neurons, CTSL in microglia, and NRP1 in endothelial cells.25 In principle therefore, a broad range of cells in the central nervous system (CNS) have a variety of receptors that may facilitate infection.
4. Laboratory testing for COVID-19 has significant limitations
In part due to the natural course of disease wherein viral titers rise over time, laboratory testing for SARS-CoV-2 does not completely exclude patient infection, complicating the neuropathologist’s management of surgical or autopsy cases. There may also be a bias towards evaluating autopsies of COVID-19 patients with severe disease and high viral loads. The analytical sensitivity of the commonly used COVID-19 reverse transcriptase polymerase chain reaction (RT-PCR) assay is excellent with a limit of detection as low as 6.25 copies/µL in a nasopharyngeal sample.30 In a clinical setting, the sensitivity of RT-PCR may be 83.3% or significantly less, and may be affected by infection phase, sample type, collection procedures, and testing platforms.31,32
COVID-19 RT-PCR testing may produce false-negative results in the initial phase of infection. In sequential testing of patients who have symptoms, suspicious chest computed tomography (CT) findings and an initial negative RT-PCR test, COVID-19 RT-PCR positivity occurs at a mean of 5.1 ± 1.5 days after the initial test.32 A patient with a negative RT-PCR test result in a nasopharyngeal swab but with a positive SARS-CoV-2 cerebrospinal fluid (CSF) test has been reported.33 While RT-PCR detection of genomic RNA is not specific for viable virus, identification of sub-genomic RNAs transcribed in infected cells have been used in clinical testing to document the presence of actively replicating virus in lieu of viral cultures.34 Although chest CT scans may detect signs of COVID-19 days before RT-PCR positivity, CT findings overlap with other viral pneumonias.35
Serologic testing has uses for contact tracing, epidemiology, and vaccine studies but is more challenging to use for primary diagnosis given the latency for development of antibodies.36–40 The most common serological assays are the rapid lateral flow assay, enzyme linked immunosorbent assay (ELISA), and virus neutralization assay. Two-step ELISA assays are quantitative and more reliable than flow assays that are easily scalable but are qualitative.40 The virus neutralization assay detects and quantifies antibodies that inhibit viral replication but is technically complex.37
5. Systemic pathophysiology of COVID-19
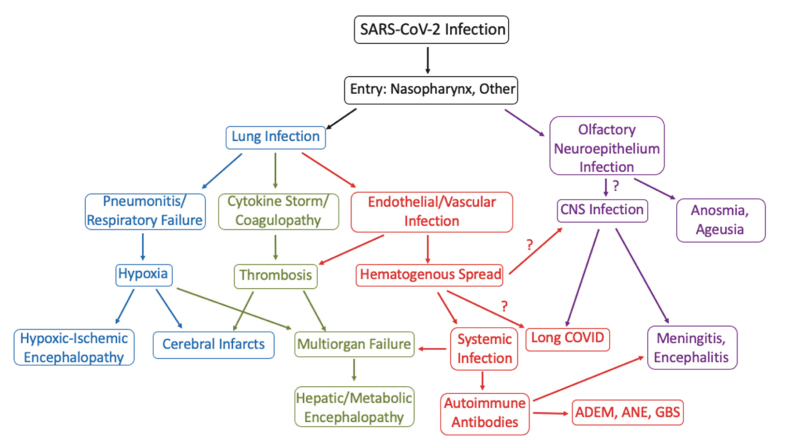
In the first stage of infection, SARS-CoV-2 targets nasal and bronchial epithelial cells as well as pneumocytes.28 As infection progresses, SARS-CoV-2 infects pulmonary endothelial cells, abrogating the epithelial-endothelial barrier.41 A subsequent ingress of neutrophils and monocytes is followed by pulmonary edema and hyaline membrane formation, a component of early acute respiratory distress syndrome (ARDS).41,42 In severe COVID-19, coagulopathy can occur, reflecting microthrombi formation secondary to endothelial cell inflammation and cytokine storms.41,43 COVID-19 associated hypercoagulability induces venous thromboembolism and arterial occlusion.41 The interplay of these systemic derangements likely contributes to the pathologic changes seen in the CNS (Figure 2).
Figure 2:
Flow chart modeling COVID-19 pathogenesis and neurological dysfunction. ADEM= Acute disseminated encephalomyelitis; ANE= Acute necrotizing encephalopathy; GBS= Guillain-Barré syndrome.
6. COVID-19 neurological manifestations
COVID-19 associated neurological manifestations range from mild symptoms such as dizziness, headache, dysgeusia, or anosmia to severe disorders such as stroke, Guillain-Barré syndrome (GBS), acute hemorrhagic necrotizing encephalopathy, meningoencephalitis, and cerebral venous thrombosis. The frequency of reported neurological signs and symptoms is variable but substantial regardless. In an early Chinese retrospective study, 36.4% of 214 COVID-19 patients had neurological symptoms which included dizziness (16.8%), headache (13.1%), impaired consciousness (7.5%), dysgeusia (5.6%), and anosmia (5.1%).44 In Western studies, dysgeusia and anosmia are reported in the majority of patients.45,46 A French study reports that 49 out of 58 (84%) COVID-19 intensive care unit (ICU) patients had neurological signs which included agitation (69%), confusion (65%), corticospinal tract signs (67%), and dysexecutive syndrome (33%).47 A study from a British referral center also describes cases of septic or para-infectious encephalopathy, autoimmune encephalitis including acute disseminated encephalomyelitis (ADEM), and GBS.48
7. Specific neurological disorders associated with COVID-19
Olfactory and gustatory dysfunction
In the aforementioned study from China, COVID-19 patients had gustatory dysfunction and olfactory dysfunction at frequencies of less than 6% each.44 In a prospective European study, 88.8% of 385 COVID-19 patients had gustatory dysfunction and 85.6% of 417 COVID-19 patients had olfactory dysfunction.45 In a California study, 71% of 59 COVID-19 patients recorded ageusia and 68% reported anosmia.46 Olfactory and gustatory dysfunction in COVID-19 typically resolves after 17 to 30 days from initial onset.49,50 Although long term follow-up is generally lacking,51 one study reports resolution rates at day 30 in home-quarantined COVID-19 patients of 87% for olfactory dysfunction and 82% for gustatory dysfunction.52 Presence of SARS-CoV-2 virions in the olfactory neuroepithelium of the nasal mucosa as well as in the olfactory bulb has been documented53,54 though the exact basis for olfactory dysfunction and recovery remains to be resolved.
Stroke
Stroke is the most common debilitating neurological disorder associated with COVID-19 and has a predilection for males and the elderly. Two retrospective New York studies reported respectively that 1.6% of 1,916 patients with hospitalizations or emergency department visits for COVID-19 and 0.9% of 3,556 hospitalized COVID-19 patients had radiologically-confirmed ischemic infarcts.55,56 Cryptogenic strokes were twice as common in hospitalized COVID-19 patients compared to either contemporary or historical controls.56 A third New York retrospective study found 1.1% of 3,218 COVID-19 patients had strokes; in the small subset with acute neuroimaging, 68.5% of strokes were ischemic (44.5% large vessel, 24% lacunar) and 24% were hemorrhagic.57 A retrospective Chinese study of 214 COVID-19 patients reported strokes in six patients (2.8%).44 A small American case series reported large vessel ischemic strokes in five COVID-19 patients younger than 50, four of whom had no prior history of stroke.58 For comparison, the average incidence of stroke among patients admitted through a U.S. emergency department prior to the pandemic was approximately 3.2%.59 Of patients who visited emergency departments or were hospitalized, only 0.2% of 1486 influenza patients had ischemic infarcts which was significantly less than COVID-19 patients even after adjusting for age, sex and, race.55
In another New York study, 33 out of 755 (4.4%) COVID-19 patients with neuroimaging had evidence of intracranial hemorrhage not associated with trauma, brain metastases, or tumor resection.60 Parenchymal hemorrhages with mass effect and herniation were present in five patients, punctate hemorrhages in seven, small to moderate sized hemorrhages in 17, and large single site hemorrhages without herniation in four.60 Twenty-six of the 33 intracranial hemorrhages occurred as a transformation of an ischemic infarct.60 Of the five patients with parenchymal hemorrhages, four had high partial thromboplastin time or anti-factor Xa in the 72 hours before the intracranial hemorrhage.60
Advanced age, cardiovascular disease, cerebrovascular disease, diabetes, chronic respiratory disease, hypertension, obesity, smoking, and cancer are risk factors for severe COVID-19 disease or poor outcome.61–67 In a Canadian study, critically ill COVID-19 patients with blood groups A and AB were more likely to require ventilation, continuous renal replacement therapy, and prolonged intensive care unit admission.68 In contrast, an earlier American COVID-19 study did not show an association between specific blood groups and either ventilation or death.69 Differences in cohorts including mortality rates may account for the contradictory findings.68 It cannot escape notice that a number of the aforementioned COVID-19 prognostic factors are associated with atherosclerosis and arteriolosclerosis that, in the setting of COVID-19 respiratory compromise and coagulopathy, may contribute to strokes.
Guillain-Barré syndrome
GBS is an uncommon, immune-mediated demyelinating disease of the peripheral nerves that often follows viral infections. Common presenting symptoms in COVID-19 patients include symmetrical flaccid quadriparesis, ataxia, facial weakness, respiratory failure, and lower paresthesia.70–72 RT-PCR of nasopharyngeal swabs tested positive for SARS-CoV-2 but CSF was negative for tested patients.73–75 GBS symptoms tend to emerge between day five to 10 after COVID-19 symptom onset.71 Over 30 cases of GBS have been reported.73–76 One proposed mechanism is an autoimmune hyperreaction, triggering release of pro-inflammatory mediators such as interleukin (IL)-6, that cause autoimmune demyelination or axonal damage. Alternatively in some cases, SARS-CoV-2 may induce production of antibodies targeting gangliosides, leading to peripheral neuropathy.72,77 COVID-19 associated Miller Fisher syndrome (MFS), a variant of GBS with ophthalmoplegia, areflexia, and ataxia, and polyneuritis cranialis (PNC), a GBS variant with multiple cranial neuropathies, have been reported.78
Meningitis and encephalitis
COVID-19 associated meningitis has only infrequently been reported but one case highlights the need for CSF testing if suspected.33 A 24-year-old Japanese male presented with headache, generalized fatigue and fever. Nine days after onset of typical COVID-19 symptoms, the patient had altered mental status, transient generalized seizures, and neck stiffness. Brain magnetic resonance imaging (MRI) showed hyperintensities along the lateral ventricle and in the medial temporal lobe including the hippocampus. SARS-CoV-2 RNA was not detected by RT-PCR in a nasopharyngeal swab but was in CSF.33 Autopsies performed on COVID-19 patients have suggested meningoencephalitis in some cases and these are discussed in section 8.79
Other disorders
A Brazilian study reported central venous thrombosis (CVT) in three previously healthy COVID-19 patients younger than 41 years, underscoring the need for awareness of a hypercoagulable state in COVID-19 even in this age group.80
Acute hemorrhagic necrotizing encephalopathy, an uncommon complication characterized by multifocal symmetric brain lesions including bilateral thalamic lesions, has been associated with COVID-19, though rarely.81,82 This complication may be caused by blood-brain-barrier disruption related to intracranial cytokine storms.81 COVID-19 associated cases of ADEM-like disease and autoimmune encephalitis have been documented.72,83
8. Neuropathological findings and neuropathogenesis of COVID-19
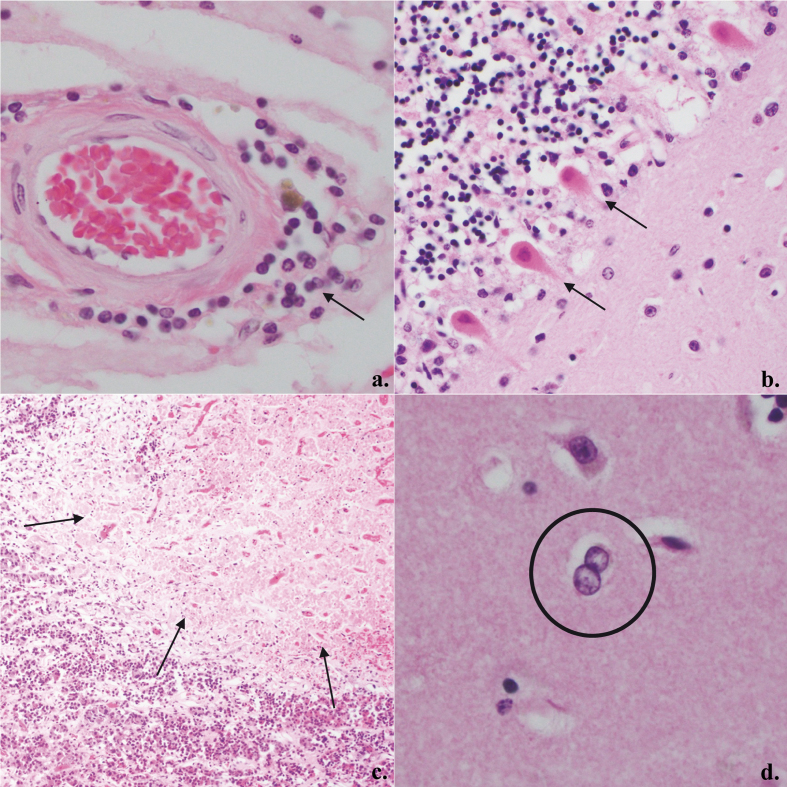
To examine the spectrum of COVID-19 neuropathology, we reviewed 20 papers encompassing 184 patients with tissue-based neuropathological analyses including 101 cases analyzed by RT-PCR for SARS-CoV-2 and 83 cases by immunohistochemistry (IHC) (Table 1).25,53,79,83–99 All cases are autopsies except for four biopsies and one case of unspecified type. The range of histologic findings is broad and in part reflects the heterogeneity of neurological findings. The most frequent findings (Table 1) include microglial activation with microglial nodules in a likely underestimated subset; lymphoid inflammation including perivascular lymphocytosis (Figure 3a), parenchymal lymphocytic infiltration, and leptomeningeal lymphocytic inflammation; hypoxic-ischemic changes (Figure 3b); astrogliosis; acute/subacute brain infarcts; primary hemorrhage; and microthrombi. It should be noted that the prevalence of detected lymphoid inflammation and microglial nodules is high in papers with immunohistochemical studies and low in those without.
Table 1:
Neuropathological findings in COVID-19 brain tissue
| Viral presence by RT-PCR | 53.5% (54/101) |
| Viral presence by IHC | 27.7% (23/83) |
| Microglial Activation | 42.9% (79/184) |
| Microglial Nodules* | 33.3% (14/42) |
| Lymphoid Inflammation | 37.5% (69/184) |
| Perivascular | 33.7% (62/184) |
| Parenchymal | 23.9% (44/184) |
| Leptomeningeal | 23.4% (43/184) |
| Acute Hypoxic-Ischemic Changes | 29.9% (55/184) |
| Astrogliosis | 27.7% (51/184) |
| Acute/Subacute Brain Infarcts | 21.2% (39/184) |
| Spontaneous Hemorrhage | 15.8% (29/184) |
| Microthrombi | 15.2% (28/184) |
| Alzheimer Type II Astrocytosis | 2.7% (5/184) |
| Neuronophagy | 2.2% (4/184) |
| Neuronal Cell Loss | 2.2% (4/184) |
| Hemorrhagic Transformation of Infarct | 2.2% (4/184) |
| Foci of Demyelination | 1.6% (3/184) |
| Vascular Neutrophilic Plugs | 1.6% (3/184) |
| Perivascular Neutrophils | 1.1% (2/184) |
| Parenchymal Neutrophilic Infiltration | 1.1% (2/184) |
| Leptomeningeal Histiocytes | 0.5% (1/184) |
| Acute Purulent Meningitis | 0.5% (1/184) |
Table 1: Some histologic findings are likely to be under-reported as reviewed studies are variable in their focus. *Only studies tabulating the prevalence of microglial nodules are included. Studies mentioning microglial nodules in their case series but not enumerating the frequency are excluded.
Figure 3:
Representative COVID-19 histopathology: a. Mild perivascular lymphoid inflammation, 400X (arrow); b. Eosinophilia in Purkinje cells compatible with acute hypoxic-ischemic change, 200X (arrows); c. Subacute infarct of anterior pituitary gland, 100X (arrows); d. Alzheimer Type II astrocytes in basal ganglia, 400X (circle).
SARS-CoV-2 is detectable in central nervous system tissue
At least three routes of CNS infection by SARS-CoV-2 have been proposed: retrograde transmission via olfactory sensory neurons, infiltration of immune cells, and entry across the blood-brain barrier.100,101 The prevalence of anosmia and ageusia in COVID-19 patients led to the theory that SARS-CoV-2 enters the brain via infection of neurons in the olfactory neuroepithelium (which resides in the mucosa of the nasal cavity) and from there to the olfactory bulb and then to other brain regions. This olfactory route is used by other coronaviruses, such as SARS-CoV102 and MERS-CoV.103 COVID-19 patients frequently display MRI hyperintensity in the olfactory cortex.104 The ACE2 receptor, used by SARS-CoV-2 for cellular entry, is expressed in sustentacular cells and stem cells of the nasal olfactory epithelium.23,105 While one study suggests olfactory neurons themselves do not express the ACE2 receptor,23 another report contradicts this finding.106 Besides technical sensitivity issues, it may be that the expression levels of the ACE2 receptor and other receptors differ under inflammatory conditions as compared to physiologic conditions. Viral particles have been detected by electron microscopy and IHC in the olfactory epithelium54 as well as by electron microscopy in olfactory neurons of the nasal mucosa at autopsy.53 Morbini and colleagues report ultrastructural evidence of SARS-CoV-2 particles in the olfactory bulb of a COVID-19 patient.54 A second theory posits that SARS-CoV-2 may infect immune cells that cross the blood-brain barrier and deliver virus into the brain. This mechanism is well described in HIV.107 White blood cells including lymphocytes and monocytes express the ACE2 receptor.108,109 Infection of immune cells by SARS-CoV-2 is an active area of study. Finally, the third theory extends the well documented behavior of encephalitic blood-borne coronaviruses to enter through the blood-brain barrier via infection of vessel wall cells.110
Histologically, on routine hematoxylin- and eosin-stained slides, neither viral inclusions nor specific cellular changes recognizable as direct viral infection have been reported. However, SARS-CoV-2 has been detected in the brain by RT-PCR, IHC, and electron microscopy. As many as 54 out of 101 (53.5%) cases were positive for SARS-CoV-2 in the brain by RT-PCR and 23 out of 83 (27.7%) cases were positive by IHC (Table 1). In some cases, copies of virus detected by RT-PCR were low in number and the detection of virus in blood or blood cells within intracerebral vasculature rather than brain cells was a possibility. However, IHC has confirmed the presence of viral antigens in autopsy brain cells. Of note, antibodies against SARS-CoV-2 spike protein were more effective in detecting viral antigens than those targeted against nucleocapsid protein25,87,97 (Supplementary Table A). Importantly, staining using both anti-spike and anti-nucleocapsid antibodies may be more sensitive than either alone as, in a few cases, nucleocapsid protein was detected while spike protein was not. Whether this finding is a technical issue related to the quality of the antibodies or the intrinsic accessibility of the relevant epitopes or both is unresolved. Staining of virus localizes in scattered cortical neurons and endothelial cells,87 as well as brainstem cranial nerve roots and isolated cells (cell type unclear) in the medulla oblongata; the images of the isolated cells had a striking lack of attendant chronic inflammation.25 This disconnect between viral infection and inflammation raises the question of immune evasion. One case exhibited viral staining around the edges of subcortical white matter microinfarcts.87 The immunostaining pattern consists of diffuse cytoplasmic and perinuclear positivity with small concentrated foci possibly representing viral inclusion bodies.87 Meinhardt and colleagues, using in situ hybridization (ISH), identified SARS-CoV-2 in the olfactory epithelium and mucus.53 Lastly, viral particles compatible with SARS-CoV-2 have been identified by electron microscopy in olfactory bulb and frontal lobe tissue.54,96 To date, regions in which SARS-CoV-2 have been detected by IHC, RT-PCR, or electron microscopy relevant to the CNS include cornea, conjunctiva, olfactory epithelium, olfactory bulb, olfactory tubercle, frontal lobe, cerebellum, medulla oblongata, cranial nerves and trigeminal ganglion. Most investigations studied limited areas of brain or did not specify the origin of cerebral cortex tissue. Additional studies are needed to better establish the frequency and extent of direct infection in the CNS. As some patients have "Long COVID” wherein they have persistent symptoms for months, whether and how SARS-CoV-2 may persist in the brain will need to be evaluated in the future.
Chronic inflammatory and reactive glial changes
Microgliosis and astrocytosis are common in COVID-19 brains including in the olfactory bulb.25 Lymphoid inflammation, which tends to be minimal or mild in many cases, is not uncommon particularly in the medulla oblongata and if IHC is used for detection.25 In many studies that did not use IHC, lymphoid inflammation was reported as absent or infrequent; this is concordant with our experience and anecdotally with that of colleagues in Asia and in the United States (personal communication). A substantial portion of these reactive changes may be secondary to systemic issues (e.g. sepsis) or other neuropathology (infarcts, hemorrhages, etc.) rather than a response to direct infection. Microgliosis and chronic inflammation did not correlate with the severity of COVID-19 disease nor were there any discernible neuropathological differences in patients from nursing homes, hospital wards, or intensive care units.25 Furthermore, microglial activation, perivascular lymphocytosis, and leptomeningeal lymphocytic infiltration is observed to similar degrees in the control brains of septic (non-COVID-19) patients when compared to COVID-19 patients.86
Some cases present findings compatible with viral meningoencephalitis including parenchymal lymphocytic clustering around microglial nodules, concomitant leptomeningeal lymphocytic inflammation, and even neuronophagy. In the study by Matschke and colleagues, four out of 16 cases immunopositive for SARS-CoV-2 had 10 to 49 CD8+ cytotoxic T lymphocytes per high power field in at least three fields in the medulla oblongata.25 The observed lymphocytes tended to cluster near activated microglial nodules,25 as commonly seen in viral encephalitis. RT-PCR detected SARS-CoV-2 RNA in three out of the four cases.25 Three studies report neuronophagy in the medulla associated with histiocytic and lymphocytic parenchymal infiltration.25,84,89 von Weyhern and colleagues report six cases of perivascular and parenchymal lymphocytosis with neuronal loss and axon degeneration in the brainstem, which the authors determined to be adequate to diagnose SARS-CoV-2 viral encephalitis.79 In the same study, five of the six cases had concomitant meningitis.79 Respiratory and cardiovascular control centers of the medulla may be affected.53 While the majority of COVID-19 patients with neurological manifestations do not have detectable SARS-CoV-2 RNA in CSF by RT-PCR,111–113 exceptions to this trend have been reported in at least four COVID-19 patients.33,114–116 Anti-SARS-CoV-2 antibodies were detected in the CSF of one COVID-19 patient suggesting an immune response to viral infection.87 It appears therefore that histologically documentable cases of SARS-CoV-2 encephalitis and/or meningitis do occur, with a tendency to involve the brainstem. As only a few series report the majority of brainstem microglial encephalitis cases, the question arises as to whether particular viral strains, therapeutic approaches, genetic background or detection methods are responsible for its apparent absence or paucity in the majority of studies.
Autoimmune mediated inflammation may also occur. Autoimmune encephalitis, ADEM, and acute necrotizing encephalopathy have also been reported clinically though histopathologic evaluation has been limited. ADEM-like pathology has been reported in one autopsy case.83
Microthrombi, infarcts, hemorrhages, and “neutrophilic plugs”
SARS-CoV-2 may induce a cytokine storm, a severe hyperimmune reaction characterized by excessive and rapid release of cytokines such as IL-6 and IL-1β into the blood.117,118 IL-6 activates the coagulation system and increases vascular permeability119 which, in combination with viral endotheliopathy,120 may account for the well documented COVID-19 associated coagulopathy.41,121 SARS-CoV-2 has been detected within cerebral endothelial cells by IHC and electron microscopy.87,96 Hypercoagulability, in turn, results in histologic findings of microthrombi, infarcts, and hemorrhages. Also, IL-1β plays a major role in triggering vascular “neutrophilic plugs” containing neutrophils and/or platelets as well as neutrophil extracellular traps, a mesh of deoxynucleic acid (DNA)-rich material coated with antimicrobials that entrap and kill microbes.122 These “neutrophilic plugs” have been found in the brain as well as the lungs, heart, kidneys, and liver of COVID-19 patients who come to autopsy.88 Approximately 1.6% of cases have “neutrophilic plugs” in the brain (Table 1) though this percentage is likely an underestimate as pathologists do not typically evaluate for these structures. Pneumonia and ARDS with resultant hypoxemia as well as pre-existing arteriosclerosis are also likely contributory to the cerebral infarcts. In addition to cerebral infarcts, we have noted occasional cases of pituitary infarcts (Figure 3c) in our COVID-19 autopsies. Numerous extramedullary megakaryocytes were present in subacute cerebral infarcts of one COVID-19 patient.84
Alzheimer Type II astrocytosis
Alzheimer Type II astrocytosis (Figure 3d) characteristic of hepatic encephalopathy has been reported.89,97 The reports do not specify whether the frequency of the Alzheimer Type II astrocytes reaches a threshold of five or more per 20 high power fields, the cutoff suggested by Agarwal and colleagues for hepatic encephalopathy.123 In a paper by Solomon and colleagues, four out of four cases with Alzheimer Type II astrocytes had chronic liver disease or alcohol use disorder.97 The relative contribution of hepatic encephalopathy to cases currently ascribed as septic or para-infectious encephalopathy remains to be seen.
9. Conclusions
Systemic dysfunction and viral infection of the CNS can cause a wide range of COVID-19 related neuropathological changes. The incidence of neuropathological findings should be viewed with caution given great variability in what tissues were studied and what types of ancillary studies were (or were not) performed. Inflammatory changes were reported in a high percentage of cases in some series but not in others, at least in part due to the use of immunostaining; however, the lack of controls in most of these studies limit interpretation of these findings. Selection or referral bias, the decedent’s co-morbidities, therapies given, patient’s genetic background, immune status, immunization status and perhaps viral strains of SARS-CoV-2 may also contribute to differences and will need to be dissected out in the future. Whether or how much of the inflammatory changes seen are due to autoimmune phenomena versus direct viral infection or other causes remains to be resolved. The presence of microglial nodules and detectable virus are indicative of viral meningoencephalitis in some cases; a predilection for the medulla perhaps with compromise of respiratory and cardiovascular control centers may compound the COVID-19 patient’s often already tenuous cardiorespiratory function. While there is evidence for an olfactory route of infection, it is unclear whether this is the major mechanism of CNS infection given that the virus is rarely detectable in the olfactory bulb. Cerebral endothelial infection is also present and a hematogenous route of infection is therefore plausible perhaps even common. Data on where the virus is detectable in the brain is limited as studies have focused on the frontal lobe and brainstem. It would not be surprising to find a greater extent of infection than currently confirmed. It should be kept in mind that the sensitivity of virus detection is suboptimal given that most samples are derived from autopsies. Lastly, it is notable that inflammation does not always coincide with virus localization, raising the concern that the virus may be evading the immune response in the CNS. Whether the CNS is a potential long-term reservoir of virus and a contributor to “Long COVID” remains to be seen. Lastly, increased risk for vascular dementia or neurodegenerative disorders are hypothetical concerns that may need to be addressed at a later time.
Funding
This work was supported in part by NIMH 2U24MH10092 and U01 MH083500 (S. Magaki, E. Singer, W. Yong) and in part by the UCLA Broad Stem Cell Research Center COVID 19 Research Award OCRC #20-70 (H.V. Vinters, S. Magaki, and T. Zhang).
Supplementary Material
References
- Naming the coronavirus disease (COVID-19) and the virus that causes it. World Health Organization. Accessed October 21, 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it
- Weekly epidemiological update - 29 December 2020. World Health Organization. Accessed December 30, 2020. https://www.who.int/publications/m/item/weekly-epidemiological-update---29-december-2020
- Ye ZW, Yuan S, Yuen KS, Fung SY, Chan CP, Jin DY. Zoonotic origins of human coronaviruses. Int J Biol Sci. 2020;16(10):1686-1697. 10.7150/ijbs.45472 [DOI] [PMC free article] [PubMed]
- Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed]
- Caly L, Druce J, Roberts J, et al. Isolation and rapid sharing of the 2019 novel coronavirus (SARS-CoV-2) from the first patient diagnosed with COVID-19 in Australia. Med J Aust. 2020;212(10):459-462. 10.5694/mja2.50569 [DOI] [PMC free article] [PubMed]
- Gulholm T, Basile K, Kok J, Chen S, Rawlinson W. Laboratory diagnosis of severe acute respiratory syndrome coronavirus 2. Pathology. 2020;52(7):745-753. 10.1016/j.pathol.2020.09.011 [DOI] [PMC free article] [PubMed]
- Koyama T, Platt D, Parida L. Variant analysis of SARS-cov-2 genomes. Bull World Health Organ. 2020;98(7):495-504. 10.2471/BLT.20.253591 [DOI] [PMC free article] [PubMed]
- Naqvi AAT, Fatima K, Mohammad T, et al. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim Biophys acta Mol basis Dis. 2020;1866(10):165878. 10.1016/j.bbadis.2020.165878 [DOI] [PMC free article] [PubMed]
- Ou X, Liu Y, Lei X, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11(1). 10.1038/s41467-020-15562-9 [DOI] [PMC free article] [PubMed]
- Pachetti M, Marini B, Benedetti F, et al. Emerging SARS-CoV-2 mutation hot spots include a novel RNA-dependent-RNA polymerase variant. J Transl Med. 2020;18(1):1-9. 10.1186/s12967-020-02344-6 [DOI] [PMC free article] [PubMed]
- Day T, Gandon S, Lion S, Otto SP. On the evolutionary epidemiology of SARS-CoV-2. Curr Biol. 2020;30(15):R849-R857. 10.1016/j.cub.2020.06.031 [DOI] [PMC free article] [PubMed]
- Rowland-Jones S, Andrews SM. Recent advances in understanding HIV evolution. F1000Research. 2017;6(0):1-7. 10.12688/f1000research.10876.1 [DOI] [PMC free article] [PubMed]
- Nobusawa E, Sato K. Comparison of the Mutation Rates of Human Influenza A and B Viruses. J Virol. 2006;80(7):3675-3678. 10.1128/jvi.80.7.3675-3678.2006 [DOI] [PMC free article] [PubMed]
- Gheblawi M, Wang K, Viveiros A, et al. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ Res. 2020;126(10):1456-1474. 10.1161/CIRCRESAHA.120.317015 [DOI] [PMC free article] [PubMed]
- Wang K, Chen W, Zhang Z, et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther. 2020;5(1):283. 10.1038/s41392-020-00426-x [DOI] [PMC free article] [PubMed]
- Fernández-Atucha A, Izagirre A, Fraile-Bermúdez AB, et al. Sex differences in the aging pattern of renin-angiotensin system serum peptidases. Biol Sex Differ. 2017;8(1). 10.1186/s13293-017-0128-8 [DOI] [PMC free article] [PubMed]
- Hu Y, Li X, Wu N, Wang N, Qui C, Li J. Study on the correlation among sex, age, and the activity of ACE, ACE2 and the ratio of ACE/ACE2. J Qiqihar Med Coll. 2018;39(8):884-887. 10.3969/j.issn.1002-1256.2018.08.005 [DOI]
- Liu Y, Zhao L, Zhang Q, Zhang L, Ren G. Effect of long-term smoking on expressiong of serum ACE and ACE2 as well as its significance. J Taishan Med Coll. 2019;40(4):258-260. 10.3969/j.issn.1004-7115.2019.04.007 [DOI]
- Smith JC, Sausville EL, Girish V, et al. Cigarette Smoke Exposure and Inflammatory Signaling Increase the Expression of the SARS-CoV-2 Receptor ACE2 in the Respiratory Tract. Dev Cell. 2020;53(5):514-529.e3. 10.1016/j.devcel.2020.05.012 [DOI] [PMC free article] [PubMed]
- Bernardi S, Toffoli B, Zennaro C, et al. High-salt diet increases glomerular ACE/ACE2 ratio leading to oxidative stress and kidney damage. Nephrol Dial Transplant. 2012;27(5):1793-1800. 10.1093/ndt/gfr600 [DOI] [PubMed]
- Lavrentyev EN, Malik KU. High glucose-induced Nox1-derived superoxides downregulate PKC-βII, which subsequently decreases ACE2 expression and ANG(1-7) formation in rat VSMCs. Am J Physiol - Hear Circ Physiol. 2009;296(1):106-118. 10.1152/ajpheart.00239.2008 [DOI] [PMC free article] [PubMed]
- Xia H, Lazartigues E. Angiotensin-converting enzyme 2 in the brain: properties and future directions. J Neurochem. 2008;107(6):1482-1494. 10.1111/j.1471-4159.2008.05723.x [DOI] [PMC free article] [PubMed]
- Brann D, Tsukahara T, Weinreb C, et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv. 2020;6(31):eabc5801. 10.1126/sciadv.abc5801 [DOI] [PMC free article] [PubMed]
- Chen M, Shen W, Rowan NR, et al. Elevated ACE2 expression in the olfactory neuroepithelium: implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. Eur Respir J. 2020;56(3):2001948. 10.1183/13993003.01948-2020 [DOI] [PMC free article] [PubMed]
- Matschke J, Lütgehetmann M, Hagel C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19(11). 10.1016/S1474-4422(20)30308-2 [DOI] [PMC free article] [PubMed]
- Wang K, Chen W, Zhou Y Sen, et al. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. bioRxiv. Published online January 1, 2020:2020.03.14.988345. 10.1101/2020.03.14.988345 [DOI]
- Cantuti-Castelvetri L, Ojha R, Pedro LD, et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and provides a possible pathway into the central nervous system. bioRxiv. Published online January 1, 2020:2020.06.07.137802. 10.1101/2020.06.07.137802 [DOI]
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271-280.e8. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed]
- Zang R, Gomez Castro MF, McCune BT, et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci Immunol. 2020;5(47):eabc3582. 10.1126/sciimmunol.abc3582 [DOI] [PMC free article] [PubMed]
- COVID-19 RT-PCR test - Emergency Use Authorization (EUA) summary. Laboratory Corporation of America. Accessed September 2, 2020. https://www.fda.gov/media/136151/download
- Long C, Xu H, Shen Q, et al. Diagnosis of the Coronavirus disease (COVID-19): rRT-PCR or CT? Eur J Radiol. 2020;126:108961. 10.1016/j.ejrad.2020.108961 [DOI] [PMC free article] [PubMed]
- Ai T, Yang Z, Hou H, et al. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020;296(2):E32-E40. 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed]
- Moriguchi T, Harii N, Goto J, et al. A first Case of Meningitis/Encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55-58. 10.1016/j.ijid.2020.03.062 [DOI] [PMC free article] [PubMed]
- Perera RAPM, Tso E, Tsang OTY, et al. SARS-CoV-2 Virus Culture and Subgenomic RNA for Respiratory Specimens from Patients with Mild Coronavirus Disease. Emerg Infect Dis J. 2020;26(11):2701. 10.3201/eid2611.203219 [DOI] [PMC free article] [PubMed]
- Dai W-C, Zhang HW, Yu J, et al. CT Imaging and Differential Diagnosis of COVID-19. Can Assoc Radiol J. 2020;71(2):195-200. 10.1177/0846537120913033 [DOI] [PMC free article] [PubMed]
- Guo L, Ren L, Yang S, et al. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin Infect Dis an Off Publ Infect Dis Soc Am. Published online March 2020. 10.1093/cid/ciaa310 [DOI] [PMC free article] [PubMed]
- Gauger PC, Vincent AL. Serum Virus Neutralization Assay for Detection and Quantitation of Serum Neutralizing Antibodies to Influenza A Virus in Swine. Methods Mol Biol. 2020;2123:321-333. 10.1007/978-1-0716-0346-8_23 [DOI] [PubMed]
- Okba NMA, Müller MA, Li W, et al. Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus Disease 2019 Patients. Emerg Infect Dis. 2020;26(7). 10.3201/eid2607.200841 [DOI] [PMC free article] [PubMed]
- Sun B, Feng Y, Mo X, et al. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg Microbes Infect. 2020;9(1):940-948. 10.1080/22221751.2020.1762515 [DOI] [PMC free article] [PubMed]
- Krammer F, Simon V. Serology assays to manage COVID-19. Science. 2020;368(6495):1060-1061. 10.1126/science.abc1227 [DOI] [PubMed]
- Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;324(8):782-793. 10.1001/jama.2020.12839 [DOI] [PubMed]
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420-422. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed]
- Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8(6):e46-e47. 10.1016/S2213-2600(20)30216-2 [DOI] [PMC free article] [PubMed]
- Mao L, Jin H, Wang M, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683-690. 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed]
- Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Oto-Rhino-Laryngology. 2020;277(8):2251-2261. 10.1007/s00405-020-05965-1 [DOI] [PMC free article] [PubMed]
- Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. 2020;10(7):806-813. 10.1002/alr.22579 [DOI] [PMC free article] [PubMed]
- Helms J, Kremer S, Merdji H, et al. Neurologic Features in Severe SARS-CoV-2 Infection. N Engl J Med. 2020;382(21):2268-2270. 10.1056/nejmc2008597 [DOI] [PMC free article] [PubMed]
- Paterson RW, Brown RL, Benjamin L, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143(10):3104-3120. 10.1093/brain/awaa240 [DOI] [PMC free article] [PubMed]
- Paolo G. Does COVID-19 cause permanent damage to olfactory and gustatory function? Med Hypotheses. 2020;143:110086. 10.1016/j.mehy.2020.110086 [DOI] [PMC free article] [PubMed]
- Hopkins C, Surda P, Whitehead E, Kumar BN. Early recovery following new onset anosmia during the COVID-19 pandemic – an observational cohort study. J Otolaryngol - Head Neck Surg. 2020;49(1):26. 10.1186/s40463-020-00423-8 [DOI] [PMC free article] [PubMed]
- Whitcroft KL, Hummel T. Olfactory Dysfunction in COVID-19: Diagnosis and Management. JAMA. 2020;323(24):2512-2514. 10.1001/jama.2020.8391 [DOI] [PubMed]
- Paderno A, Mattavelli D, Rampinelli V, et al. Olfactory and Gustatory Outcomes in COVID-19: A Prospective Evaluation in Nonhospitalized Subjects. Otolaryngol Head Neck Surg. 2020;163(6):1144-1149. 10.1177/0194599820939538 [DOI] [PMC free article] [PubMed]
- Meinhardt J, Radke J, Dittmayer C, et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. Published online 2020. 10.1038/s41593-020-00758-5 [DOI] [PubMed]
- Morbini P, Benazzo M, Verga L, et al. Ultrastructural Evidence of Direct Viral Damage to the Olfactory Complex in Patients Testing Positive for SARS-CoV-2. JAMA Otolaryngol Neck Surg. 2020;146(10):972-973. 10.1001/jamaoto.2020.2366 [DOI] [PubMed]
- Merkler AE, Parikh NS, Mir S, et al. Risk of Ischemic Stroke in Patients with Coronavirus Disease 2019 (COVID-19) vs Patients with Influenza. JAMA Neurol. 2020;77(11):1–7. 10.1001/jamaneurol.2020.2730 [DOI] [PMC free article] [PubMed]
- Yaghi S, Ishida K, Torres J, et al. SARS-CoV-2 and Stroke in a New York Healthcare System. Stroke. 2020;51(7):2002-2011. 10.1161/STROKEAHA.120.030335 [DOI] [PMC free article] [PubMed]
- Jain R, Young M, Dogra S, et al. COVID-19 related neuroimaging findings: A signal of thromboembolic complications and a strong prognostic marker of poor patient outcome. J Neurol Sci. 2020;414:116923. 10.1016/j.jns.2020.116923 [DOI] [PMC free article] [PubMed]
- Oxley TJ, Mocco J, Majidi S, et al. Large-Vessel Stroke as a Presenting Feature of COVID-19 in the Young. N Engl J Med. 2020;382(20):e60-e60. 10.1056/NEJMc2009787 [DOI] [PMC free article] [PubMed]
- National Hospital Ambulatory Medical Care Survey: 2017 Emergency Department Summary Tables. Centers for Disease Control and Prevention. Accessed January 10, 2021. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2017_ed_web_tables-508.pdf
- Dogra S, Jain R, Cao M, et al. Hemorrhagic stroke and anticoagulation in COVID-19. J Stroke Cerebrovasc Dis. 2020;29(8):104984. 10.1016/j.jstrokecerebrovasdis.2020.104984 [DOI] [PMC free article] [PubMed]
- Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. Published online February 2020. 10.1001/jama.2020.2648 [DOI] [PubMed]
- Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2020;94:91-95. 10.1016/j.ijid.2020.03.017 [DOI] [PMC free article] [PubMed]
- Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed]
- Jordan RE, Adab P, Cheng KK. COVID-19: risk factors for severe disease and death. BMJ. 2020;368:m1198. 10.1136/bmj.m1198 [DOI] [PubMed]
- Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY). 2020;12(7):6049-6057. 10.18632/aging.103000 [DOI] [PMC free article] [PubMed]
- Simonnet A, Chetboun M, Poissy J, et al. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity (Silver Spring). 2020;28(7):1195-1199. 10.1002/oby.22831 [DOI] [PMC free article] [PubMed]
- Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin Infect Dis. Published online April 2020. 10.1093/cid/ciaa415 [DOI] [PMC free article] [PubMed]
- Hoiland RL, Fergusson NA, Mitra AR, et al. The association of ABO blood group with indices of disease severity and multiorgan dysfunction in COVID-19. Blood Adv. 2020;4(20):4981-4989. 10.1182/bloodadvances.2020002623 [DOI] [PMC free article] [PubMed]
- Latz CA, DeCarlo C, Boitano L, et al. Blood type and outcomes in patients with COVID-19. Ann Hematol. 2020;99(9):2113-2118. 10.1007/s00277-020-04169-1 [DOI] [PMC free article] [PubMed]
- Padroni M, Mastrangelo V, Asioli GM, et al. Guillain-Barré syndrome following COVID-19: new infection, old complication? J Neurol. 2020;(Table 1):1-3. 10.1007/s00415-020-09849-6 [DOI] [PMC free article] [PubMed]
- Toscano G, Palmerini F, Ravaglia S, et al. Guillain–Barré Syndrome Associated with SARS-CoV-2. N Engl J Med. 2020;382(26):2574-2576. 10.1056/NEJMc2009191 [DOI] [PMC free article] [PubMed]
- Dalakas MC. Guillain-Barré syndrome: The first documented COVID-19–triggered autoimmune neurologic disease. Neurol - Neuroimmunol Neuroinflammation. 2020;7(5):e781. 10.1212/NXI.0000000000000781 [DOI] [PMC free article] [PubMed]
- Caress JB, Castoro RJ, Simmons Z, et al. COVID-19-associated Guillain-Barré syndrome: The early pandemic experience. Muscle Nerve. 2020;62(4):485-491. 10.1002/mus.27024 [DOI] [PMC free article] [PubMed]
- Trujillo Gittermann LM, Valenzuela Feris SN, von Oetinger Giacoman A. Relation between COVID-19 and Guillain-Barré syndrome in adults. Systematic review TT - Relación entre COVID-19 y síndrome de Guillain-Barré en adultos. Revisión sistemática. Neurologia. 2020;35(9):646-654. 10.1016/j.nrl.2020.07.004 [DOI] [PMC free article] [PubMed]
- Abu-Rumeileh S, Abdelhak A, Foschi M, Tumani H, Otto M. Guillain-Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J Neurol. Published online August 25, 2020:1-38. 10.1007/s00415-020-10124-x [DOI] [PMC free article] [PubMed]
- Rahimi K. Guillain-Barre syndrome during COVID-19 pandemic: an overview of the reports. Neurol Sci. 2020;41(11):3149-3156. 10.1007/s10072-020-04693-y [DOI] [PMC free article] [PubMed]
- Ehrenfeld M, Tincani A, Andreoli L, et al. COVID-19 and autoimmunity. Autoimmun Rev. 2020;19(8):102597. 10.1016/j.autrev.2020.102597 [DOI] [PMC free article] [PubMed]
- Gutiérrez-Ortiz C, Méndez A, Rodrigo-Rey S, et al. Miller Fisher Syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;241:10.1212/WNL.0000000000009619. 10.1212/wnl.0000000000009619 [DOI] [PubMed]
- von Weyhern CH, Kaufmann I, Neff F, Kremer M. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet (London, England). 2020;395(10241):e109. 10.1016/S0140-6736(20)31282-4 [DOI] [PMC free article] [PubMed]
- Cavalcanti D, Raz E, Shapiro M, et al. Cerebral Venous Thrombosis associated with COVID-19. Am J Neuroradiol. 2020;41(8):1-7. 10.3174/ajnr.a6644 [DOI] [PMC free article] [PubMed]
- Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated Acute Hemorrhagic Necrotizing Encephalopathy: Imaging Features. Radiology. 2020;296(2):E119-E120. 10.1148/radiol.2020201187 [DOI] [PMC free article] [PubMed]
- Dixon L, Varley J, Gontsarova A, et al. COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol - Neuroimmunol Neuroinflammation. 2020;7(5):e789. 10.1212/NXI.0000000000000789 [DOI] [PMC free article] [PubMed]
- Reichard RR, Kashani KB, Boire NA, Constantopoulos E, Guo Y, Lucchinetti CF. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020;140(1):1-6. 10.1007/s00401-020-02166-2 [DOI] [PMC free article] [PubMed]
- Jensen MP, Le Quesne J, Officer-Jones L, et al. Neuropathological findings in two patients with fatal COVID-19. Neuropathol Appl Neurobiol. 2020;n/a(n/a). 10.1111/nan.12662 [DOI] [PubMed]
- Jaunmuktane Z, Mahadeva U, Green A, et al. Microvascular injury and hypoxic damage: emerging neuropathological signatures in COVID-19. Acta Neuropathol. 2020;140(3):397-400. 10.1007/s00401-020-02190-2 [DOI] [PMC free article] [PubMed]
- Deigendesch N, Sironi L, Kutza M, et al. Correlates of critical illness-related encephalopathy predominate postmortem COVID-19 neuropathology. Acta Neuropathol. 2020;140(4):583-586. 10.1007/s00401-020-02213-y [DOI] [PMC free article] [PubMed]
- Song E, Zhang C, Israelow B, et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. bioRxiv. Published online September 8, 2020:2020.06.25.169946. 10.1101/2020.06.25.169946 [DOI]
- Schurink B, Roos E, Radonic T, et al. Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. The Lancet Microbe. 2020;1(7):e290-e299. 10.1016/S2666-5247(20)30144-0 [DOI] [PMC free article] [PubMed]
- Al-Dalahmah O, Thakur KT, Nordvig AS, et al. Neuronophagia and microglial nodules in a SARS-CoV-2 patient with cerebellar hemorrhage. Acta Neuropathol Commun. 2020;8(1):147. 10.1186/s40478-020-01024-2 [DOI] [PMC free article] [PubMed]
- Hernández-Fernández F, Valencia HS, Barbella-Aponte RA, et al. Cerebrovascular disease in patients with COVID-19: neuroimaging, histological and clinical description. Brain. 2020;143(10):3089-3103. 10.1093/brain/awaa239 [DOI] [PMC free article] [PubMed]
- Fabbri VP, Foschini MP, Lazzarotto T, et al. Brain ischemic injury in COVID-19-infected patients: a series of 10 post-mortem cases. Brain Pathol. Published online October 1, 2020. 10.1111/bpa.12901 [DOI] [PMC free article] [PubMed]
- Patel SD, Kollar R, Troy P, et al. Malignant Cerebral Ischemia in A COVID-19 Infected Patient: Case Review and Histopathological Findings. J Stroke Cerebrovasc Dis. 2020;29(11):105231. 10.1016/j.jstrokecerebrovasdis.2020.105231 [DOI] [PMC free article] [PubMed]
- Patel HN, Syed A, Lobel JS, et al. Cerebellar infarction requiring surgical decompression in patient with COVID 19 pathological analysis and brief review. Interdiscip Neurosurg Adv Tech case Manag. 2020;22:100850. 10.1016/j.inat.2020.100850 [DOI] [PMC free article] [PubMed]
- Bryce C, Grimes Z, Pujadas E, et al. Pathophysiology of SARS-CoV-2: targeting of endothelial cells renders a complex disease with thrombotic microangiopathy and aberrant immune response. The Mount Sinai COVID-19 autopsy experience. medRxiv. Published online January 1, 2020:2020.05.18.20099960. 10.1101/2020.05.18.20099960 [DOI]
- Remmelink M, De Mendoca R, D’Haene N, et al. Unspecific post-mortem findings despite multiorgan 1 viral spread in COVID-19 patients. Crit Care. 2020;21(1):495. 10.1186/s13054-020-03218-5 [DOI] [PMC free article] [PubMed]
- Paniz-Mondolfi A, Bryce C, Grimes Z, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol. 2020;92(7):699-702. 10.1002/jmv.25915 [DOI] [PMC free article] [PubMed]
- Solomon IH, Normandin E, Bhattacharyya S, et al. Neuropathological Features of Covid-19. N Engl J Med. 2020;383(10):989-992. 10.1056/NEJMc2019373 [DOI] [PMC free article] [PubMed]
- Hanley B, Naresh KN, Roufosse C, et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. The Lancet Microbe. 2020;1(6):e245-e253. 10.1016/S2666-5247(20)30115-4 [DOI] [PMC free article] [PubMed]
- Kantonen J, Mahzabin S, Mäyränpää MI, et al. Neuropathologic features of four autopsied COVID-19 patients. Brain Pathol. Published online August 6, 2020. 10.1111/bpa.12889 [DOI] [PMC free article] [PubMed]
- Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and Neurologic Manifestations of the Coronaviruses in the Age of Coronavirus Disease 2019: A Review. JAMA Neurol. 2020;77(8):1018-1027. 10.1001/jamaneurol.2020.2065 [DOI] [PMC free article] [PubMed]
- Iadecola C, Anrather J, Kamel H. Effects of COVID-19 on the Nervous System. Cell. 2020;183(1):16-27.e1. 10.1016/j.cell.2020.08.028 [DOI] [PMC free article] [PubMed]
- Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82(15):7264-7275. 10.1128/JVI.00737-08 [DOI] [PMC free article] [PubMed]
- Hao XY, Lv Q, Li F Di, Xu YF, Gao H. The characteristics of hDPP4 transgenic mice subjected to aerosol MERS coronavirus infection via an animal nose-only exposure device. Anim Model Exp Med. 2019;2(4):269-281. 10.1002/ame2.12088 [DOI] [PMC free article] [PubMed]
- Politi LS, Salsano E, Grimaldi M. Magnetic Resonance Imaging Alteration of the Brain in a Patient With Coronavirus Disease 2019 (COVID-19) and Anosmia. JAMA Neurol. 2020;77(8):1028-1029. 10.1001/jamaneurol.2020.2125 [DOI] [PubMed]
- Fodoulian L, Tuberosa J, Rossier D, et al. SARS-CoV-2 Receptors and Entry Genes Are Expressed in the Human Olfactory Neuroepithelium and Brain. iScience. 2020;23(12):101839. 10.1016/j.isci.2020.101839 [DOI] [PMC free article] [PubMed]
- Nampoothiri S, Sauve F, Ternier G, et al. The hypothalamus as a hub for putative SARS-CoV-2 brain infection. bioRxiv. Published online January 1, 2020:2020.06.08.139329. 10.1101/2020.06.08.139329 [DOI]
- Kim WK, Corey S, Alvarez X, Williams K. Monocyte/macrophage traffic in HIV and SIV encephalitis. J Leukoc Biol. 2003;74(5):650-656. 10.1189/jlb.0503207 [DOI] [PubMed]
- Trojanowicz B, Ulrich C, Kohler F, et al. Monocytic angiotensin-converting enzyme 2 relates to atherosclerosis in patients with chronic kidney disease. Nephrol Dial Transplant. 2017;32(2):287-298. 10.1093/ndt/gfw206 [DOI] [PMC free article] [PubMed]
- Salamanna F, Maglio M, Landini MP, Fini M. Body Localization of ACE-2: On the Trail of the Keyhole of SARS-CoV-2. Front Med. 2020;7:935. https://www.frontiersin.org/article/10.3389/fmed.2020.594495 [DOI] [PMC free article] [PubMed]
- Bergmann CC, Lane TE, Stohlman SA. Coronavirus infection of the central nervous system: host-virus stand-off. Nat Rev Microbiol. 2006;4(2):121-132. 10.1038/nrmicro1343 [DOI] [PMC free article] [PubMed]
- Neumann B, Schmidbauer ML, Dimitriadis K, et al. Cerebrospinal fluid findings in COVID-19 patients with neurological symptoms. J Neurol Sci. 2020;418:117090. 10.1016/j.jns.2020.117090 [DOI] [PMC free article] [PubMed]
- Espíndola O de M, Siqueira M, Soares CN, et al. Patients with COVID-19 and neurological manifestations show undetectable SARS-CoV-2 RNA levels in the cerebrospinal fluid. Int J Infect Dis. 2020;96:567-569. 10.1016/j.ijid.2020.05.123 [DOI] [PMC free article] [PubMed]
- Bellon M, Schweblin C, Lambeng N, et al. Cerebrospinal fluid features in SARS-CoV-2 RT-PCR positive patients. Clin Infect Dis. Published online August 8, 2020:ciaa1165. 10.1093/cid/ciaa1165 [DOI] [PMC free article] [PubMed]
- Hung ECW, Chim SSC, Chan PKS, et al. Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with severe acute respiratory syndrome. Clin Chem. 2003;49(12):2108-2109. 10.1373/clinchem.2003.025437 [DOI] [PMC free article] [PubMed]
- Zhou L, Zhang M, Wang J, Gao J. SARS-Cov-2: Underestimated damage to nervous system. Travel Med Infect Dis. 2020;36:101642. 10.1016/j.tmaid.2020.101642 [DOI] [PMC free article] [PubMed]
- Virhammar J, Kumlien E, Fällmar D, et al. Acute necrotizing encephalopathy with SARS-CoV-2 RNA confirmed in cerebrospinal fluid. Neurology. 2020;95(10):445-449. 10.1212/WNL.0000000000010250 [DOI] [PMC free article] [PubMed]
- Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620-2629. 10.1172/JCI137244 [DOI] [PMC free article] [PubMed]
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed]
- Song P, Li W, Xie J, Hou Y, You C. Cytokine storm induced by SARS-CoV-2. Clin Chim Acta. 2020;509:280-287. 10.1016/j.cca.2020.06.017 [DOI] [PMC free article] [PubMed]
- Goshua G, Pine AB, Meizlish ML, et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7(8):e575-e582. 10.1016/S2352-3026(20)30216-7 [DOI] [PMC free article] [PubMed]
- Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023-1026. 10.1111/jth.14810 [DOI] [PMC free article] [PubMed]
- Yaqinuddin A, Kvietys P, Kashir J. COVID-19: Role of neutrophil extracellular traps in acute lung injury. Respir Investig. 2020;58(5):419-420. 10.1016/j.resinv.2020.06.001 [DOI] [PMC free article] [PubMed]
- Agarwal AN, Mais DD. Sensitivity and Specificity of Alzheimer Type II Astrocytes in Hepatic Encephalopathy. Arch Pathol Lab Med. 2019;143(10):1256-1258. 10.5858/arpa.2018-0455-OA [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.