Abstract
Glycogen synthase kinase 3 (GSK3) is a monomeric serine-threonine kinase discovered in 1980 in a rat skeletal muscle. It has been involved in various cellular processes including embryogenesis, immune response, inflammation, apoptosis, autophagy, wound healing, neurodegeneration, and carcinogenesis. GSK3 exists in two different isoforms, GSK3α and GSK3β, both containing seven antiparallel beta-plates, a short linking part and an alpha helix, but coded by different genes and variously expressed in human tissues. In the current review, we comprehensively appraise the current literature on the role of GSK3 in various cancers with emphasis on ovarian carcinoma. Our findings indicate that the role of GSK3 in ovarian cancer development cannot be decisively determined as the currently available data support both prooncogenic and tumor-suppressive effects. Likewise, the clinical impact of GSK3 expression on ovarian cancer patients and its potential therapeutic implications are also limited. Further studies are needed to fully elucidate the pathophysiological and clinical implications of GSK3 activity in ovarian cancer.
Keywords: GSK3, ovarian cancer, therapeutic target
INTRODUCTION
According to the European Union (EU) statistics, almost 30,000 women died due to ovarian cancer throughout the Union in 2018. That accounts for 5% of all cancer-related deaths among women in the EU [1]. Because of lack of early symptoms and effective screening options [2], ovarian cancer is often diagnosed at advanced FIGO stages [3] and is responsible for the highest mortality rates among gynecologic malignancies, including breast cancer. Therefore, there is an unmet need to comprehensively study the molecular background of this disease in order to devise and institute targeted therapy.
Glycogen synthase kinase 3 (GSK3) is a monomeric serine-threonine kinase discovered in 1980 in a rat skeletal muscle. Although numbered 3, it is the only enzyme that has been proven to phosphorylate glycogen-synthase, whereas the enzymes previously being called GSK1 and 2 do not have that characteristic [4]. GSK3 has been found to play an important role in various cellular processes, including embryonic development, immune response, inflammation, apoptosis, autophagy, wound healing, neurodegeneration, and carcinogenesis [5-9]. GSK3 has two isoforms, GSK3α and GSK3β, both containing seven antiparallel beta-plates, a short linking part and an alpha helix, but coded by different genes and variously expressed in human tissues. GSK3β is coded by GSK3B gene on the long arm of chromosome 3 [10] and is more abundantly expressed in natural killer (NK) cells, bone marrow granulocytes, and ovaries [11]. Its molecular mass is slightly lower than that of GSK3α, as it does not contain glycine-rich N-terminal domain. It is located in both the nucleus and cytoplasm [12]. Expression of one isoform cannot compensate for the loss of another, which is especially important during embryonic development, when the loss of GSK3B gene is lethal [13].
Phosphorylation of tyrosine 216 (Y216) is responsible for the constitutive activity of GSK3β [14], which is autophosphorylation during the translation process [15]. Peculiarly, GSK3β is a tyrosine-kinase during its formation but later assumes the function of a serine-threonine kinase [16]. Other kinases, such as PYK2 and Fyn, as well as proapoptotic signals, can also complete that phosphorylation [17-19]. Although constitutively active, priming phosphorylation by other kinases is usually required for GSK3β substrates [20]. On the other hand, phosphorylation of serine 9 (S9) of the GSK3β blocks the substrate binding and inactivates the enzyme [14]. Many kinases are involved in that process. Protein-kinase A, activated by cyclic adenosine monophosphate (cAMP), Akt (protein-kinase B, PKB), activated through the PIP3/mTOR pathway, and integrin-linked kinase (ILK) are the main factors performing S9 phosphorylation. Phosphorylations of some other sites that also inhibit the enzyme activity (by extracellular signal-regulated kinase [ERK], for example) [21], as well as dephosphorylations of S9 by protein phosphatases that resume the catalytic activity [22], are possible too (Figure 1).
FIGURE 1.
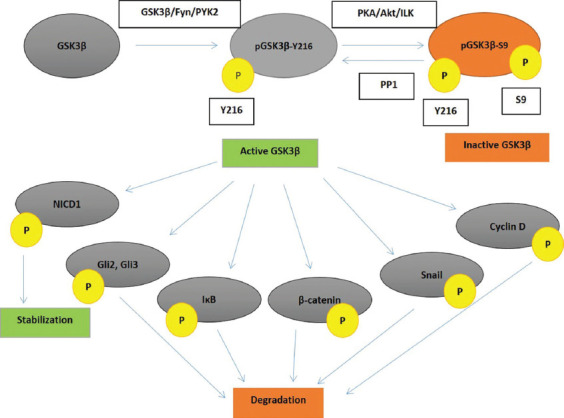
GSK3β activation, inactivation, and activity towards its key substrates. GSK3β – glycogen synthase kinase 3β; pGSK3β-Y216 – glycogen synthase kinase 3β phosphorylated at tyrosine 216; pGSK3β-S9 – glycogen synthase kinase 3β phosphorylated at serine 9; Y216 – tyrosine 216; S9 – serine 9; PKA – protein kinase A; Akt – protein kinase B; ILK – integrin-linked kinase; PP1 – protein-phosphatase 1; NICD1 – Notch 1 intracellular domain; Gli – glioma-associated oncogene; IκB – inhibitor κB; P – phosphate group; PYK2 – tyrosine protein kinase 2.
GSK3β participates in many cellular signaling pathways (Figure 1). It often phosphorylates more than one component of a pathway and has pleiotropic, often opposite, effects on that same pathway and cell proliferation.
Within the Wnt signaling pathway, GSK3 is in a protein complex with casein kinase 1 (CK1), axin, and adenomatous polyposis coli (APC). It normally phosphorylates β-catenin, having previously been phosphorylated by CK1, which is consequently ubiquitinated and degraded in the proteasome [23]. Upon Wnt pathway activation, the protein complex GSK3/CK1/axin/APC is disintegrated, and unphosphorylated β-catenin is translocated into the nucleus, where it activates the transcription of various protooncogenes, such as MYC, JUN, cyclins, matrix-metalloproteinase, and MDR1, which encodes p-glycoprotein, a transporter responsible for chemotherapy resistance [23]. It seems that GSK3 activity towards β-catenin does not depend on its phosphorylation status on S9, which appears to be a protective mechanism for this important pathway when GSK3 is aberrantly phosphorylated by some kinase [24,25]. For instance, highly active Akt does not fully inhibit GSK3β activity in pancreatic and colon cancer cells [26,27]. However, a more recent study on human colorectal cancer cell lines indicates that hyperactive Akt causes GSK3β inhibition and β-catenin accumulation [28]. Apart from phosphorylating β-catenin, GSK3 can phosphorylate low density lipoprotein receptor-related protein (LRP) molecule and thus reveal a binding site for axin on LRP, which mimics pathway activation by a Wnt ligand [29].
Full-length Gli2 and Gli3 are the key GSK3 substrates among the components of the Hedgehog signaling pathway [30]. Upon phosphorylation, Gli2 is usually completely degraded in the proteasome, whereas Gli3 is processed into a transcription repressor [31,32]. In a case of GSK3β inactivation, full-length Gli proteins are activated by other kinases and translocated into the nucleus, where they activate transcription of the Hedgehog pathway components genes GLI1 (which in turn acts as a transcription activator independent on inactivation by GSK3) and PTCH, protooncogenes IGF2 and MYC, as well as apoptosis inhibitor BCL2 [13]. Besides that, by phosphorylating suppressor of fused homolog (SuFu), GSK3β reduces SuFu’s potential to bind to Gli, which in turn accumulates in the nucleus and activates transcription [33]. Despite the traditional view of GSK3β being inactivated by S9 phosphorylation, recent studies by Trnski et al. suggest that, regarding phosphorylation of Gli3, pGSK3β-S9 is actually an active and pGSK3β-Y216 an inactive form of GSK3β [34]. Furthermore, treating colon cancer cells (both cell lines and ex vivo specimens) with GSK3β inhibitor lithium chloride (LiCl) enhances its phosphorylation on S9 and promotes the formation of Gli3/SuFu/GSK3β complex, with consequent phosphorylation and inactivation of Gli3. In contrast, GSK3 knockdown in cells causes Hedgehog pathway activation [34], which indicates that pGSK3β-S9 is not necessarily inactive.
In the NFkB signaling pathway, GSK3 activates a kinase cascade resulting in phosphorylation of inhibitory IkB and its dissolution from nuclear factor-kB (NFkB) [35], which is translocated into the nucleus and activates transcription. The effect is the same when GSK3 phosphorylates inhibitory p100 molecule in multiple myeloma cells [36]. On the other hand, phosphorylation of p65 in HeLa cervical cancer cells [37] and IkB kinase (IKK) inhibition together with IkB stabilization in human astrocytes [38] makes GSK3β also a negative regulator of this pathway.
Notch, a pathway usually associated with suppression of cell differentiation [39], is activated by the detachment of its intracellular domain (Notch intracellular domain – NICD) and its nuclear translocation. GSK3β phosphorylation in Ser/Thr – Pro – Ser/Thr regions stabilizes NCID in Notch 1 [40,41] and Notch 3 [42], and inactivates it in Notch2 [43]. Mitigating Notch1 recycling is likewise an example of negative regulation of this pathway by GSK3β [44].
In response to transforming growth factor beta (TGF-β), ERK kinase inactivates GSK3β, resulting in Snail protein accumulation [45,46] and promotion of epithelial-mesenchymal transition (EMT), which is characterized by the loss of cell polarity and serves as a pre-condition for tumor invasion and metastasis [11]. In contrast, GSK3 decreases cell susceptibility to TGF signaling by phosphorylating Smad3 [47].
Interestingly, GSK3 may act as both a proapoptotic and an antiapoptotic factor, depending on cell type and signaling environment [13]. GSK3β promotes apoptosis in conditions of hypoxia [48] and DNA damage [49] by inhibiting cell survival signals like cAMP-response element binding (CREB) protein and heat-shock protein 1 [50] and activating proapoptotic transcription factors such as p53 [49]. The relationship between GSK3 and p53 is complex. Phosphorylation of p53 (previously phosphorylated by DNA-protein-kinase) by GSK3β blocks its interactions with inhibitory mouse double minute 2 homolog (MDM2), thus contributing to the stabilization of p53 and its activity as a transcriptional activator of proapoptotic genes [51]. In addition, activity of GSK3β increases in interactions with p53 by a phosphorylation independent mechanism [49]. Moreover, inhibition of GSK3β is shown to promote GSK3β, MDM2, and p53 sequestration in the cytoplasm, where p53 cannot act as a transcriptional activator [52]. However, inhibition of GSK3β can lead to MDM2 hypophosphorylation, p53 accumulation and consequent apoptosis, which suggests an antiapoptotic activity of GSK3β. By phosphorylating Bax, GSK3β localizes it to mitochondria and induces its proapoptotic activity [53,54]; however, GSK3 inhibition also promotes apoptosis, by hypophosphorylation, and thus decreasing activity, of apoptosis regulator Bcl2 [55,56].
The effects of GSK3 on the process of autophagy are more straightforward as GSK3 opposes it not only by promoting glucose metabolism and utilization, but also by phosphorylating protein raptor (regulatory associated protein of mTOR), which activates mTORC1 complex and inhibits autophagy [57].
GSK3 is actively involved in cell cycle regulation since the key factors (cyclins, cyclin-dependent kinases, key points regulators, and transcription factors) are among its substrates [13,58]. GSK3 phosphorylates cyclins D and E, important for transition from G1 to S phase, and causes their degradation [59,60]. Transcription factors c-myc and c-fos (also S phase promoters), which are phosphorylated primarily by dual-specificity tyrosine phosphorylation-regulated kinase (DYRK), are also phosphorylated by GSK3β, which guides them toward degradation [61]. On the other hand, GSK3β inhibition in pancreatic cancer cells suppresses Rb phosphorylation by cyclin D/cyclin-dependent kinase 4 or 6 complex and its subsequent degradation [62].
EMT is characterized by the loss of functional E-cadherin (encoded by the CDH1 gene). As already mentioned, GSK3 phosphorylates and inhibits Snail protein, the CDH1 gene transcription repressor, thus indirectly increasing E-cadherin expression [63]. GSK3β also contributes to this by the degradation of β-catenin that is bound to E-cadherin [23]. GSK3β takes part in decreasing matrix-metalloproteinase expression and is therefore thought to maintain a stem cell phenotype in tumor cells, but without diminishing their metastatic potential [64].
The role of GSK3 in tumor formation and promotion is controversial, as it has diverse effects on numerous cellular processes and pathways, which can also differ among different cell types. There is a clear evidence for both prooncogenic and tumor-suppressive effects of GSK3. Several illustrative examples will be briefly described here, although the list should not be considered exhaustive.
In glioblastoma multiforme, the most common and most lethal brain tumor, GSK3 inhibitors facilitate apoptosis through inhibition of antiapoptotic mechanisms in mitochondria and inhibit the NFkB pathway that is essential for cell survival [56,65]. GSK3β also seems to be responsible for NFkB aberrant activity in pediatric acute lymphoblastic leukemia [66] and pancreatic cancer [67] cells. In renal cancer cells, GSK3 inhibitors induce cell cycle arrest, differentiation of the malignant cells, and autophagy [68].
In contrast to the neoplasms mentioned above, in skin [69], oral [70], and lung [71] cancers a high expression of inactive pGSK3β-S9 is found, which suggests tumor-suppressing effects of the enzyme in those cancers. In melanoma, microRNA miR-769 inhibits GSK3β activity during the tumor development process, which also indicates tumor-suppressing effects of GSK3 [72].
GSK3 IN OVARIAN CANCER DEVELOPMENT
Recognition that GSK3 is a factor in ovarian cancer development dates back to the late 1990s, when the analysis of 61 ovarian cancer samples of different histologic types in Japan revealed that 33% of endometrioid and 14% of mucinous carcinoma specimens had β-catenin mutations changing serine and threonine residues targeted by GSK3. The patients with these mutations had more abundant nuclear β-catenin expression and were on average 10 years younger than the mean age of all included patients [73]. Rask et al. (2003) were the first to discover GSK3β overexpression in ovarian cancer cells [74] (Table 1), with increased expression of β-catenin and lower expression of APC also described. In ovarian cancer cell lines, a nuclear localization of β-catenin has been reported, which is not the case in ovarian cancer tissue samples. That was explained by E-cadherin overexpression, which binds β-catenin into a perimembranous protein complex, which raised the question of β-catenin being included in ovarian cancer development as a transcription activator, cellular integrity stabilizer or both [74]. Further investigations by Usongo et al. revealed a perimembranous expression of β-catenin in normal ovarian epithelial cells, in contrast to those stimulated by Wnt3 or by GSK3 inhibitor LiCl, in which β-catenin was expressed in the cell nucleus. However, despite nuclear localization of β-catenin, its target genes’ transcription is not elevated [75].
TABLE 1.
A summary of various GSK3 effects on ovarian cancer cell growth
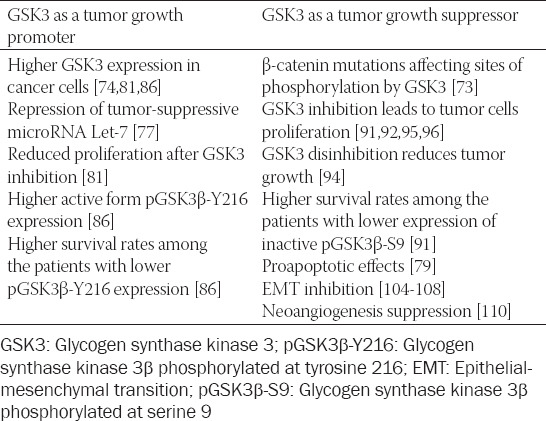
Investigations pertaining to the Hedgehog and p53 pathways have been less extensive. However, it has been found that GSK3 inhibition reduces expression of activating Gli3A and PTCH (Hedgehog pathway activity indicator) [76]. It has also been found that GSK3β reduces the expression of tumor-suppressor microRNA Let-7 in ovarian cancer cells, and it seems that p53 is involved in the process. In contrast to the expectations, p53 expression is lower when GSK3β is active and increases after GSK3β inactivation [77]. A possible explanation is that damaged protein glycosylation in cancer cells causes accumulation of those proteins and subsequent endoplasmic reticulum stress. That condition, by a still unknown mechanism, facilitates GSK3-mediated phosphorylation of p53 at serine 376, which acts as a degradation signal [78]. On the other hand, investigations of resveratrol as an apoptotic inducer through GSK3 activation, N-glycosylation suppression, and endoplasmic reticulum stress suggest a proapoptotic role of GSK3 and endoplasmic reticulum stress [79].
Wulfkuhle et al. found that GSK3β expression varies more between the specimens of the same histologic type than among different ovarian cancer histologic types, which made them emphasize an individual approach to every patient [80]. Cao et al. found a higher immunohistochemical expression of GSK3β in ovarian cancer cells, whereas expression of the enzyme phosphorylated on serine 9 (pGSK3β-S9) varied among two cell lines included in the study. The treatment with LiCl increased pGSK3β-S9 expression, while overall expression remained unchanged. The treatment with LiCl also caused a decrease in cell number, which led to the conclusion that GSK3β promotes ovarian cancer cell proliferation and tumor growth (Table 1) [81]. Cyclin D levels were also considerably lower after GSK3β inhibition, which was in contrast to the common belief that GSK3β phosphorylates cyclin D and facilitates its degradation [81]. The importance of cyclin D lies in the fact that it can induce cell cycle arrest even when cyclin E is constitutively active [82]. Cao et al. therefore suggest that GSK3β is the key driving force in ovarian cancer development, the explanation being its metabolic effects, namely decreased glycogenesis and increased glucose utilization through the process of glycolysis [81]. This view is supported by the facts that cancer cells have higher metabolic needs [83] and depend heavily on glycolysis [84]. The authors also emphasize the prooncogenic effects of GSK3β on the NFkB pathway [81], although it has previously been said that those effects could be tumor suppressive as well. Glycolysis hypothesis is also supported by the findings that glycolysis inhibitor apatinib (which also has other effects such as vascular endothelial growth factor [VEGF] receptor 2 inhibition, Akt inhibition, and GSK3 reactivation) significantly reduces ovarian cancer masses both in vitro and in mouse transplants in vivo [85]. Fu et al. confirmed GSK3β overexpression in ovarian cancer cells and found that active form pGSK3β-Y216 is also highly expressed [86], which again favors prooncogenic role of the enzyme. Furthermore, higher GSK3β levels are associated with advanced FIGO stages and elevated CA-125 levels [86]. Overexpression of pGSK3β-Y216 correlates not only with FIGO stage and CA-125 levels but also with the residual tumor masses and unfavorable chemotherapy outcomes. The survival analysis revealed that patients with lower levels of GSK3β and pGSK3β-Y216 had significantly higher overall survival. Finally, GSK3β inhibition leads to tumor growth retardation both in vitro and in vivo: whereas tumor masses in mice were significantly lower throughout the entire experiment, tumor volumes were significantly lower during most of the study, but not at the very end [86].
Findings about GSK3 overexpression and hyperactivity prompted further research on its upstream regulation, mainly protein kinase B (PKB, Akt), which is believed to phosphorylate GSK3β on S9 and cause its inactivation. Do et al. studied activin A, peptide hormone that participates in follicle development and FSH β-chain expression regulation [87,88]. It can act in both Smad-dependent (like TGF-β) and Smad-independent (through mitogen-activated protein [MAP] and phosphatidylinositol-3,4,5-trisphosphate [PIP3] kinases) way [89]. Activin A has no effect on normal ovarian cell proliferation but can boost cancer cell proliferation [90]. Do et al. showed that activin A activates Akt, which in turn phosphorylates GSK3β on serine 9 and inactivates it to a greater extent than GSK3α does. Immunohistochemical expression of pGSK3β-S9 differs depending on tumor malignant potential. Thus, in benign ovarian neoplasms, pGSK3β-S9 expression is predominantly localized (polarized) around apical gap junctions, whereas in malignant neoplasms (carcinomas) its expression is diffuse. Borderline tumors have mixed expression, both polarized and diffuse. Inactivation status of GSK3β is associated with survival, i.e., patients with lower levels of inactivated GSK3β live longer (Table 1) [91]. This study supports suppressive effects of GSK3β on ovarian cancer development, and similar findings were reported by Cianfrocca et al. when studying endothelin-1 effects. Apart from GSK3β phosphorylation through the cascade endothelin-1 receptor → β-arrestin → PIP3 kinase → ILK → Akt → GSK3β, it was shown that endothelin-1 receptor activation facilitates removal of GSK3β from the complex that phosphorylates β-catenin, which leads to its degradation [92]. Oral hypoglycemic drug metformin reduces ovarian cancer cells growth in Akt-depending manner. It activates AMP kinase [93], which phosphorylates and inhibits Akt, leading to GSK3β disinhibition. Cyclin D is then phosphorylated by GSK3β and degraded, resulting in cell cycle arrest [94]. Polyphenol resveratrol, apart from causing endoplasmic reticulum stress that has already been mentioned, inhibits both Akt and ERK, thus facilitating GSK3β reactivation on OVCAR3 cell line, with a consequent lower expression of cyclin D1 and reduced cancer cell number [95]. Other Akt inhibitors, such as GSK690693, GSK2141795, GDC-0068, MK-2206, AKT-IN-1 and AKT-IN-2, also have the potential to inhibit the growth of ovarian tumors transplanted into mice and some of them are being studied in clinical trials [96].
GSK3 IN EPITHELIAL-MESENCHYMAL TRANSITION, NEOANGIOGENESIS, AND METASTASIS
Due to their mesothelial origin, ovarian surface cells normally express mesenchymal N-cadherin, rather than epithelial E-cadherin [97]. Once affected by metaplasia, they start expressing E-cadherin, which can promote the EMT in ovarian surface cells [98]. Early carcinoma cells express E-cadherin, whereas advanced carcinoma cells, and particularly metastases, are characterized by low E-cadherin expression [99], which suggests that a loss of E-cadherin is a pre-condition for metastasis. Apart from β-catenin, it is thought that Snail protein plays a key role in EMT induction. Indeed, patients with higher Snail expressions have significantly shorter overall survivals [100]. It appears that the TGF-β and Wnt pathways, activated alone, promote EMT; however, when activated together, those pathways actually suppress EMT [101]. Moreover, GSK3β inhibitor concentration affects β-catenin level, which in turn decides the destiny of EMT. Lower doses of LiCl and lower β-catenin levels suppress EMT, while higher doses of LiCl and higher β-catenin concentrations have the opposite effects [101].
Endothelin receptor activation, besides its effects on proliferation, also promotes EMT due to decreased β-catenin and Snail degradation after Akt activation and GSK3 inactivation. In that study, E-cadherin levels were lower and N-cadherin expression reappeared [102]. Forkhead box protein C2 (FOXC2) has similar effects by activating Akt and/or ERK in platinum-resistant ovarian cancer cell lines [103]. The role of GSK3β in EMT suppression is strongly supported by the fact that compounds that reduce serine 9 phosphorylation of the enzyme (like emodin, anthraquinone derived from rhubarb and aloe, and sPSB3 molecule) inhibit EMT in ovarian cancer cell lines (Table 1) [104-106]. Several microRNAs, such as miR16 and miR203a-3p, may exhibit similar effects, although their precise mechanisms are not known yet [107,108].
The study of Burkhalter et al. showed that integrins α2, α3, and β1 have an important and complex role in EMT and β-catenin levels control: they reduce membranous E-cadherin expression, probably by E-cadherin rearrangement, as they do not reduce the overall expression of E-cadherin. That reduction promotes EMT. Also, β-catenin liberated from the complex with E-cadherin is translocated into the nucleus, where it promotes gene transcription, including those coding for matrix-metalloproteinase, which again promotes EMT and may account for Wnt hyperactivity even without genetic or epigenetic alterations of its components. On top of that, b1 integrin activates ILK kinase that inhibits GSK3β, which leads to further accumulation of β-catenin [109].
When oxygen supply is normal, GSK3β suppresses neoangiogenesis by phosphorylating hypoxia-inducible factor 1 (HIF1), which increases its affinity towards ubiquitin-ligase and its subsequent ubiquitination and degradation. In hypoxia, however, GSK3β activity decreases and stabilized HIF1 promotes the VEGF gene transcription [110]. VEGF then induces neoangiogenesis and affects neighboring cells by activating Akt and further inhibiting GSK3β [111].
THE ROLE OF GSK3 IN CHEMOTHERAPY RESISTANCE
Vincristine
A repeated exposure of SKOV3 cell line (metastatic ovarian adenocarcinoma) to vincristine revealed a subpopulation of cells resistant to vincristine. The analysis of those cells showed that the resistant cells had increased expression of Wnt family member 5a (Wnt5a), survivin, and β-catenin and elevated phosphorylation of Akt and GSK3β (on serine 9). WNT5A gene silencing, as well as PIP3 kinase inhibition, succeeded to reduce survivin and β-catenin levels and Akt and GSK3β phosphorylation. Therefore, it seems that aberrantly active Wnt pathway favors vincristine resistance through GSK3β inhibition [112].
Paclitaxel
In ovarian cancer cells resistant to paclitaxel, an increased expression of Dishevelled protein, a positive regulator of Wnt pathway, was observed. Downstream of Dishevelled, S9 phosphorylation of GSK3β and β-catenin levels were increased, as were the expression of its target genes, such as MDR1 and BCL2, which seems to be the key features of resistance to paclitaxel. Akt is again involved in GSK3β inhibition, as Akt inhibitors were able to restore GSK3β activity [113]. Another research group reported four times higher GSK3 expression in resistant cells. Since SKOV3 cells used in the experiment normally do not express GSK3α, it appears that at least part of that increase was due to GSK3β [114]. However, expressions of phosphorylated forms were not assessed, and it is therefore possible that the expression was elevated due to S9 phosphorylated, inactive, form.
Cisplatin
Ovarian cancer cells resistant to cisplatin had similar overall expression of GSK3β and of pGSK3β-Y216, but significantly lower expression of silenced pGSK3β-S9 in comparison to the source cell line. The protective role of GSK3β toward cisplatin resistance is additionally supported by the facts that the cells treated with LiCl have lower cell death rates when exposed to cisplatin, that inhibitory concentration 50 (IC50) of cisplatin for those cells is significantly higher, and that those two findings are absent in cells with constitutively active pGSK3β-S9A, not prone to serine 9 phosphorylation [115]. Tangeretin, a flavonoid from citrus fruit, succeeded to restore cisplatin sensitivity to resistant cells, but the effect was 60% lower if the cells were pre-treated with GSK3β inhibitor LiCl [116]. Another study revealed a substantially lower GSK3β activity in cisplatin resistant cells compared with the source cell line. Akt activities were similar, but ERK activities were elevated in cisplatin-resistant cells [117].
GSK3 AS A PROGNOSTIC FACTOR
The clinical data on the prognostic role of GSK3 in ovarian cancer patients are currently limited. Two studies that analyzed survival related to GSK3 status have already been presented above: Fu et al. found higher survival rates among the patients with lower GSK3β and active pGSK3β-Y216 expression, whereas Do et al. claimed survival rates to be higher among the patients with lower expression of inactive pGSK3β-S9 [86,91]. The third study, conducted by Shin et al. and restricted only to serous ovarian carcinomas, found that among patients with higher GSK3β activity there were fewer at advanced FIGO stages. However, the results did not reach the significance level [5]. The same study found that, in contrast to GSK3β expression, DNA-protein kinase, Akt3, and p53 overexpression clearly correlated with shorter survival [5].
GSK3 AS A POTENTIAL THERAPEUTIC TARGET
Since the exact function of GSK3 in ovarian cancer development is still unclear, both its activation and inhibition have been tried as therapeutic options.
Activation of GSK3 as a therapeutic option
The study in which metformin-induced AMP kinase activation caused Akt inhibition and GSK3β restitution, with subsequent cyclin D degradation and cell cycle arrest, has already been discussed above [94]. Regardless of mechanism, several studies found a positive effect of metformin on survival of ovarian cancer patients [118,119], which was confirmed in a recently published meta-analysis study [120].
Akt inhibitors disinhibit GSK3, and a number of them have been investigated in clinical studies for their therapeutic potential (Tables 2 and 3).
TABLE 2.
Akt inhibitors in current clinical trials for ovarian cancer treatment (https://clinicaltrials.gov/ct2/results?cond=Ovarian+Cancer+&term=Akt&cntry=&state=& city=&dist=)
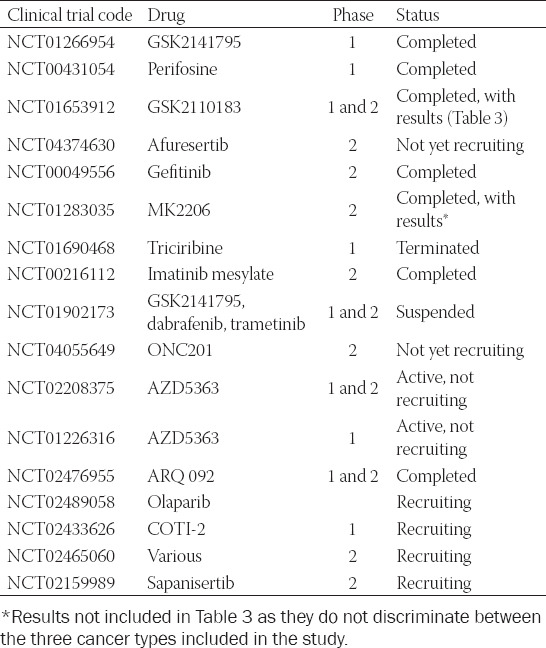
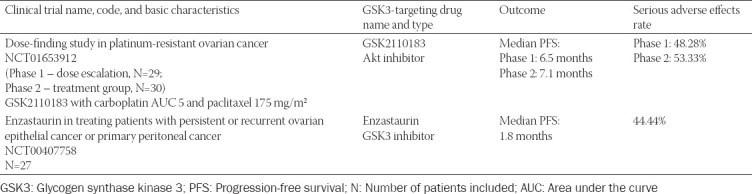
TABLE 3.
GSK3-targeted ovarian cancer therapy – clinical trials with published results
In addition to conventional drugs, some phytochemicals and Chinese traditional medicine products have also been investigated. Thus, fungus Trametes robiniophila (Chinese: Huaier) and Fuling granules (mixture of Aconitum napellus, Wolfiporia extensa, Patrinia heterophylla, and the root of Paeonia rubra), both traditional Chinese medicines, act as Akt inhibitors, reduce β-catenin expression, and induce apoptosis. Fuling granules, in addition, inhibit the TGF-β pathway and suppress EMT. Polyphenol resveratrol, a red wine component, which is an anti-inflammatory agent and protects against oxidative stress, has various effects on tumor mass reduction [121]. It can inhibit Akt and ERK and restore GSK3β activity in human ovarian cancer cell lines in vitro [95] and induce apoptosis of malignant cells [79].
Inhibition of GSK3 as a therapeutic option
Several studies took the opposite approach – to inhibit GSK3 when it is overactive.
LiCl, already discussed in this review, inhibits GSK3 in two ways: it competes with magnesium ions for the binding site on the enzyme and, as its charge is 1+ compared to magnesium’s 2+, changes charge density around the enzyme [122]. LiCl also favors S9 phosphorylation of GSK3β [123]. In contrast to Cao et al., who found reduced cell number and reduced cyclin D expression after exposure to LiCl, as well as reduced tumor mass in vivo, more recent study by Novetsky et al. found a minimal activity of LiCl toward tumor mass reduction and insignificant effects of LiCl when combined with paclitaxel or cisplatin. According to their results, only tumor cells’ metabolism reduction was significant and only after the exposure of cells to 10 mM LiCl (the concentration used by Cao et al. as well), whereas 1 mM LiCl had no effect at all [81,124].
Compound 9ING41 also fosters S9 phosphorylation but acts also as a competitive inhibitor at the GSK3 enzyme ATP binding site. An increased cyclin D1 expression was seen in 9ING41-treated SKOV3 cells, which is consistent with the hypothesis that GSK3β inhibition leads to accumulation of cyclin D1. Surprisingly, however, the proliferation rate of those cells was not elevated, and an increase in apoptosis was seen. 9ING41 treatment reduced tumor masses in mice with transplanted human ovarian cancer cells and no adverse effects were noticed. LiCl in this experiment failed to reduce tumor masses in vivo [125]. There is a clinical trial currently recruiting participants that aims to assess 9ING41 activity toward advanced cancers, including ovarian cancer: (https://clinicaltrials.gov/ct2/show/record/NCT03678883?term=GSK-3%CE%B2&cond=Ovarian+Cancer&draw=2&rank=1).
(2Z,3E)-6-bromoindirubine-3’-oxime (BIO) is a competitive GSK3β inhibitor at ATP binding site that proved to be efficient in a proliferation suppression and cancer cell number reduction in several cancer cell lines, such as breast, pancreatic, osteosarcoma, and melanoma lines [126-129]. Yu and Zhao showed that BIO exhibits the same effects on ovarian cancer cell lines, along with reduced number of cells in S and M-phase and reduced number of invasive and migrating cells. The mechanisms that can account for these effects are decreased expressions of GSK3β, β-catenin, matrix-metalloproteinase, and their corresponding mRNA molecules [130].
AZD1080, another ATP binding site inhibitor, reduces the expressions of GSK3β, cyclin D1, cyclin-dependent kinases 1 and 2, matrix-metalloproteinase 9 and antiapoptotic molecule B-cell lymphoma-extra large (Bcl-xL), and blocks proliferation, filopodia production, invasiveness, and metastasis of ovarian cancer cell lines [131].
Bearing in mind that a competitive inhibition may be overridden by increased substrate concentrations, Gao et al. developed a series of benzothiazinone uncompetitive (allosteric) GSK3β inhibitors, the majority of which expressed moderate antiproliferative activity on ovarian cancer cell lines. Their tumor-suppressive activity on A2780 and OVCA433 lines differed depending on the numbers of carbon atoms in their structures, the compound named 20 g being most specific towards GSK3β and most effective in suppressing malignant cell growth. Its effects were confirmed in vivo in mice with transplanted human cancer cells [132].
Ursolic acid from Oldenlandia diffusa induces apoptosis in leukemia, melanoma, prostatic, breast, and colon cancer cells by affecting various signaling pathways (Akt, NFkB, ERK, and c-Jun) and oxygen reactive species concentrations [133-136]. In SKOV3 and A2780 cells, ursolic acid enhances S9 phosphorylation of GSK3β and its inactivation, β-catenin stabilization and accumulation and, surprisingly, reduces expression of cell survival signals (c-myc and Bcl-xL) and matrix-metalloproteinase, which in turn facilitates apoptosis and reduces tumor invasiveness [137].
GSK3β inhibitor enzastaurin entered phase two clinical trials in three independent studies. The results of one of these studies have been published: 27 ovarian cancer patients were treated with enzastaurin, having a median overall survival of 15.1 months and median progression-free survival of just 1.8 months, whereas 12 patients experienced serious adverse effects (https://clinicaltrials.gov/ct2/show/results/NCT00407758?term=enzastaurin&cond =Ovarian+Cancer&draw= 2&rank=2; Table 3).
DISCUSION AND CONCLUSION
The role of GSK3 in ovarian cancer development cannot be decisively determined as the currently available data support both prooncogenic and tumor-suppressive effects (summarized in Table 1).
To elucidate the exact role of GSK3 in ovarian cancer development further research is necessary. In ovarian cancer cells, the impact of GSK3 on some signaling pathways (e.g., the Notch pathway) has not been studied yet, which is important because GSK3 has different effects in different cells and under different circumstances. Also, signaling pathways have been usually examined individually and separately from other pathways, thus losing the opportunity to get a broader insight into the pleiotropic effects of GSK3 and potential crosstalk between various pathways at the level of GSK3 [138].
The studies concerning GSK3 expression in ovarian cancer cells most commonly report its overexpression, independently on the cell lines used. One study also assessed the expression of the active form pGSK3β-Y216 and found its overexpression as well, which led to the conclusion that GSK3β is both overexpressed and overactivated in ovarian cancer cells [86]. However, the expression of the inactive form pGSK3β-S9 was not measured in these experiments although it can possibly account for the overexpression. Despite that, and despite the fact that overexpression does not necessarily mean overactivation, these studies clearly propose GSK3 hyperactivity in ovarian cancer cells and its role as a driving force in cancer development [74,81,86].
On the other hand, the studies concerning the upstream control of GSK3 activity, especially Akt kinase, suggest that GSK3 is a tumor growth suppressor because activated Akt inhibits GSK3 and β-catenin subsequently accumulates. These findings are supported by the fact that after Akt inhibition, GSK3 activity is reinstated and proliferation slows down [94]. In this group, the results are also consistent independently of the cell line used. However, aberrant pathway activity may be a result of the mutations of its components, regardless of GSK3 activity. For example, CTNNB1 gene mutations targeting sites phosphorylated by GSK3 on β-catenin [73], mutations of positive pathway regulators that cause its constitutive activity (such as Frizzled), mutations or epigenetic silencing of negative regulators, and mutations of proteins in a complex with GSK3 may all cause Wnt pathway hyperactivity [139]. Besides, β-catenin levels are controlled not only by GSK3 but also by E-cadherin levels [109].
Taking into account these opposite findings, it is surprising that only the study of cisplatin resistance by Cai et al. assessed the expression of both pGSK3β-S9 and pGSK3β-Y216 on the same specimens, the only difference between the cells sensitive and resistant to platinum being elevated expression of inactive pGSK3β-S9 in the subpopulation of resistant cells [115].
A more detailed mechanism of S9 phosphorylation suggests that taking pGSK3β-S9 as an active and pGSK3β-Y216 as an inactive form might be oversimplified. Phosphate from S9 lies in the site that recognizes the phosphate group of the pre-phosphorylated substrate. Thus, for substrates that have not undergone priming phosphorylation, this is not a mechanism that prevents phosphorylation by GSK3β [140]. Furthermore, this inhibition is a competition between two phosphate groups, which can then be overcome by a higher substrate concentration [140]. Finally, phosphorylation status of S9 is in a dynamic equilibrium, i.e., it fluctuates, which means that S9 phosphorylation does not completely reduce the catalytic activity of GSK3β [140]. Similar findings that S9 phosphorylation does not affect GSK3β activity on the Wnt pathway [24,25] and that “inactive” pGSK3β-S9 form is actually the one that phosphorylates Gli3 in the Hedgehog pathway [34], also support this view. It seems that GSK3β as a part of protein complexes (axine-APC-CK1-GSK3β and Gli3-GSK3β-SuFu) is not inhibited by S9 phosphorylation. Taking the aforementioned into consideration, it appears that taking S9 phosphorylation as a fixed on/off mechanism could not be flexible enough to study the role of the enzyme in cancer development and progression. Similar view is advocated by Mitra and Roy, who suggested that the cell fate depends on GSK3β inhibitor and β-catenin concentrations, where lower inhibitor concentrations suppressed invasiveness while higher concentrations facilitated it [101]. We believe that a critical concentration of β-catenin is necessary to induce EMT.
The current evidence indicates that in spite of the significance that is given to GSK3 in ovarian cancer pathogenesis, it has not been shown useful in the prognosis of the patients with ovarian cancer. The study regarding GSK3β expression found higher survival rates among the patients with lower GSK3β expression [86], whereas the study concerning Akt kinase found higher survival rates among the patients with lower expression of inhibited pGSK3β-S9 [91]. Another study found no significant correlation between survival and GSK3β expression [5].
As a therapeutic approach, both activation and inhibition have been reported successful, but further investigations of both effects are needed, to determine more detailed pharmacokinetics of new medicines and to explain dual functions of GSK3 in cancer development. In other words, caution is necessary in order to avoid tumor progression when targeting GSK3 as it has both prooncogenic and tumor-suppressive features, especially having in mind the proposed protective functions of GSK3 against EMT [104,106,108], neoangiogenesis [110], and resistance to chemotherapy [112,113,115].
In conclusion, since the exact function of GSK3 in cancer development has not been elucidated yet, further research on the regulation and effects of this enzyme is undoubtedly needed. Although some therapeutic options targeting GSK3 exhibited promising results, GSK3 as a treatment target is still in an early phase of development. Therefore, it is essential to approach every patient individually and to keep carefully assessing possible outcomes of GSK3 focused treatment strategies.
ACKNOWLEDGMENTS
This publication was co-financed by the European Union through the Europe Regional Development Fund, Operational Programme Competitiveness and Cohesion, under grant agreement No. KK.01.1.1.01.0008, Reproductive and Regenerative Medicine - Exploring New Platforms and Potentials.
Footnotes
Conflict of interest statement: The authors declare no conflict of interests
Funding: This publication was co-financed by the European Union through the Europe Regional Development Fund, Operational Programme Competitiveness and Cohesion, under grant agreement No. KK.01.1.1.01.0008, Reproductive and Regenerative Medicine - Exploring New Platforms and Potentials.
REFERENCES
- 1.Organisation for Economic Co-operation and Development. Health at a Glance. Europe: Organisation for Economic Co-operation and Development; 2018. Union E. https://doi.org/10.1007/springerreference_75881. [Google Scholar]
- 2.Force USPST. The Guide to Clinical Preventive Services 2010-2011:Recommendations of the US Preventive Services Task Force. Rockville, MD: United States Preventive Services Task Force; 2010. US Preventive Services Task Force Guides to Clinical Preventive Services. https://doi.org/10.1016/b978-1-4557-0658-7.00018-9. [Google Scholar]
- 3.Sekerija M, Bubanovi L, Novak P, Veltruski J, Glibo M, Stavinoha M, et al. Cancer Incidence in Croatia 2017. Croatian National Cancer Registry, Bulletin No. 42. 2020 [Google Scholar]
- 4.Patel P, Woodgett JR. Glycogen synthase kinase 3:A kinase for all pathways? Curr Top Dev Biol. 2017;123:277–302. doi: 10.1016/bs.ctdb.2016.11.011. https://doi.org/10.1016/bs.ctdb.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Shin K, Kim KH, Yoon MS, Suh DS, Lee JY, Kim A, et al. Expression of interactive genes associated with apoptosis and their prognostic value for ovarian serous adenocarcinoma. Adv Clin Exp Med. 2016;25(3):513–21. doi: 10.17219/acem/62540. https://doi.org/10.17219/acem/62540. [DOI] [PubMed] [Google Scholar]
- 6.Naruse K, Ueno M, Satoh T, Nomiyama H, Tei H, Takeda M, et al. A YAC contig of the human CC chemokine genes clustered on chromosome 17q11.2. Genomics. 1996;34(2):236–40. doi: 10.1006/geno.1996.0274. https://doi.org/10.1006/geno.1996.0274. [DOI] [PubMed] [Google Scholar]
- 7.Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol. 2001;69(4):513–21. [PubMed] [Google Scholar]
- 8.Goodyear A, Jones A, Troyer R, Bielefeldt-Ohmann H, Dow S. Critical protective role for MCP-1 in pneumonic Burkholderia mallei infection. J Immunol. 2010;184(3):1445–54. doi: 10.4049/jimmunol.0900411. https://doi.org/10.4049/jimmunol.0900411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujimoto H, Sangai T, Ishii G, Ikehara A, Nagashima T, Miyazaki M, et al. Stromal MCP-1 in mammary tumors induces tumor-associated macrophage infiltration and contributes to tumor progression. Int J Cancer. 2009;125(6):1276–84. doi: 10.1002/ijc.24378. https://doi.org/10.1002/ijc.24378. [DOI] [PubMed] [Google Scholar]
- 10.Zhang M, Sun H, Zhao S, Wang Y, Pu H, Wang Y, et al. Expression of PD-L1 and prognosis in breast cancer:A meta-analysis. Oncotarget. 2017;8(19):31347–54. doi: 10.18632/oncotarget.15532. https://doi.org/10.18632/oncotarget.15532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mancinelli R, Carpino G, Petrungaro S, Mammola CL, Tomaipitinca L, Filippini A, et al. Multifaceted roles of GSK-3 in cancer and autophagy-related diseases. Oxid Med Cell Longev. 2017;2017:4629495. doi: 10.1155/2017/4629495. https://doi.org/10.1155/2017/4629495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azoulay-Alfaguter I, Yaffe Y, Licht-Murava A, Urbanska M, Jaworski J, Pietrokovski S, et al. Distinct molecular regulation of glycogen synthase kinase-3alpha isozyme controlled by its N-terminal region:Functional role in calcium/calpain signaling. J Biol Chem. 2011;286(15):13470–80. doi: 10.1074/jbc.M110.127969. https://doi.org/10.1074/jbc.m110.127969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagini S, Sophia J, Mishra R. Glycogen synthase kinases:Moonlighting proteins with theranostic potential in cancer. Semin Cancer Biol. 2019;56:25–36. doi: 10.1016/j.semcancer.2017.12.010. https://doi.org/10.1016/j.semcancer.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2(10):769–76. doi: 10.1038/35096075. https://doi.org/10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 15.Goc A, Al-Husein B, Katsanevas K, Steinbach A, Lou U, Sabbineni H, et al. Targeting Src-mediated Tyr216 phosphorylation and activation of GSK-3 in prostate cancer cells inhibit prostate cancer progression in vitro and in vivo. Oncotarget. 2014;5(3):775–87. doi: 10.18632/oncotarget.1770. https://doi.org/10.18632/oncotarget.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole A, Frame S, Cohen P. Further evidence that the tyrosine phosphorylation of glycogen synthase kinase-3 (GSK3) in mammalian cells is an autophosphorylation event. Biochem J. 2004;377(Pt 1):249–55. doi: 10.1042/BJ20031259. https://doi.org/10.1042/bj20031259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCubrey JA, Steelman LS, Bertrand FE, Davis NM, Sokolosky M, Abrams SL, et al. GSK-3 as potential target for therapeutic intervention in cancer. Oncotarget. 2014;5(10):2881–911. doi: 10.18632/oncotarget.2037. https://doi.org/10.18632/oncotarget.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobs KM, Bhave SR, Ferraro DJ, Jaboin JJ, Hallahan DE, Thotala D. GSK-3b: A bifunctional role in cell death pathways. Int J Cell Biol. 2012;2012:930710. doi: 10.1155/2012/930710. https://doi.org/10.1155/2012/930710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sayas CL, Ariaens A, Ponsioen B, Moolenaar WH. GSK-3 is activated by the tyrosine kinase Pyk2 during LPA1-mediated neurite retraction. Mol Biol Cell. 2006;17(4):1834–44. doi: 10.1091/mbc.E05-07-0688. https://doi.org/10.1091/mbc.e05-07-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forde JE, Dale TC. Glycogen synthase kinase 3:A key regulator of cellular fate. Cell Mol Life Sci. 2007;64(15):1930–44. doi: 10.1007/s00018-007-7045-7. https://doi.org/10.1007/s00018-007-7045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thornton TM, Pedraza-Alva G, Deng B, Wood CD, Aronshtam A, Clements JL, et al. Phosphorylation by p38 MAPK as an alternative pathway for GSK3βeta inactivation. Science (New York) 2008;320(5876):667–70. doi: 10.1126/science.1156037. https://doi.org/10.1126/science.1156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennecib M, Gong CX, Grundke-Iqbal I, Iqbal K. Role of protein phosphatase-2A and -1 in the regulation of GSK-3, cdk5 and cdc2 and the phosphorylation of tau in rat forebrain. FEBS Lett. 2000;485(1):87–93. doi: 10.1016/s0014-5793(00)02203-1. https://doi.org/10.1016/s0014-5793(00)02203-1. [DOI] [PubMed] [Google Scholar]
- 23.Wu G, Huang H, Abreu JG, He X. Inhibition of GSK3 phosphorylation of beta-catenin via phosphorylated PPPSPXS motifs of Wnt coreceptor LRP6. PLoS One. 2009;4(3):e4926. doi: 10.1371/journal.pone.0004926. https://doi.org/10.1371/journal.pone.0004926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng SS, Mahmoudi T, Danenberg E, Bejaoui I, de Lau W, Korswagen HC, et al. Phosphatidylinositol 3-kinase signaling does not activate the Wnt cascade. J Biol Chem. 2009;284(51):35308–13. doi: 10.1074/jbc.M109.078261. https://doi.org/10.1074/jbc.m109.078261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woodgett JR. Judging a protein by more than its name:GSK-3. Sci STKE. 2001;2001(100):e12. doi: 10.1126/stke.2001.100.re12. https://doi.org/10.1126/stke.2001.100.re12. [DOI] [PubMed] [Google Scholar]
- 26.Shakoori A, Ougolkov A, Yu ZW, Zhang B, Modarressi MH, Billadeau DD, et al. Deregulated GSK3βeta activity in colorectal cancer:Its association with tumor cell survival and proliferation. Biochem Biophys Res Commun. 2005;334(4):1365–73. doi: 10.1016/j.bbrc.2005.07.041. https://doi.org/10.1016/j.bbrc.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 27.Ougolkov AV, Fernandez-Zapico ME, Savoy DN, Urrutia RA, Billadeau DD. Glycogen synthase kinase-3beta participates in nuclear factor kappaB-mediated gene transcription and cell survival in pancreatic cancer cells. Cancer Res. 2005;65(6):2076–81. doi: 10.1158/0008-5472.CAN-04-3642. https://doi.org/10.1158/0008-5472.can-04-3642. [DOI] [PubMed] [Google Scholar]
- 28.Yun SH, Park JI. PGC-1a regulates cell proliferation and invasion via AKT/GSK-3b/β-catenin pathway in human colorectal cancer SW620 and SW480 cells. Anticancer Res. 2020;40(2):653–64. doi: 10.21873/anticanres.13995. https://doi.org/10.21873/anticanres.13995. [DOI] [PubMed] [Google Scholar]
- 29.Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, et al. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438(7069):873–7. doi: 10.1038/nature04185. https://doi.org/10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skoda AM, Simovic D, Karin V, Kardum V, Vranic S, Serman L. The role of the hedgehog signaling pathway in cancer:A comprehensive review. Bosn J Basic Med Sci. 2018;18(1):8–20. doi: 10.17305/bjbms.2018.2756. https://doi.org/10.17305/bjbms.2018.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100(4):423–34. doi: 10.1016/s0092-8674(00)80678-9. https://doi.org/10.1016/s0092-8674(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 32.Pan Y, Bai CB, Joyner AL, Wang B. Sonic hedgehog signaling regulates Gli2 transcriptional activity by suppressing its processing and degradation. Mol Cell Biol. 2006;26(9):3365–77. doi: 10.1128/MCB.26.9.3365-3377.2006. https://doi.org/10.1128/mcb.26.9.3365-3377.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takenaka K, Kise Y, Miki H. GSK3βeta positively regulates Hedgehog signaling through Sufu in mammalian cells. Biochem Biophys Res Commun. 2007;353(2):501–8. doi: 10.1016/j.bbrc.2006.12.058. https://doi.org/10.1016/j.bbrc.2006.12.058. [DOI] [PubMed] [Google Scholar]
- 34.Trnski D, Sabol M, Gojević A, Martinić M, Ozretić P, Musani V, et al. GSK3β and Gli3 play a role in activation of Hedgehog-Gli pathway in human colon cancer targeting GSK3β downregulates the signaling pathway and reduces cell proliferation. Biochim Biophys Acta. 2015;1852(12):2574–84. doi: 10.1016/j.bbadis.2015.09.005. https://doi.org/10.1016/j.bbadis.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Takada Y, Fang X, Jamaluddin MS, Boyd DD, Aggarwal BB. Genetic deletion of glycogen synthase kinase-3beta abrogates activation of IkBa kinase, JNK, Akt, and p44/p42 MAPK but potentiates apoptosis induced by tumor necrosis factor. J Biol Chem. 2004;279(38):39541–54. doi: 10.1074/jbc.M403449200. https://doi.org/10.1074/jbc.m403449200. [DOI] [PubMed] [Google Scholar]
- 36.Busino L, Millman SE, Scotto L, Kyratsous CA, Basrur V, O'Connor O, et al. Fbxw7a- and GSK3-mediated degradation of p100 is a pro-survival mechanism in multiple myeloma. Nat Cell Biol. 2012;14(4):375–85. doi: 10.1038/ncb2463. https://doi.org/10.1038/ncb2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buss H, Dörrie A, Schmitz ML, Frank R, Livingstone M, Resch K, et al. Phosphorylation of serine 468 by GSK-3beta negatively regulates basal p65 NF-kappaB activity. J Biol Chem. 2004;279(48):49571–4. doi: 10.1074/jbc.C400442200. https://doi.org/10.1074/jbc.c400442200. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez JF, Sniderhan LF, Williamson AL, Fan S, Chakraborty-Sett S, Maggirwar SB. Glycogen synthase kinase 3beta-mediated apoptosis of primary cortical astrocytes involves inhibition of nuclear factor kappaB signaling. Mol Cell Biol. 2003;23(13):4649–62. doi: 10.1128/MCB.23.13.4649-4662.2003. https://doi.org/10.1128/mcb.23.13.4649-4662.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kopan R, Turner DL. The Notch pathway: Democracy and aristocracy in the selection of cell fate. Curr Opin Neurobiol. 1996;6(5):594–601. doi: 10.1016/s0959-4388(96)80090-0. https://doi.org/10.1016/s0959-4388(96)80090-0. [DOI] [PubMed] [Google Scholar]
- 40.Han X, Ju JH, Shin I. Glycogen synthase kinase 3-b phosphorylates novel S/T-P-S/T domains in Notch1 intracellular domain and induces its nuclear localization. Biochem Biophys Res Commun. 2012;423(2):282–8. doi: 10.1016/j.bbrc.2012.05.111. https://doi.org/10.1016/j.bbrc.2012.05.111. [DOI] [PubMed] [Google Scholar]
- 41.Foltz DR, Santiago MC, Berechid BE, Nye JS. Glycogen synthase kinase-3beta modulates Notch signaling and stability. Curr Biol. 2002;12(12):1006–11. doi: 10.1016/s0960-9822(02)00888-6. https://doi.org/10.1016/s0960-9822(02)00888-6. [DOI] [PubMed] [Google Scholar]
- 42.Giovannini C, Baglioni M, Toaldo MB, Ventrucci C, D'Adamo S, Cipone M, et al. Notch3 inhibition enhances sorafenib cytotoxic efficacy by promoting GSK3β phosphorylation and p21 down-regulation in hepatocellular carcinoma. Oncotarget. 2013;4(10):1618–31. doi: 10.18632/oncotarget.1221. https://doi.org/10.18632/oncotarget.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Espinosa L, Inglés-Esteve J, Aguilera C, Bigas A. Phosphorylation by glycogen synthase kinase-3 beta down-regulates Notch activity, a link for Notch and Wnt pathways. J Biol Chem. 2003;278(34):32227–35. doi: 10.1074/jbc.M304001200. https://doi.org/10.1074/jbc.m304001200. [DOI] [PubMed] [Google Scholar]
- 44.Zheng L, Conner SD. Glycogen synthase kinase 3b inhibition enhances Notch1 recycling. Mol Biol Cell. 2018;29(4):389–95. doi: 10.1091/mbc.E17-07-0474. https://doi.org/10.1091/mbc.e17-07-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marchetti A, Colletti M, Cozzolino AM, Steindler C, Lunadei M, Mancone C, et al. ERK5/MAPK is activated by TGFbeta in hepatocytes and required for the GSK-3beta-mediated snail protein stabilization. Cell Signal. 2008;20(11):2113–8. doi: 10.1016/j.cellsig.2008.08.002. https://doi.org/10.1016/j.cellsig.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Li S, Lu J, Chen Y, Xiong N, Li L, Zhang J, et al. MCP-1-induced ERK/GSK-3b/Snail signaling facilitates the epithelial-mesenchymal transition and promotes the migration of MCF-7 human breast carcinoma cells. Cell Mol Immunol. 2017;14(7):621–30. doi: 10.1038/cmi.2015.106. https://doi.org/10.1038/cmi.2015.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo X, Ramirez A, Waddell DS, Li Z, Liu X, Wang XF. Axin and GSK3- control Smad3 protein stability and modulate TGF- signaling. Genes Dev. 2008;22(1):106–20. doi: 10.1101/gad.1590908. https://doi.org/10.1101/gad.1590908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loberg RD, Vesely E, Brosius FC., 3rd Enhanced glycogen synthase kinase-3beta activity mediates hypoxia-induced apoptosis of vascular smooth muscle cells and is prevented by glucose transport and metabolism. J Biol Chem. 2002;277(44):41667–73. doi: 10.1074/jbc.M206405200. https://doi.org/10.1074/jbc.m206405200. [DOI] [PubMed] [Google Scholar]
- 49.Watcharasit P, Bijur GN, Zmijewski JW, Song L, Zmijewska A, Chen X, et al. Direct activating, interaction between glycogen synthase kinase-3beta and p53 after DNA damage. Proc Natl Acad Sci USA. 2002;99(12):7951–5. doi: 10.1073/pnas.122062299. https://doi.org/10.1073/pnas.122062299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65(4):391–426. doi: 10.1016/s0301-0082(01)00011-9. https://doi.org/10.1016/s0301-0082(01)00024-7. [DOI] [PubMed] [Google Scholar]
- 51.Turenne GA, Price BD. Glycogen synthase kinase3 beta phosphorylates serine 33 of p53 and activates p53's transcriptional activity. BMC Cell Biol. 2001;2:12. doi: 10.1186/1471-2121-2-12. https://doi.org/10.1186/1471-2121-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thotala DK, Hallahan DE, Yazlovitskaya EM. Glycogen synthase kinase 3b inhibitors protect hippocampal neurons from radiation-induced apoptosis by regulating MDM2-p53 pathway. Cell Death Differ. 2012;19(3):387–96. doi: 10.1038/cdd.2011.94. https://doi.org/10.1038/cdd.2011.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Linseman DA, Butts BD, Precht TA, Phelps RA, Le SS, Laessig TA, et al. Glycogen synthase kinase-3beta phosphorylates Bax and promotes its mitochondrial localization during neuronal apoptosis. J Neurosci. 2004;24(44):9993–10002. doi: 10.1523/JNEUROSCI.2057-04.2004. https://doi.org/10.1523/jneurosci.2057-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thotala DK, Geng L, Dickey AK, Hallahan DE, Yazlovitskaya EM. A new class of molecular targeted radioprotectors:GSK-3beta inhibitors. Int J Radiat Oncol Biol Phys. 2010;76(2):557–65. doi: 10.1016/j.ijrobp.2009.09.024. https://doi.org/10.1016/j.ijrobp.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou F, Zhang L, van Laar T, van Dam H, Ten Dijke P. GSK3β inactivation induces apoptosis of leukemia cells by repressing the function of c-Myb. Mol Biol Cell. 2011;22(18):3533–40. doi: 10.1091/mbc.E11-06-0483. https://doi.org/10.1091/mbc.11-06-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kotliarova S, Pastorino S, Kovell LC, Kotliarov Y, Song H, Zhang W, et al. Glycogen synthase kinase-3 inhibition induces glioma cell death through c-MYC, nuclear factor-kappaB, and glucose regulation. Cancer Res. 2008;68(16):6643–51. doi: 10.1158/0008-5472.CAN-08-0850. https://doi.org/10.1158/0008-5472.can-08-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Azoulay-Alfaguter I, Elya R, Avrahami L, Katz A, Eldar-Finkelman H. Combined regulation of mTORC1 and lysosomal acidification by GSK-3 suppresses autophagy and contributes to cancer cell growth. Oncogene. 2015;34(35):4613–23. doi: 10.1038/onc.2014.390. https://doi.org/10.1038/onc.2014.390. [DOI] [PubMed] [Google Scholar]
- 58.McCubrey JA, Rakus D, Gizak A, Steelman LS, Abrams SL, Lertpiriyapong K, et al. Effects of mutations in Wnt/β-catenin, Hedgehog, Notch and PI3K pathways on GSK-3 activity-diverse effects on cell growth, metabolism and cancer. Biochim Biophys Acta. 2016;1863(12):2942–76. doi: 10.1016/j.bbamcr.2016.09.004. https://doi.org/10.1016/j.bbamcr.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 59.Mishra R. Glycogen synthase kinase 3 beta:Can it be a target for oral cancer. Mol Cancer. 2010;9:144. doi: 10.1186/1476-4598-9-144. https://doi.org/10.1186/1476-4598-9-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Welcker M, Singer J, Loeb KR, Grim J, Bloecher A, Gurien-West M, et al. Multisite phosphorylation by Cdk2 and GSK3 controls cyclin E degradation. Mol Cell. 2003;12(2):381–92. doi: 10.1016/s1097-2765(03)00287-9. https://doi.org/10.1016/s1097-2765(03)00287-9. [DOI] [PubMed] [Google Scholar]
- 61.Taira N, Mimoto R, Kurata M, Yamaguchi T, Kitagawa M, Miki Y, et al. DYRK2 priming phosphorylation of c-Jun and c-Myc modulates cell cycle progression in human cancer cells. J Clin Investig. 2012;122(3):859–72. doi: 10.1172/JCI60818. https://doi.org/10.1172/jci60818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kitano A, Shimasaki T, Chikano Y, Nakada M, Hirose M, Higashi T, et al. Aberrant glycogen synthase kinase 3b is involved in pancreatic cancer cell invasion and resistance to therapy. PLoS One. 2013;8(2):e55289. doi: 10.1371/journal.pone.0055289. https://doi.org/10.1371/journal.pone.0055289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Doble BW, Woodgett JR. Role of glycogen synthase kinase-3 in cell fate and epithelial-mesenchymal transitions. Cells Tissues Organs. 2007;185(1-3):73–84. doi: 10.1159/000101306. https://doi.org/10.1159/000101306. [DOI] [PubMed] [Google Scholar]
- 64.Domoto T, Pyko IV, Furuta T, Miyashita K, Uehara M, Shimasaki T, et al. Glycogen synthase kinase-3b is a pivotal mediator of cancer invasion and resistance to therapy. Cancer Sci. 2016;107(10):1363–72. doi: 10.1111/cas.13028. https://doi.org/10.1111/cas.13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Acikgoz E, Güler G, Camlar M, Oktem G, Aktug H. Glycogen synthase kinase-3 inhibition in glioblastoma multiforme cells induces apoptosis, cell cycle arrest and changing biomolecular structure. Spectrochim Acta A Mol Biomol Spectrosc. 2019;209:150–64. doi: 10.1016/j.saa.2018.10.036. https://doi.org/10.1016/j.saa.2018.10.036. [DOI] [PubMed] [Google Scholar]
- 66.Tovar CF, Zerón HM, Romero MD, Sánchez YV, Romero IT. Glycogen synthase kinase-3b (GSK-3b) and nuclear factor kappa-B (NFKB) in childhood acute lymphoblastic leukemia. Adv Clin Exp Med. 2016;25(6):1139–47. doi: 10.17219/acem/63752. https://doi.org/10.17219/acem/63752. [DOI] [PubMed] [Google Scholar]
- 67.Garcea G, Manson MM, Neal CP, Pattenden CJ, Sutton CD, Dennison AR, et al. Glycogen synthase kinase-3 beta;a new target in pancreatic cancer? Curr Cancer Drug Targets. 2007;7(3):209–15. doi: 10.2174/156800907780618266. https://doi.org/10.2174/156800907780618266. [DOI] [PubMed] [Google Scholar]
- 68.Pal K, Cao Y, Gaisina IN, Bhattacharya S, Dutta SK, Wang E, et al. Inhibition of GSK-3 induces differentiation and impaired glucose metabolism in renal cancer. Mol Cancer Ther. 2014;13(2):285–96. doi: 10.1158/1535-7163.MCT-13-0681. https://doi.org/10.1158/1535-7163.mct-13-0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma C, Wang J, Gao Y, Gao TW, Chen G, Bower KA, et al. The role of glycogen synthase kinase 3beta in the transformation of epidermal cells. Cancer Res. 2007;67(16):7756–64. doi: 10.1158/0008-5472.CAN-06-4665. https://doi.org/10.1158/0008-5472.can-06-4665. [DOI] [PubMed] [Google Scholar]
- 70.Mishra R, Nagini S, Rana A. Expression and inactivation of glycogen synthase kinase 3 alpha/ beta and their association with the expression of cyclin D1 and p53 in oral squamous cell carcinoma progression. Mol Cancer. 2015;14:20. doi: 10.1186/s12943-015-0300-x. https://doi.org/10.1186/s12943-015-0300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng H, Saito H, Masuda S, Yang X, Takano Y. Phosphorylated GSK3βeta-ser9 and EGFR are good prognostic factors for lung carcinomas. Anticancer Res. 2007;27(5b):3561–9. [PubMed] [Google Scholar]
- 72.Qiu HJ, Lu XH, Yang SS, Weng CY, Zhang EK, Chen FC. MiR-769 promoted cell proliferation in human melanoma by suppressing GSK3β expression. Biomed Pharmacother. 2016;82:117–23. doi: 10.1016/j.biopha.2016.04.052. https://doi.org/10.1016/j.biopha.2016.04.052. [DOI] [PubMed] [Google Scholar]
- 73.Sagae S, Kobayashi K, Nishioka Y, Sugimura M, Ishioka S, Nagata M, et al. Mutational analysis of beta-catenin gene in Japanese ovarian carcinomas:Frequent mutations in endometrioid carcinomas. Jpn J Cancer Res. 1999;90(5):510–5. doi: 10.1111/j.1349-7006.1999.tb00777.x. https://doi.org/10.1111/j.1349-7006.1999.tb00777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rask K, Nilsson A, Brännström M, Carlsson P, Hellberg P, Janson PO, et al. Wnt-signalling pathway in ovarian epithelial tumours:Increased expression of beta-catenin and GSK3βeta. Br J Cancer. 2003;89(7):1298–304. doi: 10.1038/sj.bjc.6601265. https://doi.org/10.1038/sj.bjc.6601265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Usongo M, Li X, Farookhi R. Activation of the canonical WNT signaling pathway promotes ovarian surface epithelial proliferation without inducing β-catenin/Tcf-mediated reporter expression. Dev Dyn. 2013;242(3):291–300. doi: 10.1002/dvdy.23919. https://doi.org/10.1002/dvdy.23919. [DOI] [PubMed] [Google Scholar]
- 76.Ozretić P, Trnski D, Musani V, Maurac I, Kalafatić D, Orešković S, et al. Non-canonical Hedgehog signaling activation in ovarian borderline tumors and ovarian carcinomas. Int J Oncol. 2017;51(6):1869–77. doi: 10.3892/ijo.2017.4156. https://doi.org/10.3892/ijo.2017.4156. [DOI] [PubMed] [Google Scholar]
- 77.Guo R, Abdelmohsen K, Morin PJ, Gorospe M. Novel MicroRNA reporter uncovers repression of Let-7 by GSK-3b. PLoS One. 2013;8(6):e66330. doi: 10.1371/journal.pone.0066330. https://doi.org/10.1371/journal.pone.0066330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stavridi ES, Halazonetis TD. p53 and stress in the ER. Genes Dev. 2004;18(3):241–4. doi: 10.1101/gad.1181704. https://doi.org/10.1101/gad.1181704. [DOI] [PubMed] [Google Scholar]
- 79.Gwak H, Kim S, Dhanasekaran DN, Song YS. Resveratrol triggers ER stress-mediated apoptosis by disrupting N-linked glycosylation of proteins in ovarian cancer cells. Cancer Lett. 2016;371(2):347–53. doi: 10.1016/j.canlet.2015.11.032. https://doi.org/10.1016/j.canlet.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 80.Wulfkuhle JD, Aquino JA, Calvert VS, Fishman DA, Coukos G, Liotta LA, et al. Signal pathway profiling of ovarian cancer from human tissue specimens using reverse-phase protein microarrays. Proteomics. 2003;3(11):2085–90. doi: 10.1002/pmic.200300591. https://doi.org/10.1002/pmic.200300591. [DOI] [PubMed] [Google Scholar]
- 81.Cao Q, Lu X, Feng YJ. Glycogen synthase kinase-3beta positively regulates the proliferation of human ovarian cancer cells. Cell Res. 2006;16(7):671–7. doi: 10.1038/sj.cr.7310078. https://doi.org/10.1038/sj.cr.7310078. [DOI] [PubMed] [Google Scholar]
- 82.Masamha CP, Benbrook DM. Cyclin D1 degradation is sufficient to induce G1 cell cycle arrest despite constitutive expression of cyclin E2 in ovarian cancer cells. Cancer Res. 2009;69(16):6565–72. doi: 10.1158/0008-5472.CAN-09-0913. https://doi.org/10.1158/0008-5472.can-09-0913. [DOI] [PubMed] [Google Scholar]
- 83.Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol. 2005;202(3):654–62. doi: 10.1002/jcp.20166. https://doi.org/10.1002/jcp.20166. [DOI] [PubMed] [Google Scholar]
- 84.Gatenby RA, Gawlinski ET. The glycolytic phenotype in carcinogenesis and tumor invasion:Insights through mathematical models. Cancer Res. 2003;63(14):3847–54. [PubMed] [Google Scholar]
- 85.Chen L, Cheng X, Tu W, Qi Z, Li H, Liu F, et al. Apatinib inhibits glycolysis by suppressing the VEGFR2/AKT1/SOX5/GLUT4 signaling pathway in ovarian cancer cells. Cell Oncol (Dordrecht) 2019;42(5):679–90. doi: 10.1007/s13402-019-00455-x. https://doi.org/10.1007/s13402-019-00455-x. [DOI] [PubMed] [Google Scholar]
- 86.Fu Y, Wang X, Cheng X, Ye F, Xie X, Lu W. Clinicopathological and biological significance of aberrant activation of glycogen synthase kinase-3 in ovarian cancer. Onco Targets Ther. 2014;7:1159–68. doi: 10.2147/OTT.S62158. https://doi.org/10.2147/ott.s62158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thompson TB, Cook RW, Chapman SC, Jardetzky TS, Woodruff TK. Beta A versus beta B:Is it merely a matter of expression? Mol Cell Endocrinol. 2004;225(1-2):9–17. doi: 10.1016/j.mce.2004.02.007. https://doi.org/10.1016/j.mce.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 88.Pangas SA, Woodruff TK. Activin signal transduction pathways. Trends Endocrinol Metab. 2000;11(8):309–14. doi: 10.1016/s1043-2760(00)00294-0. https://doi.org/10.1016/s1043-2760(00)00294-0. [DOI] [PubMed] [Google Scholar]
- 89.Dupont J, McNeilly J, Vaiman A, Canepa S, Combarnous Y, Taragnat C. Activin signaling pathways in ovine pituitary and LbetaT2 gonadotrope cells. Biol Reprod. 2003;68(5):1877–87. doi: 10.1095/biolreprod.102.012005. https://doi.org/10.1095/biolreprod.102.012005. [DOI] [PubMed] [Google Scholar]
- 90.Choi KC, Kang SK, Nathwani PS, Cheng KW, Auersperg N, Leung PC. Differential expression of activin/inhibin subunit and activin receptor mRNAs in normal and neoplastic ovarian surface epithelium (OSE) Mol Cell Endocrinol. 2001;174(1-2):99–110. doi: 10.1016/s0303-7207(00)00447-0. https://doi.org/10.1016/s0303-7207(00)00447-0. [DOI] [PubMed] [Google Scholar]
- 91.Do TV, Kubba LA, Antenos M, Rademaker AW, Sturgis CD, Woodruff TK. The role of activin A and Akt/GSK signaling in ovarian tumor biology. Endocrinology. 2008;149(8):3809–16. doi: 10.1210/en.2007-1584. https://doi.org/10.1210/en.2007-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cianfrocca R, Rosanò L, Spinella F, Di Castro V, Natali PG, Bagnato A. Beta-arrestin-1 mediates the endothelin-1-induced activation of Akt and integrin-linked kinase. Can J Physiol Pharmacol. 2010;88(8):796–801. doi: 10.1139/Y10-052. https://doi.org/10.1139/y10-052. [DOI] [PubMed] [Google Scholar]
- 93.Pernicova I, Korbonits M. Metformin - mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. 2014;10(3):143–56. doi: 10.1038/nrendo.2013.256. https://doi.org/10.1038/nrendo.2013.256. [DOI] [PubMed] [Google Scholar]
- 94.Gwak H, Kim Y, An H, Dhanasekaran DN, Song YS. Metformin induces degradation of cyclin D1 via AMPK/GSK3β axis in ovarian cancer. Mol Carcinog. 2017;56(2):349–58. doi: 10.1002/mc.22498. https://doi.org/10.1002/mc.22498. [DOI] [PubMed] [Google Scholar]
- 95.Vergara D, Simeone P, Toraldo D, Del Boccio P, Vergaro V, Leporatti S, et al. Resveratrol downregulates Akt/GSK and ERK signalling pathways in OVCAR-3 ovarian cancer cells. Mol Biosyst. 2012;8(4):1078–87. doi: 10.1039/c2mb05486h. https://doi.org/10.1039/c2mb05486h. [DOI] [PubMed] [Google Scholar]
- 96.Song M, Bode AM, Dong Z, Lee MH. AKT as a therapeutic target for cancer. Cancer Res. 2019;79(6):1019–31. doi: 10.1158/0008-5472.CAN-18-2738. https://doi.org/10.1158/0008-5472.CAN-18-2738. [DOI] [PubMed] [Google Scholar]
- 97.Soler AP, Knudsen KA, Tecson-Miguel A, McBrearty FX, Han AC, Salazar H. Expression of E-cadherin and N-cadherin in surface epithelial-stromal tumors of the ovary distinguishes mucinous from serous and endometrioid tumors. Hum Pathol. 1997;28(6):734–9. doi: 10.1016/s0046-8177(97)90184-2. https://doi.org/10.1016/s0046-8177(97)90184-2. [DOI] [PubMed] [Google Scholar]
- 98.Auersperg N, Pan J, Grove BD, Peterson T, Fisher J, Maines-Bandiera S, et al. E-cadherin induces mesenchymal-to-epithelial transition in human ovarian surface epithelium. Proc Natl Acad Sci USA. 1999;96(11):6249–54. doi: 10.1073/pnas.96.11.6249. https://doi.org/10.1073/pnas.96.11.6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Imai T, Horiuchi A, Shiozawa T, Osada R, Kikuchi N, Ohira S, et al. Elevated expression of E-cadherin and alpha-, beta-, and gamma-catenins in metastatic lesions compared with primary epithelial ovarian carcinomas. Hum Pathol. 2004;35(12):1469–76. doi: 10.1016/j.humpath.2004.09.014. https://doi.org/10.1016/j.humpath.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 100.Blechschmidt K, Sassen S, Schmalfeldt B, Schuster T, Höfler H, Becker KF. The E-cadherin repressor Snail is associated with lower overall survival of ovarian cancer patients. Br J Cancer. 2008;98(2):489–95. doi: 10.1038/sj.bjc.6604115. https://doi.org/10.1038/sj.bjc.6604115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mitra T, Roy SS. Co-activation of TGFb and Wnt signalling pathways abrogates EMT in ovarian cancer cells. Cell Physiol Biochem. 2017;41(4):1336–45. doi: 10.1159/000464436. https://doi.org/10.1159/000464436. [DOI] [PubMed] [Google Scholar]
- 102.RosanòL, Spinella F, Di Castro V, Nicotra MR, Dedhar S, de Herreros AG, et al. Endothelin-1 promotes epithelial-to-mesenchymal transition in human ovarian cancer cells. Cancer Res. 2005;65(24):11649–57. doi: 10.1158/0008-5472.CAN-05-2123. https://doi.org/10.1158/0008-5472.can-05-2123. [DOI] [PubMed] [Google Scholar]
- 103.Li C, Ding H, Tian J, Wu L, Wang Y, Xing Y, et al. Forkhead box protein C2 promotes epithelial-mesenchymal transition, migration and invasion in cisplatin-resistant human ovarian cancer cell line (SKOV3/CDDP) Cell Physiol Biochem. 2016;39(3):1098–110. doi: 10.1159/000447818. https://doi.org/10.1159/000447818. [DOI] [PubMed] [Google Scholar]
- 104.Hu C, Dong T, Li R, Lu J, Wei X, Liu P. Emodin inhibits epithelial to mesenchymal transition in epithelial ovarian cancer cells by regulation of GSK-3b/β-catenin/ZEB1 signaling pathway. Oncol Rep. 2016;35(4):2027–34. doi: 10.3892/or.2016.4591. https://doi.org/10.3892/or.2016.4591. [DOI] [PubMed] [Google Scholar]
- 105.Lu J, Xu Y, Wei X, Zhao Z, Xue J, Liu P. Emodin inhibits the epithelial to mesenchymal transition of epithelial ovarian cancer cells via ILK/GSK-3b/Slug signaling pathway. Biomed Res Int. 2016;2016:6253280. doi: 10.1155/2016/6253280. https://doi.org/10.1155/2016/6253280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu Y, Zhou H, Zhu R, Ding F, Li Y, Cao X, et al. SPSB3 targets SNAIL for degradation in GSK-3b phosphorylation-dependent manner and regulates metastasis. Oncogene. 2018;37(6):768–76. doi: 10.1038/onc.2017.370. https://doi.org/10.1038/onc.2017.370. [DOI] [PubMed] [Google Scholar]
- 107.Li N, Yang L, Sun Y, Wu X. MicroRNA-16 inhibits migration and invasion via regulation of the Wnt/β-catenin signaling pathway in ovarian cancer. Oncol Lett. 2019;17(3):2631–8. doi: 10.3892/ol.2019.9923. https://doi.org/10.3892/ol.2019.9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu HY, Zhang YY, Zhu BL, Feng FZ, Zhang HT, Yan H, et al. MiR-203a-3p regulates the biological behaviors of ovarian cancer cells through mediating the Akt/GSK-3b/Snail signaling pathway by targeting ATM. J Ovarian Res. 2019;12(1):60. doi: 10.1186/s13048-019-0532-2. https://doi.org/10.1186/s13048-019-0532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Burkhalter RJ, Symowicz J, Hudson LG, Gottardi CJ, Stack MS. Integrin regulation of beta-catenin signaling in ovarian carcinoma. J Biol Chem. 2011;286(26):23467–75. doi: 10.1074/jbc.M110.199539. https://doi.org/10.1074/jbc.m110.199539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cassavaugh JM, Hale SA, Wellman TL, Howe AK, Wong C, Lounsbury KM. Negative regulation of HIF-1a by an FBW7-mediated degradation pathway during hypoxia. J Cell Biochem. 2011;112(12):3882–90. doi: 10.1002/jcb.23321. https://doi.org/10.1002/jcb.23321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim BR, Lee SH, Park MS, Seo SH, Park YM, Kwon YJ, et al. MARCKSL1 exhibits anti-angiogenic effects through suppression of VEGFR-2-dependent Akt/PDK-1/mTOR phosphorylation. Oncol Rep. 2016;35(2):1041–8. doi: 10.3892/or.2015.4408. https://doi.org/10.3892/or.2015.4408. [DOI] [PubMed] [Google Scholar]
- 112.Wu FL, Chen HL, Hu XW, Liang LY, Xu WL. Wnt5a modulates vincristine resistance through PI3K/Akt/GSK3β signaling pathway in human ovarian carcinoma SKOV3/VCR cells. Sheng Xue Bao. 2019;71(3):415–23. [PubMed] [Google Scholar]
- 113.Zhang K, Song H, Yang P, Dai X, Li Y, Wang L, et al. Silencing dishevelled-1 sensitizes paclitaxel-resistant human ovarian cancer cells via AKT/GSK-3b/β-catenin signalling. Cell Prolif. 2015;48(2):249–58. doi: 10.1111/cpr.12161. https://doi.org/10.1111/cpr.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fu Y, Hu D, Qiu J, Xie X, Ye F, Lu WG. Overexpression of glycogen synthase kinase-3 in ovarian carcinoma cells with acquired paclitaxel resistance. Int J Gynecol Cancer. 2011;21(3):439–44. doi: 10.1097/IGC.0b013e31820d7366. https://doi.org/10.1097/igc.0b013e31820d7366. [DOI] [PubMed] [Google Scholar]
- 115.Cai G, Wang J, Xin X, Ke Z, Luo J. Phosphorylation of glycogen synthase kinase-3 beta at serine 9 confers cisplatin resistance in ovarian cancer cells. Int J Oncol. 2007;31(3):657–62. https://doi.org/10.3892/ijo.31.3.657. [PubMed] [Google Scholar]
- 116.Arafa SA, Zhu Q, Barakat BM, Wani G, Zhao Q, El-Mahdy MA, et al. Tangeretin sensitizes cisplatin-resistant human ovarian cancer cells through downregulation of phosphoinositide 3-kinase/Akt signaling pathway. Cancer Res. 2009;69(23):8910–7. doi: 10.1158/0008-5472.CAN-09-1543. https://doi.org/10.1158/0008-5472.can-09-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yu PN, Yan MD, Lai HC, Huang RL, Chou YC, Lin WC, et al. Downregulation of miR-29 contributes to cisplatin resistance of ovarian cancer cells. Int J Cancer. 2014;134(3):542–51. doi: 10.1002/ijc.28399. https://doi.org/10.1002/ijc.28399. [DOI] [PubMed] [Google Scholar]
- 118.Kumar S, Meuter A, Thapa P, Langstraat C, Giri S, Chien J, et al. Metformin intake is associated with better survival in ovarian cancer:A case-control study. Cancer. 2013;119(3):555–62. doi: 10.1002/cncr.27706. https://doi.org/10.1002/cncr.27706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Simonelli C, Bertolotti M, Sabbatini P, Berek JS, Pfisterer J, Binaschi M, et al. Effect of metformin on recurrence-free survival and overall survival in diabetic patients affected by advanced ovarian cancer. J Clin Oncol. 2013;31(Suppl 15):5522. https://doi.org/10.1200/jco.2013.31.15_suppl.5522. [Google Scholar]
- 120.Majidi A, Na R, Dixon-Suen S, Jordan SJ, Webb PM. Common medications and survival in women with ovarian cancer:A systematic review and meta-analysis. Gynecol Oncol. 2020;157(3):678–85. doi: 10.1016/j.ygyno.2020.03.028. https://doi.org/10.1016/j.ygyno.2020.03.028. [DOI] [PubMed] [Google Scholar]
- 121.Rauf A, Imran M, Butt MS, Nadeem M, Peters DG, Mubarak MS. Resveratrol as an anti-cancer agent:A review. Crit Rev Food Sci Nutr. 2018;58(9):1428–47. doi: 10.1080/10408398.2016.1263597. https://doi.org/10.1080/10408398.2016.1263597. [DOI] [PubMed] [Google Scholar]
- 122.Ryves WJ, Harwood AJ. Lithium inhibits glycogen synthase kinase-3 by competition for magnesium. Biochem Biophys Res Commun. 2001;280(3):720–5. doi: 10.1006/bbrc.2000.4169. https://doi.org/10.1006/bbrc.2000.4169. [DOI] [PubMed] [Google Scholar]
- 123.Li X, Friedman AB, Zhu W, Wang L, Boswell S, May RS, et al. Lithium regulates glycogen synthase kinase-3beta in human peripheral blood mononuclear cells:Implication in the treatment of bipolar disorder. Biol Psychiatry. 2007;61(2):216–22. doi: 10.1016/j.biopsych.2006.02.027. https://doi.org/10.1016/j.biopsych.2006.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Novetsky AP, Thompson DM, Zighelboim I, Thaker PH, Powell MA, Mutch DG, et al. Lithium chloride and inhibition of glycogen synthase kinase 3b as a potential therapy for serous ovarian cancer. Int J Gynecol Cancer. 2013;23(2):361–6. doi: 10.1097/IGC.0b013e31827cfecb. https://doi.org/10.1097/igc.0b013e31827cfecb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hilliard TS, Gaisina IN, Muehlbauer AG, Gaisin AM, Gallier F, Burdette JE. Glycogen synthase kinase 3b inhibitors induce apoptosis in ovarian cancer cells and inhibit in-vivo tumor growth. Anti Cancer Drugs. 2011;22(10):978–85. doi: 10.1097/CAD.0b013e32834ac8fc. https://doi.org/10.1097/cad.0b013e32834ac8fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nicolaou KA, Liapis V, Evdokiou A, Constantinou C, Magiatis P, Skaltsounis AL, et al. Induction of discrete apoptotic pathways by bromo-substituted indirubin derivatives in invasive breast cancer cells. Biochem Biophys Res Commun. 2012;425(1):76–82. doi: 10.1016/j.bbrc.2012.07.053. https://doi.org/10.1016/j.bbrc.2012.07.053. [DOI] [PubMed] [Google Scholar]
- 127.Liu L, Nam S, Tian Y, Yang F, Wu J, Wang Y, et al. 6-Bromoindirubin-3'-oxime inhibits JAK/STAT3 signaling and induces apoptosis of human melanoma cells. Cancer Res. 2011;71(11):3972–9. doi: 10.1158/0008-5472.CAN-10-3852. https://doi.org/10.1158/0008-5472.can-10-3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cao H, Chu Y, Lv X, Qiu P, Liu C, Zhang H, et al. GSK3 inhibitor-BIO regulates proliferation of immortalized pancreatic mesenchymal stem cells (iPMSCs) PLoS One. 2012;7(2):e31502. doi: 10.1371/journal.pone.0031502. https://doi.org/10.1371/journal.pone.0031502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dastjerdi FV, Zeynali B, Tafreshi AP, Shahraz A, Chavoshi MS, Najafabadi IK, et al. Inhibition of GSK-3b enhances neural differentiation in unrestricted somatic stem cells. Cell Biol Int. 2012;36(11):967–72. doi: 10.1042/CBI20110541. https://doi.org/10.1042/cbi20110541. [DOI] [PubMed] [Google Scholar]
- 130.Yu AS, Zhao L. Effects of the GSK-3b inhibitor (2Z,3E)-6-bromoindirubin-3'-oxime upon ovarian cancer cells. Tumour Biol. 2016;37(4):4857–64. doi: 10.1007/s13277-015-4344-8. https://doi.org/10.1007/s13277-015-4344-8. [DOI] [PubMed] [Google Scholar]
- 131.Chen S, Sun KX, Feng MX, Sang XB, Liu BL, Zhao Y. Role of glycogen synthase kinase-3b inhibitor AZD1080 in ovarian cancer. Drug Des Dev Ther. 2016;10:1225–32. doi: 10.2147/DDDT.S102506. https://doi.org/10.2147/dddt.s102506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gao Y, Ye DY, Zhou WC, Chu Y. The discovery of novel benzothiazinones as highly selective non-ATP competitive glycogen synthase kinase 3b inhibitors for the treatment of ovarian cancer. Eur J Med Chem. 2017;135:370–81. doi: 10.1016/j.ejmech.2017.04.039. https://doi.org/10.1016/j.ejmech.2017.04.039. [DOI] [PubMed] [Google Scholar]
- 133.Shanmugam MK, Manu KA, Ong TH, Ramachandran L, Surana R, Bist P, et al. Inhibition of CXCR4/CXCL12 signaling axis by ursolic acid leads to suppression of metastasis in transgenic adenocarcinoma of mouse prostate model. Int J Cancer. 2011;129(7):1552–63. doi: 10.1002/ijc.26120. https://doi.org/10.1002/ijc.26120. [DOI] [PubMed] [Google Scholar]
- 134.Kassi E, Sourlingas TG, Spiliotaki M, Papoutsi Z, Pratsinis H, Aligiannis N, et al. Ursolic acid triggers apoptosis and Bcl-2 downregulation in MCF-7 breast cancer cells. Cancer Investig. 2009;27(7):723–33. doi: 10.1080/07357900802672712. https://doi.org/10.1080/07357900802672712. [DOI] [PubMed] [Google Scholar]
- 135.Manu KA, Kuttan G. Ursolic acid induces apoptosis by activating p53 and caspase-3 gene expressions and suppressing NF-kappaB mediated activation of bcl-2 in B16F-10 melanoma cells. Int Immunopharmacol. 2008;8(7):974–81. doi: 10.1016/j.intimp.2008.02.013. https://doi.org/10.1016/j.intimp.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 136.Andersson D, Liu JJ, Nilsson A, Duan RD. Ursolic acid inhibits proliferation and stimulates apoptosis in HT29 cells following activation of alkaline sphingomyelinase. Anticancer Res. 2003;23(4):3317–22. [PubMed] [Google Scholar]
- 137.Song YH, Jeong SJ, Kwon HY, Kim B, Kim SH, Yoo DY. Ursolic acid from Oldenlandia diffusa induces apoptosis via activation of caspases and phosphorylation of glycogen synthase kinase 3 beta in SK-OV-3 ovarian cancer cells. Biol Pharm Bull. 2012;35(7):1022–8. doi: 10.1248/bpb.b110660. https://doi.org/10.1248/bpb.b110660. [DOI] [PubMed] [Google Scholar]
- 138.Cormier KW, Woodgett JR. Recent advances in understanding the cellular roles of GSK-3. F1000Res. 2017;6:F1000. doi: 10.12688/f1000research.10557.1. https://doi.org/10.12688/f1000research.10557.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nguyen VH, Hough R, Bernaudo S, Peng C. Wnt/β-catenin signalling in ovarian cancer: Insights into its hyperactivation and function in tumorigenesis. J Ovarian Res. 2019;12(1):122. doi: 10.1186/s13048-019-0596-z. https://doi.org/10.1186/s13048-019-0596-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (GSK3):Regulation, actions, and diseases. Pharmacol Ther. 2015;148:114–31. doi: 10.1016/j.pharmthera.2014.11.016. https://doi.org/10.1016/j.pharmthera.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


