Supplemental Digital Content is available in the text.
Summary:
Systemic scleroderma is a chronic connective tissue disease characterized by internal organ and skin fibrosis. Unfortunately, there is a lack of efficacious treatments for cutaneous manifestations, and alternative interventions should be considered. Fat grafting has gained significant attention due to its regenerative properties and success in improving skin quality and volume deficits in fibrotic diseases. While some studies have investigated the efficacy of autologous fat grafting, we utilized the Coleman method for harvesting and processing to determine the efficacy of fat grafting to improve skin fibrosis in the hands and face of scleroderma patients without excess processing of adipose tissue. Patients with a diagnosis of scleroderma who underwent fat grafting between March 2015 and March 2019 at the University of Michigan were included. Ten female patients were identified that met inclusion criteria. The mean age at the time of surgery was 48.7 (± 17.6) years. An average of 53.2 (± 15.5) ml of fat was injected into the hands and 26.1 (± 16.4) ml into the face. Patients were treated with 1–4 rounds of grafting depending on the initial severity of skin fibrosis and volume deficiency. Fat grafting subjectively and qualitatively improved perioral skin quality, facial animation, hand range of motion, and hand pain for patients with systemic scleroderma. No complications were identified. Additional studies are necessary to determine the ideal volume, timing of treatments, and type of fat to optimize the efficacy of autologous fat grafting for the treatment of systemic scleroderma.
INTRODUCTION
Systemic scleroderma is a rare disease, affecting more women in their 30s–50s and resulting in as high as 68% living with disability.1,2 Systemic scleroderma is a chronic autoimmune disease characterized by vascular disruption and progressive internal organ and skin fibrosis.3 Although the presentation of internal organ fibrosis varies, all patients have cutaneous manifestations, most notably on the face and hands.3,4 Facial skin fibrosis results in limited facial expression, thinning of lips, increased perioral rhytids, microstomia, and difficulty eating.5,6 Hand manifestations include tendon retraction, joint contracture, limited range of motion, Raynaud’s phenomenon, digital ulcers, and hand pain.7 Although topical and oral treatments have been shown to limit the progression of cutaneous forms of scleroderma, these therapies are unable to reverse the skin fibrosis.8,9
Fat grafting is effective in improving contour irregularities, volume deficiency, and skin elasticity.10 Although fat grafting has been shown to improve fibrotic diseases, such as scleroderma, there is a significant variability in the processing of fat that results in the use of stromal vascular fraction and adipose stem cells that are less than minimally manipulated, which would limit its use due to FDA regulations. In the current study, we report our experience using less processed fat with the Coleman method to determine its efficacy on the skin of scleroderma patients. We describe cases to further support autologous fat grafting using the Coleman method as a feasible method for autologous fat grafting that is minimally manipulated.
PATIENTS AND METHODS
After obtaining institutional review board approval, a chart review was conducted on scleroderma patients undergoing autologous fat grafting to the face or hands, between March 2015 and March 2020 at the University of Michigan for this case series. All patients were 18 years or older and fulfilled the American College of Rheumatology criteria for scleroderma. Patients lacking complete operative data were excluded.
Surgical Technique
Fat was harvested from the abdomen or thighs, using a 3.0-mm liposuction cannula under a low pressure connected 60 ml syringe. Centrifugation was performed at 3000 rpm for 3 minutes, and the oil and debris were decanted, resulting in a homogenous mixture. The harvested fat was transferred to 3 ml syringes in preparation for injection. For the face, small incisions were made with a 11-blade along the lateral commissures as access points. Small aliquots (0.1–0.2 ml) of fat were injected with each pass. For the hands, access points were located near the wrist, and small aliquots of fat were injected over the dorsum of the hand, extending from the proximal wrist to the proximal interphalangeal joints of each digit and to the thumb interphalangeal joint.
Postoperative Care
Patients were routinely evaluated at 1, 3, 6-weeks, and 12-weeks postoperatively to address any complications and to perform subjective, qualitative interviews to evaluate the effects of autologous fat grafting. Photographs were also taken at each follow-up visit.
RESULTS
Ten women with systemic scleroderma were included in the case series. Five patients underwent fat grafting to the face only, and 5 patients underwent fat grafting to the face and hands. The mean age was 48.7 years (range 25–78 years). (See table 1, Supplemental Digital Content 1, which displays study patients. http://links.lww.com/PRSGO/B555.)
Medical history of patients is summarized in SDC1 (See table 1, Supplemental Digital Content 1, which displays study patients. http://links.lww.com/PRSGO/B555). Five patients had a history of smoking. No current smokers were included in the study. Eight patients were on immunosuppressive medications.
The total volume of fat grafted and number of treatments varied between patients and depended on skin elasticity and volume depletion at the time of presentation. The mean volume of fat injected to the face was 26.1 ml (range 7.5–71 ml), with a mean volume of 8.2ml (range 3–20 ml) to the lips, 5.8 ml (range 3–8 ml) to the nasolabial folds, 17 ml (range 6–40) to the malar regions, and 8.1 ml (range 1–23 ml) to the marionette lines. (See table 2, Supplemental Digital Content 2, which displays volume of fat injected to the face and hand. http://links.lww.com/PRSGO/B556.)
A mean total of 53.2 ml (range 30–78 ml) was injected into the bilateral hands. Several patients benefited from additional rounds of fat grafting, during which larger volumes, when necessary, could be injected without compromising the skin. No major or minor complications occurred. Minor adverse events included pain, bruising, and swelling at the donor site and minor bruising at the recipient site. All events resolved without intervention. No delayed wound healing or signs of infection were noted. Mean follow-up was 6.2 months.
All patients had subjective improvement in soft tissue volume, pliability, and skin quality based on physical examination performed by the treating surgeon. Visual qualitative examinations revealed improved facial animation, reduced perioral rhytids, limited pain, and subjectively enhanced facial aesthetics following fat grafting to the face (Fig. 1). (See figure 1, Supplemental Digital Content 3, which displays fat grafting improves facial lipoatrophy secondary to systemic scleroderma. A 60-year-old woman with deep perioral rhytides over the upper and lower lips secondary to systemic scleroderma. (a) Preoperative photographs. (b) Postoperative photographs 5 months after first round of fat grafting. A total of 34 ml of fat was injected into the face, with 6 ml into the lips, 24 ml into the malar regions, and 4 ml into the marionette lines. (c) Postoperative photographs 2 months after second round of fat grafting. A total of 35 ml of fat was injected into the face, with 7 ml into the lips, 18 ml into the malar region, and 10 ml into the marionette lines. Improvements in skin elasticity, perioral rhytids, and malar volume can be appreciated. http://links.lww.com/PRSGO/B557.) (See figure 2, Supplemental Digital Content 4, fat grafting to the hands improves skin fibrosis in the hands. (Top) Preoperative photos of a 48-year-old woman with significant loss of thickness of the dorsum of the hands bilaterally due to systemic scleroderma. (Middle) Postoperative photographs 5 months after first round of fat grafting. A total of 48 ml was injected into bilateral hands. (Bottom) Postoperative photographs 2 months after the second round of fat grafting. A total of 62 ml was injected into bilateral hands. Visible improvement in the dorsal skin thickness of bilateral hands and proximal phalanx. http://links.lww.com/PRSGO/B558.) Skin elasticity and hand volume demonstrated significant subjective, qualitative improvements with some improvement in mobility (Fig. 2).
Fig. 1.
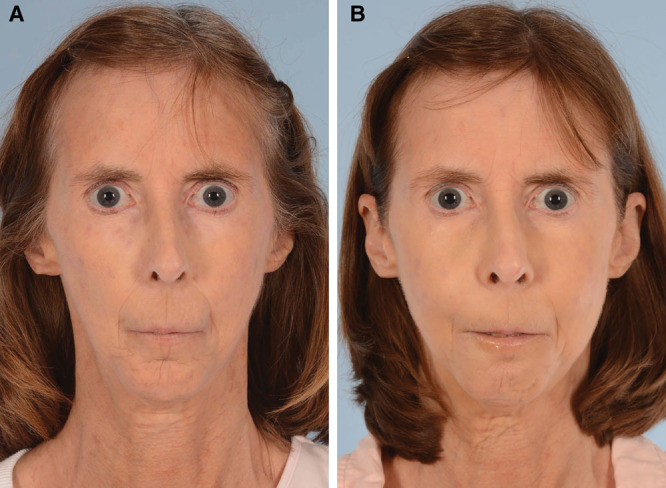
Improvement in skin fibrosis follow facial fat grafting. A, Preoperative photograph of a 60-year-old woman with classic facial manifestations of scleroderma, including skin tightening and hardening, perioral rhytids, and malar volume depletion. B, Postoperative photograph 2 months after the second round of autologous fat grafting of 7 ml into the lips, 18 ml into the malar region, and 10 ml into the marionette lines, resulting in improvement in lip contour and malar volume.
Fig. 2.
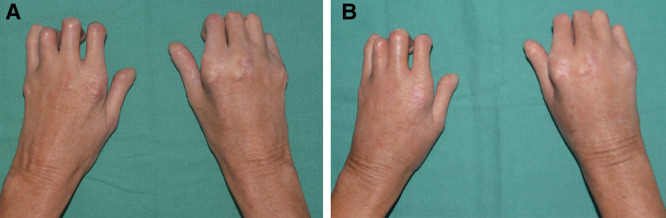
Enhanced skin elasticity following fat grafting to bilateral hands. A, Preoperative photograph of a 48-year-old woman with significant loss of thickness in the dorsal hands due to systemic scleroderma. B, Postoperative photograph 2 months after second round of fat grafting with 62 ml into bilateral hands. Visible improvement in the dorsal skin thickness and volume of bilateral hands and proximal phalanxes.
DISCUSSION
The regenerative and volume-enhancing properties of autologous fat grafting have resulted in growing interest in its use for the treatment of scleroderma.11–13 Our study findings are consistent with those of the previous studies demonstrating that fat grafting improves facial skin fibrosis and pain.14–19 Others have also shown improved facial Rodnan scores, reduced xerostomia, improved Mouth Handicap in Systemic Sclerosis scores, increased intraoral incisal distance, and improved global patient satisfaction with fat grafting.14–19 Few studies, to date, exist investigating the impact of autologous fat grafting on the hands of scleroderma patients. Studies have reported healing of chronic digital ulcers and improved hand pain secondary to scleroderma following fat grafting.20–22 We show that autologous fat grafting to the hands results in subjective, qualitative improvement in skin tightening, range of motion, and pain even in patients with late stage disease. Fat grafting at earlier stages of the disease, before restricted range of motion in the hands or limited facial expression, may limit the severity of the disease, preventing the irreversible deformity that results from the skin fibrosis and hardening. Consistent with prior studies, our study shows that autologous fat grafting has significant implications on improving the standard of living for scleroderma patients through improvements in perioral and hand function.19,21
Fat grafting for scleroderma patients requires special consideration that balances correcting the volume defect without placing tension on the fibrotic skin. Oftentimes, a second round of fat grafting leads to more favorable results, as the skin has improved with the first round to allow for larger volume fat grafting. As the current study is a case series, we are limited by retrospective, qualitative, and subjective analyses. Further evaluation of patient-reported outcomes and objective measures are needed to assess the functional and aesthetic changes to the hands and face after fat grafting in scleroderma patients. Together, these results demonstrate that autologous fat grafting merits further investigation and optimization for the treatment of scleroderma.
PATIENT CONSENT
Patients provided consent for the use of their images.
Supplementary Material
Footnotes
Published online 25 January 2021.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Odonwodo A, Badri T, Hariz A. Scleroderma (systemic sclerosis). Available at https://www.ncbi.nlm.nih.gov/books/NBK537335/. Published 2020. Accessed September 16, 2020.
- 2.Fischer A, Zimovetz E, Ling C, et al. Humanistic and cost burden of systemic sclerosis: a review of the literature. Autoimmun Rev. 2017;16:1147–1154. [DOI] [PubMed] [Google Scholar]
- 3.Cutolo M, Soldano S, Smith V. Pathophysiology of systemic sclerosis: current understanding and new insights. Expert Rev Clin Immunol. 2019;15:753–764. [DOI] [PubMed] [Google Scholar]
- 4.Czirják L, Foeldvari I, Müller-Ladner U. Skin involvement in systemic sclerosis. Rheumatology (Oxford). 2008;47(suppl 5):v44–v45. [DOI] [PubMed] [Google Scholar]
- 5.Crincoli V, Fatone L, Fanelli M, et al. Orofacial manifestations and temporomandibular disorders of systemic scleroderma: an observational study. Int J Mol Sci. 2016;17:1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jagadish R, Mehta DS, Jagadish P. Oral and periodontal manifestations associated with systemic sclerosis: a case series and review. J Indian Soc Periodontol. 2012;16:271–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young A, Namas R, Dodge C, et al. Hand impairment in systemic sclerosis: various manifestations and currently available treatment. Curr Treatm Opt Rheumatol. 2016;2:252–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearson DR, Werth VP, Pappas-Taffer L. Systemic sclerosis: current concepts of skin and systemic manifestations. Clin Dermatol. 2018;36:459–474. [DOI] [PubMed] [Google Scholar]
- 9.Vitiello M, Abuchar A, Santana N, et al. An update on the treatment of the cutaneous manifestations of systemic sclerosis: the dermatologist’s point of view. J Clin Aesthet Dermatol. 2012;5:33–43. [PMC free article] [PubMed] [Google Scholar]
- 10.Sinno S, Wilson S, Brownstone N, et al. Current thoughts on fat grafting: using the evidence to determine fact or fiction. Plast Reconstr Surg. 2016;137:818–824. [DOI] [PubMed] [Google Scholar]
- 11.Guillaume-Jugnot P, Daumas A, Magalon J, et al. State of the art. Autologous fat graft and adipose tissue-derived stromal vascular fraction injection for hand therapy in systemic sclerosis patients. Curr Res Transl Med. 2016;64:35–42. [DOI] [PubMed] [Google Scholar]
- 12.Magalon G, Daumas A, Sautereau N, et al. Regenerative approach to scleroderma with fat grafting. Clin Plast Surg. 2015;42:353–364. [DOI] [PubMed] [Google Scholar]
- 13.Daumas A, Magalon J, Delaunay F, et al. Fat grafting for treatment of facial scleroderma. Clin Plast Surg. 2020;47:155–163. [DOI] [PubMed] [Google Scholar]
- 14.Almadori A, Griffin M, Ryan CM, et al. Stem cell enriched lipotransfer reverses the effects of fibrosis in systemic sclerosis. PLoS One. 2019;14e0218068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Papa N, Caviggioli F, Sambataro D, et al. Autologous fat grafting in the treatment of fibrotic perioral changes in patients with systemic sclerosis. Cell Transplant. 2015;24:63–72. [DOI] [PubMed] [Google Scholar]
- 16.Gheisari M, Ahmadzadeh A, Nobari N, et al. Autologous fat grafting in the treatment of facial scleroderma. Dermatol Res Pract. 2018;2018:6568016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vincent C, Agard C, Barbarot S, et al. [Orofacial manifestations of systemic sclerosis: a study of 30 consecutive patients]. Rev Stomatol Chir Maxillofac. 2010;111:128–134. [DOI] [PubMed] [Google Scholar]
- 18.Blezien O, D’Andrea F, Nicoletti GF, et al. Effects of fat grafting containing stem cells in microstomia and microcheilia derived from systemic sclerosis. Aesthetic Plast Surg. 2017;41:839–844. [DOI] [PubMed] [Google Scholar]
- 19.Sautereau N, Daumas A, Truillet R, et al. Efficacy of autologous microfat graft on facial handicap in systemic sclerosis patients. Plast Reconstr Surg Glob Open. 2016;4:e660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faggioli P, Saporiti E, Falaschi M, Mazzone A, Moscatelli A. AB0720 autologous fat grafting as treatment of scleroderma-induced digital ulcers and perioral sclerosis. Experience of a single center. Ann Rheum Dis. 2015;74:1139. [Google Scholar]
- 21.Bene MD, Pozzi MR, Rovati L, et al. Autologous fat grafting for scleroderma-induced digital ulcers. An effective technique in patients with systemic sclerosis. Handchir Mikrochir Plast Chir. 2014;46:242–247. [DOI] [PubMed] [Google Scholar]
- 22.Del Papa N, Di Luca G, Andracco R, et al. Regional grafting of autologous adipose tissue is effective in inducing prompt healing of indolent digital ulcers in patients with systemic sclerosis: results of a monocentric randomized controlled study. Arthritis Res Ther. 2019;21:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


