These Guidelines were developed by the European Hematology Association (EHA) and European Society for Medical Oncology (ESMO). The 2 societies nominated authors to write the guidelines as well as reviewers to comment on them. These guidelines were approved by the EHA Board and the ESMO Guidelines Committee in November 2020.
Highlights
This EHA-ESMO Clinical Practice Guideline provides key recommendations on the management of multiple myeloma
Authorship includes a multidisciplinary group of experts from different institutions and countries in Europe
Key treatment recommendations are provided for both newly diagnosed myeloma patients and patients with relapsed/refractory disease
Recommendations for the treatment of plasma cell leukaemia, solitary plasmacytoma and smouldering myeloma are also provided
Key recommendations for myeloma complications, including bone disease and renal impairment, are included
Incidence and epidemiology
Multiple myeloma (MM) is a plasma cell neoplasm that accounts for 1%-1.8% of all cancers and is the second most common haematological malignancy with an estimated incidence in Europe of 4.5-6.0/100 000/y. Despite the significant improvement in patients’ survival over the past 20 years, only 10%-15% of patients achieve or exceed expected survival compared with the matched general population.1
Diagnosis and staging
In 2017, ESMO published clinical practice guidelines for the diagnosis, staging and definitions of progressive disease, relapse and refractoriness to therapy, which have not changed and are summarised in Supplementary Tables S1-S3, http://links.lww.com/HS/A128.2
The recommendations for the tests that are required for the diagnosis, determination of prognosis and follow-up of MM are described in Table 1.
Table 1.
Recommendations on Examinations at Diagnosis, Response Assessment, During Follow-up and at Relapse of MM.
| Tool | Diagnosis | At Response | At Follow-up | At Relapse | |
|---|---|---|---|---|---|
| Blood | Blood count and blood smear | Obligatory | Obligatory | Obligatory | Obligatory |
| Serum electrophoresis and IF | Obligatory | Obligatory (IF for CR confirmation) | Obligatory (IF for CR patients) | Obligatory | |
| Serum-free light chain | Obligatory | Obligatory to confirm sCR | Obligatory | Obligatory | |
| Serum immunoglobulin levels | Obligatory | Obligatory | Obligatory | Obligatory | |
| Renal and liver function tests | Obligatory | Obligatory | Obligatory | Obligatory | |
| Calcium | Obligatory | Obligatory | Obligatory | Obligatory | |
| Lactate dehydrogenase | Obligatory | Obligatory | Obligatory | Obligatory | |
| Albumin, β2m | Obligatory | Not required | Optional | Obligatory | |
| Flow cytometry | Optional | Not required | Not required | Optional | |
| Urine | Urine sample from 24 h urine collection to check for proteinuria and light-chain proteinuria | Obligatory | Obligatory | Obligatory | Obligatory |
| Urine electrophoresis and IF electrophoresis | Obligatory | Obligatory (IF for CR confirmation) | Obligatory (IF for CR patients) | Obligatory | |
| BM | BM cytology and biopsy to confirm plasmacytosis and monoclonality | Obligatory | Obligatory to confirm CR or for nonsecretory MM | Not required | Optional (obligatory for nonsecretory disease) |
| NGF or NGS to detect clonal plasma cells | Obligatory | Obligatory to confirm MRD negativity in CR or sCR patients | Every 12 mo in CR and/or MRD-negative patientsa | Optional | |
| Cytogenetics: karyotype and FISH for detection of del17p, t(4;14), t(14;16), ampl 1q/gain 1q, t(11;14) | Obligatory | Not required | Not required | Obligatory for del17p, ampl 1q/gain 1q and t(11;14) | |
| Advanced techniques: GEP, NGS | For clinical trials use only | For clinical trials use only | For clinical trials use only | For clinical trials use only | |
| Imaging | WBLD-CT | Obligatory | Not required | When symptomatic (or CT of the symptomatic area) | Obligatory |
| PET-CT | Optional (it may be carried out instead of WBLD-CT if available) | Obligatory to confirm imaging MRD | Every 12 mo in BM MRD-negative patientsb | Optional | |
| Whole-body MRI | Obligatory in WBLD-CT-negative cases and if PET-CT is not carried out | Not required | When symptomatic | Optional |
Adapted with permission from Caers et al.3
aSustained MRD negativity is supported by IMWG guidelines,4 although it is not fully reimbursed in several countries. In a recent “Real-World” study, MRD assessments were carried out in 139 patients before starting lenalidomide maintenance after ASCT and/or at the achievement of CR, while additional assessments were subsequently carried out on an annual basis until sustained MRD negativity was confirmed. In total, 34.3% of patients who were MRD-positive after induction treatment achieved MRD-negative status during maintenance and ultimately had improved PFS. Sequential MRD assessments identified patients with progressively decreasing MRD levels who also had better PFS outcomes, compared with patients not showing a decreasing pattern of MRD.5
bRecommended based on panel consensus in order to confirm extramedullary MRD negativity in patients who are MRD-negative in the BM.
ASCT = autologous stem cell transplantation; β2m = beta-2 microglobulin; BM = bone marrow; CR = complete response; CT = computed tomography; FISH = fluorescence in situ hybridization; GEP = gene expression profiling; IF = immunofixation; IMWG = International Myeloma Working Group; MM = multiple myeloma; MRD = minimal residual disease; MRI = magnetic resonance imaging; NGF = next-generation flow cytometry; NGS = next-generation sequencing; PET-CT = positron emission tomography-computed tomography; PFS = progression-free survival; sCR = stringent complete response; WBLD-CT = whole-body low-dose computed tomography.
Response criteria to anti-myeloma therapy
One of the most significant improvements in the response criteria is the introduction of minimal residual disease (MRD) both in the bone marrow (BM) (using either next-generation sequencing or next-generation flow [NGF] cytometry) and outside the BM (using positron emission tomography-computed tomography [PET-CT]; imaging MRD).4 MRD negativity in the BM in patients who have achieved conventional complete response (CR) consistently correlates with prolonged progression-free survival (PFS) and overall survival (OS) in both newly diagnosed MM (NDMM) and relapsed/refractory MM (RRMM) patients.6,7
MRD negativity in the BM, defined as the absence of tumour plasma cells within 1 000 000 BM cells (<10–6) shows the best results for the prediction of both PFS and OS compared with higher cutoff values (ie, 10–5).6 Outside the BM, PET-CT is able to recognise hypermetabolic areas in approximately 15%-20% of patients with MRD negativity in the BM and is considered the best method for imaging MRD to date.8
MRD has been found to be a surrogate endpoint for PFS in patients receiving first-line treatment.9 Therefore, MRD may be used as an endpoint to accelerate drug development. The use of MRD to drive treatment decisions is under investigation, for example, whether maintenance/continuous therapy in MRD-negative patients can be stopped or whether treatment needs to be changed in MRD-positive patients, especially in high-risk MM. The results of several phase III trials in the field will clarify the role of MRD in making decisions about therapy in MM.
Front-line therapy
Smouldering MM
Patients with standard- or intermediate-risk smouldering MM (SMM; see Supplementary Table S4, http://links.lww.com/HS/A128) do not need immediate therapy. Myeloma treatment should be initiated according to the International Myeloma Working Group (IMWG) recommendations.10 Regarding high-risk SMM, which is recently defined by the “20-20-20” rule (Supplementary Table S4, http://links.lww.com/HS/A128),11 2 randomised, phase III studies have shown that lenalidomide plays a significant role in prolonging PFS. In the first study, 119 patients with high-risk SMM (before the introduction of the new criteria for the definition of myeloma10) were randomly assigned either to receive treatment with the combination of lenalidomide plus dexamethasone (Rd) for 9 cycles followed by lenalidomide maintenance or to observation. At a median follow-up of 75 months, Rd improved both PFS (median PFS [mPFS] not reached versus 23 mo; P < 0.0001) and OS compared with observation (hazard ratio [HR] = 0.43; P = 0.024).12,13 However, this study was conducted several years ago and enrolled a number of patients who are considered as having MM according to the revised definition. In the second study,14 182 patients with intermediate- or high-risk SMM were randomly assigned either to receive lenalidomide monotherapy or to observation. At a median follow-up of 35 months, PFS was longer with lenalidomide (HR = 0.28; P = 0.002); this result was driven mainly by the high-risk SMM group.14 This study has not reported OS advantage for the lenalidomide arm to date. Several phase II studies using daratumumab (Dara) monotherapy,15 isatuximab (Isa) monotherapy or other Rd-based regimens (with elotuzumab [EloRd], or with ixazomib) have shown encouraging results.
All the above data suggest that high-risk SMM patients should be encouraged to participate in randomised phase III trials to reveal the best treatment that offers OS advantage. To date, no treatment has been approved for SMM.
Newly diagnosed patients who are eligible for high-dose therapy and autologous transplantation
For fit NDMM patients, aged <70 years, without comorbidities, induction followed by high-dose therapy (HDT) with autologous stem cell transplantation (ASCT) and lenalidomide maintenance is the recommended treatment. Two recent phase III trials comparing the use or not of upfront ASCT, after triplet novel agent-based induction, showed that PFS was improved in the upfront ASCT arm.16-18 The first study was conducted by the French Myeloma Study Group and included 700 patients who were randomised to receive induction therapy with 3 cycles of bortezomib, lenalidomide and dexamethasone (VRd) and then consolidation therapy with either 5 additional cycles of VRd or high-dose melphalan (HDM) plus ASCT followed by 2 additional cycles of VRd. Patients in both groups received maintenance therapy with lenalidomide for 1 year. After a median follow-up of 44 months in the VRd-alone group and 43 months in the ASCT group, the mPFS was longer in the ASCT group (50 versus 36 mo; P < 0.001). This benefit was observed across all patient subgroups, including advanced Revised International Staging System (R-ISS) and high-risk cytogenetics. OS at 4 years was not different between the ASCT and the non-ASCT groups.17
The second study was conducted by the European Myeloma Network (EMN)—EMN02/HO95 trial—and included 1503 patients who received an induction therapy with 3-4 cycles of bortezomib, cyclophosphamide and dexamethasone (VCD) followed by the first randomisation between bortezomib, melphalan and prednisone (VMP) versus ASCT. A second randomisation to consolidation therapy (2 cycles of VRd) versus no consolidation was carried out after intensification therapy, to be followed by lenalidomide maintenance until progression or toxicity in both arms. With a median follow-up from the first randomisation of 60.3 months, the mPFS was improved with ASCT compared with VMP (56.7 versus 41.9 mo; P = 0.0001).18
Induction regimen
A 3-drug combination, including at least bortezomib and dexamethasone, has been the standard of care.2,19 Bortezomib, thalidomide, dexamethasone (VTD) induction showed better response rates over VCD at the expense of a higher rate of peripheral neuropathy.20 VCD and bortezomib, doxorubicin and dexamethasone (PAd) were equally effective in terms of response but VCD was less toxic.21 In single-arm studies, VRd produced high very good partial response (VGPR), CR and MRD negativity rates, as well as prolonged PFS.6,17,22-24 However, there is no direct comparison between VTD with VRd induction before ASCT. There is only an integrated analysis of 3 randomised trials, presented in abstract form, which showed that VRd produces higher VGPR and MRD negativity rates compared with VTD.25
The introduction of monoclonal antibodies (mAbs), and especially of Dara, in the front-line setting has changed the treatment landscape in MM. In the phase III CASSIOPEIA trial, 4 cycles of induction with VTD (n = 542) were compared with 4 cycles of VTD plus Dara (DaraVTD) (n = 543); patients then received a single ASCT followed by consolidation and maintenance.26 PFS at 18 months showed the superiority of DaraVTD over VTD (93% versus 85%, P < 0.0001).27 The combination of Dara with VRd (DaraVRd) had better results. In the randomised phase II GRIFFIN study, 207 patients were randomly assigned to receive VRd ± Dara induction (4 cycles), ASCT, VRd ± Dara consolidation (2 cycles) and lenalidomide ± Dara maintenance (26 cycles). The 24-month PFS rates were 95.8% for DaraVRd and 89.8% for VRd.23
The substitution of bortezomib with the second-generation proteasome inhibitor (PI) carfilzomib (K) resulted in high sustained MRD negativity rate in carfilzomib, lenalidomide and dexamethasone (KRd) compared with VRd, especially in patients with advanced R-ISS.28 There is no direct comparison between VRd and KRd in NDMM patients who are eligible for ASCT; however, in the ENDURANCE trial (see Elderly patients’ section), which included <30% of patients who received an ASCT, there was no PFS difference between the 2 regimens.
Based on the above data, VRd is likely to offer the best risk-benefit profile to date among triplet combinations (II, B). The 4-drug combination DaraVTD is more efficacious than VTD (I, A) but comparisons are lacking versus DaraVRd or VRd (these regimens have not been approved by the European Medicines Agency [EMA]). The EMA approval of DaraVTD makes it a new standard of care for induction before ASCT. Novel studies that are ongoing compare DaraVRd versus VRd, DaraVCD versus VTD, or combinations with novel mAbs such as IsaVRd, IsaKRd or EloVRd will reveal the best induction regimen in the future.
Conditioning regimen before ASCT
HDM (200 mg/m2) remains the standard conditioning regimen before ASCT for NDMM patients. The addition of busulfan to melphalan has not shown OS benefit over HDM.29,30 The addition of bortezomib to HDM did not improve the efficacy of the conditioning regimen and had higher toxicity.31
Consolidation therapy
The EMN02/HO95 study showed that at a median follow-up of 42 months, consolidation therapy with 2 cycles of VRd improved mPFS compared with no consolidation (58.9 versus 45.5 mo; P = 0.014).18 It must be noted that induction treatment in this study included 4 cycles of VCD and not VRd or DaraVTD.
The use of a second planned ASCT as consolidation has also been tested in clinical trials. In the EMN02/HO95 study, in centres with a policy of double ASCT, patients were assigned to receive VMP, single ASCT (ASCT-1) or 2 planned ASCTs (administered 2-3 mo apart; ASCT-2) to prospectively compare ASCT-1 with ASCT-2. Patients who received ASCT-2 had a prolonged PFS compared with those who received ASCT-1: the 3-year PFS probability was 53.5% for ASCT-2 versus 44.9% for ASCT-1 group (P = 0.036), which represented a 26% reduced risk of progression or death in the ASCT-2 group. Importantly, ASCT-2 significantly improved the outcome of patients with high-risk cytogenetics (mPFS: 46 and 26.7 mo for ASCT-2 and ASCT-1, respectively; HR = 0.59; P = 0.062).16 In the same study, OS from the first randomisation was significantly prolonged with ASCT-2 compared with ASCT-1 (3-y rate: 89% versus 82%; HR = 0.52; P = 0.011); this benefit was also reported in patients with R-ISS II + III (HR = 0.48; P = 0.013) and with high-risk cytogenetics (HR = 0.52; P = 0.042).16
The phase III StaMINA study randomised 758 patients who received induction therapy for up to 12 cycles, followed by 1 ASCT versus tandem ASCT versus ASCT-1 followed by 4 subsequent cycles of VRd; all treatment groups received lenalidomide maintenance until disease progression.32 The 6-year PFS in high-risk patients was 43.6% and 26% for tandem ASCT and ASCT-1, respectively (P = 0.03).33
Finally, a study which compared tandem ASCTs with ASCT-1 followed by allogeneic SCT (allo-SCT) has recently reported the 10-year median follow-up results. In both standard-risk (n = 625) and high-risk patients (n = 85), there was no PFS or OS difference.34
Maintenance therapy
Treatment with lenalidomide maintenance after ASCT offers PFS and OS benefits over placebo as reported in 2 large randomised trials.35,36 A meta-analysis including more than 1200 patients, with a median follow-up of 79.5 months, showed that lenalidomide maintenance offers more than 2 years of PFS benefit (52.8 versus 23.5 mo) and 2.5 years of OS benefit over placebo. In this study, there was no benefit in patients with ISS-III disease or high-risk cytogenetics.37 However, the Medical Research Council (MRC) myeloma-XI trial, in which 1137 patients were assigned to lenalidomide maintenance and 834 patients to observation, showed that, in high-risk patients, the 3-year OS was 75% in the lenalidomide group compared with 64% in the observation group, and in ultra-high-risk patients it was 63% versus 43.5%, respectively.38 These results should be taken with caution as the study was not powered to show differences in the 2 sub-populations and all patients who entered the maintenance phase were immunomodulatory drug (IMiD)-exposed and -sensitive. Furthermore the definition of high-risk patients was different in the meta-analysis and in the MRC-XI trial; in the meta-analysis, high-risk cytogenetics included only t(4;14) and del17p patients while in the MRC-XI trial patients were classified into 3 cytogenetic risk groups: standard risk (no adverse cytogenetic abnormalities), high risk (1 adverse cytogenetic abnormality), or ultra-high risk (2 or more adverse cytogenetic abnormalities) [adverse cytogenetic abnormalities were defined as gain(1q), t(4;14), t(14;16), t(14;20), or del(17p)].37,38 The EMA has approved lenalidomide for maintenance therapy post-ASCT for all MM patients until progression.
Bortezomib maintenance showed PFS benefit compared with thalidomide maintenance in a randomised study, but the induction treatment was not the same between the 2 treatment groups (PAd versus vincristine, doxorubicin and dexamethasone [VAD], respectively).39 A recent double-blind, placebo-controlled, phase III trial (TOURMALINE-MM3) compared the oral PI ixazomib with placebo in 656 patients who received induction therapy plus HDM + ASCT. There was a 28% reduction in the risk of progression or death with ixazomib (mPFS: 26.5 versus 21.3 mo, respectively; P = 0.0023). In the high-risk population, ixazomib also offered similar PFS advantage over placebo (HR = 0.62, 95% confidence interval = 0.38-1.02).40 Bortezomib and ixazomib have not yet been approved by the EMA for maintenance after ASCT.
Elderly patients or patients with NDMM who are not eligible to receive HDT and autologous transplantation
Before 2019, VMP and Rd were the standards of care for NDMM patients who were not eligible for an ASCT in Europe.41,42 A phase III trial comparing VRd with Rd in 525 NDMM patients (43% were younger than 65 y old) was recently updated and showed the superiority of VRd regarding PFS (mPFS: 41 versus 29 mo; P = 0.003) and OS (median OS [mOS] not reached versus 69 mo; P = 0.0114).43 Based on these results, the EMA approved VRd in April 2019 for use in NDMM patients who are not eligible for ASCT. The substitution of bortezomib with K in the Rd combination seems not to offer better results. The ENDURANCE trial, which compared KRd versus VRd in NDMM patients without an immediate intent for ASCT, failed to show superiority of KRd regarding PFS in the study population (n = 1087), which included a low number of patients with high-risk cytogenetics.44
The addition of Dara to VMP and Rd has created 2 new standards of care. DaraVMP and DaraRd were approved by the EMA in October 2019, based on the results of 2 large phase III studies. In the ALCYONE study, 706 patients with NDMM who were ineligible for ASCT were randomised to receive 9 cycles of VMP either alone or with Dara (DaraVMP); then Dara was given until disease progression.45 At a median follow-up of 40 months, the mPFS was 36.4 versus 19.3 months for the DaraVMP and VMP arms, respectively, while the 36-month rate of OS was 78% and 68% for the 2 groups (HR = 0.60; P = 0.0003).46 In the MAIA study, 737 NDMM patients who were ineligible for ASCT were randomised to receive either DaraRd or Rd until disease progression. At a median follow-up of 28 months, the estimated PFS at 30 months was 70.6% in the DaraRd group and 55.6% in the Rd group (HR = 0.56; P < 0.001).47
Other approved regimens in this setting include bendamustine plus prednisone48 and melphalan, prednisone and lenalidomide (MPR),49 but they are not routinely used and cannot be considered as standards of care.
It is important to realise that one-third of patients are older than 75 years at diagnosis and at least 30% are frail. Please refer to Management of frail elderly patients in the Supplementary Material, http://links.lww.com/HS/A128, for consensus panel recommendations for the management of these patients.
Although maintenance is not standard for patients who are not eligible for ASCT (almost all approved regimens are used continuously until progression or unacceptable toxicity), ixazomib maintenance was tested in a phase III study which included 706 patients who received 6-12 months of standard induction before being randomised to receive either ixazomib or placebo. Ixazomib maintenance offered a PFS benefit over placebo (17.4 versus 9.4 months, HR = 0.65, P = 0.00003).50 Figure 1 depicts the first-line options for the treatment of NDMM patients.
Figure 1.
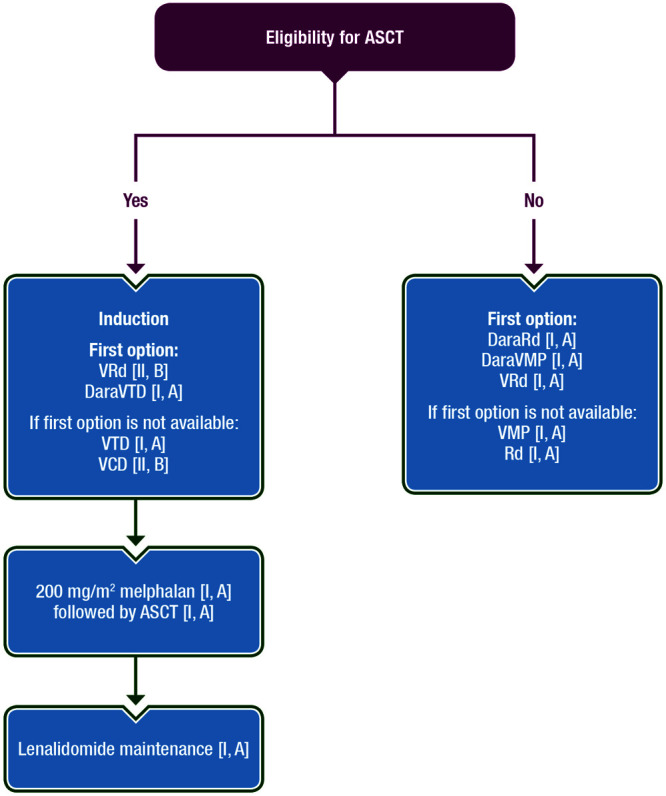
Recommendations for MM front-line therapy. ASCT = autologous stem cell transplantation; DaraRd = daratumumab/lenalidomide/dexamethasone; DaraVMP = daratumumab/bortezomib/melphalan/prednisone; DaraVTD = daratumumab/bortezomib/thalidomide/dexamethasone; MM = multiple myeloma; Rd = lenalidomide/dexamethasone; VCD = bortezomib/cyclophosphamide/dexamethasone; VMP = bortezomib/melphalan/prednisone; VRd = bortezomib/lenalidomide/dexamethasone; VTD = bortezomib/thalidomide/dexamethasone.
Recommendations
“Watch-and-wait” remains the recommended approach for SMM (II, B). High-risk patients are encouraged to participate in randomised phase III studies that are powered for OS advantage of the experimental treatment modality.
For patients <70 years without comorbidities, induction therapy followed by HDM and ASCT is the recommended treatment (I, A).
Regarding induction therapy pre-ASCT, VRd is likely to offer the best risk-benefit profile to date among triplets based on bortezomib (II, B); however, VRd lacks direct comparisons with VTD or DaraVTD and is not licensed by the EMA. The 4-drug combination DaraVTD is more efficacious than VTD (I, A) and is the new standard of care. If this is not available, VTD (I, A) or VCD (II, B) may be used. DaraVRd and Isa-VRd are under clinical investigation and may be standards of care in the near future. Induction with 4-6 cycles is the recommended approach.
HDM (200 mg/m2) is the standard conditioning regimen before ASCT (I, A).
Consolidation therapy post-ASCT has not been established to date as standard therapy; 2 cycles of VRd consolidation has to be considered in patients who receive VCD induction (II, B), while a tandem ASCT is recommended for patients with genetically defined high-risk disease (II, B) or in all patients who received VCD induction (II, B). Allo-SCT following ASCT does not offer OS benefit even in high-risk disease compared with tandem ASCT.
Maintenance with lenalidomide is considered the standard of care for all MM patients post-ASCT (I, A); bortezomib may be considered for patients with high-risk disease (II, B). Ixazomib maintenance offers PFS benefit over placebo (I, A), but has not been approved by the EMA or the US Food and Drug Administration (FDA).
For patients who are not eligible for ASCT, there are 3 new standards of care: VRd, DaraVMP and DaraRd (I, A). When DaraRd and DaraVMP are not available, VRd is the preferred option in fit patients; Rd and VMP may be considered for patients who cannot receive the previous regimens (I, A).
Treatment of relapsed/refractory patients
Patients who have received 1 prior line of therapy
Salvage ASCT may be an option for patients who have received front-line induction with bortezomib-based triplet combination followed by an ASCT. Two prospective studies of salvage ASCT have been published so far. The first included a bortezomib-based re-induction and a randomisation between salvage ASCT or cyclophosphamide, which is suboptimal for relapsed patients. Salvage ASCT significantly extended mPFS (19 versus 11 mo; P < 0.001) and OS (67 versus 52 mo; P = 0.0169).51 The second included patients with first to third relapse who were randomised to a transplant arm (n = 139) consisting of 3 Rd re-induction cycles, ASCT and lenalidomide maintenance (10 mg/d) or to a control arm (n = 138) of continuous Rd. Although there was no difference regarding PFS and OS between the 2 arms, almost 30% of the patients in the transplant arm did not receive the assigned ASCT mainly due to early disease progression. Multivariate landmark analyses from the time of ASCT showed superior PFS and OS (P = 0.0087 and P = 0.0057, respectively) in patients who received ASCT.52 The American and European Associations for Bone and Marrow Transplantation have reported that HDT and ASCT should be considered appropriate treatment of any patient relapsing after primary therapy that includes an ASCT with initial remission duration of >18 months.53 However, this recommendation was made before the broad use of lenalidomide as maintenance therapy post-ASCT. Although there is no evidence for the role of salvage ASCT in patients who received lenalidomide maintenance, the panel suggests that second-line ASCT is a logical approach for patients who relapse after primary therapy that includes an ASCT followed by lenalidomide maintenance and had an initial remission duration of ≥36 months. The use of re-induction is a matter of debate as there is no prospective study on this issue. Retrospective studies suggest that the use of re-induction does not offer survival benefit in salvage ASCT.54
In patients in whom a salvage ASCT is not considered, the second-line therapy should include an Rd-based regimen, that is, KRd, DaraRd, ixazomib/lenalidomide/dexamethasone (IRd) or EloRd for patients who received a bortezomib-based therapy upfront without lenalidomide or Dara (ie, VCD, VTD, VMP); all these combinations were found to be superior to Rd, in terms of PFS, in pivotal phase III studies.55-58 Based on both HR and absolute values of mPFS, DaraRd provides the longest PFS for patients with RRMM who have received 1-3 prior lines of therapy and have a standard-risk cytogenetic profile. KRd and EloRd have also shown OS benefit over Rd: mOS 48.3 versus 40.4 months for KRd versus Rd (HR = 0.79; P = 0.0045),59 and 48.3 versus 39.6 months for EloRd versus Rd,60 respectively. DaraRd is likely to have OS benefit over Rd, but mature data have not been presented, while IRd has no OS benefit over Rd. For relapsed patients with high-risk cytogenetics, although different cutoff values are used for the definition of del17p positivity, all above triplets have shown better results compared with Rd; however, the combination of a PI with Rd, that is, KRd or IRd, along with DaraRd seem to offer the best benefit to date.
Elderly patients who received Rd upfront without Dara61 or patients who received lenalidomide maintenance after ASCT and are progressing (lenalidomide-refractory patients), according to previous guidelines, could receive either K plus dexamethasone (Kd) or Dara and bortezomib plus dexamethasone (DaraVd) (patients treated with Kd or DaraVd had significantly improved PFS compared with Vd).62,63 Three phase III studies suggest that the combination of pomalidomide with bortezomib and dexamethasone (PomVd) and the combinations of Dara or Isa with K and dexamethasone (DaraKd or IsaKd) are new options for this setting.62-64 In the first study, PomVd (n = 278) was compared with Vd (n = 270) in RRMM patients who had received 1-3 prior lines of therapy that included lenalidomide. More than 70% of the patients were refractory to lenalidomide. After a median follow-up of 16 months, PomVd improved mPFS in the study population (11.2 versus 7.1 mo; HR = 0.61; P < 0.0001) as well as in patients refractory to lenalidomide (9.5 versus 5.6 mo; P = 0.0008) and in patients refractory to lenalidomide who received only 1 prior line of treatment (17.8 versus 9.5 mo; P = 0.03).64 PomVd was approved by the EMA in May 2019.
In the second study (CANDOR), DaraKd was compared with Kd in RRMM patients who had received 1 prior line of therapy. This study showed that the mPFS was not reached for the DaraKd group and it was 15.8 months for the Kd group (HR = 0.63; P = 0.0014). DaraKd resulted in a better PFS benefit both among lenalidomide-exposed (HR = 0.52) and lenalidomide-refractory patients (HR = 0.45).65
Finally, in the third study, which was reported at the EHA 2020 meeting, only 302 patients with RRMM and 1-3 prior lines of therapy were randomised to receive either IsaKd (n = 179) or Kd (n = 123). At a median follow-up of 20.7 months, mPFS was not reached for IsaKd whereas it was 19.1 months for Kd (HR = 0.53; P = 0.0007).66
Thus, PomVd, DaraKd and IsaKd are recommended therapies for patients who were previously exposed or are refractory to lenalidomide, while DaraKd or IsaKd can also be given in patients who are refractory to bortezomib.
The approval of Dara-based regimens (DaraVTD, DaraVMP and DaraRd) and of VRd, as first-line therapy for myeloma patients, makes the treatment of second and subsequent lines of therapy very challenging. Although there is some evidence that Dara retreatment can be efficacious in some patients,67,68 there are no data for Dara retreatment at second line.
Venetoclax is a selective Bcl-2 inhibitor that promotes MM cell apoptosis. The phase III BELLINI trial evaluated the combination of venetoclax with Vd (VenVd) compared with Vd among RRMM patients, who had received 1-3 prior lines of therapy and were PI sensitive. A significant PFS benefit was reported with VenVd among patients with t(11;14) (HR = 0.11; P = 0.004) and those with high BCL2 gene expression (HR = 0.24; P<0.0001) but no OS difference was shown in this population. On the contrary, Vd was superior to VenVd in terms of OS among patients without t(11;14) and low BCL2 gene expression (HR = 3.04; P = 0.022).69 Therefore, VenVd is an option only for patients with t(11;14) who have failed lenalidomide and are sensitive to PI. Prospective clinical trials are needed to confirm the BELLINI findings in patients with RRMM with high BCL2 gene expression. Furthermore, antibiotic prophylaxis is recommended for all patients receiving VenVd. Venetoclax is not currently licensed for treatment of MM.
Selinexor is an oral, selective inhibitor of exportin 1 protein-mediated nuclear export, leading to the reactivation of tumour-suppressor proteins. Selinexor in combination with Vd (SVd) was compared with Vd in a phase III study with 402 patients with RRMM who received 1-3 prior lines of therapy. SVd significantly prolonged mPFS compared with Vd (13.9 versus 9.4 mo, HR = 0.70, P = 0.0066), suggesting that SVd might be another option in patients who were treated with lenalidomide-based regimens upfront.70 SVd is awaiting EMA approval.
In Figure 2, the possible options for second-line therapy, taking into consideration the previous line and the refractoriness to specific agents, are described. For Dara-previously exposed or -refractory patients, the recommendations are based on panel opinion as there is no evidence for the efficacy of the approved second-line regimens in these patients to date.
Figure 2.
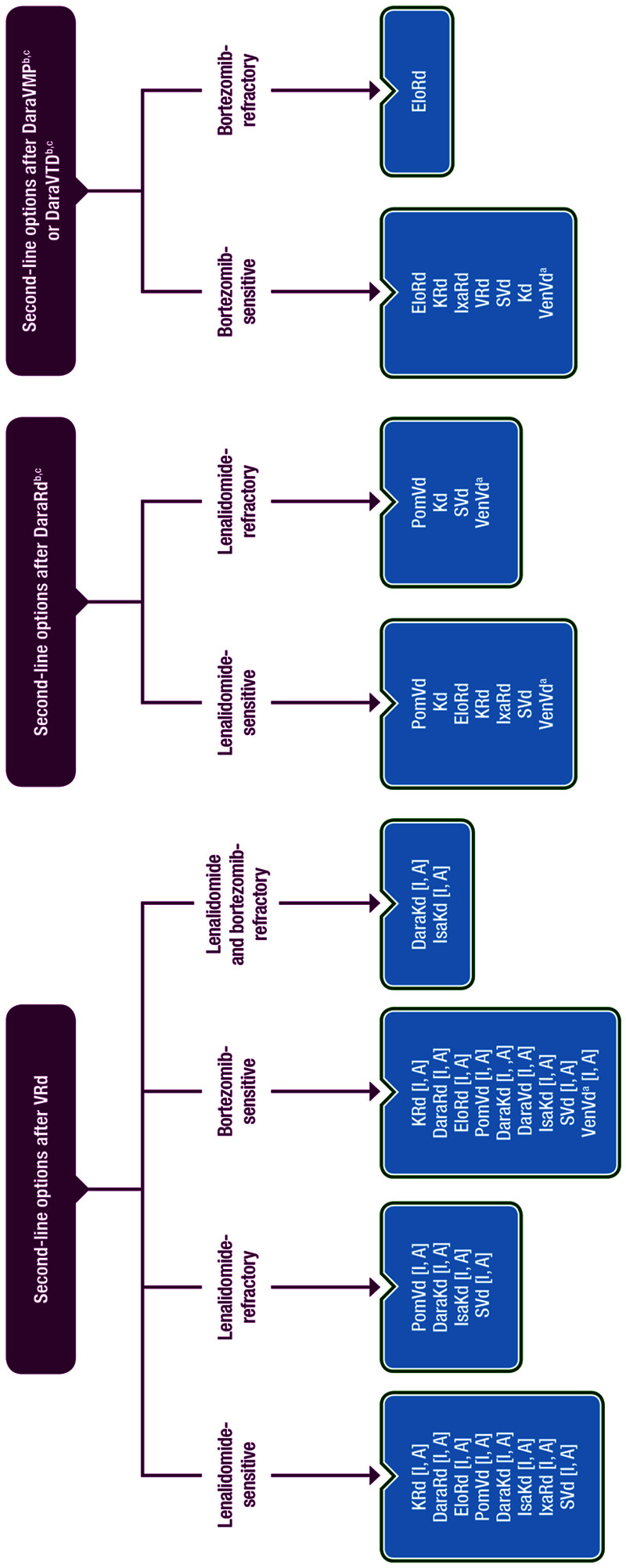
Second-line options for MM patients who received VRd and Dara-based front-line therapies. The 3 different flowcharts shown in this figure depict 3 different scenarios—depending on the first-line treatment given (from left to right): second-line options after VRd first-line treatment, second-line options after DaraRd first-line treatment, and second-line options after DaraVMP or DaraVTD first-line treatment. aPatients with t(11;14). bPatients who progress while on monthly daratumumab are considered as daratumumab-refractory. cAll recommendations for patients who receive front-line therapy with daratumumab-based therapies are based on panel consensus as there are no trials evaluating regimens in second-line therapy that include patients refractory or exposed to daratumumab. Dara = daratumumab; Elo = elotuzumab; Isa = isatuximab; Ixa = ixazomib; K = carfilzomib; Kd = carfilzomib/dexamethasone; MM = multiple myeloma; PomVd = pomalidomide/bortezomib/dexamethasone; Rd = lenalidomide/dexamethasone; S = selinexor; Vd = bortezomib/dexamethasone; VMP = bortezomib/melphalan/prednisone; VRd = bortezomib/lenalidomide/dexamethasone; Ven = venetoclax; VTD = bortezomib/thalidomide/dexamethasone.
Patients who have received 2 or more prior lines of therapy
Treatment of RRMM patients who received 2 or more prior lines of therapy is becoming challenging.71 A recent study revealed that patients who are refractory to 2 PIs, 2 IMiDs and a CD38 mAb have an mOS of 5.6 months only.72 For patients who have been exposed or are refractory to both bortezomib and lenalidomide, who have not received an mAb, DaraKd or IsaKd are suitable options. The combinations of Elo or Isa with pomalidomide and dexamethasone (EloPd and IsaPd, respectively) are suitable options for patients who have failed ≥2 lines of previous therapies, including lenalidomide and a PI, based on the results of 2 studies. The first was a phase II study, in which patients were randomly assigned to receive either EloPd (n = 60) or Pd (n = 57). After a follow-up period of 9 months, the mPFS was 10.3 months in the EloPd group and 4.7 in the Pd group (HR = 0.54; P = 0.008).73 The second was a phase III study, in which patients were randomised to receive either IsaPd (n = 154) or Pd (n = 153). At a median follow-up of 11.6 months, mPFS was 11.5 months in the IsaPd group versus 6.5 months in the Pd group (HR = 0.596; P = 0.001).74 EloPd and IsaPd were recently approved by the EMA in this setting.
The combination of Dara with pomalidomide and dexamethasone (DaraPd) has been approved by the FDA for patients who have failed ≥2 lines of previous therapies, including lenalidomide and a PI. This was based on a phase II nonrandomised study where DaraPd was given in 103 patients with RRMM. At a median follow-up of 13 months, the mPFS was 8.8 months and the mOS was 17.5 months.75 DaraPd has not yet been approved by the EMA, as the results of the phase III APOLLO study (DaraPd versus Pd) are pending.
Patients with t(11;14), who are refractory to lenalidomide and are PI-sensitive may be treated with VenVd, when this regimen is licensed, as previously discussed.
For triple-class refractory patients, selinexor-dexamethasone (Sd) or belantamab mafodotin monotherapy may be suitable options. In a phase II study with 122 RRMM patients (median number of 7 prior lines of therapy), oral selinexor was given with dexamethasone twice weekly. An mPFS of 3.7 months and an mOS of 8.6 months were reported. Fatigue, nausea and decreased appetite were common and were typically grade 1 or 2 events; grade 3 events were noted in up to 25% of patients and no grade 4 events were reported.76
Belantamab mafodotin is an antibody-drug conjugate targeting B-cell maturation antigen (BCMA). In a phase II study, 196 patients with triple-class refractory MM received 2 different doses of belantamab mafodotin (2.5 and 3.4 mg/kg). The mPFS was 2.9 and 4.9 months for the 2 doses, respectively. The most common grade 3-4 adverse events included keratopathy (27% and 21% of patients for the 2 doses, respectively), thrombocytopaenia and anaemia.77 Melflufen may also be beneficial in Dara and pomalidomide-refractory patients but the results of the phase III trial have not yet been reported.78
Figure 3 summarises the recommendations for RRMM patients who receive third or subsequent lines of therapy.
Figure 3.
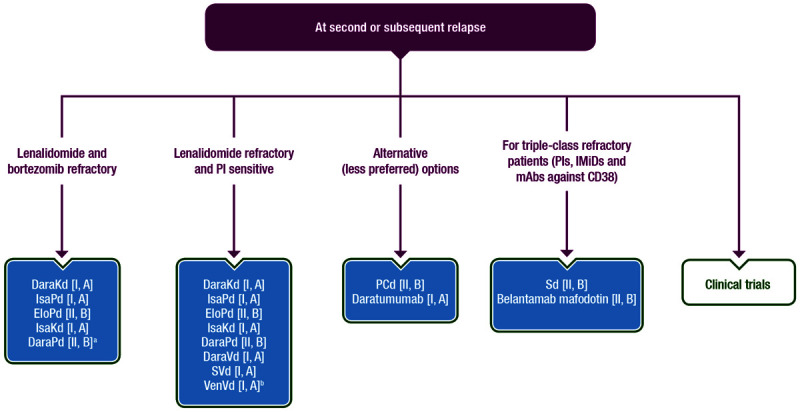
Recommendations for MM patients who received a third or subsequent line of therapy. aOnly phase IB data are published for DaraPd. Publication of phase III data are expected in 2021. bFor patients with t(11;14). Dara = daratumumab; Elo = elotuzumab; IMiD = immunomodulatory drug; Isa = isatuximab; Kd = carfilzomib/dexamethasone; mAb = monoclonal antibody; MM = multiple myeloma; PCd = pomalidomide/cyclophosphamide/dexamethasone; Pd = pomalidomide/dexamethasone; PI = proteasome inhibitor; S = selinexor; Sd = selinexor/dexamethasone; Vd = bortezomib/dexamethasone; Ven = venetoclax.
Immunotherapy strategies targeting BCMA or other antigens on the surface of myeloma cells, including bispecific T-cell engagers (BiTEs) and chimeric antigen receptor T (CAR-T) cells, are under clinical investigation in RRMM patients. Results for the first published study with a CAR-T cell product in myeloma patients showed that infusion of bb2121 in 33 consecutive patients with multirefractory disease resulted in an objective response rate of 85%, including 15 patients (45%) with CR; all were MRD-negative. The mPFS was 11.8 months. CAR-T cell expansion was associated with better responses and CAR-T cells persisted up to 1 year after the infusion. A total of 25 patients (76%) had cytokine release syndrome, while neurological toxic effects occurred in 14 patients (42%).79 Several studies using other CAR-T cell products or T-cell engagers (TCEs) were reported in the American Society of Clinical Oncology (ASCO) and EHA 2020 meetings and had similar results, suggesting that these immunotherapy techniques may increase survival of myeloma patients (see Supplementary Material, http://links.lww.com/HS/A128, section on novel immunotherapies for myeloma).
Recommendations
Patients who receive second-line therapy
Second-line ASCT is an option for patients who received primary therapy that included an ASCT followed by lenalidomide maintenance and had an initial remission duration of ≥36 months (panel consensus).
Patients who had received a bortezomib-based therapy upfront without lenalidomide or Dara should receive an Rd-based regimen, that is, KRd, DaraRd, IRd or EloRd (I, A). DaraRd provides the best PFS for these patients, while only KRd and EloRd showed an OS benefit over Rd to date.
Patients who are refractory to lenalidomide upfront could receive either PomVD, DaraKd, IsaKd or DaraVd (I, A). PomVd is the approved indication with best results, in terms of PFS, as second-line therapy in lenalidomide-refractory patients. DaraKd has given the best reported PFS to date in lenalidomide-refractory patients, but DaraKd is awaiting EMA approval. Similarly, IsaKd and SVd, which are also suitable for this setting (I, A), have not yet been approved by the EMA.
VenVd is a suitable option for patients with t(11;14) who have failed lenalidomide and are sensitive to PIs (I, A), if available.
Patients at third and subsequent lines of treatment
For patients who have been exposed or are refractory to both bortezomib and lenalidomide, DaraKd (I, A), IsaPd (I, A), IsaKd (I, A) and EloPd (II, B) are recommended.
Patients with t(11;14), who are refractory to lenalidomide and are PI-sensitive may be treated with VenVd (I, A), if available.
For triple-class refractory patients, Sd or belantamab mafodotin monotherapy is recommended (II, B), if available. Results of phase III studies of melflufen, TCEs and CAR-Ts in triple-class refractory patients are awaited.
Management of plasma cell leukaemia
Primary plasma cell leukaemia (PPCL) is a rare and aggressive variant of MM, operationally defined by the presence of 20% and/or an absolute number >2 × 109/L of clonal plasma cells in the peripheral blood without a previous history of MM.80 The cutoff value of circulating plasma cells for the definition of PCL may be reduced to 5% in the near future, as the survival of these patients is similar to those with 20% of circulating plasma cells.76 PPCL should be distinguished from secondary PCL, which generally constitutes the leukaemic evolution of a preexisting, end-stage RRMM, and from extramedullary myeloma. Diagnostic work-up and staging procedures in PPCL are similar to those applied in MM. However, they have to be implemented by peripheral blood analysis for measuring circulating PC count and PET-CT for detecting possible extramedullary lesions.81
The outcome of patients with PCL remains poor and the mOS is around 1 year.80,81 There are no precise guidelines for the treatment of PPCL due to the lack of phase III trials in this setting. Only 2 prospective phase II studies have been published so far for PPCL.82,83 Overall, treatment should be immediate and possibly oriented toward bortezomib and/or lenalidomide-based multiphase approaches in combination with chemotherapy agents, with short treatment-free intervals. It should ideally include induction, double ASCT, consolidation and maintenance (II, B). KRd may be another option for these patients84 but more data are needed before a recommendation can be made for PCL patients.
Allo-SCT should be considered in selected cases (III, C). In a recent study, 71 patients (median age 56 y) with PPCL underwent an allo-SCT and the 4-year outcomes were: nonrelapse mortality 12%, PFS 19% and OS 31%.85 Thus, in patients younger than 50 years of age with a suitable donor, a myeloablative allo-SCT can be considered. Otherwise, a tandem transplant with an ASCT followed by a reduced-intensity conditioning allo-SCT if a related or an unrelated donor is available can be considered (IV, C).
Patients not eligible for transplant procedures should preferably receive continuous treatment (III, C). In relapsed/refractory PPCL, a switch to drugs not used at diagnosis should be considered, favouring combinations of lenalidomide or pomalidomide plus dexamethasone with K or mAbs (Dara or Elo) (expert consensus). See Supplementary Table S6, http://links.lww.com/HS/A128, for summary recommendations.
Management of solitary plasmacytoma
Solitary plasmacytoma is an infrequent form of plasma cell neoplasm that presents as a single mass of monoclonal plasma cells, with either extramedullary or intraosseous location.3,86 Clonal PCs are typically absent in the BM aspirate by conventional morphology or immunohistochemistry and there are no other MM features (hypercalcaemia, anaemia or renal disease attributable to MM).10 In some patients, a BM aspiration can detect a low monoclonal plasma cell infiltration, which indicates a high risk of early progression to an overt myeloma disease.3,86 Furthermore, in a study following the use of high sensitivity flow cytometry, half of the patients showed occult BM infiltration and half of these cases progressed at 2 years.87 Thus, detection of clonal PC using sensitive techniques in the BM is suggested (II, B) and systemic treatment of myeloma should be considered in these patients (III, B). Before treatment initiation, whole-body magnetic resonance imaging (MRI) and PET-CT should be carried out to exclude the presence of multiple plasmacytomas, commending systemic treatment instead of radiotherapy (RT) (I, A).8,88 Local high-dose RT is the preferred treatment of choice (II, A), but about two-thirds of patients develop MM at 10 years’ follow-up.89 With current staging techniques, that is, NGF and PET-CT, the incidence of solitary plasmacytoma is expected to decrease and the cure rate to increase. See Supplementary Table S6, http://links.lww.com/HS/A128, for summary recommendations.
Supportive care
Recommendations for the management of myeloma complications, that is, bone disease, anaemia, BM failure, infections, vaccination strategies and renal impairment, are described in the Supplementary Material, http://links.lww.com/HS/A128, in the section on Supportive Care.
Follow-up and long-term implications
Table 1 includes all tests that have to be carried out during follow-up of myeloma patients. Full blood count, serum and urine electrophoresis and serum-free light chain (sFLC) determination, creatinine and calcium tests should be carried out monthly or at least every 3 months. sFLC should be used to detect light chain escape. In cases of relapsed patients with no positivity for del17p or add1q at diagnosis, fluorescence in situ hybridization analysis for del17p and add1q should be carried out to reveal high-risk relapse. In case of bone pain, whole-body low-dose CT (WBLD-CT), MRI or PET-CT should be carried out to detect new bone lesions.3
MM has for a long time been considered as an incurable disease. Recent trials incorporating novel agents and ASCT report a statistical cure fraction of more than 15%.1,24 The addition of quadruplet combinations including mAbs as part of front-line therapies and novel immunotherapy strategies seems to further increase this apparent cure rate.
Personalised medicine
The presence of t(11;14) in RRMM patients should be investigated to decide for venetoclax-based regimens, when available in Europe. Otherwise, no prognostic factor or staging system, including R-ISS or gene-expression profiling, is used routinely to define a risk-adapted strategy. In myeloma, more research is needed to identify molecular markers which could lead to advances in personalised medicine.
Methodology
These Clinical Practice Guidelines were developed in accordance with the ESMO standard operating procedures for Clinical Practice Guidelines development (www.esmo.org/Guidelines/ESMO-Guidelines-Methodology). An interdisciplinary panel of clinical experts on MM, members of ESMO, EHA, and EMN selected the relevant literature. Levels of evidence and grades of recommendations were assigned according to the adapted Infectious Diseases Society of America-United States Public Health Service Grading System (Supplementary Table S7, http://links.lww.com/HS/A128).90 Statements without grading were considered justified standard clinical practice by the experts.
Acknowledgments
The authors thank all those who contributed to this manuscript on behalf of the EHA and ESMO.
Sources of Funding
Production costs have been covered by EHA from central funds.
Disclosures
MAD reported consultancy and honoraria from Janssen, Celgene, Takeda, Amgen and Bristol Myers Squibb. PM reported honoraria from Celgene, Janssen, Takeda, Amgen and Abbvie. ET reported honoraria from Bristol Myers Squibb, Janssen, Celgene, Takeda, Genesis Pharma, Amgen, Sanofi and Novartis and research funding from Janssen, Amgen, Takeda, Sanofi and Genesis Pharma. MVM reported honoraria from lectures and boards from Janssen, Celgene, Amgen, Takeda, Abbvie, GlaxoSmithKline, Adaptive, Roche and Seattle Genetics. SZ reported participation in advisory boards for Takeda, Celgene, Janssen, Sanofi and Oncopeptides and research funding from Celgene, Janssen and Takeda. GC reported being a member of speaker bureau for Takeda, Bristol Myers Squibb, Celgene, Amgen, Sanofi and Janssen and has received research grants from Bristol Myers Squibb, Celgene and Takeda. MD reported honoraria from Abbvie, Adaptive Biotechnologies, Amgen, Bristol Myers Squibb, Celgene, Janssen, Karyopharm, Sanofi and Takeda and has received research funding from Bristol Myers Squibb, Celgene, Janssen and Takeda. RH reported consultant or advisory roles for Janssen, Amgen, Celgene, AbbVie, Bristol Myers Squibb, Novartis, PharmaMar and Takeda; honoraria from Janssen, Amgen, Celgene, Bristol Myers Squibb, PharmaMar and Takeda and has received research grants from Janssen, Amgen, Celgene, Bristol Myers Squibb, Novartis and Takeda. FS reported honoraria from Amgen, Celgene, Bristol Myers Squibb, Takeda, Abbvie, Janssen, Novartis, SkyliteDX, Oncopeptides, Sanofi, GlaxoSmithKline, Adaptive and Merck Sharp & Dohme. MC reported honoraria from Janssen, Celgene, Amgen, Bristol Myers Squibb, Takeda, AbbVie, Sanofi and Adaptive Biotechnologies and speaker’s bureau membership for Janssen and Celgene. HG reported grants from Amgen, Bristol Myers Squibb, Celgene, Chugai, Dietmar-Hopp-Foundation, Janssen, John Hopkins University and Sanofi; research support from Amgen, Bristol Myers Squibb, Celgene, Chugai, Janssen, Incyte, Molecular Partners, Merck Sharp & Dohme, Sanofi, Mundipharma, Takeda and Novartis; participation in advisory boards for Adaptive Biotechnology, Amgen, Bristol Myers Squibb, Celgene, Janssen, Sanofi, Takeda; honoraria from Academy2 GmbH & Co. KG, Amgen, ArtTempi, Bristol Myers Squibb, Celgene, Chop GmbH, Chugai, FomF GmbH, GlaxoSmithKline, GWT Forschung und Innovation Dresden, InVo Institut für Versorgungsforschung in der Onkologie GbR, Janssen, Kompetenznetz Maligne Lymphome (KML), MedConcept GmbH, Medical Communication GmbH, New Concept Oncology, Novartis, Omnia Med Deutschland, Sanofi. TF reported speaker and advisory roles for Janssen, Bristol Myers Squibb, Takeda, advisory role for Roche, Sanofi, Karyopharm and Oncopeptides and Speaker for Amgen. HE reported consulting and advisory roles for Bristol Myers Squibb, Celgene, Janssen, Amgen, Takeda, Sanofi and GlaxoSmithKline; research funding from Bristol Myers Squibb, Celgene, Janssen, Amgen, GlaxoSmithKline and Sanofi; honoraria from Bristol Myers Squibb, Celgene, Amgen, Takeda, Sanofi and GlaxoSmithKline. MB has received honoraria from Sanofi, Celgene, Amgen, Janssen, Novartis, Bristol Myers Squibb and AbbVie; has served on the advisory boards for Janssen and GlaxoSmithKline; has received research funding from Sanofi, Celgene, Amgen, Janssen, Novartis, Bristol Myers Squibb and Mundipharma. JS-M reported consultancy for Amgen, Bristol Myers Squibb, Celgene, Janssen, Merck Sharp & Dohme, Novartis, GlaxoSmithKline, Takeda, Sanofi and Roche. PS reported honoraria and advisory roles for Celgene, Janssen, Amgen, Takeda, Bristol Myers Squibb and Skyline and research funding from Celgene, Amgen, Janssen and Takeda. UM reported honoraria from Celgene, Janssen, Amgen, Takeda, AbbVie, Bristol Myers Squibb and Sanofi.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
References
- 1.Usmani SZ, Hoering A, Cavo M, et al. Clinical predictors of long-term survival in newly diagnosed transplant eligible multiple myeloma - an IMWG research project. Blood Cancer J. 2018; 8:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moreau P, San Miguel J, Sonneveld P, et al. Multiple myeloma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017; 28suppl_4iv52–iv61 [DOI] [PubMed] [Google Scholar]
- 3.Caers J, Garderet L, Kortüm KM, et al. European Myeloma Network recommendations on tools for the diagnosis and monitoring of multiple myeloma: what to use and when. Haematologica. 2018; 103:1772–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016; 17:e328–e346 [DOI] [PubMed] [Google Scholar]
- 5.Alonso R, Cedena MT, Wong S, et al. Prolonged lenalidomide maintenance therapy improves the depth of response in multiple myeloma. Blood Adv. 2020; 4:2163–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perrot A, Lauwers-Cances V, Corre J, et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood. 2018; 132:2456–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munshi NC, Avet-Loiseau H, Rawstron AC, et al. Association of minimal residual disease with superior survival outcomes in patients with multiple myeloma: a meta-analysis. JAMA Oncol. 2017; 3:28–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavo M, Terpos E, Nanni C, et al. Role of 18F-FDG PET/CT in the diagnosis and management of multiple myeloma and other plasma cell disorders: a consensus statement by the International Myeloma Working Group. Lancet Oncol. 2017; 18:e206–e217 [DOI] [PubMed] [Google Scholar]
- 9.Avet-Loiseau H, Ludwig H, Landgren O, et al. Minimal residual disease status as a surrogate endpoint for progression-free survival in newly diagnosed multiple myeloma studies: a meta-analysis. Clin Lymphoma Myeloma Leuk. 2020; 20:e30–e37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014; 15:e538–e548 [DOI] [PubMed] [Google Scholar]
- 11.Rajkumar SV, Landgren O, Mateos MV. Smoldering multiple myeloma. Blood. 2015; 125:3069–3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mateos MV, Hernández MT, Giraldo P, et al. Lenalidomide plus dexamethasone for high-risk smoldering multiple myeloma. N Engl J Med. 2013; 369:438–447 [DOI] [PubMed] [Google Scholar]
- 13.Mateos MV, Hernández MT, Giraldo P, et al. Lenalidomide plus dexamethasone versus observation in patients with high-risk smouldering multiple myeloma (QuiRedex): long-term follow-up of a randomised, controlled, phase 3 trial. Lancet Oncol. 2016; 17:1127–1136 [DOI] [PubMed] [Google Scholar]
- 14.Lonial S, Jacobus S, Fonseca R, et al. Randomized trial of lenalidomide versus observation in smoldering multiple myeloma. J Clin Oncol. 2020; 38:1126–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landgren CO, Chari A, Cohen YC, et al. Daratumumab monotherapy for patients with intermediate-risk or high-risk smoldering multiple myeloma: a randomized, open-label, multicenter, phase 2 study (CENTAURUS). Leukemia. 2020; 34:1840–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cavo M, Gay F, Patriarca F, et al. Double autologous stem cell transplantation significantly prolongs progression-free survival and overall survival in comparison with single autotransplantation in newly diagnosed multiple myeloma: an analysis of phase 3 EMN02/HO95 study. Blood. 2017; 130suppl 1401 [Google Scholar]
- 17.Attal M, Lauwers-Cances V, Hulin C, et al. ; IFM 2009 Study. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017; 376:1311–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavo M, Gay F, Beksac M, et al. Autologous haematopoietic stem-cell transplantation versus bortezomib-melphalan-prednisone, with or without bortezomib-lenalidomide-dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): a multicentre, randomised, open-label, phase 3 study. Lancet Haematol. 2020; 7:e456–e468 [DOI] [PubMed] [Google Scholar]
- 19.Sonneveld P, Goldschmidt H, Rosiñol L, et al. Bortezomib-based versus nonbortezomib-based induction treatment before autologous stem-cell transplantation in patients with previously untreated multiple myeloma: a meta-analysis of phase III randomized, controlled trials. J Clin Oncol. 2013; 31:3279–3287 [DOI] [PubMed] [Google Scholar]
- 20.Moreau P, Hulin C, Macro M, et al. VTD is superior to VCD prior to intensive therapy in multiple myeloma: results of the prospective IFM2013-04 trial. Blood. 2016; 127:2569–2574 [DOI] [PubMed] [Google Scholar]
- 21.Mai EK, Bertsch U, Dürig J, et al. Phase III trial of bortezomib, cyclophosphamide and dexamethasone (VCD) versus bortezomib, doxorubicin and dexamethasone (PAd) in newly diagnosed myeloma. Leukemia. 2015; 29:1721–1729 [DOI] [PubMed] [Google Scholar]
- 22.Rosiñol L, Oriol A, Rios R, et al. Bortezomib, lenalidomide, and dexamethasone as induction therapy prior to autologous transplant in multiple myeloma. Blood. 2019; 134:1337–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voorhees PM, Kaufman JL, Laubach J, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 2020; 136:936–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joseph NS, Kaufman JL, Dhodapkar MV, et al. Long-term follow-up results of lenalidomide, bortezomib, and dexamethasone induction therapy and risk-adapted maintenance approach in newly diagnosed multiple myeloma. J Clin Oncol. 2020; 38:1928–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosinol L, Hebraud B, Oriol A, et al. Integrated analysis of bortezomib- lenalidomide-dexamethasone vs bortezomib-thalidomide-dexamethasone in transplant-eligible newly diagnosed myeloma. Clin Lymphoma Myeloma Leuk. 2019; 19supplE1–E230396823 [Google Scholar]
- 26.Moreau P, Attal M, Hulin C, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 2019; 394:29–38 [DOI] [PubMed] [Google Scholar]
- 27.Moreau P, Attal M, Hulin C, et al. Phase 3 randomized study of daratumumab + bortezomib/thalidomide/dexamethasone (D-VTd) vs VTd in transplant-eligible newly diagnosed multiple myeloma: CASSIOPEIA part 1 results. J Clin Oncol. 2019; 37suppl 158003 [Google Scholar]
- 28.Gay F, Cerrato C, Petrucci M, et al. Efficacy of carfilzomib lenalidomide dexamethasone (KRd) with or without transplantation in newly diagnosed myeloma according to risk status: results from the FORTE trial. J Clin Oncol. 2019; 37suppl 158002 [Google Scholar]
- 29.Blanes M, Lahuerta JJ, González JD, et al. Intravenous busulfan and melphalan as a conditioning regimen for autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: a matched comparison to a melphalan-only approach. Biol Blood Marrow Transplant. 2013; 19:69–74 [DOI] [PubMed] [Google Scholar]
- 30.Blanes M, Lorenzo JI, Ribas P, et al. Intravenous busulfan plus melphalan versus melphalan alone as conditioning regimen for patients with multiple myeloma. Ann Hematol. 2019; 98:2013–2015 [DOI] [PubMed] [Google Scholar]
- 31.Roussel M, Hebraud B, Lauwers-Cances V, et al. Bortezomib and high-dose melphalan vs. high-dose melphalan as conditioning regimen before autologous stem cell transplantation in de novo multiple myeloma patients: a phase 3 study of the Intergroupe Francophone Du Myelome (IFM 2014-02). Blood. 2017; 130suppl 1398. [DOI] [PubMed] [Google Scholar]
- 32.Stadtmauer EA, Pasquini MC, Blackwell B, et al. Autologous transplantation, consolidation, and maintenance therapy in multiple myeloma: results of the BMT CTN 0702 trial. J Clin Oncol. 2019; 37:589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hari P, Pasquini M, Stadtmauer E, et al. Long-term follow-up of BMT CTN 0702 (STaMINA) of postautologous hematopoietic cell transplantation (autoHCT) strategies in the upfront treatment of multiple myeloma (MM). J Clin Oncol. 2020; 38suppl 158506 [Google Scholar]
- 34.Giralt S, Costa LJ, Maloney D, et al. Tandem autologous-autologous versus autologous-allogeneic hematopoietic stem cell transplant for patients with multiple myeloma: long-term follow-up results from the Blood and Marrow Transplant Clinical Trials Network 0102 Trial. Biol Blood Marrow Transplant. 2020; 26:798–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012; 366:1782–1791 [DOI] [PubMed] [Google Scholar]
- 36.McCarthy PL, Owzar K, Hofmeister CC, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012; 366:1770–1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017; 35:3279–3289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jackson GH, Davies FE, Pawlyn C, et al. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019; 20:57–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldschmidt H, Lokhorst HM, Mai EK, et al. Bortezomib before and after high-dose therapy in myeloma: long-term results from the phase III HOVON-65/GMMG-HD4 trial. Leukemia. 2018; 32:383–390 [DOI] [PubMed] [Google Scholar]
- 40.Dimopoulos MA, Gay F, Schjesvold F, et al. Oral ixazomib maintenance following autologous stem cell transplantation (TOURMALINE-MM3): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2019; 393:253–264 [DOI] [PubMed] [Google Scholar]
- 41.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008; 359:906–917 [DOI] [PubMed] [Google Scholar]
- 42.Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014; 371:906–917 [DOI] [PubMed] [Google Scholar]
- 43.Durie BGM, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017; 389:519–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar S, Jacobus S, Cohen AD, et al. Carfilzomib lenalidomide, and dexamethasone (KRd) versus bortezomib, lenalidomide, and dexamethasone (VRd) for initial therapy of newly diagnosed multiple myeloma (NDMM): results of ENDURANCE (E1A11) phase III trial. J Clin Oncol. 2020; 38(no. 18_suppl):Abstract LBA3 [Google Scholar]
- 45.Mateos MV, Dimopoulos MA, Cavo M, et al. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med. 2018; 378:518–528 [DOI] [PubMed] [Google Scholar]
- 46.Mateos MV, Cavo M, Blade J, et al. Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): a randomised, open-label, phase 3 trial. Lancet. 2020; 395:132–141 [DOI] [PubMed] [Google Scholar]
- 47.Facon T, Kumar S, Plesner T, et al. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med. 2019; 380:2104–2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pönisch W, Mitrou PS, Merkle K, et al. Treatment of bendamustine and prednisone in patients with newly diagnosed multiple myeloma results in superior complete response rate, prolonged time to treatment failure and improved quality of life compared to treatment with melphalan and prednisone–a randomized phase III study of the East German Study Group of Hematology and Oncology (OSHO). J Cancer Res Clin Oncol. 2006; 132:205–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palumbo A, Hajek R, Delforge M, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med. 2012; 366:1759–1769 [DOI] [PubMed] [Google Scholar]
- 50.Dimopoulos M, Špička I, Quach H, et al. Ixazomib as postinduction maintenance for patients with newly diagnosed multiple lyeloma not undergoing autologous stem cell transplantation: the phase III TOURMALINE-MM4 trial J Clin Oncol. 2020; :4030–4041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cook G, Ashcroft AJ, Cairns DA, et al. The effect of salvage autologous stem-cell transplantation on overall survival in patients with relapsed multiple myeloma (final results from BSBMT/UKMF Myeloma X Relapse [Intensive]): a randomised, open-label, phase 3 trial. Lancet Haematol. 2016; 3:e340–e351 [DOI] [PubMed] [Google Scholar]
- 52.Goldschmidt H, Baertsch MA, Schlenzka J, et al. Salvage autologous transplant and lenalidomide maintenance vs. lenalidomide/dexamethasone for relapsed multiple myeloma: the randomized GMMG phase III trial ReLApsE. Leukemia. 2020July 21. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 53.Giralt S, Garderet L, Durie B, et al. American Society of Blood and Marrow Transplantation, European Society of Blood and Marrow Transplantation, Blood and Marrow Transplant Clinical Trials Network, and International Myeloma Working Group consensus conference on salvage hematopoietic cell transplantation in patients with relapsed multiple myeloma. Biol Blood Marrow Transplant. 2015; 21:2039–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller KC, Gertz MA, Buadi FK, et al. The impact of re-induction prior to salvage autologous stem cell transplantation in multiple myeloma. Bone Marrow Transplant. 2019; 54:2039–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015; 372:142–152 [DOI] [PubMed] [Google Scholar]
- 56.Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016; 375:1319–1331 [DOI] [PubMed] [Google Scholar]
- 57.Moreau P, Masszi T, Grzasko N, et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016; 374:1621–1634 [DOI] [PubMed] [Google Scholar]
- 58.Lonial S, Dimopoulos M, Palumbo A, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015; 373:621–631 [DOI] [PubMed] [Google Scholar]
- 59.Siegel DS, Dimopoulos MA, Ludwig H, et al. Improvement in overall survival with carfilzomib, lenalidomide, and dexamethasone in patients with relapsed or refractory multiple myeloma. J Clin Oncol. 2018; 36:728–734 [DOI] [PubMed] [Google Scholar]
- 60.Dimopoulos M, Weisel K, Lonial S, et al. Elotuzumab plus lenalidomide/dexamethasone for relapsed/refractory multiple myeloma: final overall survival results from the phase 3 ELOQUENT-2 trial. 17th International Myeloma Workshop, September 12–15, 2019, Boston, MA. Abstract OAB-021 [Google Scholar]
- 61.Harousseau JL, Dimopoulos MA, Wang M, et al. Better quality of response to lenalidomide plus dexamethasone is associated with improved clinical outcomes in patients with relapsed or refractory multiple myeloma. Haematologica. 2010; 95:1738–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dimopoulos MA, Goldschmidt H, Niesvizky R, et al. Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): an interim overall survival analysis of an open-label, randomised, phase 3 trial. Lancet Oncol. 2017; 18:1327–1337 [DOI] [PubMed] [Google Scholar]
- 63.Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016; 375:754–766 [DOI] [PubMed] [Google Scholar]
- 64.Richardson PG, Oriol A, Beksac M, et al. Pomalidomide, bortezomib, and dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with lenalidomide (OPTIMISMM): a randomised, open-label, phase 3 trial. Lancet Oncol. 2019; 20:781–794 [DOI] [PubMed] [Google Scholar]
- 65.Usmani S, Quach H, Mateos MV, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for the treatment of patients with relapsed or refractory multiple myeloma (RRMM): primary analysis results from the randomized, open-label, phase 3 study Candor (NCT03158688). Blood. 2019; 134:LBA-6 [Google Scholar]
- 66.Moreau P, Dimopoulos MA, Mikhael J, et al. Isatuximab plus carfilzomib and dexamethasone vs carfilzomib and dexamethasone in relapsed/refractory multiple myeloma (IKEMA): interim analysis of a phase 3, randomized, open-label study. Presented at: The European Hematology Association 25th Annual Congress, June 11–21, 2020. Virtual Congress: Abstract LBA2603 [Google Scholar]
- 67.Gavriatopoulou M, Kastritis E, Ntanasis-Stathopoulos I, et al. The addition of IMiDs for patients with daratumumab-refractory multiple myeloma can overcome refractoriness to both agents. Blood. 2018; 131:464–467 [DOI] [PubMed] [Google Scholar]
- 68.Nooka AK, Joseph NS, Kaufman JL, et al. Clinical efficacy of daratumumab, pomalidomide, and dexamethasone in patients with relapsed or refractory myeloma: utility of re-treatment with daratumumab among refractory patients. Cancer. 2019; 125:2991–3000 [DOI] [PubMed] [Google Scholar]
- 69.Kumar S, Harrison S, Cavo M, et al. Venetoclax or placebo in combination with bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma (BELLINI): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2020;21:1630–1642. [DOI] [PubMed] [Google Scholar]
- 70.Dimopoulos M, Delimpasi S, Simonova M, et al. Weekly selinexor, bortezomib, and dexamethasone (SVd) versus twice weekly bortezomib and dexamethasone (Vd) in patients with multiple myeloma (MM) after one to three prior therapies: initial results of the phase III BOSTON study. J Clin Oncol. 2020; 38no.15_supplAbstract 8501 [Google Scholar]
- 71.Dimopoulos MA, Richardson PG, Moreau P, et al. Current treatment landscape for relapsed and/or refractory multiple myeloma. Nat Rev Clin Oncol. 2015; 12:42–54 [DOI] [PubMed] [Google Scholar]
- 72.Gandhi UH, Cornell RF, Lakshman A, et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia. 2019; 33:2266–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dimopoulos MA, Dytfeld D, Grosicki S, et al. Elotuzumab plus pomalidomide and dexamethasone for multiple myeloma. N Engl J Med. 2018; 379:1811–1822 [DOI] [PubMed] [Google Scholar]
- 74.Attal M, Richardson PG, Rajkumar SV, et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet. 2019; 394:2096–2107 [DOI] [PubMed] [Google Scholar]
- 75.Chari A, Suvannasankha A, Fay JW, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017; 130:974–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chari A, Vogl DT, Gavriatopoulou M, et al. Oral selinexor-dexamethasone for triple-class refractory multiple myeloma. N Engl J Med. 2019; 381:727–738 [DOI] [PubMed] [Google Scholar]
- 77.Lonial S, Lee HC, Badros A, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020; 21:207–221 [DOI] [PubMed] [Google Scholar]
- 78.Richardson PG, Bringhen S, Voorhees P, et al. Melflufen plus dexamethasone in relapsed and refractory multiple myeloma (O-12-M1): a multicentre, international, open-label, phase 1-2 study. Lancet Haematol. 2020; 7:e395–e407 [DOI] [PubMed] [Google Scholar]
- 79.Raje N, Berdeja J, Lin Y, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019; 380:1726–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fernandez de Larrea C, Kyle RA, Durie BG, et al. Plasma cell leukemia: consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group. Leukemia. 2013; 27:780–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gavriatopoulou M, Musto P, Caers J, et al. European Myeloma Network recommendations on diagnosis and management of patients with rare plasma cell dyscrasias. Leukemia. 2018; 32:1883–1898 [DOI] [PubMed] [Google Scholar]
- 82.Musto P, Simeon V, Martorelli MC, et al. Lenalidomide and low-dose dexamethasone for newly diagnosed primary plasma cell leukemia. Leukemia. 2014; 28:222–225 [DOI] [PubMed] [Google Scholar]
- 83.Royer B, Minvielle S, Diouf M, et al. Bortezomib, doxorubicin, cyclophosphamide, dexamethasone induction followed by stem cell transplantation for primary plasma cell leukemia: a prospective phase II study of the Intergroupe Francophone du Myélome. J Clin Oncol. 2016; 34:2125–2132 [DOI] [PubMed] [Google Scholar]
- 84.Van De Donk NWCJ, van der Holt B, Schjesvold FH, et al. Treatment of primary plasma cell leukemia with carfilzomib and lenalidomide-based therapy: results of the first interim analysis of the phase 2 EMN12/HOVON129 study. Blood. 2019; 134suppl_1693 [Google Scholar]
- 85.Dhakal B, Patel S, Girnius S, et al. Hematopoietic cell transplantation utilization and outcomes for primary plasma cell leukemia in the current era. Leukemia. 2020; 34:3338–3347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dimopoulos MA, Hamilos G. Solitary bone plasmacytoma and extramedullary plasmacytoma. Curr Treat Options Oncol. 2002; 3:255–259 [DOI] [PubMed] [Google Scholar]
- 87.Paiva B, Chandia M, Vidriales MB, et al. Multiparameter flow cytometry for staging of solitary bone plasmacytoma: new criteria for risk of progression to myeloma. Blood. 2014; 124:1300–1303 [DOI] [PubMed] [Google Scholar]
- 88.Dimopoulos MA, Hillengass J, Usmani S, et al. Role of magnetic resonance imaging in the management of patients with multiple myeloma: a consensus statement. J Clin Oncol. 2015; 33:657–664 [DOI] [PubMed] [Google Scholar]
- 89.Tsang RW, Campbell BA, Goda JS, et al. Radiation therapy for solitary plasmacytoma and multiple myeloma: guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2018; 101:794–808 [DOI] [PubMed] [Google Scholar]
- 90.Dykewicz CA; Centers for Disease Control and Prevention (U.S.); Infectious Diseases Society of America; American Society of Blood and Marrow Transplantation. Summary of the guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Clin Infect Dis. 2001; 33:139–144(Adapted from: Gross PA, Barrett TL, Dellinger EP, et al. Purpose of quality standards for infectious diseases. Clin Infect Dis. 1994;18:421.) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


