Abstract
The benefits of athletic activity may be attenuated by sport-related head impacts, including soccer-related concussion and subconcussive events. The purpose of this study is to characterize the specific effects of soccer heading on white matter microstructure and cognitive function, independent of concussion, relative to non-athlete controls and relative to active athletes who are not involved in collision sports. 246 amateur soccer players, 72 non-contact/non-collision sports athletes and 110 healthy,non-athlete controls were included in the study, and underwent cognitive testing and 3T diffusion tensor imaging (DTI). Voxelwise linear regression, comparing soccer players and non-contact/non-collision sports athletes healthy,non-athlete controls, identified regions of abnormally low and high fractional anisotropy (FA), axial diffusivity (AD), radial diffusivity (RD) and mean diffusivity (MD) in athlete participants. Generalized estimating equations were used to examine the effects of 2 week and 1 year heading exposure quartile on cognitive performance and on the volume of each high and each low DTI parameter. Athletes with no or lower exposure to repetitive heading exhibited greater expression of low RD, greater expression of high FA and better performance on tasks of attention, processing speed, verbal memory, and working memory compared to non-athletes. Soccer players with the highest exposure to repetitive head impacts, however, did not differ significantly from healthy, non-athletes on either micro-structural features or cognitive performance, findings not explained by concussion history or demographic factors. These results are consistent with the notion that beneficial effects of athletic conditioning or training on brain structure and function may be attenuated by exposure to repeated subconcussive head impacts.
Introduction
Athletic activity confers beneficial effects on brain health (Forbes et al. 2013; Hillman et al. 2008; Callisaya and Nosaka 2017), a factor to consider in the overall risk and benefit equation for sports entailing head injury and other risks. On the one hand, benefits of athletics may be attenuated by sport-related head impacts, including soccer-related concussion (mild head injury resulting in loss of consciousness less than 30 min, post-traumatic amnesia less than 24 h, and GCS 13–15) and subconcussive events (head injury that does not result in clinical diagnosis of concussion(Bailes et al. 2013), which pose risks for transient (Straume-Næsheim et al. 2009) and potentially persistent adverse effects on brain structure and function(Bunc et al. 2017; Stålnacke et al. 2004). On the other hand, the beneficial effects of athletics, known to enhance cardiovascular and overall health, may contribute to brain reserve that buffers the adverse effects of repetitive head impacts (RHI). Adverse effects of head impacts in collision sports like soccer may thus be underestimated when active athletes are compared to non-athlete controls.
Soccer, the most popular sport worldwide with 224 million currently active players across 204 countries (Bunc et al. 2017; Kunz 2007), is associated with adverse effects of head impacts from heading and from collisions. Higher levels of heading are related to persistent white matter microstructural changes and lower performance on tests of cognitive function (Levitch et al. 2018; Lipton et al. 2013). Moreover, unrecognized injury due to repetitive heading may account for the dominant share of adverse cognitive effects, rather than recognized concussion (Stewart et al. 2018). In addition to functional effects, few studies have identified effects of heading, independent of concussion, on white matter (Tarnutzer et al. 2016; Rubin et al. 2018). An important limitation of most previous studies is that they largely address the effects of heading within groups of soccer players, without comparison to a control group. As a consequence, the beneficial effects of athletic activity on the brain cannot be parsed from adverse effects of heading (Tarnutzer et al. 2016).
The purpose of this study is to characterize the specific effects of soccer heading on white matter microstructure and cognitive function, independent of recognized concussion: (1) relative to non-athlete controls and (2) relative to active athletes who have no exposure to collision sports (i.e., non-contact/non-collision sports athletes).
Methods
Overall design
We separately examined effects of heading over the two distinct timeframes on diffusion tensor imaging (DTI) and cognitive performance. Two-week and 12-month heading exposure were studied as two separate categorical variables. For each variable, individual participants (healthy, non-athlete; non-contact/non-collision sports athletes athlete or soccer) were assigned to one of 6 groups. Group 1 included healthy, non-athletes only, Group 2 included non-contact/non-collision sports athletes only and Groups 3–6 comprised soccer players stratified by level of heading. Second, we determined, in each non-contact/non-collision sports athleteathlete and in each soccer player, the brain regions where each differed significantly from the non-athletes. We summarized abnormalities, in each individual, as the total volume of white matter exhibiting abnormally low DTI parameters and, separately, the total volume of white matter exhibiting abnormally high DTI parameters. Both low and high abnormalities can coexist in the same individual at discrete brain locations. This step yielded two summary measures (high and low) for each of 4 DTI parameters (FA, RD, AD, MD) in each non-contact/non-collision sports athleteand in each soccer player. These measures as well as cognitive test scores served as outcome variables.
Subject enrollment
The Einstein Soccer study is an ongoing longitudinal study examining the impact of heading in a group of adult amateur soccer players; subjects undergo imaging and other assessments at the beginning and end of a two-year period. The study complied with the Health Insurance Portability and Accountability Act, was approved by the institutional review board, and all subjects provided written, informed consent. Amateur soccer players, ages 18–55, were recruited via local advertisement and social media. Inclusion criteria included English language fluency, soccer play for at least 5 years, and current active soccer play for at least 6 months per year.
Exclusion criteria included history of Bipolar Disorder, Schizophrenia, neurological disorder, contraindication to MRI or recreational drug use within previous thirty days.
Non-contact/non-collision sport athletes met the above inclusion and exclusion criteria (active play at least 6 months per year for at least 5 years) with respect to a non-collision sport. “Non-collision” sports included baseball, swimming, tennis, running/track, gymnastics, rowing/crew, cycling, dancing, figure skating. Sports specifically excluded were basketball, football, soccer, hockey, wrestling, lacrosse, volleyball, rugby, boxing and martial arts. Healthy non-athletes comprised healthy individuals aged 18–55 years. Exclusion criteria for non-athletes included history of head injury, psychiatric disease (Bipolar Disorder, Schizophrenia, Anxiety, Depression), neurological disease, diabetes, heart disease, hypertension, contraindication to MRI or recreational drug use within previous thirty days.
All participants completed a baseline study visit that included the same data collection protocol for MRI and cognitive assessment. The initial visit also included collection of demographic features. Some of the soccer players completed an additional identical visit two years after initial enrollment. Handedness was assessed at the first visit using the Edinburgh Handedness Inventory (Oldfield 1971), which generates a laterality index on a continuous scale. The laterality index ranges from + 1 (strongly right-handed) to −1 (strongly left-handed).
Heading assessment
Heading was assessed at the time of enrollment using “HeadCount,” a structured, web-based questionnaire, the details and validation of which have been previously described (Catenaccio et al. 2016; Lipton et al. 2018). In brief, HeadCount-12m estimates heading over the prior 12 months and HeadCount-2w estimates heading over the prior 2 weeks based on a structured questionnaire that assesses exposure during outdoor practice, outdoor games, indoor practice and indoor games (Catenaccio et al. 2016). Twelve-month and two-week heading estimates served as the exposure measures of interest in this study.
Image acquisition
Imaging was performed at time of enrollment (V0) and at two years (V24) using a 3.0T Philips Achieva TX scanner (Philips Medical Systems, Best, The Netherlands) with a 32-channel head coil. T1-weighted 3D magnetization-prepared rapid acquisition of gradient echo imaging was performed with TR/TE/TI = 9.9/4.6/900 ms, flip angle 8°, 1mm3 isotropic resolution, 240 × 188 × 220 matrix. Diffusion tensor imaging was performed using 2D single-shot EPI with 32 diffusion encoding directions, b-value = 800 s/mm2, TR = 10 s, TE = 65 ms, 2 mm3 isotropic resolution, 128 × 120 matrix, 70 slices.
Image processing, analysis and imaging variable calculation
Image processing was performed using a high-performance computing system running the Community Enterprise Operating System (CentOS) Linux distribution and utilized the FSL software package (FSL v2.0.18, https://fsl.fmrib.ox.ac.uk/). In brief, the 32 diffusion-weighted image sets (32 b = 800 s/mm2 images) were corrected for head motion and eddy current effects by using an affine registration algorithm, with the b = 0 s/mm2 image as the target volume. Brain extraction was performed using Brain Extraction Tool (BET), and a white matter mask was generated with FAST, to limit subsequent analyses to white matter voxels only. Tensor fitting was performed at each voxel using the FMRIB Diffusion Toolbox (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FDT).
To minimize the impact of potential registration errors, subject-based registration (SURE-Quant) was used to define subject-specific abnormalities, as described previously (Suri et al. 2015). Eddy current correction and tensor fitting was performed using the FSL software package. Field map-based EPI distortion correction of each diffusion tensor MRI dataset was performed using FSL-FUGUE. The distortion-corrected EPI volume thus produced was then registered to the patient’s T1W volume, using FLIRT to perform a rigid body transformation. Diffusion parameter images for each control participant were transformed, using the nonlinear procedure within the Automated Registration Toolbox (ART) package, to match each individual athlete participant (soccer player, non-contact/non-collision sport athlete) according to the methods described by Suri et al. (2015) Voxelwise linear regression analysis, incorporating age and sex as covariates, was performed, comparing each participant to the group of 110 healthy controls, to identify subject-specific abnormalities. Regions were considered significant where more than 100 contiguous voxels, each meeting a threshold of P = 0.01, formed a contiguous cluster.
The statistical images from each participant, comprising clusters where FA, AD, RD or MD differed from controls, were each divided into two separate maps: (1) all clusters exhibiting diffusion parameter values > 0 and (2) all clusters exhibiting diffusion parameter values < 0. Total volume (number of 1-mm3 voxels) of all clusters meeting criteria for significance (above) was then computed for each map, for each subject, to generate the following eight imaging variables of interest for each subject: volume of all clusters where FA, AD, RD or MD > 0 and volume of all clusters where FA, AD, RD or MD < 0.
Neuropsychological assessment
Cognitive testing was performed at both study visits using Cogstate® (Cogstate, Ltd., NY, USA), a valid and reliable computer-administered battery (Maruff et al. 2009). Neuropsychological domains tested included: verbal learning and memory, psychomotor speed, attention, and working memory. The International Shopping List—Immediate (ISL) and International Shopping List—Delayed Recall tasks (ISRL) measured verbal learning and memory abilities, respectively (score reflects number of correct responses). The Groton Maze Chase Test (GMCT) measured psychomotor speed (score reflects total number of correct moves per second). The identification (IDN) and One Back Test (ONB) measure attention (scores reflect reaction time and accuracy). The Two Back Test (TWOB) measured working memory (score reflects number of correct responses). In addition, the WRAT4 Reading subtest was administered as a measure of premorbid verbal IQ (Bright et al. 2002).
Statistical analysis
Statistical analyses were performed using IBM SPSS (Statistical Package for the Social Sciences, IBM SPSS, Inc, Chicago, IL: Versions 24 and 25). Because distributions of both two-week and 12-month heading totals were positively skewed, to mitigate the high leverage of subjects with extreme high heading counts in the regression, each heading exposure variable was transformed into ordinal-categorical variables of four approximately equal size quartiles, with the lowest exposure group (i.e., Q1) having de minimis heading exposure (see Table 1). Cognitive test scores were treated as a continuous measure utilizing raw scores from each of the domain-specific tasks. Higher score reflects better performance on all tasks except for the IDN, where the score reflects reaction time and higher score therefore indicates worse performance. Data were included from multiple visits per subject such that data collected during a single session was the unit of analysis, where each soccer player could contribute one or two units depending on whether they completed one or two study visits.
Table 1.
Exposure characteristics of the soccer player group
| Longer-Term Heading Quartiles (HeadCount-12m) | ||||||
| Total number of reports n = 357 | Q1 | Q2 | Q3 | Q4 | ||
| n = 89 | n = 90 | n = 89 | n = 89 | |||
| Years of heading | 12.1 (7.2) | 12.4 (8.2) | 12.8(8.1) | 12.0 (7.07) | 12.0 (6.3) | |
| Heading-12m | Min | 0 | 0 | 169 | 586 | 1529 |
| Max | 139561 | 165 | 574 | 1500 | 139561 | |
| Mean | 2661.82 | 57.93 | 357.2 | 951.11 | 6701.01 | |
| Median | 790.5 | 44 | 335 | 887 | 2737 | |
| Recent Heading Quartiles (HeadCount-2w) | ||||||
| Total number of reports n = 357 | Q1 | Q2 | Q3 | Q4 | ||
| n=104 | n = 87 | n = 83 | n = 83 | |||
| Heading - 2w | Min | 0 | 0 | 1 | 11 | 40 |
| Max | 635 | 0 | 10 | 39 | 635 | |
| Mean | 37.89 | 0 | 5.71 | 21.43 | 135.54 | |
| Median | 9 | 0 | 6 | 20 | 97 | |
| Lifetime concussion history | 0 | 163 | 59 | 54 | 62 | 67 |
| 1 | 36 | 17 | 18 | 10 | 8 | |
| 2+ | 46 | 13 | 18 | 17 | 13 | |
| Days of soccer play | Outdoor Games | 2.19 | 1.61 | 1.94 | 2.12 | 2.92 |
| Outdoor Practice | 2.89 | 1.91 | 2.11 | 2.82 | 4.28 | |
| Indoor Games | 1.49 | 1.04 | 1.39 | 1.3 | 1.81 | |
| Indoor Practice | 1.38 | 0.88 | 0.98 | 1.15 | 2.03 | |
We leveraged repeated measures within subjects across multiple visits, applying generalized estimating equations (GEE) (Hanley et al. 2003; Hardin 2005), to examine the effects of heading exposure on cognitive performance and on the volume of each high and each low DTI parameter. We also tested the relationship of each low and high DTI parameter with cognitive performance. GEE explicitly account for repeated measures from the same subject and appropriately adjust the standard errors of the parameter estimates for the within-subject correlation in the data. Data from repeated visits were pooled in order to increase the power of the study to assess the association of soccer heading with white matter microstructure and cognitive function. For each model, age, sex, years of education, concussion history (Y/N), and verbal IQ were assessed as potential covariates. Those covariates demonstrating significance at p < 0.1 were included in the final model. Given the large number of statistical tests performed, Bonferroni correction was employed to control the familywise false positive error rate for all analyses involving DTI metrics.
Results
Subject characteristics
246 amateur soccer players (“soccer” group) were included in the study; 111 returned for a two-year follow-up visit, for a total of 357 assessments included in the analyses. Among soccer players, total number of heads per year ranged from 0 to 139,561 (mean = 2661.82 heads/ year, median = 790.5 heads/year). Total number of heads per two-week time period ranged from 0 to 635 (mean = 37.9 heads/2 weeks, median = 9 heads/2 weeks). Participants enrolled in the study were adult amateur recreational soccer players participating in leagues that are active for most if not all of the year. Players’ active play period was thus not restricted to a specific season or timeframe.
Table 1 shows heading characteristics by quartile for both the 12 month and 2 week heading assessments. At the outset, we did not necessarily expect consistency of heading quartile assignment defined by HeadCount-2w and HeadCount-12m, as the two heading estimates address two different timeframes. For example, a player with high heading activity over the prior year might be assessed at a time when they happened to not have played much during the prior two weeks. Notwithstanding these expectations, we in fact found a high degree of consistency for quartile assignment by HeadCount-2w and HeadCount-12m. In 44% of cases, quartile assignment was exactly matched. In 40%, quartile assignment differed by only one rank, which is likely to occur due to slight variation of exposure that just crosses the quartile cutoff. 12% of cases showed higher quartile assignment of more than 1 rank for the 12 month measure compared to the 2 week measure. This is consistent with the scenario described above. Notably, only 4% of reports yielded a lower quartile assignment of more than 1 rank for the 12 month measure compared to the 2 week measure.
72 non-contact/non-collision sport athletes and 110 healthy, non-athletes were included in the analysis. Demographic characteristics of the soccer players, non-collision athletes, and non-athletes are shown in Table 2. There were no significant differences in years of education and WRAT score between the three groups, but there were a significant differences in other demographic characteristics between the three groups: age (p < 0.001; non-contact/non-collision sport athletes were younger than the soccer players and healthy, non-athlete controls, soccer players were younger than healthy, non-athletes), sex at birth (p < 0.001; there were significantly more males among the soccer players as compared to the non-contact/non-collision sport athletes and healthy, non-athletes), handedness (p = 0.048; there was a trend toward right-handedness among non-contact/non-collision athletes compared to healthy, non-athletes) and race (p = 0.005; there were significantly more ‘Whites’ among the non-contact/non-collision sport athletes and significantly more ‘Non-Whites’ among the healthy, non-athletes). Significantly more soccer players reported history of sports-related concussion, compared to non-contact/non-collision sport athlete (p < 0.001). Healthy, non-athletes had no history of concussion (this was an exclusion criterion for the healthy, non-athlete group).
Table 2.
Baseline demographic characteristics. Continuous variables are reported as mean (standard deviation). Categorical variables are reported as frequency (%)
| Soccer Players (N = 246) | Non-Collision Athletes (N = 72) | Non-Athletic Controls (N= 116) | P value | |
|---|---|---|---|---|
| Variable (continuous) | ||||
| Age | 25.48 (7.2) | 22.76 (5.2) | 28.96 (11.1) | P < 0.001 |
| Education (years) | 15.6 (2.2) | 15.14 (1.9) | 15.38 (3.6) | P = 0.369 |
| IQ (WRAT Score) | 104.61 (14.6) | 107.6 (10.4) | 106.1 (17.8) | P = 0.291 |
| Handedness | 0.787 (0.4) | 0.850 (0.33) | 0.702 (0.46) | p = 0.048 |
| Years of Play at Similar Frequency | 12.11 (7.2) | |||
| Age started (years) | 7.70 (4.1) | |||
| Variable (categorical) | ||||
| Gender | P < 0.001 | |||
| Male | 174 (70.7) | 30 (41.7) | 63 (54.3) | |
| Female | 72 (29.3) | 42 (58.3) | 53 (45.7) | |
| Concussion History | P < 0.001 | |||
| Yes | 163 (66.3) | 6 (8.3) | ||
| No | 83 (33.7) | 66 (91.7) | ||
| Race | P = 0.005 | |||
| White | 154 (62.6) | 50 (69.4) | 54 (46.6) | |
| Non-White | 66 (26.8) | 14 (19.4) | 50 (43.1) |
Heading and DTI parameters
Voxelwise linear regression, adjusted for age and sex at birth, comparing soccer players and non-contact/non-collision sport athletes to healthy, non-athletes, identified regions of abnormally low and high FA, AD, RD and MD in most, but not all, athlete participants (Table 3).
Table 3.
Presence of abnormal DTI measures in soccer players and non-collision athletes relative to a group of healthy controls. Volume indicates mean volume of the specified abnormal parameter across all white matter voxels in the group. Percent indicates prevalence of abnormality within each group
| Soccer Players | Non-Collision Athletes | |||||||
|---|---|---|---|---|---|---|---|---|
| Supranormal | Subnormal | Supranormal | Subnormal | |||||
| Volume (mean, mm3) | Percent (N = 357) | Volume (mean, mm3) | Percent (N = 357) | Volume (mean, mm3) | Percent (N = 72) | Volume (mean, mm3) | Percent (N = 72) | |
| FA | 858 | 76.5% | 589 | 39.5% | 1403 | 86.1% | 2109 | 36.1% |
| AD | 964 | 78.7% | 379 | 42.6% | 4524 | 69.4% | 3096 | 59.7% |
| RD | 740 | 63.0% | 902 | 66.1% | 10759 | 45.8% | 1682 | 80.6% |
| MD | 1127 | 66.9% | 1208 | 63.9% | 7552 | 54.2% | 2105 | 83.3% |
12-Month Heading
Expression of abnormally low RD did not differ between non-contact/non-collision sport athlete and soccer players with less exposure to heading (Q1-Q2), but soccer players with high levels of exposure to heading (Q3-Q4) showed a significantly greater expression of low RD as compared to non-contact/non-collision sport athletes (Fig. 1). Expression of high FA was similar among non-contact/non-collision sport athletes and soccer players with less exposure to heading (Q1-Q3) but soccer players with the highest level of exposure (Q4) showed significantly less expression of high FA as compared to non-contact/non-collision sport athletes (Fig. 1). A similar trend was observed for the relationship of heading exposure with subnormal MD. No differences were observed among non-contact/non-collision sport athletes and Q1 and Q2 soccer heading groups on low RD or high FA. Volume of subnormal FA, subnormal AD or high RD, MD or AD were not significantly different among participant groups. None of the covariates were significant predictors of the imaging measures and were therefore not included in the final models. Table 4 displays quartile-specific regression coefficient (β) and significance (p) for each participant group.
Fig. 1.

DTI Parameters and Heading. Box and whisker plots show median and interquartile ranges for: a Low RD and long-term (12-month) heading quartiles. b Low RD and short-term (2-week) heading quartiles. c High FA and long-term (12-month) heading quartile. Red indicates reference group. * Indicates significant difference between the athlete quartile and the reference group
Table 4.
Relationship of longer-term heading and DTI measures
| Athlete Quartile (Longer Term Heading) | N | High AD | Low AD | High MD | Low MD | High RD | Low RD | High FA | Low FA (covariate: age) |
|---|---|---|---|---|---|---|---|---|---|
| Test of Model Effect raw p (adjusted p value) | - | 0.204 (1.632) | 0.214 (1.712) | 0.216 (1.728) | 0.012 (0.096) | 0.477 3.816) | 0.000000208 (0.0000016) | 0.004 (0.032) | 0.394 (3.152) |
| Q4 B(p) | 89 | −478.00 (0.40) | −764.63 (0.04) | −641.77 (0.49) | −1067.76 (0.00) | −1383.19 (0.28) | −1037.48 (0.00) | −886.73 (0.00) | −929.07 (0.61) |
| Q3 B(p) | 91 | −1.82 (0.99) | −714.28 (0.05) | −246.50 (0.79) | −717.18 (0.03) | 1122.30 (0.38) | −560.23 (0.03) | −479.34 (0.09) | −1801.32 (0.23) |
| Q2 B(p) | 88 | −35.5 (0.43) | −594.7 (0.12) | −663.24 (0.47) | −342.94 (0.34) | −1393.72 (0.27) | −423.22 (0.10) | −456.34 (0.11) | −775.06 (0.6) |
| Q1 B(p) | 89 | −504.00 (0.35) | −529.63 (0.19) | −899.01 (0.32) | −93.15 (0.87) | −1435.64 (0.26) | −209.66 (0.58) | −356.38 (0.28) | −1614.34 (0.28) |
2-Week heading
Expression of abnormally low RD did not differ between non-contact/non-collision sport athletes and soccer players with less exposure to heading (Q1-Q3); however, there was greater expression of abnormally low RD in soccer players with highest level of heading exposure (Q4) as compared to non-contact/non-collision sport athletes (Fig. 1). Volume of subnormal FA, MD or AD and high FA, RD, MD or AD were not significantly different among participant groups. None of the covariates were significant predictors of the imaging measures and were therefore not included in the final models. Table 5 displays quartile-specific regression coefficient (β) and significance (p) for each group.
Table 5.
Relationship of recent heading and DTI measures
| Recent Heading | N | High AD | Low AD | High MD | Low MD (Covariate: WRAT) | High RD | Low RD (Covariate: Age) | High FA | Low FA |
|---|---|---|---|---|---|---|---|---|---|
| Test of Model Effect raw p (adjusted P) | - | 0.773 (5.864) | 0.163 (1.304) | 0.442 (3.536) | 0.097 (0.48) | 0.291 (2.328) | 0.002 (0.016) | 0.036 (0.288) | 0.090 (0.72) |
| Q4 B(p) | 104 | −361.57 (0.53) | −798.48 (0.03) | −474.03 (0.61) | −933.27 (0.01) | −1233.32 (0.33) | −972.66 (0.00) | −800.48 (0.00) | −197.55 (0.20) |
| Q3 B(p) | 87 | −311.91 (0.57) | −640.31 (0.09) | −352.98 (0.70) | −412.02 (0.23) | 110.05 (0.37) | −672.91 (0.01) | −558.29 (0.04) | −1033.01 (0.56) |
| Q2 B(p) | 83 | −511.99 (0.35) | −718.50 (0.05) | −819.69 (0.36) | −36.28 (0.05) | 1470.19 (0.25) | −672.56 (0.01) | −528.68 (0.05) | −2032.15 (0.18) |
| Q1 B(p) | 83 | −265.95 (0.63) | −494.72 (0.22) | −799.08 (0.38) | −186.61 (0.72) | 1484.9 (0.24) | −346.14 (0.32) | −341.56 (0.29) | −1246.72 (0.45) |
Heading and cognitive performance
12-Month Heading
Non-contact/non-collision sport athletes and soccer players with low levels of heading exposure performed significantly better than healthy, non-athletes on tasks of attention (IDN; Fig. 2), processing speed (GMCT; Fig. 2) and verbal memory (ISRL; Fig. 2), whereas soccer players with higher levels of heading exposure did not differ from non-contact/non-collision sport athletes in performance on these tasks. No differences were observed between groups for performance on verbal learning (ISL), working memory (TWOB), or attention (ONB). None of the covariates were significant predictors of cognitive performance and were therefore not included in the final models. Table 6 shows quartile-specific regression coefficient (β) and significance (p) for each group.
Fig. 2.
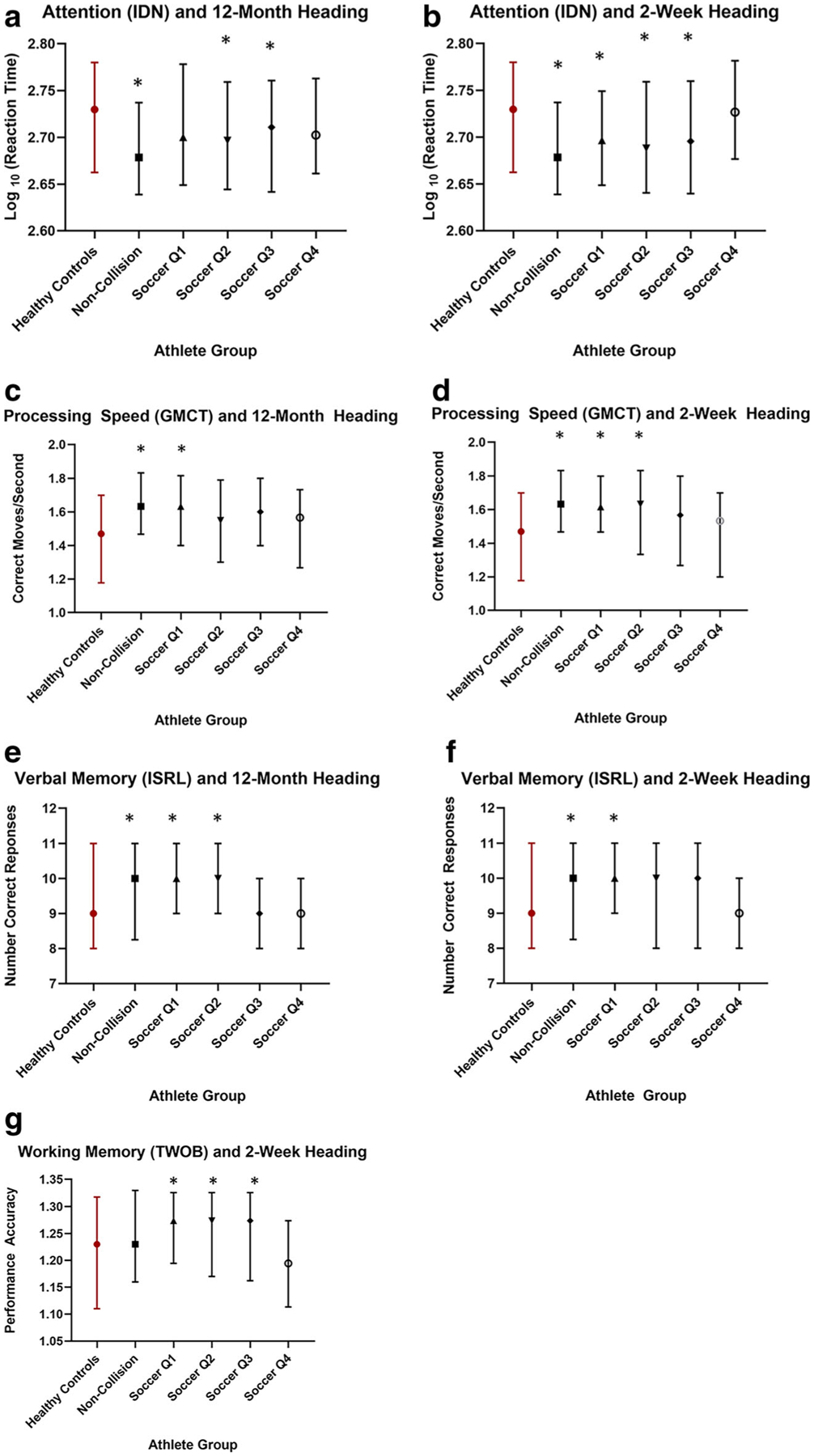
Cognitive Function and heading. a Attention (IDN) and long-term (12-month) heading quartiles b Attention (IDN) and short-term (2-week) heading quartiles. c Processing Speed (GMCT) and long-term (12-month) heading quartiles. d Processing Speed (GMCT) and short-term (2-week) heading quartiles. e Verbal Memory (ISRL) and long-term (12-month) heading quartiles and f Verbal Memory (ISRL) and short-term (2-week) heading quartiles. g Working memory (TWOB) and short-term (2-week) heading quartiles. Box and whisker plots show median and interquartile ranges. Red indicates reference group. * Indicates significant difference between the athlete quartile and the reference group
Table 6.
Relationship of longer-term heading and cognitive performance
| Task | Attention (IDN) | Procesing Speed (GMCT) (covariate: age) | Verbal Learning (ISL) (covariate: gender, education, WRAT) | Verbal Memory (ISRL) | Attention (ONB) (covariate: WRAT) | Working Memory (TWOB) (covariate: age, WRAT) |
|---|---|---|---|---|---|---|
| Test of Model Effect | 0.003 | 0.01 | 0.068 | 0.019 | 0.795 | 0.070 |
| Q4 B (p value) | −0.048 (0.142) | 0.031 (0.610) | −0.584 (0.320) | 0.012 (0.964) | 0.002 (0.938) | 0.063 (0.106) |
| Q3 B (p value) | −0.026 (0.030) | 0.085 (0.188) | −0.489 (0.357) | −0.021 (0.934) | −0.006 (0.814) | 0.048 (0.205) |
| Q2 B (p value) | −0.141 (0.020) | 0.093 (0.098) | 0.595 (0.282) | 0.640 (0.008) | −0.002 (0.957) | 0.070 (0.100) |
| Q1 B (p value) | −0.077 (0.082) | 0.127 (0.045) | 0.707 (0.211) | 0.552 (0.028) | 0.046 (0.188) | 0.120 (0.002) |
| Athlete | −0.038 (0.001) | 0.172 (0.001) | 0.758 (0.192) | 0.532 (0.033) | 0.010 (0.695) | 0.067 (0.103) |
2-Week Heading
Non-contact/non-collision sport athletes and soccer players with low levels of heading exposure performed significantly better than healthy, non-athletes on tasks of attention (IDN; Fig. 2b), processing speed (GMCT; Fig. 2d), verbal memory (ISRL; Fig. 2f), and working memory (TWOB; Fig. 2g). No differences were observed among groups for verbal learning (ISL) or attention (ONB). None of the covariates were significant predictors of cognitive performance and were therefore not included in the final models. Table 7 displays quartile-specific regression coefficient (β) and significance (p) for each group.
Table 7.
Relationship of recent heading and cognitive performance
| Attention (IDN) | Processing Speed (GMCT) (covariate: age) | Verbal Learning (ISL) (covariate: gender, education, WRAT) | Verbal Memory (ISRL) | Attention (ONB) (covariate: WRAT) | Working Memory (TWOB) (covariate: Age) | |
|---|---|---|---|---|---|---|
| Test of Model Effect | 0.003 | 0.00003 | 0.122 | 0.030 | 0.133 | 0.018 |
| Q4 B (p value) | −0.061 (0.197) | −0.043 (0.500) | −0.407 (0.465) | −0.087 (0.750) | −0.054 (0.125) | 0.006 (0.898) |
| Q3 B (p value) | −0.124 (0.030) | 0.040 (0.544) | −0.511 (0.386) | 0.178 (0.477) | 0.012 (0.699) | 0.089 (0.022) |
| Q2 B (p value) | −0.091 (0.044) | 0.140 (0.021) | 0.313 (0.579) | 0.384 (0.144) | 0.027 (0.420) | 0.089 (0.020) |
| Q1 B (p value) | −0.027 (0.019) | 0.178 (0.001) | 0.643 (0.225) | 0.607 (0.008) | 0.045 (0.076) | 0.105 (0.006) |
| Athlete | −0.038 (0.001) | 0.176 (0.001) | 0.762 (0.190) | 0.535 (0.032) | 0.011 (0.684) | 0.069 (0.091) |
Relationship of DTI parameters with cognition
There were significant associations between volume of low and high DTI measures and cognition. Greater volume of low AD was significantly related to better verbal memory performance (ISRL) (raw p = 0.0004, adjusted p value = 0.02), and greater volume of low MD was associated with better processing speed (GMCT) (raw p = 0.0003, adjusted p value = 0.01) and attention performance (ONB) (raw p = 0.001, adjusted p value = 0.048). Greater volume of low RD was associated with better processing speed (GMCT) (raw p = 0.006, adjusted p value = 0.288) and attention performance (ONB) (raw p = 0.041, adjusted p value = 1). There were no significant associations of low FA or high FA, AD, RD or MD with cognitive performance. Table 8 shows raw and adjusted p values for the linear regression analyses.
Table 8.
Relationship of volume DTI parameter abnormality and cognitive performance
| Cognitive Measure | Imaging Measure | Raw P-value | Adjusted P-value |
|---|---|---|---|
| ISL | hFA | 0.554 | 26.592 |
| ISRL | hFA | 0.820 | 39.36 |
| ONB | hFA | 0.477 | 22.896 |
| TWOB | hFA | 0.772 | 37.056 |
| IDN | hFA | 0.059 | 2.832 |
| GMCT | hFA | 0.674 | 32.352 |
| ISL | lFA | 0.078 | 3.744 |
| ISRL | lFA | 0.320 | 15.36 |
| ONB | lFA | 0.369 | 17.712 |
| TWOB | lFA | 0.610 | 29.28 |
| IDN | lFA | 0.230 | 11.04 |
| GMCT | lFA | 0.526 | 25.248 |
| ISL | hRD | 0.142 | 6.816 |
| ISRL | hRD | 0.00001577445690 | 0.000757 |
| ONB | hRD | 0.324 | 15.552 |
| TWOB | hRD | 0.852 | 40.896 |
| IDN | hRD | 0.666 | 31.968 |
| GMCT | hRD | 0.923 | 44.304 |
| ISL | lRD | 0.947 | 45.456 |
| ISRL | lRD | 0.904 | 43.392 |
| ONB | lRD | 0.041 | 1.968 |
| TWOB | lRD | 0.472 | 22.656 |
| IDN | lRD | 0.068 | 3.264 |
| GMCT | lRD | 0.006 | 0.288 |
| ISL | hMD | 0.883 | 42.384 |
| ISRL | hMD | 0.131 | 6.288 |
| ONB | hMD | 0.191 | 9.168 |
| TWOB | hMD | 0.425 | 20.4 |
| IDN | hMD | 0.547 | 26.256 |
| GMCT | hMD | 0.526 | 25.248 |
| ISL | lMD | 0.582 | 27.936 |
| ISRL | lMD | 0.640 | 30.72 |
| ONB | lMD | 0.001 | 0.048 |
| TWOB | lMD | 0.052 | 2.496 |
| IDN | lMD | 0.072 | 3.456 |
| GMCT | lMD | 0.000309 | 0.014832 |
| ISL | hAD | 0.959 | 46.032 |
| ISRL | hAD | 0.088 | 4.224 |
| ONB | hAD | 0.139 | 6.672 |
| TWOB | hAD | 0.291 | 13.968 |
| IDN | hAD | 0.451 | 21.648 |
| GMCT | hAD | 0.460 | 22.08 |
| ISL | lAD | 0.240 | 11.52 |
| ISRL | lAD | 0.00041803350800000 | 0.020066 |
| ONB | lAD | 0.137 | 6.576 |
| TWOB | lAD | 0.142 | 6.816 |
| IDN | lAD | 0.189 | 9.072 |
| GMCT | lAD | 0.039 | 1.872 |
Discussion
Our comparison of healthy non-athletes and non-contact/non-collision sport athletes with soccer players reveals an interesting divergence of microstructural features and cognitive performance. Athletes with no or lower exposure to repetitive heading exhibited both a higher degree of white matter anisotropy and better cognitive performance compared to non-athletes. Soccer players with the highest exposure to repetitive head impacts, however, did not differ significantly from non-athletes on either micro-structural features or cognitive performance. Statistically significant differences were not explained by concussion history or demographic factors. These results lead us to hypothesize that beneficial effects of athletic conditioning or training on brain structure and function might be attenuated by exposure to repeated subconcussive head impacts, a proposition that can motivate future investigation.
It is known that physical activity, and organized sports participation in particular, reduce all-cause mortality, as well as diabetes, cardiovascular disease, depression, dementia, breast cancer, and colon cancer (Khan et al. 2012); the observed benefit has been specifically linked to sports-related exercise (Sabia et al. 2012). It is postulated that beneficial effects of exercise are mediated through processes such as angiogenesis, neurogenesis, synaptogenesis, release of neurotrophins (Hötting and Röder 2013), and myelination (Kim and Sung 2017). In contrast to prior studies showing reduced mortality in American football players as compared to general United States population (Nguyen et al. 2019), Nguyen, et al. demonstrated elevated mortality among National Football League (NFL) players compared with Major League Baseball (MLB) players. The authors attribute this apparent discrepancy in findings to the choice of controls (Nguyen et al. 2019). If we had only compared soccer players to healthy non-athletes, potential beneficial effects of participation in athletics or training effects related to soccer might have masked these potentially adverse structural and functional effects of heading. We therefore compared soccer players to a similarly active group of non-contact/non-collision sport athletes in order to begin to disentangle potential opposing effects of athletics/training vs. RHI on brain structure and function.
The potential adverse consequences of subconcussive impacts must also be considered in context of other sources of impact, such as recognized concussion. We have consistently found that prior concussion does not explain symptoms (Stewart et al. 2017), imaging abnormalities (Lipton et al. 2013; Koerte et al. 2012) or cognitive performance (Stewart et al. 2018), in adult amateur soccer players, whereas heading does (Lipton et al. 2013; Stewart et al. 2017, 2018; Koerte et al. 2012). Moreover, worse cognitive performance in soccer players is predicted by higher levels of heading, but not by subconcussive unintended impacts, such as collisions (Stewart et al. 2017). Many studies addressing subconcussive impact effects explicitly exclude subjects with history of concussion (Koerte et al. 2012; Davenport et al. 2016; McAllister and McCrea 2017; Miller et al. 2007). While this approach yields a potentially more homogeneous sample, it limits power and opens the door to selection bias. Players who experience concussion might exhibit patterns of play and heading behaviors that differ from concussion-naïve individuals. We therefore include players in the current study regardless of concussion history and characterize the role of concussion as a covariate in our models.
We examined associations of both shorter-term (two-week) and intermediate-term (one-year) exposure to heading with microstructure (DTI) measures. Greater heading over both timeframes was associated with lesser expression of low RD, a parameter shown to reflect myelin integrity (Winklewski et al. 2018), but only 12-month heading was associated with corresponding lesser expression of high anisotropy (FA). This pattern suggests that longer-term exposure to heading may be more robustly associated with adverse effects on microstructure, a pattern consistent with accumulating pathology in response to ongoing exposure to repeated head impacts. The pattern of findings is also concordant with Bartnik-Olson et al. (2014) who found that soccer players without cognitive impairment following concussion expressed lower RD and higher FA in the posterior limb of the internal capsule compared to controls, whereas those with cognitive impairment did not. Similarly, Bahrami et al. (2016) found lesser increase of FA in subjects with greater cumulative head impact exposure over a single season of American football compared to those with less exposure to head Koerte et al. (2012) reported higher RD in concussion-naive soccer players as compared to swimmers; this might be consistent with our findings that non-collision athletes express more low RD as compared to soccer players with high exposure to heading.
Several studies have reported an adverse association of heading with cognitive performance, most commonly affecting memory, attention and executive function (Straume-Næsheim et al. 2009; Levitch et al. 2018; Lipton et al. 2013; Downs and Abwender 2002; Forbes et al. 2016; Janda et al. 2002; Kontos et al. 2011; Matser et al. 2001; Rutherford et al. 2005; Salinas et al. 2009; Stephens et al. 2010; Jones et al. 2013). However, many relied on non-validated self-report measures of exposure to heading (Tarnutzer et al. 2016), a limitation addressed in this study by using a validated, structured heading exposure assessment tool. Our findings in this large sample of amateur soccer players and non-athletes, whom we compare to non-collision athletes, not only reproduce the adverse associations of heading with cognitive performance that we previously reported in a smaller independent sample of players (Levitch et al. 2018), but further illustrates that this adverse effect may represent an attenuation of expected beneficial effects of athletic participation. Findings of Koerte et al. (2017) where adolescent non-contact sport athletes demonstrated improvement of cognitive performance over the course of a training season, whereas soccer players did not, are also consistent with the attenuation of an exercise- or training-related brain benefit among soccer players with high levels of heading exposure that we suggest based on our findings. We found recent heading was associated with working memory, but one-year heading was not. This suggests that effects of heading on working memory may be transient, similar to previous findings by Levitch et al. (2018).
The preliminary findings related to the association between volume of abnormal DTI parameters and cognitive function suggest that microstructural alterations reflected by DTI may index pathologic changes underpinning adverse functional effects. The significant association between greater volume of subnormal MD and improved performance on tasks of executive function is consistent with McAllister et al. (2014). We detected a trend association of more subnormal RD with better performance on tasks of executive function (GMCT), a task on which players with greater exposure to heading performed worse as compared to non-contact/non-collision athletes. These intriguing preliminary findings should motivate further investigation into potential mediating roles of imaging measures in the relationship of RHI with cognitive performance.
Our findings should be considered in context of several limitations. We did not explicitly test for a beneficial effect of athletics on brain function, but indirectly infer that such an effect might explain the relationships we describe. Thus, the notion that RHI attenuates the beneficial effect of sport participation represents a hypothesis that derives from our findings but remains to be definitively addressed by explicit testing. We cannot completely exclude the possibility that the non-athlete controls participated in athletics in the past. In any case, this would represent an unlikely source of bias because these same individuals serve as the reference group for both the non-collision athletes and soccer players. The cross-sectional analysis we report is limited in its ability to support causal relationships and to differentiate between shorter- versus longer-term heading exposure. For instance, it is plausible that individuals with poor brain health or lower baseline cognitive function are more inclined towards extreme levels of heading. We did not exclude soccer athletes who played other collision sports. If injury occurred while engaged in another sport, that contribution could not be disentangled from the effects of heading. For subjects with history of prior concussion, we did not have access to reliable data on time of concussion and tested the presence/absence of concussion history as a covariate. The potential role of recent concussion cannot therefore be addressed. The higher number of head impacts reported by soccer athletes included in the sample compared to some prior reports in the literature may reflect the generally fewer restrictions on adult recreational play and the fact that the players in our sample were not restricted to play during a circumscribed active season of play as well as other factors. However, these differences may also make our sample more representative of the more than 256 million players worldwide, compared to participants in professional, elite and educational settings.” Finally, reporting bias and reverse causation cannot be absolutely excluded, but are unlikely explanations of our findings, which converge across distinct timeframes, exposures and outcomes.
In conclusion, our findings add to existing knowledge that athletic participation confers beneficial effects on brain structure and function and raises the possibility that these effects may be attenuated by exposure to high levels of subconcussive soccer heading over the shorter- and longer-terms. Further study is warranted to further characterize risk of repetitive subconcussive impacts in sports toward developing approaches for its mitigation.
Funding information
This study was funded by the National Institutes of Health (R01NS082432) and the Dana Foundation.
Richard Lipton receives research support from the NIH: 2PO1 AG003949 (mPI), 5U10 NS077308 (PI), RO1 NS082432 (Investigator), 1RF1 AG057531 (Site PI), RF1 AG054548 (Investigator), 1RO1 AG048642 (Investigator), R56 AG057548 (Investigator), K23 NS09610 (Mentor), K23AG049466 (Mentor), 1K01AG054700 (Mentor). He also receives support from the Migraine Research Foundation and the National Headache Foundation. He serves on the editorial board of Neurology, senior advisor to Headache, and associate editor to Cephalalgia. He has reviewed for the NIA and NINDS, holds stock options in eNeura Therapeutics and Biohaven Holdings; serves as consultant, advisory board member, or has received honoraria from: American Academy of Neurology, Alder, Allergan, American Headache Society, Amgen, Autonomic Technologies, Avanir, Biohaven, Biovision, Boston Scientific, Dr. Reddy’s, Electrocore, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Merck, Pernix, Pfizer, Supernus, Teva, Trigemina, Vector, Vedanta. He receives royalties from Wolff’s Headache 7th and 8th Edition, Oxford University Press, 2009, Wiley and Informa.
Michael L. Lipton receives research funding from the National Institutes of Health (R01NS082432), the Dana Foundation and Guerbet and royalties from Springer.
Footnotes
Conflict of Interest Sara B. Strauss declares that she has no conflict of interest.
Roman Fleysher declares that he has no conflict of interest.
Chloe Ifrah declares that she has no conflict of interest.
Liane Hunter declares that she has no conflict of interest.
Kenny Ye declares that he has no conflict of interest.
Molly Zimmerman declares that she has no conflict of interest.
Walter Stewart declares that he has no conflict of interest.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Mimi Kim does consulting for Celgene and Eli Lilly on clinical trials, unrelated to the work in the paper.
References
- Bahrami N, Sharma D, Rosenthal S, Davenport EM, Urban JE, Wagner B, Jung Y, Vaughan CG, Gioia GA, & Stitzel JD (2016). Subconcussive head impact exposure and white matter tract changes over a single season of youth football. Radiology, 281, 919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailes JE, Petraglia AL, Omalu BI, Nauman E, & Talavage T (2013). Role of subconcussion in repetitive mild traumatic brain injury: a review. Journal of Neurosurgery, 119, 1235–1245. [DOI] [PubMed] [Google Scholar]
- Bartnik-Olson BL, Holshouser B, Wang H, Grube M, Tong K, Wong V, & Ashwal S (2014). Impaired neurovascular unit function contributes to persistent symptoms after concussion: a pilot study. Journal of Neurotrauma, 31, 1497–1506. [DOI] [PubMed] [Google Scholar]
- Bright P, Jaldow E, & Kopelman MD (2002). The National Adult Reading Test as a measure of premorbid intelligence: a comparison with estimates derived from demographic variables. Journal of the International Neuropsychological Society, 8, 847–854. [DOI] [PubMed] [Google Scholar]
- Bunc G, Ravnik J, & Velnar T (2017). May heading in soccer result in traumatic brain injury? A review of literature. Medical Archives, 71, 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callisaya M, & Nosaka K (2017). Effects of exercise on type 2 diabetes mellitus-related cognitive impairment and dementia. Journal of Alzheimer’s Disease, 59, 503–513. [DOI] [PubMed] [Google Scholar]
- Catenaccio E, Caccese J, Wakschlag N, Fleysher R, Kim N, Kim M, et al. (2016). Validation and calibration of HeadCount, a self-report measure for quantifying heading exposure in soccer players. Research in sports medicine, 24, 416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport EM, Urban JE, Mokhtari F, Lowther EL, Van Horn JD, Vaughan CG, Gioia GA, Whitlow CT, Stitzel JD, & Maldjian JA (2016). Subconcussive impacts and imaging findings over a season of contact sports, Concussion, 1 CNC19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs D, & Abwender D (2002). Neuropsychological impairment in soccer athletes. Journal of sports medicine and physical fitness, 42, 103. [PubMed] [Google Scholar]
- Forbes D, Thiessen EJ, Blake CM, Forbes SC, & Forbes S (2013). Exercise programs for people with dementia. Cochrane Database Syst Rev, 12, 0. [DOI] [PubMed] [Google Scholar]
- Forbes CR, Glutting JJ, & Kaminski TW (2016). Examining neurocognitive function in previously concussed interscholastic female soccer players. Applied Neuropsychology: Child, 5, 14–24. [DOI] [PubMed] [Google Scholar]
- Hanley JA, Negassa A, & Forrester JE (2003). Statistical analysis of correlated data using generalized estimating equations: an orientation. American journal of epidemiology, 157, 364–375. [DOI] [PubMed] [Google Scholar]
- Hardin JW (2005). Generalized estimating equations (GEE), Encyclopedia of statistics in behavioral science. [Google Scholar]
- Hillman CH, Erickson KI, & Kramer AF (2008). Be smart, exercise your heart: exercise effects on brain and cognition. Nature Reviews Neuroscience, 9, 58. [DOI] [PubMed] [Google Scholar]
- Hötting K, & Röder B (2013). Beneficial effects of physical exercise on neuroplasticity and cognition. Neuroscience & Biobehavioral Reviews, 37, 2243–2257. [DOI] [PubMed] [Google Scholar]
- Janda DH, Bir CA, & Cheney AL (2002). An evaluation of the cumulative concussive effect of soccer heading in the youth population. Injury Control and Safety Promotion, 9, 25–31. [DOI] [PubMed] [Google Scholar]
- Jones SAV, Breakey RW, Evans PJ (2013). Heading in football, long-term cognitive decline and dementia: evidence from screening retired professional footballers. British Journal of Sports Medicine. [DOI] [PubMed] [Google Scholar]
- Khan KM, Thompson AM, Blair SN, Sallis JF, Powell KE, Bull FC, & Bauman AE (2012). Sport and exercise as contributors to the health of nations. The Lancet, 380, 59–64. [DOI] [PubMed] [Google Scholar]
- Kim T-W, & Sung Y-H (2017). Regular exercise promotes memory function and enhances hippocampal neuroplasticity in experimental autoimmune encephalomyelitis mice. Neuroscience, 346, 173–181. [DOI] [PubMed] [Google Scholar]
- Koerte IK, Ertl-Wagner B, Reiser M, Zafonte R, & Shenton ME (2012). White matter integrity in the brains of professional soccer players without a symptomatic concussion. JAMA, 308, 1859–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerte IK, Nichols E, Tripodis Y, Schultz V, Lehner S, Igbinoba R, et al. (2017). Impaired cognitive performance in youth athletes exposed to repetitive head impacts. Journal of Neurotrauma, 34, 2389–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontos AP, Dolese A, Elbin R III., Covassin T, & Warren BL (2011). Relationship of soccer heading to computerized neurocognitive performance and symptoms among female and male youth soccer players. Brain Injury, 25, 1234–1241. [DOI] [PubMed] [Google Scholar]
- Kunz M (2007). 265 million playing football. FIFA Magazine, 7, 11–15. [Google Scholar]
- Levitch CF, Zimmerman ME, Lubin N, Kim N, Lipton RB, Stewart WF, Kim M, & Lipton ML (2018). Recent and long-term soccer heading exposure is differentially associated with neuropsychological function in amateur players. Journal of the International Neuropsychological Society, 24, 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton ML, Kim N, Zimmerman ME, Kim M, Stewart WF, Branch CA, & Lipton RB (2013). Soccer heading is associated with white matter microstructural and cognitive abnormalities. Radiology, 268, 850–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton ML, Ifrah C, Stewart WF, Fleysher R, Sliwinski MJ, Kim M, & Lipton RB (2018). Validation of HeadCount-2w for estimation of two-week heading: Comparison to daily reporting in adult amateur player. Journal of Science and Medicine in Sport, 21, 363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruff P, Thomas E, Cysique L, Brew B, Collie A, Snyder P, & Pietrzak RH (2009). Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Archives of Clinical Neuropsychology, 24, 165–178. [DOI] [PubMed] [Google Scholar]
- Matser J, Kessels A, Lezak M, & Troost J (2001). A dose-response relation of headers and concussions with cognitive impairment in professional soccer players. Journal of Clinical and Experimental Neuropsychology, 23, 770–774. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Ford JC, Flashman LA, Maerlender A, Greenwald RM, Beckwith JG, Bolander RP, Tosteson TD, Turco JH, & Raman R (2014). Effect of head impacts on diffusivity measures in a cohort of collegiate contact sport athletes. Neurology, 82, 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister T, & McCrea M (2017). Long-term cognitive and neuropsychiatric consequences of repetitive concussion and head-impact exposure. Journal of Athletic Training, 52, 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JR, Adamson GJ, Pink MM, & Sweet JC (2007). Comparison of preseason, midseason, and postseason neurocognitive scores in uninjured collegiate football players. The American Journal of Sports Medicine, 35, 1284–1288. [DOI] [PubMed] [Google Scholar]
- Nguyen VT, Zafonte RD, Chen JT, Kponee-Shovein KZ, Paganoni S, Pascual-Leone A, et al. (2019). Mortality among professional American-style football players and professional American baseball players. JAMA Network Open, 2, e194223–e194223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Rubin TG, Catenaccio E, Fleysher R, Hunter LE, Lubin N, Stewart WF, Kim M, Lipton RB, & Lipton ML (2018). MRI-defined white matter microstructural alteration associated with soccer heading is more extensive in women than men. Radiology, 289, 478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford A, Stephens R, Potter D, & Fernie G (2005). Neuropsychological impairment as a consequence of football (soccer) play and football heading: preliminary analyses and report on university footballers. Journal of Clinical and Experimental Neuropsychology, 27, 299–319. [DOI] [PubMed] [Google Scholar]
- Sabia S, Dugravot A, Kivimaki M, Brunner E, Shipley MJ, & Singh-Manoux A (2012). Effect of intensity and type of physical activity on mortality: results from the Whitehall II cohort study. American Journal of Public Health, 102, 698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas CM, Webbe FM, & Devore TT (2009). The epidemiology of soccer heading in competitive youth players. Journal of Clinical Sport Psychology, 3, 15–33. [Google Scholar]
- Stålnacke B-M, Tegner Y, & Sojka P (2004). Playing soccer increases serum concentrations of the biochemical markers of brain damage S-100B and neuron-specific enolase in elite players: a pilot study. Brain Injury, 18, 899–909. [DOI] [PubMed] [Google Scholar]
- Stephens R, Rutherford A, Potter D, & Fernie G (2010). Neuropsychological consequence of soccer play in adolescent UK school team soccer players. The Journal of Neuropsychiatry and Clinical Neurosciences, 22, 295–303. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Kim N, Ifrah CS, Lipton RB, Bachrach TA, Zimmerman ME, et al. (2017). Symptoms from repeated intentional and unintentional head impact in soccer players. Neurology. 10.1212/WNL.0000000000003657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart WF, Kim N, Ifrah C, Sliwinski M, Zimmerman ME, Kim M, Lipton RB, & Lipton ML (2018). Heading frequency is more strongly related to cognitive performance than unintentional head impacts in amateur soccer players, Frontiers in Neurology, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straume-Næsheim TM, Andersen TE, Holme IMK, McIntosh AS, Dvorak J, & Bahr R (2009). Do minor head impacts in soccer cause concussive injury? A prospective case-control study. Neurosurgery, 64, 719–725. [DOI] [PubMed] [Google Scholar]
- Suri AK, Fleysher R, & Lipton ML (2015). Subject based registration for individualized analysis of diffusion tensor MRI. PloS One, 10, e0142288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnutzer AA, Straumann D, Brugger P, Feddermann-Demont N (2016). Persistent effects of playing football and associated (subconcussive) head trauma on brain structure and function: a systematic review of the literature. British Journal of Sports Medicine. [DOI] [PubMed] [Google Scholar]
- Winklewski PJ, Sabisz A, Naumczyk P, Jodzio K, Szurowska E, & Szarmach A (2018). Understanding the physiopathology behind axial and radial diffusivity changes—What do we know? Frontiers in Neurology, 9, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]


