Supplemental Digital Content is available in the text.
Background.
Rapid excess weight gain and metabolic complications contribute to poor outcomes following liver transplant care. Providing specialist lifestyle intervention with equitable access is a challenge for posttransplant service delivery.
Methods.
This study investigated the feasibility of a 12-wk telehealth delivered lifestyle intervention for liver transplant recipients (randomized controlled trial with a delayed intervention control group). The intervention included 14 group sessions facilitated by nutrition and exercise specialists via video streaming telehealth and participants used their own devices. Feasibility was assessed across session attendance, the adequacy, acceptability, and confidence with the telehealth technology and adherence to diet (Mediterranean Diet Adherence Score). Secondary pooled analysis of effectiveness was determined from changes in quality of life and metabolic syndrome severity score.
Results.
Of the 35 participants randomized, dropout was 22.8% (n = 8) and overall session attendance rate was 60%. Confidence with and adequacy of home technology was rated high in 96% and 91% of sessions, respectively. Participants randomized to the intervention significantly improved Mediterranean Diet Adherence Score (2-point increase [95% confidence interval, 1.5-3.4] versus control 0 point change [95% confidence interval, –1.4 to 1.2]; P = 0.004). Intervention (within group) analysis found the intervention significantly decreased the metabolic syndrome severity score (–0.4 [95% confidence interval, –0.6 to –0.1] P = 0.01), and improved mental health-related quality of life (2.5 [95% confidence interval, 0.4-4.6] P = 0.03).
Conclusions.
A cardioprotective lifestyle intervention delivered via telehealth is feasible for liver transplant recipients and may improve access to specialist care to support metabolic health and wellness after transplant.
INTRODUCTION
Liver transplant restores lives; however, rapid excess weight gain and related metabolic complications negatively impact long-term outcomes.1 Optimizing cardiometabolic health for solid organ transplant recipients is crucial for improving survival,1 but to date, clinic-based weight loss programs have generally appeared ineffective for liver transplant recipients (LTRs).2
The Queensland Liver Transplant Service services a vast geographical area, more than twice that of Texas. Telehealth options are an obvious means of delivering services to LTRs who live remotely from their transplant center,3 as well as offering greater flexibility for those unable to attend the hospital because of work or other commitments. There is also evidence of the benefits of providing telemedicine options for outpatient care of complex conditions,4 as well as keen interest from LTRs themselves for this model of care,5 but the safety and acceptability of remote support for lifestyle intervention remains untested.
There is substantial evidence that improving diet quality, in particular a Mediterranean dietary pattern, has cardioprotective benefits for chronic disease6; however, given the unique metabolic disruption observed in LTRs,1 investigation of the benefits in this population remains warranted. Exercise capacity and muscle strength of LTRs are significantly lower than age- and sex-matched people without solid organ transplant.7-9 We have shown that although activities of daily living increase posttransplant, LTRs are apprehensive about exercise safety,5 with vigorous activity not initiated, even years after transplant.10 Although exercise capacity and body composition can be improved in LTRs with combined nutrition and exercise intervention,11 these programs typically rely on frequent face-to-face contact to access specialist support, resulting in poor attendance5,11 and unintentionally perpetuating inequalities in healthcare access.
In view of the changing landscape of service delivery, the primary aim of this study was to assess the safety and feasibility of telehealth delivery of a 12-wk cardioprotective lifestyle intervention (Mediterranean-style eating pattern and exercise prescription). The secondary aim was to assess the preliminary effectiveness of the intervention (versus usual care) on metabolic syndrome, diet quality, fitness, and health-related quality of life.
STUDY DESIGN
This pilot study was a randomized controlled trial, with a delayed-intervention control group, conducted at a tertiary hospital in Brisbane, Australia, between September 2017 and April 2018 (LIFE study; Australia and New Zealand Clinical Trials Register: ACTRN12617001260314). The Metro South Health Human Research Ethics Committee approved the study (HREC/17/QPAH/208), and all participants provided written informed consent.
PARTICIPANTS
All eligible LTRs scheduled for a medical outpatient review during the recruitment period (September 2017–January 2018) were sent a letter, followed by a telephone call, notifying them of the study, and inviting them to participate. LTRs, who did not have scheduled appointments during the recruitment period but resided locally (within a 100-km radius of the clinic), were similarly invited to attend an appointment with the investigators. The study was described as an opportunity to pilot a new telehealth service delivery model, not as a trial of a treatment related specifically to their personal health status. LTRs were eligible if they met the following inclusion criteria: >6 mo postliver transplant; expected survival >1 y; and with current access to a device with a camera or computer hardware with internet access and a webcam. Exclusion criteria were: LTRs with dietary restrictions, where in Mediterranean-style diet (MedDiet) is contraindicated; physical condition whereby exercise training would be inappropriate; deemed unsafe to participate by their hepatologist; or non-English speaking and unable to read English. Participants completed a health history questionnaire to screen for any cardiovascular or musculoskeletal conditions. Patient medical records were also reviewed, and any potential participant that had a contraindication to exercise training was referred to their transplant specialist for a decision regarding inclusion/exclusion.
STUDY GROUPS
Following baseline data collection, participants were randomized (2:1) to the intervention (MedDiet+E) or delayed intervention control (CON-DI) arm, using a computer-generated randomization sequence (REDCap Research Management System), stratified for sex, by the trial coordinator (research team blinded) (see Figure 1). Randomization ratio was designed to support greater number of participants being exposed to the intervention being assessed for feasibility.
FIGURE 1.
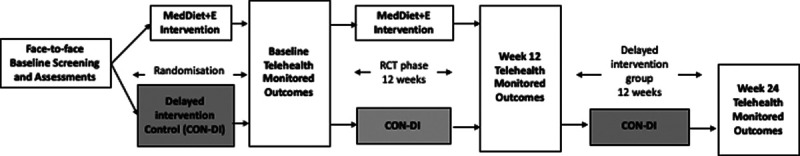
Study Schema: Face-to-face screening and assessments performed by health practitioners before randomization into either MedDiet+E (intervention) or CON-DI. Baseline telehealth monitored outcomes were then collected from both groups before 12-wk RCT phase, in which MedDiet+E received the telehealth delivered program and CON-DI received usual care. Both groups repeated telehealth monitored outcome measures at wk 12. CON-DI then went on to receive the telehealth intervention for 12 wk followed by another repeat telehealth monitored outcome assessment on completion. CON-DI, delayed intervention control; MedDiet+E, Mediterranean diet + exercise Intervention group; RCT, randomized controlled trial.
Participants allocated to the intervention arm (MedDiet+E) received a 12-wk, telehealth group-based exercise, and dietary intervention. The intervention was informed by our previous telehealth coaching program,12 and consumer engagement.5 All intervention sessions were delivered via synchronous videoconferencing streamed to participants remotely in real time, via a secure internet platform hosted by the tertiary health service (CISCO Tanberg C20, Advanced Encryption Standard-128). Participants received a workbook containing information on goal setting, the MedDiet, exercise training heart rate targets, resistance training exercises, and telehealth troubleshooting advice, along with a MedDiet recipe book and a “starter pack” of food products (Supplementary Material, SDC, http://links.lww.com/TXD/A309). Participants received an aerobic stepper (Kmart Australia Limited, Australia), resistance TheraBands (Weechee, China), and an exercise mat (Kmart Australia Limited, Australia).
Before the group-based telehealth sessions, participants completed individual telehealth assessments with an Accredited Practicing Dietitian (APD; approximately 45 min) and Accredited Exercise Physiologist (AEP; approximately 60 min), which included screening for musculoskeletal limitations.13 Following the individual sessions, participants received weekly group-based telehealth sessions alternating between APD-delivered diet education sessions (30–45 min, 6 sessions total) and AEP-delivered exercise training sessions (45–60 min, 8 sessions total). Over the 12-wk program, participants received a maximum of 14 telehealth contacts. A variety of days and times were available for group sessions including an “after-hours” option. If participants were unable to attend an APD telehealth session, then a telephone review was offered; telephone sessions were not offered for missed AEP telehealth sessions. Participants received up to 3 text messages between telehealth sessions (opt in/out process based on participant preference) that provided diet and exercise content and motivational tips (Table S1, SDC, http://links.lww.com/TXD/A309). Prerecorded online education content on a private YouTube channel, such as short videos with exercise tips, exercise sessions, and recipe demonstrations, for viewing outside of appointment times was available. Email reminders were sent with links to online content at various times during the program.
Participants were educated on MedDiet principles to improve diet quality (Supplementary Material, SDC, http://links.lww.com/TXD/A309). No specific advice was provided on calorie restriction. Participants were recommended to increase their physical activity to a minimum of 150 min moderate-to-vigorous aerobic activity per week plus muscle strength training on 2 d per week, consistent with national guidelines14 (see Supplementary Material, SDC, http://links.lww.com/TXD/A309). The group-based exercise sessions involved aerobic and resistance exercises using the equipment provided. The telehealth sessions allowed the AEP to monitor and modify exercises in real time allowing both participants and practitioners to provide immediate feedback. At the end of each session, participants received advice on achieving their exercise goals along with an individualized exercise prescription to complete unsupervised until the next exercise session (see Supplementary Material, SDC, http://links.lww.com/TXD/A309).
Participants randomized to the CON-DI arm received usual care over the first 12 wk, which included routine care from their treating physician(s). Structured allied health support is not usual care beyond 3-mo posttransplant. CON-DI participants received the same MedDiet+E telehealth program after completion of the week 12 data collection (see Figure 1).
OUTCOME MEASURES
Feasibility and Safety
A number of indicators were used to assess feasibility: recruitment rate (% of eligible patients;); APD and AEP sessions completed (%), retention rate (%) and reasons for attrition and missing data; staff-reported adequacy of technology (yes/no) at the completion of each session; participant-rated confidence with using technology (1 “not at all confident” to 10 “very confident”) and adequacy of their technology (1 “not at all adequate” to 10 “completely adequate”) at the completion of each session. A participant was deemed “lost to follow-up,” if there was no response to 3 phone calls and 3 email requests from the research team to reschedule a missed appointment. Any adverse events that occurred during telehealth sessions were recorded. Participants were asked to report their perception of safety while exercising at each session (patient reported; yes/no) and any previous adverse events between sessions were recorded.
Secondary Outcomes
Data on secondary outcomes were collected at baseline and 12 wk (and at 24 wk in CON-DI arm) for dietary adherence, exercise capacity, quality of life, and metabolic syndrome.
Dietary adherence was assessed using the Mediterranean Diet Adherence Screener (MEDAS),15 with a possible score ranging from 0 to 14 points (higher scores indicate greater MedDiet adherence). LTRs are advised to avoid alcohol after liver transplant so although still included in the survey, a score of 0 was anticipated for the alcohol component of the score. A 2-point increase in MEDAS score was deemed clinically significant.16 Proportion of participants meeting cutoff for each food group criteria in the MEDAS tool was determined before and after the intervention. In addition, to assess for any unintended consequences on dietary costs, the cost of dietary intake was estimated at each time point (methods and data in Supplementary Material, SDC, http://links.lww.com/TXD/A309).
Exercise capacity was assessed using the 6-min walk test (6MWT).17 At baseline, the test was conducted on a 10-m track within an indoor corridor, following a standardized published protocol.17 A premeasured piece of string marked the 10-m track and turn around points. The trained clinician used a lap counter and calculated the number of meters walked to the nearest 10 m. Participants practiced using the lap counter during the baseline test and were provided with the test script, string, lap counter, and stopwatch for repeat home testing.
Trained health professionals performed clinical and functional assessments at the baseline face-to-face clinic appointment on all participants. During this appointment, participants were instructed on how to perform each assessment at home and were provided with written instructions and links to online tutorials. Equipment to conduct all functional measures was provided. Self-measured baseline clinical and functional assessments were requested to be undertaken by all participants at home, unsupervised, within 1 wk of baseline clinic assessment (Figure 1). Results were recorded and sent to investigators via email or verbally transcribed at the next telehealth appointment. Reliability of home-based assessments compared with clinician-measured assessments in this group have been previously reported.18 At week 12, all participants repeated self-measured clinical and functional assessments. CON-DI arm participants repeated home-based assessments again at week 24 (postintervention). At each time point, participants received up to 3 phone call reminders to complete home-based assessments.
Clinician-assessed body weight (SECA scales Robusta 813, Hamburg, Germany) was recorded to the nearest 0.5 kg. Participants used their own personal scales for home-based weight assessment (various brands, not recorded). Waist circumference was measured midway between the lower rib margin and iliac crest, to the nearest 1 cm. Identical tape measures were provided to all participants. Height was measured (once only) with a wall-mounted stadiometer.19 Clinician-assessed systolic and diastolic blood pressure were performed seated (after at least 5 min resting) using Welch Allyn Cerner Vital Signs Monitor with an appropriate size cuff. An average of 3 measures were taken and recorded to the nearest 1 mm Hg. Participants were instructed to either use home blood pressure machines (if available) or attend a local pharmacy to have blood pressure performed by a pharmacist (various brands, not recorded). Home- or pharmacy-based blood pressure readings were taken twice on the same arm, with at least 1 min between readings after sitting quietly for a minimum of 5 min. If a >5 mm Hg difference between the first and second reading was observed, a third measure was taken. Participants reported all BP results and investigators calculated the average. Blood samples were collected at participants’ local pathology center after an overnight fast (at least 8 h). Participants returned to the same pathology center for each blood collection. A metabolic syndrome severity score (MetSSS) was calculated.20
Health-related quality of life was assessed using the 12-item Short Form Health Survey (SF-12v2).21 Summary physical (physical component score 12) and mental (mental component score for SF-12v2) components were constructed using the standard SF-12 version 2,22 with a higher score indicated better quality of life.
Statistical Analysis
Data are presented as mean (SD) or median (interquartile range [IQR]) after assessing for normality. Demographic data, clinician-derived anthropometric, and blood pressure data and clinical characteristics of participants at baseline were compared between MedDiet+E and CON-DI arms. All subsequent clinical data analyzed for the randomized controlled trial (RCT) and pooled (within intervention group) analysis included patient-derived prepost data. Between-group differences in change scores at 12 wk were examined using ANCOVA adjusting for baseline value of the outcome.
As a secondary exploratory analysis, data from immediately preintervention and immediately postintervention in the MedDiet+E (ie, baseline and wk 12) and CON-DI (ie, wk 12 and 24) arms were pooled to assess the clinical impact of the intervention exposure in all participants. Within-group comparisons were conducted using paired t-tests or Wilcoxon signed rank tests. One outlier in the intervention group was removed for all waist circumference analyses (recorded as 31 cm decrease in waist circumference and deemed implausible). Linear regression was performed to identify predictors of change in dietary adherence. All analyses were intention-to-treat, in which missing data were imputed using last observation carried forward23 or the mean value where in baseline data was missing (9%). Statistical significance was reported where P < 0.05, and all analyses were performed using SPSS (Version 25.0, New York, IBM Corp.).
RESULTS
Recruitment
Of the 131 LTRs eligible to participate, 35 (26%) enrolled in the study and were randomized to the intervention (MedDiet+E n = 23) or control (CON-DI n = 12) groups; reasons for refusal are shown in Figure 2. Recruited participants were 21–70 y of ages and primarily male (71%), which was the representative of a typical Australian transplant population.24 Participants were a median of 4 (IQR, 2–6) y posttransplant, with 9 (26%) living in regional areas (>50 km from metropolitan transplant center). There were no clinical differences between study groups in baseline demographic, clinical, or anthropometric variables, except for total cholesterol and low-density lipoprotein cholesterol, which were ~25% lower in the control group (Table 1).
FIGURE 2.
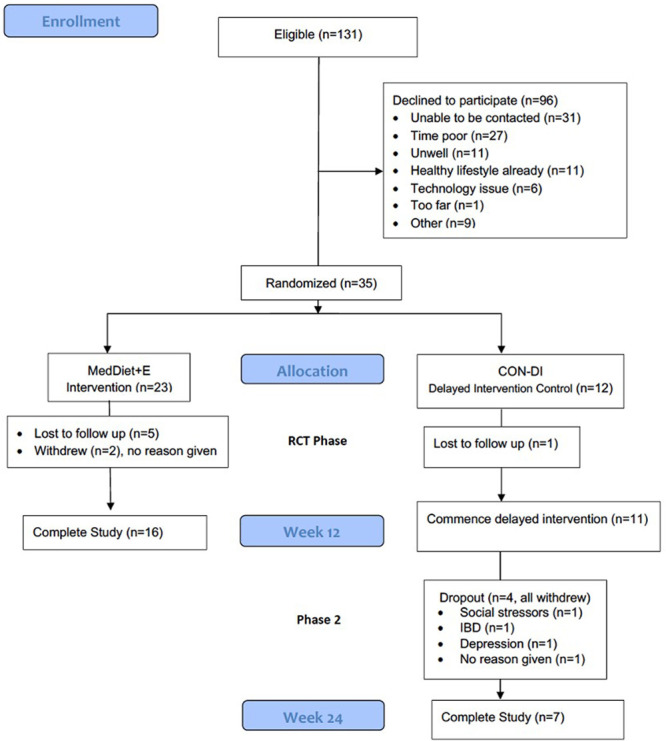
Consort diagram. CON-DI, delayed intervention control; MedDiet+E, Mediterranean-style diet + exercise intervention group.
Table 1.
Demographic, anthropometric and clinical characteristics of participants at baseline
| CON-DI (n = 12) | MedDiet+E intervention (n = 23) | |
|---|---|---|
| Sex, male (%) | 10 (83%) | 15 (65%) |
| Age, y | 50 (15) | 51 (15) |
| Years since transplant | 3 [1, 10] | 4 (2, 6) |
| Weight, kg | 87.0 (22.1) | 81.0 (17.8) |
| Body mass index, kg/m2 | 29.2 (8.4) | 27.6 (8.4) |
| Waist circumference, cm | 102 (94) | 97 (14) |
| Systolic blood pressure, mm Hg | 138 (14) | 133 (18) |
| Diastolic blood pressure, mm Hg | 85 (9) | 83 (10) |
| Glucose, mmol/L | 6.0 (1.6) | 5.4 (0.8) |
| Total cholesterol, mmol/L | 3.8 (1.2) | 4.9 (0.9) |
| Triglycerides, mmol/L | 1.1 (0.5) | 1.3 (1.0) |
| LDL cholesterol, mmol/L | 2.1 (0.9) | 3.0 (0.9) |
| HDL cholesterol, mmol/L | 1.3 (0.4) | 1.3 (0.4) |
Data are mean (SD) for continuous variables or median [IQR] if not normally distributed. Data for clinician-measured weight, body mass index, waist circumference, and blood pressure are presented. Missing data for glucose (n = 7), total cholesterol (n = 9), triglycerides (n = 8), LDL cholesterol (n = 9), and HDL cholesterol (n = 9).
CON-DI, delayed intervention control; HDL, high-density lipoprotein cholesterol; IQR, interquartile range; LDL, low-density lipoprotein cholesterol; MedDiet+E, Mediterranean diet + exercise Intervention group.
Attendance, Attrition, and Missing Data
Of the 35 participants randomized, 8 participants (22.9%) withdrew (n = 2) or were lost to follow-up (n = 6) during the RCT phase (Figure 2) after completing an average of 1 session each for nutrition and exercise. An additional 4 CON-DI arm participants withdrew during their intervention phase. The participants who completed the intervention participated in an average of 4.3 ± 1.8 dietetics sessions (71% of the 6 intended) and 4.1 ± 2.2 exercise sessions (52% of the 8 intended). Overall, 21% of data for outcome measures was missing (22% for the MedDiet+E group and 18% for the CON-DI group). Most missing data were due to attrition or participants not completing online questionnaires or tests at home with missing data for 9% of baseline clinician measures versus 30% of home-based follow-up measures. Based on type of measure, the highest proportions of missing data were for blood tests (29%), physical function tests (21%), and blood pressure measurements (17%), followed by anthropometry, MEDAS score, and quality of life score (each 15%).
Technology Access and Connectivity
Audio/visual “dropouts” or connection errors occurred in 20% of sessions (44/212 sessions) but only ever impacted an individual within a group (where in the remainder of group remained unaffected) and was not related to connection type. Staff-reported technology was adequate for 90% of the sessions. Participants rated ≥8 of 10 for their confidence level with home technology in 96% of the sessions and for adequacy of the technology in 91% of sessions.
Safety
Five participants (14%) required clearance from a transplant specialist to commence exercise. One patient was deemed unsuitable for exercise because of uncontrolled hypertension. There were no study-related adverse events. No participant reported feeling unsafe while exercising.
Dietary Adherence
There was a greater improvement in the MEDAS in the MedDiet+E compared with CON-DI group (2.4 [1.5, 3.4] versus –0.1 [–1.4, 1.2], P = 0.004; Table 2) with mean adherence score reaching 7.7 ± 2.5 by end of study in the MedDiet+E group. Additionally, there was a greater proportion meeting at least a 2-point increase in score in the MedDiet+E group compared with CON-DI at 12 wk (14 of 23 [61%] versus 1 of 12 [8%], P < 0.001). Cost analysis revealed the daily cost of dietary intake was not different from before to during the intervention (data not shown, see Supplementary Material results, SDC, http://links.lww.com/TXD/A309).
Table 2.
Dietary, anthropometric, clinical and functional outcomes for control and MedDiet+E intervention groups
| Control (n = 12) | Intervention (n = 23) | Control BL vs wk 12 estimated marginal means (95% CI) | Intervention BL vs wk 12 estimated marginal means (95% CI) | Pa | |||
|---|---|---|---|---|---|---|---|
| BL mean (SD) | Week 12 mean (SD) | BL mean (SD) | Week 12 mean (SD) | ||||
| Mediterranean diet (MEDAS) | 6.0 (2.1) | 5.9 (3.2) | 5.3 (1.4) | 7.7 (2.5) | –0.1 (–1.4, 1.2) | 2.4 (1.5, 3.4) | 0.004 |
| Waist circumference (cm) | 101 (18) | 100 (18) | 97 (14) | 94 (12) | –0.5 (–4.8, 3.7) | –4.5 (–7.6, –1.5) | 0.132 |
| Weight (kg) | 86.3 (21.4) | 86.3 (21.6) | 80.7 (18.1) | 79.0 (17.3) | 0.1 (–1.5, 1.7) | –1.8 (–2.9, –0.6) | 0.061 |
| Body mass index (kg/m2) | 28.6 (8.3) | 28.6 (8.3) | 27.5 (5.5) | 26.8 (5.3) | 0.0 (–0.5, 0.5) | –0.6 (–1.0, 0–.3) | 0.050 |
| Systolic blood pressure (mm Hg) | 134 (15) | 131 (12) | 131 (17) | 128 (16) | –2.3 (–9.2, 4.7) | –2.8 (–7.8, 2.2) | 0.901 |
| Diastolic blood pressure (mm Hg) | 84 (11) | 83 (9) | 82 (12) | 81 (11) | –0.9 (–4.3, 2.5) | –0.9 (–3.3, 1.6) | 0.985 |
| Glucose (mmol/L) | 5.9 (1.4) | 5.5 (1.0) | 5.4 (0.7) | 5.4 (0.7) | –2.4 (–0.7, 0.2) | –0.1 (–0.4, 0.2) | 0.491 |
| Total cholesterol (mmol/L) | 3.9 (1.1) | 4.0 (1.0) | 4.7 (0.8) | 4.6 (0.8) | –0.0 (–0.2, 0.1) | –0.1 (–0.2, 0.0) | 0.570 |
| Triglycerides (mmol/L) | 1.1 (0.5) | 1.6 (1.6) | 1.3 (0.9) | 1.3 (0.7) | 0.4 (–0.2, 1.0) | 0.0 (–0.4, 0.4) | 0.247 |
| LDL (mmol/L) | 2.1 (0.8) | 2.1 (0.8) | 2.9 (0.8) | 2.8 (0.6) | –0.2 (–0.4, 0.0) | 0.0 (–0.1, 0.1) | 0.124 |
| HDL (mmol/L) | 1.2 (0.3) | 1.2 (0.3) | 1.2 (0.5) | 1.3 (0.4) | 0.0 (–0.1, 0.1) | 0.1 (–0.1, 0.1) | 0.531 |
| MetSSS | 2.8 (2.3) | 2.2 (2.3) | 2.1 (1.8) | 1.5 (1.7) | –0.5 (–1.1, 0.0) | –0.7 (–1.1, –0.3) | 0.625 |
| 6-min walk test (m) | 436 (83) | 425 (71) | 448 (139) | 465 (125) | –15 (–71, 41) | 19 (–22, 60) | 0.323 |
| PCS-12 | 39.8 (7.5) | 42.7 (8.4) | 48.0 (8.1) | 49.3 (8.0) | 1.1 (–2.7, 5.0) | 2.2 (–0.6, 4.9) | 0.681 |
| MCS-12 | 50.5 (8.3) | 48.3 (9.1) | 49.7 (9.2) | 52.6 (7.9) | –2.0 (–5.4, 1.4) | 2.8 (0.3, 5.2) | 0.026 |
Data are mean (SD) and estimated marginal means for change in endpoints.
Bolded P values represent statistical significance.
aComparison of change between groups using ANCOVA adjusting for baseline values.
BL, baseline; CI, confidence interval; HDL, high-density lipoprotein cholesterol.; LDL, low-density lipoprotein cholesterol; MCS-12, mental component score for SF12v2; MEDAS, Mediterranean Dietary Adherence Screener; MetSSS, metabolic syndrome severity score; PCS, physical component score.
Effectiveness
RCT Intention-to-treat Analysis
There was no difference between groups for change in anthropometry, clinical, or functional endpoints preintervention to postintervention (Table 2). A statistically and clinically meaningful improvement in the mental component summary score in the MedDiet+E group (49.7 ± 9.2 to 52.6 ± 7.9) versus the CON-DI group (50.5 ± 8.3 to 48.3 ± 9.1) was observed, whereas no differences were observed between groups for the physical component of quality of life.
Preintervention and Postintervention—Pooled Analysis
Table 3 summarizes the within-group intervention changes on clinical, biochemical, and functional outcomes. From preintervention to postintervention, there was a significant reduction in waist circumference (–1.9 [IQR, –3.8 to –0.1] cm), body mass index (–0.5 [IQR, –0.8 to –0.1] kg/m2) and MetSSS (–0.4 [IQR, –0.6 to –0.1]) and an improvement in mental component summary score (+2.5 [IQR, 0.4–4.6]). A significant improvement was also observed in diet quality with an increase in the MEDAS score (6 points [IQR, 4–7] versus 8 points [IQR, 6–10], P < 0.001; Figure 3), with 19 participants (54%) achieving a clinically significant increase of at least 2 points.
Table 3.
Intervention (within group) analysis of anthropometric, clinical and functional outcomes preintervention and postintervention (n = 35)
| Preintervention | Postintervention | Mean difference (95% CI) | Pa | |
|---|---|---|---|---|
| Waist circumference (cm) | 98.0 (15.0) | 96.2 (14.7) | –1.9 (–3.8, –0.1) | 0.032 |
| Weight (kg) | 82.6 (19.3) | 81.2 (19.2) | –1.3 (–2.3, –0.3) | 0.011 |
| Body mass index (kg/m2) | 27.8 (6.5) | 27.3 (6.6) | –0.5 (–0.8, –0.1) | 0.009 |
| Systolic blood pressure (mm Hg)b | 131 (15) | 129 (15) | –1.8 (–5.5, 1.8) | 0.311 |
| Diastolic blood pressure (mm Hg)b | 82 (11) | 81 (10.0) | –1.6 (–3.7, 0.6) | 0.150 |
| Glucose | 5.4 (0.8) | 5.4 (0.8) | 0.0 (–0.2, 0.1) | 0.833 |
| Total cholesterol (mmol/L)b | 4.5 (1.0) | 4.5 (1.0) | 0.0 (–0.2, 0.1) | 0.642 |
| Triglycerides (mmol/L) | 1.4 (1.2) | 1.2 (1.2) | –0.2 (–0.4, 0.0) | 0.056 |
| LDL (mmol/L)b | 2.6 (0.8) | 2.6 (0.8) | 0.0 (–0.2, 0.1) | 0.814 |
| HDL (mmol/L) | 1.2 (0.4) | 1.3 (0.4) | 0.0 (0.0, 0.1) | 0.735 |
| MetSSS | 2.2 (1.9) | 1.8 (1.9) | –0.4 (–0.6, –0.1) | 0.014 |
| 6-min walk test (m)b | 440 (119) | 463 (108) | 23 (–17, 62) | 0.248 |
| PCS-12 | 46.6 (8.4) | 48.1 (7.4) | 1.5 (–0.6, 3.5) | 0.135 |
| MCS-12 | 49.8 (8.6) | 52.3 (7.6) | 2.5 (0.4, 4.6) | 0.028 |
Data are mean (SD) for the intention-to-treat population.
Bolded P values represent statistical significance.
aPre and postintervention compared using Wilcoxon signed rank test for most variables ^.
bData was normally distributed and dependent t-tests were used.
CI, confidence interval; HDL, high-density lipoprotein cholesterol.; LDL, low-density lipoprotein cholesterol; MCS-12, mental component score for SF12v2; MetSSS, metabolic syndrome severity score; PCS, physical component score.
FIGURE 3.
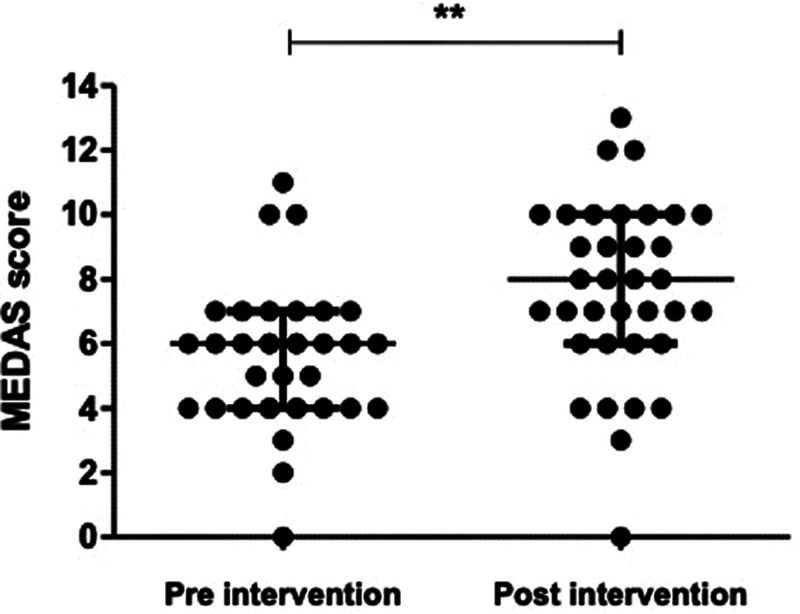
Mediterranean Diet Adherence Screener (MEDAS) scores (out of possible 14) preintervention and postintervention (n = 35) **P < 0.001, Wilcoxon signed rank test.
Linear regression analysis was performed to predict change in MEDAS based on demographic variables. Female gender significantly predicted change in MEDAS (F (1, 33) = 4.287; P = 0.046), but there were no significant relationships with age (P = 0.487) or time since transplant (P = 0.586). There was a significant positive correlation between change in MEDAS and attendance at dietary sessions (r = 0.478; P = 0.018). When examining the aspects of the MedDiet that changed in response to the intervention, more individuals achieved the criteria, compared with baseline, for the amount of olive oil (P = 0.001), fish (P = 0.039), commercial sweets/pastries (P = 0.008), nuts (P = 0.004), and Sofrito (P = 0.039) consumed (Figure 4).
FIGURE 4.
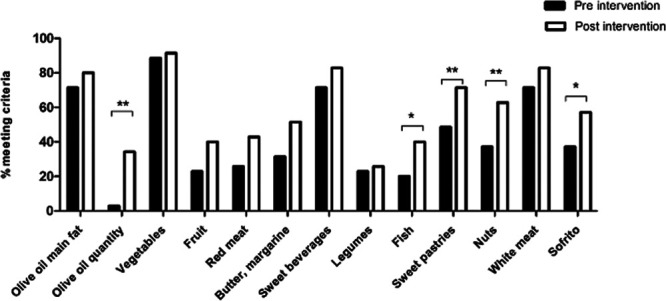
Proportion of participants meeting the criteria for individual Mediterranean Diet Adherence Screener components (n = 35) *P < 0.05; **P < 0.01. To achieve a score for each question, participants were required to (1) use olive oil as the main source of fat for cooking, (2) include ≥4 tablespoons of olive oil a d, (3) include ≥2 servings of vegetables a d, (4) include ≥3 pieces of fruit per d, (5) include <1 serve of red meat or sausages per d, (6) include <1 serve butter/cream per d, (7) include <100 mL of sugar-sweetened beverages per d, (8) include ≥3 serves of legumes/pulses per wk, (9) include ≥3 serves of fish or shellfish per wk, (10) include <3 commercial sweets/pastries per wk, (11) include ≥3 serves of nuts a wk, (12) preferentially consume chicken, turkey, or rabbit instead of veal, pork, hamburger, or sausage, and (13) prepare ≥2 or more meals per wk seasoned with sofrito (sauce of tomatoes and onion, garlic, or leek, sautéed in olive oil).
DISCUSSION
In this pilot study, we demonstrated for the first time that a telehealth-delivered diet and exercise intervention is acceptable and safe for LTRs and may effectively target cardiometabolic risk factors and improve mental components of quality of life. LTRs enrolled in this study successfully used a range of personal technologies to video link with clinicians for individualized and group sessions for diet and exercise support, and both LTRs and staff found telehealth delivery for diet and exercise a highly acceptable method of receiving care.
Telehealth attendance to group sessions was acceptable (50%–70%) albeit lower for exercise sessions compared with dietary sessions, and similar rates for telephone coaching programs tested in other conditions.12,25 An intervention with a similar style of remote exercise supervision achieved 83% attendance in a cardiac rehab setting.26 The lower attendance rate observed in this trial compares with other lifestyle interventions in LTRs, which have achieved only 15%–37% face-to-face attendance.11,27 Although participants could dial in to a dietary session from practically any location (eg, at work, or while away on holidays), participating in an exercise session requires additional preparation around the practicalities of exercising such as appropriate location, clothing and footwear, and access to exercise equipment. These barriers, common also for face-to-face exercise sessions, remain relevant for telehealth exercise appointments.
The program focused on diet quality as a targeted cardiovascular risk factor. The relatively low MEDAS score at baseline and lack of dietary pattern change in the control group indicates that, without specialized support, communities following a Western-style diet are unlikely to naturally adopt this cardioprotective dietary pattern after liver transplant. Diet quality improved after telehealth, similarly to other face-to-face interventions in Australian cohorts28,29 with >50% of participants achieving ≥2-point increase in MEDAS score. Adopting a MedDiet outside of the Mediterranean region has its challenges,30-33 but with appropriate support, we found the MedDiet was an acceptable strategy to improve diet quality in an Australian liver transplant population.34 The acceptability of the MedDiet pattern in Western societies and to what degree of adherence to “traditional” MedDiet principles is necessary for clinical effect is an ongoing scientific debate.35 Nonetheless, with increasing clinical interest in shifting dietetic services to facilitate more cardioprotective diet patterns in liver clinics,36 considerations of multicultural patient groups are a welcome development to better translate the evidence for MedDiet effectiveness for those living outside of the Mediterranean.35,37,38 Importantly, this study indicated a shift to a greater Mediterranean style of eating could be done in a cost-neutral manner, reflecting the results of cost effectiveness evaluations in other Australian settings.39,40
Despite no specific advice regarding calorie restriction, after exposure to the intervention, participants experienced a reduction in body mass and waist circumference, as is consistent with other MedDiet trials.41,42 This improvement was the main factor driving the improvement of MetSSS in the pooled analysis, along with a trend to improvements in serum triglyceride. This aligns with results from short term43,44 and larger, long-term (>12 mo) studies of the MedDiet.31,45 In highly supported environments, an improvement in metabolic syndrome with adherence to MedDiet has been demonstrated for other solid organ transplant recipients.46 This study is the first to demonstrate preliminary data for cardiometabolic benefit related to telehealth delivered dietary support for LTRs. In addition, a service such as this which engages consumers in the codesign,5 highlights the value that health and social support can have on improving mental components of quality of life.47
In the absence of specific clinical guidelines for posttransplant exercise prescription, the study targeted general health prescription14 and included bouts of moderate and vigorous-intensity aerobic exercise and resistance exercises. The exercise sessions with telehealth supervision were safe, with no adverse events in well-screened participants. This is consistent with previous work showing specialist-prescribed exercise is safe before48,49 and after transplant.11,27,50,51 Although in-person supervised exercise can lead to significant improvements in fitness and global strength with low dropout, it has been shown that a significant proportion of patients (45% in a recent study) are unable to participate because they live too far away from their transplant center.52 This study provides support for greater investment in telehealth care, particularly in settings like Australia, where centralized specialist services have vast geographical outreach.
The study recruitment rate of 25% was similar to other studies of transplant recipients where in telephone recruitment was linked with upcoming clinic visits50 and was representative of the typical demographics of the transplant service. However, a greater uptake may be desired if rolled out into usual care. Time since transplant may impact recruitment as Krasnoff et al11 achieved almost twice the recruitment rate when they invited patients within 2 mo of transplant. LTRs from our center have indicated they want support early after transplant,5 and this may prevent excess weight gain within the first 6 mo posttransplant.10 This feasibility study was offered to patients as a pilot of new service delivery, rather than a direct personal care need. It is likely that uptake may be greater if eligible LTR are offered to link with telehealth service before hospital discharge. Studies of diet and exercise for weight reduction are typically burdened with dropout rates of 20%–40% due to high demands on time and behavior changes of participants.53,54 Across the limited number of studies that have tested diet and exercise interventions in LTRs, all within supervised clinical settings, dropout rates have ranged between 25% and 40%.11,55 It was an important observation for feasibility assessment that dropout rates of this study were similar or better than for those interventions delivered face to face.
Relying on remote monitoring of outcomes is an ongoing dilemma for telehealth services. In this study, missing data were mostly related to participants not completing or reporting their home-based assessments within the required time frame. This was despite online data entry, alleviating the need for postage or clinic attendance. Investing in partnerships with LTRs and highlighting the value of their participatory role in their posttransplant care is a cultural shift that may improve confidence and participation in self-monitoring. Modest financial incentives have been shown to facilitate health behavior changes such as increased walking in solid organ transplant recipients, and although this is not a traditional strategy, it is an example of how to improve LTRs engagement with health monitoring and reporting.50
Other lessons from this study were that adequate orientation of patients to the technology was crucial to ensure confidence and literacy in an unsupervised setting, as was training of clinical staff to troubleshoot technical connection issues in real time. This requires diversion of some staff time to technology-related tasks. Modeling has indicated that although eHealth delivery modes for dietetic intervention have a cost to establish, the recurring costs are lower than face-to-face care.56 In addition, qualitative analysis from participants indicated that the cost savings to patients through reduced travel time and lost work hours associated with face-to-face appointments were highly valued.34 Experience with rapid disruptions to telehealth delivery of care during the COVID-19 pandemic57 is likely to drive demand for this transition for many services.
A strength of this study is the LTR codesign of the intervention.5 We were able to demonstrate clinical benefit with greater convenience and no perceptible increase in cost for LTRs, without sacrificing the rapport and trust that comes from engaging with a specialist center.34 Inclusion of LTRs from regional and metropolitan areas captured the feasibility of telehealth connections across a range of locations. Recent experiences with Covid-19 disruptions to service delivery reflected the experience of this study whereby the cost of or access to technology is rarely a barrier to engaging with telehealth, however reliable and strong internet connection can at times be an issue for rural communities in Australia. Although the study participants were broadly representative of our typical patient cohort, results may not be generalizable to other transplant centers. Participants were not severely metabolically impaired at baseline, and metabolic outcomes were only evaluated short term. Although metabolic benefit of the Mediterranean diet has been demonstrated within 12 wk in other studies,43,44 larger, long-term studies in more specific populations, are needed to adequately address the potential metabolic confounders on clinical complications such as steroid use, nutritional status, and excess weight gain early posttransplant. Implementation processes that assist uptake of services in the peritransplant phase, before patients returning to competing life commitments, may be beneficial to enhance adoption.
CONCLUSION
Telehealth delivery of diet and exercise facilitated sessions that target cardiometabolic risk factors in LTRs is safe and feasible; however, remote outcome monitoring remains a challenge for some patients. Using technology to disrupt traditional service delivery models may improve access of care and may be one strategy for supporting metabolic health and wellness of LTRs.
ACKNOWLEDGMENTS
Thank you to Nicholas Matigian from the MetroSouth Biostatistics Clinic; Sean O’Halloran from the Princess Alexandra Hospital UQ Telehealth Centre; patients and staff of the Queensland Liver Transplant Service; and Victoria Williams, Ilaria Croci, and Keira Murray who assisted data collection and data entry. Thank you to companies who donated food product and equipment for the participant “starter packs” including Cobram Estate Olive Oil, Almond Board of Australia, Heinz Australia, Safcol, Simplot Australia, Woolworths (Buranda), and Diabetes Queensland.
Supplementary Material
Footnotes
Published online 4 February, 2021.
Project funding was received from the Princess Alexandra Hospital Research Support Scheme, Princess Alexandra Hospital Research Foundation, and the Allied Health Professionals Office of Queensland.
The authors disclose no conflict of interest
I.J.H. participated in research design, participated in the writing of the article, participated in the performance of the research, participated in data analysis, and approved final version of article. A.K.H., H.E.J., and L.E.-W. participated in the writing of the article, participated in the performance of the research, participated in data analysis, and approved final version of article. H.L.M. and H.M.S. participated in the writing of the article, participated in data analysis, and approved final version of article. A.B. participated in the writing of the article, participated in the performance of the research, and approved final version of article. R.S. participated in the writing of the article, participated in data analysis, and approved final version of article. C.S. participated in the performance of the research, participated in data analysis, and approved final version of article. M.J. participated in the performance of the research and approved final version of article. M.M.R. and J.S.C. participated in research design, participated in the writing of the article, approved final version of article. K.L.C. participated in research design, participated in the writing of the article, participated in data analysis, and approved final version of article. S.E.K. participated in research design, participated in the writing of the article, participated in the performance of the research, participated in data analysis, and approved final version of article. G.A.M.: Participated in research design, participated in the writing of the article, participated in data analysis, and approved final version of article.
Clinical Trial Registry: Australia and New Zealand Clinical Trials Register: ACTRN12617001260314.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.European Association for the Study of the Liver. EASL clinical practice guidelines: liver transplantation. J Hepatol. 2016;64:433–485. [DOI] [PubMed] [Google Scholar]
- 2.Patel SS, Siddiqui MB, Chadrakumaran A, et al. Office-based weight loss counseling is ineffective in liver transplant recipients. Dig Dis Sci. 2020;65:639–646. [DOI] [PubMed] [Google Scholar]
- 3.Leimig R, Gower G, Thompson DA, et al. Infection, rejection, and hospitalizations in transplant recipients using telehealth. Prog Transplant. 2008;18:97–102. [DOI] [PubMed] [Google Scholar]
- 4.Flodgren G, Rachas A, Farmer AJ, et al. Interactive telemedicine: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2015;2015:CD002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hickman IJ, Coran D, Wallen MP, et al. ‘Back to life’-using knowledge exchange processes to enhance lifestyle interventions for liver transplant recipients: a qualitative study. Nutr Diet. 2019;76:399–406. [DOI] [PubMed] [Google Scholar]
- 6.Dinu M, Pagliai G, Casini A, et al. Mediterranean diet and multiple health outcomes: an umbrella review of meta-analyses of observational studies and randomised trials. Eur J Clin Nutr. 2018;72:30–43. [DOI] [PubMed] [Google Scholar]
- 7.De Bona M, Ponton P, Ermani M, et al. The impact of liver disease and medical complications on quality of life and psychological distress before and after liver transplantation. J Hepatol. 2000;33:609–615. [DOI] [PubMed] [Google Scholar]
- 8.Lemyze M, Dharancy S, Nevière R, et al. Aerobic capacity in patients with chronic liver disease: very modest effect of liver transplantation. Presse Med. 2010;39:e174–e181. [DOI] [PubMed] [Google Scholar]
- 9.Ratcliffe J, Longworth L, Young T, et al. ; Cost-Effectiveness of Liver Transplantation Team. Assessing health-related quality of life pre- and post-liver transplantation: a prospective multicenter study. Liver Transpl. 2002;8:263–270. [DOI] [PubMed] [Google Scholar]
- 10.McCoy SM, Campbell KL, Lassemillante AM, et al. Changes in dietary patterns and body composition within 12 months of liver transplantation. Hepatobiliary Surg Nutr. 2017;6:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krasnoff JB, Vintro AQ, Ascher NL, et al. A randomized trial of exercise and dietary counseling after liver transplantation. Am J Transplant. 2006;6:1896–1905. [DOI] [PubMed] [Google Scholar]
- 12.Whelan ME, Goode AD, Eakin EG, et al. Feasibility, effectiveness and cost-effectiveness of a telephone-based weight loss program delivered via a hospital outpatient setting. Transl Behav Med. 2016;6:386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson PD, Arena R, Riebe D, et al. ; American College of Sports Medicine. ACSM’s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription, ninth edition. Curr Sports Med Rep. 2013;12:215–217. [DOI] [PubMed] [Google Scholar]
- 14.Brown WJ, Bauman AE, Bull FC, et al. Development of evidence-based physical activity recommendations for adults (18-64 years). Report prepared for the Australian Government Department of Health; 2012. Available at https://www1.health.gov.au/internet/main/publishing.nsf/Content/health-pubhlth-strateg-phys-act-guidelines. Accessed July 1, 2020.
- 15.Papadaki A, Johnson L, Toumpakari Z, et al. Validation of the English version of the 14-item mediterranean diet adherence screener of the PREDIMED study, in people at high cardiovascular risk in the UK. Nutrients. 2018;10:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martínez-González MA, García-Arellano A, Toledo E, et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: the PREDIMED trial. PLoS One. 2012;7:e43134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coombes J, Skinner T. ESSA’s Student Manual for Health, Exercise and Sport Assessment. Elsevier Health Sciences; 2014. [Google Scholar]
- 18.Keating SE, Barnett A, Croci I, et al. Agreement and reliability of clinician-in-clinic versus patient-at-home clinical and functional assessments: implications for telehealth services. Arch Rehabil Res Clin Transl. 2020;2:100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.International Society for the Advancement of Kinanthropometry. International Standards for Anthropometric Assessment. International Society for the Advancement of Kinanthropometry; 2001. [Google Scholar]
- 20.Wiley JF, Carrington MJ. A metabolic syndrome severity score: a tool to quantify cardio-metabolic risk factors. Prev Med. 2016;88:189–195. [DOI] [PubMed] [Google Scholar]
- 21.Ware J, Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 22.Jayasinghe UW, Harris MF, Taggart J, et al. Gender differences in health-related quality of life of Australian chronically-ill adults: patient and physician characteristics do matter. Health Qual Life Outcomes. 2013;11:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu-Seifert H, Zhang S, D’Souza D, et al. A closer look at the baseline-observation-carried-forward (BOCF). Patient Prefer Adherence. 2010;4:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Australia and New Zealand Liver and Intestinal Transplant Registry. ANZLITR 30th Annual Report 2020. 2020. Available at https://www3.anzltr.org/. Accessed July 1, 2020.
- 25.Viglione C, Bouwman D, Rahman N, et al. A technology-assisted health coaching intervention vs. enhanced usual care for primary care-based obesity treatment: a randomized controlled trial. BMC Obes. 2019;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang R, Bruning J, Morris NR, et al. Home-based telerehabilitation is not inferior to a centre-based program in patients with chronic heart failure: a randomised trial. J Physiother. 2017;63:101–107. [DOI] [PubMed] [Google Scholar]
- 27.Totti V, Tamè M, Burra P, et al. Physical condition, glycemia, liver function, and quality of life in liver transplant recipients after a 12-month supervised exercise program. Transplant Proc. 2019;51:2952–2957. [DOI] [PubMed] [Google Scholar]
- 28.Mayr HL, Tierney AC, Kucianski T, et al. Australian patients with coronary heart disease achieve high adherence to 6-month Mediterranean diet intervention: preliminary results of the AUSMED Heart Trial. Nutrition. 2019;61:21–31. [DOI] [PubMed] [Google Scholar]
- 29.Davis C, Hodgson J, Bryan J, et al. Older Australians can achieve high adherence to the Mediterranean diet during a 6 month randomised intervention; results from the Medley Study. Nutrients. 2017;9:534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haigh L, Bremner S, Houghton D, et al. Barriers and facilitators to Mediterranean diet adoption by patients with nonalcoholic fatty liver disease in Northern Europe. Clin Gastroenterol Hepatol. 2019;17:1364–1371.e3. [DOI] [PubMed] [Google Scholar]
- 31.Echeverría G, Dussaillant C, McGee EE, et al. Promoting and implementing the Mediterranean diet in the southern hemisphere: the Chilean experience. Eur J Clin Nutr. 2019;72(Suppl 1):38–46. [DOI] [PubMed] [Google Scholar]
- 32.Thodis A, Itsiopoulos C, Kouris-Blazos A, et al. Observational study of adherence to a traditional Mediterranean diet, sociocultural characteristics and cardiovascular disease risk factors of older Greek Australians from MEDiterranean ISlands (MEDIS-Australia Study): protocol and rationale. Nutr Diet. 2018;75:44–51. [DOI] [PubMed] [Google Scholar]
- 33.Jacobs DR, Jr, Petersen KS, Svendsen K, et al. Considerations to facilitate a US study that replicates PREDIMED. Metabolism. 2018;85:361–367. [DOI] [PubMed] [Google Scholar]
- 34.Barnett A, Campbell KL, Mayr HL, et al. Liver transplant recipients’ experiences and perspectives of a telehealth-delivered lifestyle programme: a qualitative study. J Telemed Telecare. [Epub ahead of print. January 27, 2020]. doi: 10.1177/1357633X19900459. [DOI] [PubMed] [Google Scholar]
- 35.Martínez-González MÁ, Hershey MS, Zazpe I, et al. Transferability of the Mediterranean diet to non-Mediterranean countries. What is and what is not the Mediterranean diet. Nutrients. 2017;9:1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray E, McCoy S, Campbell K, et al. Modern day challenges for the nutrition management of liver transplant recipients. Nutr Diet. 2014;71:86–91. [Google Scholar]
- 37.Radd-Vagenas S, Fiatarone Singh MA, Daniel K, et al. Validity of the Mediterranean diet and culinary index (MediCul) for online assessment of adherence to the ‘traditional’ diet and aspects of cuisine in older adults. Nutrients. 2018;10:1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knight A, Bryan J, Murphy K. Is the Mediterranean diet a feasible approach to preserving cognitive function and reducing risk of dementia for older adults in Western countries? New insights and future directions. Ageing Res Rev. 2016;25:85–101. [DOI] [PubMed] [Google Scholar]
- 39.Segal L, Twizeyemariya A, Zarnowiecki D, et al. Cost effectiveness and cost-utility analysis of a group-based diet intervention for treating major depression - the HELFIMED trial. Nutr Neurosci. 2020;23:770–778. [DOI] [PubMed] [Google Scholar]
- 40.Chatterton ML, Mihalopoulos C, O’Neil A, et al. Economic evaluation of a dietary intervention for adults with major depression (the “SMILES” trial). BMC Public Health. 2018;18:599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esposito K, Kastorini CM, Panagiotakos DB, et al. Mediterranean diet and weight loss: meta-analysis of randomized controlled trials. Metab Syndr Relat Disord. 2011;9:1–12. [DOI] [PubMed] [Google Scholar]
- 42.Bendall CL, Mayr HL, Opie RS, et al. Central obesity and the Mediterranean diet: a systematic review of intervention trials. Crit Rev Food Sci Nutr. 2018;58:3070–3084. [DOI] [PubMed] [Google Scholar]
- 43.Estruch R, Martínez-González MA, Corella D, et al. ; PREDIMED Study Investigators. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. 2006;145:1–11. [DOI] [PubMed] [Google Scholar]
- 44.Properzi C, O’Sullivan TA, Sherriff JL, et al. Ad libitum Mediterranean and low-fat diets both significantly reduce hepatic steatosis: a Randomized Controlled Trial. Hepatology. 2018;68:1741–1754. [DOI] [PubMed] [Google Scholar]
- 45.Babio N, Toledo E, Estruch R, et al. ; PREDIMED Study Investigators. Mediterranean diets and metabolic syndrome status in the PREDIMED randomized trial. CMAJ. 2014;186:E649–E657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Entwistle TR, Green AC, Fildes JE, et al. Adherence to Mediterranean and low-fat diets among heart and lung transplant recipients: a randomized feasibility study. Nutr J. 2018;17:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Entwistle VA, Cribb A, Owens J. Why health and social care support for people with long-term conditions should be oriented towards enabling them to live well. Health Care Anal. 2018;26:48–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams FR, Vallance A, Faulkner T, et al. Home-based exercise in patients awaiting liver transplantation: a Feasibility Study. Liver Transpl. 2019;25:995–1006. [DOI] [PubMed] [Google Scholar]
- 49.Wallen MP, Keating SE, Hall A, et al. Exercise training is safe and feasible in patients awaiting liver transplantation: a Pilot Randomized Controlled Trial. Liver Transpl. 2019;25:1576–1580. [DOI] [PubMed] [Google Scholar]
- 50.Serper M, Barankay I, Chadha S, et al. A randomized, controlled, behavioral intervention to promote walking after abdominal organ transplantation: results from the LIFT study. Transpl Int. 2020;33:632–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beyer N, Aadahl M, Strange B, et al. Improved physical performance after orthotopic liver transplantation. Liver Transpl Surg. 1999;5:301–309. [DOI] [PubMed] [Google Scholar]
- 52.Moya-Nájera D, Moya-Herraiz Á, Compte-Torrero L, et al. Combined resistance and endurance training at a moderate-to-high intensity improves physical condition and quality of life in liver transplant patients. Liver Transpl. 2017;23:1273–1281. [DOI] [PubMed] [Google Scholar]
- 53.Huys N, Van Stappen V, Shadid S, et al. ; Feel4Diabetes-study group. Effectiveness of a family-, school- and community-based intervention on physical activity and its correlates in Belgian families with an increased risk for type 2 diabetes mellitus: the Feel4Diabetes-study. BMC Public Health. 2020;20:1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Di Noia J, Schultz S, Monica D. Recruitment and retention of WIC participants in a longitudinal dietary intervention trial. Contemp Clin Trials Commun. 2019;16:100438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Totti V, Campione T, Mosconi G, et al. Promotion of pre- and post-transplant physical exercise in the Emilia-Romagna region: the network of the program “Transplantation, Physical Activity, and Sport”. Transplant Proc. 2019;51:2902–2905. [DOI] [PubMed] [Google Scholar]
- 56.Rollo ME, Burrows T, Vincze LJ, et al. Cost evaluation of providing evidence-based dietetic services for weight management in adults: In-person versus eHealth delivery. Nutr Diet. 2018;75:35–43. [DOI] [PubMed] [Google Scholar]
- 57.Webster P. Virtual health care in the era of COVID-19. Lancet. 2020;395:1180–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


