Abstract
BACKGROUND
Efforts to prevent Clostridioides difficile infection continue to expand across the health care spectrum in the United States. Whether these efforts are reducing the national burden of C. difficile infection is unclear.
METHODS
The Emerging Infections Program identified cases of C. difficile infection (stool specimens positive for C. difficile in a person ≥1 year of age with no positive test in the previous 8 weeks) in 10 U.S. sites. We used case and census sampling weights to estimate the national burden of C. difficile infection, first recurrences, hospitalizations, and in-hospital deaths from 2011 through 2017. Health care–associated infections were defined as those with onset in a health care facility or associated with recent admission to a health care facility; all others were classified as community-associated infections. For trend analyses, we used weighted random-intercept models with negative binomial distribution and logistic-regression models to adjust for the higher sensitivity of nucleic acid amplification tests (NAATs) as compared with other test types.
RESULTS
The number of cases of C. difficile infection in the 10 U.S. sites was 15,461 in 2011 (10,177 health care–associated and 5284 community-associated cases) and 15,512 in 2017 (7973 health care–associated and 7539 community-associated cases). The estimated national burden of C. difficile infection was 476,400 cases (95% confidence interval [CI], 419,900 to 532,900) in 2011 and 462,100 cases (95% CI, 428,600 to 495,600) in 2017. With accounting for NAAT use, the adjusted estimate of the total burden of C. difficile infection decreased by 24% (95% CI, 6 to 36) from 2011 through 2017; the adjusted estimate of the national burden of health care–associated C. difficile infection decreased by 36% (95% CI, 24 to 54), whereas the adjusted estimate of the national burden of community-associated C. difficile infection was unchanged. The adjusted estimate of the burden of hospitalizations for C. difficile infection decreased by 24% (95% CI, 0 to 48), whereas the adjusted estimates of the burden of first recurrences and in-hospital deaths did not change significantly.
CONCLUSIONS
The estimated national burden of C. difficile infection and associated hospitalizations decreased from 2011 through 2017, owing to a decline in health care–associated infections. (Funded by the Centers for Disease Control and Prevention.)
The incidence of Clostridioides difficile (formerly Clostridium difficile) infection and associated hospitalizations increased in the 2000s,1,2 largely because of the emergence of the epidemic strain ribotype 027.3,4 The introduction of more sensitive C. difficile assays in the late 2000s, such as nucleic acid amplification tests (NAATs), probably contributed to the rising incidence of C. difficile infection.5,6 Concerns have been increasing about the potential overdiagnosis of C. difficile infection by NAAT, since this test detects the gene encoding the toxin rather than the actual toxin; these concerns have called into question the appropriate approach for diagnosis of C. difficile infection. Although in the United States there is currently no consensus on the best laboratory test for C. difficile infection, whether an institution or laboratory has prespecified criteria for C. difficile testing can help guide the choice of testing method.7 The higher sensitivity of NAATs as compared with other assays should be taken into account when estimating the burden of and trends in C. difficile infection.
Although traditionally considered an infection affecting patients in health care facilities, C. difficile infection is increasingly identified in the community among persons with no recent hospitalizations or stays in a long-term care facility.8,9 To monitor the changing epidemiology of C. difficile infection and generate national estimates of disease burden, the Centers for Disease Control and Prevention (CDC) Emerging Infections Program (EIP) has been conducting population-based surveillance of C. difficile infection in 10 U.S. sites since 2011.10 Using EIP data, we previously estimated that 453,000 episodes of C. difficile infection (95% confidence interval [CI], 397,100 to 508,500) occurred in the United States in 2011.11 Those data were based on a 2011 rate of NAAT use of 52%, which we have since updated to 55%.
Prevention of C. difficile infection has long been a national priority,12 as efforts to improve infection prevention and antibiotic stewardship continue to expand across the health care spectrum. To assess national progress in reducing C. difficile infection, we used EIP data to determine trends in the national estimates of the burden and incidence of C. difficile infection and associated outcomes from 2011 through 2017.
METHODS
SURVEILLANCE POPULATION
EIP surveillance of C. difficile infection involves 35 counties in 10 states (California, Colorado, Connecticut, Georgia, Maryland, Minnesota, New Mexico, New York, Oregon, and Tennessee), with a surveillance population of more than 12 million persons in 2017. A total of 34 of these counties have participated in surveillance of C. difficile infection since 2011. The surveillance protocol underwent ethical review by the CDC and all EIP sites and either was deemed to be a nonresearch activity or received institutional review board approval with a waiver of informed consent.
We defined an incident case as a positive result on a C. difficile toxin or molecular assay of a stool specimen obtained from a person at least 1 year of age with no positive test in the previous 8 weeks. This definition did not require the presence of diarrhea. Laboratories that serve the surveillance areas reported positive C. difficile tests to EIP staff and were audited at least annually for case ascertainment.
DATA COLLECTION
Data-collection methods have been described previously.10,13 EIP staff performed chart reviews on all cases at eight EIP sites and on a random sample of cases at two EIP sites (Colorado and Georgia) to collect selected data, including hospitalization on the day of or in the 6 calendar days after diagnosis of C. difficile infection. Cases were initially classified as having had onset in the community or onset associated with a health care facility (positive stool collected >3 days after hospital admission or from a resident of a long-term care facility) (see the Supplementary Appendix, available with the full text of this article at NEJM.org). All community-onset cases and a 10% random sample of cases that had an onset associated with a health care facility underwent subsequent chart review for additional data, including first recurrent episode (a positive stool specimen within 2 to 8 weeks after the last positive test) and in-hospital death. Community-onset cases were further classified as community-associated if there was no documented admission to a health care facility in the preceding 12 weeks; all other community-onset cases, along with cases that had an onset associated with a health care facility, were classified as health care–associated. We surveyed laboratories annually regarding their C. difficile testing method to determine the percentage of cases diagnosed by NAAT (used alone or in a multistep algorithm in which NAAT was almost always the last test performed).
ISOLATE CHARACTERIZATION
A convenience sample of stool specimens (see the Supplementary Appendix) was cultured for C. difficile at either the Edward Hines, Jr. Veterans Affairs Hospital or the Minnesota Department of Health Public Health Laboratory. Recovered isolates underwent strain typing at the CDC, with the use of capillary-based polymerase-chain-reaction ribotyping starting in 201214; results were analyzed against a library of standard profiles with the use of BioNumerics software (Applied Maths). C. difficile isolates that were obtained in 2011 underwent a different typing method (pulsed-field gel electrophoresis) and have been described previously11; therefore, we focused primarily on the isolates from 2012 through 2017.
STATISTICAL ANALYSIS
In cases of C. difficile infection in which the patient’s race was unknown (18.7%) or the epidemiologic class was unknown (0.9%), we performed multiple imputation separately for each site and year on the basis of the distribution of known values stratified according to age and sex. At the two EIP sites where sampling was conducted, we estimated nonsampled cases that were aggregated according to age, sex, race, and epidemiologic class through domain analysis. We used the estimated cases to build two random-intercept models with negative binomial distribution to account for site clustering and overdispersion, one for community-associated cases and another for health care–associated cases, adjusting for age, sex, race, and the percentage of cases diagnosed by means of NAAT. Because NAAT is more sensitive than other test types and its use increased from 2011 through 2017, to appropriately compare burden estimates over time, we used the NAAT coefficients from the models to arbitrarily fix NAAT use at the 2011 rate of 55% (as if NAAT use had remained constant since 2011) in order to perform trend analyses.
For cases that did not undergo additional chart review, we performed another domain analysis to estimate the following outcomes, stratified according to the patient’s age, sex, and race: first recurrence of C. difficile infection, hospitalization, and in-hospital death. We used cases with complete data to build a separate community-associated and health care–associated logistic-regression model for each of these outcomes, adjusting for age, sex, race, and diagnostic method (NAAT vs. other test types), and used the NAAT coefficients from these models to adjust NAAT use to 55% for each year to perform trend analyses.
The calculation of sampling weights for generating national estimates of the burden of C. difficile infection and associated outcomes and for performing trend analyses is described in the Supplementary Appendix. We also performed a sensitivity analysis to estimate the 2017 national burden of C. difficile infection according to different levels of NAAT use.
RESULTS
BURDEN AND INCIDENCE OF C. DIFFICILE INFECTION
The percentage of EIP cases that were diagnosed by means of NAAT increased from 55% in 2011 to 84% in 2016, then decreased to 83% in 2017 (Fig. 1). The number of reported EIP cases was 15,461 in 2011 (10,177 health care–associated and 5284 community-associated cases) and 15,512 in 2017 (7973 health care–associated and 7539 community-associated cases) (Table 1).
Figure 1. Percentage of Cases of Clostridioides difficile Infection (CDI) Diagnosed by Means of NAAT at 10 U.S. Emerging Infections Program Sites, 2011–2017.
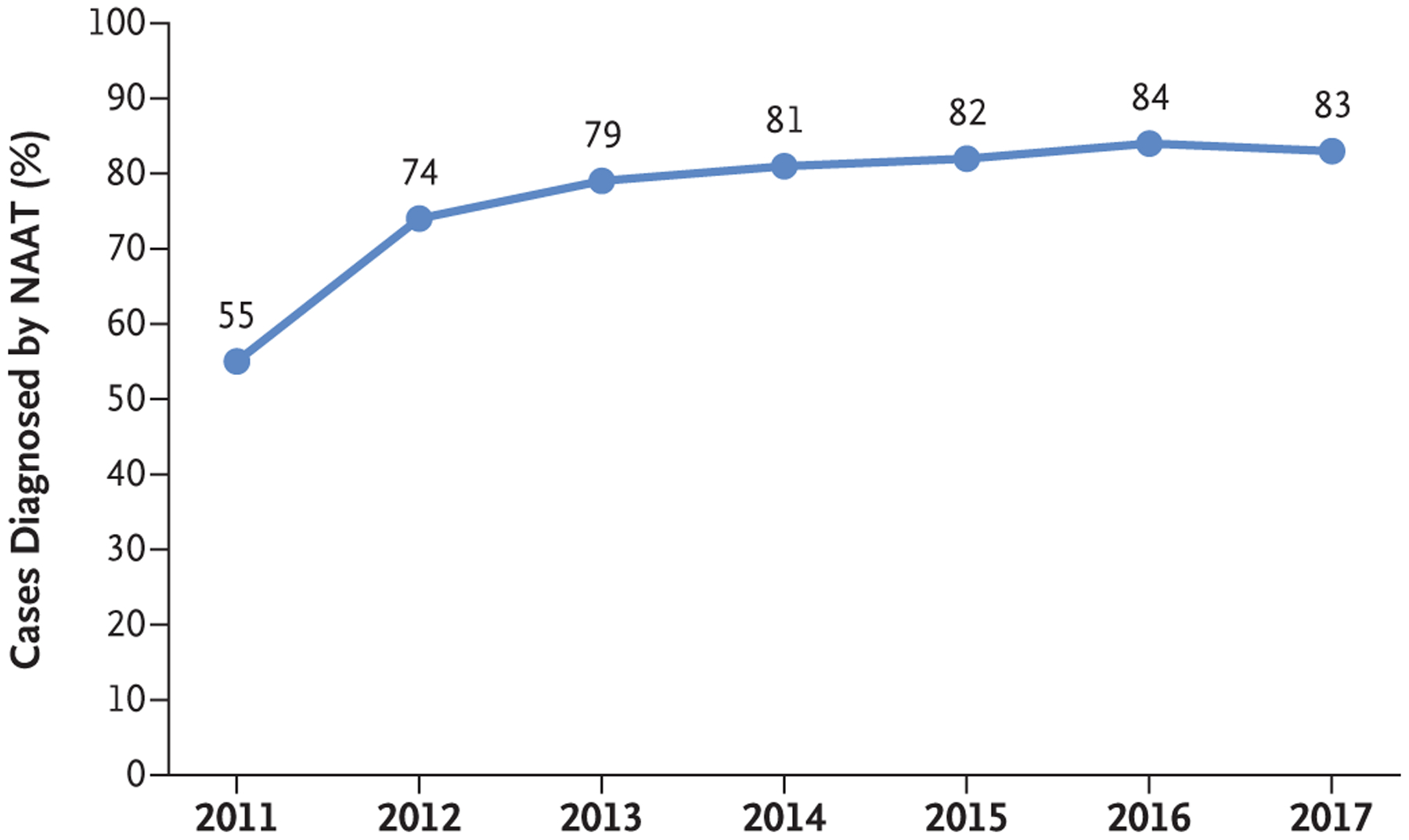
Diagnosis of CDI by nucleic acid amplification test (NAAT) was defined as a positive C. difficile stool test conducted with the use of NAAT alone or with a multistep testing algorithm in which NAAT was almost always the last test performed. The non-NAAT methods that were used by participating laboratories consisted primarily of an enzyme immunoassay for toxins, used either alone or in combination with an enzyme immunoassay for glutamate dehydrogenase. Less than 1% of participating laboratories used other methods, such as the cell-culture cytotoxicity neutralization assay.
Table 1.
Reported Cases of Clostridioides difficile Infection (CDI) and Crude Incidence, According to Epidemiologic Class, at 10 U.S. Emerging Infections Program Sites, 2011–2017.*
| Surveillance Year | Population ≥1 Yr of Age | Community-Associated CDI | Health Care-Associated CDI | All CDI | |||
|---|---|---|---|---|---|---|---|
| No. of Cases | Incidence per 100,000 Persons | No. of Cases | Incidence per 100,000 Persons | No. of Cases | Incidence per 100,000 Persons | ||
| no. | |||||||
| 2011 | 10,971,319 | 5284 | 48.16 | 10,177 | 92.76 | 15,461 | 140.92 |
| 2012† | 11,283,326 | 5967 | 52.88 | 10,482 | 92.90 | 16,449 | 145.78 |
| 2013 | 11,552,955 | 6441 | 55.75 | 9,938 | 86.02 | 16,379 | 141.77 |
| 2014 | 11,533,856 | 6669 | 57.82 | 9,662 | 83.77 | 16,331 | 141.59 |
| 2015 | 11,682,427 | 7697 | 65.89 | 9,655 | 82.65 | 17,352 | 148.53 |
| 2016 | 11,777,482 | 7915 | 67.20 | 8,881 | 75.41 | 16,796 | 142.61 |
| 2017 | 11,906,512 | 7539 | 63.32 | 7,973 | 66.96 | 15,512 | 130.28 |
The population for each surveillance year is based on estimates from the U.S. Census Bureau. The weighted frequency of cases in Colorado and Georgia was based on 33% random sampling for cases in persons 18 years of age or older.
Data presented in the table exclude cases from Olmsted County, Minnesota, where CDI surveillance began midyear. The total number of reported CDI cases in 2012 would be 16,564 if CDI cases from Olmsted County, Minnesota, were included.
Without adjustment for NAAT use, the estimated total national burden of C. difficile infection was 476,400 cases (95% CI, 419,900 to 532,900) in 2011, with an estimated incidence of 154.9 (95% CI, 136.5 to 173.3) per 100,000 population, and 462,100 cases (95% CI, 428,600 to 495,600) in 2017, with an estimated incidence of 143.6 (95% CI, 133.2 to 154.0) per 100,000 population (Fig. 2). The estimated national burden of health care–associated C. difficile infection was 306,500 cases (95% CI, 269,000 to 343,900) in 2011, with an estimated incidence of 99.6 (95% CI, 87.5 to 111.8) per 100,000 population, and 235,700 cases (95% CI, 221,700 to 249,700) in 2017, with an estimated incidence of 73.3 (95% CI, 68.9 to 77.6) per 100,000 population. The estimated national burden of community-associated C. difficile infection was 170,000 cases (95% CI, 150,900 to 189,000) in 2011, with an estimated incidence of 55.3 (95% CI, 49.1 to 61.4) per 100,000 population, and 226,400 cases (95% CI, 206,900 to 245,900) in 2017, with an estimated incidence of 70.4 (95% CI, 64.3 to 76.4) per 100,000 population.
Figure 2 (facing page). U.S. National Estimates of the Burden and Incidence of Community-Associated CDI, Health Care–Associated CDI, and Total CDI, 2011–2017.
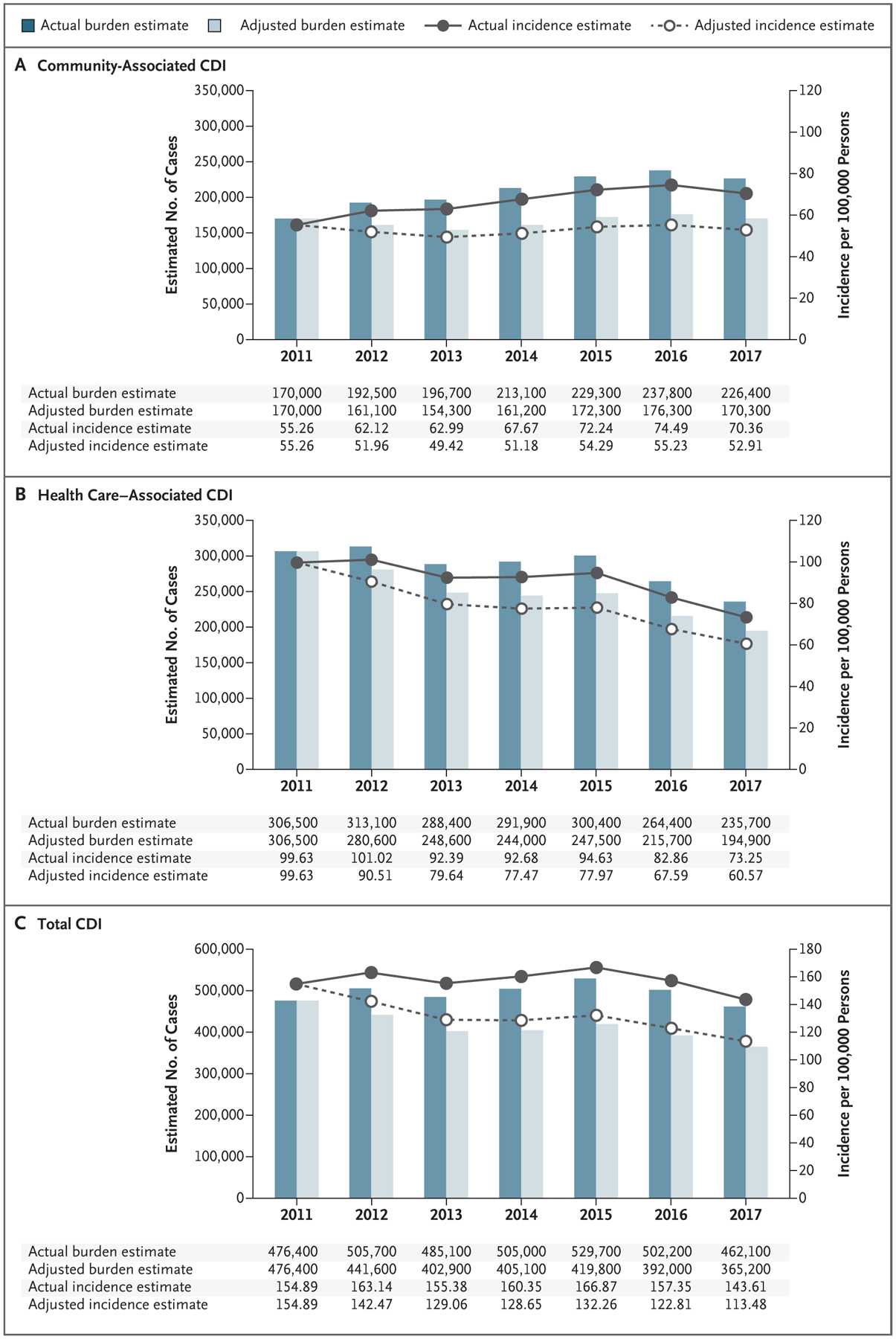
The estimates of the actual national burden and incidence for each year are based on the actual rate of use of the nucleic acid amplification test (NAAT) in that year, with adjustment for the age, sex, and race of the U.S. population. The estimates of the adjusted national burden and incidence for each year are based on an adjustment of NAAT use to the 2011 rate of 55%, with additional adjustment for the age, sex, and race of the U.S. population. The estimates of the national burden of community-associated CDI and health care–associated CDI do not always add up to the estimated total burden of CDI because of rounding.
After adjustment of NAAT use to the 2011 rate of 55%, the estimated burden of C. difficile infection decreased from 2012 through 2017 (Fig. 2). Trend analyses showed the adjusted estimate of the total national burden of C. difficile infection changed annually by −4% (95% CI, −1 to −6), which resulted in a decrease of 24% (95% CI, 6 to 36) from 2011 through 2017. The adjusted estimate of the national burden of health care–associated C. difficile infection changed annually by −6% (95% CI, −4 to −9), which resulted in a decrease of 36% (95% CI, 24 to 54) from 2011 through 2017, whereas there was no change in the adjusted estimate of the national burden of community-associated C. difficile infection (0%; 95% CI, −2 to 3). In the sensitivity analysis, the estimated national burden of C. difficile infection in 2017 ranged from 244,600 cases (95% CI, 227,300 to 261,800) if no laboratories used NAAT to 508,900 cases (95% CI, 472,600 to 545,200) if all laboratories used NAAT (Fig. S1 in the Supplementary Appendix).
C. DIFFICILE RECURRENCES, HOSPITALIZATIONS, AND IN-HOSPITAL DEATHS
The frequency of first recurrence of C. difficile infection, hospitalizations for C. difficile infection (with onset of infection in either the community or the hospital), and in-hospital deaths at the 10 EIP sites fluctuated from 2011 through 2017 and was higher among health care–associated infections than among community-associated infections (Table 2). A similar pattern was observed with the estimates of the national incidence and burden of first recurrence of C. difficile infection, hospitalizations, and in-hospital deaths (Table 3). When the rate of NAAT use was held at 55%, there were no significant changes in the adjusted estimates of the national burden of first recurrences and in-hospital deaths. In contrast, the adjusted estimate of the total national burden of hospitalizations for C. difficile infection changed annually by −4% (95% CI, −8 to 0), which resulted in a decrease of 24% (95% CI, 0 to 48) from 2011 through 2017. The adjusted estimate of the national burden of hospitalizations among health care–associated infections changed annually by −5% (95% CI, −1 to −9), but there was no significant change among community-associated infections.
Table 2.
Estimates of First Recurrences, Hospitalizations, and In-Hospital Deaths Associated with CDI, According to Epidemiologic Class, at 10 U.S. Emerging Infections Program Sites, 2011–2017.*
| Surveillance Year | Estimated First Recurrences | Estimated Hospitalizations | Estimated In-Hospital Deaths | |||
|---|---|---|---|---|---|---|
| Community-Associated CDI | Health Care-Associated CDI | Community-Associated CDI | Health Care-Associated CDI | Community-Associated CDI | Health Care-Associated CDI | |
| 2011 | 678 | 1959 | 1691 | 6145 | 159 | 889 |
| 2012 | 703 | 1848 | 2130 | 6527 | 161 | 571 |
| 2013 | 879 | 1665 | 2259 | 6398 | 202 | 581 |
| 2014 | 843 | 1690 | 2320 | 6537 | 142 | 670 |
| 2015 | 1066 | 1740 | 2633 | 6643 | 198 | 812 |
| 2016 | 1092 | 1380 | 2740 | 6024 | 190 | 750 |
| 2017 | 967 | 1221 | 2505 | 5483 | 164 | 573 |
The frequency of cases was weighted to account for sampling in two of the Emerging Infections Program sites (Colorado and Georgia) and sampling of health care–associated CDI cases. Recurrence refers to the first recurrent episode, defined as a positive stool specimen within 2 to 8 weeks after the initial positive test. Hospitalization includes admission on the day of or in the 6 calendar days after diagnosis of CDI. In-hospital deaths refer to deaths that occurred during hospitalization.
Table 3.
U.S. National Estimates of First Recurrences, Hospitalizations, and In-Hospital Deaths Associated with CDI, According to Epidemiologic Class, 2011–2017.*
| Variable | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|---|---|
| Estimated first recurrences | |||||||
| Community-associated CDI | |||||||
| No. (95% CI) | 24,100 (19,000–29,300) | 23,400 (19,500–27,400) | 28,800 (24,900–32,800) | 29,300 (24,100–34,500) | 35,300 (29,700–40,900) | 33,600 (29,000–38,300) | 31,300 (26,600–36,000) |
| Incidence per 100,000 persons (95% CI) | 7.9 (6.2–9.5) | 7.6 (6.3–8.8) | 9.2 (8.0–10.5) | 9.3 (7.7–11.0) | 11.1 (9.4–12.9) | 10.5 (9.1–12.0) | 9.7 (8.3–11.2) |
| Health care-associated CDI | |||||||
| No. (95% CI) | 60,500 (50,100–70,800) | 53,900 (44,100–63,700) | 48,500 (41,800–55,100) | 55,600 (46,800–64,400) | 58,100 (51,300–64,900) | 43,500 (37,500–49,400) | 38,500 (32,100–44,800) |
| Incidence per 100,000 persons (95% CI) | 19.7 (16.3–23.0) | 17.4 (14.2–20.6) | 15.5 (13.4–17.7) | 17.7 (14.9–20.5) | 18.3 (16.2–20.5) | 13.6 (11.8–15.5) | 12.0 (10.0–13.9) |
| Estimated hospitalizations | |||||||
| Community-associated CDI | |||||||
| No. (95% CI) | 52,500 (43,800–61,100) | 61,800 (54,200–69,500) | 66,000 (58,900–73,200) | 71,600 (61,200–81,900) | 76,300 (68,200–84,300) | 79,100 (68,000–90,300) | 69,900 (61,100–78,600) |
| Incidence per 100,000 persons (95% CI) | 17.1 (14.3–19.9) | 20.0 (17.5–22.4) | 21.2 (18.9–23.5) | 22.7 (19.4–26.0) | 24.0 (21.5–26.6) | 24.8 (21.3–28.3) | 21.7 (19.0–24.4) |
| Health care-associated CDI | |||||||
| No. (95% CI) | 186,600 (155,500–217,600) | 189,600 (165,700–213,500) | 178,400 (158,300–198,500) | 190,200 (171,400–209,000) | 196,000 (179,700–212,300) | 173,100 (158,900–187,200) | 154,100 (140,700–167,400) |
| Incidence per 100,000 persons (95% CI) | 60.7 (50.6–70.8) | 61.2 (53.5–68.9) | 57.2 (50.7–63.6) | 60.4 (54.4–66.4) | 61.8 (56.6–66.9) | 54.2 (49.8–58.7) | 47.9 (43.7–52.0) |
| Estimated in-hospital deaths | |||||||
| Community-associated CDI | |||||||
| No. (95% CI) | 5000 (3200–6700) | 5100 (3000–7100) | 5700 (3600–7700) | 5800 (3800–7900) | 5800 (3900–7700) | 7200 (3400–11,000) | 4300 (2300–6300) |
| Incidence per 100,000 persons (95% CI) | 1.6 (1.0–2.2) | 1.6 (1.0–2.3) | 1.8 (1.2–2.5) | 1.9 (1.2–2.5) | 1.8 (1.2–2.4) | 2.3 (1.1–3.4) | 1.3 (0.7–2.0) |
| Health care-associated CDI | |||||||
| No. (95% CI) | 25,600 (18,200–33,000) | 15,300 (11,000–19,700) | 16,700 (12,400–21,100) | 19,000 (14,500–23,400) | 23,200 (19,800–26,600) | 19,500 (15,800–23,200) | 16,200 (13,300–19,200) |
| Incidence per 100,000 persons (95% CI) | 8.3 (5.9–10.7) | 5.0 (3.6–6.4) | 5.4 (4.0–6.8) | 6.0 (4.6–7.5) | 7.3 (6.2–8.4) | 6.1 (5.0–7.3) | 5.0 (4.1–6.0) |
Recurrence refers to the first recurrent episode, defined as a positive stool specimen within 2 to 8 weeks after the initial positive test. Hospitalization includes admission on the day of or in the 6 calendar days after diagnosis of CDI. In-hospital deaths refer to deaths that occurred during hospitalization.
ISOLATE CHARACTERIZATION
From 2012 through 2017, a total of 969 to 1443 C. difficile isolates were submitted annually to the CDC (Table S1), consisting of 275 distinct community-associated and 227 distinct health care-associated strain types. Ribotypes 027, 106, 014, 002, and 020 were the most common community-associated and health care–associated ribotypes identified each year, except in 2017, when ribotype 020 was replaced by 076 as one of the top health care–associated ribotypes. From 2012 through 2017, ribotype 027 decreased significantly among health care–associated isolates (21% vs. 15%; P = 0.02) and community-associated isolates (17% vs. 6%; P<0.001). Including the 2011 isolates,10 there was an even greater decline in the prevalence of the North American Pulsed Field 1 (NAP1) strain (which represents mostly ribotype 27) or of ribotype 027 among health care–associated isolates (31% in 2011 vs. 15% in 2017; P<0.001) and among community-associated isolates (19% in 2011 vs. 6% in 2017; P<0.001).
Because ribotype 027 is more likely than other strains to be detected by enzyme immunoassays for toxins in stool because of its increased toxin production15 and because the decreased use of enzyme immunoassays for toxins might affect its prevalence, we analyzed data from laboratories that used NAAT and found a similar decreasing trend of ribotype 027 during this period. Despite its decline, ribotype 027 remained the predominant health care–associated ribotype during this period, whereas ribotype 106 has been the most prevalent community-associated ribotype since 2014.
DISCUSSION
The estimated burden of C. difficile infection in the United States declined from 2011 through 2017, despite the increasing use of the more sensitive diagnostic test of NAAT. When NAAT use was held constant over this period, we observed a significant decrease in the adjusted estimate of the national burden of C. difficile infection, which supports a true decline in C. difficile infection. This decrease was driven by the decline in health care–associated infections, which decreased annually by an adjusted 6%, whereas there was no change in the adjusted estimate of the burden of community-associated infections. In contrast, a 2015 U.S. acute care point-prevalence survey indicated that the prevalence of health care–associated C. difficile infection had not changed as compared with 2011; however, the survey did not account for increased NAAT use, which could have masked a true reduction in C. difficile infection.16
The decrease in health care–associated C. difficile infection probably reflects a decline in both cases with an onset associated with a long-term care facility and hospital-onset cases. We previously observed a 55% decrease in the adjusted incidence of C. difficile infection with an onset associated with a long-term care facility across EIP sites from 2011 through 2015.17 According to the CDC National Healthcare Safety Network, the C. difficile standardized infection ratio, which is a risk-adjusted measure of hospital-onset C. difficile infection, declined by 20% from 2015 through 2017.18 Several factors probably contributed to the decrease in health care–associated C. difficile infection, including a decline in ribotype 027, which might be partly driven by reduced fluoroquinolone use in U.S. hospitals.17,19 The association between fluoroquinolones and C. difficile infection is well described,3,4,20 and in one study, hospitals that achieved a 30% reduction in fluoroquinolone use observed a 19% reduction in hospital-onset C. difficile infection.21 In England, the restriction of fluoroquinolone prescribing probably contributed to the drastic reduction of C. difficile infection through a deselection of fluoroquinolone-resistant strains, including ribotype 027.22 Adherence to recommended infection-prevention practices may have also decreased health care–associated infections, as shown by several successful local and regional initiatives for the prevention of C. difficile infection.23,24 Public reporting of C. difficile infection and pay-for-performance programs probably increased prevention efforts. With the rise in NAAT use and the potential for overdiagnosis of C. difficile infection, there has been a greater emphasis on diagnostic stewardship to counter the effects of the increased sensitivity of NAAT by reducing inappropriate testing,25 which may have also reduced health care–associated infections.
In contrast, estimates of community-associated C. difficile infection did not change after accounting for NAAT use. Several factors might have contributed to the high burden of community-associated C. difficile infection, such as changes in testing practices. This may include increased frequency of C. difficile testing owing to increased awareness among outpatient providers. Diagnostic stewardship efforts have primarily targeted inpatient settings, and little is known regarding the extent to which overtesting might be occurring in outpatient settings. High use of outpatient antibiotics is another contributing factor. In studies of community-associated C. difficile infection, approximately 60% of patients had recent antibiotic use.8,26 Although U.S. outpatient antibiotic prescription rates decreased from 2011 to 2016,27 at least 30% of outpatient antibiotic prescriptions are estimated to be unnecessary,28 which highlights the need to improve outpatient prescribing.
Other contributing factors might be related to community exposures, given that 18% of patients with community-associated C. difficile infection had no recent outpatient health care exposures.8,26 C. difficile transmission among households and between humans and domestic and farm animals has been reported,29,30 and although a direct foodborne link has not been established, toxigenic C. difficile has been cultured from retail meat and vegetables.31,32 A distinct pattern of genetic relatedness between some C. difficile isolates has been observed in Europe that does not show the country, regional, or within-hospital clustering that would suggest local person-to-person transmission but instead seems to suggest dissemination through other routes, such as the food chain.33
We observed a decrease in the adjusted estimate of the burden of hospitalizations among health care–associated C. difficile infections, which resulted in a 24% decrease in the total adjusted burden of all hospitalizations for C. difficile infection, as included in the CDC’s Antibiotic Resistance Threats in the United States, 2019.34 This is not surprising, given that health care–associated C. difficile infection has declined, thereby reducing the burden of hospitalizations. The adjusted estimate of the burden of in-hospital deaths did not change; however, we did not assess for attributable mortality. The adjusted estimate of the burden of first recurrences also did not change, which underscores the need to improve the identification of patients at high risk for recurrence to help target prevention strategies.
Although community-associated strains were more diverse than health care–associated strains, the most common ribotypes were the same in both groups and have been frequently observed in Europe.33 In a finding consistent with data from the U.S. Veterans Health Administration and from other countries,35,36 ribotype 027 decreased significantly from 2011 through 2017, even among isolates from laboratories that used NAAT, which indicates that the decrease is not likely to be due to decreased use of enzyme immunoassays for toxins. Although ribotype 027 could have declined naturally, the widespread decrease in fluoroquinolone use was probably a factor.17,19 Other strains that are associated with severe disease, such as ribotypes 078 and 244,37,38 have rarely been identified among U.S. clinical isolates.
This analysis has several limitations. The case definition was based on a laboratory diagnosis, and it is possible that some identified cases were only colonized with C. difficile. We attempted to identify every case occurring in catchment-area residents, but it is possible that some cases were missed (e.g., in residents hospitalized outside the catchment area). Additional limitations are discussed in the Supplementary Appendix.
The estimated burden of C. difficile infection in the United States decreased by an adjusted 24% from 2011 through 2017, owing to a decline in health care–associated infections. Community-associated infections did not decrease and contributed to nearly 50% of the burden of C. difficile infection in 2017. Continued efforts are needed to improve infection prevention and diagnostic and antibiotic stewardship in both inpatient and outpatient settings. The CDC Targeted Assessment for Prevention Strategy helps facilities target wards with the highest rates of C. difficile infection to improve infection-prevention practices.39 The CDC Core Elements of Antibiotic Stewardship provide a framework to improve antibiotic use across the health care spectrum.40 Additional efforts include incentivizing facilities to work collaboratively across a region and improving our understanding of the role of non-health care reservoirs in C. difficile transmission. The development of a C. difficile vaccine and exploration of innovative strategies, such as gut microbiome restoration for primary prevention of C. difficile infection, might also further reduce the burden of C. difficile infection.
Supplementary Material
Acknowledgments
Supported by the Centers for Disease Control and Prevention. Dr. Dumyati reports receiving fees for serving on a drug safety monitoring board from Seres Therapeutics; Dr. Kainer, receiving fees for serving on a board and travel support from Infectious Disease Consulting, fees for the production of CME materials and travel support from WebMD, and consulting fees and travel support from Pfizer; and Dr. Gerding, receiving advisory board fees from Ferring Pharmaceuticals, Rebiotix, Merck, Da Volterra, Summit Therapeutics, and Actelion, receiving consulting fees from Pfizer, Sanofi Pasteur, MGB Biopharma, and Medpace, and holding patents (US No. 6,635,260, CAN No. 2,232,001, and EU No. 0952773) on methods and compositions for the prevention and treatment of Clostridium difficile-associated diseases. No other potential conflict of interest relevant to this article was reported.
We thank the following persons for their contribution to the Emerging Infections Program C. difficile infection surveillance activity: Fernanda Lessa, Jessica Cohen, and Jamie Barnes from the Centers for Disease Control and Prevention; Erin Parker, Joelle Nadle, and Karen Click from the California Emerging Infections Program; James Hadler from the Connecticut Emerging Infections Program; Wendy Baughman, Leigh Ann Clark, Olivia Almendares, Zirka Smith, and Andrew Revis from the Georgia Emerging Infections Program and the Atlanta Research and Education Foundation; Ruth Lynfield from the Minnesota Department of Health; Nicole Kenslow and Kristina Flores from the University of New Mexico and the New Mexico Emerging Infections Program; Corinne Davis and Rose Devasia from the Tennessee Department of Health; and Susan Sambol and Laurica Petrella-Zitco from the Edward Hines, Jr. Veterans Affairs Hospital.
Appendix
The authors’ full names and academic degrees are as follows: Alice Y. Guh, M.D., M.P.H., Yi Mu, Ph.D., Lisa G. Winston, M.D., Helen Johnston, M.P.H., Danyel Olson, M.S., M.P.H., Monica M. Farley, M.D., Lucy E. Wilson, M.D., Stacy M. Holzbauer, D.V.M., M.P.H., Erin C. Phipps, D.V.M., M.P.H., Ghinwa K. Dumyati, M.D., Zintars G. Beldavs, M.S., Marion A. Kainer, M.B., B.S., M.P.H., Maria Karlsson, Ph.D., Dale N. Gerding, M.D., and L. Clifford McDonald, M.D.
The authors’ affiliations are as follows: the Division of Healthcare Quality Promotion (A.Y.G., Y.M., M.K., L.C.M.) and the Career Epidemiology Field Officer Program (S.M.H.), Centers for Disease Control and Prevention, Emory University School of Medicine (M.M.F.), and the Veterans Affairs Medical Center (M.M.F.) — all in Atlanta; the University of California, San Francisco, School of Medicine, San Francisco (L.G.W.); the Colorado Department of Public Health and Environment, Denver (H.J.); the Connecticut Emerging Infections Program, Yale School of Public Health, New Haven (D.O.); the University of Maryland Baltimore County and the Maryland Department of Health, Baltimore (L.E.W.); the Minnesota Department of Health, St. Paul (S.M.H.); the University of New Mexico, New Mexico Emerging Infections Program, Albuquerque (E.C.P.); the New York Emerging Infections Program and University of Rochester Medical Center, Rochester (G.K.D.); the Oregon Health Authority, Portland (Z.G.B.); the Tennessee Department of Health, Nashville (M.A.K.); and Stritch School of Medicine, Loyola University Chicago, Maywood, and the Edward Hines, Jr. Veterans Affairs Hospital, Hines — both in Illinois (D.N.G.).
Footnotes
The authors’ full names, academic degrees, and affiliations are listed in the Appendix.
The members of the Emerging Infections Program Clostridioides difficile Infection Working Group are listed in the Supplementary Appendix, available at NEJM.org.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Elixhauser A, Jhung MA. Clostridium difficile-associated disease in U.S. hospitals, 1993–2005: Healthcare Cost and Utilization Project statistical brief #50. Rockville, MD: Agency for Healthcare research and Quality, April 2008. (http://www.hcup-us.ahrq.gov/reports/statbriefs/sb50.pdf). [Google Scholar]
- 2.Layton BA, McDonald LC, Gerding DN, Liedtke LA, Strausbaugh LJ. Perceived increases in the incidence and severity of Clostridium difficile disease: an emerging threat that continues to unfold. In: Proceedings of 15th Annual Scientific Meeting of the Society for Healthcare Epidemiology of America, Los Angeles, April 9–12, 2005 abstract. [Google Scholar]
- 3.Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile–associated diarrhea with high morbidity and mortality. N Engl J Med 2005; 353: 2442–9. [DOI] [PubMed] [Google Scholar]
- 4.McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med 2005; 353: 2433–41. [DOI] [PubMed] [Google Scholar]
- 5.Gould CV, Edwards JR, Cohen J, et al. Effect of nucleic acid amplification testing on population-based incidence rates of Clostridium difficile infection. Clin Infect Dis 2013; 57: 1304–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moehring RW, Lofgren ET, Anderson DJ. Impact of change to molecular testing for Clostridium difficile infection on healthcare facility-associated incidence rates. Infect Control Hosp Epidemiol 2013; 34: 1055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66(7): e1–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chitnis AS, Holzbauer SM, Belflower RM, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med 2013; 173: 1359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerding DN, Lessa FC. The epidemiology of Clostridium difficile infection inside and outside health care institutions. Infect Dis Clin North Am 2015; 29: 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clostridioides difficile infection (CDI) tracking. Atlanta: Centers for Disease Control and Prevention, July 2019. (https://www.cdc.gov/hai/eip/cdiff-tracking.html). [Google Scholar]
- 11.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372: 825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Department of Health and Human Services. National action plan to prevent health care-associated infections. October 2016. (https://health.gov/hcq/prevent-hai-action-plan.asp#actionplan_development).
- 13.Lessa FC, Mu Y, Winston LG, et al. Determinants of Clostridium difficile infection incidence across diverse United States geographic locations. Open Forum Infect Dis 2014; 1(2): ofu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fawley WN, Knetsch CW, MacCannell DR, et al. Development and validation of an internationally-standardized, high-resolution capillary gel-based electrophoresis PCR-ribotyping protocol for Clostridium difficile. PLoS One 2015; 10(2): e0118150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tenover FC, Novak-Weekley S, Woods CW, et al. Impact of strain type on detection of toxigenic Clostridium difficile: comparison of molecular diagnostic and enzyme immunoassay approaches. J Clin Microbiol 2010; 48: 3719 high-resolution 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magill SS, O’Leary E, Janelle SJ, et al. Changes in prevalence of health care-associated infections in U.S. hospitals. N Engl J Med 2018; 379: 1732–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guh AY, Mu Y, Baggs J, et al. Trends in incidence of long-term-care facility onset Clostridium difficile infections in 10 US geographic locations during 2011–2015. Am J Infect Control 2018; 46: 840–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. 2017. National and state healthcare-associated infections progress report. March 2019 (https://www.cdc.gov/hai/data/archive/2017-HAI-progress-report.html).
- 19.Baggs J, Fridkin SK, Pollack LA, Srinivasan A, Jernigan JA. Estimating national trends in inpatient antibiotic use among US hospitals from 2006 to 2012. JAMA Intern Med 2016; 176: 1639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pépin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis 2005; 41: 1254–60. [DOI] [PubMed] [Google Scholar]
- 21.Kazakova SV, Baggs J, McDonald LC, et al. Association between antibiotic use and hospital-onset Clostridioides difficile infection in US acute care hospitals, 2006–2012: an ecologic analysis. Clin Infect Dis 2020; 70: 11–8. [DOI] [PubMed] [Google Scholar]
- 22.Dingle KE, Didelot X, Quan TP, et al. Effects of control interventions on Clostridium difficile infection in England: an observational study. Lancet Infect Dis 2017; 17: 411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koll BS, Ruiz RE, Calfee DP, et al. Prevention of hospital-onset Clostridium difficile infection in the New York metropolitan region using a collaborative intervention model. J Healthc Qual 2014; 36: 35–45. [DOI] [PubMed] [Google Scholar]
- 24.Rizzo KR, Yi SH, Garcia EP, Zahn M, Epson E. Reduction in Clostridium difficile infection rates following a multifacility prevention initiative in Orange County, California: a controlled interrupted time series evaluation. Infect Control Hosp Epidemiol 2019; 40: 872–9. [DOI] [PubMed] [Google Scholar]
- 25.Yen C, Holtom P, Butler-Wu SM, Wald-Dickler N, Shulman I, Spellberg B. Reducing Clostridium difficile colitis rates via cost-saving diagnostic stewardship. Infect Control Hosp Epidemiol 2018; 39: 734–6. [DOI] [PubMed] [Google Scholar]
- 26.Guh AY, Adkins SH, Li Q, et al. Risk factors for community-associated Clostridium difficile infection in adults: a case-control study. Open Forum Infect Dis 2017; 4(4): ofx171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King LM, Bartoces M, Fleming-Dutra KE, Roberts RM, Hicks LA. Changes in US outpatient antibiotic prescriptions from 2011–2016. Clin Infect Dis 2020; 70: 370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016; 315: 1864–73. [DOI] [PubMed] [Google Scholar]
- 29.Loo VG, Brassard P, Miller MA. House-hold transmission of Clostridium difficile to family members and domestic pets. Infect Control Hosp Epidemiol 2016; 37: 1342–8. [DOI] [PubMed] [Google Scholar]
- 30.Knetsch CW, Connor TR, Mutreja A, et al. Whole genome sequencing reveals potential spread of Clostridium difficile between humans and farm animals in the Netherlands, 2002 to 2011. Euro Surveill 2014; 19: 20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Songer JG, Trinh HT, Killgore GE, Thompson AD, McDonald LC, Limbago BM. Clostridium difficile in retail meat products, USA, 2007. Emerg Infect Dis 2009; 15: 819–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez-Palacios A, Ilic S, LeJeune JT. Clostridium difficile with moxifloxacin/clindamycin resistance in vegetables in Ohio, USA, and prevalence meta-analysis. J Pathog 2014; 2014: 158601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eyre DW, Davies KA, Davis G, et al. Two distinct patterns of Clostridium difficile diversity across Europe indicating contrasting routes of spread. Clin Infect Dis 2018; 67: 1035–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States. 2019. (https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf).
- 35.Giancola SE, Williams RJ II, Gentry CA. Prevalence of the Clostridium difficile BI/NAP1/027 strain across the United States Veterans Health Administration. Clin Microbiol Infect 2018;24: 877–81. [DOI] [PubMed] [Google Scholar]
- 36.Katz KC, Golding GR, Choi KB, et al. The evolving epidemiology of Clostridium difficile infection in Canadian hospitals during a postepidemic period (2009–2015). CMAJ 2018; 190(25): E758–E765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goorhuis A, Bakker D, Corver J, et al. Emergence of Clostridium difficile infection due to a new hypervirulent strain, polymerase chain reaction ribotype 078. Clin Infect Dis 2008; 47: 1162–70. [DOI] [PubMed] [Google Scholar]
- 38.Lim SK, Stuart RL, Mackin KE, et al. Emergence of a ribotype 244 strain of Clostridium difficile associated with severe disease and related to the epidemic ribotype 027 strain. Clin Infect Dis 2014; 58: 1723–30. [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention. Healthcare-associated infections: the Targeted Assessment for Prevention (TAP) strategy. June 2019. (https://www.cdc.gov/hai/prevent/tap.html).
- 40.Centers for Disease Control and Prevention. Antibiotic prescribing and use: appropriate antibiotic use. November 2019. (https://www.cdc.gov/antibiotic-use/index.html).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


