Abstract
Background
To mitigate the COVID-19 pandemic, countries worldwide have enacted unprecedented movement restrictions, physical distancing measures, and face mask requirements. Until safe and efficacious vaccines or antiviral drugs become widely available, viral testing remains the primary mitigation measure for rapid identification and isolation of infected individuals. We aimed to assess the economic trade-offs of expanding and accelerating testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) across the USA in different transmission scenarios.
Methods
We used a multiscale model that incorporates SARS-CoV-2 transmission at the population level and daily viral load dynamics at the individual level to assess eight surveillance testing strategies that varied by testing frequency (from daily to monthly testing) and isolation period (1 or 2 weeks), compared with the status-quo strategy of symptom-based testing and isolation. For each testing strategy, we first estimated the costs (incorporating costs of diagnostic testing and admissions to hospital, and salary lost while in isolation) and years of life lost (YLLs) prevented under rapid and low transmission scenarios. We then assessed the testing strategies across a range of scenarios, each defined by effective reproduction number (Re), willingness to pay per YLL averted, and cost of a test, to estimate the probability that a particular strategy had the greatest net benefit. Additionally, for a range of transmission scenarios (Re from 1·1 to 3), we estimated a threshold test price at which the status-quo strategy outperforms all testing strategies considered.
Findings
Our modelling showed that daily testing combined with a 2-week isolation period was the most costly strategy considered, reflecting increased costs with greater test frequency and length of isolation period. Assuming a societal willingness to pay of US$100 000 per YLL averted and a price of $5 per test, the strategy most likely to be cost-effective under a rapid transmission scenario (Re of 2·2) is weekly testing followed by a 2-week isolation period subsequent to a positive test result. Under low transmission scenarios (Re of 1·2), monthly testing of the population followed by 1-week isolation rather than 2-week isolation is likely to be most cost-effective. Expanded surveillance testing is more likely to be cost-effective than the status-quo testing strategy if the price per test is less than $75 across all transmission rates considered.
Interpretation
Extensive expansion of SARS-CoV-2 testing programmes with more frequent and rapid tests across communities coupled with isolation of individuals with confirmed infection is essential for mitigating the COVID-19 pandemic. Furthermore, resources recouped from shortened isolation duration could be cost-effectively allocated to more frequent testing.
Funding
US National Institutes of Health, US Centers for Disease Control and Prevention, and Love, Tito's.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to threaten the health, economy, and stability of the world. The virus emerged from Wuhan, China, at the end of 2019 and a pandemic was declared by WHO on March 11, 2020.1 As of Jan 23, 2021, the COVID-19 burden in the USA has surpassed 24 million confirmed cases and 400 000 deaths.2 Globally, as of this same date, over 96 million cases and more than 2 million deaths have been reported.2 Moreover, the estimated economic costs exceeded US$21 trillion in 2020.3
As of December, 2020, two SARS-CoV-2 vaccines that completed phase 3 trials had been approved for broad use in the USA.4 However, even the most optimistic projections for high-income countries suggest that herd immunity acquired through infection or afforded by vaccination might not be attainable until at least the third quarter of 2021.5 Numerous SARS-CoV-2 antiviral drugs are also under assessment, which aim to reduce the severity of COVID-19 and provide prophylaxis from infection.6 Until such medical countermeasures become widely available, the world is primarily combating SARS-CoV-2 through unprecedented non-pharmacological interventions including the wearing of face masks, travel restrictions, and physical distancing measures that can have dire socioeconomic costs.7 The contribution of asymptomatic and presymptomatic cases towards transmission makes control via non-pharmacological interventions challenging.8 Therefore, symptom-based testing and isolation strategies might not be sufficient to curtail the pandemic.8 Although mass diagnostic testing, contact tracing, and isolation can substantially mitigate spread,9 only a few countries have been able to scale such programmes to the levels required to contain pandemic waves.7 For instance, in October, 2020, the Chinese port city of Qingdao took the unprecedented step of testing all 9·5 million of its residents,10 and in late October, 2020, Slovakia tested 3·62 million people in a weekend from a population of 5·4 million, representing 67% of the total population or 82% of the adult population.11
Research in context.
Evidence before this study
We searched PubMed with no language restrictions on Nov, 28, 2020, for publications since database inception focusing on the cost-effectiveness of expanding COVID-19 testing in the USA using the search terms (“Economic”)[Title/Abstract] AND (“Testing”[Title/Abstract] OR “Test”)[Title/Abstract] AND (“SARS-CoV-2”[Title/Abstract] OR “COVID-19”)[Title/Abstract] AND (“United States”[Title/Abstract] OR “US”[Title/Abstract] OR “America”[Title/Abstract] OR “U.S.”)[Title/Abstract]. We found only one article that addressed the cost-effectiveness of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing strategies in the USA, which specifically focused on use of PCR testing to bolster endoscopy practices. Five articles have estimated the impact of social distancing measures on the economic burden of COVID-19 in the USA (eg, city lockdown, contact tracing, emergency sick leave, and household quarantine). However, we did not find any articles that provide estimates for the cost-effectiveness of vastly expanding testing using the affordable 15-min SARS-CoV-2 antigen test approved by the US Food and Drug Administration on Aug 26, 2020.
Added value of this study
Using a data-driven model of SARS-CoV-2 transmission that incorporates daily viral load dynamics of infected individuals, we assessed the economic trade-offs of expanding and accelerating SARS-CoV-2 testing with surveillance testing. To our knowledge, this study is the first to identify strategies that are expected to be cost-effective, depending on the local transmission rate of the virus, the costs of SARS-CoV-2 testing and admissions to hospital and the societal willingness to pay for averting COVID-19 deaths. Given the epidemiological and economic conditions in the USA as of December, 2020, the optimal strategy depends on the level of the transmission in the community. Under rapid transmission scenarios (effective reproduction number [R e] of 2·2), weekly testing of the entire population followed by a 2-week isolation period is advised; under lower transmission rates (R e of 1·2), staggered monthly testing followed by a 1-week isolation is advised.
Implications of all the available evidence
Despite the intimidating upfront costs, mass testing with rapid surveillance tests coupled with strict but relatively short isolation of confirmed cases is recommended to health authorities and local governments as a cost-effective strategy for mitigating the unprecedented threat of the COVID-19 pandemic, before safe and efficacious vaccines can be widely administered or efficacious drugs become available.
The first and most widely used SARS-CoV-2 tests have applied RT-PCR to identify viral particles swabbed from the nose or throat of a patient.12 In the USA, restricted availability and slow laboratory turnaround times have impeded the use of testing to slow viral spread.13 However, as the pandemic continues, cheaper and faster testing technologies are becoming increasingly common.13 In August, 2020, the US Food and Drug Administration (FDA) approved both a point-of-care saliva-based PCR test (SalivaDirect, Yale School of Public Health, Department of Epidemiology of Microbial Diseases, New Haven, CT, USA) providing results in 48 h14 and a 15-min SARS-CoV-2 antigen test (BinaxNOW COVID-19 Ag Card, Abbott Diagnostics, Scarbourgh, ME, USA).15 The new PCR tests, which require only a small sample of saliva, have a sensitivity of 94% and specificity of 100% at a price of $1·21–4·39 per test.16 Antigen tests, which detect viral surface proteins, can provide a rapid and accurate indication of active infection. For example, the BinaxNOW assays, which cost roughly $5 per test, provide a sensitivity of 97·1% and specificity of 98·5%.13, 15 The accuracy of other antigen tests produced commercially by Beijing Savant (Beijing China), Shenzhen Bioeasy (Shenzhen, China), Coris BioConcept (Gemboux Belgium), Liming Bio-Products (Tempe, AZ, USA), and RapiGEN (Gunpo-si, South Korea) varies widely, with an average sensitivity of 56·2% and specificity of 98·9%.17 Additionally, serological tests can identify SARS-CoV-2-specific antibodies starting a week after infection and possibly for months or years after recovery, with an estimated sensitivity of 90% and specificity of 99%.13, 18 At roughly $50 per test, serological surveys are providing retrospective insight into the spread of the pandemic throughout the USA.19
As affordable and rapid SARS-CoV-2 tests become more widely available,15 mass testing will become an increasingly economically viable strategy for slowing the spread of the virus and averting large pandemic waves. Using a network-based mathematical model of within-host and between-host SARS-CoV-2 infection dynamics, we aimed to assess the use of cheap and fast antigen tests in the USA under a range of testing approaches reflecting the heterogeneous implementation of non-pharmacological interventions across the USA and the world and to identify the most cost-effective testing and isolation strategies in varied transmission scenarios.
Methods
Study design and epidemic model
We used a stochastic individual-based chain-binomial model of SARS-CoV-2 transmission that incorporates household-specific and age-stratified heterogeneities and temporal changes in viral load that affect diagnostic sensitivity to compare the cost-effectiveness of eight testing strategies, which vary by testing frequency and isolation period for confirmed cases, with the status-quo strategy (ie, a symptom-based testing and isolation). Our assessment of cost-effectiveness takes into account the direct costs of testing, admissions to hospital, salary lost during isolation, and the economic burden of COVID-19 quantified as societal willingness to pay for years of life lost (YLLs) prevented.
We simulated epidemic outbreaks for 150 days in contact networks with nodes representing individuals and edges representing epidemiologically relevant contacts between individuals. We implemented the stochastic individual-based model using the parameters given in the appendix (pp 3–4). Each individual can be in one of 14 states that reflect both the progression of infection and testing (figure 1 ). Upon SARS-CoV-2 infection, an individual remains in a non-infectious incubation compartment for 1/σ days, where σ is the transition rate from the non-infectious stage to the presymptomatic stage, after which they enter either the asymptomatic or presymptomatic infectious state with probabilities of 1 – p sym and p sym. Asymptomatic cases transition to the recovered state after an average asymptomatic infectious period of 1/, where is the recovery rate for asymptomatic cases. Presymptomatic cases become symptomatic at a rate of ε and then recover at a rate of γ. Recovered individuals are assumed to be immunised against future infection for the duration of the simulation. For each infected individual, the model tracks the number of days since infection to determine antigen test sensitivity.
Figure 1.
Schematic of the individual-based SARS-CoV-2 infection dynamic model
Upon infection, susceptible individuals (S) progress to being exposed individuals (E), where they are neither infectious nor symptomatic. A fraction of cases become asymptomatic infectious (A) with lower infectiousness before recovering (R); the remaining cases progress to presymptomatic (P), where they are moderately infectious but not yet symptomatic, followed by symptomatic infectious (Y) and then either recover (R) or are admitted to hospital (H). Individuals admitted to hospital are assumed to be fully isolated and progress either to recovered (R) or deceased (D). Recovered individuals remain protected from future infection for the duration of the 150-day simulation. The various testing strategies assume that individuals are tested at a specified frequency, ranging from daily to monthly, according to an evenly staggered testing schedule, regardless of their disease state. Those testing positive and all members of their household proceed to isolate or quarantine for the specified period (either 7 or 14 days). After isolation, individuals return to non-isolated states corresponding to the current state of their health but no longer participate in surveillance testing. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
The infectiousness of a case (ie, an infected individual) is governed by both infection status (ie, presymptomatic, asymptomatic, or symptomatic) and type of contact (ie, household or non-household). The infectiousness of asymptomatic cases is reduced by the factor and the infectiousness of presymptomatic cases is reduced by the factor ω relative to symptomatic cases. To determine the baseline infectiousness of symptomatic cases, we first calibrated the within-household transmission rate, βh, to produce a household secondary attack rate of 35%, as reported elsewhere.20 We then calibrated the non-household transmission rate, βnh, to produce the specified effective reproduction number, R e, using an interior-point algorithm that minimises the mean square error between the mean R e across 100 simulations and the desired value. In each simulation, we simulated the first 100 infections assuming a status-quo testing strategy in which 29·4% of symptomatic cases are tested and isolated for 2 weeks beginning an average of 2 days after symptom onset. We estimated R e to be the ratio between the number of people being infected and the number who are infectious in our simulations.
Testing strategies
We compared eight testing strategies, in which all individuals are tested at a frequency ranging from daily to monthly (ie, every 1, 7, 14, and 28 days), coupled with either a 1-week or 2-week isolation period for confirmed cases. Specifically, we modelled rapid antigen testing of the entire population at different frequencies, where d test indicates the interval between tests in days. For example, d test = 7 days means that each individual is tested once every 7 days, with tests distributed equally across each 7-day period. Test outcomes depend on the infection status of the tested individual and the sensitivity and specificity of the test. Infected and newly recovered individuals can test positive for up to 41 days, based on days since infection and the sensitivity of the test on that day (appendix p 5). Susceptible individuals and recovered individuals (at least 42 days after infection) are assumed to test positive on the basis of the false-positive rate of the test.15 After a positive test, an individual is permanently released from future testing. We also did a sensitivity analysis to consider a scenario in which testing resumes 30 days after a positive test to guard against false positives.
Individuals who test positive and all members of their household move into their corresponding isolation or quarantine state for a specified isolation period (d iso), where they are unable to infect others outside of their household (ie, susceptible-isolated, exposed-isolated, presymptomatic-isolated, asymptomatic-isolated, symptomatic-isolated, or recovered-isolated individuals; figure 1). We assumed that household transmission can still occur and that, after isolation, individuals progress to a non-isolated state corresponding to their current infectious or non-infectious state.
During isolation, all household members who have not already tested positive continue testing according to the current regimen. If any member tests positive during isolation, the d iso-day isolation clock restarts for the entire household. At the end of the isolation period, all members of the household who did not test positive during isolation are tested again. If any are positive, the clock restarts; if none are positive, the entire household is released.
To determine the public health benefits of each strategy, we also modelled a status-quo strategy that assumes a baseline level of symptomatic testing, with isolation upon confirmation of a positive test, without additional surveillance testing.
Individual-based network
Our individual-based SARS-CoV-2 infection dynamic model assumes that the virus spreads through a fixed contact network consisting of 2019 individuals and 25 428 contacts between those individuals. We populated our network by first constructing 1000 households. The size and age composition of each household was based on a randomly sampled household from among the 129 697 households included in the 2017 US National Household Travel Survey.21 We assumed that households are fully connected (ie, all nodes in the same household are linked by edges). We assumed that our model represents the household structure, contact patterns, and SARS-CoV-2 transmission dynamics of typical US communities, and directly scaled our results from the 2019 individuals in the model to the 328 million residents of the USA.22, 23 We constructed random links between individuals in different households on the basis of reported age-specific contact rates in the USA, stratified into age bins of 5–17, 18–49, 50–64, and 65 years and older.22 Specifically, to determine the number of contacts that a node in age group a i has with nodes in age group a j, we drew random deviates from Poisson distributions centred at the mean number of contacts between a i and a j. The resulting network includes 1000 households, 2019 nodes (people), and degrees (numbers of edges per node) that roughly follow a gamma distribution with shape 3·69 and scale 3·41.
Estimating YLLs averted and monetary costs of a strategy
For each scenario, we ran 1000 rounds of simulations for the nine candidate testing strategies (including the status-quo strategy). All parameters were identical except for those governing testing. For each round, we determined the averted YLLs for each strategy τ as follows.
First, we calculated the difference in incidence by age group as Δa,τ = I a,0 – I a,τ, where I a,0 is the total incidence of infection in age group a produced by the status-quo simulation and I a,τ is the total incidence of infection in age group a produced by the strategy τ simulations.
Second, we estimated the YLLs prevented by the testing strategy as defined by
where λa denotes the future-discounted life expectancy for individuals of age a, and δa denotes the age-specific case-fatality rate for COVID-19.24
Similarly, we determined the incremental monetary costs for each strategy as given by
where T τ is the total number of tests administered in the strategy (τ) simulation, T 0 is the total number of tests administered in the status-quo simulation, c T is the price of administering a single test, and Q τ,a and Q 0,a are the total people-weeks of isolation or quarantine in age group a in each simulation, s a is the average weekly salary for age group a, and H τ,a and H 0,a are the total number of admissions to hospital due to COVID-19 in age group a in each simulation, and c H,a is the median cost of admission to hospital for COVID-19 for age group a. The cost parameter values are given in the appendix (p 6).
Estimating the cost-effectiveness acceptability frontier
The willingness to pay per YLL averted is the theoretical maximum price that a society is willing to pay to prevent the loss of 1 year of life. Health economists have inferred from health-care expenditure that in the USA the willing to pay is $100 000–200 000 per quality-adjusted life-year (QALY),25 of which YLL is one component. For a given willingness to pay for a YLL averted (θ), we calculated the net monetary benefit, NMB, of a strategy as:
We determined the optimal testing and isolation strategy across a range of scenarios, each defined by R e, willingness to pay per YLL averted, and cost of a test. For each scenario, we ran 1000 rounds of parallel simulations for each of the nine candidate testing strategies (including the status-quo strategy). For each of the 1000 rounds of nine simulations, we identified the strategy with the highest net monetary benefit. We then estimated the probability that a particular strategy had the greatest net benefit of all strategies by the proportion of simulation rounds in which it resulted in the highest net benefit. For a given scenario, the strategy with the highest probability of having the highest net monetary benefit was considered optimal.
Using this approach, we first assumed a price of $5 per test and determined optimal strategies across a range of willingness to pay per YLL averted up to $200 000. We then fixed the willingness to pay per YLL averted to $100 000 and determined optimal strategies for a range of testing prices up to $200 per test, given that tests are widely available for under $200 as of October, 2020.26
Uncertainty quantification, scenario analyses, and sensitivity analyses
To quantify uncertainty, we visualised individual simulation results and reported the probability that the chosen strategy was suboptimal to one or more of the alternative strategies (ie, probability of error). The primary source of uncertainty in our analyses was microstochasticity in our probabilistic agent-based model. For example, the duration of time that an infected individual spends in any given disease state is an exponential random number governed by transition rates (as shown in the appendix [pp 3–4]); furthermore, infection events occur probabilistically between contacts and testing schedules are assigned randomly.
Because the transmission rate of SARS-CoV-2 will vary depending on the extent of community mitigation and the accumulation of immunity via infection and vaccination, we determined the optimal strategy across a range of transmission scenarios, with R e ranging from 1·1 to 3. For each scenario, we assumed a willingness to pay per YLL averted of US$100 000 and determined the best testing strategy at a price of $5 per test and a threshold test price at which the status-quo strategy outperforms all testing strategies considered.
We also assessed robustness of the results with respect to several structural features of the model. First, we increased the population size from 1000 to 5000 households. Second, we relaxed the assumption that individuals perfectly comply with isolation and assumed instead that isolated individuals reduce their non-household transmission by a random fraction, uniformly distributed between zero and one. Third, we modified our immunity passport model as follows: individuals who test positive are not permanently exempted from future testing, but rather re-join the surveillance testing programme 30 days after their positive test. Fourth, we added a 10-day isolation period in addition to 1-week and 2-week options and determined the optimal strategy across all 12 testing plus isolation combinations. Finally, we extended the cost-effectiveness calculations to include the loss of QALYs associated with admissions to hospital due to COVID-19 (parameters used for morbidity associated with COVID-19 hospital admissions are given in the appendix [p 6]).
We did all statistical analyses using Matlab R2020a.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
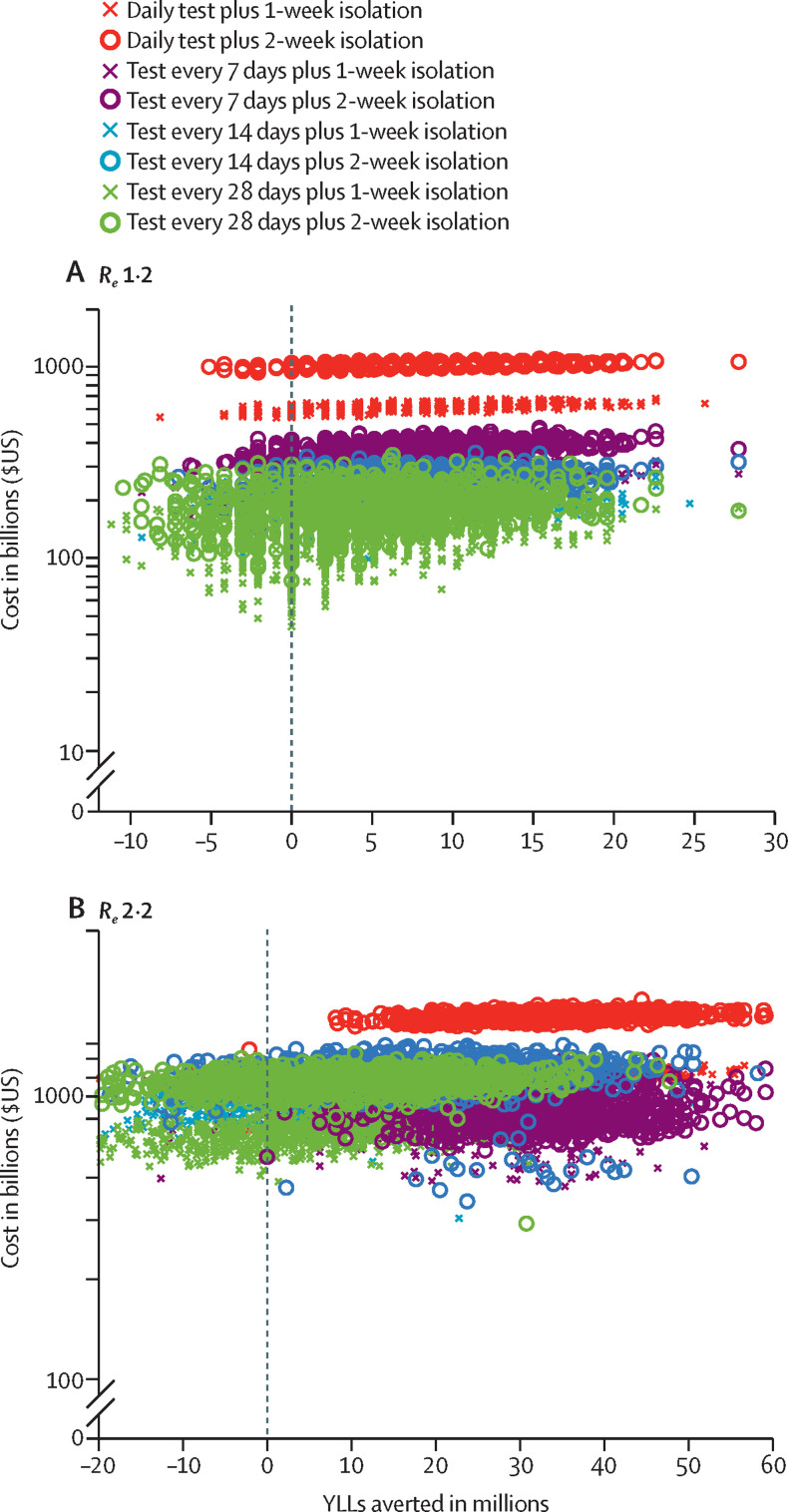
We estimated the health outcomes (YLLs prevented) and economic outcomes (costs of diagnostic testing, admissions to hospital due to COVID-19, and salary lost while in isolation) associated with each testing strategy, conditional on the transmission rate for the virus (R e; figure 2 ). As expected, both costs and YLL averted tend to increase with the frequency of testing and the length of isolation period. Generally, the most costly option we considered was daily testing coupled with a 2-week isolation period for confirmed cases.
Figure 2.
Estimated costs of testing strategies and YLLs averted, assuming US$5 tests and an Re of 1·2 (A) or 2·2 (B)
Each datapoint corresponds to one of 1000 stochastic simulations for the specified testing strategy, under parameters given in the appendix (pp 3–4). Costs include the price of administering tests, salary lost during isolation after a positive test result, and costs associated with admission to hospital due to COVID-19; YLLs averted considers mortality due to COVID-19. The costs and YLLs averted are all scaled assuming a US population of 328·2 million people, as estimated in 2019.23Re=effective reproduction number. YLLs=years of life lost.
We then identified the optimal strategy—ie, the strategy most likely to provide the greatest net monetary benefit for a given testing price and societal willingness to pay per YLL averted. At the base case, with a price of $5 per test and willingness to pay per YLL averted of $100 000, the optimal strategy under high transmission scenarios (R e of 2·2) would be weekly testing coupled with 2-week isolation for confirmed cases (figure 3 ; appendix p 8). This strategy is expected to be optimal at all willingness to pay per YLL averted thresholds above $10 000 (figure 3). Conversely, maintaining a willingness to pay per YLL averted of $100 000,25 this strategy is expected to be optimal for prices under $400 per test (table ).
Figure 3.
Cost-effectiveness acceptability frontier, assuming an Re of 1·2 (A, B) or 2·2 (C, D)
(A, C) Assuming each test costs US$5, the probability that a candidate strategy has the greatest net benefit under a given willingness to pay per YLL averted (x axis) is based on 1000 rounds of stochastic simulations. In each round, every strategy is simulated and the one resulting in the largest net monetary benefit is deemed optimal. (B, D) Assuming a willingness to pay of $100 000 per YYL averted, the same procedure is applied across a range of test prices (x axis). The graphs depict the best strategy—ie, the one that most often yielded the highest expected net monetary benefit across the 1000 sets of simulation. On appendix p 8, we depict the cost-effectiveness acceptability curves for the top three performing strategies. Re=effective reproduction number. YLLs=years of life lost.
Table.
Optimal testing and isolation strategies and price thresholds for cost-effective testing for each potential Re for SARS-CoV-2 transmission
|
Optimal strategy (assuming $5 per test) |
Testing threshold (cost per test) | |||
|---|---|---|---|---|
| Testing frequency (days between tests)* | Isolation period | Probability of error | ||
| 1·1 | 28 | 1 week | 0·65 | $75 |
| 1·2 | 28 | 1 week | 0·66 | $125 |
| 1·3 | 14 | 1 week | 0·69 | $175 |
| 1·4 | 14 | 1 week | 0·65 | $350 |
| 1·5 | 7 | 1 week | 0·72 | $325 |
| 1·6 | 7 | 1 week | 0·63 | $375 |
| 1·7 | 7 | 1 week | 0·62 | $425 |
| 1·8 | 7 | 1 week | 0·62 | $475 |
| 1·9 | 7 | 2 weeks | 0·45 | $450 |
| 2·0 | 7 | 2 weeks | 0·40 | $375 |
| 2·1 | 7 | 2 weeks | 0·40 | $350 |
| 2·2 | 7 | 2 weeks | 0·43 | $400 |
| 2·5 | 1 | 2 weeks | 0·43 | $400 |
| 3 | 1 | 2 weeks | 0·18 | $275 |
The middle columns give the optimal testing and isolation strategies and probability of error for each Re scenario, assuming that each test costs US$5 and assuming a societal willingness to pay per YLL averted of $100 000. The rightmost column gives a threshold price above which the status-quo strategy (ie, symptom-based testing and isolation) is expected to be more cost-effective than all eight testing strategies considered. The threshold value was identified by assessing all strategies across a range of costs per test up to $2000 at $25 increments. The low transmission and high transmission scenarios in Figure 2, Figure 3 correspond to Re of 1·2 for low transmission and 2·2 for high transmission. Re=effective reproduction number. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. YLLs=years of life lost.
A 7-day testing frequency means that individuals are tested once every 7 days, on a rotating basis.
The optimality of this strategy is robust to changes in several model assumptions. Specifically, weekly testing combined with 2-week isolation remains the preferred strategy under high transmission scenarios (R e of 2·2) for larger population sizes, if individuals are assumed to resume testing 30 days after a positive test, when using QALYs to assess COVID-19 morbidity averted in addition to YLL averted, and even when the strategy options are extended to include 10-day in addition to 1-week and 2-week isolation periods (appendix p 7). However, the optimal frequency of testing increased from weekly to daily if individual compliance with isolation is imperfect (appendix p 7). Assuming a test cost of $5 and willingness to pay per YLL averted of $100 000, testing every 14 days with 1-week isolation is optimal for moderate transmission rates (R e 1·3–1·4), and monthly testing with 1-week isolation is optimal for lower transmission scenarios (R e 1·1–1·2; table; appendix p 8). We identified threshold test prices above which the status-quo strategy is expected to outperform all eight candidate strategies and found that expanded surveillance is more cost-effective than the status-quo scenario if the price per test is less than $75 across all transmission rates (table).
Discussion
Aggressive and sustainable SARS-CoV-2 testing programmes have potential to substantially mitigate the threat of COVID-19 in susceptible communities and ensure the integrity of our health-care systems, while bolstering our societies and economies. As the cost of testing for SARS-CoV-2 is rapidly decreasing in the USA, the optimal strategy will depend on the transmission rate of the virus. Our modelling data indicate that in communities where the virus is spreading rapidly, weekly testing coupled with a 2-week isolation period after a positive test is advisable. Where non-pharmacological measures are substantially curtailing the spread of the virus, monthly testing with a 1-week isolation period after a positive test is expected to be the most optimal strategy, according to our data. Although considerable uncertainty exists regarding the optimal choice among these strategies, surveillance testing at least monthly is preferred to the status quo of symptom-based testing as long as the R e of the virus is slightly above 1 and tests cost less than $75 to administer. As R e increases, so does our certainty that frequent surveillance testing would be an efficient use of resources.
Because the infectious period is estimated to last 7–10 days,27 many governments and public health agencies have recommended that people with a confirmed infection isolate for 10–20 days after symptom onset.28 In our assessment of different isolation periods, we started the isolation clock from the date of testing, which might occur before or after symptom onset. Notably, at low-to-moderate rates of SARS-CoV-2 transmission (R e <1·9), we found that a 1-week period of isolation for people who test positive and quarantining of their household contacts is expected to be more efficient than a 10-day or 2-week isolation period. Roughly speaking, loss of salary in the second week outweighs the costs of infections that occur during the second week after testing.
Until a safe and efficacious SARS-CoV-2 vaccine can be widely administered, sustained periods of rapid transmission might continue to threaten the global population. As of December, 2020, numerous cities are facing overwhelming surges in admissions to hospital due to COVID-19 and are struggling to slow the spread of the virus.29 Transmission rates might continue to increase as socioeconomic and political pressures force the relaxation of mitigation policies in some countries, public compliance deteriorates, and winter conditions in the northern hemisphere amplify transmission. Surveillance testing offers a cost-effective strategy to mitigate risks where non-pharmacological measures are falling short or as a proactive path towards relaxing strict measures that are effective but socioeconomically burdensome.
The optimal strategies we identified here might not yet be logistically feasible everywhere. They require large quantities of low-cost rapid SARS-CoV-2 antigen tests and multifaceted distribution plans that potentially combine school, university, and workplace testing; delivery of home test kits; and widely accessible public testing sites. Monthly testing across the USA would require 12 million tests per day. On Sept 28, 2020, the US Federal Government began distributing 150 million of the FDA-approved 15-min Abbot BinaxNOW SARS-CoV-2 antigen tests to State Governments, local health departments, schools, and nursing homes around the country.30 Abbott anticipated producing 100 million of these tests per month by the end of 2020.31 Numerous schools, universities, long-term care facilities, correctional facilities, health-care systems, and other large employers have already implemented high-frequency surveillance testing programmes. Despite the enormous gap between currently available tests and the optimal testing strategies, these efforts indicate that a rapid roll-out might be possible. In the meantime, our findings suggest that even suboptimal levels of surveillance testing are generally better than the status quo and that testing efforts will be most cost-effective in communities with rapidly escalating pandemic waves.
Our economic calculations have a restricted scope because we consider only the expense of testing and the loss of salary during isolation and household quarantine. Testing might lead to additional expense because asymptomatic or so-called paucisymptomatic individuals (ie, those with few or very mild symptoms) who test positive might seek health care when they would otherwise not have suspected themselves to be infected. Conversely, preventing infectious spread averts the costs associated with unrealised symptomatic COVID-19 illness. Likewise, we quantified the economic benefits of averting mortality and the cost savings of averting admissions to hospital, but did not consider the prevention of non-fatal morbidity caused by SARS-CoV-2 infection, which is substantial, or the indirect health and mental health consequences of the pandemic.32 Finally, we assessed testing strategies across a range of reproduction numbers, but did not account for the direct or indirect costs of the non-pharmacological interventions enacted to slow transmission in the milder scenarios.
The extent and duration of immunity after infection with SARS-CoV-2 is unclear. Immunity has been found to wane over several months after recovery in some individuals, but can last for more than 6 months in others.33 Because of the 5-month timeframe of our projections and our focus on immediate policy guidance for mitigating risks until vaccines are widely available, we made the simplifying assumption that recovered individuals cannot be re-infected. If immunity is more transient and re-infections are more common than assumed, we speculate that the reproduction number of the virus and thus the cost-effectiveness of surveillance testing would increase, assuming that individuals who test positive resume surveillance testing after recovery.
We note two additional sources of empirical uncertainty in translating these results to policy. First, our understanding of the relative infectiousness of asymptomatic and presymptomatic individuals is still evolving. Second, the optimal strategy depends on the transmission rate of the virus. Our results indicate that the effect of frequent testing and extended isolation increases with the intensity of transmission. However, local transmission rates vary due to a myriad of factors including population density, mitigation policies, and pre-existing immunity. Nonetheless, we found that for a wide range of model parameter values, more frequent testing combined with reduced duration of isolation has a greater impact and is more cost-effective.
Despite the intimidating upfront costs, we found that ramping-up mass asymptomatic testing for SARS-CoV-2 across the USA is a cost-effective and impactful strategy for mitigating the unprecedented threat of the COVID-19 pandemic. When coupled with an expansion of contact-tracing programmes, testing can be instrumental in averting pandemic waves and allowing the relaxation of costly travel restrictions and physical distancing measures.7 If COVID-19 remains a persistent threat, simultaneous surveillance testing for SARS-CoV-2 and seasonal influenza viruses might provide additional public health and economic benefits.8
Data sharing
The computer code and simulated data will be made available to anyone for any purpose on request to the corresponding author after publication.
Acknowledgments
Acknowledgments
Financial support was provided by US National Institutes of Health (grant numbers U01 GM087719 and K01 AI141576) and US Centers for Disease Control and Prevention COVID Supplement (grant numbers U01P001136-01-01 and CDC-HHS-6U01IP001137-01). ZD, ML, and LAM acknowledge a donation from Love, Tito's (the philanthropic arm of Tito's Homemade Vodka, Austin, TX, USA) to the University of Texas to support the modeling of COVID-19 transmission and mitigation strategies.
Contributors
ZD, AP, YB, MCF, ML, AV, BJC, APG, and LAM conceived the study, designed the statistical methods, did analyses, interpreted results, and wrote and revised the manuscript. MC and APyP did the data analysis, generated the age-stratified contact patterns of the USA, and revised the manuscript. ZD and LAM accessed and verified the underlying data. All authors had full access to all of the data in the study and the corresponding author had final responsibility for the decision to submit for publication.
Declaration of interests
APyP and AV report grants from Metabiota outside of the submitted work. All other authors declare no competing interests.
Supplementary Material
References
- 1.Wan W. The Washington Post; March 11, 2020. WHO declares a pandemic of coronavirus disease COVID-19.https://www.washingtonpost.com/health/2020/03/11/who-declares-pandemic-coronavirus-disease-covid-19/ [Google Scholar]
- 2.COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6
- 3.O'Neill M. Economic pain: COVID-19 pandemic will cost global economy $21 trillion. https://scitechdaily.com/economic-pain-covid-19-pandemic-will-cost-global-economy-21-trillion/
- 4.Zimmer C, Corum J, Wee S-L. The New York Times; June 10, 2020. Coronavirus vaccine tracker.https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html [Google Scholar]
- 5.Wang W, Wu Q, Yang J. Global, regional, and national estimates of target population sizes for COVID-19 vaccination: descriptive study. BMJ. 2020;371 doi: 10.1136/bmj.m4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Regan H, McKeehan B, Woodyatt A, Wagnert M, Macaya M. CNN; Sept 4, 2020. September 4 coronavirus news.https://www.cnn.com/world/live-news/coronavirus-pandemic-09-04-20-intl/index.html [Google Scholar]
- 7.Han E, Tan MMJ, Turk E. Lessons learnt from easing COVID-19 restrictions: an analysis of countries and regions in Asia Pacific and Europe. Lancet. 2020;396:1525–1534. doi: 10.1016/S0140-6736(20)32007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moghadas SM, Fitzpatrick MC, Sah P. The implications of silent transmission for the control of COVID-19 outbreaks. Proc Natl Acad Sci USA. 2020;117:17513–17515. doi: 10.1073/pnas.2008373117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aleta A, Martín-Corral D, Pastore Y, Piontti A. Modelling the impact of testing, contact tracing and household quarantine on second waves of COVID-19. Nat Hum Behav. 2020;4:964–971. doi: 10.1038/s41562-020-0931-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Associated Press The latest: 3 million tested for coronavirus in Chinese city. U.S. News & World Report. Oct 12, 2020. https://www.usnews.com/news/health-news/articles/2020-10-12/the-latest-china-to-test-city-of-9-million-amid-new-cases
- 11.The Slovak Spectator; Nov 1, 2020. More than 3.6 million people tested during the weekend.https://spectator.sme.sk/c/22525342/coronavirus-in-slovakia-nationwide-testing-final-results.html [Google Scholar]
- 12.Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020;323:2249–2251. doi: 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- 13.Ducharme J. Time; Aug 20, 2020. What to know about COVID-19 tests, from PCR to antigen to antibody.https://time.com/5880255/covid-19-tests-types/ [Google Scholar]
- 14.Office of the Commissioner Emergency use authorization. US Food and Drug Administration. https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization
- 15.Park A. Time; Aug 27, 2020. For $5 and in 15 minutes you can learn if you have COVID-19.https://time.com/5884012/15-minute-coronavirus-test/ [Google Scholar]
- 16.Greenwood M. Yale School of Medicine; Sept 3, 2020. SalivaDirect: what you need to know about the new COVID-19 test.https://medicine.yale.edu/news-article/27120/ [Google Scholar]
- 17.Dinnes J, Deeks JJ, Adriano A. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2020;8 doi: 10.1002/14651858.CD013705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.US Centers for Disease Control and Prevention; Aug 1, 2020. Interim guidelines for COVID-19 antibody testing.https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html [Google Scholar]
- 19.US Centers for Disease Control and Prevention; Oct 3, 2020. Commercial laboratory seroprevalence surveys.https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/commercial-lab-surveys.html [Google Scholar]
- 20.Grijalva CG, Rolfes MA, Zhu Y. Transmission of SARS-CoV-2 infections in households - Tennessee and Wisconsin, April-September 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1631–1634. doi: 10.15585/mmwr.mm6944e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.US Department of Transportation Federal Highway Administration 2017 summary statistics for demographic characteristics and travel. https://nhts.ornl.gov/
- 22.Mistry D, Litvinova M, Pastore y Piontti A. Inferring high-resolution human mixing patterns for disease modeling. Nat Commun. 2021;21:323. doi: 10.1038/s41467-020-20544-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.United States Census Bureau; June 17, 2020. National population by characteristics: 2010–2019.https://www.census.gov/data/tables/time-series/demo/popest/2010s-national-detail.html [Google Scholar]
- 24.Verity R, Okell LC, Dorigatti I. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371:796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 26.Kliff S. The New York Times; June 16, 2020. Most coronavirus tests cost about $100. Why did one cost $2,315?https://www.nytimes.com/2020/06/16/upshot/coronavirus-test-cost-varies-widely.html [Google Scholar]
- 27.He X, Lau EHY, Wu P. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 28.US Centers for Disease Control and Prevention; Sept 10, 2020. Duration of isolation and precautions for adults with COVID-19.https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html [Google Scholar]
- 29.US COVID map & risk levels. COVID Act Now. 2021. https://covidactnow.org/?s=1538484
- 30.President Trump gives update on COVID-19 testing, C-Span. Sept 28, 2020. https://www.c-span.org/video/?476324-1/president-trump-announces-plan-distribute-150-million-rapid-coronavirus-tests
- 31.Guarascio F, Miller J. Reuters; Oct 27, 2020. EU weighs buying Roche, Abbott rapid COVID tests amid limited supplies.https://www.reuters.com/article/us-health-coronavirus-eu-rapid-tests-idUSKBN27C1GH [Google Scholar]
- 32.US Centers for Disease Control and Prevention; Sept 10, 2020. COVID-19 pandemic planning scenarios.https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html [Google Scholar]
- 33.de Jesus EG. ScienceNews; Nov 24, 2020. Immunity to COVID-19 may persist six months or more.https://www.sciencenews.org/article/covid-19-immunity-antibodies-persist-six-months-coronavirus [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The computer code and simulated data will be made available to anyone for any purpose on request to the corresponding author after publication.