Abstract
Convalescent plasma has emerged as a promising therapeutic agent for patients with coronavirus disease 2019 (COVID-19), has received emergency use authorization, and is being widely used during the COVID-19 pandemic. Passive antibody therapy via plasma or serum has been successfully used to treat infectious diseases for more than a century. Passive antibody administration is based on the presumption that convalescent plasma or serum contains therapeutic antibodies that can be passively transferred to the plasma recipient. There are numerous examples in which convalescent plasma has been used successfully as post-exposure prophylaxis and treatment of infectious diseases, including previous coronavirus outbreaks. In the context of the COVID-19 pandemic, convalescent plasma was demonstrated to be safe and potentially effective among patients infected with COVID-19. This review provides an overview of the historical uses of convalescent plasma therapy, summarizes current evidence for convalescent plasma use for COVID-19, and highlights future antibody therapies.
Introduction
The first Nobel Prize in Physiology or Medicine in 1901 was awarded to Emil Adolf von Behring “for his work on serum therapy, especially its application against diphtheria, by which he has opened a new road in the domain of medical science and thereby placed in the hands of the physician a victorious weapon against illness and deaths” [1].
Emerging and epidemic infectious disease outbreaks represent a significant threat to global public health [2]. On 31 December 2019, the World Health Organization (WHO) became aware of a cluster of zoonotic viral pneumonia cases linked to a wet animal seafood and wholesale market in Wuhan, China [3]. The new pathogen rapidly spread worldwide, and within 3 months was declared a pandemic by the WHO, on 11 March 2020 [4, 5]. The causative agent was a novel strain of coronavirus (CoV) belonging to the same family of viruses that cause severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), and was dubbed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [6]. The disease caused by SARS-CoV-2 was named coronavirus disease 19 (COVID-19). By November 2020, more than 50 million people had been afflicted worldwide and nearly 1.3 million had died due to SARS-CoV-2 [7].
In response to the pandemic, clinical research has focused on evaluating the effectiveness of open-label COVID-19 therapies [8]. Initial efforts focused on repurposing existing antiviral drugs, with limited success except for remdesivir. Among hospitalized patients with COVID-19, hydroxychloqoruine [9], lopinavir/ritonavir [10]. and interferon [11] had little or no clinical or mortality benefit. However, dexamethasone therapy was shown to have a mortality benefit among patients receiving respiratory support via supplemental oxygen or mechanical ventilation [12], and remdesivir had a clinical benefit among patients not receiving respiratory support [13]. In light of the lack of definitive treatments for hospitalized patients with COVID-19, current medical management remains largely supportive. Although vaccines are under investigation and two vaccines received Emergency Use Authorization (EUA) in the US and conditional approval in several other countries in late 2020, there are significant barriers to rapid implementation, including regulatory standards, logistic hurdles, and intrinsic properties of product storage (i.e., cold storage) that preclude immediate, rapid distribution and administration en masse [14, 15].
In contrast, convalescent plasma therapy is likely a readily implementable [16], safe [17, 18], and effective stopgap treatment for COVID-19 [19, 20] until the “COVID-19 vaccine cavalry” arrives. Importantly, passive immunity therapies are potential long-term immunization and treatment strategies for patients who are unable to receive a vaccine. In this context, this review briefly describes SARS-CoV-2 and its clinical implications. Subsequently, we discuss the mechanisms of passive immunotherapy and outline the historical precedent for antibody-based therapies. Finally, we conclude with a summary of evidence behind the use of convalescent plasma for treatment of COVID-19.
SARS-CoV-2 and Clinical Implications
SARS-CoV-2
CoVs are large, enveloped, single-stranded RNA viruses that are usually present in animals or humans [6, 21]. With the emergence of SARS-CoV-2, seven CoV species are now known to cause human disease. Four viruses (HKU1, OC43, 229E, and NL63) are prevalent in humans, causing only mild to moderate upper respiratory symptoms, similar to the common cold in immunocompetent recipients [6, 21]. The remaining three strains—MERS-CoV, SARS-CoV-1, and the newly discovered SARS-CoV-2—can cause fatal pneumonia and have led to major epidemics and pandemics [6, 21].
SARS-CoV-2 has multiple unique characteristics, including being highly transmissible during asymptomatic infection, which has contributed to its rapid and pandemic worldwide spread [22]. The spike proteins of CoVs have a region called the receptor-binding domain (RBD) that is required for entry into human cells [23]. Similar to those of other CoVs, the SARS-CoV-2 RBD is effective at invading cells in the upper respiratory tract (e.g., sinuses). However, SARS-CoV-2 is more efficient than other CoVs at infecting cells in the lower respiratory tract (e.g., lungs). Moreover, SARS-CoV-2 binds to the ACE2 receptor with high affinity and is uniquely equipped for forcing entry into host cells. Relative to SARS-CoV-1, SARS-CoV-2 is 10 to 20 times more likely to bind ACE2 and has an RBD that is particularly close fitting [23]. These unique characteristics of SARS-CoV-2 contribute to the variability of clinical presentations and high mortality rates among patients with COVID-19 [24].
COVID-19 can present with a wide range of clinical manifestations, varying from an asymptomatic carrier state to severe, multi-organ failure requiring intensive care unit level of care [6, 21]. The virus is primarily transmitted via respiratory droplets from face-to-face contact and to a lesser extent via aerosols and contaminated surfaces [25]. The mean incubation period of COVID-19 is approximately 5 days, with more than 95% of patients showing symptoms within 11 days of infection [6, 21]. Among patients with COVID-19 who require hospitalization, the average interval from symptom onset to hospital admission is 7 days. The median age of hospitalized patients ranges between 47 and 73 years old, and most (~60%) hospitalized patients are male [6, 21].
Convalescent plasma therapy
Since the 1890s, passive antibody therapy has been successfully used to treat infectious diseases [19]. Prior to the availability of monoclonal antibodies and gamma globulin products, passive immunization therapy relied on use of convalescent or immune blood products (i.e., plasma or serum) collected from recovered donors (or animals) as a therapeutic agent for at-risk or infected patients for the purpose of prophylaxis or treatment of a specific pathogen [26]. Contrary to active immunization therapy (vaccination), which requires an extended time to elicit an immune response and can display a wide range of clinical variability among recipients [26], passive antibody administration involves the transfer of pre-formed antibodies and is the only effective strategy that confers immediate protection in susceptible individuals. Hence, until an effective vaccine becomes widely available, convalescent plasma has the potential to confer immunity among at-risk or infected patients, reducing the societal disease burden during large-scale pandemics [26].
Historically, the use of passive immunotherapy has involved different formulations, including whole blood, pooled human immunoglobulin, convalescent blood products, antibodies harvested from animals such as horses and rabbits, and, more recently, monoclonal or polyclonal antibodies [27]. Plasma collection by apheresis with subsequent convalescent plasma transfusion has been the most widely used passive immunotherapy strategy during prior pandemics [27]. Practically, an individual who has recovered from an infectious disease has a blood product withdrawn via venipuncture, and the blood product is screened for neutralizing antibodies (see below) specific to the causative pathogen. Ideally, high-titer neutralizing antibody convalescent plasma is used for therapy to maximize biologic activity. Convalescent plasma may be transfused to non-infected individuals to provide passive immunity to the recipient or to ameliorate the disease course in infected individuals [27, 28].
Convalescent plasma confers immunity or is therapeutically active in patients with disease primarily via neutralizing antibodies against a specific infectious agent [29]. Neutralizing antibodies that bind to a pathogen restrict entry of the pathogen into host cells and enhance clearance of the pathogen via antibody-dependent phagocytosis, antibody-dependent cellular toxicity, and/or complement activation [29]. Additional bioactive agents in convalescent plasma may also contribute to the reduction in disease burden, including anti-inflammatory cytokines, pentraxin, natural antibodies, defensins, ensodomes, and other agents [29]. Direct and indirect humoral and cellular immune mechanisms by which convalescent plasma acts against pathogens have been also described [30, 31, 32]. Ultimately, the general goals of convalescent plasma therapy are to initiate or augment the humoral immune response, mitigate the potential cytokine storm, improve the disease course, and reduce disease progression [33].
A fundamental principle of convalescent plasma therapy is that to maximize clinical or mortality benefit, the plasma must be given early in the course of the infectious disease [34]. This fundamental principal has been repeatedly recapitulated over the past century, notably including during the meningococcal meningitis epidemic in 1913 and for diphtheria infections in children in 1940.
Historical Framework of Convalescent Plasma
Broad use for infectious diseases
Convalescent plasma therapy is reported back to the late 1800s, during which time it was the primary means of treating many infectious diseases prior to the development of antimicrobial therapy in the 1930s [19]. In 1890, Emil Adolf von Behring and Shibasaburo Kitasato used immune serum to treat tetanus and diphtheria [35], and it was particularly effective at both preventing and treating diphtheria [35]. The use of immune serum garnered support worldwide and became a revolutionary treatment. In light of these discoveries in immune serum therapy, Emil Adolf von Behring was awarded the first Nobel Prize in Physiology or Medicine in 1901 [35]. Notably, for bacterial diseases, therapy relied on the use of serum from immunized animals, while for viral diseases, physicians relied on human convalescent sera, given that virology was in its infancy and it was not possible to obtain virus for immunization studies. Convalescent blood product therapy became the basis for prevention and treatment of a myriad of infectious diseases during the 20th century, also serving as the foundation for vaccine development. Historical data from convalescent plasma trials for infectious diseases are summarized in Table 1 .
Table 1.
Summary of clinical outcomes following treatment with convalescent blood products
| Disease/outbreak | Study description | Finding |
|---|---|---|
| Diphtheria (Corynebacterium diphtheria) [35] | Case series of 220 children diagnosed with diphtheria |
|
| Pneumonia (pneumococcal pneumonia) [19] | Aggregation of 13 non-randomized studies |
|
| Meningitis (meningococcal bacteria and viruses) [101] | Review of several meningitis epidemics where convalescent serum therapy was employed |
|
| Chickenpox (varicella-zoster virus) [102] | Post-exposure prophylaxis case series study of immunocompromised patients exposed to varicella | Transfused patients rate of developing varicella infection: 32% (10/31) |
| Measles (Morbillivirus) [103] | Post-exposure prophylaxis case series study of patients exposed to measles | Transfused patients rate of developing measles infection: 10% (10/102) |
| 1918 Influenza pandemic (influenza A H1N1 virus) [36] | Meta-analysis of eight matched-control studies |
|
| Argentine hemorrhagic fever (arenavirus) [42] | Double-blind randomized clinical trial |
|
| 2003 SARS epidemic (SARS-CoV-1) [104] | Matched-control study |
|
| 2009-2010 influenza pandemic (influenza A H1N1 virus) [44] | Matched-control study |
|
| 2012-2015 MERS epidemics (MERS-CoV) [59] | Case series study | Transfused patient overall mortality rate: 0% (0/3) |
| 2013 Ebola epidemic (Ebola virus) [45] | Matched-control study |
|
| COVID-19 pandemic (SARS-CoV-2) [92] | Meta-analysis of 17 studies (13 matched-control, four randomized clinical trials) |
|
The Spanish influenza (1918 to 1920), caused by an H1N1 influenza virus of avian origin, was the first reported pandemic for which convalescent blood products were used as therapeutic agents. A meta-analysis of eight studies from the Spanish influenza pandemic evaluated the use of convalescent plasma to treat 1,703 patients and provided evidence that infected patients who received convalescent blood products had 21% lower mortality than patients not treated with convalescent plasma [36]. Interestingly, the greatest clinical and mortality benefits were noted among patients receiving convalescent blood products in early stages of the disease course [36].
During the first half of the 20th century, the therapeutic role of convalescent blood products extended to other viral conditions, such as mumps [37], polio [38], and measles [39], and bacterial infections, including Haemophilus influenzae B [40], pneumococcus, and meningococcus [41] infections. Nevertheless, the use of convalescent plasma therapy to treat bacterial infections markedly declined following the discovery of antibiotics in the 1930s and was largely abandoned by the mid-1940s.
In the post-antibiotic era, the interest in antibodies as therapeutic agents for infectious diseases has been notable but has generally been restricted to replacement therapy for patients with immunoglobulin deficiencies [19] or in the context of epidemics or pandemics. During the intervals between infectious disease outbreaks, however, support for convalescent plasma therapy appears to wane, only to wax during an ensuing infectious disease outbreak.
Between 1974 and 1978, a double-blind, randomized clinical trial in patients with Argentine hemorrhagic fever treated with convalescent plasma within 8 days of disease onset revealed a 15.4% lower mortality rate compared to patients who received control plasma lacking neutralizing antibodies to Argentine hemorrhagic fever virus [42]. Comparable results were described in subsequent outbreaks of Argentine hemorrhagic fever [43]. Similarly, during the 2009-2010 H1N1 influenza pandemic, convalescent plasma was used to treat individuals with severe H1N1 infections requiring intensive care [44]. Patients treated with convalescent plasma had reduced respiratory viral burden, reduced serum cytokine responses, and reduced mortality [44]. During the 2013 West African Ebola epidemic, a small nonrandomized study in Sierra Leone revealed significantly longer survival for patients who were treated with convalescent whole blood compared to patients receiving standard treatment [45]. Two patients with Ebola who were transferred to the U.S., were treated with a combination of convalescent plasma and an experimental drug (TKM-100802), and both survived their infections [46]. There is also anecdotal evidence from the H5N1 [47, 48] and H7N9 [49] avian flu outbreaks that use of convalescent plasma was effective, with all patients treated with convalescent plasma surviving. Although each viral disease and epidemic is unique, these experiences provide important historical precedents supporting convalescent blood products as “empiric” therapies that should be readily implemented early during a pandemic and disease course for patients.
Use of convalescent plasma for treatment of CoVs
Convalescent plasma is not a new therapy in the management of CoVs [50]. During the 21st century, there have been two major epidemics caused by CoVs that were associated with high mortality: the 2003 SARS-CoV-1 epidemic originating in Hong Kong and the 2012 MERS-CoV epidemic, which originated in Saudi Arabia. In both outbreaks, the high mortality and absence of effective therapies engendered the use of convalescent plasma.
The initial studies supporting the use of convalescent plasma for treatment of SARS-CoV-1 were limited to case reports [51, 52] and case series [53, 54]. Multiple subsequent non-randomized and retrospective studies added more robust evidence to the efficacy of convalescent plasma for SARS-CoV-1. A study by Soo et al. compared 21 patients treated with steroids (methylprednisolone) to 19 patients receiving convalescent plasma [55]. The number of patients discharged by day 22 of hospitalization was higher for patients treated with convalescent plasma than for those in the steroid group (74% versus 19%, respectively). Additionally, the mortality rate was lower in the convalescent plasma group (0 deaths) than in the steroid group (5 deaths) [55].
The largest investigation of convalescent plasma during the SARS-CoV-1 outbreak involved 80 patients with SARS in Hong Kong [56]. In a retrospective analysis, 80 patients who received convalescent plasma were dichotomized into early and late transfusion groups, using 14 days between the onset of symptoms and the transfusion date as the cut point [56]. Compared to the late transfusion group, the early group had improved prognosis, as evidenced by a higher rate of hospital discharge by day 22 (58% versus 16%). These data suggest that convalescent plasma is an effective treatment for CoV infections and are consistent with the notion that the optimal use of convalescent plasma involves early administration. In addition, patients who were RT-PCR positive and seronegative for CoV at the time of therapy had improved prognosis [56], consistent with the notion that early use, prior to host development of an immune response, was most effective. A meta-analysis including eight observational studies and 214 patients with SARS demonstrated a mortality benefit following transfusion of convalescent plasma [57].
The initial case reports and case series in the MERS epidemic failed to show a clinical benefit for patients who were transfused with convalescent plasma containing uncharacterized neutralizing antibody titers [58]. In line with the notion that neutralizing antibody titers are a marker of convalescent plasma potency, a subsequent study provided evidence that transfusion of convalescent plasma containing a high MERS-CoV neutralizing antibody titer resulted in seroconversion of the recipient post-transfusion. However, seroconversion was not achieved among patients transfused with convalescent plasma containing a low neutralizing antibody titer [59]. These findings highlight a challenge for the therapeutic use of convalescent plasma, namely, that recovered survivors of viral diseases may not produce high-titer neutralizing antibody [60].
Use of Convalescent Plasma for Treatment of COVID-19
Characterization of COVID-19 convalescent plasma
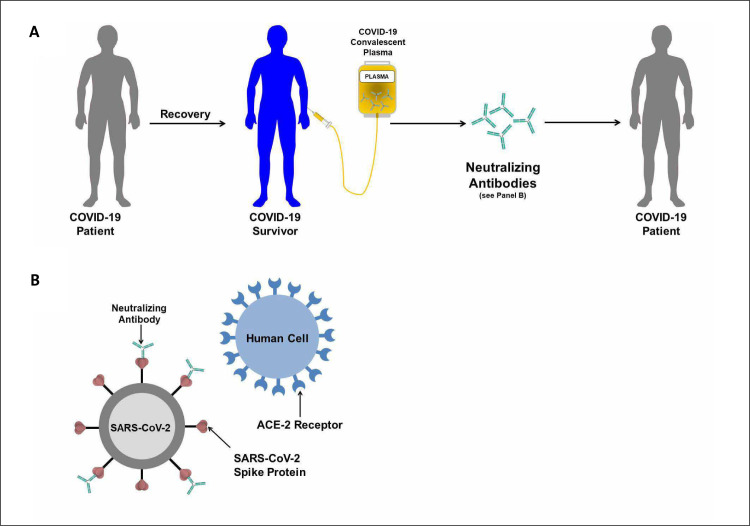
In the current COVID-19 pandemic, blood collection centers from around the world have established programs for recovered survivors to donate COVID-19 convalescent plasma, and regulatory agencies in the U.S. have provided widespread access to convalescent plasma for emergency use in hospitalized patients with COVID-19. Convalescent plasma may be obtained from recovered COVID-19 survivors via apheresis or separated from whole blood collected as a standard blood donation (Fig. 1 ). Apheresis collection is strongly preferred because it yields more units of convalescent plasma per donation and for a given donor it may be performed more frequently than standard blood donation [61].
Figure 1.
Schematic illustrating the use of convalescent plasma for COVID-19. An individual who was sick with COVID-19 and currently recovered (COVID-19 Survivor) has blood drawn and screened for virus neutralizing antibodies. Following identification of those with high levels of neutralizing antibody, plasma containing these virus neutralizing antibodies can be administered to individuals currently sick with COVID-19. (Adapted from [26].)
There were several barriers to recruitment of potential convalescent plasma donors during the COVID-19 pandemic primarily due to public health interventions to mitigate the spread of COVID-19, including physical distancing, restricted traveling and public transit, and imposed lockdowns [62]. Strategies that have been used to successfully recruit convalescent plasma donors include donor self-identification, based on public awareness following social media campaigns and campaigns through formal media outlets, as well clinician referral of patients who previously tested positive for SARS-CoV-2 infection [63].
Early work from the current CoV pandemic suggests that SARS-CoV-2 elicits a robust immune response with high levels of antibodies, including immunoglobulins (IgM and IgG), for months after the onset of COVID-19, suggesting a relatively large window of time and high probability of successful extraction of high-titer anti-SARS-CoV-2 plasma [64, 65, 66, 67, 68]. Subsequent studies have highlighted several nuances in the neutralizing antibody response; levels have been found to be higher following more severe disease [69] and to decrease substantially within the first 90 days after symptom onset in individuals with mild disease [70]. Neutralizing antibody levels can be measured in donors or convalescent plasma units indirectly, using enzyme-linked immunosorbent assays or pseudovirus neutralization assays, or directly, using live SARS-CoV-2 neutralization assays performed under biosafety level 3 conditions. Under the U.S. emergency use authorization for use of convalescent plasma to treat COVID-19 [71] issued on 23 August 2020, convalescent plasma units were dichotomized as low or high antibody titer based on results from a qualitative chemiluminescent immunoassay for detection of IgG against spike protein.
COVID-19 convalescent plasma routinely undergoes standard infectious disease screening for donated blood products but is not routinely tested for SARS-CoV-2, as respiratory viruses are not known to be transmitted by transfusion [72]. ABO and Rhesus blood type are determined to facilitate compatible plasma transfusion.
Safety profile of COVID-19 convalescent plasma
In interim reports from a large U.S. national registry including over 100,000 hospitalized adults with COVID-19, data from the first 5,000, and 20,000 patients transfused with COVID-19 convalescent plasma demonstrated low incidences of transfusion reactions (<1% of patients) [17, 18]. These interim reports provide evidence that among hospitalized patients with COVID-19, transfusion of convalescent plasma is safe and carries no excess risk of complications beyond what may be expected from fresh frozen plasma use in critically ill patients [18]. The safety of convalescent plasma treatment for COVID-19 is further supported by data from a randomized clinical trial comparing convalescent plasma transfusion to fresh frozen plasma transfusion [73]. In this trial, events were seen at comparably low rates between the control (7%) and convalescent plasma (4%) arms, suggesting that the safety profile of convalescent plasma transfusion is similar to the known safety profile of fresh frozen plasma transfusion.
Efficacy signals for COVID-19 convalescent plasma
There have been several randomized, controlled trials investigating convalescent plasma treatment in patients hospitalized for severe or life-threatening COVID-19 [74, 75, 76, 77, 78]. Four of these trials found a (non-significant) reduction in mortality following treatment with convalescent plasma [74, 75, 76, 77] versus control, whereas one trial found no mortality benefit [78, 79]. However, in the latter study, by Agarwal et al. [78], a positive effect of convalescent plasma on clinical symptoms and viral clearance was still evident, despite treatment being late in the disease course (median time to treatment = 8 days after symptom onset). Furthermore, reduced mortality from COVID-19 with convalescent plasma treatment has been observed consistently in matched-control studies [68, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91]. When pooling data across all randomized trials and controlled studies, convalescent plasma treatment was shown to be associated with a significant reduction in mortality (mortality in convalescent plasma group, 31%; versus mortality in control group, 19%; odds ratio, 0.5; 95% confidence interval (CI), 0.40 to 0.69; P < 0.001) [92].
Analyses from the U.S. COVID-19 convalescent plasma expanded access program (EAP) have revealed a dose-dependent response between the neutralizing antibody titer in donor convalescent plasma and COVID-19 mortality, where patients who received convalescent plasma with higher neutralizing antibody titers had lower mortality than patients who received convalescent plasma with lower neutralizing antibody titers (7-day mortality, ~9% versus ~12%) [93]. A smaller cohort study (n = 49) by Maor et al. [94] similarly found that a larger proportion of patients who received convalescent plasma containing high levels of virus-specific antibodies improved within 14 days compared to those receiving low-IgG convalescent plasma (~61% versus ~37%). A dose-dependent response between mortality and IgG antibody levels was also apparent in an Argentine randomized control trial of early plasma use in elderly patients [95]. The existence of a dose-response relationship is a particularly strong piece of evidence for plasma efficacy, which directly implicates specific antibodies to SARS-Cov-2 as the active agents in convalescent plasma.
Data from the EAP cohort also revealed that patients who were transfused with convalescent plasma within 3 days of COVID-19 diagnosis versus 4 or more days after diagnosis had reduced mortality (7-day mortality, ~9% versus ~12%) [96]. A single-center, propensity score-matched cohort study including 353 COVID-19 patients also assessed the relationship between the timing of convalescent plasma transfusion and mortality. Salazar et al. [91] found that 60-day mortality was not different between patients transfused with convalescent plasma >72 hours after admission and controls, whereas mortality was significantly decreased in patients who received high-antibody titer convalescent plasma within 72 hours of admission (convalescent plasma, ~6% versus control, ~11% mortality). In fact, these investigators found that the greatest efficacy of convalescent plasma was associated with administration in the first 44 hours of hospitalization [97]. Overall, these results provide evidence that the mortality benefit of convalescent plasma is most apparent in patients transfused with plasma containing high antibody levels early in the disease course, consistent with historical precedents of convalescent plasma use in prior infectious disease outbreaks [36, 56, 59].
Framework for the Future
In the context of COVID-19, the available data support the notion that convalescent plasma provides clinical and mortality benefits for hospitalized patients and that the benefit of convalescent plasma is most apparent in patients transfused with plasma containing high anti-SARS-CoV-2 antibody levels early in the disease course. The observed dose-response relationship between antibody levels and mortality suggests that neutralizing antibodies are an active agent in convalescent plasma and that antibody activity is a marker of convalescent plasma potency. Based on the notion that virus-neutralizing antibodies are an effective treatment, monoclonal antibody treatments are being developed and have demonstrated encouraging signs of effectiveness in reducing symptoms and viral load in early studies [98, 99, 100]. In line with the development of vaccines subsequent to convalescent plasma treatment in the early 20th century, immunization is anticipated to ultimately provide substantial protection against COVID-19.
The historical experiences and current evidence during the COVID-19 pandemic described here provide a compelling rationale for convalescent blood products to remain a “weapon against illness and deaths in the defense against infectious diseases” [1]. Although there are uncertainties and limitations regarding the use of convalescent blood products in the context of any pandemic [27], convalescent blood products should be considered an “empiric” therapy and a first-line defense against novel infectious diseases. Convalescent blood products may continue to be an effective stopgap therapeutic until the vaccine cavalry arrives. Experiences from the COVID-19 pandemic may serve as a model for future responses to outbreaks of novel viral diseases among humans.
References
- 1.The Nobel Prize in Physiology or Medicine 1901. In: 2020. NMA, editor. NobelPrize.org.
- 2.Rojek AM, Horby PW. Modernising epidemic science: enabling patient-centred research during epidemics. BMC Med. 2016;14:212. doi: 10.1186/s12916-016-0760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montelongo-Jauregui D, Vila T, Sultan AS, Jabra-Rizk MA. Convalescent serum therapy for COVID-19: A 19th century remedy for a 21st century disease. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 7.Ni L, Ye F, Cheng ML, Feng Y, Deng YQ, Zhao H. Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity. 2020;52:971–7 e3. doi: 10.1016/j.immuni.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burki TK. Completion of clinical trials in light of COVID-19. Lancet Respir Med. 2020;8:1178–1180. doi: 10.1016/S2213-2600(20)30460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Group RC, Horby P, Mafham M, Linsell L, Bell JL, Staplin N. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. 2020;383:2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horby PW, Mafham M, Bell JL, Linsell L, Staplin N, Emberson J. Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020;396:1345–1352. doi: 10.1016/S0140-6736(20)32013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Consortium WHOST, Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V. Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med. 2020 doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Group RC, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med. 2020 [Google Scholar]
- 13.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmer C, Thomas K. New York Times Company; New York City: 2020. Pfizer's Covid Vaccine: 11 Things You Need to Know. The New York Times. [Google Scholar]
- 15.Ducharme J. TIME USA, LLC; New York City: 2020. Why You May Not Be Able to Get Pfizer's Frontrunner COVID-19 Vaccine. TIME. [Google Scholar]
- 16.Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, Winters JL. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020 doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joyner MJ, Bruno KA, Klassen SA, Kunze KL, Johnson PW, Lesser ER. Safety Update: COVID-19 Convalescent Plasma in 20,000 Hospitalized Patients. Mayo Clin Proc. 2020;95:1888–1897. doi: 10.1016/j.mayocp.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joyner MJ, Wright RS, Fairweather D, Senefeld JW, Bruno KA, Klassen SA. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130:4791–4797. doi: 10.1172/JCI140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casadevall A, Scharff MD. Return to the past: the case for antibody-based therapies in infectious diseases. Clin Infect Dis. 1995;21:150–161. doi: 10.1093/clinids/21.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joyner MJ, Carter RE, Senefeld JW, Klassen SA, Mills JR, Johnson PW. Association of Convalescent Plasma Antibodies with Covid-19 Mortality. In review. 2020 [Google Scholar]
- 21.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 25.Jayaweera M, Perera H, Gunawardana B, Manatunge J. Transmission of COVID-19 virus by droplets and aerosols: A critical review on the unresolved dichotomy. Environ Res. 2020;188 doi: 10.1016/j.envres.2020.109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casadevall A, Pirofski LA. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130:1545–1548. doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marano G, Vaglio S, Pupella S, Facco G, Catalano L, Liumbruno GM. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus. 2016;14:152–157. doi: 10.2450/2015.0131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casadevall A, Pirofski LA. The convalescent sera option for containing COVID-19. J Clin Invest. 2020 doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garraud O, Heshmati F, Pozzetto B, Lefrere F, Girot R, Saillol A. Plasma therapy against infectious pathogens, as of yesterday, today and tomorrow. Transfus Clin Biol. 2016;23:39–44. doi: 10.1016/j.tracli.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naranjo-Gomez M, Lambour J, Piechaczyk M, Pelegrin M. Neutrophils are essential for induction of vaccine-like effects by antiviral monoclonal antibody immunotherapies. JCI Insight. 2018:3. doi: 10.1172/jci.insight.97339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pelegrin M, Naranjo-Gomez M, Piechaczyk M. Antiviral Monoclonal Antibodies: Can They Be More Than Simple Neutralizing Agents? Trends Microbiol. 2015;23:653–665. doi: 10.1016/j.tim.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nasser R, Pelegrin M, Plays M, Gros L, Piechaczyk M. Control of regulatory T cells is necessary for vaccine-like effects of antiviral immunotherapy by monoclonal antibodies. Blood. 2013;121:1102–1111. doi: 10.1182/blood-2012-06-432153. [DOI] [PubMed] [Google Scholar]
- 33.Xi Y. Convalescent plasma therapy for COVID-19: a tried-and-true old strategy? Signal Transduct Target Ther. 2020;5:203. doi: 10.1038/s41392-020-00310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cecil RL. Effects of Early Serum Treatment on Pneumococcus Type I Pneumonia. Trans Am Clin Climatol Assoc. 1936;52:52–63. [PMC free article] [PubMed] [Google Scholar]
- 35.Kaufmann SH. Remembering Emil von Behring: from Tetanus Treatment to Antibody Cooperation with Phagocytes. mBio. 2017:8. doi: 10.1128/mBio.00117-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145:599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- 37.Rambar AC. Mumps; use of convalescent serum in the treatment and prophylaxis of orchitis. Am J Dis Child. 1946;71:1–13. [PubMed] [Google Scholar]
- 38.Hammon WM, Coriell LL, Wehrle PF, Stokes J., Jr. Evaluation of Red Cross gamma globulin as a prophylactic agent for poliomyelitis. IV. Final report of results based on clinical diagnoses. J Am Med Assoc. 1953;151:1272–1285. [PubMed] [Google Scholar]
- 39.Janeway CA. Use of Concentrated Human Serum gamma-Globulin in the Prevention and Attenuation of Measles. Bull N Y Acad Med. 1945;21:202–222. [PMC free article] [PubMed] [Google Scholar]
- 40.Alexander HE, Leidy G. Hemophilus influenzae meningitis treated with streptomycin. JAMA. 1946;132:434–440. doi: 10.1001/jama.1946.02870430014005. [DOI] [PubMed] [Google Scholar]
- 41.Casadevall A, Scharff MD. Serum therapy revisited: animal models of infection and development of passive antibody therapy. Antimicrob Agents Chemother. 1994;38:1695–1702. doi: 10.1128/aac.38.8.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maiztegui JI, Fernandez NJ, de Damilano AJ. Efficacy of immune plasma in treatment of Argentine haemorrhagic fever and association between treatment and a late neurological syndrome. Lancet. 1979;2:1216–1217. doi: 10.1016/s0140-6736(79)92335-3. [DOI] [PubMed] [Google Scholar]
- 43.Ruggiero HA, Pérez Isquierdo F, Milani HA, Barri A, Val A, Maglio F. Treatment of Argentine hemorrhagic fever with convalescent's plasma. 4433 cases. Presse Med. 1986;15:2239–2242. [PubMed] [Google Scholar]
- 44.Hung IF, To KK, Lee CK, Lee KL, Chan K, Yan WW. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52:447–456. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sahr F, Ansumana R, Massaquoi TA, Idriss BR, Sesay FR, Lamin JM. Evaluation of convalescent whole blood for treating Ebola Virus Disease in Freetown, Sierra Leone. J Infect. 2017;74:302–309. doi: 10.1016/j.jinf.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kraft CS, Hewlett AL, Koepsell S, Winkler AM, Kratochvil CJ, Larson L. The Use of TKM-100802 and Convalescent Plasma in 2 Patients With Ebola Virus Disease in the United States. Clin Infect Dis. 2015;61:496–502. doi: 10.1093/cid/civ334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kong LK, Zhou BP. Successful treatment of avian influenza with convalescent plasma. Hong Kong Med J. 2006;12:489. [PubMed] [Google Scholar]
- 48.Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med. 2007;357:1450–1451. doi: 10.1056/NEJMc070359. [DOI] [PubMed] [Google Scholar]
- 49.Wu XX, Gao HN, Wu HB, Peng XM, Ou HL, Li LJ. Successful treatment of avian-origin influenza A (H7N9) infection using convalescent plasma. Int J Infect Dis. 2015;41:3–5. doi: 10.1016/j.ijid.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan HC, Roback JD. Convalescent Plasma: Therapeutic Hope or Hopeless Strategy in the SARS-CoV-2 Pandemic. Transfus Med Rev. 2020;34:145–150. doi: 10.1016/j.tmrv.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong VW, Dai D, Wu AK, Sung JJ. Treatment of severe acute respiratory syndrome with convalescent plasma. Hong Kong Med J. 2003;9:199–201. [PubMed] [Google Scholar]
- 52.Kong L. Severe acute respiratory syndrome (SARS) Transfus Apher Sci. 2003;29:101. doi: 10.1016/S1473-0502(03)00109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou XZ, Zhao M, Wang FS, Jiang TJ, Li YG, Nie WM. Epidemiologic features, clinical diagnosis and therapy of first cluster of patients with severe acute respiratory syndrome in Beijing area. Zhonghua Yi Xue Za Zhi. 2003;83:1018–1022. [PubMed] [Google Scholar]
- 54.Yeh KM, Chiueh TS, Siu LK, Lin JC, Chan PK, Peng MY. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother. 2005;56:919–922. doi: 10.1093/jac/dki346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soo YO, Cheng Y, Wong R, Hui DS, Lee CK, Tsang KK. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect. 2004;10:676–678. doi: 10.1111/j.1469-0691.2004.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng Y, Wong R, Soo YO, Wong WS, Lee CK, Ng MH. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw FM, Lim WS. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Min CK, Cheon S, Ha NY, Sohn KM, Kim Y, Aigerim A. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci Rep. 2016;6 doi: 10.1038/srep25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ko JH, Seok H, Cho SY, Ha YE, Baek JY, Kim SH. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience. Antivir Ther. 2018;23:617–622. doi: 10.3851/IMP3243. [DOI] [PubMed] [Google Scholar]
- 60.Arabi YM, Hajeer AH, Luke T, Raviprakash K, Balkhy H, Johani S. Feasibility of Using Convalescent Plasma Immunotherapy for MERS-CoV Infection, Saudi Arabia. Emerg Infect Dis. 2016;22:1554–1561. doi: 10.3201/eid2209.151164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Food and Drug Administration . Department of Health and Human Services; Silver Springs, Maryland: 2019. Food and drugs chapter I - part 640 - Additional standards for human blood and blood products. [Google Scholar]
- 62.Budhai A, Wu AA, Hall L, Strauss D, Paradiso S, Alberigo J. How did we rapidly implement a convalescent plasma program? Transfusion. 2020;60:1348–1355. doi: 10.1111/trf.15910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Andersen KJ, Klassen SA, Larson KF, Ripoll JG, Senefeld JW, Clayburn AJ. Recruitment Strategy for Potential COVID-19 Convalescent Plasma Donors. Mayo Clin Proc. 2020;95:2343–2349. doi: 10.1016/j.mayocp.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease, 2019. medRxiv. 2020 doi: 10.1093/cid/ciaa344. 2020.03.02.20030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dong C, Ni L, Ye F, Chen M-L, Feng Y, Deng Y-Q. Characterization of anti-viral immunity in recovered individuals infected by SARS-CoV-2. medRxiv. 2020 2020.03.17.20036640. [Google Scholar]
- 66.Wajnberg A, Amanat F, Firpo A, Altman DR, Bailey MJ, Mansour M. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370:1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Amanat F, Stadlbauer D, Strohmeier S, Nguyen THO, Chromikova V, McMahon M. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;26:1033–1036. doi: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duan K, Liu B, Li C, Zhang H, Yu T, Qu J. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klein SL, Pekosz A, Park H-S, Ursin RL, Shapiro JR, Benner SE. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J Clin Invest. 2020;130:6141–6150. doi: 10.1172/JCI142004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ibarrondo FJ, Fulcher JA, Goodman-Meza D, Elliott J, Hofmann C, Hausner MA. Rapid Decay of Anti–SARS-CoV-2 Antibodies in Persons with Mild Covid-19. N Eng J Med. 2020;383:1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hilton DM. Food and Drug Administration; Silver Spring, Maryland: 2020. Emergency Use Authorization (EUA) for emergency use of COVID-19 convalescent plasma forthe treatment of hos pitalized patients with Coronavirus Disease 2019 (COVID-19) pp. 1–7. [Google Scholar]
- 72.Katz LM. Is SARS-CoV-2 transfusion transmitted? Transfusion. 2020;60:1111–1114. doi: 10.1111/trf.15831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bajpai M, Kumar S, Maheshwari A, Chhabra K, kale P, Gupta A. Efficacy of Convalescent Plasma Therapy compared to Fresh Frozen Plasma in Severely ill COVID-19 Patients: A Pilot Randomized Controlled Trial. medRxiv. 2020 2020.10.25.20219337. [Google Scholar]
- 74.Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA. 2020;324:460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gharbharan A, Jordans CCE, GeurtsvanKessel C, den Hollander JG, Karim F, Mollema FPN. Convalescent Plasma for COVID-19. A randomized clinical trial. medRxiv. 2020 2020.07.01.20139857. [Google Scholar]
- 76.Rasheed AM, Fatak DF, Hashim HA, Maulood MF, Kabah KK, Almusawi YA. The therapeutic potential of convalescent plasma therapy on treating critically-ill COVID-19 patients residing in respiratory care units in hospitals in Baghdad, Iraq. Infez Med. 2020;28:357–366. [PubMed] [Google Scholar]
- 77.Avendano-Sola C, Ramos-Martinez A, Munez-Rubio E, Ruiz-Antoran B, Malo de Molina R, Torres F. Convalescent Plasma for COVID-19: A multicenter, randomized clinical trial. medRxiv. 2020 2020.08.26.20182444. [Google Scholar]
- 78.Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ (Clinical research ed.) 2020;371 doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Simonovich VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone MG, Vazquez C. A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia. N Engl J Med. 2020 doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Perotti C, Baldanti F, Bruno R, Del Fante C, Seminari E, Casari S. Mortality reduction in 46 severe Covid-19 patients treated with hyperimmune plasma. A proof of concept single arm multicenter trial. Haematologica. 2020 doi: 10.3324/haematol.2020.261784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Omrani AS, Zaqout A, Baiou A, Daghfal J, Elkum N, Alattar RA. Convalescent plasma for the treatment of patients with severe coronavirus disease 2019: A preliminary report. J Med Virol. 2020 doi: 10.1002/jmv.26537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hegerova L, Gooley TA, Sweerus KA, Maree C, Bailey N, Bailey M. Use of convalescent plasma in hospitalized patients with COVID-19: case series. Blood. 2020;136:759–762. doi: 10.1182/blood.2020006964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zeng QL, Yu ZJ, Gou JJ, Li GM, Ma SH, Zhang GF. Effect of Convalescent Plasma Therapy on Viral Shedding and Survival in Patients With Coronavirus Disease 2019. J Infect Dis. 2020;222:38–43. doi: 10.1093/infdis/jiaa228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Donato M, Park S, Baker M, Korngold R, Morawski A, Geng X. Clinical and laboratory evaluation of patients with SARS-CoV-2 pneumonia treated with high-titer convalescent plasma: a prospective study. medRxiv. 2020 doi: 10.1172/jci.insight.143196. 2020.07.20.20156398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu STH, Lin HM, Baine I, Wajnberg A, Gumprecht JP, Rahman F. Convalescent plasma treatment of severe COVID-19: a propensity score-matched control study. Nat Med. 2020 doi: 10.1038/s41591-020-1088-9. [DOI] [PubMed] [Google Scholar]
- 86.Salazar MR, González SE, Regairaz L, Ferrando NS, González Martínez VV, Carrera Ramos PM. Effect of convalescent plasma on mortality in patients with COVID-19 pneumonia. medRxiv. 2020 2020.10.08.20202606. [Google Scholar]
- 87.Xia X, Li K, Wu L, Wang Z, Zhu M, Huang B. Improved clinical symptoms and mortality among patients with severe or critical COVID-19 after convalescent plasma transfusion. Blood. 2020;136:755–759. doi: 10.1182/blood.2020007079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abolghasemi H, Eshghi P, Cheraghali AM, Imani Fooladi AA, Bolouki Moghaddam F, Imanizadeh S. Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: Results of a multicenter clinical study. Transfus Apher Sci. 2020 doi: 10.1016/j.transci.2020.102875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rogers R, Shehadeh F, Mylona EK, Rich J, Neill M, Touzard-Romo F. Convalescent plasma for patients with severe COVID-19: a matched cohort study. 2020 doi: 10.1093/cid/ciaa1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Altuntas F, Ata N, Yigenoglu TN, Bascı S, Dal MS, Korkmaz S. Convalescent plasma therapy in patients with COVID-19. Transfus Apher Sci. 2020 doi: 10.1016/j.transci.2020.103017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Salazar E, Christensen PA, Graviss EA, Nguyen DT, Castillo B, Chen J. Significantly Decreased Mortality in a Large Cohort of Coronavirus Disease 2019 (COVID-19) Patients Transfused Early with Convalescent Plasma Containing High-Titer Anti-Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Spike Protein IgG. Am J Pathol. 2021;191:90–107. doi: 10.1016/j.ajpath.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Klassen SA, Senefeld JW, Johnson PW, Carter RE, Wiggins CC, Shoham S. Evidence favoring the efficacy of convalescent plasma for COVID-19 therapy. medRxiv. 2020 2020.07.29.20162917. [Google Scholar]
- 93.Administration US-FD. Updated Evidence to Support the Emergency Use of COVID-19 Convalescent Plasma – as of 9/23/2020. 2020.
- 94.Maor Y, Cohen D, Paran N, Israely T, Ezra V, Axelrod O. Compassionate use of convalescent plasma for treatment of moderate and severe pneumonia in COVID-19 patients and association with IgG antibody levels in donated plasma. EClinicalMedicine. 2020;26 doi: 10.1016/j.eclinm.2020.100525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Libster R, Marc GP, Wappner D, Coviello S, Bianchi A, Braem V. Prevention of severe COVID-19 in the elderly by early high-titer plasma. medRxiv. 2020 2020.11.20.20234013. [Google Scholar]
- 96.Joyner MJ, Senefeld JW, Klassen SA, Mills JR, Johnson PW, Theel ES. Effect of Convalescent Plasma on Mortality among Hospitalized Patients with COVID-19: Initial Three-Month Experience. medRxiv. 2020 2020.08.12.20169359. [Google Scholar]
- 98.Regeneron. Regeneron's REGN-COV2 Antibody Cocktail Reduced Viral Levels and Improved Symptoms in Non-Hospitalized COVID-19 Patients, 2020.
- 99.Lilly. Lilly announces proof of concept data for neutralizing antibody LY-CoV555 in the COVID-19 outpatient setting, 2020.
- 100.Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19. N Eng J Med. 2020 doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Flexner S. The Results of the Serum Treatment in Thirteen Hundred Cases of Epidemic Meningitis. J Exp Med. 1913;17:553–576. doi: 10.1084/jem.17.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Balfour HH, Jr., Groth KE, McCullough J, Kalis JM, Marker SC, Nesbit ME. Prevention or modification of varicella using zoster immune plasma. Am J Dis Child. 1977;131:693–696. doi: 10.1001/archpedi.1977.02120190087020. [DOI] [PubMed] [Google Scholar]
- 103.Zingher A. Convalescent Whole Blood, Plasma and Serum in Prophylaxis of Measles. JAMA. 1924;82:1180–1187. doi: 10.1002/rmv.480. [DOI] [PubMed] [Google Scholar]
- 104.Soo YOY, Cheng Y, Wong R, Hui DS, Lee CK, Tsang KKS. Wiley Online Library; 2004. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clinical microbiology and infection; pp. 676–678. [DOI] [PMC free article] [PubMed] [Google Scholar]