Supplemental Digital Content is available in the text.
Background.
Liver splitting allows the opportunity to share a deceased graft between 2 recipients but remains underutilized. We hypothesized that liver splitting during continuous dual hypothermic oxygenated machine perfusion (DHOPE) is feasible, with shortened total cold ischemia times and improved logistics. Here, we describe a left lateral segment (LLS) and extended right lobe (ERL) liver split procedure during continuous DHOPE preservation with subsequent transplantation at 2 different centers.
Methods.
After transport using static cold storage, a 51-year-old brain death donor liver underwent end-ischemic DHOPE. During DHOPE, the donor liver was maintained <10 °C and oxygenated with a Po2 of >106 kPa. An ex situ ERL/LLS split was performed with continuing DHOPE throughout the procedure to avoid additional ischemia time.
Results.
Total cold ischemia times for the LLS and ERL were 205 minutes and 468 minutes, respectively. Both partial grafts were successfully transplanted at 2 different transplant centers. Peak aspartate aminotransferase and alanine aminotransferase were 172 IU/L and 107 IU/L for the LLS graft, and 839 IU/L and 502 IU/L for the ERL graft, respectively. The recipient of the LLS experienced an episode of acute cellular rejection. The ERL transplantation was complicated by severe acute pancreatitis with jejunum perforation requiring percutaneous drainage and acute cellular rejection. No device-related adverse events were observed.
Conclusions.
Liver splitting during continuous DHOPE preservation is feasible, has the potential to substantially shorten cold ischemia time and may optimize transplant logistics. Therefore liver splitting with DHOPE can potentially improve utilization of split liver transplantation.
With an ongoing disparity between supply and demand for transplantable livers, particularly for pediatric recipients, it has become increasingly important to find new methods of both expanding the donor pool and improving graft quality.1 One method for increasing the number of available grafts is to split the deceased donor liver and share the graft between 2 recipients.2 For the majority of pediatric recipients, a left lateral segment (LLS) split suffices, while the extended right lobe (ERL) can be transplanted into an adult recipient. Liver splitting typically takes place on the back table with the graft immersed in ice-cold preservation solution. The splitting procedure itself, as well as subsequent transport of the ERL to a remote transplant center prolongs cold ischemia time (CIT), which may negatively affect patient outcome after transplantation.3 As a solution, in situ liver splitting, similar to that seen in living donor procedures, was developed as a way to reduce CITs.4 This, however, prolongs operation time during organ retrieval and may complicate logistics.
End-ischemic ex situ hypothermic oxygenated machine perfusion has seen increasing utilization in recent years due to its ability to mitigate ischemia/reperfusion injury (IRI) in donation after circulatory death liver transplantation.5 This method can be applied to single hypothermic machine perfusion through the portal vein (HOPE) or dual perfusion through the portal vein and hepatic artery (dual hypothermic oxygenated machine perfusion [DHOPE]). The application of end-ischemic dynamic machine preservation by DHOPE during split liver procedures could provide an interesting strategy to reduce prolonged CITs associated with ex situ liver splitting. Furthermore, replenishment of ATP during DHOPE and maintaining a constantly stable temperature during the split may attenuate IRI, improving organ quality and outcome in both partial grafts.6 The combination of above-mentioned advantages may feasibly improve transplant logistics.
Here, we present a case report demonstrating the technical aspects of liver splitting during dynamic machine preservation with DHOPE, after which both partial grafts were successfully transplanted at 2 different centers.
MATERIALS AND METHODS
DHOPE is implemented as standard practice in our center for donation after circulatory death liver transplantation and can be applied for logistical reasons such as expected prolonged CITs (eg, in case of retransplantation or ex situ split). No formal medical ethical committee approval was obtained for this case. The Declaration of Helsinki and the Declaration of Istanbul were adhered to.
A liver graft was accepted from a 51-year-old brain death donor in a regional hospital who suffered from cerebrovascular bleeding. The donor weight was 70 kg, height 192 cm, and had a calculated body mass index of 19 kg/m2. The Eurotransplant donor risk index was 1.62. Organ procurement was performed in a standard fashion. During procurement, the donor organs were flushed via the cannulated aorta using 5 liters of cold, heparinized (25 000 IU) modified University of Wisconsin (UW) preservation solution. Hepatectomy was completed after 38 minutes from start of cold perfusion, and after an additional portal back table flush with 2 liters of UW solution, the liver was placed in static cold storage (SCS) for transport to the splitting center. A 5 cm cylindrical segment of supratruncal aorta was left attached to the celiac trunk during procurement for cannulation purposes.
Upon arrival at the splitting center (full timeline represented in Figure 1), the liver was immersed in ice-cold UW solution for back table procurement to prepare for dual cannulation allowing DHOPE preservation using the portal vein for portal perfusion and the supratruncal aorta for arterial perfusion, as described previously.7 In brief, the liver was placed in supine position in the reservoir of a LiverAssist device (Organ Assist, Groningen, The Netherlands), after which the 24F portal vein and hepatic artery cannulas were subsequently connected to the perfusion system. A continuous portal flow was provided with a pressure of 3 mm Hg. Hepatic artery pressure was set and maintained at 25 mm Hg with pulsatile flow of 60/min throughout the perfusion. Temperature was maintained at <10 °C throughout the perfusion. The perfusion solution comprised 4 L UW machine perfusion solution (PumpProtect; Carnamedica, Warsaw, Poland) and was oxygenated (100% oxygen at 1 L/min) with a Po2 of >106 kPa.
FIGURE 1.
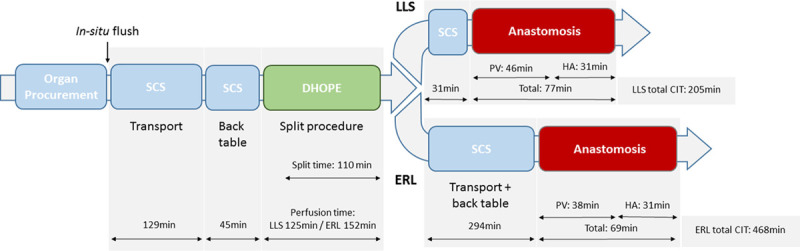
Timeline of dual hypothermic oxygenated machine perfusion (DHOPE) split liver procedure into the left lateral segment (LLS) and extended right lobe (ERL). CIT, cold ischemia time; HA, hepatic artery; PV, portal vein; SCS, static cold storage.
Portal venous and hepatic arterial flow and pressure parameters were maintained and recorded every 15 minutes. Perfusate analysis was performed every 30 minutes using an ABL90 FLEX blood gas analyzer (Radiometer, Denmark).
Ex situ LLS and ERL split was performed in the LiverAssist reservoir during continuous DHOPE throughout the procedure (Figure 2A–D) by 2 surgeons assisted by a surgical nurse (Video, SDC, http://links.lww.com/TXD/A308). Surgeon 1, standing at the front of the LiverAssist, used the Cavitron ultrasonic surgical aspirator device (Excel+; Integra LifeSciences, Tullamore, Ireland), with simultaneous ligation and cutting of exposed microvasculature/bile ducts in the parenchymal transection plane performed by surgeon 2, standing at the back of the LiverAssist. A gauze was placed underneath the liver to prevent any Cavitron ultrasonic surgical aspirator-related tissue debris from entering the perfusion system and preclude potential obstruction of the oxygenators. Additionally, a piece of silicone tubing was placed underneath the transection plane and connected to the rim of the reservoir to establish a reversed hanging maneuver, “folding” the graft open along the transection plane like a book, allowing for improved visualization. The left hepatic vein was separated from the middle hepatic vein and caval vein, and good venous outflow from both LLS and ERL was observed. After parenchymal transection, both LLS and ERL remained adequately perfused via the portal and arterial branches, indicated by stable perfusion parameters (Figure 2A–E). Finally, the hilar plate (including the bile duct) was identified and divided at the plane between S4 and S2/3, resulting in complete division except for the hepatic arteries and portal veins. Because the LLS was allocated to a pediatric recipient at the splitting center, the timing of vascular division was performed in accordance with the surgical team of the pediatric recipient to minimize the second SCS time. When the recipient went anhepatic, the left portal vein and left hepatic artery of the donor graft were divided and the stump to the main portal vein and proper hepatic artery were over sewn. The LLS was removed, immediately immersed in ice-cold UW solution, and transferred to the recipient operating room, while the ERL remained in the reservoir with continuing machine perfusion (Figure 2E). Subsequently, the ERL was removed from the device, immediately immersed in ice-cold UW solution, and packed in polystyrene box with ice for transportation to the second transplant center.
FIGURE 2.
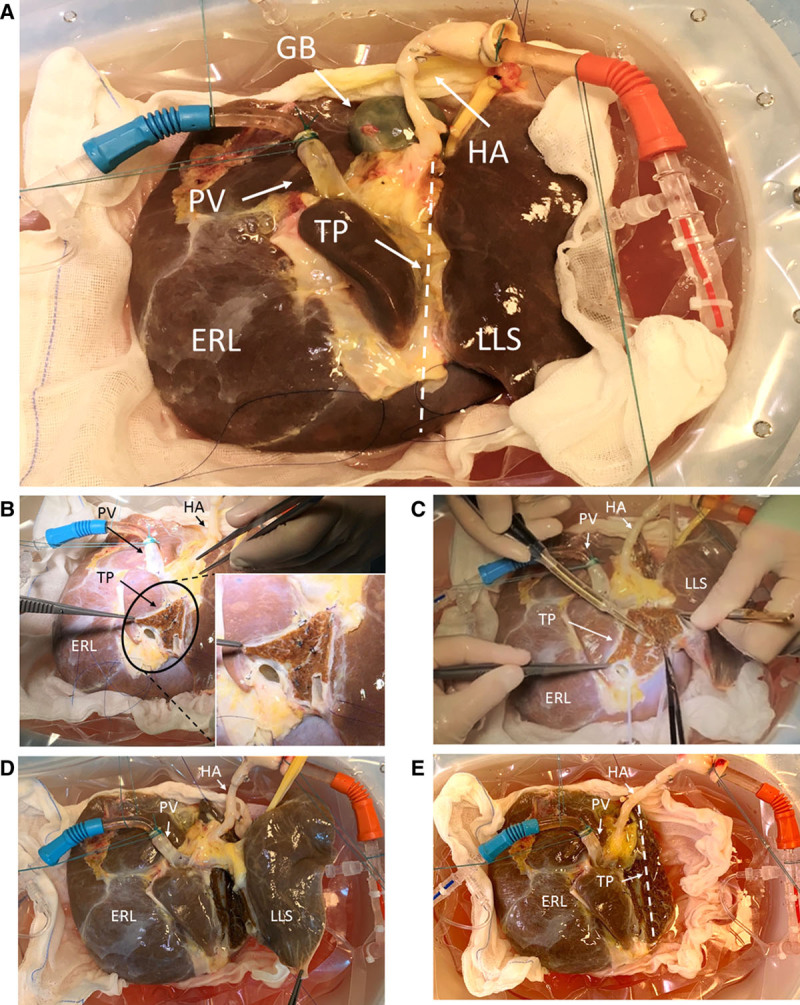
The progression of the split procedure is observable from (A) start of dual hypothermic oxygenated machine perfusion (DHOPE), (B) start of left lateral segment (LLS)/extended right lobe (ERL) liver split with division of the middle and left hepatic vein with magnification of the transection plane, (C) midway through parenchymal liver split using the CUSA device, (D) demonstrating full parenchymal separation of the LLS from the ERL, and (E) showing dual perfusion of the ERL only, after the LLS has been fully removed. CUSA, Cavitron ultrasonic surgical aspirator; GB, gall bladder; HA, hepatic artery; PV, portal vein; TP, transection plane.
RESULTS
The first CIT for the SCS-preserved organ was 174 minutes, including 129 minutes of transport to our center, followed by a back-table procedure and cannulation for another 45 minutes (Figure 1).
During DHOPE, hepatic arterial and portal venous flow rates were 50–60 mL/min at 25 mm Hg and 80–120 mL/min at 3 mm Hg, respectively. Slight fluctuations in flow occurred due to manipulation of the liver during the split procedure. DHOPE preservation time for the LLS was 125 minutes. The ERL remained on the pump for a further 27 minutes while the LLS was being prepared for implantation and transported to the recipient operating room. The duration of the splitting procedure during DHOPE preservation was 110 minutes (Figure 1). Total preservation time for the LLS was 355 minutes. Postperfusion weight was 216 g for the LLS and 1082 g for the ERL.
Implantation of the LLS and portal reperfusion required 46 minutes. The hepatic arterial anastomosis required an additional 31 minutes, giving a total anastomosis time of 77 minutes. Perioperative blood loss was 3.4 liters. The recipient was supplemented with 5 units of red blood cells, 2 units of plasma, 1.2 g fibrinogen, and 200 mg tranexamic acid. The postoperative course was complicated by a reoperation for removal of a hematoma at postoperative day 3 and a grade 2 (biopsy-proven) episode of acute cellular rejection treated by high dose steroids after 27 days. LLS recipient initially experienced a peak-rise of both aspartate aminotransferase (172 IU/L) and alanine aminotransferase (107 IU/L), followed by a decrease in the first 4 days posttransplant. The second increase from day 4 to beyond day 7 of both markers was most likely related to the grade 2 acute cellular rejection (Figure 3A). On day 7, bilirubin was 2.46 mg/dL and international normalized ratio was 1.3.
FIGURE 3.
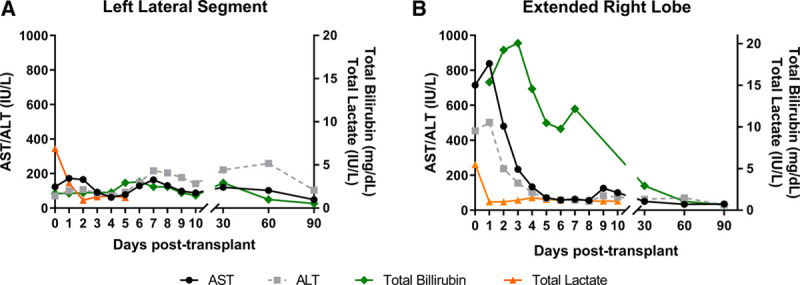
Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, and total lactate levels in the recipients of (A) left lateral segment (LLS) and (B) extended right lobe (ERL) during the first 3 mo after transplantation. The increase in AST and ALT 5 d posttransplant seen in the LLS graft recipient is reflective of an episode of (biopsy-proven) acute rejection.
The total CIT for the ERL was 468 minutes, and a total preservation time of 622 minutes (Figure 1). The ERL was reperfused via the portal vein after 38 minutes and subsequently via the hepatic artery after 31 minutes. Perioperative blood loss was 6.2 liters. The recipient was supplemented with 4 units of red blood cells, 4 units of plasma, and 1 unit of platelets. The postoperative course was complicated by severe acute pancreatitis with jejunum perforation requiring percutaneous drainage and acute cellular rejection (biopsy-proven) treated with increased immunosuppression. Peak of both aspartate aminotransferase (839 IU/L) and alanine aminotransferase (502 IU/L) in the ERL recipient was seen on day 2 posttransplant followed by a decrease of transaminases in the subsequent 5 days (Figure 3B). On day 7, bilirubin was 12.2 mg/dL and international normalized ratio was 1.4.
At 6-month follow-up, both LLS and ERL recipients are at home with good functioning grafts. No device-related adverse events were observed during follow-up.
DISCUSSION
Liver splitting has the opportunity to expand 1 scarce resource into 2, thereby adding great value in liver transplantation for vulnerable recipients such as pediatrics and small adults. Despite improvements in surgical techniques and expertise, split liver transplantation remains underutilized.8 Typically, split liver procedures take place on the back-table under ischemic SCS conditions. One major advantage of DHOPE is the protective mechanisms induced by keeping the organ both cold and oxygenated during the split procedure. This substantially shortens the ischemic SCS time. End-ischemic DHOPE resuscitates mitochondria, leading to ATP replenishment during dynamic preservation. Subsequently, the production of reactive oxygen species after reperfusion in the recipient is reduced, mitigating IRI.5
Another advantage of dual perfusion is that potential variation in the arterial anatomy becomes more obvious. Arteries are filled with pulsatile flow, leading to better visualization. This also makes it easier to identify leaks from arterial branches that may not have been ligated. A potential disadvantage is that the current LiverAssist device does not allow performance of an intraoperative cholangiogram in the context of bile duct division planning during perfusion. However, if necessary, this can theoretically be accomplished by extended tubing and using a radiolucent bowl.
At present, only 1 other case report of ex situ liver splitting with concurrent DHOPE exists, where a 19-year-old brain death donor liver was split for implantation into 2 pediatric recipients, with a hyperreduction of the LLS to S2 for transplant to a neonate. The authors demonstrated positive results, with mild IRI and no device-related adverse events.9 Both grafts were transplanted at the same center, and therefore, no second transportation was involved; however, they report a total CIT of 11 and 14 hours for LLS and ERL, respectively. In our study, DHOPE allowed for a substantial reduction in CIT, particularly in the case of the ERL, where total CIT was reduced to <8 hours even with the addition of a second transport time to a second center (294 min transport and back table).
Liver splitting during normothermic machine perfusion (NMP) has previously been demonstrated as a proof of concept on human grafts rejected for transplant.10-12 These studies proposed splitting during NMP as a method of viability assessment, logistical improvement, and of potential benefit to the graft by reducing ischemia times. Although functional assessment is not possible at hypothermic temperatures, in optimal, high-quality grafts such as the 1 reported here, functional assessment and viability testing are not necessary. Liver splitting during NMP may add increased risk of injury through additional and unnecessary rewarming steps, increasing warm ischemia times. ERL grafts traveling to another recipient hospital after splitting will also undergo an additional episode of cooling, SCS, and rewarming. The effects of repeat cycles of rewarming on liver grafts are unknown.
DHOPE has several advantages when compared with NMP for liver splitting. Firstly, there is no recooling phase between end of NMP and SCS for transport, and thus additional injury from temperature change is avoided. Second, DHOPE poses a lower risk to the organ should there be a technical issue with the perfusion machine. In the event of such an issue, the graft is simply returned to SCS conditions without the need for rapid cooling and flushing that would be necessary during NMP. Furthermore, the liver is under minimal metabolic demand during DHOPE preservation. The split graft to be transplanted in the splitting center is in optimal condition for implantation due to resuscitation from end-ischemic DHOPE. The split graft traveling to a separate transplant center is subjected to a second phase of CIT after initial the split, however, does benefit from a shorter ischemic preservation time and from the oxygenated resuscitation during the split procedure. This is preferable over end-ischemic NMP, where the organ is not resuscitated before perfusion at normothermia (37 °C). Finally, the combination of the discussed advantages above may feasibly improve logistical obstacles.
Our technique of vascular splitting at the level of the left portal vein and left hepatic artery allowed continuing DHOPE preservation of the ERL graft. There is currently no evidence that HOPE is inferior to DHOPE, meaning that HOPE with portal vein perfusion could be continued in cases where the proper hepatic artery is used for the LLS. This, however, would not be possible with NMP, as sufficient oxygenation of the bile ducts via the hepatic artery is essential at 37 °C. Therefore, (D)HOPE may facilitate sequential liver transplantation of the ERL graft at the same center.9 Additionally, our report provides further evidence that DHOPE for split liver transplantation is feasible and can be an attractive therapeutic solution to grafts with expected prolonged CIT.
We propose that the technique of liver splitting during continuous DHOPE has the potential to improve logistics and utilization of split liver transplantation and could be a useful strategy to shorten ischemic SCS time and mitigate subsequent IRI.
Supplementary Material
Footnotes
Published online 4 February, 2021.
The authors declare no funding or conflicts of interest.
A.M.T., H.H., W.G.P., R.J.P., and V.E.d.M. participated in writing of the article. A.M.T. and V.E.d.M. participated in data analysis. R.J.P. and V.E.d.M. participated in study design. All authors participated in performance of the study. All authors have read the article, contributed with critical revisions, and have approved the final draft.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Wertheim JA, Petrowsky H, Saab S, et al. Major challenges limiting liver transplantation in the United States. Am J Transplant. 2011; 11:1773–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pichlmayr R, Ringe B, Gubernatis G, et al. [Transplantation of a donor liver to 2 recipients (splitting transplantation)–a new method in the further development of segmental liver transplantation]. Langenbecks Arch Chir. 1988; 373:127–130 [PubMed] [Google Scholar]
- 3.Mogul DB, Luo X, Garonzik-Wang J, et al. Expansion of the liver donor supply through greater use of split-liver transplantation: identifying optimal recipients. Liver Transpl. 2019; 25:119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rogiers X, Malago M, Habib N, et al. In situ splitting of the liver in the heart-beating cadaveric organ donor for transplantation in two recipients. Transplantation. 1995; 59:1081–1083 [PubMed] [Google Scholar]
- 5.van Rijn R, van Leeuwen OB, Matton APM, et al. Hypothermic oxygenated machine perfusion reduces bile duct reperfusion injury after transplantation of donation after circulatory death livers. Liver Transpl. 2018; 24:655–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Meijer VE, Fujiyoshi M, Porte RJ. Ex situ machine perfusion strategies in liver transplantation. J Hepatol. 2019; 70:203–205 [DOI] [PubMed] [Google Scholar]
- 7.van Rijn R, Karimian N, Matton APM, et al. Dual hypothermic oxygenated machine perfusion in liver transplants donated after circulatory death. Br J Surg. 2017; 104:907–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ge J, Perito ER, Bucuvalas J, et al. Split liver transplantation is utilized infrequently and concentrated at few transplant centers in the United States. Am J Transplant. 2020; 20:1116–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spada M, Angelico R, Grimaldi C, et al. The new horizon of split-liver transplantation: ex situ liver splitting during hypothermic oxygenated machine perfusion. Liver Transpl. 2020; 26:1363–1367 [DOI] [PubMed] [Google Scholar]
- 10.Brockmann JG, Vogel T, Coussios C, et al. Liver splitting during normothermic organ preservation. Liver Transpl. 2017; 23:701–706 [DOI] [PubMed] [Google Scholar]
- 11.Stephenson BTF, Bonney GK, Laing RW, et al. Proof of concept: liver splitting during normothermic machine perfusion. J Surg Case Rep. 2018; 2018:rjx218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Leeuwen OB, Fujiyoshi M, Ubbink R, et al. Ex situ machine perfusion of human donor livers via the surgically reopened umbilical vein: a proof of concept. Transplantation. 2019; 103:2130–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


