Abstract
The dominant theory of Alzheimer disease (AD) has been that amyloid-β (Aβ) accumulation in the brain is the initial cause of the degeneration leading to cognitive and functional deficits. Autosomal dominant Alzheimer disease (ADAD), in which pathologic mutations of the amyloid precursor protein (APP) or presenilins (PSENs) genes are known to cause abnormalities of Aβ metabolism, should thus offer perhaps the best opportunity to test anti-Aβ drugs. Two long-term preventive studies (Dominantly Inherited Alzheimer Network Trials Unit Adaptive Prevention Trial [DIAN-TU-APT] and Alzheimer Preventive Initiative–ADAD) were set up to evaluate the efficacy of monoclonal anti-Aβ antibodies (solanezumab, gantenerumab, and crenezumab) in carriers of ADAD, but the results of the DIAN-TU-APT study have shown that neither solanezumab nor gantenerumab slowed cognitive decline in 144 subjects with ADAD followed for 4 years, despite one of the drugs (gantenerumab) significantly affected biomarkers relevant to their intended mechanism of action. Surprisingly, solanezumab significantly accelerated cognitive decline of both asymptomatic and symptomatic subjects. These failures further undermine the Aβ hypothesis and could support the suggestion that ADAD is triggered by accumulation of other APP metabolites, rather than Aβ.
Alzheimer disease (AD) is a chronic neurodegenerative disease with an insidious onset, which usually progresses with increasing rapidity. It accounts for around 70% of dementia diagnoses. There are 2 forms of AD: the most common is called sporadic AD (SAD) because it is not caused by a specific gene, although genetic risk factors have been identified, the most important being APOE, CLU, CR1, and PICALM.1 SAD generally appears after age 65 years, with most cases occurring after age 80 years.2 It is very common, affecting more than 50 million people worldwide. The other type of AD is quite rare (1%–3% of all AD cases) and has a genetic cause. It is called dominantly inherited AD, familial AD, or autosomal dominant AD (ADAD). ADAD is caused by mutations in the amyloid precursor protein (APP) or presenilins (PSEN1 and PSEN2) genes that cause abnormalities in Aβ metabolism. ADAD generally has an early onset (as young as age 30 years) but can occur at late ages.3 APP is the precursor of amyloid-β (Aβ), and presenilin-1 is the protease element of the γ-secretase enzyme complex responsible for the final release of Aβ from APP. ADAD bears neuropathologic and biomarker features that are similar to those of SAD, but they usually occur at a younger age4—it therefore offers a potentially valuable setting in which to test the efficacy of drugs targeting Aβ.
Pathophysiology and Anti-Aβ Therapeutic Approaches to AD
The presence of intracellular neurofibrillary tangles and extracellular plaques in the brain is the histologic hallmark of AD. Plaques are mainly composed of Aβ, a 40–42–amino acid peptide with established roles in the mediation of neuronal homeostasis. Neurofibrillary tangles comprise aggregated hyperphosphorylated tau, a protein normally active in axonal microtubular stabilization. Also recognized as histopathologic markers are microglial dysfunction, astrocytic activation, and neuritic dystrophy. The Aβ cascade hypothesis of AD asserts that Aβ accumulation in the brain is the initial pathologic event, starting 15–20 years before the disease presents clinically. The autosomal dominant forms of AD arise following point mutations of APP and the enzymes involved in its processing (PSEN1 and PSEN2), which lead to altered Aβ production. It is noteworthy that another specific mutation of APP (A673T) is recognized to give protection against AD in cognitively healthy elderly individuals.5 The altered amino acid is close to the β-site APP cleaving enzyme-1 (BACE1) cleavage site and reduces Aβ production in vitro by ∼40%. In late-onset SAD, faulty Aβ clearance and/or increased BACE1 activity are held responsible for Aβ accumulation. Such accumulation has also been linked to the APOE4 allele, making it the most leading genetic risk factor for SAD. Broad acceptance of the amyloid hypothesis has driven the intensive research efforts of the last 20 years to develop compounds that counter Aβ accumulation—an objective mainly pursued through 2 approaches: either the reduction of Aβ production by inhibition of the enzymes (BACE1 and γ-secretase) that cleave APP to generate Aβ or the enhancement of Aβ clearance by active or passive immunotherapy. Neither of these approaches has been shown to have therapeutic effects in patients with AD, even in the very early stages.6
Prevention Studies in Patients With SAD
Over the last 10 years, the scientific community has realized that the mild-to-moderate or even early stages of AD are too late for anti-Aβ drugs to reverse or halt disease progression. About 25% of subjects enrolled in clinical trials in which AD diagnosis was based on neuropsychological and clinical testing do not have objective evidence of Aβ brain deposition.7 New AD diagnostic criteria were proposed to define AD-related dementia based on biomarker evidence of brain amyloidosis, thus enabling the identification of preclinical stages of AD and allowing studies of earlier pharmacologic intervention.8
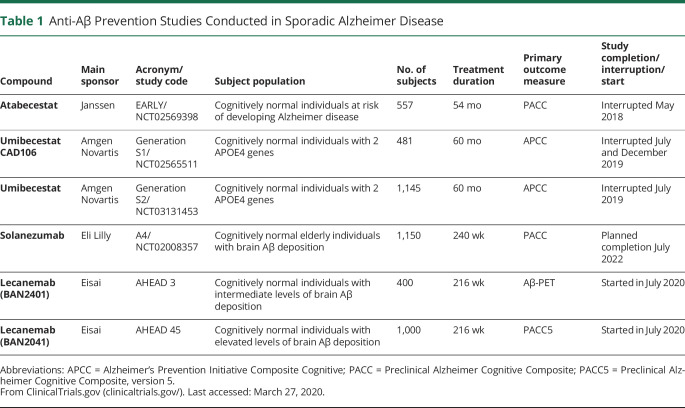
However, to date, prevention studies with anti-Aβ drugs have failed to show lower rates of cognitive decline in cognitively normal subjects at risk of developing AD (table 1). These prevention studies tested 2 BACE1 inhibitors (atabecestat and umibecestat), and an active anti-Aβ vaccine (CAD106) in different, cognitively unimpaired populations. The EARLY study, which studied atabecestat, enrolled 557 cognitively normal subjects at risk of developing AD because of positive family history of dementia, signs of brain Aβ accumulation, or having an APOE4 gene. The study was initially terminated early because of serious liver enzyme elevations—subsequently, however, it was revealed that the drug had worsened cognitive performance compared with placebo.9 Two large studies (Generation 1 and Generation 2) both tested umibecestat (a selective BACE1 inhibitor) and CAD106 (an active Aβ immunotherapy) in 1,626 cognitively normal subjects without evidence of Aβ brain deposition but carrying 2 APOE4 alleles.10 In July 2019, the umibecestat arms of the 2 studies were prematurely interrupted because of worsening cognitive function, and in December 2019, the CAD106 vaccine arms were also stopped.11
Table 1.
Anti-Aβ Prevention Studies Conducted in Sporadic Alzheimer Disease
This leaves 3 major preventive trials ongoing in SAD. The first is the antiamyloid treatment in asymptomatic AD (A4) study of solanezumab in cognitively normal elderly subjects with signs of amyloid accumulation. This trial, which started in 2014, is not scheduled to complete until late 2022, reflecting the prolonged follow-up required in this stage of AD development. Recently, 2 other prevention studies with lecanemab (BAN2041) were launched in cognitively normal individuals with intermediate (AHEAD 3 study) and elevated (AHEAD 45 study) levels of brain Aβ deposition (table 1).
Prevention Studies in Patients With Autosomal Dominant AD
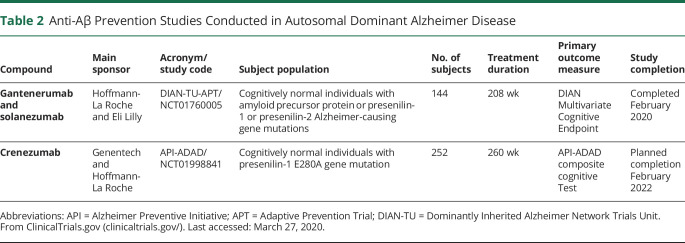
In 2008 and 2011, 2 international network of research centers, the Dominantly Inherited Alzheimer Network (DIAN) and the Alzheimer Preventive Initiative (API), were launched to establish international, multicenter registries of individuals with ADAD and to facilitate recruitment to observational and therapeutic studies on these subjects. The DIAN-TU-APT trial was set up to test solanezumab (a humanized immunoglobulin G1 [IgG1] monoclonal antibody that recognizes soluble monomeric form of Aβ) and gantenerumab (a fully human IgG1 monoclonal antibody that mainly recognizes fibrillary forms of Aβ) in presymptomatic subjects with ADAD. The API-ADAD trial was set up to test crenezumab (a fully humanized IgG4 monoclonal antibody selective for oligomeric and fibrillar forms of Aβ) in cognitively normal subjects with ADAD (table 2).
Table 2.
Anti-Aβ Prevention Studies Conducted in Autosomal Dominant Alzheimer Disease
The DIAN-TU-Adaptive Prevention Trial
The DIAN-TU-Adaptive Prevention Trial (APT) trial was a double-blind, placebo-controlled study intended to investigate whether gantenerumab or solanezumab could slow cognitive decline in presymptomatic or mildly symptomatic subjects who carried ADAD genetic mutations (PSEN1, PSEN2, or APP).12 The study started in 2012 and followed subjects for an average of around 5 years (maximum 7 years). Subjects were expected to develop symptoms within 15 years of enrollment based on the time of disease onset in their parents or already had mild symptoms of cognitive decline/memory loss at study entry. The trial was undertaken in Australia, Canada, France, Spain, the United Kingdom, and the United States across 24 sites. Originally, the study was a 2-year biomarker target engagement trial, but was later modified to be a full efficacy study measuring a cognitive primary end point following at least 4 years of treatment. In summer 2017, midway through the trial, the dose of gantenerumab was increased fivefold from 225 to 1,200 mg subcutaneously every 4 weeks, whereas the dose of solanezumab was increased fourfold from 400 to 1,600 mg IV every 4 weeks. The primary outcome measure of efficacy was the DIAN Multivariate Cognitive Endpoint, a composite comprised of the delayed recall score from the International Shopping List Test (episodic memory), the Logical Memory delayed recall score from the Wechsler Memory Scale–Revised (executive functioning), the Digit Symbol Coding test total score from the Wechsler Adult Intelligence Scale–Revised (processing speed), and the MMSE total score (global mental status). These measures were selected because of their advantageous psychometric characteristics, namely their reduced ceiling and floor effects, relatively low variability, sensitivity to subtle declines occurring before clinical diagnosis, and face validity as indicators of the cognitive phenotype of AD. The DIAN-TU composite is purported to be sensitive to decline and to produce feasible sample size requirements to detect the appropriate effect sizes. For example, with 60 actively treated mutation carrier subjects and 40 placebo-treated mutation carrier participants, the power to identify a 30% slowing in disease progression at 4 years would be 0.90 using the ADAD disease progression model, while permitting participants to continue in the study until the last 1 reaches 4 years.12 To enlarge the placebo data set, the trial had included natural history data from 49 mutation carriers enrolled in the DIAN observational study, which is collecting comparable progression data as the DIAN-TU-APT.
Full results of the DIAN-TU study were presented at the Alzheimer's Association International Conference 2020 (July 26–30, Amsterdam).13,14 One hundred forty-four mutation carriers were enrolled in the study: 52 on solanezumab, 52 on gantenerumab, and 40 on placebo. Compared with baseline, treatment with solanezumab significantly increased CSF levels of Aβ42 but did not significantly affected brain Aβ load, as determined by Pittsburgh compound B –PET. Surprisingly, the drug significantly increased CSF concentrations of neurofilament light (NfL), a marker of neurodegeneration. Importantly, solanezumab treatment significantly accelerated cognitive deterioration in the AD mutation carriers compared with placebo (cognitive progression ratio = 1.255, 95% confidence interval: 1.136–1.376).13 The detrimental effects were visible both in asymptomatic and symptomatic subjects. Compared with baseline, gantenerumab treatment significantly lowered brain amyloid burden and increased CSF Aβ42 levels. The antibody treatment significantly decreased CSF levels of total tau, p-tau181, and NfL, effects considered positive, but which did not translate to a cognitive benefit (cognitive progression ratio = 1.063, 95% confidence interval: 0.949–1.180).14 The failure of high doses of gantenerumab to produce cognitive benefit in asymptomatic or symptomatic subjects with ADAD, despite significant effects on central AD biomarkers, may be ascribed to the late introduction of the substantially higher dose levels, an insufficient observation period (4 years), or to a limited sample size. On the other hand, recent animal work has demonstrated that deficiency of p-tau205 is sufficient to impair memory function in the absence of Aβ pathology,15 raising key questions about the contribution of tau phosphorylation to the development of AD. The significant detrimental effects on cognition caused by treatment with high doses of solanezumab could be explain by the high affinity of this antibody for monomeric Aβ that could have physiologic role as opposed to the high affinity of gantenerumab for Aβ aggregates or oligomeric species. On the other hand, looking at the lower 95% confidence interval of the primary end point of the trial (0.949), we should not expect to see breakthrough positive effects of gantenerumab in these subjects.
The API-ADAD Trial
The API-ADAD trial started in 2013 and is being conducted in cognitively normal individuals (aged 30–60 years) living in Antioquia, Colombia, and bearing the PSEN1 E280A mutation that leads to early cerebral Aβ deposition followed at around age 50 years by a progressive decline in cognition and clinical function.16 In the API-ADAD trial, 169 PSEN1 mutation carriers are receiving crenezumab (undisclosed dose) or placebo as fortnightly subcutaneous or monthly IV injections for at least 5 years. In addition, 83 unrandomized noncarriers are blindly receiving placebo to protect study participants from knowledge of the presence of the pathogenic mutation. The study is scheduled to complete in early 2022.
Reconsidering the Pathologic Role of Familial AD Mutations
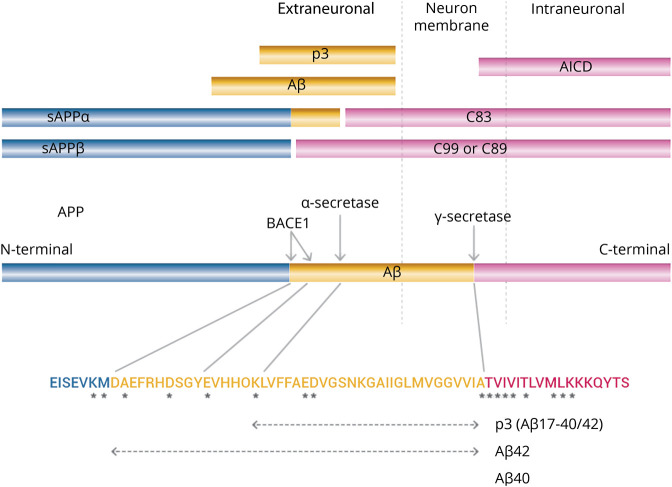
ADAD is linked to specific mutations of APP and presenilins, whereas no mutations of BACE1 are known to cause AD. Figure provides a schematic illustration of the main metabolic pathway of APP and aminoacidic positions of main APP mutations linked to ADAD. α-Secretase cleaves the α-site of APP releasing N-terminal fragments sAPPα and the C-terminal fragment C83. The γ-secretase complex then cleaves C83 releasing p3 extraneuronally and APP intracellular domain (AICD) intraneuronally. BACE1 cleaves APP at the β-cleavage site (Met671-Asp672) releasing the N-terminal fragment sAPPβ596 and the C-terminal fragment C99 (CTFβ). sAPPβ596 is secreted, whereas C99 is cleaved by the γ-secretase complex releasing Aβ extraneuronally and AICD intraneuronally. BACE1 is also known to cleave APP at a less well-characterized β′-cleavage site (Tyr681-Glu682). Cleavage of APP at the β′-cleavage site generates C89 and sAPPβ606. sAPPβ606 is secreted, and C89 is subsequently cleaved by γ-secretase to generate truncated Aβ11–40/42.17 In the normal physiologic state, α-secretase cleaves ≥90% of APP, and the remainder is cleaved by BACE1. The major products in this APP metabolic pathway are thus sAPPα, C83, p3, and AICD (which is rapidly degraded)—Aβ is normally a minor product. The mutations found in familial AD, especially presenilin mutations, may thus affect the formation and processing of a variety of products. These pathogenic mutations cluster near the α-secretase, BACE1, and γ-secretase cleavage sites (figure) and cause accumulation of APP C-terminal fragments18–20; such accumulation has also been found in SAD.21 Furthermore, mutations in presenilin, the proteolytic element of the γ-secretase complex, reduce γ-secretase activity.22–26 These studies indicate that ADAD mutations can cause complete loss of presenilin-1 function in vivo. Clinically, presenilin mutations are almost always present in a heterozygous state implying that γ-secretase activity is not completely lost. However, some authors have shown that PSEN1 mutations interfere with γ-secretase activity in a dominant-negative manner,27 suggesting that clinical presenilin mutations may produce familial AD through a loss-of-function mechanism. The fact that all the hundreds of presenilin familial AD mutations cause production of a transcript with a full-length open reading frame strongly indicates that the mutant presenilin proteins have a dominant activity that is not simply a haploinsufficiency effect.28 The rare cases of homozygosity for PSEN1 familial AD mutations involve alleles that do not have complete loss of γ-secretase function, thus preserving Notch signaling29 that is essential for embryonic development.
Figure. Scheme of the Structural and Functional Relationships of APP.
Asterisks indicate pathogenic APP mutations that have been identified in familial AD, which cluster near the α-secretase, β-secretase (BACE1), and γ-secretase cleavage sites. AD = Alzheimer disease; APP = amyloid precursor protein; BACE1 = β-site APP cleaving enzyme-1.
A decrease in the catalytic capacity of γ-secretase, which would lead to an increase in APP C-terminal fragments, facilitates the pathogenesis in familial AD30,31—thus familial AD should be considered a disease characterized by a primary accumulation of C-terminal fragments of APP, in addition to an accumulation of Aβ. The therapeutic approach of using BACE1 inhibitors would result in accumulation of C83 C-terminal fragment, whereas presenilin dysfunction would result in accumulation of the C99 C-terminal fragment. It is widely recognized that C99 accumulation induces neuronal toxicity.32 Neuropathologic studies in patients with AD have shown that C99 accumulates in vulnerable neurons, and its levels correlate with the degree of cognitive impairment in patients having AD. In contrast, Aβ levels are increased in both vulnerable and resistant brain areas.33 C99 is consistently detected much earlier than Aβ, suggesting that this APP metabolite could be an early contributor to AD pathology.34 C99 accumulates principally within endolysosomal and autophagic structures, where it is accompanied by C99-derived C83 accumulation within the same intracellular organelles. Both these C-terminal fragments of APP dimerize, leading to the generation of higher molecular weight species.34 In AD animal models, increases in C99 provoke the upregulation of cholesterol internalization and its delivery to the endoplasmic reticulum, which in turn result in the loss of lipid homeostasis and the appearance of AD signatures, such as higher production of longer forms of Aβ.35 The therapeutic use of γ-secretase inhibitors mimics the malfunctioning of presenilin in patients with ADAD with an accumulation of C99.36 Overall, these observations would explain why γ-secretase and BACE1 inhibitors have both produced detrimental effects on cognition6 and behavior37 in patients with AD. It is interesting to note that the E682K Leuven mutation of APP appears to drive AD by inhibiting cleavage at the β′-cleavage site of APP.38 The inhibition by BACE1 inhibitors of this alternative β′-cleavage site may be another reason why BACE1 inhibitors worsen cognition in clinical trials.
Similarly, monoclonal antibodies specifically directed at Aβ would not work because they do not ameliorate the pathologic accumulation of the C-terminal fragments of APP.39 However, because Aβ is included in the C99 fragment, there is the theoretical possibility that some anti-Aβ antibodies may react also with C99. Is should be interesting to verify whether monoclonal antibodies, which have shown some hints of clinical efficacy in AD, like aducanumab and lecanemab (BAN2401),40 show cross-reactivity against C99.
Recent Studies Suggest Culprits Other Than Aβ as the Initial Cause of AD
Several recent studies have suggested that an increase in brain Aβ concentration may be not the initial step of the AD process. A neuropathology study in 5,007 subjects has shown that the APOE2/2 allele is significantly associated with an exceptionally low AD dementia risk odds ratio (OR) compared with both APOE2/3 (OR = 0.34) and APOE4/4 (OR = 0.004).41 Another important brain imaging study in 489 subjects showed that APOEε4 is associated with increased tau-PET uptake in the entorhinal cortex and hippocampus, independent of its link with Aβ accumulation.42 A recent case report described a PSEN1 mutation carrier who did not develop mild cognitive impairment until her seventies, 3 decades after the expected age at clinical onset.43 The individual had 2 copies of the APOE3 Christchurch (R136S) mutation, high brain amyloid levels, and limited tau and neurodegenerative biomarkers. Although the Christchurch mutation may have protected the PSEN1 carrier from Aβ-induced dysfunction through an unidentified as yet mechanism, collectively, these findings indicate that APOE may be implicated in the pathogenesis of AD through an effect on tau and that this effect is mediated by microglia.
A recent neuropathology study has shown that necroptosis, a programmed form of necrosis characterized by assembly of the necrosome complex composed of phosphorylated proteins (pRIPK1, pRIPK3, and pMLKL), is associated with neuronal loss in the AD brain.44 Another study has shown that a mutation (rs72824905-G) in the PLCG2, the gene encoding for Cγ2 (a phospholipase involved in the transmembrane transduction of immune signals), reduces the risk of AD.45 Another study has found that the ovary-orientated protein ovarian carcinoma immunoreactive antigen domain containing 1 (OCIAD1) is a neurodegeneration-associated factor for AD.46 High levels of OCIAD1 were found in vulnerable brain areas and dystrophic neurites and correlated with disease severity. This study suggests that OCIAD1 contributes to neurodegeneration in AD by impairing mitochondria function, leading to neuronal vulnerability and synaptic damage. Collectively, these studies suggest that neuronal death in AD may be linked to Aβ-independent mechanisms.
A recent study in 1,289 cognitively normal participants has shown that subjects with untreated diabetes displayed greater tau pathology than both treated patients with diabetes and subjects with normal glycemia and that they progressed to dementia at higher rates than the control group (hazard ratio = 1.602)47—suggesting that abnormal glucose metabolism may drive AD pathogenesis.48
Finally, a recent study found that DNA from various Gram-positive and Gram-negative bacteria results in tau misfolding, especially DNA extracted from certain bacterial species previously detected in the brain, CSF, or oral cavity of patients with AD.49 These findings indicate that microbial DNA may play a previously overlooked role in the propagation of tau protein misfolding and AD pathogenesis. They strengthen the hypothesis that compromised blood-brain and intestinal barriers represent an important source of microbial DNA in the CNS, opening novel opportunities for therapeutic interventions.50
Although these studies did not establish whether Aβ is the inciting event of neuronal injury and that multiple mechanisms can occur simultaneously, they point out that the initial cause of AD may be more complex than was initially thought. Given this, the clinical question is whether anti-Aβ antibodies still work in patients with the disease? We believe that potent anti-Aβ drugs, including anti-Aβ antibodies, should at least slow the initially subtle cognitive decline that can be detected by sophisticated composite cognitive scales in asymptomatic or presymptomatic patients at risk of developing AD—but this has not been the case up to now, as studies listed in tables 1 and 2 clearly show. It is essentially contradictory that high doses of solanezumab in the DIAN-TU study significantly accelerated cognitive decline in both asymptomatic and symptomatic subjects with ADAD, the prototypical clinical condition to test the Aβ hypothesis of AD. This is not the only trial in which worsening of cognition has been seen after anti-Aβ therapy6 and such findings should tell us that something is wrong in our understanding of the AD pathophysiology process.
Conclusions
The Aβ hypothesis has dominated AD research since 1991.51 It proposes that brain accumulation of the Aβ peptide triggers the formation of tau aggregates, which kill neurons causing neuroinflammation and ultimately leading to dementia. ADAD is caused by mutations in APP or PSENs genes, both implicated in the generation of the Aβ peptide. Studies from families with ADAD have been considered critical to supporting the amyloid cascade hypothesis that underpins the current development of amyloid-based disease-modifying therapies in SAD. In the last 5 years, many pharmaceutical companies have abandoned targeting Aβ to treat AD as a consequence of a long series of major setbacks in clinical studies, both at early and mild-to-moderate stages—alternative targets such as tau accumulation, neuroinflammation, or microbioma are now being pursued.
A fully successful DIAN-TU study in subjects with ADAD would have revived the Aβ hypothesis of AD and spurred interest in prevention studies in cognitively normal people without pathologic genetic mutations but at high risk of developing AD—whether through carriage of a risk-associated allele of the APOE4 gene, parental or family history of AD, or biomarker evidence of brain deposition in the brain. This, however, has not occurred, and the mixed results raise more questions than they answer.
The long list of negative anti-Aβ trials in AD,6 including apparently this last one in the genetic form of the disease, suggests the alternate hypothesis that the observed overproduction of Aβ in AD might simply reflect a form of synaptic plasticity attempting to compensate for neuronal dysfunction. Aβ has important physiologic roles in brain function, including synaptic plasticity, memory formation, and neurogenesis.52 The idea that Aβ overexpression is a compensatory mechanism is in line with observations that anti-Aβ drugs like BACE1 and γ-secretase inhibitors may induce or worsen cognitive performance and psychiatric disturbances in patients with AD even during the early stages of the disease.9,53
Another fascinating hypothesis generated by experimental, genetic, and epidemiologic data is that amyloidogenesis in the AD brain could be linked to the antimicrobial role of Aβ and that innate immune-mediated inflammation propagates neurodegeneration.54 Amyloid deposition is normally countered by microglial phagocytosis, which clears the amyloid, cellular debris, and dead neurons. In the long term, microglia may switch function and kill neurons as amyloid induced tau aggregation and tangles accumulate. This reactive gliosis and neuroinflammation results in debilitating neural damage and dementia. In 2008, Bertram et al. reported the first gene associated with neuroinflammation in AD, CD33.55 When highly expressed, CD33 turns the microglial response from protective to pathologic, creating the runaway inflammation that is a distinguishing characteristic of AD. Subsequent research has shown that CD33 inhibits microglial uptake of Aβ56 and that triggering receptor expressed on myeloid cells 2 (TREM2) is working downstream of CD33 to control neuroinflammation.57 Studies in a tauopathy mouse model have shown that APOE, the strongest genetic risk factor for AD, regulates neurodegeneration predominantly by modulating microglial activation.58 The discovery of these innate immunity genes associated with AD suggests that the innate immune response could work for decades before symptoms arise in people who carry genetic risk factors for the disease.
The failure to achieve the primary end point of the DIAN-TU study necessitates a reconsideration of the role of APP and PSENs mutations in familial AD. It may be hypothesized that these mutations trigger AD pathogenesis through abnormal APP metabolism and accumulation of APP C-terminal fragments rather than Aβ production and Aβ plaque formation. The overproduction of Aβ could be nonspecific, whereas other metabolites of APP (i.e., C99 and C83) could be the real culprits in neuronal death and, as such, should be targeted. Indeed, all attempts to develop Aβ-targeting drugs to treat AD have ended in failure, and recent findings indicate that the main factor underlying the development and progression of AD could be tau, not Aβ. Therefore, AD could be a disorder that is triggered by impairment of APP metabolism and progresses through tau pathology, not Aβ. Aβ may just be one character in a complex story that has been too simplistically interpreted for too long. It is now time to reconsider this story from a different perspective to fit all the clues into a coherent and plausible representation.
Glossary
- AD
Alzheimer disease
- ADAD
autosomal dominant AD
- AICD
APP intracellular domain
- API
Alzheimer Preventive Initiative
- APP
amyloid precursor protein
- APT
Adaptive Prevention Trial
- BACE1
β-site APP cleaving enzyme-1
- DIAN-TU
Dominantly Inherited Alzheimer Network Trials Unit
- IgG
immunoglobulin G
- NfL
neurofilament light
- OCIAD1
ovarian carcinoma immunoreactive antigen domain containing 1
- OR
odds ratio
- SAD
sporadic AD
Appendix. Authors
Study Funding
No targeted funding reported.
Disclosure
B.P. Imbimbo is an employee at Chiesi Farmaceutici; he is listed among the inventors of a number of Chiesi Farmaceutici patents of anti-Alzheimer drugs. U. Lucca and M. Watling have no conflict of interest to declare. Go to Neurology.org/NG for full disclosures.
References
- 1.Apostolova LG, Risacher SL, Duran T, et al. Associations of the top 20 Alzheimer disease risk variants with brain amyloidosis. JAMA Neurol 2018;75:328–341.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucca U, Tettamanti M, Tiraboschi P, et al. Incidence of dementia in the oldest-old and its relationship with age: the Monzino 80-plus population-based study. Alzheimers Dement 2020;16:472–481. [DOI] [PubMed] [Google Scholar]
- 3.Day GS, Musiek ES, Roe CM, et al. Phenotypic similarities between late-onset autosomal dominant and sporadic Alzheimer disease: a single-family case-control study. JAMA Neurol 2016;73:1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quiroz YT, Sperling RA, Norton DJ, et al. Association between amyloid and tau accumulation in young adults with autosomal dominant Alzheimer disease. JAMA Neurol 2018;75;548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jonsson T, Atwal JK, Steinberg S, et al. A mutation in APP protects against Alzheimer's disease and age-related cognitive decline. Nature 2012;488:96–99. [DOI] [PubMed] [Google Scholar]
- 6.Panza F, Lozupone M, Logroscino G, Imbimbo BP. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat Rev Neurol 2019;15:73–88. [DOI] [PubMed] [Google Scholar]
- 7.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med 2016;8:595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henley D, Raghavan N, Sperling R, Aisen P, Raman R, Romano G. Preliminary results of a trial of atabecestat in preclinical Alzheimer's disease. N Engl J Med 2019;380:1483–1485. [DOI] [PubMed] [Google Scholar]
- 10.Lopez Lopez C, Tariot PN, Caputo A, et al. The Alzheimer's Prevention Initiative Generation Program: study design of two randomized controlled trials for individuals at risk for clinical onset of Alzheimer's disease. Alzheimers Dement 2019;5:216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graf A, Borowsky B, Tariot P, et al. Alzheimer's Prevention Initiative Generation Program: update and next steps. J Prev Alzheimers Dis 2019;6(suppl 1):S12–S13. [Google Scholar]
- 12.Bateman RJ, Benzinger TL, Berry S, et al. The DIAN-TU Next Generation Alzheimer's prevention trial: adaptive design and disease progression model. Alzheimers Dement 2017;13:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farlow MR DIAN-TU Prevention Trial: solanezumab in-depth outcomes. Alzheimer's Association International Conference (AAIC) 2020; July 26–30, 2020; Amsterdam. Available at: aaic2020.vfairs.com/en/hall#topics-tab. Accessed August 9, 2020.
- 14.Salloway SP DIAN-TU Prevention Trial: gantenerumab in-depth outcomes. Alzheimer's Association International Conference (AAIC) 2020; July 26–30, 2020; Amsterdam. Available at: aaic2020.vfairs.com/en/hall#topics-tab. Accessed August 9, 2020.
- 15.Ittner A, Asih PR, Tan ARP, et al. Reduction of advanced tau-mediated memory deficits by the MAP kinase p38γ. Acta Neuropathol 2020;140:279–294. [DOI] [PubMed] [Google Scholar]
- 16.Rios-Romenets S, Giraldo-Chica M, López H, et al. The value of pre-screening in the Alzheimer's Prevention Initiative (API) Autosomal Dominant Alzheimer's Disease trial. J Prev Alzheimers Dis 2018;5:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng Y, Wang Z, Wang R, et al. Amyloid-β protein (Aβ) Glu11 is the major β-secretase site of β-site amyloid-β precursor protein-cleaving enzyme 1 (BACE1), and shifting the cleavage site to Aβ Asp1 contributes to Alzheimer pathogenesis. Eur J Neurosci 2013;37:1962–1969. [DOI] [PubMed] [Google Scholar]
- 18.Tesco G, Ginestroni A, Hiltunen M, et al. APP substitutions V715F and L720P alter PS1 conformation and differentially affect Aβ and AICD generation. J Neurochem 2005;95:446–456. [DOI] [PubMed] [Google Scholar]
- 19.Wiley JC, Hudson M, Kanning KC, Schecterson LC, Bothwell M. Familial Alzheimer's disease mutations inhibit γ-secretase-mediated liberation of β-amyloid precursor protein carboxy-terminal fragment. J Neurochem 2005;94:1189–1201. [DOI] [PubMed] [Google Scholar]
- 20.Xu TH, Yan Y, Kang Y, Jiang Y, Melcher K, Xu HE. Alzheimer's disease-associated mutations increase amyloid precursor protein resistance to γ-secretase cleavage and the Aβ42/Aβ40 ratio. Cell Discov 2016;2:16026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pera M, Alcolea D, Sánchez-Valle R, et al. Distinct patterns of APP processing in the CNS in autosomal-dominant and sporadic Alzheimer disease. Acta Neuropathol 2013;125:201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen F, Gu Y, Hasegawa H, et al. Presenilin 1 mutations activate γ42-secretase but reciprocally inhibit ε-secretase cleavage of amyloid precursor protein (APP) and S3-cleavage of notch. J Biol Chem 2002;277:36521–36526. [DOI] [PubMed] [Google Scholar]
- 23.Walker ES, Martinez M, Brunkan AL, Goate A. Presenilin 2 familial Alzheimer's disease mutations result in partial loss of function and dramatic changes in Aβ 42/40 ratios. J Neurochem 2005;92:294–301. [DOI] [PubMed] [Google Scholar]
- 24.Bentahir M, Nyabi O, Verhamme J, et al. Presenilin clinical mutations can affect γ-secretase activity by different mechanisms. J Neurochem 2006;96:732–742. [DOI] [PubMed] [Google Scholar]
- 25.Shen J, Kelleher RJ III. The presenilin hypothesis of Alzheimer's disease: evidence for a loss-of-function pathogenic mechanism. Proc Natl Acad Sci USA 2007;104:403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia D, Watanabe H, Wu B, et al. Presenilin-1 knockin mice reveal loss-of-function mechanism for familial Alzheimer's disease. Neuron 2015;85:967–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heilig EA, Gutti U, Tai T, et al. Trans-dominant negative effects of pathogenic PSEN1 mutations on γ-secretase activity and Aβ production. J Neurosci 2013;33:11606–11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jayne T, Newman M, Verdile G, et al. Evidence for and against a pathogenic role of reduced γ-secretase activity in familial Alzheimer's disease. J Alzheimers Dis 2016;52:781–799. [DOI] [PubMed] [Google Scholar]
- 29.Tambini MD, D'Adamio L. Knock-in rats with homozygous PSEN1 L435F Alzheimer mutation are viable and show selective γ-secretase activity loss causing low Aβ40/42 and high Aβ43. J Biol Chem 2020;295:7442–7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svedružić ŽM, Popović K, Šendula-Jengić V. Decrease in catalytic capacity of γ-secretase can facilitate pathogenesis in sporadic and Familial Alzheimer's disease. Mol Cell Neurosci 2015;67:55–65. [DOI] [PubMed] [Google Scholar]
- 31.Kametani F, Hasegawa M. Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer's disease. Front Neurosci 2018;12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lauritzen I, Pardossi-Piquard R, Bourgeois A, et al. Intraneuronal aggregation of the β-CTF fragment of APP (C99) induces Aβ-independent lysosomal-autophagic pathology. Acta Neuropathol 2016;132:257–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pulina MV, Hopkins M, Haroutunian V, Greengard P, Bustos V. C99 selectively accumulates in vulnerable neurons in Alzheimer's disease. Alzheimers Dement 2020;16:273–282. [DOI] [PubMed] [Google Scholar]
- 34.Lauritzen I, Pardossi-Piquard R, Bourgeois A, Bécot A, Checler F. Does intraneuronal accumulation of carboxyl-terminal fragments of the amyloid precursor protein trigger early neurotoxicity in Alzheimer's disease? Curr Alzheimer Res 2019;16:453–457. [DOI] [PubMed] [Google Scholar]
- 35.Montesinos J, Pera M, Larrea D, et al. The Alzheimer's disease-associated C99 fragment of APP regulates cellular cholesterol trafficking. EMBO J 2020;39:e103791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lauritzen I, Bécot A, Bourgeois A, et al. Targeting γ-secretase triggers the selective enrichment of oligomeric APP-CTFs in brain extracellular vesicles from Alzheimer cell and mouse models. Transl Neurodegener 2019;8:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panza F, Lozupone M, Bellomo A, Imbimbo BP. Do anti-amyloid-β drugs affect neuropsychiatric status in Alzheimer's disease patients? Ageing Res Rev 2019;55:100948. [DOI] [PubMed] [Google Scholar]
- 38.Zhou L, Brouwers N, Benilova I, et al. Amyloid precursor protein mutation E682K at the alternative β-secretase cleavage β'-site increases Aβ generation. EMBO Mol Med 2011;3:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunys J, Valverde A, Checler F. Are N- and C-terminally truncated Aβ species key pathological triggers in Alzheimer's disease? J Biol Chem 2018;293:15419–15428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panza F, Lozupone M, Seripa D, Imbimbo BP. Amyloid-β immunotherapy for Alzheimer's disease: is it now a long shot…? Ann Neurol 2019;85:303–315. [DOI] [PubMed] [Google Scholar]
- 41.Reiman EM, Arboleda-Velasquez JF, Quiroz YT, et al. Exceptionally low likelihood of Alzheimer's dementia in APOE2 homozygotes from a 5,000-person neuropathological study. Nat Commun 2020;11:667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Therriault J, Benedet AL, Pascoal TA, et al. Association of apolipoprotein E ε4 with medial temporal tau independent of amyloid-β. JAMA Neurol 2020;77:470–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arboleda-Velasquez JF, Lopera F, O'Hare M, et al. Resistance to autosomal dominant Alzheimer's disease in an APOE3 Christchurch homozygote: a case report. Nat Med 2019;25:1680–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koper MJ, Van Schoor E, Ospitalieri S, et al. Necrosome complex detected in granulovacuolar degeneration is associated with neuronal loss in Alzheimer's disease. Acta Neuropathol 2020;139:463–484. [DOI] [PubMed] [Google Scholar]
- 45.van der Lee SJ, Conway OJ, Jansen I, et al. A nonsynonymous mutation in PLCG2 reduces the risk of Alzheimer's disease, dementia with Lewy bodies and frontotemporal dementia, and increases the likelihood of longevity. Acta Neuropathol 2019;138:237–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X, Wang L, Cykowski M, et al. OCIAD1 contributes to neurodegeneration in Alzheimer's disease by inducing mitochondria dysfunction, neuronal vulnerability and synaptic damages. EBioMedicine 2020;51:102569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McIntosh EC, Nation DA; Alzheimer's Disease Neuroimaging Initiative. Importance of treatment status in links between type 2 diabetes and Alzheimer's disease. Diabetes Care 2019;42:972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuehn BM In Alzheimer research, glucose metabolism moves to center stage. JAMA 2020;323:297–299. [DOI] [PubMed] [Google Scholar]
- 49.Tetz G, Pinho M, Pritzkow S, Mendez N, Soto C, Tetz V. Bacterial DNA promotes Tau aggregation. Sci Rep 2020;10:2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Panza F, Lozupone M, Solfrizzi V, Watling M, Imbimbo BP. Time to test antibacterial therapy in Alzheimer's disease. Brain 2019;142:2905–2929. [DOI] [PubMed] [Google Scholar]
- 51.Makin S The amyloid hypothesis on trial. Nature 2018;559:S4–S7. [DOI] [PubMed] [Google Scholar]
- 52.Gulisano W, Melone M, Ripoli C, et al. Neuromodulatory action of picomolar extracellular Aβ42 oligomers on presynaptic and postsynaptic mechanisms underlying synaptic function and memory. J Neurosci 2019;39:5986–6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Egan MF, Kost J, Voss T, et al. Randomized trial of verubecestat for prodromal Alzheimer's disease. N Engl J Med 2019;380:1408–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moir RD, Lathe R, Tanzi RE. The antimicrobial protection hypothesis of Alzheimer's disease. Alzheimers Dement 2018;14:1602–1614. [DOI] [PubMed] [Google Scholar]
- 55.Bertram L, Lange C, Mullin K, et al. Genome-wide association analysis reveals putative Alzheimer's disease susceptibility loci in addition to APOE. Am J Hum Genet 2008;83:623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Griciuc A, Serrano-Pozo A, Parrado AR, et al. Alzheimer's disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron 2013;78:631–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Griciuc A, Patel S, Federico AN, et al. TREM2 acts downstream of CD33 in modulating microglial pathology in Alzheimer's disease. Neuron 2019;103:820–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi Y, Manis M, Long J, et al. Microglia drive APOE-dependent neurodegeneration in a tauopathy mouse model. J Exp Med 2019;216:2546–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]