Abstract
In 1994 Corner published five new species within the genus Psathyrella, all having been collected on the Malay Peninsula between 1929 and 1930. Three of these species belong to the genus Hebeloma and with their vinaceous colored lamellae and spore print, when fresh, they belong to H. sect. Porphyrospora. Of these three species, only one, P. flavidifolia, was validly published and thus we herewith recombine it as H. flavidifolium. The other two species, P. splendens and P. verrucispora, are synonyms of H. parvisporum and H. lactariolens, respectively. We also describe a new Malayan species, H. radicans, which also belongs to H. sect. Porphyrospora. These findings confirm the western Pacific Rim as a diversity hotspot for H. sect. Porphyrospora. The records described within this paper, represent the first recognition that the genus Hebeloma, and indeed that members of the ectomycorrhizal Hymenogastraceae, are present on the Malay Peninsula.
Keywords: Anamika , Agaricales , Dipterocarpaceae , ectomycorrhiza, Fagaceae , taxonomy, tropical forests
Introduction
Only a small number of Hebeloma species have been described from Asia, most recently H. parvisporum from Laos (Eberhardt et al. 2020). In the same paper, H. sect. Porphyrospora was proposed to include species originally described in Anamika. This decision was based on morphological and molecular data. The most distinctive features of the section are the predominantly dry cap surface and a spore deposit that is vinaceous red when fresh, but changes to brown without any reddish hue within one year in the herbarium. This color of the fresh spores, and as a result of the spore deposit, also normally causes the fresh mature lamellae to exhibit at least tinges of vinaceous red. This spore deposit color, and the subsequent color change when dried, appears to be restricted to this one section within Hebeloma.
The geographical distribution of species within H. sect. Porphyrospora is remarkable. The majority of the species occur in the western Pacific Rim region, with the exception of two species, H. porphyrosporum, to date only known from Europe, and H. sarcophyllum, to date only recorded from eastern North America (Beker et al. 2016; Eberhardt et al. 2020).
During the course of this research, and our efforts to find relevant information about Hebeloma recorded from the Malay Peninsula, we came across a paper by E.J.H. Corner (1993), effectively published 1994 (Corner 1994 [“1993”]), where he described five new Psathyrella species from the Malay Peninsula. These taxa have ornamented spores, and Corner followed Pegler and Young (1992), who also included a few species with ornamented spores in Psathyrella. Furthermore, P. splendens has a membranous persisting veil forming a conspicuous annulus, a feature excluding its position in the current circumscription of genus Psathyrella (Örstadius et al. 2015). This and two other of the new species, according to Corner (1994 [“1993”]), do not fit well within the genus. On the one hand, their robust stature might suggest they should be placed in Lacrymaria Pat. (which Voto (2019) did for all five of the Corner (1994 [“1993”]) taxa), but while the spores of Lacrymaria are black or certainly very dark in mass, at least the three collections with ornamented spores have fuscous purple or vinaceous brown spores. Also, Lacrymaria spores have a germ pore, not seen in these collections.
It is now clear that these three species belong to the genus Hebeloma. Based on the spore color in fresh material, they are members of H. sect. Porphyrospora. Unfortunately, the publication of two of these species is invalid under Art. 40.7 of the International Code (Turland et al. 2018), as the published description does not specify the herbarium in which the types are conserved.
It does appear that two of these three species, Psathyrella splendens and P. verrucispora, have been described and classified within Hebeloma since Corner’s publication, as H. parvisporum and H. lactariolens, originally published as Alnicola lactariolens. The third taxon, P. flavidifolia is recombined here as H. flavidifolium. Within this paper we cite seven new Hebeloma collections from the Malay Peninsula, collected by one of the authors (E. H.) during 2009 and 2010. Three of these collections are referred to H. lactariolens, one to H. parvisporum, two to H. flavidifolium and one to a species here described as new, H. radicans. All collections are from mixed tropical lowland forests dominated by Dipterocarpus, Quercus and Lithocarpus.
Corner (1994 [“1993”]) published detailed descriptions and excellent drawings of P. splendens and P. verrucispora. However, his description of P. flavidifolia is rather brief and has very little microscopic detail. He writes: “P. flavidifolia, is imperfectly known from one collection and is included in order that it may be rediscovered”. He goes on to say: “I describe this fungus, even though my notes on microscopic details are so imperfect, because it indicates an ally of P. splendens. It may be rare because I found it but once and, then, it puzzled me and became Hebeloma in my notes”. It appears that Corner already guessed that perhaps this taxon belonged within Hebeloma. Based on two new collections from the Malay Peninsula, we can now provide a much more detailed description and photographs of this mushroom. The description of Hebeloma radicans is based on a single collection. Although this is unfortunate, we have decided to go ahead with the description of this new species, anticipating that the knowledge of this species will advance its rediscovery and that of related taxa.
Materials and methods
Basidiomes were collected, dried and accessioned at the fungus herbarium of the Forest Research Institute Malaysia (FRIM) with duplicates in the collection of E. Horak at the herbarium of the Eidgenössische Technische Hochschule Zürich (ZT). Type material of the Corner species was obtained from the herbarium of the Royal Botanic Garden of Edinburgh (E).
Sequence data were obtained from dried specimens by direct sequencing following methods detailed in Eberhardt et al. (2016) and Cripps et al. (2019) for ITS and Vesterholt et al. (2014) for MCM7 (a DNA replication licensing factor). Sequence data were generated by LGC Genomics (Berlin, Germany). Sequences were edited using Sequencher vs. 4.8 (Gene Codes Corp., Ann Arbor, Michigan). Newly generated sequences were accessioned to GenBank (MT832016–MT832022 and MT832328–MT832331).
Flammula alnicola was used for rooting, and two species of Alnicola [Naucoria fide Species Fungorum (Index Fungorum Partnership 2019) accessed 13 Dec 2019] (A. amarescens and A. salicis) were used as additional outgroups. Members of the genus Hebeloma are represented by material, including type material, used in earlier publications (Beker et al. 2016; Eberhardt et al. 2020) and listed in Table 1. Material of all sequenced collections (apart from MEL 2382694) was available for examination.
Table 1.
Sequences used in the analysis. Herbarium abbreviations follow Index Herbariorum and are given in capital letters followed by a space or hyphen and the herbarium number. Private collections are indicated by the lack of a space between the letters and numbers. MO refers to https://mushroomobserver.org/
| Species | Country | HJB database reference | Voucher | GenBank acc. no. ITS | GenBank acc. no. MCM7 |
|---|---|---|---|---|---|
| Alnicola amarescens (Quél.) R. Heim & Romagn. | Switzerland | HJB11116 | HJB11116 | MK961996† | MK961952† |
| Alnicola salicis (P.D. Orton) Bon | U.K. | HJB14745 | HJB14745 | MK962001† | MK961960† |
| Flammula alnicola (Fr.) P. Kumm. | Germany | – | GLM-F045994 | MK957190† | MK961971† |
| Hebeloma aestivale Vesterh. | U.K. | HJB9291 | HJB9291 | KT218221‡ | MK961944† |
| H. alboerumpens Vila & al. | Spain | HJB13021 | JVG1090114-15 | JQ751220§ | JQ751104§ |
| H. alpinum (J. Favre) Bruchet | Switzerland | HJB11132 | HJB11132 | KM390590| | KM390046| |
| H. aminophilum R.N. Hilton & O.K. Mill. | New Zealand | HJB10682 | PDD 102982 (PL14504) | MK961993† | MK961949† |
| H. aminophilum | Australia | HJB16823 | HO 586929 | MK962007† | MK961966† |
| H. aminophilum f. hygrosarx B.J. Rees | Australia | HJB1000297 | PERTH 06659152 | MK962016† | MK961969† |
| H. angustilamellatum (Zhu L. Yang & Z.W. Ge) B.J. Rees | China | HJB1000408 | HKAS 42927 | AY575919¶ | – |
| H. angustilamellatum | Thailand | HJB12251 | GENT RW07-470 | MK961997† | MK961953† |
| H. angustilamellatum | Laos | HJB14851 | HNL 501000 | MK962003† | MK961962† |
| H. angustilamellatum | Laos | HJB17006 | HNL 501053 | MK962010† | – |
| H. bulbiferum Maire | Croatia | HJB13083 | TUR-A 177060 | KT218422‡ | MK961956† |
| H. cavipes Huijsman | Spain | HJB9433 | HJB9433 | KT217362# | KT216685# |
| H. celatum Grilli, U. Eberh. & Beker | Germany | HJB13621 | BR 5020184119676 | KT218446‡ | MK961957† |
| H. crustuliniforme (Bull.) Quél. | Spain | HJB11237 | HJB11237 | JN943870†† | KF309440| |
| H. cylindrosporum Romagn. | Spain | HJB11427 | C-F-44748 | FJ769365‡‡ | MT832328 |
| H. cylindrosporum | France | HJB12763 | HJB12763 | JQ751210§ | JQ751106§ |
| H. dunense L. Corb. & R. Heim | Belgium | HJB14141 | AdH11031 | KY271835§§ | MK961959† |
| H. flavidifolium | Malaysia | HJB13504 | E. Horak 13404 (ZT) | MT832021 | – |
| H. flavidifolium | Malaysia | HJB13505 | E. Horak 13406 (ZT) | MT832022 | – |
| H. ifeleletorum Kropp | American Samoa | HJB1000386 | UTC 00235643 | MK962019† | MK961970† |
| H. indicum (K.A. Thomas & al.) B.J. Rees | India | HJB1000384 | IB 19971307 | AF407163|| | – |
| H. indicum | India | HJB12902 | IB 19991200 | MK961999† | MK961955† |
| H. khogianum Bresinsky | New Caledonia | HJB1000388 | M-0124631 | GU591635¶¶ | – |
| H. lactariolens Clémençon & Hongo) B.J. Rees & Orlovich | Japan | – | LAU HC88/95 | AY818352¶ | – |
| H. lactariolens | China | – | HMAS 280191 | KX513590††† | – |
| H. lactariolens | Malaysia | HJB13363 | E. Horak 12796 (ZT) | MT832017 | MT832330 |
| H. lactariolens | Malaysia | HJB13365 | E. Horak 13287 (ZT) | MT832019 | – |
| H. lactariolens | Malaysia | HJB13503 | E. Horak 13381 (ZT) | MT832020 | MT832331 |
| H. laterinum (Batsch) Vesterh. | France | HJB13703 | HJB13703 | MK962000† | MK961958† |
| H. mediorufum Soop | New Zealand | HJB10689 | PDD 102983 (PL51404) | KM390552| | KM390037| |
| H. mediorufum | New Zealand | HJB10688 | PDD102995 (PL167404) | KM390572| | KM390042| |
| H. mesophaeum (Pers.) Quél. | Iceland | HJB11050 | HJB11050 | MK961995† | MK961951† |
| H. parvisporum Sparre Pedersen & al. | Laos | HJB14850 | HNL 501009 | MK962002† | MK961961† |
| H. parvisporum | Laos | HJB14852 | HNL 500968 | MK962004† | MK961963† |
| H. parvisporum | Laos | HJB17004 | HNL 500914 | MK962008† | – |
| H. parvisporum | Laos | HJB17005 | HNL 500984 | MK962009† | – |
| H. parvisporum | Laos | HJB17007 | HNL 500884 | MK962011† | – |
| H. parvisporum | Malaysia | HJB13362 | E. Horak 12795 (ZT) | MT832016 | – |
| H. plesiocistum Beker & al. | Spain | HJB11514 | JVG1021214-5 | EU570170‡‡‡ | JQ751115§ |
| H. porphyrosporum Maire | Italy | HJB10344 | HJB10344 | MK961992† | MK961947† |
| H. porphyrosporum | Spain | HJB10767 | HJB10767 | MK961994† | MK961950† |
| H. radicans | Malaysia | HJB13364 | E. Horak 13265 (ZT) | MT832018 | – |
| H. radicosum (Bull.) Ricken | Belgium | HJB10262 | HJB10262 | MK961990† | MK961945† |
| H. radicosum | Italy | HJB10314 | HJB10314 | MK961991† | MK961946† |
| H. sarcophyllum (Peck) Sacc. | U.S.A. | HJB15696 | DPL 10569 | MK962005† | MK961964† |
| H. sarcophyllum | U.S.A. | HJB17783 | MO301904 | MK962014† | – |
| H. sinapizans (Paulet) Gillet | U.K. | HJB10628 | HJB10628 | JQ751191§ | JQ751119§ |
| H. sinapizans | U.K. | HJB10751 | HJB10751 | JQ751193§ | JQ751121 |
| H. subvictoriense B.J. Rees | Australia | HJB1000299 | MEL 2331640 | MK962017† | – |
| H. syrjense (P. Karst.) P. Karst. | France | HJB12064 | HJB12064 | JQ751206§ | JQ751122§ |
| H. syrjense | Finland | HJB12396 | C 26197F | JQ751218§ | JQ751123§ |
| H. theobrominum Quadr. | Estonia | HJB10009 | HJB10009 | EU570181‡‡‡ | JQ751124 |
| H. theobrominum | Belgium | HJB10063 | HJB10063 | FJ816623§§§ | JQ751125§ |
| H. vaccinum Romagn. | Belgium | HJB9965 | HJB9965 | KT217371# | KT216689# |
| H. velutipes Bruchet | France | HJB10547 | HJB10547 | EU570174‡‡‡ | MK961948† |
| H. velutipes | U.K. | HJB10483 | HJB10483 | EU570175‡‡‡ | MT832329 |
| H. vesterholtii Beker & U. Eberh. | Italy | HJB10339 | HJB10339 | FJ816629, FJ816630§§§ | JQ751132 |
| H. vesterholtii | Italy | HJB11869 | HJB11869 | FJ943239, FJ943240§§§ | JQ751135§ |
| H. victoriense A.A. Holland & Pegler | New Zealand | HJB12401 | PDD 93802 (PL3408) | MK961998† | MK961954† |
| H. victoriense | Australia | HJB16704 | HO 586713 | MK962006† | MK961965† |
| H. vinosophyllum Hongo | Japan | HJB17411 | MO287712 (UK323) | MK962012† | MK961967† |
| H. vinosophyllum | Japan | HJB17413 | MO299315 (UK347) | MK962013† | MK961968† |
| H. westraliense Bougher & al. | Australia | HJB1000134 | PERTH 01012665 | MK962015† | – |
| H. youngii B.J. Rees | Australia | – | MEL 2382694 | KP012873||| | – |
| H. youngii | Australia | HJB1000343 | BRI AQ669300 | MK962018† | – |
† Eberhardt et al. (2020); ‡ Grilli et al. (2016); § Eberhardt et al. (2013); | Eberhardt et al. (2015); ¶ Yang et al. (2005); # Eberhardt et al. (2016); †† Schoch et al. (2012); ‡‡ Vesterholt et al. (2009); §§ Beker et al. (2018); || Thomas et al. (2002); ¶¶ Rees et al. (2013); ††† Wei et al. 06 Jul 2016, no reference found; ‡‡‡ Eberhardt et al. (2009); §§§ Eberhardt and Beker (2010); ||| Bonito et al. 19 Oct 2014, no reference found.
Sequence alignments were done online in mafft using the E-INS-i option (Katoh et al. 2017) for ITS and ‘auto’ for MCM7 data. Alignments were viewed and reformatted using aliview 1.24 (Larsson 2014). Maximum likelihood (ML) analyses of single locus alignments were calculated in raxml 8.2.10 (Stamatakis 2014) using the raxml-Gui interface 2.0 (Silvestro and Michalak 2012; Edler et al. 2019), with the GTRGAMMA option, 10 searches for the best ML tree, using the MRE option to limit the number of rapid bootstrap replicates.
The compatibility of the two loci was accessed following the principle of Kauff and Lutzoni (2002), assuming a conflict to be significant if two different relationships for the same set of taxa, one being monophyletic and the other non-monophyletic, are supported by bootstrap with more than 75% in ML analyses.
The datasets were then concatenated and subdivided into five partitions, ITS and four MCM7 partitions, the exon in three partitions by codon position and the intron. In IQ tree 2.0.6, the best partitioning scheme and the best likelihood models were determined under the Bayesian information criterion (Lanfear et al. 2012, 2014: Kalyaanamoorthy et al. 2017). This scheme and the selected models were used for ML tree construction (Nguyen et al. 2015; Chernomor et al. 2016). A bootstrap analysis was run in 500 replicates.
A Bayesian inference (BI) analysis was run with mrbayes 3.2.6 (Ronquist et al. 2012) on CIPRES (Miller et al. 2012). The BI analysis was done unpartitioned in two runs with four chains including one heated chain each using the GTRINVGAMMA model and a uniform prior and sampling one tree of each run every 10,000 generations. The analysis was stopped automatically after 4.28 mio generations. The first 25% of trees were discarded as burnin for calculating posterior probabilities.
Trees were visualized using FigTree 1.4.4 (Rambaut 2006–2018) and submitted to TreeBASE (http://purl.org/phylo/treebase/phylows/study/TB2:S26715). Relationships between species are termed “fully supported”, if bootstrap support is 100% or posterior probability is 1, respectively; and “supported” if bootstrap support ≥ 75% and posterior probabilities ≥ 0.95.
Details of morphological analyses were provided in Beker et al. (2016). For each collection at least 50 spores were measured in Melzer’s reagent, excluding the apiculus. The maximum length and width of each spore was measured, and its Q value (ratio of length to width) calculated. Average length, width, and Q value were calculated and recorded alongside the median, standard deviation, and 5% and 95% percentiles. The assessment and coding of spore characters followed Beker et al. (2016) and Vesterholt (2005). The average width of the widest part of the cheilocystidium in the vicinity of the apex appears to be an important character in the separation of species within Hebeloma (Vesterholt 2005). It is also important, when determining this average width near the apex, not to be selective with regard to the cystidia chosen for measurement. To determine the average width at the apex, about 100 cheilocystidia were measured on the lamella edge. For other measurements, around 20 cheilocystidia, separated from the lamella edge, were measured from each collection. Because of the complex shapes of the cheilocystidia, four measurements were made: length, width at apex (A), width at narrowest point in central region (M), and maximum width in lower half (B). The measurements were given in this order, and an average value was calculated for each of these measurements. For each cheilocystidium the ratios A/M, A/B, and B/M were calculated and averaged across all cheilocystidia measured. Measurements were made in 5% KOH and Melzer’s reagent. For all other details with regard to our methodology, see Beker et al. (2016). Each collection studied has a database record number associated with that collection; we give these numbers as we intend to make the database publicly available.
Results
We obtained ITS data for all recent collections from Malaysia and in addition MCM7 data for Malaysian H. lactariolens. No sequence information could be obtained from Corner’s material. The datasets included 68 ITS and 49 MCM7 sequences (Table 1). Bootstrap support was based on 350 or 300 replicates, respectively. The single locus ML results obtained under the GTRGAMMA model (See TreeBase submission) were fully compatible.
The concatenated dataset included 1439 sites that were analyzed in three partitions with three different models (ITS: GTR+F+I+G4; MCM7 1st and 3rd position: K3P+I; MCM7 2nd and intron: K2P+I) in the ML tree reconstruction. Bootstrap support was based on 500 replicates. The topology of the ML tree is shown in Fig. 1. The consensus tree resulting from the BI analysis differed from the depicted ML tree only at few supported parts of the tree (see TreeBase submission). Posterior probabilities were based on 642 trees and included in Fig. 1.
Figure 1.
ML topology of concatenated ITS and MCM7 sequences of Hebeloma and Alnicola. Flammula alnicola is used for rooting purposes. Bootstrap support based on 500 replicates and posterior probabilities based on a BI analysis are indicated at the branches. Assignment of species to sections follows Beker et al. (2016). Sequences in red are from Malaysian collections discussed in this paper. T indicates type collections. Thick branches indicate full support. AS – Asia, EU – Europe, NA – North America, O – Oceania, gr. – group.
All of the Malaysian collections are included in the clade corresponding to H. sect. Porphyrospora and there within the western Pacific rim clade. The clade of the species H. flavidifolium received full bootstrap and posterior probability support as does the clade of H. parvisporum. In the ML reconstruction, Hebeloma lactariolens is paraphyletic in relation to the sequences of the Oceanic species H. youngii, which are monophyletic and receive full support. In the BI result, H. lactariolens is monophyletic, but unsupported and in a weakly (0.96 posterior probability) supported sister clade relationship with the clade of H. angustilamellatum, H. flavidifolium, H. ifeleleretorum and the H. indicum clade. The Malaysian collections that we refer to as H. flavidifolium, H. lactariolens, and H. parvisporum (Fig. 2) are morphologically and molecularly congruous with each other and other collections from the respective species. The only representative of H. radicans is morphologically and molecularly incongruous with all other known species of fungi.
Figure 2.
Macroscopic features AHebeloma flavidifolium (E. Horak 13406) BH. lactariolens (E. Horak 13381) CH. parvisporum (E. Horak 12796) DH. radicans holotype (E. Horak 13265). Photographs E. Horak.
Taxonomy
We include four species collected from the Malay Peninsula. Three of these have previously been described as Psathyrella. Two of these species, P. splendens and P. verrucispora, were invalidly published but have since been validly published within Hebeloma, as H. parvisporum and H. lactariolens, respectively. The third of these species, Psathyrella flavidifolia was validly published and here we recombine it as a Hebeloma. Finally, we describe a fourth Hebeloma from the Malay Peninsula, Hebeloma radicans, as new.
Hebeloma flavidifolium
(Corner) Beker & U. Eberh. comb. nov.
81AA558B-2F48-5316-A0E1-A9741CE7BD6C
838406
Figure 3.
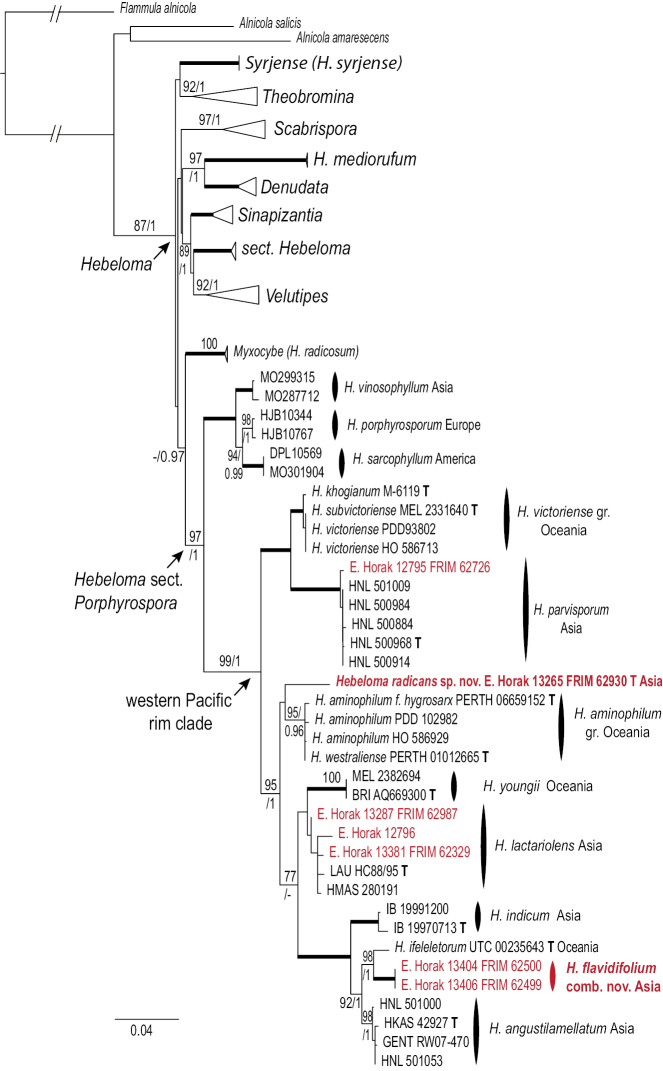
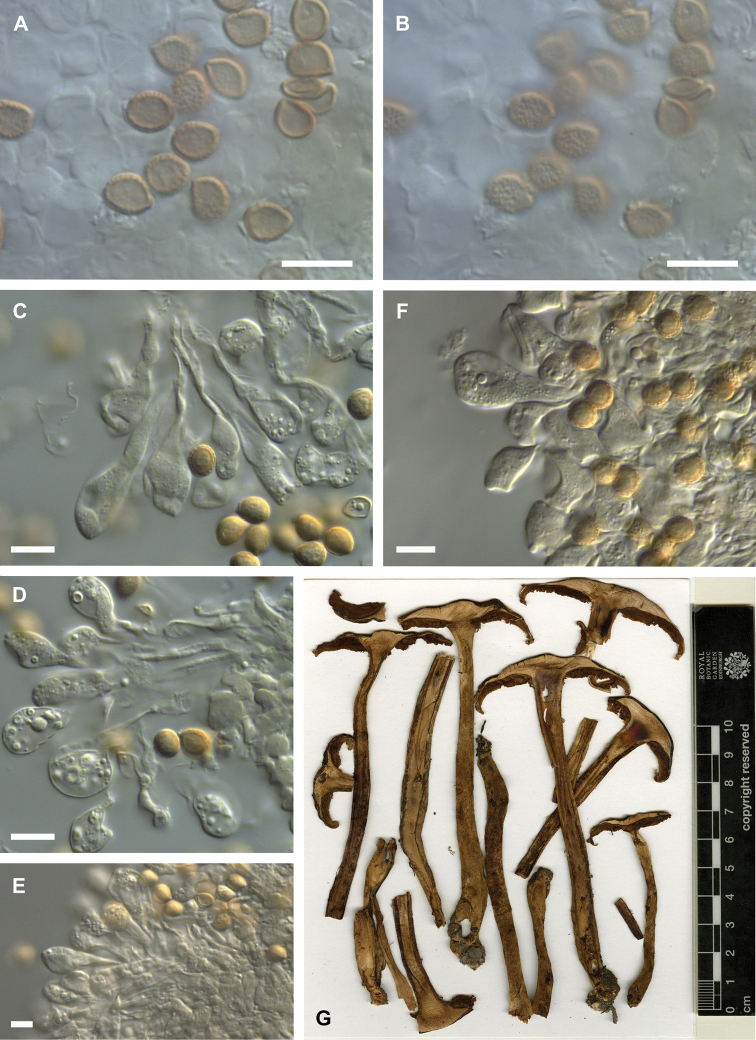
Microscopic features of Hebeloma flavidifolium holotype (E 00204812) A spores in Melzer’s reagent ×1600 B spore ornamentation in Melzer’s reagent ×1600 C cheilocystidia in Melzer’s reagent ×500 D cheilocystidia in Melzer’s reagent ×1000 E cheilocystidia in KOH ×1000 F pleurocystidia in KOH ×1000. Scale bars: 10 µm (A–F). Photographs H.J. Beker. G Exsiccata (a section of photograph from http://data.rbge.org.uk/herb/ E 00204812 provided by the Royal Botanic Garden Edinburgh).
Figure 4.
Microscopic features of Hebeloma flavidifolium (E. Horak 13406) A spores in Melzer’s reagent ×1600 B spore ornamentation in Melzer’s reagent ×1600 C cheilocystidia in KOH ×500 D cheilocystidia in KOH ×1000 E basidium in KOH ×1000 F pleurocystidia in KOH ×1000 G caulocystidium in KOH ×1000 H ixocutis section (showing thin gelatinous epicutis) in KOH ×125 I epicutis hyphae in KOH ×500 J subcutis below epicutis in KOH ×500. Scale bars: 10 µm, 100 µm (H). Photographs H.J. Beker.
Figure 5.
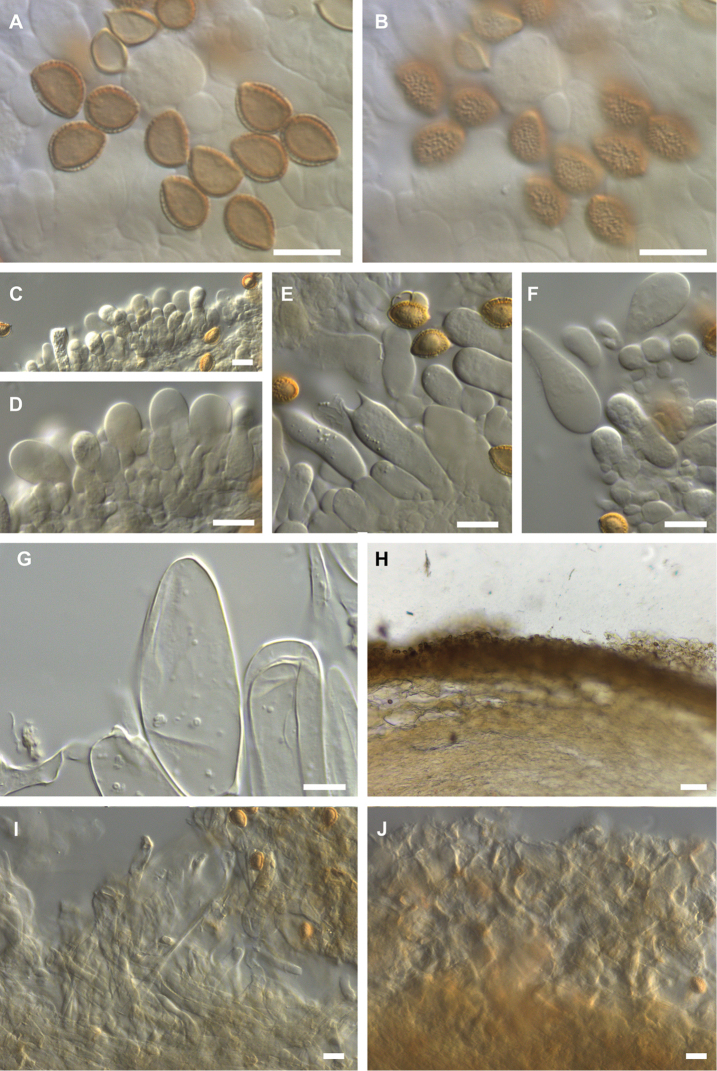
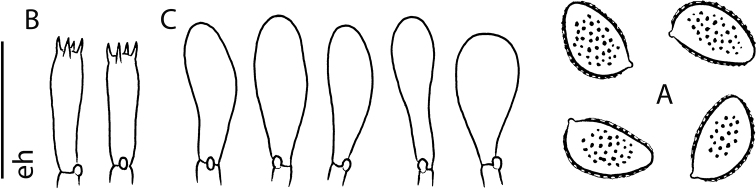
Microscopic features of Hebeloma flavidifolium (E. Horak 13406) A spores ×2000 B basidia ×1000 C cheilocystidia ×1000 D pleurocystidia ×1000 E pileipellis (section of subcutis below epicutis) ×500. Scale bar: 10 µm ×2000, 20 µm ×1000 and 40 µm ×500. Drawing E. Horak.
Basionym.Psathyrella flavidifolia Corner, Gdns’ Bull., Singapore 45(2): 339 (1994) [“1993”].
Homotypic synonym.
Lacrymaria flavidifolia (Corner) Voto, Boll. Assoc. micol. ecol. Romana 107(2): 94 (2019).
Type.
Malaysia. Pahang State: Raub district, Bukit Fraser (Fraser’s Hill), ca. 1200 m a.s.l., Quercus woodland, 25 Nov 1930, E.J.H. Corner (holotype: E! [E 00204812]; database reference HJB19600).
Description.
Basidiomes scattered. Pileus 35–105 mm wide, convex to broadly umbonate; surface dry, sometimes rugulose, occasionally striate at the margin, usually with veil remnants on the margin; cuticle color predominantly cinnamon brown to orange brown (6C5, 7C7) in the center with paler margin, dark beige to tan (5B3); pileus margin strongly involute when young, hygrophanous. Lamellae adnate, often with decurrent tooth, 2–3 mm broad, crowded, thin, with approx. 80–90 full length lamellae and 2–3 lamellules between the lamellae, off-white to cream or yellow-grey when young, later becoming more pinkish or grayish red to purplish and eventually vinaceous to purple-brown or brown following spore maturity; edges weakly fimbriate and white; the white edge remains when the basidiome is dried but the reddish brown color of the lamellae disappears with time. Stipe 50–120 mm long and with central width 5–12 mm, cylindrical sometimes tapering or clavate towards the base, not rooting, occasionally with mycelial cords at the base; white or alutaceous; surface dry, fibrillose, pruinose in the upper part, not discoloring with handling, becoming hollow with age. Flesh whitish, hardly discoloring where bruised. Odor indistinct to raphanoid; taste bitter. Spore vinaceous cinnamon becoming chocolate brown. Exsiccata with no particular characteristics.
Basidiospores based on at least 50 spores from each of three collections, 5% to 95% percentile range 8.9–11.4 × 5.6–7.1 µm, with median 9.7–10.6 × 6.1–6.7 µm and av. 9.6–10.6 × 6.1–6.6 µm with av. S. D. length 0.47 µm and width 0.33 µm; Q value 5% to 95% percentile range 1.43–1.72, with median 1.53–1.58 and av. 1.53–1.59 with av. S. D. 0.07; amygdaloid, occasionally limoniform with small apiculus and rounded apically, with a distinct thinning of the apical wall, without guttules, usually very strongly ornamented, warty, with a strongly and distinctly loosening perispore on almost every mature spore and strongly dextrinoid, becoming medium brown in Melzer’s reagent, sometimes deep brown, ((O3) O4; P3; D3 (D4)); spore color under the light microscope distinctly brown. Basidia av. dimensions 19–33 × 6–9 µm, cylindrical to clavate, without pigmentation, 4-spored. Cheilocystidia irregular, cylindrical to ventricose, often pyriform or napiform often mucronate or rostrate, even lanceolate (as shown in Fig. 3c for example) sometimes septate with width near apex (excluding any rostrum) 5% to 95% percentile range 5.4–10.2 µm, with median 5.6–8.4 µm and av. 5.7–8.6 µm with av. S.D. 0.94; and av. overall measurements 26–29 × 5.7–8.6 × 6.6–9.7 × 5.8–7.5 µm av. Cheilocystidium av. ratios A/M: 0.9–0.91, A/B: 0.77–1.6, B/M: 0.61–1.35. Pleurocystidia present, and abundant, and similar to cheilocystidia, but more often mucronate. Caulocystidia resembling the cheilocystidia but tending to be more cylindrical and longer up to 60 µm. Pileipellis an ixocutis with a very thin epicutis only about 30 µm thick, with gelatinized hyphae, sometimes encrusted, up to 6 µm wide. Subcutis, below the epicutis, orange-brown and the trama below the cutis made up of isodiametric cells up to 17 µm wide. Clamp connections at septa present throughout the basidiome.
Distribution.
So far known only from Bukit Fraser (Fraser’s Hill), Malaysia.
Ecology.
The recent collections were found scattered in lowland dipterocarp-oak woodland on the side of the path in tropical rain forest with Quercus.
Additional material examined.
Malaysia. Pahang State: Raub district, Bukit Fraser (Fraser’s Hill), Jalan Girdle, ca. 1000 m a.s.l., 3.71°N, 101.74°E, Quercus woodland, 26 Apr. 2010, E. Horak 13406 (collection E. Horak at ZT, FRIM [FRIM 62499]; database reference HJB13505); Pahang State: Raub district, Bukit Fraser (Fraser’s Hill), Jalan Girdle, ca. 1000 m alt., 3.71°N, 101.74°E, Quercus woodland, 26 Apr. 2010, E. Horak 13404 (collection E. Horak at ZT, FRIM [FRIM 62500]; database reference HJB13504).
Remarks.
Given Corner’s original description almost totally lacked any microscopic information, we present a full description here based on the holotype plus two more recent collections from roughly the same location, both collected by E. Horak. Morphologically, this species most closely resembles Hebeloma angustilamellatum, originally described from the Yunnan province of China (Yang et al. 2005) and also recorded from northern Thailand and Laos (Table 1, Fig. 1), from which it can be distinguished morphologically by the very strongly ornamented spores (O4), conspicuous even without immersion (those of H. angustilamellatum are O3, so distinctly ornamented but not conspicuous without immersion) and the less conspicuous annulus on the fibrillose stipe of mature basidiomes (H. angustilamellatum has a more persistent annulus, always present, and a stipe, with scattered fibrillose scales, consistently present.) Phylogenetically, based on ITS and MCM7, H. flavidifolium is a sister species of H. ifeleleretorum described from Samoa, but all three form a cluster in Fig. 1 that received full posterior probability and 92% bootstrap support.
Hebeloma lactariolens
(Clémençon & Hongo) B.J. Rees & Orlovich, Mycologia 105: 1055 (2013).
A5E58920-E487-555D-ACED-55A522F00CB0
Figure 6.
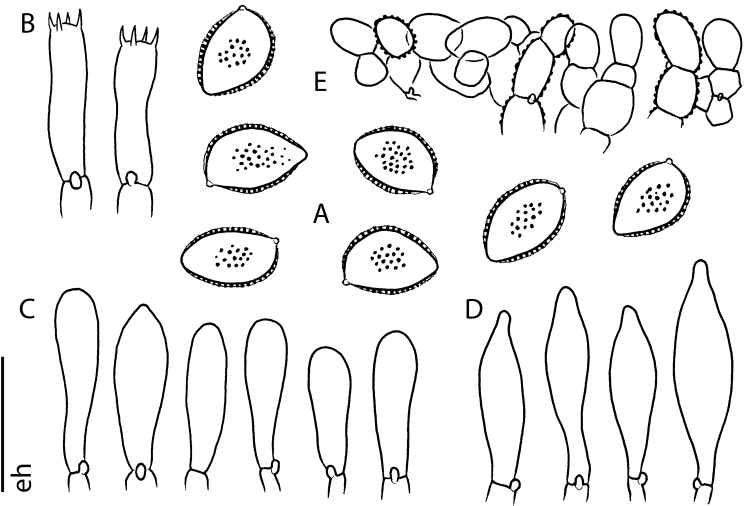
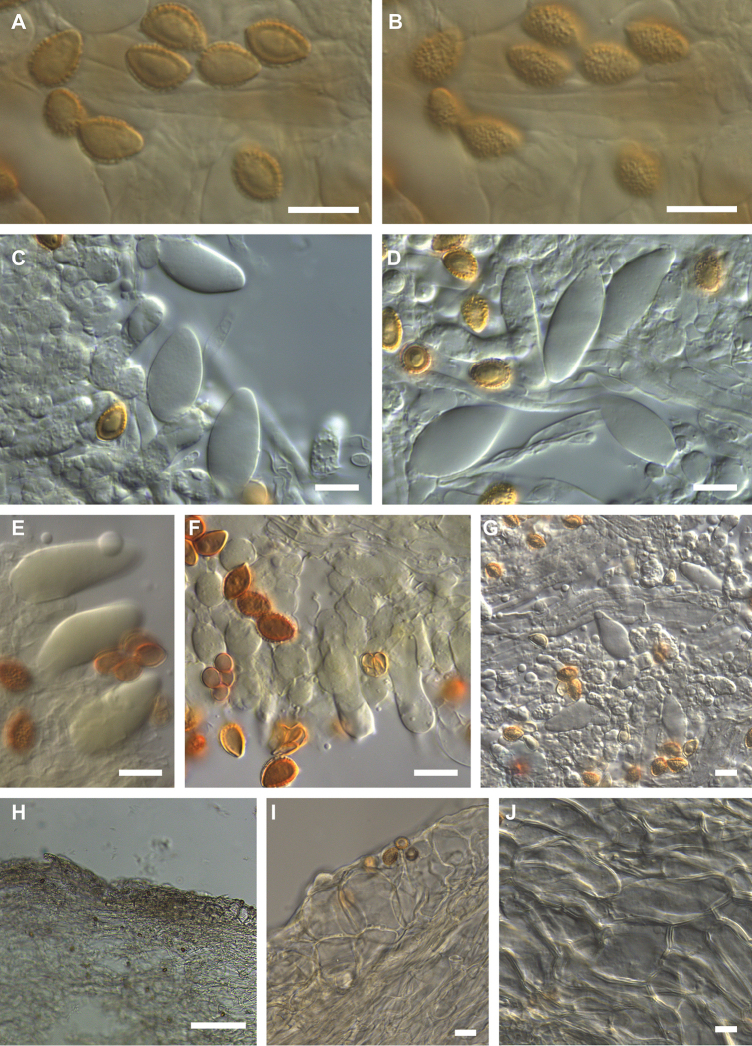
Microscopic features of Hebeloma lactariolens (E 00204780); intended holotype of Psathyrella verrucispora nom. inval.) A spores in Melzer’s reagent ×1600 B spore ornamentation in Melzer’s reagent ×1600 C basidium in KOH ×1000 D cheilocystidia in KOH ×1000 E, F pleurocystidium in KOH ×1000 G caulocystidia in KOH ×500 H sectional view of cutis below the gelatinous epicutis in KOH ×500 I sectional view of ixocutis showing thin gelatinous epicutis in KOH ×125. Scale bars: 10 µm (A–H), 100 µm (I). Photographs H.J. Beker. J Exsiccata (a section of photograph from http://data.rbge.org.uk/herb/E00204780 provided by the Royal Botanic Garden Edinburgh).
Type.
Japan. Shiga-ken: Otsu-shi, Tomikawa, ca. 180 m a.s.l., 34.9001°N, 135.9489°E, Pinus sp., Quercus sp., 15 Aug 1988, T. Hongo, H. Clémençon HC88/95 (holotype TNS! [TNS-F-237670]; isotype LAU; database reference HJB1000383; ITS GenBank acc. no. AY818352).
Homotypic synonyms.
Alnicola lactariolens Clémençon & Hongo, Mycoscience 35(1): 25 (1994). Anamika lactariolens (Clémençon & Hongo) Matheny, Mycol. Res. 109(11): 1262 (2005).
Heterotypic synonyms.
Psathyrella verrucispora Corner, Gdns’Bull., Singapore 45(2): 344 (1994) [1993], nom. inval., Art. 40.7 ≡ Lacrymaria verrucispora (Corner) Voto, Boll. Assoc. micol. ecol. Romana 107(2): 95 (2019), nom. inval., Art. 40.7. Type: Singapore. Malay Peninsula, Aug. 1929, E.J.H. Corner (holotype E! [E 00204780]; database reference HJB19598).
Other material examined.
Malaysia. Johor State: Mersing district, Endau-Rompin Selai, Endau-Rompin (Johor) National Park, Camp Lubuk Tapah, ca. 130 m a.s.l., 2.2976°N, 103.1351°E, with Dipterocarpus, 19 Mar. 2009, E. Horak 12796 (collection E. Horak at ZT, FRIM [FRIM 62726]; database reference HJB13363); Johor State: Kluang district, Endau-Rompin Peta, Endau-Rompin (Johor) National Park, trail to Upeh Guling, ca. 40 m a.s.l., 2.5230°N, 103.3611°E, in woodland with Dipterocarpus and Quercus, 4 Sept. 2009, E. Horak 13287 (collection E. Horak at ZT, FRIM [FRIM 62987]; database reference HJB13365); Negeri Sembilan State: Jelebu district, Simpang Pertang, Pasoh Forest Reserve, ca. 165 m a.s.l., 2.7264°N, 102.0783°E, in woodland, 20 Apr. 2010, E. Horak 13381 (collection E. Horak at ZT, FRIM [FRIM 62329]; database reference HJB13503). SINGAPORE. Malay Peninsula, (E! [E 002048240]; database reference HJB19652), this is just a spore print collected by E.J.H. Corner that may be from the intended type of Psathyrella verrucispora.
Remarks.
Clémençon and Hongo (1994) originally published this taxon as Alnicola lactariolens in the April issue of Mycoscience, apparently published on 1 Apr 1994; it appears Corner had effectively published the paper including the same taxon one day earlier, on 31 Mar 1994 as Psathyrella verrucispora. Both are morphologically clearly members of Hebeloma section Porphyrospora. The authors of both papers comment on the purple-brown (vinaceous) spore print, Corner (1994 [“1993”], p. 345) notes that the spore deposit color is fuscous purple, which is why he described his species in Psathyrella rather than Lacrymaria. Clémençon and Hongo (1994) commented on the spore deposit being a dark purple-brown color, an unknown feature of Alnicola. In Yang et al. (2005)Alnicola lactariolens was recombined into Anamika and later by Rees et al. (2013) into Hebeloma. The spore deposit color and its typical color change upon storage is the most striking feature of members of H. sect. Porphyrospora (Eberhardt et al. 2020). Good descriptions and further illustrations of H. lactariolens can be found in Corner (1994 [“1993”]) and Clémençon and Hongo (1994). Figure 6, shows various macro and micro characters of Corner’s intended type of Psathyrella verrucispora.
This species is rather variable molecularly and in the ML reconstruction forms a clade together with H. youngii, an Australian species growing with Eucalyptus and Corymbia, to our knowledge only known from the type locality (Rees et al. 2013). Even though the monophyly of H. lactariolens in relation to H. youngii is not bootstrap-supported within this analysis (Fig. 1), although it is in the BI results (see TreeBase), the molecular distance, the occurrence on different continents, the different host associations, and morphologically, the cheilocystidia which for H. youngii are more consistently lanceolate and the number of full length lamellae which for H. youngii is in the range 50–60 while for H. lactariolens is always less than 40, clearly separate these taxa. The Malaysian and Singapore records are from lowland tropical forests while the type has been described from a subtropical habitat from Japan, thus hinting at a wide climatic and geographical range. Hebeloma lactariolens is according to observations of S. S. L. not uncommon in Malaysia. The FRIM database includes additional records of this species (not studied) from Hutan Simpan Semangkuk, Fraser’s Hill, Pahang and the Pasoh Forest Reserve, Negeri Sembilan, from hill respective lowland dipterocarp forests.
Hebeloma parvisporum
Sparre Pedersen, Læssøe, Beker & U. Eberh., Mycologia 112: 179 (2020)
3FDD4FFC-1DEF-51C1-875B-8C00369F76F2
Figure 7.
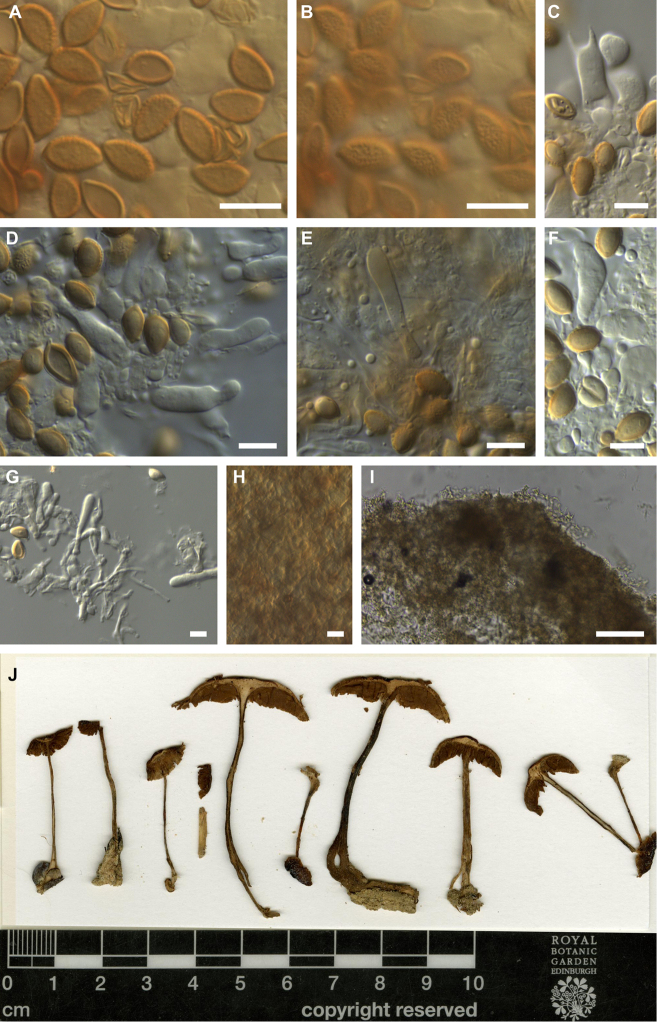
Microscopic features of Hebeloma parvisporum (E 00204835; intended holotype of Psathyrella splendens nom. inval.) A spores in Melzer’s reagent ×1600 B spore ornamentation in Melzer’s reagent ×1600 C, D cheilocystidia in KOH ×1000 E cheilocystidia and basidium in KOH ×500 F caulocystidia, in KOH ×1000. Scale bars: 10 µm. Photos H.J. Beker. G Exsiccata (a section of photograph from http://data.rbge.org.uk/herb/E00204835, provided by the Royal Botanic Garden Edinburgh).
Type.
Laos. Xieng Khouang: Phoukhout, Laethong, ca. 1135 m a.s.l., 19.742408°N, 103.258102°E, on soil under Fagaceae, 18 Aug 2015, T. Læssøe, O.S. Pedersen (holotype: HNL [HNL 500968]; isotype: C! [C-F-122153]; database reference HJB14852; ITS GenBank Acc. No.: MK962004).
Heterotypic synonyms.
Psathyrella splendens Corner, Gdns’ Bull., Singapore 45(2): 341 (1994) [“1993”], nom. inval., Art. 40.7 ≡ Lacrymaria splendens (Corner) Voto, Boll. Assoc. micol. ecol. Romana 107: 95 (2019), nom. inval., Art. 40.7. Type. Singapore. Malay Peninsula, 9. Mar 1930, E.J.H. Corner (holotype: E! [E 00204835]; database reference HJB19597).
Other material examined.
Laos. Xiang Khouang: Khoun, Thoum, ca.1130 m a.s.l., 19.314945°N, 103.409749°E, under Fagaceae, 20 Aug. 2015, T. Læssøe, O.S. Pedersen (HNL [HNL 501009]; database reference HJB14850); Xiang Khouang: Paek, Phonekham, ca.1125 a.s.l., 19.494286°N, 103.269110°E, under Fagaceae, 16 Aug. 2015, T. Læssøe, O.S. Pedersen (HNL [HNL 500914]; database reference HJB17004); Xieng Khouang, Phoukhout, Ban Bong, ca.1150 m a.s.l., 19.672180°N, 103.135841°S, under Fagaceae 15 Aug. 2015, T. Læssøe, O.S. Pedersen (HNL [HNL 500884]; database reference HJB17007); Xieng Khouang, Phoukhout, Sui, ca. 1150 m a.s.l., 19.530514°N, 102.8659°E, under Fagaceae, 19 Aug. 2015, T. Læssøe, O.S. Pedersen (HNL [HNL 500984]; database reference HJB17005). MALAYSIA. Johor State, Mersing district, Endau-Rompin Selai, Endau-Rompin (Johor) National Park, Camp Lubuk Tapah, ca. 130 m alt., 2.2976°N, 103.1351°E, with Dipterocarpus, 19 Mar 2009, E. Horak 12795 (collection E. Horak at ZT; database reference HJB13362).
Remarks.
The description of this species (Eberhardt et al. 2020) was based upon the above collections from Laos. The intended holotype of P. splendens was examined and is micro- and macromorphologically in agreement with H. parvisporum; this is illustrated in Fig. 7 which shows the main micro characters of Corner’s intended type. The collection from Malaysia is monophyletic with the Laos material. Molecularly, the species is most closely related to the Australian/New Zealand H. victoriense species group.
The collection cited as holotype for P. splendens was collected in Singapore while Corner also cites other collections from Singapore and Malaysia (Corner 1994 [“1993”]), to which we can add the Malaysian collection above. Plate 3 of Corner (1994 [“1993”]) illustrates the species macroscopically; Lee (2017) includes a photograph of P. splendens from the FRIM forest and comments that it often grows in large clusters and is common in the FRIM forest and other parts of the country. The FRIM database includes additional records of this species (not studied) from: Endau-Rompin National Park, Johor; Fraser’s Hill, Pahang; the FRIM grounds, Kepong, Selangor; Pasoh, Negeri Sembilan and Tasik Bera, Pahang from lowland and hill dipterocarp forests and a planted dipterocarp forest. S.S.L. observed this species also in degraded hill dipterocarp forest in Janda Baik, Pahang. The species is not listed on the checklist of mushrooms in Thailand (Chandrariskul et al. 2011), but Felix Hampe (oral communication, 21 Jan 2020) reported it from Thailand (Chiang Mai Prov.). Thus, it appears that this species may be widespread within tropical Asia, associated with Fagaceae and dipterocarps (Dipterocarpus). In Laos, H. parvisporum is found for sale in the local markets for human consumption, but its synonym P. splendens is not listed among the species consumed in Malaysia (Chang and Lee 2004; Samsudin and Abdullah 2019).
Hebeloma radicans
E. Horak, Beker & U. Eberh. sp. nov.
E0EC68CD-6813-5608-8200-137758EACEF6
838407
Figure 8.
Microscopic features of Hebeloma radicans holotype (E. Horak 13265) A spores in KOH ×1600 B spore ornamentation in KOH ×1600 C cheilocystidia and basidium in KOH ×1000 D cheilocystidia and basidium in KOH ×1000 E pleurocystidia in Melzer’s reagent ×1000 F basidia in KOH ×1000 G pleurocystidia in KOH ×500 H sectional view of ixocutis showing thin gelatinous epicutis in KOH ×125 I sectional view of subcutis and trama below subcutis in KOH ×500 J sectional view of trama below subcutis in KOH ×500. Scale bars: 10 µm, 100 µm (H). Photographs H.J. Beker.
Figure 9.
Microscopic features of Hebeloma radicans holotype (E. Horak 13265) A spores ×2000 B basidia ×1000 C cheilocystidia ×1000. Scale bar: 10 µm ×2000, 20 µm ×1000 and 40 µm ×500. Drawing E. Horak.
Diagnosis.
The combination of a deeply rooting stipe, about 60 full length lamellae (from stipe to margin of pileus) and spores where almost every spore has a strongly loosening perispore forming a clear layer around the spore, separate this taxon from all other members of H. sect. Porphyrospora, as does the ITS-sequence.
Type.
Malaysia. Johor State: Kluang district, Endau-Rompin Peta, Endau-Rompin (Johor) National Park, Kampung-Peta, trail to Kuala Marong, ca. 50 m a.s.l., 2.52°N, 103.36°E, on soil in lowland dipterocarp-oak forest, 3 Sept 2009, E. Horak, 13265 (holotype: collection E. Horak at ZT; isotype: FRIM [FRIM 62930]; database reference HJB13364, ITS GenBank Acc. No.: MT832018).
Description.
Basidiomes scattered. Pileus 37–64 mm wide, convex to broadly umbonate; surface dry or slightly viscid, without veil remnants on the pileus; cuticle color predominantly cream to pale buff (4A3, 4A4) in the center with paler margin, off-white to pale cream (4A2); pileus margin entire, hygrophanous. Lamellae adnate, moderately dense, thin, with approx. 60 full length lamellae and 2–3 lamellulae between the lamellae, off-white to cream when young, later pinkish or grayish red to purplish and eventually vinaceous to purple-brown following spore maturity; edges weakly fimbriate and white; the white edge remains when the basidiome is dried but the reddish brown color of the lamellae disappears with time. Stipe 160–194 mm long (including the ‘root’) and with central width 4–9 mm, cylindrical, distinctly and deeply rooting, white or alutaceous; surface dry, fibrillose, pruinose in the upper part, discoloring with handling and age. Flesh whitish, hardly discoloring where bruised. Smell fragrant; taste bitter. Spore deposit porphyry-brown (10E4). Exsiccata with no particular characteristics.
Basidiospores based on n = 94 spores of the holotype, 5% to 95% percentile range 8.7–10.2 × 5.6–6.6 µm, with median 9.5 × 6.2 µm and av. 9.5 × 6.2 µm with S. D. length 0.47 µm and width 0.34 µm; Q value 5% to 95% percentile range 1.43–1.65, with median 1.53 and av. 1.54 with S. D. 0.07; amygdaloid, with small apiculus and rounded apically, with a distinct thinning of the apical wall and never any sign of papilla, without guttules, usually very strongly ornamented, warty, with a strongly and distinctly loosening perispore on almost every mature spore (almost forming a uniform layer around the spore and making measurement quite difficult at times) and very strongly dextrinoid, immediately becoming deep and intensely red-brown in Melzer’s reagent, (O4; P3; D4); spore color under the light microscope distinctly brown. Basidia 21–29 × 6–8 µm, with av. 24.3 × 7.2 µm, cylindrical to clavate, without pigmentation, 4-spored. Cheilocystidia ventricose, primarily pyriform often mucronate or rostrate with width near apex (excluding any rostrum) 5% to 95% percentile range 5–8 µm, with median 6.4 µm and av. 6.5 µm with S.D. 1.06; and av. overall measurements 24 × 6.5 × 9.9 × 8.3 µm av. Cheilocystidium av. ratios A/M: 0.66, A/B: 0.79, B/M: 0.84. Pleurocystidia present, and abundant, and similar to cheilocystidia. Caulocystidia resembling the pleurocystidia but tending to be more cylindrical and longer. Pileipellis an ixocutis with a very thin epicutis only about 20 µm thick, with gelatinized hyphae up to 5 µm wide. The cutis below the epicutis is orange-brown and the trama below the cutis is made up of isodiametric cells up to 25 µm wide. Clamp connections at septa present throughout the basidiome.
Distribution.
Only known from the type locality in Endau-Rompin (Johor) National Park, Malaysia.
Ecology.
Scattered in lowland dipterocarp-oak woodland on the side of the path.
Etymology.
From ‘radicans’, meaning rooting, to emphasize this character of the species.
Remarks.
Hebeloma radicans with its vinaceous colored lamellae when mature and the porphyry colored spore print which turns brown with time, is a typical member of H. sect. Porphyrospora. The highly ornamented and highly dextrinoid spores are often seen in taxa of this section; while the consistently loosening perispore is also a common feature of a number of the taxa within this section, the regularity and presentation of the perispore is atypical and very distinctive. The rooting stipe is also unusual; while we have recorded rooting stipes in other members of this section, namely: H. lactariolens, H. parvisporum, and H. victoriense, in these cases it is a shallow root occurring infrequently and not on every basidiome. The rooting stipe of H. radicans is deep and more reminiscent of H. radicosum. This long rooting stipe should be sufficient to distinguish this species from other described members of this section, but taken together with the spore properties and also the moderately dense (but not crowded) lamellae (approx. 60 full length lamellae), assuming these characters are constant, this taxon is clearly distinct. In Fig. 1 as in the BI reconstruction, H. radicans is sister to the Oceanic H. aminophilum group clade, but this relationship is not supported. The ITS differs by at least 2.2% from other members of H. sect. Porphyrospora; there are many species in Hebeloma that are less distant from each other (Beker et al. 2016).
While, to date, we only have one collection of this species, given its morphological differences and molecular distinctness, we are confident that this taxon is different from any other described within Hebeloma and we hope that its publication will encourage its rediscovery. It is of course possible that it has been confused with other genera, e.g. Psathyrella, as was the case with other Malay Peninsula collections as described here, but thus far we have not been able to find any evidence of this.
Discussion
Had the describers of Hebeloma parvisporum been aware of Psathyrella splendens, they would have used that epithet for H. parvisporum. When describing the species, other genera like Alnicola, Naucoria, and even Pholiota were checked for misplaced Hebeloma species (Eberhardt et al. 2020), but it did not occur to the authors to investigate Psathyrella names – nor, it seems, to the authors who reclassified Alnicola lactariolens (Yang et al. 2005; Rees et al. 2013) without referring to P. verrucispora.
We here demonstrate the presence of four, presumably endogenous species, of Hebeloma in tropical forests of the Malay Peninsula, a genus previously overlooked in this region. In the checklist for Malaysia (Lee et al. 2012) the genus Hebeloma is missing. In fact, the entire group of ectomycorrhizal Hymenogastraceae is missing, unless one considers Naucoria periniana, adopted from Chipp’s checklist for the Malay Peninsula (Chipp 1921). This species was recombined into Galerina by Pegler (1975), thus outside of the ectomycorrhizal Hymenogastraceae, although it appears unlikely that Pegler and Chipp refer to the same taxon (Chipp, 1921 p. 383, “King’s collector”). Hebeloma is also missing from checklists for Singapore fungi (Tham and Watling 2017a–d). The ectomycorrhizal Hymenogastraceae are missing, if assuming that Wakefieldia striaespora, described from Singapore, does not represent the same genus as the Greek collections referred to as Wakefieldia macrospora (Kaounas et al. 2011), which are members of the Hymenogastraceae and have been sequenced from ectomycorrhizal root samples (Tedersoo and Smith 2013; Richard et al. 2011). Hebeloma and Hymenogaster records from Thailand (Chandrasrikul et al. 2011) appear to be from northern Thailand and are comprised of names of species that are presumably not native to Thailand (H. albidulum, H. crustuliniforme, H. hiemale, H. radicosum, H. sacchariolens, H. sarcophyllum); the single record of Hymenogaster cf. albellus (originally described from Tasmania by Massee 1898) would currently be referred to as Descolea albella and was moved to the Bolbitaceae (Kuhar et al. 2017). The cited collection of H. angustilamellatum from Thailand is not from the Malay Peninsula (the species is not listed by Chandrasrikul et al. 2011). Thus, it is a safe assumption that these are the first literature records of Hebeloma under this name from the Malay Peninsula, almost certainly endogenous species, and possibly also the first reliable records of ectomycorrhizal Hymenogastraceae. Hebeloma is considered rare in tropical forests. Apart from the records presented here, the only confirmed record is of H. ifeleleretorum (American Samoa, Kropp 2015).
Having said this, it should be noted that the authors of checklists for the Malay Peninsula (Lee et al. 2012; Tham and Watling 2017a–d) do state that these lists are not exhaustive, but represent the state of knowledge at the time of publication. Lack of opportunity and the generally overwhelming biodiversity has often prevented the investigation of less commercially important and generally less well-studied fungi. Those of us with field experience in the area have been aware of the presence of members of Alnicola, Hebeloma and Hymenogaster (probably also in the strict sense) on the Malay Peninsula for some time.
The molecular results support earlier results of Eberhardt et al. (2020) that the members of H. sect. Porphyrospora, originating from the western Pacific Rim, apart from H. vinosophyllum, form a well-supported clade. Within this clade, however, closely related species may be of Oceanic or southeast Asian origin, and may be associated with Fagaceae and/or dipterocarps or Myrtaceae. How this pattern came about, and even whether it will be supported when more data become available, is at this point an open question.
Supplementary Material
Acknowledgements
The collections were made in the context of the Forest Research Institute Malaysia (FRIM) project “Survey, Inventory and Documentation of the Flora and Fauna Biological Diversity of Malaysia” funded by the Malaysian government. We are very much obliged to A. Bogaerts and P. Ballings of the Botanic Garden Meise (BR) for help with handling various loans from a variety of herbaria. We also thank these herbaria for their help: C, E, HNL, IB, TNS and ZT. The Royal Botanic Garden Edinburgh is acknowledged for allowing us to reproduce photographs. We are grateful for the help of K. Bensch of Mycobank in a number of nomenclatural and taxonomic questions. We thank the reviewers for their improvements of the manuscript.
Citation
Eberhardt U, Schütz N, Beker HJ, Lee SS, Horak E (2021) Hebeloma in the Malay Peninsula: Masquerading within Psathyrella. MycoKeys 77: 117–141. https://doi.org/10.3897/mycokeys.77.57394
Funding Statement
Privately funded; institutional funding of the Staatliches Museum Stuttgart; Malaysian Government
References
- Beker HJ, Eberhardt U, Vesterholt J. (2016) Hebeloma (Fr.) P. Kumm. Fungi Europaei 14. Edizioni Tecnografica, Lomazzo, 1232 pp. [Google Scholar]
- Beker HJ, Eberhardt U, Schütz N, Gulden G. (2018) A review of the genus Hebeloma in Svalbard. Mycoscience 59: 303–309. 10.1016/j.myc.2017.12.001 [DOI] [Google Scholar]
- Chandrasrikul A, Suwanarit P, Sangwanit U, Lumyong P, Payapanon A, Sanoamuang N, Pukahuta C, Petcharat V, Sardsud U, Duengkae K, Klinhom U, Thongkantha S, Thongklam S. (2011) Checklist of mushrooms (Basidiomycetes) in Thailand. Office of Natural Resources and Environmental Policy and Planning, Bangkok. https://www.phakhaolao.la/en/publications/check-list-mushrooms-basidiomycetes-thailand
- Chang YS, Lee SS. (2004) Utilisation of macrofungi species in Malaysia. Fungal Diversity 15: 15–22. http://www.fungaldiversity.org/fdp/sfdp/15-2.pdf [Google Scholar]
- Chernomor O, von Haeseler A, Minh BQ. (2016) Terrace aware data structure for phylogenomic inference from supermatrices. Systematic Biology 65: 997–1008. 10.1093/sysbio/syw037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipp TF. (1921) A list of the fungi of the Malay Peninsula. The Gardens’ Bulletin Straits Settlements 2: 311–418. [Google Scholar]
- Clémençon H, Hongo T. (1994) Notes on three Japanese Agaricales. Mycoscience 35: 21–27. 10.1007/BF02268524 [DOI] [Google Scholar]
- Corner EJH. (1994 [“1993”]) Psathyrella (Agaricales) with ornamented spores in the Malay Peninsula. Gardens Bulletin Singapore 45: 337–357. https://www.nparks.gov.sg/sbg/research/publications/gardens-bulletin-singapore/-/media/sbg/gardens-bulletin/4-4-45-2-02-y1993-v45p2-gbs-pg-337.pdf
- Cripps C, Eberhardt U, Schütz N, Beker HJ, Evenson VS, Horak E. (2019) The genus Hebeloma in the Rocky Mountain alpine zone. MycoKeys 46: 1–54. 10.3897/mycokeys.46.32823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardt U, Beker HJ. (2010) Hebeloma vesterholtii, a new species in section Theobromina. Mycological Progress 9: 215–223. 10.1007/s11557-009-0627-z [DOI] [Google Scholar]
- Eberhardt U, Beker HJ, Schütz N, Pedersen OS, Sysouphanthong P, Læssøe T. (2020) Adventurous cuisine in Laos: Hebeloma parvisporum, a new species in Hebeloma section Porphyrospora. Mycologia 112(1): 172–184. 10.1080/00275514.2019.1680220 [DOI] [PubMed] [Google Scholar]
- Eberhardt U, Beker HJ, Vesterholt J, Schütz N. (2016) The taxonomy of the European species of Hebeloma section Denudata subsections Hiemalia, Echinospora subsect. nov. and Clepsydroida subsect. nov. and five new species. Fungal Biology 120: 72–103. 10.1016/j.funbio.2015.09.014 [DOI] [PubMed] [Google Scholar]
- Eberhardt U, Beker HJ, Vesterholt J. (2015) Decrypting the Hebeloma crustuliniforme complex: European species of Hebeloma section Denudata subsection Denudata. Persoonia 35: 101–147. 10.3767/003158515X687704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardt U, Beker HJ, Vesterholt J, Dukik K, Walther G, Vila J, Fernández Brime S. (2013) European species of Hebeloma section Theobromina. Fungal Diversity 58: 103–126. 10.1007/s13225-012-0188-3 [DOI] [Google Scholar]
- Eberhardt U, Beker HJ, Vila J, Vesterholt J, Llimona X, Gadjieva R. (2009) Hebeloma species associated with Cistus. Mycological Research 113: 153–162. 10.1016/j.mycres.2008.09.007 [DOI] [PubMed] [Google Scholar]
- Edler D, Klein J, Antonelli A, Silvestro D. (2019) raxmlGUI 2.0 beta: a graphical interface and toolkit for phylogenetic analyses using RAxML. bioRxiv. 10.1101/800912 [DOI]
- Grilli E, Beker HJ, Eberhardt U, Schütz N, Leonardi M, Vizzini A. (2016) Unexpected species diversity and contrasting evolutionary hypotheses in Hebeloma sections Sinapizantia and Velutipes in Europe. Mycological Progress 15: 1–46. 10.1007/s11557-015-1148-6 [DOI] [Google Scholar]
- Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. (2017) Fast model selection for accurate phylogenetic estimates. Nature Methods 14: 587–589. 10.1038/nmeth.4285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaounas V, Assyov B, Alvarado P. (2011) New data on hypogeous fungi from Greece with special reference to Wakefieldia macrospora (Hymenogasteraceae, Agaricales) and Geopora clausa (Pyronemataceae, Pezizales). Mycologia Balcanica 8: 105–113. [Google Scholar]
- Katoh K, Rozewicki J, Yamada KD. (2017) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics bbx 108: 1–7. 10.1093/bib/bbx108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauff F, Lutzoni F. (2002) Phylogeny of the Gyalectales and Ostropales (Ascomycota, Fungi): among and within order relationships based on nuclear ribosomal RNA small and large subunits. Molecular Phylogenetics and Evolution 25: 138–156. 10.1016/S1055-7903(02)00214-2 [DOI] [PubMed] [Google Scholar]
- Kropp BR. (2015) A Samoan Hebeloma with phylogenetic ties to the western Pacific. Mycologia 107: 149–156. 10.3852/14-047 [DOI] [PubMed] [Google Scholar]
- Kuhar F, Smith ME, Mujic A, Truong C, Nouhra E. (2017) A systematic overview of (Agaricales) in the Nothofagaceae forests of Patagonia. Fungal Biology 121: 876–889. 10.1016/j.funbio.2017.06.006 [DOI] [PubMed] [Google Scholar]
- Lanfear R, Calcott B, Kainer D, Mayer C, Stamatakis A. (2014) Selecting optimal partitioning schemes for phylogenomic datasets. BMC Evolutionary Biology14: e82. 10.1186/1471-2148-14-82 [DOI] [PMC free article] [PubMed]
- Lanfear R, Calcott B, Ho SYW, Guindon S. (2012) PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution 29: 1695–1701. 10.1093/molbev/mss020 [DOI] [PubMed] [Google Scholar]
- Larsson A. (2014) AliView: a fast and lightweight alignment viewer and editor for large data sets. Bioinformatics 30: 3276–3278. 10.1093/bioinformatics/btu531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS. (2017) A Field Guide to the Larger Fungi of FRIM. Forest Research Institute Malaysia, Kepong, Selangor, Malaysia.
- Lee SS, Alias SA, Jones EGB, Zainuddin N, Chan HT. (2012) Checklist of Fungi from Malaysia. Forest Research Institute Malaysia, Kepong, Selangor.
- Massee GE. (1898) Fungi exotici, I. Bulletin of Miscellaneous Informations of the Royal Botanical Gardens Kew 1898: 113–136. 10.2307/4115483 [DOI] [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. (2010) Creating the CIPRES science gateway for inference of large phylogenetic trees. In: Xavier J (Ed.) 2010 Gateway Computing Environments Workshop (GCE) Proceedings of a meeting held 14 Nov 2010, New Orleans, Louisiana, USA. Institute of Electrical and Electronics Engineers (IEEE), Piscataway, New Jersey, 8 pp 10.1109/GCE.2010.5676129 [DOI] [Google Scholar]
- Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. (2015) IQ-TREE: A fast and effective stochastic algorithm for estimating Maximum Likelihood phylogenies. Molecular Biology and Evolution 32: 268–274. 10.1093/molbev/msu300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Örstadius L, Ryberg M, Larsson E. (2015) Molecular phylogenetics and taxonomy in Psathyrellaceae (Agaricales) with focus on psathyrelloid species: introduction of three new genera and 18 new species. Mycological Progress 14(25): 1–42. 10.1007/s11557-015-1047-x [DOI] [Google Scholar]
- Paul V, Sudin M, Fui FS, Kassim MHS, Seelan JSS. (2019) Macrofungi of Imbak Canyon-Batu Timbang Area, Sabah. Journal of Tropical Biology and Conservation 16: 107–117. https://jurcon.ums.edu.my/ojums/index.php/jtbc/article/view/2031/1325 [Google Scholar]
- Pegler DN. (1975) A revision of the Zanzibar Agaricales described by Berkeley. Kew Bulletin 30: 429–442. 10.2307/4103067 [DOI] [Google Scholar]
- Rambaut A. (2006–2018) FigTree. Tree figure drawing tool version 14.4.4. Institute of Evolutionary Biology, University of Edinburgh, Edinburgh. http://tree.bio.ed.ac.uk/software/figtree/
- Rees BJ, Midgley DJ, Marchant A, Lerkins A, Orlovich DA. (2013) Morphological and molecular data for Australian Hebeloma species do not support the generic status of Anamika. Mycologia 105: 1043–1058. 10.3852/12-404 [DOI] [PubMed] [Google Scholar]
- Richard F, Roy M, Shahin O, Sthultz C, Duchemin M, Joffre R, Selosse M-A. (2011) Ectomycorrhizal communities in a Mediterranean forest ecosystem dominated by Quercus ilex: seasonal dynamics and response to drought in the surface organic horizon. Annals of Forest Science 68: 57–68. 10.1007/s13595-010-0007-5 [DOI] [Google Scholar]
- Ronquist F, Huelsenbeck JP, Teslenko M. (2011) Draft MrBayes version 3.2 Manual: Tutorials and Model Summaries. http://mrbayes.sourceforge.net/mb3.2_manual.pdf
- Ronquist F, Teslenko M, Van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Sucharard MA, Huelsenbeck JP. (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsudin NIP, Abdullah N. (2019) Edible mushrooms from Malaysia; a literature review on their nutritional and medicinal properties. International Food Research Journal 26: 11–31. http://www.ifrj.upm.edu.my/ [Google Scholar]
- Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W, Fungal Barcoding Consortium. (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proceedings of the National Academy of Sciences 109: 6241–6246. 10.1073/pnas.1117018109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestro D, Michalak I. (2012) raxmlGUI: a graphical front-end for RAxML. Organisms Diversity and Evolution 12: 335–337. 10.1007/s13127-011-0056-0 [DOI] [Google Scholar]
- Stamatakis A. (2014) RAxML Version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedersoo L, Smith ME. (2013) Lineages of ectomycorrhizal fungi revisited: Foraging strategies and novel lineages revealed by sequences from belowground. Fungal Biology 27: 83–99. 10.1016/j.fbr.2013.09.001 [DOI] [Google Scholar]
- Tham FY, Watling R. (2017a) Annotated checklist of macrofungi recorded from Singapore: Singapore Botanic Gardens. The authors, Singapore.
- Tham FY, Watling R. (2017b) Annotated checklist of macrofungi recorded from Singapore: MacRitchie-Pierce. The authors, Singapore.
- Tham FY, Watling R. (2017c) Annotated checklist of macrofungi recorded from Singapore: Bukit Timah. The authors, Singapore.
- Tham FY, Watling R. (2017d) Annotated checklist of macrofungi recorded from Singapore: Mandai-Seletar. The authors, Singapore.
- Thomas KA, Peintner U, Moser MM, Manimohan P. (2002) Anamika, a new mycorrhizal genus of Cortinariaceae from India and its phylogenetic position based on ITS and LSU sequences. Mycological Research 106: 245–251. 10.1017/S0953756201005445 [DOI] [Google Scholar]
- Turland NJ, Wiersema JH, Barrie FR, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Kusber W-H, Li D-Z, Marhold K, May TW, McNeill J, Monro AM, Prado J, Price MJ, Smith GF [Eds] (2018) International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Koeltz Botanical Books, Glashütten. 10.12705/Code.2018 [DOI]
- Vesterholt J. (2005) The Genus Hebeloma Fungi of Northern Europe 3. Svampetryk, Tilst.
- Vesterholt J, Eberhardt U, Beker HJ. (2014) Epitypification of Hebeloma crustuliniforme. Mycological Progress 13: 553–562. 10.1007/s11557-013-0938-y [DOI] [Google Scholar]
- Vesterholt J, Gryta H, Marmeisse R, Beker HJ, Eberhardt U, Grilli E, Boyle H. (2009) (1899) Proposal to conserve the name Hebeloma cylindrosporum against Hebeloma angustispermum (Basidiomycota). Taxon 58: 1005. 10.1002/tax.583034 [DOI]
- Voto P. (2019) Novelties in the family Psathyrellaceae. Part I. Rivista Micologica Romana, Bolletino dell’Associazione Micologica Ecologica Romana 107: 94–95. [Google Scholar]
- Yang ZL, Matheny PB, Ge Z-W, Slot JC, Hibbett DS. (2005) New Asian species of the genus Anamika (Euagarics, hebelomatoid Clade) based on morphology and ribosomal DNA sequences. Mycological Research 109: 1259–1267. 10.1017/S0953756205003758 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.