Abstract
Drought is one of the major environmental stresses that negatively affect the maize (Zea mays L.) growth and production throughout the world. Foliar applications of plant growth regulators, micronutrients or osmoprotectants for stimulating drought-tolerance in plants have been intensively reported. A controlled pot experiment was conducted to study the relative efficacy of salicylic acid (SA), zinc (Zn), and glycine betaine (GB) foliar applications on morphology, chlorophyll contents, relative water content (RWC), gas-exchange attributes, activities of antioxidant enzymes, accumulations of reactive oxygen species (ROS) and osmolytes, and yield attributes of maize plants exposed to two soil water conditions (85% field capacity: well-watered, 50% field capacity: drought stress) during critical growth stages. Drought stress significantly reduced the morphological parameters, yield and its components, RWC, chlorophyll contents, and gas-exchange parameters except for intercellular CO2 concentration, compared with well water conditions. However, the foliar applications considerably enhanced all the above parameters under drought. Drought stress significantly (p < 0.05) increased the hydrogen peroxide and superoxide anion contents, and enhanced the lipid peroxidation rate measured in terms of malonaldehyde (MDA) content. However, ROS and MDA contents were substantially decreased by foliar applications under drought stress. Antioxidant enzymes activity, proline content, and the soluble sugar were increased by foliar treatments under both well-watered and drought-stressed conditions. Overall, the application of GB was the most effective among all compounds to enhance the drought tolerance in maize through reduced levels of ROS, increased activities of antioxidant enzymes and higher accumulation of osmolytes contents.
Subject terms: Plant sciences, Plant stress responses, Drought
Introduction
Maize (Zea mays L.) is one of the most important cereal crops globally. Maize is as the major source of food, feed, and bio-fuel and therefore, consumption and demand of maize is increasing worldwide1. In China, maize is the third-most important food crop after rice and wheat. The cultivated area for maize in China is estimated at 42.42 million ha with yield of about 259.23 million tonnes year1,2. However, it is an extremely sensitive cereal crop to drought stress, especially during critical growth stages. It has been well reported that maize is comparatively more vulnerable to drought stress compared with other cereals such as wheat3.
Globally, the demands for food crops are projected to be doubled by 2050 because of the ever increasing and burgeoning population4. The irrigation water is considered a major resource to crop production, but it is scarce and expensive in many regions of the world, as a result, maize production is often restricted. Universally, the agriculture sector utilizes around 70% of freshwater for irrigation to the farming lands5. Approximately 40% of the total food is produced from about 17% of the cropped land area by irrigated agriculture6. With the continued increase of world population, industrialization, and urbanization, the competition for fresh water will be highly increased among agriculture and other sectors4,7.
Drought stress severely hampers the growth and productivity of maize8–10. It triggers different changes in crop plants through various morphological, physiological, and biochemical responses11–13. Drought stress causes oxidative damage in plants through higher production of ROS, nevertheless, plants possess the antioxidant defense system and enhanced synthesis of antioxidants such as the ascorbate peroxidase (APX), glutathione reductase (GR), peroxidase (POD), superoxide dismutase (SOD), and catalase (CAT) helps in quenching of ROS produced during drought stress 10,13–17.The exogenous applications of plant growth regulators, micronutrients, or osmoprotectants can play a significant role in improving drought-resistance at various plant growth stages18,19. Salicylic acid (SA) is a natural phenolic compound that can effectively alleviate the harmful effects caused by abiotic environmental stresses20,21. Moreover, it can play important role in modulating growth, and physiological and biochemical characteristics in plants17,22–24. The SA has been reported to be beneficial in relieving the negative effects of drought stress by improving the seedling growth, leaf gas-exchange traits, and activities of APX, CAT and SOD enzymes, while decreasing the MDA and H2O2 contents25,26. Sharma et al.27 suggested that SA markedly increased drought-tolerance and could be used for increasing and stabilizing crop production under stress conditions. Zinc (Zn) is an essential micronutrient that is involved in physiological functions and structure of the regulatory cofactor of many enzymes, carbohydrate and chlorophyll production, pollen development, fertilization, RNA and DNA metabolism, and protein synthesis28,29. Zn plays a critical role in improving resistance against drought stress by detoxifying ROS generation and increasing antioxidant enzymes30,31. Glycine betaine (GB) is naturally occurring osmoprotectant compound that accumulates in numerous plants under drought stress. Iqbal et al.32 reported that foliar application of GB improved turgor potential and sunflower yield under drought stress. Raza et al.33 indicated that the growth parameters and yield components were reduced under drought stress in wheat plants, while exogenous application of GB was effective in mitigating the detrimental effects of drought. Hasanuzzaman et al.34 recorded that the GB played an important role in improving the detoxification of ROS, hence recovering photosynthesis and decreasing oxidative damage.
Nevertheless, no study has been conducted to appraise the relative effect of these compounds (SA, Zn, or GB) in improving maize tolerance against drought stress. The present study was conducted to assess the possible role of SA, Zn, anGB betaine treatments in improving drought-tolerance in maize under critical growth stages, based on changes in growth, yield, and physiological and biochemical features. It was hypothesized that the exogenous applications of these compounds could mitigate the negative effects of drought stress in maize from the fourteenth leaf (V14) until blister (R2) growth stages by regulating antioxidant enzymes. To verify this hypothesis, the maize plants were sprayed with SA, Zn, and GB under well-watered and drought-stressed conditions. The specific objectives were to (1) examine the response of maize growth characters, yield, and its components to the foliar application of SA, Zn, and GB under different soil water conditions during critical growth stages; (2) investigate the effect of respective spraying applications on chlorophyll contents, RWC, leaf gas-exchange attributes, ROS accumulation, MDA content, activity of antioxidant enzymes, and osmolytes accumulation under different soil water conditions; and (3) compare the relative efficacy of different spraying applications for ameliorating the harmful effects of drought stress by enhancing the preceding parameters in maize.
Results
Growth parameters, yield, and its components
Drought stress significantly (p < 0.05) disrupted the maize growth parameters, yield, and its components in terms of plant height, fresh weight plant−1, dry weight plant−1, the number of leaves plant−1, leaf area plant−1, the number of grains plant−1, 100-grain weight, biological yield plant−1, grain yield plant−1, and harvest index. However, the results showed that the growth parameters, yield, and its components were improved by SA, Zn, and GB spraying treatments under well-watered and drought-stressed conditions (Tables 1, 2, 3). As predicted, one or more of spraying treatments statistically improved all the above parameters in both soil water conditions except for plant height and harvest index under the well-watered condition as compared with the control treatment. Under the drought-stressed condition, the SA, Zn, or GB spray increased the plant height by 7.32%, 5.50%, and 10.07%, fresh weight by 23.08%, 16.82%, and 25.34%, dry weight by 14.31%, 12.28%, and 22.55%, number of leaves by 14.35%, 8.65%, and 25.56%, leaf area by 17.22%, 11.54%, and 22.81%, number of grains by 16.03%, 2.48%, and 29.51%, 100-grain weight by 20.83%, 16.30%, and 23.46%, biological yield by 27.54%, 12.48%, and 41.10%, grain yield by 40.52%, 19.98%, and 60.49%, and harvest index by 10.12%, 6.46%, and 13.65%, respectively, as compared to the values of control. Generally, the maximum growth parameters, yield, and its components were recorded from the plants treated with GB followed by SA and Zn spraying treatments under two soil water conditions.
Table 1.
Effect of soil water conditions and foliar treatments on plant height, fresh weight, dry weight, number of leaves, and leaf area.
| Soil water conditions | Foliar treatments | Plant height (cm) | Fresh weight plant−1 (g) | Dry weight plant−1 (g) | No. of leaves plant−1 | Leaf area plant−1 (cm2) |
|---|---|---|---|---|---|---|
| WW | CK | 181.00ab ± 5.69 | 337.87b ± 19.49 | 63.71bc ± 2.83 | 12.89bc ± 0.49 | 5237.47bc ± 125.44 |
| SA | 186.67a ± 5.24 | 362.89a ± 16.10 | 68.16ab ± 2.16 | 14.44ab ± 0.29 | 5775.23a ± 145.49 | |
| Zn | 184.33a ± 6.37 | 350.74ab ± 11.55 | 66.91ab ± 2.09 | 14.33ab ± 0.33 | 5605.47ab ± 147.14 | |
| GB | 187.67a ± 3.72 | 368.65a ± 22.74 | 70.63a ± 2.53 | 14.85a ± 0.46 | 5969.70a ± 234.39 | |
| Means | 184.91 | 355.03 | 67.35 | 14.13 | 5646.96 | |
| WD | CK | 109.32d ± 4.49 | 191.64e ± 8.89 | 37.86 fg ± 1.21 | 9.82e ± 0.61 | 3703.93f. ± 180.88 |
| SA | 117.33c ± 3.29 | 235.88c ± 11.73 | 43.28de ± 2.14 | 11.23 cd ± 0.62 | 4341.98de ± 229.12 | |
| Zn | 115.34 cd ± 3.93 | 223.88 cd ± 10.90 | 42.51ef ± 2.41 | 10.67de ± 0.33 | 4131.53e ± 271.97 | |
| GB | 120.33c ± 3.18 | 240.21c ± 9.90 | 46.40d ± 2.53 | 12.33c ± 0.67 | 4548.87d ± 165.13 | |
| Means | 115.58 | 222.90 | 42.51 | 11.01 | 4181.57 |
Values are means (± SE) of three replicates. For L.S.D.’s results, means with different letters indicate that means are different at 95% confidence level.
WW, well-watered; WD, water-deficient; CK, control (double distilled water); SA, salicylic acid; Zn, Zinc; GB, Glycine betaine.
Table 2.
Effect of soil water conditions and foliar treatments on the number of grains, 100-grain weight, biological yield, grain yield, and harvest index.
| Soil water conditions | Foliar treatments | No. of grains plant−1 | 100-grain weight (g) | Biological yield plant−1 (g) | Grain yield plant−1 (g) | Harvest index (%) |
|---|---|---|---|---|---|---|
| WW | CK | 294.65bc ± 15.25 | 31.18b ± 0.98 | 256.35c ± 4.04 | 91.72bc ± 3.93 | 35.76a ± 1.20 |
| SA | 312.10ab ± 15.14 | 32.55b ± 0.64 | 273.13ab ± 5.20 | 101.40ab ± 2.99 | 37.12a ± 0.84 | |
| Zn | 302.80b ± 16.30 | 32.22b ± 1.25 | 265.07bc ± 5.82 | 97.15b ± 1.65 | 36.71a ± 1.37 | |
| GB | 324.19a ± 25.93 | 33.20a ± 2.13 | 284.32a ± 6.01 | 106.53a ± 1.28 | 37.48a ± 0.34 | |
| Means | 308.43 | 32.28 | 269.71 | 99.19 | 36.76 | |
| WD | CK | 215.78ef ± 14.79 | 24.72 cd ± 1.04 | 163.33 g ± 3.78 | 53.05f. ± 1.70 | 32.51b ± 1.16 |
| SA | 250.36d ± 12.43 | 29.87bc ± 1.01 | 208.32e ± 5.12 | 74.55d ± 1.80 | 35.80a ± 0.51 | |
| Zn | 221.13e ± 5.99 | 28.75c ± 0.76 | 183.71f. ± 5.87 | 63.65e ± 3.11 | 34.61ab ± 0.63 | |
| GB | 279.46c ± 10.02 | 30.52b ± 0.93 | 230.46d ± 4.71 | 85.14c ± 2.01 | 36.95a ± 0.50 | |
| Means | 241.68 | 28.46 | 196.45 | 69.09 | 34.96 |
Values are means (± SE) of three replicates. For L.S.D.’s results, means with different letters indicate that means are different.
at 95% confidence level.
WW, well-watered; WD, water-deficient; CK, control (double distilled water); SA, salicylic acid; Zn, Zinc; GB, Glycine betaine.
Table 3.
p-values of the two-way factorial analysis of growth, yield, and physiological and biochemical parameters of maize as influenced by various foliar treatments under both soil water conditions.
| Parameters | Main factors effects | Interaction effects | |
|---|---|---|---|
| S | F | S × F | |
| Plant height | < 0.0001 | < 0.0293 | < 0.0491 |
| Fresh weight | < 0.0001 | < 0.0069 | < 0.0426 |
| Dry weight | < 0.0001 | < 0.0281 | < 0.0487 |
| Number of leaves | < 0.0001 | < 0.0060 | < 0.0268 |
| Leaf area | < 0.0001 | < 0.0030 | < 0.0376 |
| Number of grains | < 0.0001 | < 0.0340 | < 0.0473 |
| 100-grain weight | < 0.0003 | < 0.0204 | < 0.0342 |
| Biological yield | < 0.0001 | < 0.0001 | < 0.0073 |
| Grain yield | < 0.0001 | < 0.0001 | < 0.0152 |
| Harvest index | < 0.0113 | < 0.0197 | < 0.0466 |
| Chlorophyll a content | < 0.0022 | < 0.0014 | < 0.0327 |
| Chlorophyll b content | < 0.0001 | < 0.0078 | < 0.0254 |
| Total chlorophyll content | < 0.0001 | < 0.0001 | < 0.0426 |
| RWC | < 0.0001 | < 0.0273 | < 0.0305 |
| Net photosynthesis rate | < 0.0001 | < 0.0001 | < 0.0254 |
| Transpiration rate | < 0.0001 | < 0.0001 | < 0.0159 |
| Stomatal conductance | < 0.0001 | < 0.0001 | < 0.0289 |
| Intercellular CO2 concentration | < 0.0001 | < 0.0001 | < 0.0186 |
| APX activity | < 0.0001 | < 0.0001 | < 0.0008 |
| GR activity | < 0.0001 | < 0.0001 | < 0.0001 |
| POD activity | < 0.0003 | < 0.0001 | < 0.0376 |
| CAT activity | < 0.0001 | < 0.0001 | < 0.0226 |
| SOD activity | < 0.0001 | < 0.0039 | < 0.0288 |
| MDA content | < 0.0001 | < 0.0001 | < 0.0342 |
| H2O2 content | < 0.0001 | < 0.0001 | < 0.0245 |
| O2− content | < 0.0001 | < 0.0001 | < 0.0076 |
| Free proline content | < 0.0001 | < 0.0001 | < 0.0008 |
| Total soluble sugar | < 0.0001 | < 0.0001 | < 0.0287 |
‘S’: effect of soil water conditions; ‘F’: effect of foliar treatments; S × F, effect of the interaction between two variables.
p-values are regarded as significant (p < 0.05, n = 3) and highly significant (p < 0.01, n = 3).
Chlorophyll contents and RWC
Drought stress statistically (p < 0.05) reduced the chlorophyll (Chl.) contents of maize leaves and relative water content (RWC). However, the results revealed that the Chl. a, Chl. b, and total Chl. contents and RWC were improved by SA, Zn, and GB spraying treatments in both soil water conditions (Fig. 1 and Table 3). Compared with control, Chl. a content was significantly increased by spraying treatments under the drought-stressed condition. Chl. b content was substantially enhanced by GB treatment under both soil water conditions and by SA treatment under the drought-stressed condition. Total Chl. content was statistically influenced by spraying treatments under both soil water conditions except for Zn treatment under the well-watered condition. RWC was statistically affected by GB and SA treatments under the drought-stressed condition. Under the drought-stressed condition, the concerned spraying treatments improved Chl. a content by 22.45%, 19.78%, and 24.59%, Chl. b content by 8.00%, 6.40%, and 10.40%, total Chl. content by 16.98%, 14.74%, and 18.91%, and RWC by 19.44%, 6.75%, and 23.42%, respectively, as compared to the values of the control treatment. Overall, maximum chlorophyll contents and RWC were registered from the plants treated with GB and followed by SA and Zn spraying treatments under both soil water conditions.
Figure 1.
Effect of soil water conditions and foliar treatments on chlorophyll a, chlorophyll b, total chlorophyll, and relative water contents. Every column in each graph represents the mean (± SE) of three replicates. Different letters above columns indicate that means are different at 95% confidence level. WW, well-watered; WD, water-deficient; CK, control (double distilled water); SA, salicylic acid; Zn, Zinc; GB, Glycine betaine.
Leaf gas-exchange attributes
Drought stress significantly (p < 0.05) influenced the net photosynthesis rate (Pn), transpiration rate (Tr), stomatal conductance (Gs), and intercellular CO2 concentration (Ci). However, results noticed that the spraying treatments of SA, Zn, and GB increased the above leaf gas-exchange parameters except for Ci (Fig. 2 and Table 3). Pn and Gs were significantly increased by spraying treatments under both soil water conditions except for Zn treatment under the well-watered condition. Tr was statistically enhanced by GB treatment under both soil water conditions and by SA treatment under the drought-stressed condition. Ci was statistically decreased by spraying treatments under both soil water conditions except for Zn treatment under the well-watered condition. Under the drought-stressed condition, the respective spraying treatments promoted the Pn by 30.48%, 17.09%, and 45.77%, Tr by 32.25%, 19.35%, and 40.32%, and Gs by 66.66%, 33.33%, and 66.66%; while they decreased the Ci by 15.91%, 7.59%, and 26.62%, respectively, as compared to the values of the control. Overall, the spraying treatments improved the leaf gas-exchange attributes under the drought-stressed condition more than the well-watered condition. The GB treatment was more effective than SA and Zn treatments as compared with control under both soil water conditions.
Figure 2.
Effect of soil water conditions and foliar treatments on net photosynthesis rate (Pn), transpiration rate (Tr), stomatal conductance (Gs), and intercellular CO2 concentration (Ci). Every column in each graph represents the mean (± SE) of three replicates. Different letters above columns indicate that means are different at 95% confidence level. WW, well-watered; WD, water-deficient; CK, control (double distilled water); SA, salicylic acid; Zn, Zinc; GB, Glycine betaine.
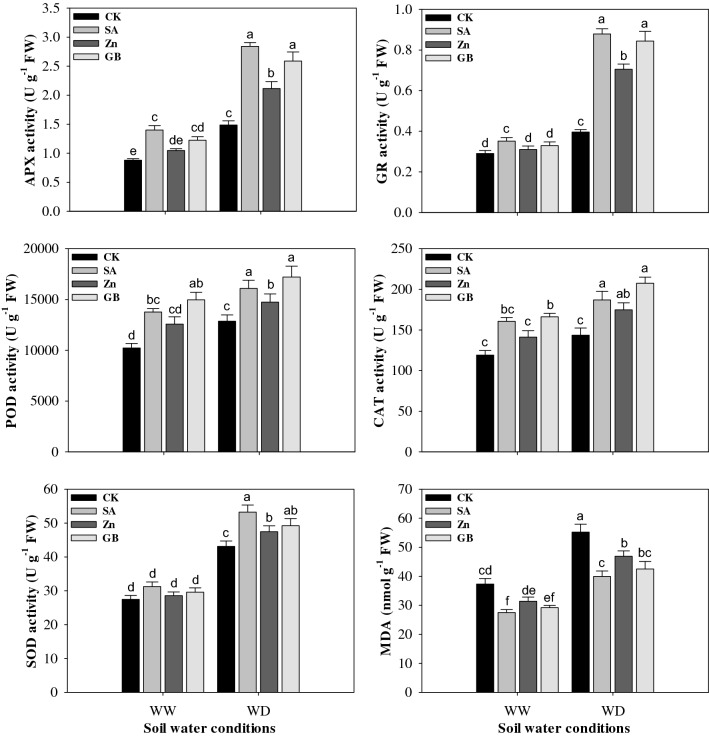
Antioxidant enzymes activity and MDA content
Drought stress significantly (p < 0.05) increased the MDA content and triggered the activities of APX, GR, POD, CAT, and SOD. However, application of SA, Zn, and GB increased the activities of APX, GR, POD, CAT, and SOD, but reduced the content of MDA under both soil water conditions (Fig. 3 and Table 3). Compared with control, the activities of APX and POD were statistically increased by spraying treatments under both soil water conditions except for Zn treatment under the well-watered condition, the GR activity was significantly enhanced by spraying treatments under the drought-stressed condition and by SA treatment under the well-watered condition, the CAT activity was statistically improved by spraying treatments under the drought-stressed condition and by GB treatment under the well-watered condition, the SOD activity was statistically increased by spraying treatments under the drought-stressed condition, but the MDA content was significantly decreased by spraying treatments under both soil water conditions except for Zn treatment under the well-watered condition. Under the drought-stressed condition, the concerned spraying treatments improved the activities of APX by 91.89%, 42.56%, and 74.32%, GR by 125.64%, 79.48%, and 115.38%, POD by 25.16%, 14.63%, and 33.91%, CAT by 30.23%, 21.80%, and 44.54%, SOD by 23.54%, 10.06%, and 14.17%, but reduced the MDA content by 27.70%, 15.13%, and 23.01%, respectively, as compared to the values of the control. Overall, the respective spraying treatments enhanced all the antioxidant enzymes activity and reduced the MDA content under the drought-stressed condition more than the well-watered condition. Results noticed that the SA treatment was the most effective and followed by GB and Zn spraying treatments in decreasing the content of MDA and increasing the activities of APX, SOD, and GR enzymes; while the GB treatment had higher values more than other spraying treatments in raising the activity of POD and CAT enzymes as compared with control treatment under both soil water conditions.
Figure 3.
Effect of soil water conditions and foliar treatments on the activities of ascorbate peroxidase (APX), glutathione reductase (GR), peroxidase (POD), catalase (CAT) and superoxide dismutase (SOD), and malonaldehyde (MDA) content. Every column in each graph represents the mean (± SE) of three replicates. Different letters above columns indicate that means are different at 95% confidence level. WW, well-watered; WD, water-deficient; CK, control (double distilled water); SA, salicylic acid; Zn, Zinc; GB, Glycine betaine.
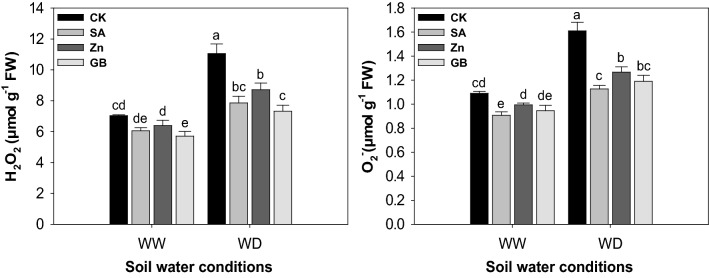
Accumulation of reactive oxygen species
Drought stress significantly (p < 0.05) increased the level of ROS accumulation. However, the spraying treatments of SA, Zn, and GB reduced the contents of hydrogen peroxide (H2O2) and superoxide anion (O2−) in both soil water conditions (Fig. 4 and Table 3). The H2O2 content was significantly decreased by spraying treatments under the drought-stressed condition and by GB treatment under the well-watered condition. The O2− content was statistically declined by spraying treatments under the drought-stressed condition and by SA treatment under the well-watered condition. Under the drought-stressed condition, application of SA, Zn, and GB decreased the contents of H2O2 by 28.92%, 21.18%, and 33.81%, and O2− by 30.43%, 21.73%, and 26.08%, respectively relative to the values of control. Overall, the positive effect of spraying treatments was better under drought-stressed than well-watered conditions.
Figure 4.
Effect of soil water conditions and foliar treatments on hydrogen peroxide (H2O2) and superoxide anion (O2−) contents. Every column in each graph represents the mean (± SE) of three replicates. Different letters above columns indicate that means are different at 95% confidence level. WW, well-watered; WD, water-deficient; CK, control (double distilled water); SA, salicylic acid; Zn, Zinc; GB, Glycine betaine.
Accumulation of osmolytes
Drought stress significantly (p < 0.05) affected the free proline content and total soluble sugar. However, the spraying treatments of SA, Zn, and GB enhanced the proline content and soluble sugar under both soil water conditions (Fig. 5 and Table 3). Compared with control, the free proline content and total soluble sugar were significantly increased by spraying treatments under two soil water conditions except for Zn treatment under the well-watered condition. Under the drought-stressed condition, the concerned spraying treatments enhanced the free proline content by 27.32%, 11.25%, and 57.08%, and total soluble sugar by 45.23%, 27.53%, and 61.23%, respectively, as compared to the values of the control. Overall, the positive effects of spraying treatments were better under the drought-stressed condition than the well-watered condition; the GB treatment was more effective in increasing the proline content and soluble sugar than other spraying treatments as compared with control treatment under both soil water conditions.
Figure 5.
Effect of soil water conditions and foliar treatments on the accumulations of free proline content and total soluble sugar. Every column in each graph represents the mean (± SE) of three replicates. Different letters above columns indicate that means are different at 95% confidence level. WW, well-watered; WD, water-deficient; CK, control (double distilled water); SA, salicylic acid; Zn, Zinc; GB, Glycine betaine.
Discussion
Drought stress is a key agricultural threat that negatively impacts the crop production. It may lead to an imbalance between the ROS accumulation and defense systems of antioxidant, resulting in oxidative damage17,35–38. Drought stress may inhibit the growth and development of numerous crops, yet the reproductive growth phases are highly sensitive by drought stress conditions38–40. In the present study, results (Tables 1, 2, 3) indicated that drought stress significantly (p < 0.05) disrupted the maize growth parameters, yield, and yield components. However, one or more spraying treatments statistically enhanced the growth parameters, yield, and its components except for the plant height and harvest index. Previously, Anjum et al.41 reported that the progressive drought condition significantly diminished the plant height, number of ears, fresh and dry weight of shoot, and grain yield when compared with well-watered plants in two cultivars of maize. Ullaha et al.42 concluded that the application of adequate Zn treatment significantly increased the area of leaves and dry weight of seedlings of chickpea under drought stress. Furthermore, Osman43 reported that the growth parameters, yield, and yield-related components were considerably reduced under drought stress; while the exogenous application of GB statistically stimulated the leaves number plant−1, fresh leaf weight plant−1, pods number plant−1, and green pods yield of pea under drought stress when compared with control. Ghazi44 recorded that the drought stress statistically decreased the growth and yield parameters; while the exogenous of SA treatment increased the plant height, fresh and dry weight of flag leaf, cob length, grains number, 100-grain weight, and grain, straw and cob yields under drought stress as compared with the untreated plants with SA. In our study, the decreases in maize growth parameters, yield, and yield components under drought stress might be attributed to the overproduction of ROS (H2O2 and O2−) which caused oxidative damage to the membranes, lipids and elevated the content of MDA (Figs. 3, 4). Several previous studies have documented that drought stress leads to an increase in the ROS production that destroys the cell membrane, causes damage to lipids, proteins, and chlorophylls, and finally decreases plant biomass accumulation15,45. However, the present study showed that the exogenous applications of SA, Zn, and GB could improve drought-tolerance in maize and the improvement might be attributed to increase in Chl. a, Chl. b, and total Chl., and RWC (Fig. 1), enhanced leaf gas-exchange attributes (Fig. 2), improved antioxidant enzymes activities (Fig. 3), reduced MDA, H2O2, and O2− contents (Figs. 3, 4), and increased accumulation of osmolytes (Fig. 5), particularly under the drought-stressed condition. Growth improvement and yield enhancement by these spraying applications under drought stress condition are considered an external indicator of metabolism modifications in the cells of plants. Previously, several studies have reported the damaging effects of drought on the growth parameters and yields of many crop plants25,39,46–48. Nevertheless, the range of losses under drought-stressed condition varied with the severity of stress and plant growth phases. Moreover, it has also been reported that SA, Zn and GB applications may play important role in improving the growth characters, yield, and yield-related components under diverse stresses in various plant species 17,23,27,35,49.
In the present study, the chlorophyll contents and RWC were significantly affected in maize plants under drought stress. Nevertheless, the foliar application of SA, Zn, and GB treatments improved these parameters under both soil water condition (Fig. 1). Several previous researches have demonstrated that the reduction in chlorophyll contents is one of the important essential factors which could restrict the photosynthesis process under drought stress38,50–52. Rahmani et al.53 mentioned that the RWC and chlorophyll content were decreased under drought, but the foliar application of Zn statistically improved the drought-tolerance by promotion in the RWC and chlorophyll content under water deficit condition during the seed-filling stage in safflower plants. The decreases in chlorophyll contents under the drought-stressed condition in the current investigation are consistent with Yavas and Unay54 who indicated that the drought stress at the grain-filling stage considerably decreased chlorophyll contents and RWC, while the foliar applications of Zn and SA had a positive impact on RWC and chlorophyll content and mitigated the damaging effects of drought stress on plants. The RWC is considered a useful variable to appraise the physiological water status in the leaves of plants55. Moharramnejad et al.52 indicated that drought stress remarkably decreased the contents of Chl. a and total Chl., and RWC when compared to the normal condition. Previously, many studies reported that the exogenous applications of SA, Zn, and GB treatments enhanced the Chl. a, Chl. b and total Chl. contents, and leaf RWC in different crops35,42,46,56.
In the current study, the leaf gas-exchange attributes were substantially affected under the drought-stressed condition. All photosynthetic gas-exchange parameters were increased by SA, Zn, and GB foliar applications except for the intercellular CO2 concentration (Fig. 2). The net photosynthesis rate, transpiration rate, and stomatal conductance were declined under drought stress, while the spraying applications of GB, Zn, and SA increased CO2 assimilation, enhanced physiological water status, and improved the synthesis of metabolites in many crop plants51,57,58. Habibi46 demonstrated that the net photosynthetic rate, transpiration rate, and stomatal conductance were significantly reduced under drought stress, but the spraying application of SA treatment statistically increased them as compared to control in drought-stressed barley plants. The application of adequate Zn substantially enhanced the leaf CO2 net assimilation rate and the efficiency of photosystem II under drought stress in chickpea plants42.
In this study, drought stress statistically increased the activity of antioxidant enzymes including APX, GR, POD, CAT, and SOD (Fig. 3). Nevertheless, the increases in the activity of antioxidant enzymes were not sufficient to protect against the accumulation of ROS and were not adequate to reform the injuries of the oxidative stress caused by the drought stress. These antioxidant enzymes were increased by the foliar application of SA, Zn, and GB treatments under the drought-stressed condition in maize plants (Fig. 3). Dianata et al.59 revealed that the exogenous treatments of SA significantly enhanced the activities of antioxidant enzymes (SOD, POD, and CAT) under drought stress as compared with no SA treatment. However, Ma et al.37 found that the relative gene expression levels of APX, GR, CAT, and APX enzymes were significantly increased, but the content of MDA was substantially decreased by the application of Zn under moderate and severe droughts for 10 and 20 days after initiation of stress conditions in wheat flag leaves. The exogenous applications could amend enzymatic antioxidants in maize plants under different soil water conditions and could efficiently scavenge harmful ROS which was showed by a noticeable decrease in the contents of MDA, H2O2, and O2− in these plants (Figs. 3, 4). Previously, many studies also recorded that enzymatic and non-enzymatic antioxidants were elevated, but the H2O2, O2−, and MDA contents were reduced by spraying applications under abiotic stresses in various crops31,52,60–62. The SOD enzyme is regarded as the first line in protecting against ROS accumulation, which converts the superoxide radical to oxygen and hydrogen peroxide63. Asada64 illustrated that the enzyme of CAT induced the conversion of hydrogen peroxide to water and molecular oxygen, where hydrogen peroxide was regarded as a powerful and harmful oxidizing agent. Mittler45 indicated that the MDA content was considered an adequate indicator of lipid peroxidation in membranes of cells. In this study, the content of MDA in maize leaves was statistically elevated by drought stress and it was concomitant with increased contents of H2O2 and O2−, while exogenous applications of SA, Zn or GB reduced the contents of MDA, H2O2, and O2− (Figs. 3, 4). The present results indicated that the foliar applications gave plants a high ability to deal with oxidative stress and could enhance the drought-tolerance in plants. Consistently, several previous studies have documented that the foliar application of different compounds increased the antioxidant enzymes and decreased the content of MDA in various crops 17,42,56,65.
The results indicated that the accumulations of ROS were significantly increased under drought stress but they were reduced by the foliar SA, Zn, and GB treatments (Fig. 4). Our results are consistent with many previous studies. who revealed that the accumulations of ROS were substantially elevated under drought stress, but the foliar applications of SA, Zn, and GB treatments reduced the accumulations of ROS in different crop plants31,41,62,66–68.
In addition to the defense system of antioxidant enzymes, osmoregulation is strongly involved in drought amelioration through adjusting the osmotic stress. The compounds of proline and soluble sugar are very important for the osmoregulation process in plants under drought stress. In the current study, proline and soluble sugar in maize leaves were substantially increased under the drought stress and they were statistically increased by foliar application of SA, Zn, and GB (Fig. 5). This phenomenon could be considered as a mechanism that water loss in plants was inhibited by modification of the osmotic condition. These results are in harmony with previous researchers51,59,68–70, who found that free proline content and total soluble sugar were increased under drought stress in different crop plants. Upadhyaya et al.48 documented that Zn treatment significantly increased the leaf proline content under drought stress when compared with the control plants. Supporting our findings, Osman43 pronounced that the foliar application of GB considerably promoted the accumulations of total soluble sugars and free amino acids under drought stress at different growth phases in pea plants. Also, Aldesuquy et al.71 mentioned that the exogenous treatment of SA statistically increased soluble sugars and proline content in flag leaf during the reproductive stage under drought stress conditions when compared to untreated wheat plants.
Based on all the above results, the beneficial effects of SA, Zn, and GB treatments could be ascribed to decrease the accumulation of H2O2, O2−, and MDA contents, perhaps by increasing the activity of antioxidant enzymes and enhancing the osmolytes accumulation (Fig. 6). Moreover, the exogenous applications of SA, Zn, and GB were effective in improving the leaf gas-exchange attributes and RWC, and stabilizing chlorophyll pigments (Fig. 6). These mechanisms are very important to sustain maize production in water deficit conditions.
Figure 6.
The different mechanisms of exogenous salicylic acid (SA), zinc (Zn), and glycine betaine (GB) applications mediated drought-tolerance in maize crop. ROS, reactive oxygen species; APX, ascorbate peroxidase; GR, glutathione reductase; POD, peroxidase; CAT, catalase; SOD, superoxide dismutase; MDA, malonaldehyde; RWC, relative water content.
Materials and methods
Experimental design and plant growth conditions
The controlled pot trial was carried out during the summer growing season of 2019 at the glasshouse of the College of Agronomy and Biotechnology (CAB), Southwest University (SWU), Chongqing, China. The experimental area lies at longitude 106◦ 26′ 02′' E, latitude 29◦ 49′ 32′' N, and altitude 220 m. During the growing season, the average minimum and maximum temperatures were 24 °C and 35 °C, and the relative humidity was between 76 and 84%. The experiment was performed in a completely randomized design (CRD) with two factors: two soil water conditions and four spraying treatments. The experiment comprised eight treatments, and each treatment contained ten pots and three replications. Summer maize cultivar Xinzhongyu 801, a commonly cultivated hybrid in China, was selected for this study. It has high-yield, stability, and adaptability and is widely grown in south-west China. Each plastic pot (30 cm diameter, 35 cm depth) was filled with 15 kg air-dried and sieved (0.5 mm) soils, which was collected from the experimental station at the CAB, SWU. Experimental soil was clay loam, and had the following physical and chemical properties: pH of 6.25, organic matter of 12.58 g kg−1, electrical conductivity (EC) of 0.45 ds m−1, bulk density of 1.44 g cm−3, soil water content at field capacity (FC) of 24.35%, total N of 0.98 g kg−1, available phosphorous of 15.53 mg kg−1, and available potassium of 86.11 mg kg−1. During the time of soil filling, the fertilizers including 5.4 g controlled-release urea (44.6% N), 10 g calcium superphosphate (12% P2O5), and 2 g potassium chloride (60% K2O) were applied to each pot. Five uniform grains were manually cultivated on the 10th of April in all pots at a depth of 4–5 cm. Thinning processes were performed after one week from germination, and two uniform seedlings pot−1 were selected for the subsequent studies. Thus, each treatment had 20 plants. All pots were irrigated to 85% FC from the tap water till the start of drought stress treatments.
Soil water conditions
The plants were subjected to two soil water conditions for 28 days, from the fourteenth leaf (V14) until blister (R2) growth stages of maize: well-watered condition (85% of field capacity; WW) and drought-stressed condition (50% of field capacity; WD). During the drought period, the pots were weighed every day to keep the required water levels in the soils by adding proper water volumes. Soil water contents for 85% and 50% field capacity were 22.5% and 11.25%, respectively. The weights similar to each of the following soil water contents (22.5% and 11.25%) are 17.45 and 16.30 kg/pot, respectively. These two field capacities are very important and we applied 85% as a normal condition treatment which maintains soil moisture near-maximum water-holding capacity and 50% as a drought condition which is near-minimum water-holding capacity.
Soil water content (SWC) was computed by using the following equation: SWC % = [(FW-DW)/DW] × 100, where FW was the fresh weight of soil sample from the inner area of each pot and DW was the dry weight of soil sample after oven drying at 85 °C for 3 days72.
Spraying treatments
After 7 and 14 days of drought imposition, the maize plants under each soil water condition were sprayed with double distilled water (CK), 140 mg l−1 SA (2-hydroxybenzoic acid, C7H6O3, MW = 138.12 g mol−1, pH: 5.8), 4 g l−1 Zn (zinc sulfate heptahydrate, ZnSO4 ‧7H2O, MW = 287.54 g mol−1), and 11.5 g l−1 GB (betaine, C5H11NO2, MW = 117.14 g mol−1). SA was added in ethanol to increase the solubility in water (1 g 10 ml−1)73. Treatments were applied on all plants at the sixteenth leaf (V16) and eighteenth leaf (V18) growth stages. We finished the spraying of treatments before tasseling stage. Tween-20 (0.05%) was added with spraying treatments as a surfactant at the time of applications. we sprayed two times to ensure absorption spraying treatments into plants leaves after imposition of drought. The effective concentrations of these spraying treatments were selected based on results of previous studies carried out under drought stress17,33,54,56,74–76.
Plant sampling and analyses
Maize plants were sampled after 14 days of spraying applications (28 days of drought imposition) to measure growth parameters, chlorophyll contents, RWC, leaf gas-exchange attributes, and biochemical features. Completely expanded, undamaged, and healthy maize plant leaves (3rd leaf from the top) were sampled from all repetitions. After cleaning, the leaves of maize plants were frozen with liquid N2 immediately and stored at -80 °C for biochemical analyses, and the kits were purchased from Sino Best Biological Co., Ltd., China. The yield and its components were recorded at harvest.
Growth parameters, yield, and its components
Six maize plants were randomly selected to measure plant height, fresh weight plant−1, dry weight plant−1, number of leaves plant−1, and leaf area plant−1. Plant height was measured by a meter scale, biomass accumulation was determined by an electronic weighing balance, and total leaf area plant−1 was measured by using a LI-3100 laser area meter (Li-COR, CID, Inc., USA). The dry weight plant−1 was estimated following oven drying at 85 °C for 48 h. At full maturity (plants at 120-days old), six maize plants were randomly harvested to measure 100-grain weight (g), the number of grains plant−1, biological yield (g plant−1), grain yield (g plant−1), and harvest index (HI). The HI was computed as the percent ratio of grain yield and biological yield according to Donald77.
Chlorophyll contents and RWC
Contents of chlorophyll (Chl. a, Chl. b and total Chl.) in the 3rd leaf from the top were determined according to Peng and Liu78. Extraction of a 250 mg leaf blade sample was done with 10 ml ethanol-acetone (vol. 1:2 ratio), and the extract was moved to a 15 ml centrifuge tube. The tubes were put in the dark to avoid from light for 24 h until the sample changed into a white color. The chlorophyll contents were calculated by the following equations: Chlorophyll a content (mg/g tissue) = (12.7D663—2.69D645) × V/ (1000 × W), Chlorophyll b content (mg/g tissue) = (22.7 D645—4.68D663) × V/ (1000 × W), and total chlorophyll content (mg/g tissue) = (20.2 D645 + 8.02 D663) × V/ (1000 × W), where, D663 and D645 are the corresponding wavelengths of the optical density value (nm), V is the volume of extracting liquid (cm3) and W is the weight of fresh leaf (mg). The relative water content (RWC) of maize leaves was measured according to Barrs and Weatherley79. Fresh maize leaves were cut into small segments (1.5 cm length), and weighed fresh weight (FW, mg); Later these leaves were floated in distilled water for 4 h under low light to register saturated weight (SW, mg), and then dried in an oven until constant weight at 80 °C for 24 h to record dry weight (DW, mg). RWC was computed as: RWC (%) = (FW—DW)/ (SW—DW) × 100%.
Leaf gas-exchange attributes
Photosynthesis characteristics including the net photosynthesis rate (Pn), stomatal conductance (Gs), transpiration rate (Tr), and intercellular CO2 concentration (Ci) were recorded using a portable infrared gas analyser based photosynthesis system (LI-6400; LiCor, Inc., Lincoln, NE, USA) at 09:30–11:30 am from the fully expanded leaf (3rd leaf from top). Air relative humidity and ambient CO2 concentration were about 78% and 370 µmol CO2 mol−1, respectively during collecting the data.
Assay of antioxidant enzymes activity and lipid peroxidation
The activities of different enzymatic antioxidants in maize leaves were recorded using commercial kits as per the manufacturer’s instructions. The kits for superoxide dismutase (SOD-A500), catalase (CAT-A501), ascorbate peroxidase (APX-A304), glutathione reductase (GR-A111), and peroxidase (POD-A502) were purchased from the same company as indicated above. The absorbance readings of SOD, CAT, APX, GR, and POD were detected at 560 nm, 240 nm, 290 nm, 340 nm, and 470 nm, respectively using an ultraviolet (UV)-visible spectrophotometer, and the activities of these enzymes were expressed as units per fresh weight (U g−1 FW). The units of the antioxidant enzymes activity were defined as follows: “one unit of GR activity was expressed as the amount of enzyme depleting 1 µmol NADPH in 1 min, one unit of SOD activity was defined as the amount of enzyme needed to reduce the reference rate to 50% of maximum inhibition, one unit of CAT activity was measured as the amount of enzyme that decomposes 1 nmol H2O2 at 240 nm min−1 in 1 g fresh weight, one unit of APX was estimated as the amount of enzyme required for catalyzing 1 μmol ASA at 290 nm 2 min−1 of 1 g fresh weight in 1 ml of a reaction mixture, and one unit of POD activity was demonstrated as the absorbance change of 0.01 at 470 nm min−1 for 1 g fresh weight in 1 ml of a reaction mixture”80–82. Lipid peroxidation was assayed as MDA content in maize leaves, through thiobarbituric (TBA) method using MDA Detection Kit (A401), obtained from the same company as indicated above. The absorbance for MDA was recorded at 532 and 600 nm and expressed as nmol g−1 fresh weight.
Estimation of reactive oxygen species accumulation
The contents of hydrogen peroxide (H2O2) and superoxide anion radical (O2−) in the leaves of maize were recorded using the commercial ‘H2O2 Detection Kit (A400)’, and ‘O2− Detection kit (A407)’, respectively, being purchased from the same company as indicated above. H2O2 content was estimated at 415 nm and represented as μmol g−1 fresh weight. Super oxygen anion serotonin reacted with hydrochloride to produce NO2−. The NO2− entered with the interaction of amino benzene and alpha-pyridoxine to the production of red compounds at 530 nm which had a characteristic absorption peak. The content of O2− was measured at 530 nm, and expressed as μmol g−1 fresh weight.
Determination of osmolytes accumulation
The contents of proline and soluble sugar in maize leaves were determined using commercial kits according to the manufacturer’s instructions. The kits for proline (PRO, A605), and soluble sugar contents (SSC, B602) were purchased from the same company as indicated above. The absorbance readings of the toluene layer were read on a spectrophotometer at 520 nm. Proline (Sigma, St Louis, MO, USA) was used as a standard curve. Proline content was expressed as µg g−1 fresh weight. The absorbance readings of SSC was detected at 620 nm using an ultraviolet (UV) visible spectrophotometer. Soluble sugar content was articulated as mg g−1 fresh weight.
Statistical analysis
Data were statistically analyzed following the analysis of variance (ANOVA) technique according to the Two-way factorial Design using Statistical Software Package MSTAT-C83. Least significant differences test (L.S.D) at 5% probability was used to test the significance among mean values of each treatment84. Ten pots and three replications were used for each treatment and 20 plants were grown for each treatment. Sigma Plot 10.0 (Systat Software Inc., San Jose, CA, USA) was used for graphical presentation of the data.
Conclusion
This experiment revealed that the spraying application of 140 mg l−1 SA, 4 g l−1 Zn, and 11.5 g l−1 GB improved drought-tolerance in maize crop through increased photosynthesis pigments and RWC, improved leaf gas-exchange attributes, enhanced activities of antioxidant enzymes, reduced of MDA, H2O2, and O2− contents, and higher accumulation of osmolytes in drought-stressed plants. Thus, it might be considered as an important strategy to improve the plant growth parameters, yield, and its components under drought-stressed condition. Overall, GB application was the most effective followed by SA and Zn applications to alleviate the injurious effects in maize triggered by drought.
Acknowledgements
The present work is part of Ph.D. dissertation of first author. The first author gives his gratitude to the teams of Key Laboratory of Eco-environments in Three Gorges Reservoir Region, and Engineering Research Center of South Upland Agriculture, Ministry of Education, China for their help and support in the experiment.
Author contributions
R.S, L.W, and E.M.S.G conceived and designed the research. R.S, R.W, H.A.H, and L.C performed the experiment. R.S wrote the initial draft of the manuscript. R.S, R.W, K.Z, and S.Z. contributed to reagents, materials, and analysis tools. H.A.H, S.H, M.I and L.W revised subsequent versions of the manuscript. All the authors have read and approved the manuscript.
Funding
Authors give their gratitude to the financial support of the China Scholarship Council (CSC No. 2017GBJ001939), Special Fund for Agro-Scientific Research in the Public Interest (No. 201503127) and the Natural Science Foundation Project of China (No. 31871583).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hafiz Athar Hussain, Email: atharhussainswu@yahoo.comf.
Longchang Wang, Email: wanglc@swu.edu.cn.
References
- 1.Cassman KG, Dobermann A, Walters DT, Yang H. Meeting cereal demand while protecting natural resources and improving environmental quality. Annu. Rev. Environ. Resour. 2003;28:315–358. doi: 10.1146/annurev.energy.28.040202.122858. [DOI] [Google Scholar]
- 2.FAOSTAT. Population data. Food and Agricultural Organization of United Nation, Roma.http://Faostat.fao.org/download/O/OA/E. (2017).
- 3.Daryanto S, Wang L, Jacinthe PA. Global synthesis of drought effects on maize and wheat production. PLoS ONE. 2016;11:e0156362. doi: 10.1371/journal.pone.0156362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tilman D, Balzer C, Hill J, Befort BL. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA. 2011;108:20260–20264. doi: 10.1073/pnas.1116437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen YJ, Ok T, Utsumi N, Kanae S, Hanasaki N. Projection of future world water resources under SRES scenarios: water withdrawal. Hydrol. Sci. J. 2008;53:11–33. doi: 10.1623/hysj.53.1.11. [DOI] [Google Scholar]
- 6.Fereres, E. & Connor, D. J. Sustainable water management in agriculture (2004).
- 7.Godfray HCJ, et al. Food security: the challenge of feeding 9 billion people. Science. 2010;327:812–818. doi: 10.1126/science.1185383. [DOI] [PubMed] [Google Scholar]
- 8.Liu, Y., Li, S., Chen, F., Yang, S. & Chen, X. Soil water dynamics and water use efficiency in spring maize (Zea mays L.) fields subjected to different water management practices on the Loess Plateau. China Agric. Water. Manag.97, 769–775 (2010).
- 9.Ge, T. D., Sui, F. G., Bai, L. P., Tong, C. L. & Sun, N. B. Effects of water stress on growth, biomass partitioning, and water-use efficiency in summer maize (Zea mays L.) throughout the growth cycle. Acta Physiol. Plant.34, 1043–1053 (2012).
- 10.Talaat, N. B., Shawky, B. T. & Ibrahim, A. S. Alleviation of drought-induced oxidative stress in maize (Zea mays L.) plants by dual application of 24-epibrassinolide and spermine. Environ. Exp. Bot.113, 47–58 (2015).
- 11.Ahammed GJ, et al. The SlWRKY81 transcription factor inhibits stomatal closure by attenuating nitric oxide accumulation in the guard cells of tomato under drought. Plantarum: Physiol; 2020. [DOI] [PubMed] [Google Scholar]
- 12.Jan S, Abbas N, Ashraf M, Ahmad P. Roles of potential plant hormones and transcription factors in controlling leaf senescence and drought tolerance. Protoplasma. 2019;256:313–329. doi: 10.1007/s00709-018-1310-5. [DOI] [PubMed] [Google Scholar]
- 13.Hussain HA, et al. Maize tolerance against drought and chilling stresses varied with root morphology and antioxidative defense system. Plants. 2020;9:720. doi: 10.3390/plants9060720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hussain HA, et al. Chilling and drought stresses in crop plants: implications, cross talk, and potential management opportunities. Front. Plant Sci. 2018;9:393. doi: 10.3389/fpls.2018.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010;33:453–467. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- 16.Scheibe, R. & Beck, E. Drought, Desiccation, and Oxidative Stress (Ecological Studies, 2011).
- 17.Sohag AAM, et al. Exogenous salicylic acid and hydrogen peroxide attenuates drought stress in rice. Plant Soil Environ. 2020;66:7–13. doi: 10.17221/472/2019-PSE. [DOI] [Google Scholar]
- 18.Li X, et al. RBOH1-dependent apoplastic H2O2 mediates epigallocatechin-3-gallate-induced abiotic stress tolerance in Solanum lycopersicum L. Environ. Exp. Bot. 2019;161:357–366. doi: 10.1016/j.envexpbot.2018.11.013. [DOI] [Google Scholar]
- 19.Ahammed GJ, et al. Tomato WRKY81 acts as a negative regulator for drought tolerance by modulating guard cell H2O2–mediated stomatal closure. Environ. Exp. Bot. 2020;171:103960. doi: 10.1016/j.envexpbot.2019.103960. [DOI] [Google Scholar]
- 20.Li, X. et al. Salicylic acid acts upstream of nitric oxide in elevated carbon dioxide-induced flavonoid biosynthesis in tea plant (Camellia sinensis L.). Environ. Exp. Bot.161, 367–374 (2019).
- 21.Kaya C, Ashraf M, Alyemeni MN, Corpas FJ, Ahmad P. Salicylic acid-induced nitric oxide enhances arsenic toxicity tolerance in maize plants by upregulating the ascorbate-glutathione cycle and glyoxalase system. J. Hazard. Mater. 2020;399:123020. doi: 10.1016/j.jhazmat.2020.123020. [DOI] [PubMed] [Google Scholar]
- 22.Hayat Q, Hayat S, Irfan M, Ahmad A. Effect of exogenous salicylic acid under changing environment: a review. Environ. Exp. Bot. 2010;68:14–25. doi: 10.1016/j.envexpbot.2009.08.005. [DOI] [Google Scholar]
- 23.Noreen S, Fatima K, Athar HUR, Ahmad S, Hussain K. Enhancement of physio-biochemical parameters of wheat through exogenous application of salicylic acid under drought stress. J. Anim. Plant Sci. 2017;27:153–163. [Google Scholar]
- 24.Fardus J, Matin MA, Hasanuzzaman M, Hossain MA, Hasanuzzaman M. Salicylic acid-induced improvement in germination and growth parameters of wheat under salinity stress. J. Anim. Plant Sci. 2018;28:197–207. [Google Scholar]
- 25.Farooq M, Wahid A, Lee DJ, Cheema SA, Aziz T. Comparative time course action of the foliar applied glycinebetaine, salicylic acid, nitrous oxide, brassinosteroids and spermine in improving drought resistance of rice. J. Agron. Crop Sci. 2010;196:336–345. doi: 10.1111/j.1439-037X.2010.00422.x. [DOI] [Google Scholar]
- 26.Maruri-López I, Aviles-Baltazar NY, Buchala A, Serrano M. Intra and extracellular journey of the phytohormone salicylic acid. Front. Plant Sci. 2019 doi: 10.3389/fpls.2019.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma M, et al. Salicylic acid mediated growth, physiological and proteomic responses in two wheat varieties under drought stress. J. Proteom. 2017;163:28–51. doi: 10.1016/j.jprot.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Khan, H. R., McDonald, G. K. & Rengel, Z. Zinc fertilization and water stress affects plant water relations, stomatal conductance and osmotic adjustment in chickpea (Cicer arietinum L.). Plant and Soil.267, 271–284 (2004).
- 29.Nasiri Y, Najafi N. Effect of soil and foliar application of zinc and iron on flowering and essential oil of chamomile at green house conditions. Acta Agric. Slov. 2015;105:1–4. doi: 10.14720/aas.2015.105.1.04. [DOI] [Google Scholar]
- 30.Wang, H. & Jin, J. Effects of zinc deficiency and drought on plant growth and metabolism of reactive oxygen species in maize (Zea mays L.). Agric. Sci. China.6, 988–995 (2007).
- 31.Sofy MR. Application of salicylic acid and zinc improves wheat yield through physiological processes under different levels of irrigation intervals. Int. J. Plant Res. 2015;5:136–156. [Google Scholar]
- 32.water relations and yield Iqbal, N., Ashraf, M. & Ashraf, M. Y. Glycinebetaine, an osmolyte of interest to improve water stress tolerance in sunflower (Helianthus annuus L.) S. Afr. J. Bot. 2008;74:274–281. doi: 10.1016/j.sajb.2007.11.016. [DOI] [Google Scholar]
- 33.Raza MAS, Saleem MF, Shah GM, Khan IH, Raza A. Exogenous application of glycinebetaine and potassium for improving water relations and grain yield of wheat under drought. J. Soil Sci. Plant Nut. 2014;14:348–364. [Google Scholar]
- 34.Hasanuzzaman M, et al. Targeting glycinebetaine for abiotic stress tolerance in crop plants: physiological mechanism, molecular interaction and signaling. Phyton Int. J. Exp. Bot. 2019;88:185–221. [Google Scholar]
- 35.Raza, M. A. S., Saleem, M. F., Ashraf, M. Y., Ali, A. & Asghar, H. N. Glycinebetaine applied under drought improved the physiological efficiency of wheat (Triticum aestivum L.) plant. Soil Environ.31, 67–71 (2012).
- 36.Anjum SA, et al. Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front. Plant Sci. 2017;8:69. doi: 10.3389/fpls.2017.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma D, et al. Physiological responses and yield of wheat plants in zinc-mediated alleviation of drought stress. Front. Plant Sci. 2017;8:860. doi: 10.3389/fpls.2017.00860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdel-Motagally FMF, El-Zohri M. Improvement of wheat yield grown under drought stress by boron foliar application at different growth stages. J. Saudi Soc. Agric. Sci. 2018;17:178–185. [Google Scholar]
- 39.Zhang B, Li W, Chang X, Li R, Jing R. Effects of favorable alleles for water-soluble carbohydrates at grain filling on grain weight under drought and heat stresses in wheat. PLoS ONE. 2014;9:e102917. doi: 10.1371/journal.pone.0102917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hussain HA, et al. Interactive effects of drought and heat stresses on morpho-physiological attributes, yield, nutrient uptake and oxidative status in maize hybrids. Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-018-37186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anjum SA, et al. Effect of progressive drought stress on growth, leaf gas exchange, and antioxidant production in two maize cultivars. Environ. Sci. Pollut. Res. 2016;23:17132–17141. doi: 10.1007/s11356-016-6894-8. [DOI] [PubMed] [Google Scholar]
- 42.Ullaha A, Romdhaneb L, Rehmanc A, Farooq M. Adequate zinc nutrition improves the tolerance against drought and heat stresses in chickpea. Plant Physiol. Biochem. 2019;143:11–18. doi: 10.1016/j.plaphy.2019.08.020. [DOI] [PubMed] [Google Scholar]
- 43.Osman HS. Enhancing antioxidant-yield relationship of pea plant under drought at different growth stages by exogenously applied glycine betaine and proline. Ann. Agric. Sci. 2015;60:389–402. doi: 10.1016/j.aoas.2015.10.004. [DOI] [Google Scholar]
- 44.Ghazi, D. A. Impact of drought stress on maize (Zea mays) plant in presence or absence of salicylic acid spraying. J. Soil Sci. Agric. Eng. Mansoura Univ.8, 223–229 (2017).
- 45.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 46.Habibi G. Exogenous salicylic acid alleviates oxidative damage of barley plants under drought stress. Acta Biol. Szeged. 2012;56:57–63. [Google Scholar]
- 47.Rollins, J. A. et al. Leaf proteome alterations in the context of physiological and morphological responses to drought and heat stress in barley (Hordeum vulgare L.). J. Exp. Bot.64, 3201–3212 (2013). [DOI] [PMC free article] [PubMed]
- 48.Upadhyaya H, Dutta BK, Panda SK. Zinc modulates drought-induced biochemical damages in tea [Camellia sinensis (L) O Kuntze] J. Agric. Food Chem. 2013;61:6660–6670. doi: 10.1021/jf304254z. [DOI] [PubMed] [Google Scholar]
- 49.Shahbaz, M., Masood, Y., Perveen, S. & Ashraf, M. Is foliar-applied glycinebetaine effective in mitigating the adverse effects of drought stress on wheat (Triticum aestivum L.). J. Appl. Bot. Food Qual.84, 192–199 (2011).
- 50.Miri, H. R. & Armin, M. The interaction effect of drought and exogenous application of glycine betaine on corn (Zea mays L.). Euro. J. Exp. Biol.3, 197–206 (2013).
- 51.Naeem M, et al. Foliar calcium spray confers drought stress tolerance in maize via modulation of plant growth, water relations, proline content and hydrogen peroxide activity. Arch. Agron. Soil Sci. 2018;64:116–131. doi: 10.1080/03650340.2017.1327713. [DOI] [Google Scholar]
- 52.Moharramnejad S, et al. Response of maize to field drought stress: oxidative defense system, osmolytes’ accumulation and photosynthetic pigments. Pak. J. Bot. 2019;51:799–807. doi: 10.30848/PJB2019-3(1). [DOI] [Google Scholar]
- 53.Rahmani F, Sayfzadeh S, Jabbari H, Valadabadi SA, Masouleh EH. Alleviation of drought stress effects on safflower yield by foliar application of zinc. Int. J. Plant Prod. 2019;13:297–308. doi: 10.1007/s42106-019-00055-7. [DOI] [Google Scholar]
- 54.Yavas I, Unay A. Effects of zinc and salicylic acid on wheat under drought stress. J. Anim. Plant Sci. 2016;26:1012–1018. [Google Scholar]
- 55.Kadioglu A, Saruhan N, Saglam A, Terzi R, Acet T. Exogenous salicylic acid alleviates effects of long term drought stress and delays leaf rolling by inducing antioxidant system. Plant Growth Regul. 2011;64:27–37. doi: 10.1007/s10725-010-9532-3. [DOI] [Google Scholar]
- 56.Sedaghat M, Sarvestani ZT, Emam Y, Bidgoli AM. Physiological and antioxidant responses of winter wheat cultivars to strigolactone and salicylic acid in drought. Plant Physiol. Biochem. 2017;119:59–69. doi: 10.1016/j.plaphy.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 57.Muhammad, A. et al. Nitrogen nutrition improves the potential of wheat (Triticum aestivum L.) to alleviate the effects of drought stress during vegetative growth periods. Front. Plant Sci.7, 981 (2016). [DOI] [PMC free article] [PubMed]
- 58.Cui G, et al. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 2017;118:138–149. doi: 10.1016/j.plaphy.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 59.effects on biochemical traits and essential oil yield Dianata, M., Saharkhiza, M. J. & Tavassolian, I. Salicylic acid mitigates drought stress in Lippia citriodora L. Biocatal. Agric. Biotechnol. 2016;8:286–293. doi: 10.1016/j.bcab.2016.10.010. [DOI] [Google Scholar]
- 60.Chen YE, et al. Effect of salicylic acid on the antioxidant system and photosystem II in wheat seedlings. Biol. Plant. 2016;60:139–147. doi: 10.1007/s10535-015-0564-4. [DOI] [Google Scholar]
- 61.Nasrin R, Ali E, Jahanfar D, Soodabeh J. Salicylic acid induced changes on antioxidant capacity, pigments and grain yield of soybean genotypes in water deficit condition. J. Plant Interact. 2017;12:457–464. doi: 10.1080/17429145.2017.1392623. [DOI] [Google Scholar]
- 62.Akter, S. et al. Effect of polyamine on pigmentation, reactive oxidative species and antioxidant under drought in maize (Zea mays L.). Turk. J. Agric. Food Sci. Technol.6, 799–811 (2018).
- 63.Alscher PG, Erturk N, Heath LS. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002;53:1331–1341. doi: 10.1093/jexbot/53.372.1331. [DOI] [PubMed] [Google Scholar]
- 64.Asada K. Ascorbate peroxidase-a hydrogen peroxide-scavenging enzyme in plants. Physiol. Plant. 1992;85:235–241. doi: 10.1111/j.1399-3054.1992.tb04728.x. [DOI] [Google Scholar]
- 65.Bathula, S., Jayalalitha, K., Ashoka, R. Y., Sree, R. M. & Ali, M. A. Zinc induced osmoregulation and antioxidant defence system in mungbean (Vigna radiata L.) under drought stress. J. Pharmacogn. Phytochem.8, 1511–1514 (2019).
- 66.Tartoura, K. A. H. Alleviation of oxidative-stress induced by drought through application of compost in wheat (Triticum aestivum L.) plants. Am. Euras. J. Agric. Environ. Sci.9, 208–216 (2010).
- 67.Datir SS, Inamdar A. Biochemical responses of wheat cultivars to peg-induced drought stress. Russ. Agric. Sci. 2019;45:5–12. doi: 10.3103/S1068367419010038. [DOI] [Google Scholar]
- 68.Parveen A, et al. Silicon priming regulates morpho-physiological growth and oxidative metabolism in maize under drought stress. Plants. 2019;8:431. doi: 10.3390/plants8100431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahmad ST, Haddad R. Study of silicon effects on antioxidant enzyme activities and osmotic adjustment of wheat under drought stress. Czech J. Genet. Plant Breed. 2011;54:17–27. doi: 10.17221/92/2010-CJGPB. [DOI] [Google Scholar]
- 70.Marček T, Hamow KÁ, Végh B, Janda T, Darko E. Metabolic response to drought in six winter wheat genotypes. PLoS ONE. 2019;14:e0212411. doi: 10.1371/journal.pone.0212411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aldesuquy, H. S., Ibraheem, F. L. & Ghanem, H. E. Comparative effects of salicylic acid and/or trehalose on osmotic adjustment and solutes allocation of two droughted wheat (Triticum aestivum L.) cultivars. Adv. Agric. Tech. Plant Sci.1, 180001 (2018).
- 72.Coombs J, Hall DO, Long SP, Scurlock JMO. Techniques in Bioproductivity and Photosynthesis. Oxford.: Pergamon; 1987. [Google Scholar]
- 73.Williams M, Senaratna T, Dixon K, Sivasithamparam K. Benzoic acid induces tolerance to biotic stress caused by Phytophthora cinnamomi in banksai attenuate. Plant Growth Regul. 2003;41:89–91. doi: 10.1023/A:1027355604096. [DOI] [Google Scholar]
- 74.Hussain M, Malik MA, Farooq M, Ashraf MY, Cheema MA. Improving drought tolerance by exogenous application of glycinebetaine and salicylic acid in sunflower. J. Agron. Crop Sci. 2008;194:193–199. doi: 10.1111/j.1439-037X.2008.00305.x. [DOI] [Google Scholar]
- 75.Yang Z, Yu J, Merewitz E, Huang B. Differential effects of abscisic acid and glycine betaine on physiological responses to drought and salinity stress for two perennial grass species. J. Am. Soc. Hort. Sci. 2012;137:96–106. doi: 10.21273/JASHS.137.2.96. [DOI] [Google Scholar]
- 76.Hafez EM. Influence of salicylic acid on ion distribution, enzymatic activity and some agromorphological characteristics of wheat under salt-affected soil. Egypt. J. Agron. 2016;38:455–469. doi: 10.21608/agro.2016.1284. [DOI] [Google Scholar]
- 77.Donald CM. In search of yield. J. Aust. Inst. Agric. Sci. 1962;28:171–178. [Google Scholar]
- 78.Peng YS, Liu E. A comparative study of methods of extracting chlorophyll. Acta Agric. Univ. Pekinensis. 1992;18:247–250. [Google Scholar]
- 79.Barrs HD, Weatherley PE. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962;15:413–428. doi: 10.1071/BI9620413. [DOI] [Google Scholar]
- 80.Khan F, et al. Coordinated effects of lead toxicity and nutrient deprivation on growth, oxidative status, and elemental composition of primed and non-primed rice seedlings. Environ. Sci. Pollut. Res. 2018;25:21185–21194. doi: 10.1007/s11356-018-2262-1. [DOI] [PubMed] [Google Scholar]
- 81.Hussain S, Khan F, Cao W, Wu L, Geng M. Seed priming alters the production and detoxification of reactive oxygen intermediates in rice seedlings grown under sub-optimal temperature and nutrient supply. Front. Plant Sci. 2016;7:439. doi: 10.3389/fpls.2016.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khan, F., Hussain, S., Khan, S. & Geng, M. Seed priming improved antioxidant defense system and alleviated Ni-induced adversities in rice seedlings under N, P, or K deprivation. Front. Plant Sci.11 (2020). [DOI] [PMC free article] [PubMed]
- 83.University, M. S . MSTAT-C: Micro-computer Statistical Program, Version 2. Michigan: Michigan State University; 1990. [Google Scholar]
- 84.Steel RC, Torrie S. H. Mc Grauc Hill Book Company, New York: Principles and Procedures of Statistics; 1997. [Google Scholar]