Abstract
Non-invasive brain stimulation techniques including repetitive transcranial magnetic stimulation (rTMS), continuous theta-burst stimulation (cTBS), paired associative stimulation (PAS), and transcranial direct current stimulation (tDCS) have been applied over the cerebellum to induce plasticity and gain insights into the interaction of the cerebellum with neo-cortical structures including the motor cortex. We compared the effects of 1 Hz rTMS, cTBS, PAS and tDCS given over the cerebellum on motor cortical excitability and interactions between the cerebellum and dorsal premotor cortex / primary motor cortex in two within subject designs in healthy controls. In experiment 1, rTMS, cTBS, PAS, and tDCS were applied over the cerebellum in 20 healthy subjects. In experiment 2, rTMS and PAS were compared to sham conditions in another group of 20 healthy subjects. In experiment 1, PAS reduced cortical excitability determined by motor evoked potentials (MEP) amplitudes, whereas rTMS increased motor thresholds and facilitated dorsal premotor-motor and cerebellum-motor cortex interactions. TDCS and cTBS had no significant effects. In experiment 2, MEP amplitudes increased after rTMS and motor thresholds following PAS. Analysis of all participants who received rTMS and PAS showed that MEP amplitudes were reduced after PAS and increased following rTMS. rTMS also caused facilitation of dorsal premotor-motor cortex and cerebellum-motor cortex interactions. In summary, cerebellar 1 Hz rTMS and PAS can effectively induce plasticity in cerebello-(premotor)-motor pathways provided larger samples are studied.
Subject terms: Neurology, Motor cortex, Cerebellum, Premotor cortex
Introduction
The cerebellum is an important relay in motor networks and has a crucial role in movement execution and control, not only by modulating primary motor cortex (M1) output through cerebello-thalamo-cortical pathways1, but also via basal ganglia2,3 and brainstem connections4. In particular, actions can be modulated by the cerebellum through corrective signals to the brainstem to alter motor execution or via thalamo-cortical projections to modulate motor preparation, because the cerebellum balances motor intention with motor execution4. Thus, studies have shown that error-based adaptation tasks are cerebellar dependent5. Furthermore, a di-synaptic pathway between the cerebellum and the basal ganglia has shown to link cerebellar error-based motor learning to reinforcement motor learning mediated by the basal ganglia6. Another key player in the motor network is the (dorsal) premotor cortex (PMd) due to its major role in movement preparation and shaping, as well as the execution of externally guided movements7. Studies based on retrograde transneuronal virus tracing8, computational modeling9, and TMS10 suggest a connection between the cerebellum and the premotor cortex.
Cerebellar connectivity with cortical motor areas can be measured non-invasively using transcranial magnetic stimulation (TMS), for instance by pairing magnetic pulses applied over the cerebellum and these areas. Activation of the dentato-thalamo-cortical tract increases M1 excitability11. Purkinje cell activation in turn inhibits this pathway11. Therefore, cerebellar TMS, supposedly activating Purkinje cells12,13, is expected to cause a net inhibition of M1. This has indeed been documented. Conditioning TMS pulses applied to the cerebellum 5 to 6 ms prior to M1 stimulation, reduce motor evoked potential (MEP) amplitudes as a measure of cortico-spinal excitability14. This has been referred to as cerebellar brain inhibition (CBI) and has been studied both in healthy subjects5,15–17 and patients with neurological diseases18–23. CBI has also been shown to have an effect on intracortical excitability such as short-interval intracortical inhibition (SICI)21. Furthermore, single-pulse cerebellar TMS has been shown to reduce contralateral silent periods at interstimulus intervals (ISI) between 20 and 40 ms24. Whether activation of premotor cortical areas contributes to CBI has not been investigated as yet.
Since interactions between cerebellum, PMd, and M1 (Cerebello-PMd-M1) may underlie motor network plasticity processes such as motor sequence learning25 and visuomotor adaptation26, triple-pulse TMS seems to be a promising tool to gain further insights into cerebello-PMd-M1 connectivity. PMd-M1 interaction has been shown to depend on the trimming of pulses, the intensity of the PMd conditioning pulse and ISI between PMd and M1 pulses. MEP amplitude reductions were induced by PMd conditioning pulses with an intensity of 90% of active motor threshold (AMT) at ISIs of 4–6 ms27 in younger healthy controls, whereas higher intensities were needed in older subjects28. Whether cerebellar influence on M1 is mediated through potentially time-sensitive connections with PMd is unknown. How cerebellar excitability changes could contribute to PMd-M1 connectivity is also unclear.
Non-invasive brain stimulation (NIBS) such as repetitive TMS (rTMS) or transcranial direct current stimulation (tDCS) can be used to induce such longer-lasting excitability changes, i.e. plasticity, in different areas of the brain, including the cerebellum29. Only few cerebellar NIBS plasticity protocols have been tested and results of these studies were variable30. TDCS applied over the motor cortex is considered to affect cortical excitability through alterations of the resting membrane potential30 as a function of polarity with decreased excitability after cathodal and increased excitability after anodal tDCS31. Administered over the cerebellum, anodal tDCS has been shown to decrease the threshold for inducing CBI, which was interpreted as increased excitability of the cerebellar cortex, since M1 excitability was not affected. Cathodal stimulation resulted in opposite effects32,33. Studies in patients with ataxia showed clinical improvement after anodal tDCS34,35. The influence of cerebellar tDCS on CBI is equivocal with some studies showing decreased15,36 CBI and others, as pointed out above, reduced thresholds to induce CBI33 after anodal tDCS.
Conventional low-frequency rTMS, e.g. 1 Hz, has consistently been shown to reduce M1 excitability when applied to M137–41. Application over the cerebellum however led to MEP facilitation, probably due to a transiently reduced excitability of Purkinje cells and an increased excitability of spinal alpha-motorneurons42,43. A continuous theta-burst stimulation (cTBS) protocol, in which rapid trains of TMS pulses are given at an interpulse interval of 50 Hz, has on the other hand been shown to decrease MEP amplitudes when applied over M144, as well as over the cerebellum16. However, the effect of cTBS is subject to high inter-subject-variability45–47. Another plasticity inducing technique is paired associative stimulation (PAS). Originally, TMS stimulation over M1 was combined with electrical stimulation of the contralateral median nerve. This combination of somatosensory input and activation of M1 resulted in Hebbian plasticity and changes of corticospinal excitability depending on the ISI48. Using a PAS protocol where cerebellar TMS stimulation was coupled with M1 TMS pulses, MEP inhibition occurred when M1 TMS pulses were preceded by cerebellar pulses by 6 ms17.
A large number of studies using cerebellar NIBS with different protocols have shown variable effects on motor cortical excitability and intracortical interactions in healthy controls and patients30,49. Whereas reviews have contrasted different NIBS protocols across studies30,49, such comparisons have the disadvantage that NIBS protocols have been applied in different populations. The main aim of the present study was a direct comparisons of different NIBS techniques in the same group of participants. To the best of our knowledge, this is the first study directly comparing the effects of rTMS, cTBS, tDCS, PAS, and sham stimulation in the same group of probands. Here, we investigated the effect of the aforementioned cerebellar plasticity protocols on M1 excitability, as well as PMd-M1 and cerebello-PMd-M1 connectivity, in healthy controls, using multi-coil, paired-pulse TMS with the aim to determine the effectiveness of these measures and their potential usefulness as treatment tools in patients with neurological disorders.
Methods
Participants and study design
In experiment 1, we investigated 20 healthy righthanded subjects (13 female, mean age 27 ± 2 years standard error of mean), who did not report any neurological disorders or symptoms, by comparing four different cerebellar plasticity induction techniques that have been effective in other studies, i.e. 1 Hz rTMS50, PAS17, cTBS51 and tDCS32. The order of the four plasticity techniques was randomized and they were applied at least one week apart from each other. Pre and post plasticity induction, cortical excitability was probed by single-pulse TMS determining resting motor threshold (RMT), AMT and MEPs, as well as dual-pulse TMS protocols for charting left PMd-M1 and cerebello-M1 excitability. Moreover, a triple-pulse TMS paradigm was used to investigate cerebello-PMd-M1 interactions.
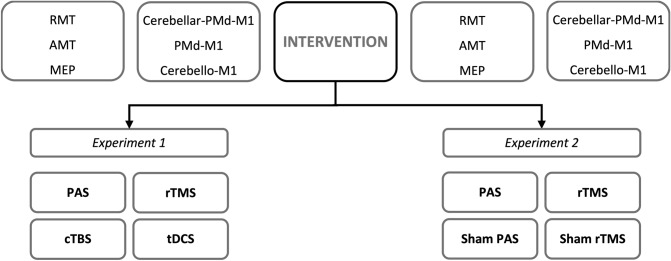
In experiment 2, we re-evaluated the efficacy of 1 Hz rTMS and PAS, which turned out to induce significant effects in experiment 1, complemented by sham stimulation as control conditions, i.e. rTMS sham and PAS sham. In experiment 2, another group of 20 healthy controls (13 female, mean age 27 ± 1.93 years standard error of mean) were investigated of whom two participants already took part in experiment 1. Pre and post plasticity measurements were identical in experiments 1 and 2 (Fig. 1).
Figure 1.
Study design. Probands were investigated in experiments 1 or 2 using four separate sessions with one of the four different plasticity intervention. Before and after the intervention the same single- and multi-pulse TMS paradigms were applied. RMT resting motor threshold, AMT active motor threshold, MEP motor evoked potential, PMd dorsal premotor cortex, M1 primary motor cortex, PAS paired associated stimulation, rTMS repetitive transcranial magnetic stimulation, cTBS continuous theta-burst, tDCS transcranial direct current stimulation.
Experimental setup
The experimental setup was similar to our previous studies52,53. Electromyography was measured over the right first dorsal interosseus muscles (FDI) by Ag/Ag–Cl disc surface electrodes in a belly tendon montage. Electromyography signal was filtered and amplified by a D360 amplifier (Digitimer Limited, Welwyn Garden City, Hertfordshire, UK) and subsequently digitized and recorded by a laboratory interface (Micro 1401; Cambridge Electronics Design (CED), Cambridge, UK) and SIGNAL software (Cambridge Electronic Devices, Cambridge, UK).
Neuronavigation
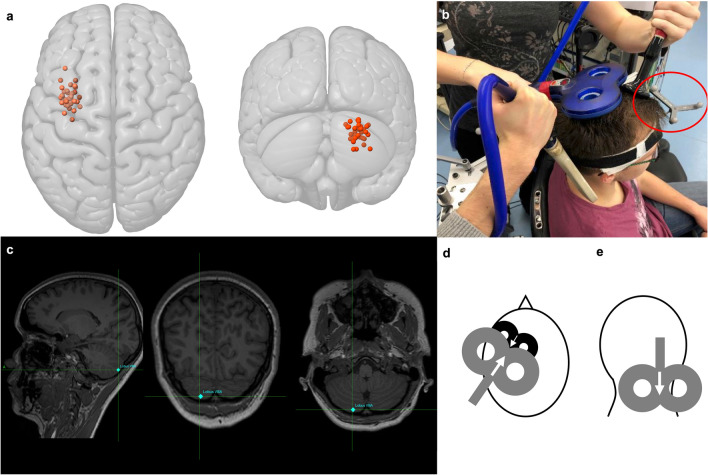
Neuronavigation was used to track the TMS coils and the subjects head to mark targets previously identified in the MRI on the scalp. The Brainsight neuronavigation system (Rogue Research, Montreal, Canada) was used in combination with the Polaris camera (Northern Digital, Ontario, Canada). Target regions for left PMd and M1 as well as for right cerebellum were identified anatomically by using an individual T1-weighted MRI for each subject. PMd was located in the gyrus anterior of the hand knob and lateral of the sulcus frontalis superior, in close proximity to the anatomical M1 (hand knob). The location of M1 was verified by identifying the “motor hot spot”, the location where a TMS pulse administered at a supra-threshold intensity produced continuously the highest MEP. Due to the close proximity of PMd and M1, the stimulation site of PMd had to be adjusted and moved anterior in some individuals, since placement of one coil on the previously identified PMd site and another coil close enough to the “motor hot spot” to generate a sufficient MEP was not feasible. The adjusted PMd location was recorded using the brain sight software.
For cerebellar stimulation, we chose lobus VIIIA, as it was reachable via TMS (Fig. 2c), has been used as a target in other TMS studies54 and is important for the execution of motor tasks and learning processes55. All stimulation sites are shown in Fig. 2a.
Figure 2.
Coil positions and stimulation sites. (a) Individual stimulation sites for right lobule VIIIA (red) and dorsal premotor cortex (PMd) (orange) using the Montreal Neurological Institute and Hospital (MNI) stimulation coordinates in anatomical convention. (b) Neuronavigated triple-coil transcranial magnetic stimulation (TMS) experimental setup. The red circle marks the position of the tracer with three reflecting spheres on the subject’s head (staff member, that gave permission to publish the picture). TMS coils are located over PMd (small 25 mm black coil) and cerebellum stimulation site (grey figure of eight coil). Blue 70 mm coil is located over the primary motor cortex (M1) hot spot. (c) Magnetic resonance T1-weighted image (MRI) of one participant with targeted stimulation spot in the right lobule VIIIA at the cerebellar surface using radiological convention. (d) View from above. The black coil symbolizes the 25 mm branding-iron-coil positioned over the left PMd. White arrow indicates TMS current flow in anterior-to-posterior direction. Grey coil symbolized 70 mm coil positioned over M1 and slightly overlapping with PMd coil. White arrow indicates TMS current flow in posterior-to-anterior direction. (e) View from behind. The grey coil symbolizes the 70 mm coil positioned over the cerebellum (lobus VIIIA) with handle pointing cranially.
Transcranial magnetic stimulation
Single- and multi-pulse TMS
TMS pulses were generated by two Magstim 2002 and one Magstim 200 magnetic stimulator (Magstim Company, Whitland, Dyfed, UK). Left M1 was stimulated by a 70 mm figure-of-eight-shaped coil and left PMd by a 25 mm, branding-iron-style, figure-of-eight-shaped coil (‘‘baby coil”; Magstim Company, Whitland, Dyfed, UK) (Fig. 2b, d)52,53. The right cerebellum was stimulated by a 70 mm figure-of-eight-shaped coil23, positioned tangentially to the scalp with the handle directed upwards (Fig. 2b, e). We did not opt for a double-cone coil used to stimulate the cerebellum in some studies56–59, since five probands in a pilot study did not tolerate the stimulation due to pain and discomfort, and a figure-of-eight-shaped coil was frequently used in previous CBI studies18,20,23,54,60–62.
MEPs were generated by a supra-threshold intensity of 120% RMT and evoked an MEP of about 1 mV. Post-intervention MEPs were registered at the same stimulator output. RMT was identified as the intensity required to produce 5 out of 10 MEPs with an amplitude between 50 and 100 µV at a resting FDI for the 70 mm coil. AMT was identified as the intensity required to produce 5 out of 10 MEPs at > 150 µV at an activated FDI with 10% of maximum voluntary contraction using a Martin-Balloon-Vigorimeter (KLS Martin, Tuttlingen, Germany) for both the 70 mm and 25 mm coils. PMd-M1 interaction was probed with an ISI of 6 ms and an intensity of the conditioning pulse of 90% AMT28,63 and cerebello-M1 interaction at an ISI of 5 ms and an intensity of the conditioning pulse of 90% RMT20,23,60,61. Cerebello-PMd-M1 triple-pulse was administered with a PMd-M1 ISI of 6 ms and cerebello-M1 ISIs of 5, 7–10 and 12 ms using the same intensities for the conditioning pulse as stated above. Regarding the dual- and triple-pulse unconditioned MEPs, the test pulse intensity was adjusted to produce an amplitude of 1 mV pre and post plasticity induction.
Induction of plasticity
To induce plasticity, we used procedures previously described to be effective in inducing plasticity in the cerebellum. In experiment 1, 1 Hz rTMS50, PAS17, cTBS44,51, and anodal tDCS64–66 was used. 1 Hz rTMS was administered with 90% RMT for 20 min using a Magstim Rapid magnetic stimulator (Magstim Company, Whitland, Dyfed, UK)50.
PAS was performed for 30 min with an ISI of 6 ms between cerebellar and M1 TMS using an intensity of 110% RMT over the cerebellum and test pulse intensity over M1 as previously established17. The intertrial interval was 5 s.
CTBS was performed at 50 Hz for 40 s. at 80% AMT using a MagVenture MagOption (MagVenture, Lucernemarken, Denmark)44,51.
Anodal tDCS was administered with the anode placed 3 cm from the inion towards the right mastoid and the cathode on the right mandibula64, a montage frequently used in the past65 and shown to generate an efficient electric-field in simulations67. Stimulation was performed with a DC-Stimulator plus (neuroCare, Munich, Germany) for 20 min with an intensity of 1 mA (current density: 0.11 mA/cm2, total current of 2.2 mA/cm266.
In experiment 2, 1 Hz rTMS and PAS were performed as in experiment 1. Additionally, a rTMS sham condition was performed using a sham coil. Sham PAS was administered for 30 min as established before17 with alternating ISIs of 2 and 10 ms between cerebellar and M1 stimulation using intensities as for real PAS.
Data analysis and statistical analysis
Peak-to-peak amplitudes were measured for each trial. Conditioned MEPs were expressed as a percentage of unconditioned MEPs. For statistical analysis, multifactorial analysis of variance with repeated measures (ANOVA) using the factors INTERVENTION, TIME and ISI were performed. Greenhouse–Geisser correction was used to correct for non-sphericity. Post hoc student t-tests were performed using Bonferroni–Holm-correction if ANOVA resulted in a significant F value with p ≤ 0.05 for a main effect or interaction. In addition to separate analyses of experiments 1 and 2, a combined analysis of the 38 participants (two participants took part in both experiments) who received 1 Hz rTMS and PAS in experiments 1 or 2 was carried out. Factors used for the multifactorial analysis of variance with repeated measures (ANOVA) were INTERVENTION (experiment 1: rTMS, PAS, cTBS, tDCS; experiment 2: rTMS, sham rTMS, PAS, sham PAS), TIME (pre and post intervention) and ISI (for cerebellar-PMd-M1 triple-pulse only with ISIs of 5 ms, 7 ms, 8 ms, 9 ms, 10 ms, and 12 ms). For a combined analysis of rTMS and PAS effect, analysis was carried out as described above with the factors INTERVENTION using only rTMS and PAS.
For both types of stimulation, the mean stimulation effect was used as the basis for a sample size estimation for a potential group comparison study in patients using G*power68,69. We used usual values for the avoidance of a type 1 or type 2 error with an alpha error of 0.05, a power (1-beta) of 0.8 and an allocation ratio of 1:1. For the comparison group, it was postulated that there was no stimulation effect and that the variance of the effects would be equal to the group investigated in this study. Data are given as mean ± standard error of mean.
Ethical statement
The study was approved by the ethic committee of the University of Lübeck and in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed and written consent of all participants was obtained.
Results
Preintervention baseline data did not differ (p > 0.1) between sessions in any of the paradigms in either experiment (unconditioned MEPs, PMd-M1, cerebello-M1, cerebello-PMd-M1).
Experiment 1—Comparison of four cerebellar plasticity protocols
Effects on unconditioned MEPs and motor thresholds
Effects on unconditioned MEPs
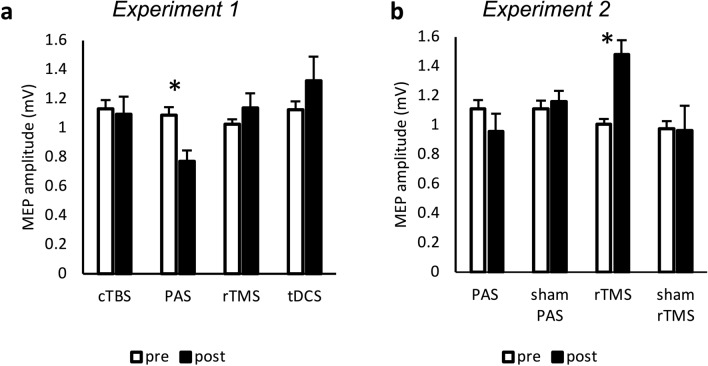
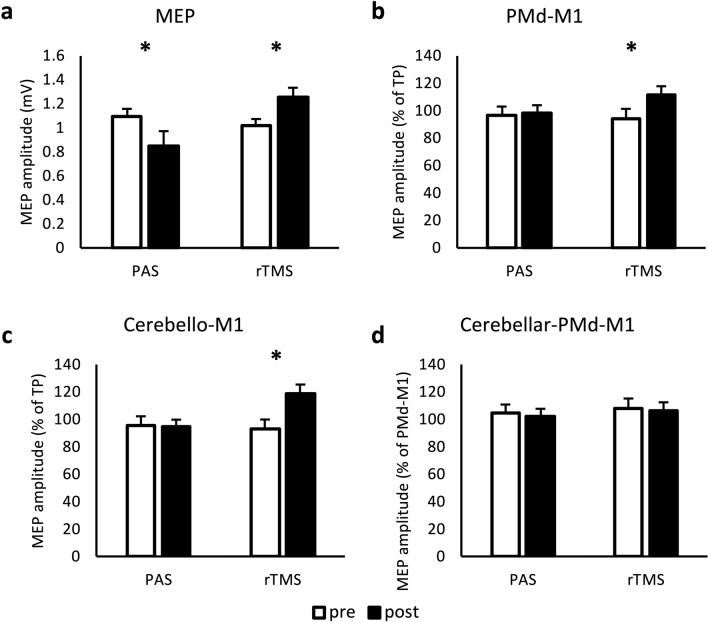
Analysis on unconditioned MEP amplitude using a multifactorial ANOVA showed a main effect for INTERVENTION (F(2.11,40.05) = 3.56, p = 0.036, = 0.158) and an interaction of INTERVENTION and TIME (F(3,57) = 3.23, p = 0.029, = 0.145). There was no main effect for TIME. Post hoc t-tests revealed a decrease of unconditioned MEP amplitudes following the PAS intervention (t = 2.62, p = 0.044) (Fig. 3a). TDCS, cTBS or rTMS had no significant effects on unconditioned MEP amplitudes (p > 0.1).
Figure 3.
Analysis of MEPs (experiments 1 and 2). MEPs were measured peak-to-peak and are indicated in mV. Error bars indicate standard error of mean. Graphs are marked with * when comparison of values with student-t-test resulted in p value < 0.05 (Bonferroni–Holm-corrected). (a) Significant decrease of MEP amplitude after PAS intervention (p = 0.044). (b) Significant increase of MEP amplitude after rTMS intervention (p = 0.004). MEP motor evoked potential, PAS paired associated stimulation, rTMS repetitive transcranial magnetic stimulation, cTBS continuous theta-burst, tDCS transcranial direct current stimulation.
Effects on motor thresholds
Analysis of motor thresholds revealed an interaction between INTERVENTION and TIME for the AMT determined with the 25 mm coil (F(1.85,35.12) = 12.27, p < 0.001, = 0.392) and the 70 mm coil (F(3,57) = 10.99, p < 0.001, = 0.336) without a main effect for either TIME or INTERVENTION. Post hoc testing showed an increase after PAS intervention (25 mm coil: t = − 6.13, p < 0.001, (44% ± 2.5% versus 47% ± 2.2% of maximum stimulator output (MSO)); 70 mm coil: t = − 4.99, p < 0.001, 28% ± 1.4% versus 30% ± 1.3% MSO)) and a slight but significant decrease after rTMS intervention (only 70 mm coil: t = 3.17, p = 0.008, 28% ± 1.4% versus 27% ± 1.5% MSO). Analysis of RMT revealed a main effect of TIME (F(1,19) = 8.08, p = 0.010, = 0.298) without interaction of INTERVENTION and TIME or a main effect of INTERVENTION.
Effects on conditioned MEP amplitudes
Effects on PMd-M1 interaction and on cerebello-M1 interaction
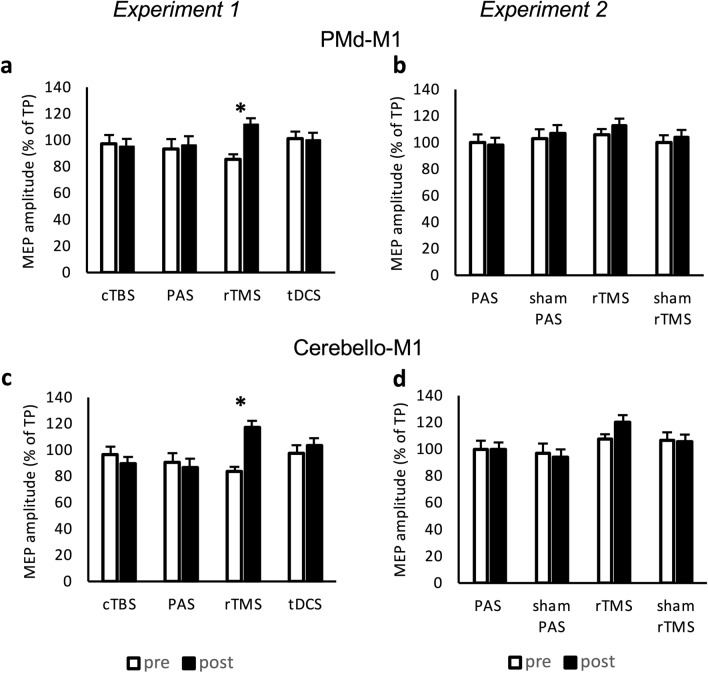
Analyzing conditioned MEPs in the PMd-M1 paradigm (relative MEP amplitudes at an ISI of 6 ms) ANOVA revealed an interaction between INTERVENTION and TIME (F(3,57) = 3.27, p = 0.028, = 0.147) without a main effect of either INTERVENTION or TIME. The increase of relative MEP amplitudes following PMd conditioning after rTMS was significant in the post hoc test (t = − 3.64, p = 0.004) (Fig. 4a).
Figure 4.
Analysis of dual-pulse TMS (experiments 1 and 2). MEP amplitudes were measured peak-to-peak and indicated in percentage to the MEP generated while only stimulating the primary motor cortex (M1) with a test pulse (TP). Conditioning pulses over PMd were administered before the TP over M1 within a dual-pulse PMd-M1 paradigm (a and b). Conditioning pulses were given over the cerebellum prior to TP M1 stimulation within the dual-pulse cerebello-M1 paradigm (c and d). Error bars indicate standard error of mean. Graphs are marked with * when comparison of values with student t-test resulted in p value < 0.05 (Bonferroni–Holm-corrected). (a) Significant increase of PMd-M1 interaction only after rTMS intervention (p = 0.004). (b) Increase of PMd-M1 interaction after rTMS intervention without significance (p = 0.250). (c) Significant increase of cerebello-M1 interaction after rTMS intervention (p < 0.001) (d) Increase of cerebello-M1 interaction after rTMS intervention with a trend to significance (p = 0.057). MEP motor evoked potential, TP test pulse over primary motor cortex, PMd dorsal premotor cortex, M1 primary motor cortex, PAS paired associative stimulation, rTMS repetitive transcranial magnetic stimulation, cTBS continuous theta-burst, tDCS transcranial direct current stimulation, ISI interstimulus interval.
Analysis of conditioned MEPs in the cerebello-M1 paradigm (relative MEP amplitudes at an ISI of 5 ms) revealed an interaction of INTERVENTION and TIME (F(3,57) = 6.54, p < 0.001, = 0.256). The facilitatory effect after rTMS was significant in the post hoc test (t = −4.42, p < 0.001) (Fig. 4c).
Effects on conditioned MEP amplitudes
Effects on cerebello-PMd-M1 interaction
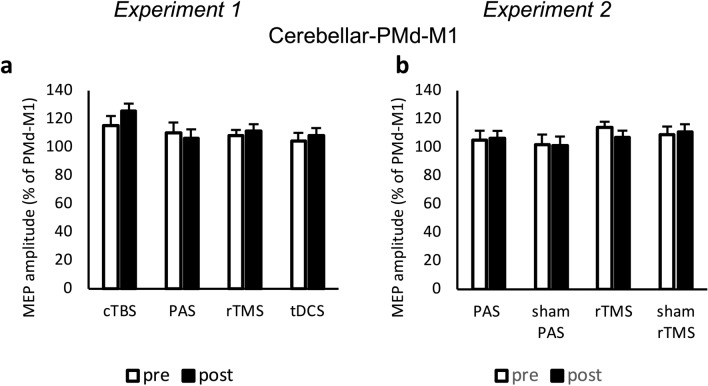
Analysis of conditioned MEP for cerebello-PMd-M1 triple-pulses (relative MEP amplitudes at ISIs between cerebellar and M1 stimulation of 5 ms, 7 ms, 8 ms, 9 ms, 10 ms, and 12 ms to PMd-M1 at 6 ms) did not show any main effects or interaction (Fig. 5a).
Figure 5.
Analysis of triple-pulse TMS (experiments 1 and 2). MEP amplitudes were measured peak-to-peak and indicated in percentage to the MEP generated due to premotor cortex (PMd)-M1 interaction. Both PMd and cerebellar conditioning pulses were given in addition to M1 stimulation within the triple-pulse paradigm. Error bars indicate standard error of mean. (a and b) There was no significant change after any of the interventions. MEP motor evoked potential, TP test pulse over primary motor cortex, PMd dorsal premotor cortex, M1 primary motor cortex, PAS paired associative stimulation, rTMS repetitive transcranial magnetic stimulation, cTBS continuous theta-burst, tDCS transcranial direct current stimulation, ISI interstimulus interval.
Taken together, in experiment 1, we found an inhibitory effect following PAS on unconditioned MEP amplitudes and a facilitatory effect of cerebellar 1 Hz rTMS on cerebello-M1 and PMd-M1 interactions.
Experiment 2—Real versus sham rTMS and PAS
Effects on unconditioned MEPs and motor thresholds
Effects on unconditioned MEPs
Analysis of unconditioned MEP amplitudes showed a main effect for INTERVENTION (F(3,57) = 5.22, p = 0.003, = 0.216), an interaction between INTERVENTION and TIME (F(3,57) = 6.36, p < 0.001, = 0.251) but no main effect of TIME. Post hoc analysis revealed an increase of MEP amplitudes after rTMS (t = − 4.31, p < 0.001) (Fig. 3b).
Effects on motor thresholds
In contrast to experiment 1, analysis of AMT values revealed a main effect of TIME for the 25 mm coil (F(1,19) = 9.84, p = 0.005, = 0.341) but no interaction. There were no significant differences in AMT determined with the 70 mm coil.
For RMT, the main effect for INTERVENTION (F(3,57) = 3.62, p = 0.018, = 0.160) and the interaction between INTERVENTION and TIME (F(3,57) = 3.73, p = 0.016, = 0.164) were significant. Post hoc analysis for RMT showed a slight but significant increase of RMT after PAS (t = 3.39, p = 0.004, 39% ± 1.4% versus 40% ± 1.5%).
Effects on conditioned MEP amplitudes
Effects on PMd-M1 and on cerebello-M1 interactions
Analyzing conditioned MEPs for PMd-M1 and cerebello-M1 interaction revealed no main effects nor interaction (p > 0.1) (Fig. 4b, d).
Effects on cerebello-PMd-M1 interaction
Analysis of conditioned MEP for cerebello-PMd-M1 triple-pulse did not show any main effects or interaction (Fig. 5b).
Taken together, in experiment 2, we found a facilitatory effect following 1 Hz rTMS on unconditioned MEP amplitudes, but no significant effects on conditioned MEPs by rTMS or PAS. Sham protocols did not show any effect.
Combined analysis of rTMS and PAS effects from experiments 1 and 2
Effects on unconditioned MEPs and motor thresholds
Effects on unconditioned MEPs
ANOVA of unconditioned MEPs showed a main effect for INTERVENTION (F(1,37) = 12.26, p = 0.001, = 0.249) and an interaction between INTERVENTION and TIME (F(1,37) = 19.98, p < 0.001, = 0.351). There was no main effect for TIME. Post hoc analysis revealed a decrease of MEP amplitude following PAS (t = 2.73, p = 0.031) and an increase after rTMS (t = − 3.48, p = 0.003) (Fig. 6a).
Figure 6.
Analysis of MEPs and multi-pulse TMS in 38 participants (experiments 1 and 2). MEP amplitudes were measured peak-to-peak and are indicated in mV (a), in percentage to the MEP generated while only stimulating the primary motor cortex (M1) with a test pulse (TP) (b and c) or in percentage to the MEP generated due to premotor cortex (PMd)-M1 interaction (d). Conditioning pulses were administered before the TP on PMd (b), cerebellum (c) or both (d). Error bars indicate standard error of mean. Graphs are marked with * when comparison of values with student t-test resulted in p value < 0.05 (Bonferroni–Holm-corrected). (a) Significant decrease of MEP amplitude after PAS intervention (p = 0.005) and significant increase of MEP amplitude after rTMS intervention (p = 0.002). (b) Significant increase of PMd-M1 interaction after rTMS intervention (p = 0.002). (c) Significant increase of cerebello-M1 interaction after rTMS intervention (p < 0.001) (d) No significant change. MEP motor evoked potential, TP test pulse over primary motor cortex, PMd dorsal premotor cortex, M1 primary motor cortex, PAS paired associative stimulation, rTMS repetitive transcranial magnetic stimulation.
Effects on motor thresholds
ANOVA of motor thresholds revealed a main effect of TIME for AMT determined with the 25 mm coil (F(1,37) = 17.24, p < 0.001, = 0.381) and RMT (F(1,37) = 16.39, p < 0.001, = 0.307). There was no main effect of INTERVENTION or interaction between INTERVENTION and TIME for AMT determined both with the 25 mm and 70 mm coil as well as for RMT. Also, there was no main effect for TIME for AMT determined with the 70 mm coil.
Effects on conditioned MEP amplitudes
Effects on PMd-M1 and cerebello-M1 interactions
ANOVA on conditioned MEP amplitudes for PMd-M1 interaction showed a main effect for TIME (F(1,37) = 8.25, p = 0.007, = 0.182) and an interaction between INTERVENTION and TIME (F(1,37) = 5.47, p = 0.025, = 0.129). Post hoc analysis showed facilitation of PMd-M1 interaction after rTMS (t = −3.70, p = 0.002) (Fig. 6b).
Analysis of cerebello-M1 interaction revealed a main effect of TIME (F(1,37) = 5.95, p = 0.020, = 0.154) and INTERVENTION (F(1,37) = 6.73, p = 0.014, = 0.139) and an interaction between INTERVENTION and TIME (F(1,37) = 13.17, p < 0.001, = 0.263). Post hoc test showed a significant facilitation after rTMS (t = −4.32, p < 0.001) (Fig. 6c).
Effects on cerebello-PMd-M1 interaction
Analysis of conditioned MEP for cerebello-PMd-M1 triple-pulse did not show any main effects or interactions of factors (Fig. 6d).
Taken together, in the combined analysis of experiment 1 and 2, we found a facilitatory effect following 1 Hz rTMS on unconditioned MEP amplitudes and on cerebello-M1 and PMd-M1 interactions, as well as an inhibitory effect following PAS on unconditioned MEP amplitudes.
Sample size calculation for future group comparisons studies
In experiments 1 and 2, a total of 38 subjects were stimulated over the cerebellum with 1 Hz rTMS and PAS. This number of cases allowed us to estimate the sample size for potential comparative group studies with patients based on the stimulation effects on the MEP amplitudes found here. For the rTMS intervention, the change in MEP amplitude was 0.29 ± 0.59 mV and for PAS − 0.24 ± 0.44 mV. For a potential comparison group, we assumed that there were no stimulation effects in this group resulting in an effect size of dz = 0.49 for rTMS and dz = 0.55 for PAS. Based on these data, the required group size would be 66 participants per group for an rTMS intervention and 54 participants per group for the PAS intervention.
Discussion
The first main finding of the present study is that 1 Hz rTMS over the right cerebellar lobule VIIIA increases corticospinal excitability as reflected by MEP amplitudes, whereas cerebellar PAS using two TMS pulses, one over the target area VIIIA followed by the second over M1, decreases MEPs. The second finding is that 1 Hz rTMS of right cerebellar lobule VIIIA also facilitates PMd-M1 and cerebello-M1 interactions. Similar to previous studies, this was not the case for the cerebellar PAS protocol17. Neither cTBS nor tDCS were effective in inducing plasticity supporting the results of previous studies, which also did not find effects on cortical excitability by cTBS70 or anodal tDCS33,71. As a third finding, we show that our results became clear and robust only with a sample size of 38, whereas results were quite variable and equivocal when only 20 participants were studied. This finding has considerable consequences for future studies using either cerebellar rTMS or cerebellar PAS as a treatment in clinical studies.
In line with our results, previous studies also found an increase of corticospinal excitability measured by MEPs after low-frequency rTMS stimulation over the cerebellum42,43,72, as well as abolishment of cerebello-M1 inhibition70. While our study focused on PMd-M1 and cerebello-M1 interaction, other studies investigated cortico-cortical interactions after cerebellar rTMS and found decreased intracortical facilitation at an ISI of 10 ms72 and an increase of intracortical facilitation at an ISI of 15 ms42. Both studies did not find alteration of SICI.
Probably, the mechanism of action of 1 Hz rTMS over the cerebellum is induction of plasticity resulting in transiently reduced excitability of Purkinje cells42, which leads to a decreased inhibitory effect on the dentate nucleus and therefore an increased excitatory output from the dentate nucleus to M1, an effect similar to long-term potentiation30. Based on single neuron and local field potential studies in cats and monkeys, it has been shown that the excitatory output of the cerebellum from the dentate nucleus projects onto excitatory as well as inhibitory interneurons in M112,73,74. Previous studies suggested that interneuron population in M1 can be activated specifically by TMS, resulting in intracortical inhibition or facilitation75,76. The finding that cerebellar rTMS leads to both an increase of corticospinal excitability (reflected by an increase of unconditioned MEP amplitudes) and facilitation of PMd-M1 interactions suggests a specific effect on certain intracortical interneurons located in M1 but inter-connected with intracortical neurons located in the PMd. The increase of cerebello-M1 excitability after cerebellar rTMS could result from disinhibition of cerebellar to motor cortex pathway but also from remote effect on M1 interneurons. The fact that the combination of conditioned stimulation sites (cerebellum and PMd) had no additional effect on M1 or PMd-M1 excitability might be explained by both conditioning effects being mediated by the same interneuron network that is already optimally stimulated, i.e. might be caused by a ceiling effect.
Cerebellar PAS as used here apparently causes long-term depression-like effects reflected by an inhibition of corticospinal excitability (inhibition of MEPs and thresholds). PAS did not affect CBI or PMd-M1 interactions. Previous work has likewise found a clear effect of cerebellar PAS on corticospinal excitability, but no effects on CBI and SICI17,77. These results can be interpreted such that cerebellar PAS as used in our study with coupling of (preceding) cerebellar and M1 stimulation predominantly acts where the induced action potentials collide, i.e. within M1.
Importantly, rTMS and PAS effects in the present study were consistently present, either as a significant finding or as trends near significance in experiments 1 and 2, where 20 subjects each were studied, but became robust and unequivocal only with a group size of 38.
The effects of NIBS on the cerebellum have not been studied as extensively as those on other brain regions. Reported effects of interventions are partly contradictory30,49,78,79. One possible explanation is related to the anatomy of the cerebellum with proximity to neck muscles and a relatively large distance from the scalp to cerebellar Purkinje cells that are presumably the most relevant target for NIBS. This makes painless supra-threshold stimulation of the cerebellum difficult. Also, the size of the cerebellum renders specific stimulation of certain parts problematic. The exact stimulation regions are not specified in many studies50,72,77,80–83. Also, certain measurements such as CBI cannot be elicited in every person, or are not tolerated, so that these participants had to be excluded in previous studies. CBI is also very sensitive towards higher test pulse amplitudes that can reduce CBI. Given these drawbacks, information on criteria for the exclusion or inclusion of participants is important.
We tried to overcome the problem of spatial inaccuracy by using neuronavigation allowing us to more accurately define our target region, i.e. area VIIIA on the right cerebellar hemisphere. Moreover, we used a test pulse intensity of 120% RMT resulting in approximately 1 mV MEPs amplitudes. To increase specificity of our findings, we used four different NIBS techniques in experiment 1 and additionally sham stimulation in experiment 2. To reduce variability, our sample size was relatively large.
There are some limitations of our study. A limitation when studying intra-hemispheric PMd-M1 interaction is the close proximity between PMd and M1. Due to the size of the TMS coils optimal positioning over both targets is challenging. To avoid an anterior shift of the PMd coil, we first identified the individual anatomical PMd position in each subject and targeted the PMd using a 25 mm coil. Given the position of the PMd coil we adapted the angulation of the TMS coil and the stimulation intensity for M1 stimulation if necessary to keep the PMd conditioning accurate. Targeting the PMd is reliable on the basis of structural MRI. Therefore, we opted for anatomical targeting and recorded both positions (PMd and M1, Fig. 2). Alternatively, brain regions of interest can also be targeted on the basis of individual functional activation during tasks. For instance, previous studies on intra-hemispheric connections between M1 and the parietal cortex targeted TMS stimulation sites on the basis of brain activation during motor imagery84.
Another limitation is that we focused on PMd-M1 circuitry but did not examine other nodes or pathways that are also relevant for the understanding of plasticity in cerebellar-cortical networks including, for instance, the ventral premotor cortex, the supplementary motor area, and the posterior parietal cortex. These should be included in future studies.
Prior to the rTMS intervention, MEPs were not modulated by PMd or cerebellar conditioning. Unconditioned MEP amplitudes increased following rTMS, albeit non-significantly, which might nonetheless have influenced rTMS effects on PMd-M1 or cerebello-M1 interaction, i.e. facilitation.
We used the data of the change in corticospinal excitability following rTMS and PAS to estimate the number of cases needed to confirm or refute a stimulation effect with sufficient statistical power in a between-group comparisons, e.g. patients vs. healthy controls. We assumed that the findings of the present study would differ, i.e. would not be present, in a patient group. Therefore, 66 participants per group would be needed for a cerebellar rTMS and 54 for a cerebellar PAS intervention. In our calculation, we assumed the main effect to be quite large compared to a presumed zero change of MEP amplitudes after the intervention in a patient group. The variance of the effect was based on the data of this study, which we consider to be reliable, since we have chosen a non-selected group of subjects and have optimally controlled the stimulation conditions by means of neuronavigation.
Since the calculation of the required sample size depends on the assumed effect, e. g. the difference between groups, as well as the variance of the main effect within a given group, any change of these values would also affect the calculated number of cases needed., i.e. the number of required cases would be considerably larger if group differences of main stimulation effects were smaller.
These data suggest that the heterogeneity of published data on cerebellar NIBS could, at least partly, be explained by small numbers in many previous studies. It thus appears plausible and advisable to generally study larger cohorts in neurophysiological studies using rTMS and PAS as possible treatment options.
In summary, cerebellar 1 Hz rTMS increases net corticospinal excitability and facilitatory interactions in cerebello-M1 and PMd-M1 pathways, whereas cerebellar PAS reduces corticospinal excitability. Most likely, rTMS effects are mediated by long-term potentiation-like mechanism in cerebello-thalamo-cortical pathways projecting onto intracortical interneurons inter-connected with other cortical areas, whereas PAS probably leads to long-term depression-like effect in cerebello-thalamo-cortical pathways connected with neural elements primarily influencing pyramidal corticospinal output.
Acknowledgements
This study was supported by the Deutsche Forschungsgemeinschaft (DFG, SFB 936, project C5).
Abbreviations
- AMT
Active motor threshold
- ANOVA
Analysis of variance
- cTBS
Continuous theta-burst stimulation
- FDI
First dorsal interosseus muscles
- ISI
Interstimulus interval
- M1
Primary motor cortex
- MEP
Motor evoked potential
- MSO
Maximum stimulator output
- NIBS
Non-invasive brain stimulation
- PAS
Paired associated stimulation
- PMd
Dorsal premotor cortex
- RMT
Resting motor threshold
- rTMS
Repetitive transcranial magnetic stimulation
- SICI
Short-interval intracortical inhibition
- tDCS
Transcranial direct current stimulation
- TMS
Transcranial magnetic stimulation
Author contributions
M.G.P., A.M., T.B., and A.W. conceived the study design. M.G.P., A.S., F.H., C.B., and A.W. acquired the data. M.G.P. analyzed the data. M.G.P., A.S., F.H., C.B., A.M., T.B., and A.W. interpreted the data. M.G.P. drafted the manuscript. M.G.P., A.S., F.H., C.B., E.T., A.M., T.B., and A.W. revised the manuscript. All authors approved the final version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. MGP, AS, FH and CB did not receive funding. ET is supported by the German Research Foundation (DFG, TZ 85/1-1). AM: Commercial research support: Pharm Allergan, Ipsen, Merz Pharmaceuticals, Actelion; Honoraria for lectures: Pharm Allergan, Ipsen, Merz Pharmaceuticals, Actelion, GlaxoSmithKline, Desitin, Teva, Takeda; Consultancies: Desitin, Merz Pharmaceuticals, Admedicum; Support from Foundations: Possehl-Stiftung (Lübeck, Germany), Margot und Jürgen Wessel Stiftung (Lübeck, Germany), Tourette Syndrome Association (Germany), Interessenverband Tourette Syndrom (Germany), CHDI, Damp-Stiftung; Academic research support: Deutsche Forschungsgemeinschaft (DFG): projects 1692/3-1, 4-1, SFB 936, and FOR 2698 (project numbers 396914663, 396577296, 396474989), Innovationsausschuss of the Gemeinsamer Bundesausschuss: Translate NAMSE (structural support for the Lübeck Center for Rare Diseases); European Reference Network—Rare Neurological Diseases (ERN—RND); Royalties for the book Neurogenetics (Oxford University Press); Advisory Boards: German Tourette syndrome Association; Alliance of patients with chronic rare diseases. TB Commercial research support: Pharm Allergan, Ipsen, Merz Pharmaceuticals, Honoraria for lectures: Pharm Allergan, Ipsen Pharma, Merz Pharmaceuticals, Consultancies: Pharm Allergan, Ipsen Pharma, Merz Pharmaceuticals. Support from Foundations: Possehl-Stiftung (Lübeck, Germany). Academic research support: Deutsche Forschungsgemeinschaft (DFG): projects SFB 936, and FOR 2698. AW receives funding from the German Research Foundation (DFG, WE 5919/2-1) and the Else Kröner-Fresenius Foundation (2018_A55).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Tobias Bäumer and Anne Weissbach.
References
- 1.Itō M. The Cerebellum and Neural Control. London: Raven Press; 1984. [Google Scholar]
- 2.Milardi D, et al. The cortico-basal ganglia-cerebellar network: Past, present and future perspectives. Front. Syst. Neurosci. 2019;13:61. doi: 10.3389/fnsys.2019.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quartarone A, et al. New insights into cortico-basal-cerebellar connectome: Clinical and physiological considerations. Brain. 2020;143:396–406. doi: 10.1093/brain/awz310. [DOI] [PubMed] [Google Scholar]
- 4.D’Angelo E. Physiology of the Cerebellum. Amsterdam: Elsevier; 2018. [Google Scholar]
- 5.Spampinato DA, Block HJ, Celnik PA. Cerebellar–M1 connectivity changes associated with motor learning are somatotopic specific. J. Neurosci. 2017;37:2377–2386. doi: 10.1523/JNEUROSCI.2511-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bostan AC, Strick PL. The basal ganglia and the cerebellum: Nodes in an integrated network. Nat. Rev. Neurosci. 2018;19:338–350. doi: 10.1038/s41583-018-0002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Picard N, Strick PL. Imaging the premotor areas. Curr. Opin. Neurobiol. 2001;11:663–672. doi: 10.1016/S0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- 8.Dum RP, Strick PL. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J. Neurophysiol. 2003;89:634–639. doi: 10.1152/jn.00626.2002. [DOI] [PubMed] [Google Scholar]
- 9.Aberra AS, Wang B, Grill WM, Peterchev AV. Simulation of transcranial magnetic stimulation in head model with morphologically-realistic cortical neurons. Brain Stimul. 2020;13:175–189. doi: 10.1016/j.brs.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spampinato DA, Celnik PA, Rothwell JC. Cerebellar–motor cortex connectivity: One or two different networks? J. Neurosci. 2020;40:4230–4239. doi: 10.1523/JNEUROSCI.2397-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groiss, S. J. & Ugawa, Y. Cerebellum. in Handbook of Clinical Neurology vol. 116 643–653 (Elsevier, Amsterdam, 2013). [DOI] [PubMed]
- 12.Holdefer RN, Miller LE, Chen LL, Houk JC. Functional connectivity between cerebellum and primary motor cortex in the awake monkey. J. Neurophysiol. 2000;84:585–590. doi: 10.1152/jn.2000.84.1.585. [DOI] [PubMed] [Google Scholar]
- 13.Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation over the cerebellum in humans. Ann. Neurol. 1995;37:703–713. doi: 10.1002/ana.410370603. [DOI] [PubMed] [Google Scholar]
- 15.Doeltgen SH, Young J, Bradnam LV. Anodal direct current stimulation of the cerebellum reduces cerebellar brain inhibition but does not influence afferent input from the hand or face in healthy adults. Cerebellum. 2016;15:466–474. doi: 10.1007/s12311-015-0713-5. [DOI] [PubMed] [Google Scholar]
- 16.Koch G, et al. Changes in intracortical circuits of the human motor cortex following theta burst stimulation of the lateral cerebellum. Clin. Neurophysiol. 2008;119:2559–2569. doi: 10.1016/j.clinph.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Lu M-K, Tsai C-H, Ziemann U. Cerebellum to motor cortex paired associative stimulation induces bidirectional STDP-like plasticity in human motor cortex. Front. Hum. Neurosci. 2012;6:260. doi: 10.3389/fnhum.2012.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benussi A, et al. Stimulation over the cerebellum with a regular figure-of-eight coil induces reduced motor cortex inhibition in patients with progressive supranuclear palsy. Brain Stimul. 2019;12:1290–1297. doi: 10.1016/j.brs.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 19.Bonnì S. Cerebellar theta burst stimulation in stroke patients with ataxia. Funct. Neurol. 2014;29:41–45. [PMC free article] [PubMed] [Google Scholar]
- 20.Brusa L, et al. Theta burst stimulation modulates cerebellar-cortical connectivity in patients with progressive supranuclear palsy. Brain Stimul. 2014;7:29–35. doi: 10.1016/j.brs.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Daskalakis ZJ, et al. Exploring the connectivity between the cerebellum and motor cortex in humans. J. Physiol. 2004;557:689–700. doi: 10.1113/jphysiol.2003.059808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koch G, et al. Cerebellar magnetic stimulation decreases levodopa-induced dyskinesias in Parkinson disease. Neurology. 2009;73:113–119. doi: 10.1212/WNL.0b013e3181ad5387. [DOI] [PubMed] [Google Scholar]
- 23.Koch G, et al. Effects of two weeks of cerebellar theta burst stimulation in cervical dystonia patients. Brain Stimul. 2014;7:564–572. doi: 10.1016/j.brs.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Matsugi A, et al. Cerebellar transcranial magnetic stimulation reduces the silent period on hand muscle electromyography during force control. Brain Sci. 2020;10:63. doi: 10.3390/brainsci10020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tzvi E, Stoldt A, Witt K, Krämer UM. Striatal-cerebellar networks mediate consolidation in a motor sequence learning task: An fMRI study using dynamic causal modelling. Neuroimage. 2015;122:52–64. doi: 10.1016/j.neuroimage.2015.07.077. [DOI] [PubMed] [Google Scholar]
- 26.Tzvi E, Koeth F, Karabanov AN, Siebner HR, Krämer UM. Cerebellar—premotor cortex interactions underlying visuomotor adaptation. Neuroimage. 2020;220:117142. doi: 10.1016/j.neuroimage.2020.117142. [DOI] [PubMed] [Google Scholar]
- 27.Civardi C, Cantello R, Asselman P, Rothwell JC. Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. Neuroimage. 2001;14:1444–1453. doi: 10.1006/nimg.2001.0918. [DOI] [PubMed] [Google Scholar]
- 28.Ni Z, et al. Reduced dorsal premotor cortex and primary motor cortex connectivity in older adults. Neurobiol. Aging. 2015;36:301–303. doi: 10.1016/j.neurobiolaging.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 29.Huang Y-Z, et al. Plasticity induced by non-invasive transcranial brain stimulation: A position paper. Clin. Neurophysiol. 2017;128:2318–2329. doi: 10.1016/j.clinph.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Tremblay S, Austin D, Hannah R, Rothwell JC. Non-invasive brain stimulation as a tool to study cerebellar-M1 interactions in humans. Cerebellum Ataxias. 2016;3:19. doi: 10.1186/s40673-016-0057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nitsche MA, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Grimaldi G, et al. Cerebellar transcranial direct current stimulation (ctDCS): A novel approach to understanding cerebellar function in health and disease. Neuroscientist. 2016;22:83–97. doi: 10.1177/1073858414559409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galea JM, Jayaram G, Ajagbe L, Celnik P. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J. Neurosci. 2009;29:9115–9122. doi: 10.1523/JNEUROSCI.2184-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benussi A, Pascual-Leone A, Borroni B. Non-invasive cerebellar stimulation in neurodegenerative ataxia: A literature review. Int. J. Mol. Sci. 2020;21:1948. doi: 10.3390/ijms21061948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orrù G, Cesari V, Conversano C, Gemignani A. The clinical application of transcranial direct current stimulation in patients with cerebellar ataxia: A systematic review. Int. J. Neurosci. 2020 doi: 10.1080/00207454.2020.1750399. [DOI] [PubMed] [Google Scholar]
- 36.Batsikadze G, et al. Effects of cerebellar transcranial direct current stimulation on cerebellar-brain inhibition in humans: A systematic evaluation. Brain Stimul. 2019;12:1177–1186. doi: 10.1016/j.brs.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 37.Chen R, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/WNL.48.5.1398. [DOI] [PubMed] [Google Scholar]
- 38.Touge T, Gerschlager W, Brown P, Rothwell JC. Are the after-effects of low-frequency rTMS on motor cortex excitability due to changes in the efficacy of cortical synapses? Clin. Neurophysiol. 2001;112:2138–2145. doi: 10.1016/S1388-2457(01)00651-4. [DOI] [PubMed] [Google Scholar]
- 39.Tsuji T, Rothwell JC. Long lasting effects of rTMS and associated peripheral sensory input on MEPs, SEPs and transcortical reflex excitability in humans. J. Physiol. 2002;540:367–376. doi: 10.1113/jphysiol.2001.013504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin. Neurophysiol. 2000;111:800–805. doi: 10.1016/S1388-2457(99)00323-5. [DOI] [PubMed] [Google Scholar]
- 41.Muellbacher W, Ziemann U, Boroojerdi B, Hallett M. Effects of low-frequency transcranial magnetic stimulation on motor excitability and basic motor behavior. Clin. Neurophysiol. 2000;111:1002–1007. doi: 10.1016/S1388-2457(00)00284-4. [DOI] [PubMed] [Google Scholar]
- 42.Oliveri M, Koch G, Torriero S, Caltagirone C. Increased facilitation of the primary motor cortex following 1Hz repetitive transcranial magnetic stimulation of the contralateral cerebellum in normal humans. Neurosci. Lett. 2005;376:188–193. doi: 10.1016/j.neulet.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 43.Gerschlager W, Christensen LOD, Bestmann S, Rothwell JC. rTMS over the cerebellum can increase corticospinal excitability through a spinal mechanism involving activation of peripheral nerve fibres. Clin. Neurophysiol. 2002;113:1435–1440. doi: 10.1016/S1388-2457(02)00156-6. [DOI] [PubMed] [Google Scholar]
- 44.Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 45.Rocchi L, et al. Variability and predictors of response to continuous theta burst stimulation: A TMS-EEG study. Front. Neurosci. 2018;12:400. doi: 10.3389/fnins.2018.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jannati A, Block G, Oberman LM, Rotenberg A, Pascual-Leone A. Interindividual variability in response to continuous theta-burst stimulation in healthy adults. Clin. Neurophysiol. 2017;128:2268–2278. doi: 10.1016/j.clinph.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hordacre B, et al. Variability in neural excitability and plasticity induction in the human cortex: A brain stimulation study. Brain Stimul. 2017;10:588–595. doi: 10.1016/j.brs.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 48.Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123(Pt 3):572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- 49.Behrangrad S, Zoghi M, Kidgell D, Jaberzadeh S. Does cerebellar non-invasive brain stimulation affect corticospinal excitability in healthy individuals? A systematic review of literature and meta-analysis. Neurosci. Lett. 2019;706:128–139. doi: 10.1016/j.neulet.2019.05.025. [DOI] [PubMed] [Google Scholar]
- 50.Olfati N, et al. Cerebellar repetitive transcranial magnetic stimulation (rTMS) for essential tremor: A double-blind, sham-controlled, crossover, add-on clinical trial. Brain Stimul. 2020;13:190–196. doi: 10.1016/j.brs.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 51.Erkelens IM, et al. A differential role for the posterior cerebellum in the adaptive control of convergence eye movements. Brain Stimul. 2020;13:215–228. doi: 10.1016/j.brs.2019.07.025. [DOI] [PubMed] [Google Scholar]
- 52.Weissbach A, et al. Premotor-motor excitability is altered in dopa-responsive dystonia: Premotor–motor excitability. Mov. Disord. 2015;30:1705–1709. doi: 10.1002/mds.26365. [DOI] [PubMed] [Google Scholar]
- 53.Weissbach A, et al. Abnormal premotor–motor interaction in heterozygous Parkin—and Pink1 mutation carriers. Clin. Neurophysiol. 2017;128:275–280. doi: 10.1016/j.clinph.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 54.Popa T, et al. Cerebellar processing of sensory inputs primes motor cortex plasticity. Cereb. Cortex. 2013;23:305–314. doi: 10.1093/cercor/bhs016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stoodley C, Schmahmann J. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. Neuroimage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 56.Strzalkowski NDJ, Chau AD, Gan LS, Kiss ZHT. Both 50 and 30 Hz continuous theta burst transcranial magnetic stimulation depresses the cerebellum. Cerebellum. 2019;18:157–165. doi: 10.1007/s12311-018-0971-0. [DOI] [PubMed] [Google Scholar]
- 57.Fernandez L, Major BP, Teo W-P, Byrne LK, Enticott PG. Assessing cerebellar brain inhibition (CBI) via transcranial magnetic stimulation (TMS): A systematic review. Neurosci. Biobehav. Rev. 2018;86:176–206. doi: 10.1016/j.neubiorev.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 58.Naro A, et al. Does transcranial alternating current stimulation induce cerebellum plasticity? Feasibility, safety and efficacy of a novel electrophysiological approach. Brain Stimul. 2016;9:388–395. doi: 10.1016/j.brs.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 59.Hardwick RM, Lesage E, Miall RC. Cerebellar transcranial magnetic stimulation: The role of coil geometry and tissue depth. Brain Stimul. 2014;7:643–649. doi: 10.1016/j.brs.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benussi A, et al. Cerebello-spinal tDCS in ataxia: A randomized, double-blind, sham-controlled, crossover trial. Neurology. 2018;91:e1090–e1101. doi: 10.1212/WNL.0000000000006210. [DOI] [PubMed] [Google Scholar]
- 61.Carrillo F, et al. Study of cerebello-thalamocortical pathway by transcranial magnetic stimulation in Parkinson’s disease. Brain Stimul. 2013;6:582–589. doi: 10.1016/j.brs.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 62.Kishore A, et al. Cerebellar sensory processing alterations impact motor cortical plasticity in Parkinson’s disease: Clues from dyskinetic patients. Cereb. Cortex. 2014;24:2055–2067. doi: 10.1093/cercor/bht058. [DOI] [PubMed] [Google Scholar]
- 63.Weissbach A, et al. Single-pulse subthalamic deep brain stimulation reduces premotor-motor facilitation in Parkinson’s disease. Parkinsonism. Relat. Disord. 2019;66:224–227. doi: 10.1016/j.parkreldis.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 64.Wessel MJ, et al. Enhancing consolidation of a new temporal motor skill by cerebellar noninvasive stimulation. Cereb. Cortex. 2016;26:1660–1667. doi: 10.1093/cercor/bhu335. [DOI] [PubMed] [Google Scholar]
- 65.Ferrucci R, Cortese F, Priori A. Cerebellar tDCS: How to do it. Cerebellum. 2015;14:27–30. doi: 10.1007/s12311-014-0599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liebrand M, et al. Beneficial effects of cerebellar tDCS on motor learning are associated with altered putamen-cerebellar connectivity: A simultaneous tDCS-fMRI study. Neuroimage. 2020;223:117363. doi: 10.1016/j.neuroimage.2020.117363. [DOI] [PubMed] [Google Scholar]
- 67.Gomez-Tames J, et al. Group-level and functional-region analysis of electric-field shape during cerebellar transcranial direct current stimulation with different electrode montages. J. Neural Eng. 2019;16:036001. doi: 10.1088/1741-2552/ab0ac5. [DOI] [PubMed] [Google Scholar]
- 68.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39:175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 69.Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 70.Popa T, Russo M, Meunier S. Long-lasting inhibition of cerebellar output. Brain Stimul. 2010;3:161–169. doi: 10.1016/j.brs.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 71.Craig CE, Doumas M. Anodal transcranial direct current stimulation shows minimal, measure-specific effects on dynamic postural control in young and older adults: A double blind, sham-controlled study. PLoS ONE. 2017;12:e0170331. doi: 10.1371/journal.pone.0170331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fierro B, et al. Modulatory effects of 1 Hz rTMS over the cerebellum on motor cortex excitability. Exp. Brain Res. 2007;176:440–447. doi: 10.1007/s00221-006-0628-y. [DOI] [PubMed] [Google Scholar]
- 73.Ando N, Izawa Y, Shinoda Y. Relative contributions of thalamic reticular nucleus neurons and intrinsic interneurons to inhibition of thalamic neurons projecting to the motor cortex. J. Neurophysiol. 1995;73:2470–2485. doi: 10.1152/jn.1995.73.6.2470. [DOI] [PubMed] [Google Scholar]
- 74.Na J, Kakei S, Shinoda Y. Cerebellar input to corticothalamic neurons in layers V and VI in the motor cortex. Neurosci. Res. 1997;28:77–91. doi: 10.1016/S0168-0102(97)00031-X. [DOI] [PubMed] [Google Scholar]
- 75.Kujirai T, et al. Corticocortical inhibition in human motor cortex. J. Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J. Physiol. 1996;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lu M-K, et al. Impaired cerebellum to primary motor cortex associative plasticity in Parkinson’s disease and spinocerebellar ataxia type 3. Front. Neurol. 2017;8:445. doi: 10.3389/fneur.2017.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miterko LN, et al. Consensus paper: Experimental neurostimulation of the cerebellum. Cerebellum. 2019;18:1064–1097. doi: 10.1007/s12311-019-01041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Dun K, Bodranghien F, Manto M, Mariën P. Targeting the cerebellum by noninvasive neurostimulation: A review. Cerebellum. 2017;16:695–741. doi: 10.1007/s12311-016-0840-7. [DOI] [PubMed] [Google Scholar]
- 80.Dale ML, DeVries WH, Mancini M, George MS. Cerebellar rTMS for motor control in progressive supranuclear palsy. Brain Stimul. 2019;12:1588–1591. doi: 10.1016/j.brs.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 81.Koch G, et al. Improving visuo-motor learning with cerebellar theta burst stimulation: Behavioral and neurophysiological evidence. Neuroimage. 2020;208:116424. doi: 10.1016/j.neuroimage.2019.116424. [DOI] [PubMed] [Google Scholar]
- 82.Koch G, et al. Effect of cerebellar stimulation on gait and balance recovery in patients with hemiparetic stroke: A randomized clinical trial. JAMA Neurol. 2019;76:170. doi: 10.1001/jamaneurol.2018.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sasegbon A, et al. Cerebellar repetitive transcranial magnetic stimulation restores pharyngeal brain activity and swallowing behaviour after disruption by a cortical virtual lesion. J. Physiol. 2019;597:2533–2546. doi: 10.1113/JP277545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lebon F, Lotze M, Stinear CM, Byblow WD. Task-dependent interaction between parietal and contralateral primary motor cortex during explicit versus implicit motor imagery. PLoS ONE. 2012;7:e37850. doi: 10.1371/journal.pone.0037850. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.