Abstract
Cardiovascular diseases (CVDs) are the number one cause of death globally, and the early identification of high risk is crucial to prevent the disease and to reduce healthcare costs. Short life expectancy and increased mortality among the Roma are generally accepted (although not indeed proven by mortality analyses) which can be partially explained by the high prevalence of cardiovascular risk factors (CVRF) among them. This study aims to elaborate on the prevalence of the most important CVD risk factors, assess the estimation of a 10-year risk of development of fatal and nonfatal CVDs based on the most used risk assessment scoring models, and to compare the Hungarian general (HG) and Roma (HR) populations. In 2018 a complex health survey was accomplished on the HG (n = 380) and HR (n = 347) populations. The prevalence of CVRS was defined and 10-year cardiovascular risk was estimated for both study populations using the following systems: Framingham Risk Score for hard coronary heart disease (FRSCHD) and for cardiovascular disease (FRSCVD), Systematic COronary Risk Evaluation (SCORE), ACC/AHA Pooled Cohort Equations (PCE) and Revised Pooled Cohort Equations (RPCE). After the risk scores had been calculated, the populations were divided into risk categories and all subjects were classified. For all CVD risk estimation scores, the average of the estimated risk was higher among Roma compared to the HG independently of the gender. The proportion of high-risk group in the Hungarian Roma males population was on average 1.5–3 times higher than in the general one. Among Roma females, the average risk value was higher than in the HG one. The proportion of high-risk group in the Hungarian Roma females population was on average 2–3 times higher compared to the distribution of females in the general population. Our results show that both genders in the Hungarian Roma population have a significantly higher risk for a 10-year development of cardiovascular diseases and dying from them compared to the HG one. Therefore, cardiovascular interventions should be focusing not only on reducing smoking among Roma but on improving health literacy and service provision regarding prevention, early recognition, and treatment of lipid disorders and diabetes among them.
Subject terms: Cardiology, Population screening
Introduction
Of the 55.9 million deaths worldwide in 2017, non-communicable diseases (NCDs) accounted for 73.4%. Among NCDs, the largest number of deaths (17.8 million worldwide) were estimated for cardiovascular diseases (CVDs)1. Each year CVDs (mainly ischaemic heart disease and stroke) cause about 3.9 million deaths in Europe, over 1.8 million deaths in the European Union (EU). Although the CVD burden showed a steady decrease during the last few decades in the EU countries, a severe East–West gap in mortality still exists. Central and Eastern Europe (CEE) including the eleven post-communist countries joining the EU since 2004 is the region with the highest CVD burden in world2. The standardized death rate for CVDs—the leading cause of death in the country—was 588.15 per 100,000 inhabitants in 2017 in Hungary (5th worst among the EU-28 countries), 1.6 times higher than the EU average (369.46 deaths per 100,000)1. Life expectancy increased between 2000 and 2017 (from 71.9 to 76.0 years) in Hungary; a phenomenon that can be explained primarily by the decrease in deaths due to cardiovascular diseases, and especially to stroke. Although the IHD-related death rate decreased by 12% in Hungary between 2000 and 2016, it is still significantly lower than the EU average decrease of more than 40%.
It is reasonable to suppose that the unfavourable CVD mortality figures are at least partly linked with the high representation of the Roma population in the majority of CEE countries3. With an estimated number of 10–12 million people, the Roma population is the largest ethnic group in Europe. They live concentrated in the countries of the CEE region (in Bulgaria, Hungary, Slovakia, and Romania)4 and their representation in Hungary is about 8–10% of the total population5,6. They are concentrated in the Southwest and the Northeast regions of the country, where they frequently live in segregated colonies with severe environmental problems, such as the lack of sewage and gas mains, garbage deposits, waterlogged soil, and lack of water mains7. In addition to segregation and deprived living conditions low educational attainment and labour market barriers also measurably exist8. These factors combined adversely affect their health indicators. Estimating the scale of the problem is further encumbered by the fact that the collection of data related to health and health-care utilization on an ethnic basis is difficult in countries where Roma live9. One-off surveys indicate that they suffer from poor health and have only limited access to healthcare10–12.
Increased mortality and short life expectancy of the Roma is not proven (the ethnicity is not recorded in the mortality statistics), but suspected on the basis of the high prevalence of health risk factors among them. Several studies have examined the prevalence of cardiovascular risk factors among the Roma minority and compared it with that of the general population13–15. Obesity (France16, Hungary17, Romania15, Slovakia18–20, Spain21), diabetes (France16, Hungary17, Serbia22, Slovakia19), insulin resistance (Slovakia20), smoking (Croatia23, France16, Romania15,24, Slovakia20,25), physical inactivity (Romania26, Slovakia27), hypertension (France16, Hungary17, Slovakia19), abnormal lipid profile (Hungary17, Romania15, Slovakia19), and metabolic syndrome (Hungary17, Slovakia14,19) have high prevalence and were significantly more common among the Roma, regardless of the country in which they live. Roma CHD patients have a worse risk profile at the entry of care and seem to be undertreated compared with non-Roma CHD patients28.
The high representation of CVD risk factors can be explained by the impact of environmental factors above mentioned and also by genetic causes (such as in the case of type 2 diabetes mellitus29). Based on our previous studies, we can state that the higher risk/prevalence of the venous thrombosis30 and reduced high-density lipoprotein cholesterol levels31–33 are determined by genetic factors, whereas the high risk/prevalence of type 2 diabetes34 and obesity35 are influenced by environmental factors in the Hungarian Roma population. Although several studies have examined the prevalence of CVD risk factors in the Roma population, currently, there are no studies focusing on their combined effect in CVD morbidity or mortality risk prediction models.
About 360 predictive estimation models have been identified by Damen et al.36 that might be used to develop targeted primary prevention and intervention against the development of CVDs. The most widely used tools for estimating cardiovascular risk in a population are the Framingham Risk Score (FRS37), the Systematic COronary Risk Evaluation (SCORE38), the Pooled Cohort Equations (PCE39) and Revised Pooled Cohort Equations (RPCE40) introduced by American College of Cardiology (ACC) and American Heart Association (AHA). Currently, the most widely praised clinical practice guidelines (Canadian Cardiovascular Society41, European Society of Cardiology/European Society of Hypertension42, ACC/AHA43, Joint British Societies recommendations on the prevention of Cardiovascular Disease44) are used to estimate the future risk of CVD applying the total/global/absolute risk score for CVDs. Over the past two decades, several randomised control trials have examined the effectiveness of CVD risk scores45–47. These charts include traditional cardiovascular risk factors, such as age, gender, smoking status, presence of diabetes, blood pressure, high-density lipoprotein- (HDL-C), and total cholesterol (TC) levels.
The present study was conducted in order to compare the 10-year cardiovascular risk prediction scores in the Hungarian general and Roma populations and to identify high-risk individuals who might be targeted by cost-effective cardiovascular interventions. In order to ensure the comparability of our data with findings obtained in other studies, we used the three most common risk estimation models/methods (FRS, SCORE, and ACC/AHA PCE) and their revised version for our study.
Material and methods
Study populations
Sample representative of the Hungarian Roma (HR) population living in segregated colonies in Northeast Hungary
The Hungarian Roma sample population was enrolled from Hajdú-Bihar and Szabolcs-Szatmár-Bereg counties in Northeast Hungary. The majority of the segregated Hungarian Roma population lives in these two counties. There were 92 segregated colonies identified and 25 of them were selected randomly using general practitioners' (GPs) validated household lists. Twenty individuals (one person per household between the ages of 20 and 64) were randomly chosen from each segregated colony and each of them was interviewed face-to-face by Roma ethnic university students under the supervision of a public health coordinator. In addition to this, they were invited to visit a GP for a physical examination and blood collection. The ethnicity of the study participants was identified based on self-declaration.
Sample representative of the Hungarian general (HG) population living in Northeast Hungary
The Hungarian general sample population consisted of people randomly selected from the same counties as the Hungarian Roma one. These people are between 20 and 64 years of age and were registered by GPs involved in the General Practitioners’ Morbidity Sentinel Stations Programme (GPMSSP). The GPMSSP was established in 1998 to monitor the incidence and prevalence of chronic non-communicable diseases of major public health importance48. Twenty GPs were randomly chosen and 25 individuals from each GP were included in the study. If a person could not be reached, a new one was included instead, but is someone refused to respond, no substitute person could be drawn. Further details on the study design and sample populations can be read in our previous research paper49. Briefly, based on the results obtained in a complex three pillars (questionnaire, physical and laboratory examinations) health (interview and examination) survey a database was created, which—among others—consists all the data necessary the CVD risk calculations in the models we used.
Regarding both study populations known pregnancy was an exclusion criterion during sample collection. Individuals diagnosed with any form of cardiovascular diseases (coronary heart disease, cerebrovascular disease, peripheral arterial disease, rheumatic heart disease, congenital heart disease, deep vein thrombosis and pulmonary embolism) were also excluded from the CVD risk score calculations.
Ethics declarations
All procedures performed in studies involving human participants were carried out by the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments. This study was approved by the Ethical Committee of the University of Debrecen, Medical Health Sciences Centre (reference No. 2462-2006), and by the Ethics Committee of the Hungarian Scientific Council on Health (reference No. 61327-2017/EKU). All participants provided written informed consent to participate in the study. This article does not contain any studies with animals performed by any of the authors.
Cardiovascular risk stratification models in our study
In the present study, we estimate the cardiovascular risk of Hungarian general and Roma populations in Northeast Hungary using the three most commonly used risk estimation models (FRS, SCORE, PCE) and their revised version. For more detailed characteristics of the cardiovascular risk assessment models used in the study see Table 1.
Table 1.
Characteristics of cardiovascular risk assessment models used in the study.
| Framingham Risk Scores (FRSs) | Systematic COronary Risk Evaluation (SCORE) | American College of Cardiology/American Heart Association Pooled Cohort Equations | |||
|---|---|---|---|---|---|
| Characteristics | ATPIII hard coronary heart disease (FRSCHD) | Cardiovascular diseases generally (FRSCVD) | High-risk algorithm | Original (PCE) | Revised (RPCE) |
| Year of development | 2002 | 2008 | 2003 | 2013 | 2018 |
| Age group for which applicable (years) | 30–75 | 30–75 | 40–65 | 40–79 | 40–79 |
| Populations for which was validated | Predominantly individuals of Western European descent living in United States of America | European countries with high CVD risk (e.g.: Hungary) | Multiethnic cohorts living in United States of America | ||
| Parameters included | Age, gender, total cholesterol, HDL-cholesterol, systolic blood pressure, BP treatment, and smoking status | Age, gender, total cholesterol, HDL-cholesterol, systolic blood pressure, BP treatment, diabetes, and smoking status | Age, gender, total cholesterol, HDL-cholesterol, systolic blood pressure, smoking status | Age, gender, race, total cholesterol, HDL-cholesterol, systolic blood pressure, BP treatment, diabetes, and smoking status | |
| Parameters excluded | Diabetes status, family history of CVD | Family history of CVD | BP treatment, diabetes status, family history of CVD | Family history of CVD | |
| Endpoints | CHD death and nonfatal MI | CHD death, nonfatal myocardial infarction, coronary insufficiency or angina, fatal or nonfatal stroke, and heart failure | Fatal atherosclerotic CVD events (including CHD, arrhythmia, heart failure, stroke, aortic aneurysm, and peripheral vascular disease) | CHD death, nonfatal MI, fatal stroke, nonfatal stroke | |
Framingham risk score for ATPIII hard coronary heart disease (FRSCHD) and for cardiovascular disease generally (FRSCVD)
The Framingham Risk Score calculation as a gender-specific algorithm was first developed to assess the 10-year risk of the development of coronary heart disease (CHD) for individuals with different combinations of risk factors based on data obtained from the Framingham Heart Study50. The Framingham Risk Score was modified (in 2002) by the third Adult Treatment Panel (ATP III) by the elimination of diabetes from the algorithm since it was considered to be a coronary heart disease (CHD) equivalent; broadening of the age range, by the inclusion of hypertension treatment, age-specific points for smoking and total cholesterol51. In our study, the ATPIII based Framingham Risk Score to estimate the 10-year risk of hard coronary heart disease was used.
The original 1998 and revised 2002 Framingham Risk Scores do not include all of the potential manifestations and adverse consequences of atherosclerosis, such as stroke, transient ischemic attack, claudication, and heart failure (although manifestations of aortic atherosclerosis were omitted). These patient-important vascular outcomes were included in the development of the 2008 Framingham general cardiovascular disease risk score, which was shown to have a reliable predictive ability37. The estimated risk of developing a cardiovascular event was higher when this risk score was used than in the case of those that predict only CHD events.
The analyses were performed for participants of the study populations aged 30–64, which were divided into the following 10-year risk categories: low (< 10%), intermediate (10–20%), or high (≥ 20%) categories.
Systematic COronary Risk Evaluation (SCORE)
SCORE, recommended in the 2007 European Society of Cardiology guidelines on cardiovascular disease prevention in clinical practice, included data on more than 205,178 patients pooled from cohort studies in 12 European countries, 3 million person-years of observation and 7,934 fatal CV events38,52. The SCORE model has been calibrated according to each European country’s mortality statistics. A unique aspect of SCORE is that separate risk scores were calculated for high- and low-risk regions of Europe. The predictive value of SCORE was high in each study cohort. Hungary is a high-risk country, and there is no separate data available for the Roma population, so we used the high-risk formula for both populations in our calculations.
SCORE differs from the earlier Framingham risk models (and others) in two important ways: it estimates the 10-year risk of any first fatal atherosclerotic event (e.g. stroke or ruptured abdominal aneurysm), not only CHD-related deaths, and it estimates CVD mortality. SCORE was counted by an online calculator tool53.
The analyses were performed for study populations aged 40–64, which were divided into the following categories: low (< 2%), intermediate (2–5%), or high (≥ 5%) SCORE risk category.
American College of Cardiology/American Heart Association Pooled Cohort Equations (PCE) and Revised Pooled Cohort Equations (RPCE)
For decades, FRS was the most widely used to estimate 10-year CVD risk in asymptomatic individuals. Nevertheless, FRS underestimates lifetime risk, especially in younger individuals with multiple risks and in women; moreover, it does not predict the risk of stroke54,55, which has made the introduction of the ACC/AHA Pooled Cohort Risk Equations necessary in order to overcome these limitations. In this system, diabetes mellitus was included as a predictor variable, and fatal and nonfatal stroke was added to the CVD endpoints39,56.
The 2013 American College of Cardiology/American Heart Association (ACC/AHA) pooled cohort equations model was developed from several United States cohorts and it includes different calculators for Caucasian and African American populations43. Outcomes are limited to both fatal and nonfatal CHD and stroke. From 2013, the PCE risk score has been recommended for use in the United States39,43.
Several cohorts of patients were used to develop the 2013 ACC/AHA cardiovascular risk calculator57, the first risk model which includes data from large populations of both Caucasian and African American patients. The model is based on the same parameters as the 2008 Framingham general CVD model, but in contrast to the 2008 Framingham model, it includes only hard endpoints (fatal and nonfatal MI and stroke). However, while the calculator appears to be well-calibrated in populations similar to those for which the calculator was developed, it has not been that accurate in other populations58.
A potential limitation of the ACC/AHA calculator is that a family history of premature CVD is not included in the model. This may result in underestimation of risk in patients with very strong family histories of cardiovascular events. Additionally, the ACC/AHA includes diabetes only as a yes/no choice. Issues that may affect the risk of diabetes include patient age, sex, other cardiovascular risk factors, duration of diabetes, and whether the patient has type 1 or type 2 diabetes mellitus are not considered.
The 2013 PCE for predicting 10-year atherosclerotic CVD risk has been criticized for overestimating risk. Outdated population data and statistical methods were considered as the main weaknesses of PCE. In 2018, a modified version was released that was described using other statistical methods and new populations. This Revised Pooled Cohort Equations model estimates the 10-year risk for atherosclerotic cardiovascular disease which is defined as coronary death or nonfatal myocardial infarction, or fatal or nonfatal stroke40.
The analyses we have performed for study populations aged 40–64, which were divided into the following categories: low (< 5%), borderline (5–7.49%), intermediate (7.5–19.99%), or high (≥ 20%) risk category for both risk models.
Statistical analyses
All statistical tests were conducted using IBM SPSS version 26 (IBM Company, Armonk, NY, USA) software. Mann–Whitney U tests were used to compare the age, systolic, and diastolic blood pressure, total cholesterol and, HDL-C level of the study populations. Mean and 95% confidence interval (95% CI) were used to describe continuous variables. Frequencies of categorical variables were statistically compared by using the chi-squared test. Generally, the conventional p threshold of 0.05 was used.
The following risk estimation models were calculated based on their formula using Microsoft Excel 2013 programme: FRSCHD, FRSCVD, PCE, and RPCE. For SCORE, the HeartScore online calculator was used53. The age group corresponding to the risk calculation model was selected specifically for each study population. Individuals diagnosed with any form of cardiovascular diseases (coronary heart disease, cerebrovascular disease, peripheral arterial disease, rheumatic heart disease, congenital heart disease, deep vein thrombosis and pulmonary embolism) were also excluded from the CVD risk score calculations. To avoid distorting effects the study populations were matched for age.
Results
Process of sample selection and prevalence of cardiovascular diseases in the Hungarian general and Hungarian Roma populations
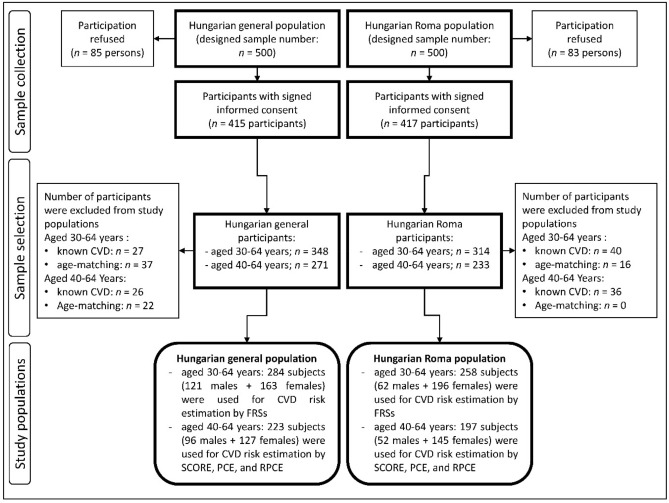
The prevalence of cardiovascular diseases was significantly higher in the HR population compared with the HG one (HR: 12.74% vs HG: 7.76%, p = 0.034) in the age group of 30–64 years. Significant difference (p = 0.046) was also observed in the 40–64-year-old age group, 18.27% had CVD in the Hungarian Roma and 10.61% in the Hungarian general population. After exclusion of individuals with any form of CVD (27 persons in the 30–64 age group and 26 persons in the 40–64 age group from the HG population; 40 persons in the 30–64 age group and 36 persons in the 40–64 age group from the HR one) during the process of age matching, 16 h and 37 HG individuals were excluded from the 30–64 age group, while 22 HG were excluded from the 40–64 age group. For more details on the process of sample selection see Fig. 1.
Figure 1.
Flowchart showing the process of sample selection for study populations.
Characteristics of study populations by age groups
Characteristics of 30–64-year-old age group
The characteristics of 30–64-year-old male populations significantly differed in the prevalence of smoking (HG: 35.54% vs. HR: 58.06%, p = 0.004, while female populations showed a significant difference in HDL-C levels (HG: 1.47 vs. HR: 1.29, p < 0.001), the prevalence of smoking (HG: 33.74% vs. HR: 68.37%, p < 0.001), and the prevalence of diabetes (HG: 8.59% vs. HR: 16.33%, p = 0.029). The prevalence of lipid-lowering therapy, indicating indirectly an increased risk of CVD, was higher in both sexes in the Roma population (HR: 12.90% vs. HG: 4.96%, p = 0.056 in males; HR: 11.22% vs. HG: 4.91%, p = 0.031 in females). See more detailed population characteristics in Table 2.
Table 2.
Characteristics of 30–64-year-old study populations involved in the Framingham Risk Score for hard coronary heart disease (FRSCHD) and Framingham Risk Score for cardiovascular disease (FRSCVD) calculations by ethnicity and sex.
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Hungarian general (n = 121) | Hungarian Roma (n = 62) | p-value | Hungarian general (n = 163) | Hungarian Roma (n = 196) | p-value | |
| Mean (95% CI) | Mean (95% CI) | |||||
| Age (years) | 47.98 (46.35–49.60) | 49.60 (47.11–52.08) | 0.244 | 47.31 (45.85–48.76) | 46.29 (45.03–47.55) | 0.274 |
| Total cholesterol levels (mmol/L) | 5.13 (4.96–5.31) | 5.05 (4.79–5.32) | 0.563 | 5.11 (4.94–5.28) | 5.13 (4.97–5.28) | 0.931 |
| HDL-C levels (mmol/L) | 1.31 (1.24–1.38) | 1.23 (1.14–1.33) | 0.211 | 1.47 (1.41–1.53) | 1.29 (1.24–1.34) | < 0.001 |
| Systolic blood pressure (mmHg) | 130.76 (128.43–133.08) | 130.98 (126.24–135.73) | 0.370 | 124.86 (122.65–127.07) | 124.94 (122.49–127.40) | 0.479 |
| Prevalence (%) | p-value | Prevalence (%) | p-value | |||
|---|---|---|---|---|---|---|
| Smokers | 35.54 (27.43–44.33) | 58.06 (45.65–69.75) | 0.004 | 33.74 (26.82–41.24) | 68.37 (61.62–74.57) | < 0.001 |
| Treated for high blood pressure | 25.62 (18.48–33.91) | 37.10 (25.88–49.49) | 0.107 | 31.90 (25.11–39.33) | 35.20 (28.77–42.07) | 0.510 |
| Diabetesa | 10.74 (6.16–17.18) | 9.68 (4.14–18.86) | 0.823 | 8.59 (5.01–13.62) | 16.33 (11.66–21.98) | 0.029 |
| Receiving lipid-lowering therapyb | 4.96% (2.10–9.93) | 12.90% (6.29–22.87) | 0.056 | 4.91 (2.35–9.05) | 11.22 (7.38–16.21) | 0.031 |
Significant differences in mean or prevalence rates are highlighted in bold.
aUsed only in the FRSCVD calculation.
bNot used for risk calculation in any risk assessment models.
Characteristics of 40–64-year-old age group
The characteristics of 40–64-year-old male populations of the HG and HR samples significantly differed in the prevalence of smoking (HG: 35.42% vs. HR: 57.69%, p = 0.009), while the female populations in HDL-C levels (HG: 1.48 vs. HR: 1.31, p < 0.001) and the prevalence of smoking (HG: 36.22% vs. HR: 65.52%, p < 0.001). The prevalence of lipid-lowering therapy, indicating indirectly an increased risk of CVD, was higher in both sexes in the Roma population (HR: 15.38% vs. HG: 6.25%, p = 0.069 in males; HR: 14.48% vs. HG: 6.30%, p = 0.029 in females). See more detailed population characteristics in Table 3.
Table 3.
Characteristics of 40–64-year-old study populations involved in the calculation of SCORE, PCE and, RPCE by ethnicity and sex.
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Hungarian general (n = 96) | Hungarian Roma (n = 52) | p-value | Hungarian general (n = 127) | Hungarian Roma (n = 145) | p-value | |
| Mean (95% CI) | Mean (95% CI) | |||||
| Age (years) | 51.36 (50.02–52.71) | 52.69 (50.69–54.70) | 0.292 | 51.02 (49.81–52.24) | 50.24 (49.14–51.35) | 0.354 |
| Total cholesterol levels (mmol/L) | 5.21 (5.00–5.41) | 5.12 (4.82–5.41) | 0.476 | 5.22 (5.04–5.40) | 5.31 (5.13–5.50) | 0.613 |
| HDL-C levels (mmol/L) | 1.33 (1.25–1.41) | 1.25 (1.14–1.35) | 0.212 | 1.48 (1.41–1.55) | 1.31 (1.25–1.37) | < 0.001 |
| Systolic blood pressure (mmHg) | 131.06 (128.51–133.61) | 133.08 (127.19–138.52) | 0.981 | 127.19 (124.69–129.70) | 128.73 (125.88–131.58) | 0.967 |
| Prevalence (%) | p-value | Prevalence (%) | p-value | |||
|---|---|---|---|---|---|---|
| Smokers | 35.42 (26.40–45.30) | 57.69 (44.15–70.40) | 0.009 | 36.22 (28.25–44.81) | 65.52 (57.53–72.89) | < 0.001 |
| Treated for high blood pressurea | 30.21 (21.71–39.88) | 48.46 (26.15–52.02) | 0.308 | 37.01 (28.98–45.62) | 40.69 (32.94–48.80) | 0.534 |
| Diabetesa | 12.50 (7.02–20.20) | 11.54 (4.96–22.24) | 0.864 | 11.02 (6.46–17.34) | 18.62 (12.94–25.54) | 0.081 |
| Receiving lipid-lowering therapyb | 6.25 (2.65–12.43) | 15.38 (7.55–26.94) | 0.069 | 6.30 (3.02–11.53) | 14.48 (9.48–20.89) | 0.029 |
Significant differences in mean or prevalence rates are highlighted in bold.
aNot used for SCORE chart calculation.
bNot used for risk calculation in any risk assessment models.
Comparison of estimated 10-year risk based on Framingham Risk Score for ATPIII hard coronary heart disease (FRSCHD) and for cardiovascular disease (FRSCVD) between Hungarian general and Roma populations
The average 10-year risk of CHD based on the calculated FRSCHD shows a significant difference between the Hungarian general and Roma males (HG: 7.23% vs. HR: 9.42%, p = 0.023) and females (HG: 1.79% vs. HR: 2.85%, p < 0.001; respectively) populations. In general, the average 10-year risk of CVD is significantly different based on the FRSCVD between the two observed female populations (HG: 6.38% vs. HR: 8.43%, p = 0.035) but not between the males (HG: 13.29% vs. HR: 16.88%, p = 0.075). See more details in Table 4.
Table 4.
Results obtained by Framingham Risk Score for hard coronary heart disease (FRSCHD) and Framingham Risk Score for cardiovascular disease (FRSCVD) calculations in the 30–64-year-old Hungarian general and Hungarian Roma populations.
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Hungarian general (n = 121) | Hungarian Roma (n = 62) | p-value | Hungarian general (n = 163) | Hungarian Roma (n = 196) | p-value | |
| Mean (95% CI) | Mean (95% CI) | |||||
| FRSCHD | 7.23 (6.20–8.25) | 9.84 (8.00–11.68) | 0.023 | 1.79 (1.43–2.16) | 2.85 (2.38–3.31) | < 0.001 |
| FRSCVD | 13.29 (11.45–15.13) | 16.88 (13.52–20.24) | 0.075 | 6.38 (5.57–7.18) | 8.43 (7.27–9.60) | 0.035 |
Significant differences in mean or prevalence rates are highlighted in bold.
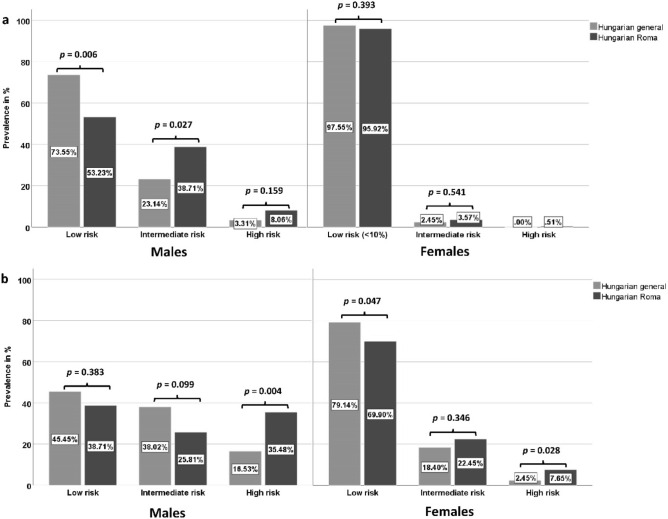
The relative frequency of low-, intermediate-, and high-risk subjects in the studied male populations differed significantly in cases of both Framingham Risk Score calculations (FRSCHD: p = 0.026; FRSCVD: p = 0.020). In case of FRSCHD, the representation of low-risk individuals was significantly lower (HG: 73.55% vs. HR: 53.23%, p = 0.006), while the intermediate- (HG: 23.14% vs. HR: 38.71%, p = 0.027) and high-risk (HG: 3.31% vs. HR: 8.06%, p = 0.159) subjects were more frequent in the Hungarian Roma male population than in the Hungarian general. In case of the FRSCVD, people with low- and intermediate cardiovascular risk were more frequent in the Hungarian general male population, while the prevalence of high-risk individuals was 2.15 times higher in the Roma population (HG: 16.53% vs. 35.48%, p = 0.004). The relative frequency of low-, intermediate-, and high-risk subjects in the studied female populations differed significantly in cases of the FRSCVD (p = 0.017) but not in the FRSCHD (p = 0.500), for more details see Fig. 2.
Figure 2.
Distribution of subjects by risk categories defined by Framingham Risk Score for hard coronary heart disease (FRSCHD-a) and Framingham Risk Score for cardiovascular disease (FRSCVD-b) calculations in the 30–64-year-old Hungarian general and Hungarian Roma populations.
Comparison of estimated 10-year risk based on Systematic COronary Risk Evaluation (SCORE) between Hungarian general and Roma populations
The average 10-year risk based on SCORE calculated by the HearthScore online tool is significantly different between Hungarian general and Hungarian Roma male populations (HG: 3.83% vs. HR: 5.35%, p = 0.036). No significant difference in risk was detected between the female populations (see more details in Table 5).
Table 5.
Results of SCORE in the 40–64-year-old Hungarian general and Hungarian Roma populations.
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Hungarian general (n = 96) | Hungarian Roma (n = 52) | p-value | Hungarian general (n = 127) | Hungarian Roma (n = 145) | p-value | |
| Mean (95% CI) | Mean (95% CI) | |||||
| SCORE | 3.83 (2.22–4.45) | 5.35 (3.97–6.72) | 0.036 | 1.28 (1.00–1.55) | 1.88 (1.50–2.25) | 0.098 |
Significant differences in mean or prevalence rates are highlighted in bold.
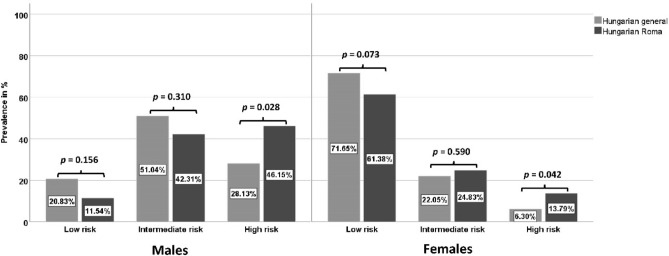
The relative frequency of risk categories in the studied male populations non-significantly differed (p = 0.068). The representation of the high-risk group was more than 1.5 times higher (HG: 28.13% vs. HR: 46.15%, p = 0.028) in the Hungarian Roma male population than in the general one. No significant difference was detected in the distribution profile of risk frequencies between the female populations (p = 0.082), however, there are significantly more people belonging to the high-risk group among Roma females than among Hungarians (HG: 6.30% vs. HR: 13.79%, p = 0.042), for more details see Fig. 3.
Figure 3.
Distribution of SCORE risk categories in the 40–64-year-old Hungarian general and Hungarian Roma populations.
Comparison of estimated 10-year risk based on ACC/AHA Pooled Cohort Equations (PCE) and Revised Pooled Cohort Equations (RPCE) between Hungarian general and Roma populations
The average 10-year risk based on PCE and RPCE show a significant difference between the Hungarian general and Roma male populations (PCE: HG: 7.81% vs. HR: 10.39%, p = 0.012; RPCE: HG: 6.64% vs. HR: 9.48%, p = 0.020). Estimated cardiovascular risk also showed significant difference in case of the study female populations (PCE: HG: 3.55% vs. 5.59%, p < 0.001; RPCE: 2.43% vs. 4.16%, p = 0.004). See more details in Table 6.
Table 6.
Results obtained by using ACC/AHA pooled cohort equations (PCE) and revised pooled cohort equations (RPCE) in the 40–64-year-old Hungarian general and Hungarian Roma populations.
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Hungarian general (n = 96) | Hungarian Roma (n = 52) | p-value | Hungarian general (n = 127) | Hungarian Roma (n = 145) | p-value | |
| Mean (95% CI) | Mean (95% CI) | |||||
| PCE | 7.81 (6.55–9.07) | 10.39 (8.38–12.41) | 0.012 | 3.55 (3.01–4.09) | 5.59 (4.75–6.43) | < 0.001 |
| RPCE | 6.64 (5.61–7.68) | 9.48 (7.34–11.63) | 0.020 | 2.43 (2.02–2.84) | 4.16 (3.31–5.00) | 0.004 |
Significant differences in mean or prevalence rates are highlighted in bold.
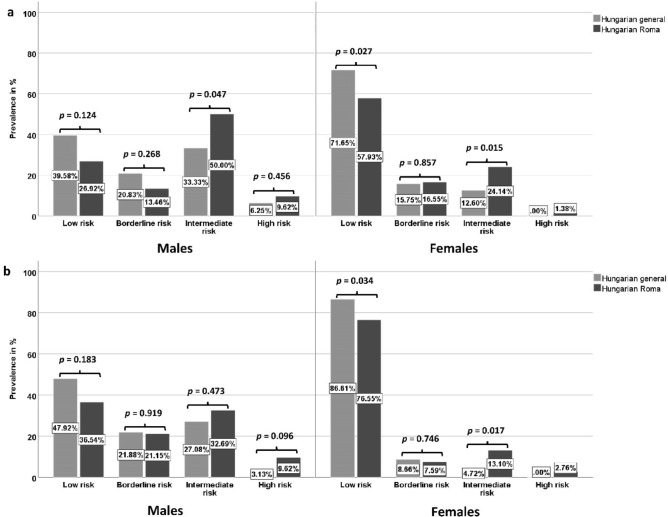
The relative frequency of low-, intermediate-, and high-risk subjects in the studied male populations did not differ significantly in either score calculations (PCE: p = 0.142 or RPCE: p = 0.262). The prevalence of the high-risk group was 1.5 times higher in case of PCE (HG: 6.25% vs. HR: 9.62%, p = 0.456) and three times in case of the RPCE (HG: 3.13 vs HR: 9.62, p = 0.096) in the Hungarian Roma male population than in the general one. There were significant differences in the relative frequency of risk groups between the female populations in the case of both versions of the Pooled Cohort Equations (PCE: p = 0.036 and RPCE: p = 0.022). The prevalence of the intermediate-risk group was significantly 2 times higher in case of PCE (HG: 12.60% vs. HR: 24.14%, p = 0.015) and 2.8 times in case of the RPCE (HG: 4.72% vs HR: 13.10%, p = 0.017) in the Hungarian Roma male population than in the general one. A high-risk individual occurred only in the Hungarian Roma male population (PCE: 1.38%; RPCE: 2.76%). See more details in Fig. 4.
Figure 4.
Distribution of subjects by risk categories defined by ACC/AHA pooled cohort equations (PCE-a) and revised pooled cohort equations (RPCE-b) in the 40–64-year-old Hungarian general and Hungarian Roma populations.
Discussion
The incidence and prevalence of cardiovascular diseases, as well as the mortality caused by them in the Hungarian population, are extremely high compared to other EU member states2. It is reasonable to suppose that the unfavourable morbidity and mortality figures strongly related to the fact that about 8–10% of the Hungarian population belongs to the Roma ethnic group. It is well documented that the prevalence of traditional cardiovascular risk factors is higher among the Roma than in the general population. Although previous studies have already estimated the frequency of cardiovascular risk factors among the Roma, no study has yet been conducted with the aim of examining the combined effects of these risk factors by using any of the CVD risk assessment models.
At present, there are multiple risk stratification algorithms in use to estimate the cardiovascular risk at both an individual and a population level. Our main goal was to perform a cardiovascular risk assessment in the Hungarian general and Roma populations and compare the results obtained.
To achieve this goal, the 10-year risk of developing coronary artery disease (based on Framingham Risk Scores), fatal atherosclerotic events (based on Systematic COronary Risk Evaluation), fatal coronary heart disease, nonfatal myocardial infarction, and fatal and non-fatal stroke (based on Pooled Cohort Equations) were estimated in both study populations.
Approximately half of all deaths in Hungary can be attributed to behavioural risk factors, such as alcohol consumption, smoking, poor diet, and low physical activity59. The proportion of these factors is well above the EU average of 39%. In 2014, the daily smoking rate was 25%, making it the third highest in the EU. Approximately every fifth death was attributable to tobacco consumption (direct or second-hand smoking). In 2017, every fifth adult was obese in Hungary, making adult obesity one of the highest in the EU. The proportion of the obese in the population has been increasing over the past decade. Overweight and obesity among children is also a growing problem, with nearly one in five 15-year-olds being overweight or obese in 2013 and 201460. In 2017, dietary risks (high sugar and salt intake, low fruit and vegetable consumption) were also responsible for nearly 28% (~ 34 000) of total mortality, which is 10% higher than the EU average59.
Our study confirms the fact that the prevalence of cardiovascular risk factors are higher among Roma. In the Roma population, we observed a significantly higher prevalence of smoking regardless of sex or age which is partly attributable to the fact that smoking is a part of the traditional Roma lifestyle61,62. The proportion of Roma females being under antidiabetic therapy (30–64-year old: HG: 8.59% vs. HR: 16.33% p = 0.029; 40–64-year old: HG: 11.02% vs. HR: 18.62%, p = 0.081) was higher furthermore the HDL-C level was significantly lower (30–64-year old: HG: 1.47 vs. HR: 1.29, p < 0.001; 40–64-year old: HG: 1.48 vs. HR: 1.31, p < 0.001) in both age groups. According to our previous research, the higher prevalence of low HDL-C levels among Roma is clearly due to genetic causes31–33, while the factors behind diabetes are due to ethnicity related ones34. Our results that smoking and low HDL-C levels are more common among Roma than in the general population are in harmony with those previously published on the topic13,15,16,20,23,25.
In our study, it was found that the 10-year cardiovascular risk of the Roma population independently from the gender is significantly higher compared to that of the Hungarian general one regardless of the risk estimator model used.
Classifying the population as low-, intermediate-, or high-risk groups from a cardiovascular point of view is an important strategy to identify individuals susceptible to serious cardiovascular events. It is also a useful way of identifying people whose health may be improved by preventive measures. Apart from being time- and cost-effective, this minor intervention can further reduce the chances of developing a cardiovascular disease63,64, therefore, decreases cardiovascular mortality. Hence, we can shift our focus on primary prevention methods (e.g.: reduction of smoking, taking aspirin, antihypertensive and cholesterol-lowering preventive medication in the indicated groups, and/or reducing body weight) instead of treating CVD65,66.
In the case of both sexes, it can be said that the share of individuals belonging to the high-risk group was significantly higher in the case of the Roma population compared to the Hungarian general one. In the case of the Roma population, the proportion of the high-risk group was on average 1.5–3 times higher than that of the Hungarian general one.
Our current study has its strengths and limitations. On the one hand, the accurate identification of ethnicity is a common challenge for studies like ours. Due to the criteria of sample selection used in our present study (individuals were excluded during the age-matching process and if they were diagnosed with any form of CVD), the sample population cannot be interpreted as a representative sample for the whole Hungarian general or Roma population. Given that the Hungarian general population may also include Roma individuals, the difference between the two groups may be underestimated. Risk assessment models used in the study include only traditional cardiovascular risk factors, in other words, age, gender, smoking and diabetic status, blood pressure, and cholesterol levels. This represents a major limitation when applying these equations to genetically susceptible people. The risk assessment models were validated/tested neither for the Hungarian general nor for the Roma population, so the reliability of our results might be questioned.
On the other hand, the study has several strengths. This is the first study to compare the cardiovascular risk burden of the Hungarian general and Roma populations. In our study samples were collected from the same geographical area, hence significant confounding factors on CVD risk, such as access to health care or specific environmental exposures can be excluded.
It is clear from our results that, regardless of the risk calculation method used, higher cardiovascular risk can be detected among the Hungarian Roma compared to the general population. The Roma living in Hungary should be considered highly endangered also from a cardiovascular point of view, and this should be taken into account especially when developing targeted cardiovascular interventions. The extremely high overall cardiovascular risk among Roma men should also be highlighted, which is in harmony with the high prevalence of premature mortality caused by CVDs among Roma67,68.
Author contributions
Conceptualization, P.P. and R.A.; data curation, P.P.; formal analysis, P.P.; methodology, P.P. and R.A.; supervision, R.A.; writing—original draft, P.P.; figures and tables preparation: P.P.; writing—review and editing, R.A.; project administration, Z.K., J.S. and R.A. All authors have read and agreed to the published version of the manuscript.
Funding
The project was co-financed by the European Union under European Regional Development Fund (GINOP-2.3.2-15-2016-00005), as well as by the Hungarian Academy of Sciences (TK2016-78). P.P. is recipient of a fellowship from the New National Excellence Program of the Ministry for Innovation and Technology (ÚNKP-19-3).
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to privacy/ethical restrictions but are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Collaborators, G. B. D. C. o. D. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Movsisyan NK, Vinciguerra M, Medina-Inojosa JR, Lopez-Jimenez F. Cardiovascular diseases in central and eastern Europe: A call for more surveillance and evidence-based health promotion. Ann. Glob. Health. 2020;86:21. doi: 10.5334/aogh.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roma equality, inclusion and participation in the EU: The new strategic framework for the equality, inclusion and participation of Roma in EU countries and preparation of the post-2020 initiative (European Commission, Brussels, 2020).
- 4.Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee, and the Committee of the Regions: An EU Framework for National Roma Integration Strategies up to 2020 (Belgium, 2011).
- 5.Population Census of Hungary in 2011 (Hungarian Central Statistical Office, Budapest, Hungary, 2011).
- 6.Estimates of Roma population in European Countries (Council of Europe, Strasbourg, France, 2014).
- 7.Kosa K, Darago L, Adany R. Environmental survey of segregated habitats of Roma in Hungary: A way to be empowering and reliable in minority research. Eur. J. Public Health. 2011;21:463–468. doi: 10.1093/eurpub/ckp097. [DOI] [PubMed] [Google Scholar]
- 8.Poverty and employment: The situation of Roma in 11 EU Member States (Publications Office of the European Union, Luxembourg, 2016).
- 9.Adany R. Roma health is global ill health. Eur. J. Public Health. 2014;24:702–703. doi: 10.1093/eurpub/cku143. [DOI] [PubMed] [Google Scholar]
- 10.Kosa K, Adany R. Studying vulnerable populations: Lessons from the Roma minority. Epidemiology. 2007;18:290–299. doi: 10.1097/01.ede.0000258919.15281.4f. [DOI] [PubMed] [Google Scholar]
- 11.Voko Z, et al. Does socioeconomic status fully mediate the effect of ethnicity on the health of Roma people in Hungary? J. Epidemiol. Community Health. 2009;63:455–460. doi: 10.1136/jech.2008.079715. [DOI] [PubMed] [Google Scholar]
- 12.Foldes ME, Covaci A. Research on Roma health and access to healthcare: State of the art and future challenges. Int. J. Public Health. 2012;57:37–39. doi: 10.1007/s00038-011-0312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobranici M, Buzea A, Popescu R. The cardiovascular risk factors of the Roma (gypsies) people in Central-Eastern Europe: A review of the published literature. J. Med. Life. 2012;5:382–389. [PMC free article] [PubMed] [Google Scholar]
- 14.Fedacko J, et al. Prevalence of cardiovascular risk factors in relation to metabolic syndrome in the Roma population compared with the non-Roma population in the eastern part of Slovakia. Cent. Eur. J. Public Health. 2014;22(Suppl):S69–74. doi: 10.21101/cejph.a3904. [DOI] [PubMed] [Google Scholar]
- 15.Weiss E, Japie C, Balahura AM, Bartos D, Badila E. Cardiovascular risk factors in a Roma sample population from Romania. Rom. J. Intern. Med. 2018;56:193–202. doi: 10.2478/rjim-2018-0010. [DOI] [PubMed] [Google Scholar]
- 16.Papon C, Delarche N, Le Borgne C, Bauduer F. Assessment of cardiovascular risk factors in a Roma community from Southwestern France. Am. J. Hum. Biol. 2017 doi: 10.1002/ajhb.22895. [DOI] [PubMed] [Google Scholar]
- 17.Kosa Z, et al. Prevalence of metabolic syndrome among Roma: A comparative health examination survey in Hungary. Eur. J. Pub. Health. 2015;25:299–304. doi: 10.1093/eurpub/cku157. [DOI] [PubMed] [Google Scholar]
- 18.Dolinska S, Kudlackova M, Ginter E. The prevalence of female obesity in the world and in the Slovak Gypsy women. Bratisl. Lek. Listy. 2007;108:207–211. [PubMed] [Google Scholar]
- 19.de Courten BV, et al. Higher prevalence of type 2 diabetes, metabolic syndrome and cardiovascular diseases in gypsies than in non-gypsies in Slovakia. Diabetes Res. Clin. Pract. 2003;62:95–103. doi: 10.1016/s0168-8227(03)00162-1. [DOI] [PubMed] [Google Scholar]
- 20.Krajcovicova-Kudlackova M, Blazicek P, Spustova V, Valachovicova M, Ginter E. Cardiovascular risk factors in young Gypsy population. Bratisl. Lek. Listy. 2004;105:256–259. [PubMed] [Google Scholar]
- 21.Carrasco-Garrido P, de Andres AL, Barrera VH, Jimenez-Trujillo I, Jimenez-Garcia R. Health status of Roma women in Spain. Eur. J. Public Health. 2011;21:793–798. doi: 10.1093/eurpub/ckq153. [DOI] [PubMed] [Google Scholar]
- 22.Zivkovic TB, et al. Screening for diabetes among Roma people living in Serbia. Croat. Med. J. 2010;51:144–150. doi: 10.3325/cmj.2010.51.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeljko H, et al. Traditional CVD risk factors and socio-economic deprivation in Roma minority population of Croatia. Coll. Antropol. 2008;32:667–676. [PubMed] [Google Scholar]
- 24.Enache G, et al. Prevalence of overweight and obesity in a roma population from Southern Romania-Calarasi County. Acta Endocrinol. (Buchar.) 2018;14:122–130. doi: 10.4183/aeb.2018.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hujova Z, et al. The prevalence of cigarette smoking and its relation to certain risk predictors of cardiovascular diseases in central-Slovakian Roma children and adolescents. Cent. Eur. J. Public Health. 2011;19:67–72. doi: 10.21101/cejph.a3621. [DOI] [PubMed] [Google Scholar]
- 26.Szabó MI, Balázs A, Máté B, Kelemen P. Low level of physical activity in two roma subgroups compared to non-Roma Population in Niraj Valley, Transylvania. J. Interdiscip. Med. 2019;4(1):20–28. doi: 10.2478/jim-2019-0002. [DOI] [Google Scholar]
- 27.Babinska I, et al. Does the population living in Roma settlements differ in physical activity, smoking and alcohol consumption from the majority population in Slovakia? Cent. Eur. J. Public Health. 2014;22(Suppl):S22–27. doi: 10.21101/cejph.a3897. [DOI] [PubMed] [Google Scholar]
- 28.Sudzinova A, et al. Roma coronary heart disease patients have more medical risk factors and greater severity of coronary heart disease than non-Roma. Int. J. Public Health. 2013;58:409–415. doi: 10.1007/s00038-013-0462-5. [DOI] [PubMed] [Google Scholar]
- 29.Piko P, Werissa NA, Fiatal S, Sandor J, Adany R. Impact of genetic factors on the age of onset for type 2 diabetes mellitus in addition to the conventional risk factors. J. Pers. Med. 2020 doi: 10.3390/jpm11010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiatal S, Piko P, Kosa Z, Sandor J, Adany R. Genetic profiling revealed an increased risk of venous thrombosis in the Hungarian Roma population. Thromb. Res. 2019;179:37–44. doi: 10.1016/j.thromres.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 31.Piko P, Fiatal S, Kosa Z, Sandor J, Adany R. Genetic factors exist behind the high prevalence of reduced high-density lipoprotein cholesterol levels in the Roma population. Atherosclerosis. 2017;263:119–126. doi: 10.1016/j.atherosclerosis.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 32.Piko P, Fiatal S, Kosa Z, Sandor J, Adany R. Generalizability and applicability of results obtained from populations of European descent regarding the effect direction and size of HDL-C level-associated genetic variants to the Hungarian general and Roma populations. Gene. 2019;686:187–193. doi: 10.1016/j.gene.2018.11.067. [DOI] [PubMed] [Google Scholar]
- 33.Piko P, et al. The effect of haplotypes in the CETP and LIPC genes on the triglycerides to HDL-C ratio and its components in the Roma and Hungarian general populations. Genes (Basel) 2020 doi: 10.3390/genes11010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Werissa NA, et al. SNP-based genetic risk score modeling suggests no increased genetic susceptibility of the roma population to type 2 diabetes mellitus. Genes (Basel) 2019 doi: 10.3390/genes10110942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagy K, Fiatal S, Sandor J, Adany R. Distinct penetrance of obesity-associated susceptibility alleles in the Hungarian General and Roma Populations. Obes. Facts. 2017;10:444–457. doi: 10.1159/000478094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Damen JA, et al. Prediction models for cardiovascular disease risk in the general population: Systematic review. BMJ. 2016;353:i2416. doi: 10.1136/bmj.i2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D'Agostino RB, Sr, et al. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 38.Conroy RM, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: The SCORE project. Eur. Heart J. 2003;24:987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 39.Karmali KN, Goff DC, Jr, Ning H, Lloyd-Jones DM. A systematic examination of the 2013 ACC/AHA pooled cohort risk assessment tool for atherosclerotic cardiovascular disease. J. Am. Coll. Cardiol. 2014;64:959–968. doi: 10.1016/j.jacc.2014.06.1186. [DOI] [PubMed] [Google Scholar]
- 40.Yadlowsky S, et al. Clinical implications of revised pooled cohort equations for estimating atherosclerotic cardiovascular disease risk. Ann. Intern. Med. 2018;169:20–29. doi: 10.7326/M17-3011. [DOI] [PubMed] [Google Scholar]
- 41.Anderson TJ, et al. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can. J. Cardiol. 2013;29:151–167. doi: 10.1016/j.cjca.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 42.Piepoli MF, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur. Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goff DC, Jr, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 44.Board, J. B. S. Joint British Societies' consensus recommendations for the prevention of cardiovascular disease (JBS3) Heart. 2014;100(Suppl 2):1–67. doi: 10.1136/heartjnl-2014-305693. [DOI] [PubMed] [Google Scholar]
- 45.Garg N, et al. Comparison of different cardiovascular risk score calculators for cardiovascular risk prediction and guideline recommended statin uses. Indian Heart J. 2017;69:458–463. doi: 10.1016/j.ihj.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Findlay SG, Kasliwal RR, Bansal M, Tarique A, Zaman A. A comparison of cardiovascular risk scores in native and migrant South Asian populations. SSM Popul. Health. 2020;11:100594. doi: 10.1016/j.ssmph.2020.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bansal M, Kasliwal RR, Trehan N. Comparative accuracy of different risk scores in assessing cardiovascular risk in Indians: A study in patients with first myocardial infarction. Indian Heart J. 2014;66:580–586. doi: 10.1016/j.ihj.2014.10.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Szeles G, et al. A preliminary evaluation of a health monitoring programme in Hungary. Eur. J. Pub. Health. 2005;15:26–32. doi: 10.1093/eurpub/cki107. [DOI] [PubMed] [Google Scholar]
- 49.Adany R, et al. Prevalence of insulin resistance in the Hungarian general and Roma populations as defined by using data generated in a complex health (interview and examination) survey. Int. J. Environ. Res. Public Health. 2020 doi: 10.3390/ijerph17134833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson PW, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 51.National Cholesterol Education Program Expert Panel on Detection, E. & Treatment of High Blood Cholesterol in, A Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106:3143–3421. doi: 10.1161/circ.106.25.3143. [DOI] [PubMed] [Google Scholar]
- 52.Graham I, et al. European guidelines on cardiovascular disease prevention in clinical practice: Executive summary: Fourth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by representatives of nine societies and by invited experts) Eur. Heart J. 2007;28:2375–2414. doi: 10.1093/eurheartj/ehm316. [DOI] [PubMed] [Google Scholar]
- 53.HeartScore: The interactive tool for predicting and managing the risk of heart attack and stroke (European Society of Cardiology, Brussels, Belgium, 2020).
- 54.Schlendorf KH, Nasir K, Blumenthal RS. Limitations of the Framingham risk score are now much clearer. Prev. Med. 2009;48:115–116. doi: 10.1016/j.ypmed.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 55.Marma AK, Lloyd-Jones DM. Systematic examination of the updated Framingham heart study general cardiovascular risk profile. Circulation. 2009;120:384–390. doi: 10.1161/CIRCULATIONAHA.108.835470. [DOI] [PubMed] [Google Scholar]
- 56.Preiss D, Kristensen SL. The new pooled cohort equations risk calculator. Can. J. Cardiol. 2015;31:613–619. doi: 10.1016/j.cjca.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 57.Heart Risk Calculator: Cardiovascular risk assessment in adults (10-year, ACC/AHA 2013) (Symcat, United States of America, 2017).
- 58.Lloyd-Jones DM, et al. Correction. J. Am. Coll. Cardiol. 2019;73:3234. doi: 10.1016/j.jacc.2019.05.010. [DOI] [Google Scholar]
- 59.Global Burden of Disease Study 2017 (Global Burden of Disease Collaborative Network, Institute for Health Metrics and Evaluation, Seattle, United States of America, 2018).
- 60.OECD, Systems, E. O. o. H. & Policies. Hungary: Country Health Profile 2019 (2019).
- 61.Paulik E, Nagymajtenyi L, Easterling D, Rogers T. Smoking behaviour and attitudes of Hungarian Roma and non-Roma population towards tobacco control policies. Int. J. Public Health. 2011;56:485–491. doi: 10.1007/s00038-011-0252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Petek D, Pavlic DR, Svab I, Lolic D. Attitudes of Roma toward smoking: Qualitative study in Slovenia. Croat. Med. J. 2006;47:344–347. [PMC free article] [PubMed] [Google Scholar]
- 63.Heidenreich PA, et al. Forecasting the future of cardiovascular disease in the United States: A policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 64.Teo KK, et al. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: A case–control study. Lancet. 2006;368:647–658. doi: 10.1016/S0140-6736(06)69249-0. [DOI] [PubMed] [Google Scholar]
- 65.Kahn R, Robertson RM, Smith R, Eddy D. The impact of prevention on reducing the burden of cardiovascular disease. Circulation. 2008;118:576–585. doi: 10.1161/CIRCULATIONAHA.108.190186. [DOI] [PubMed] [Google Scholar]
- 66.Akosah KO, Schaper A, Cogbill C, Schoenfeld P. Preventing myocardial infarction in the young adult in the first place: How do the National Cholesterol Education Panel III guidelines perform? J. Am. Coll. Cardiol. 2003;41:1475–1479. doi: 10.1016/s0735-1097(03)00187-6. [DOI] [PubMed] [Google Scholar]
- 67.Kohler IV, Preston SH. Ethnic and religious differentials in Bulgarian mortality, 1993–1998. Popul. Stud. (Camb.) 2011;65:91–113. doi: 10.1080/00324728.2010.535554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bogdanovic D, et al. Mortality of Roma population in Serbia, 2002–2005. Croat. Med. J. 2007;48:720–726. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to privacy/ethical restrictions but are available from the corresponding author on reasonable request.