Abstract
Due to the limited utility of Bacillus Calmette–Guérin (BCG), the only approved vaccine available for tuberculosis, there is a need to develop a more effective and safe vaccine. We evaluated the safety and efficacy of a dry powder aerosol (DPA) formulation of BCG encapsulated alginate particle (BEAP) and the conventional intradermal BCG immunization in infant rhesus macaques (Macaca mulatta). The infant macaques were immunized intratracheally with DPA of BEAP into the lungs. Animals were monitored for their growth, behaviour, any adverse and allergic response. The protective efficacy of BEAP was estimated by the ex-vivo H37Rv infection method. Post-immunization with BEAP, granulocytes count, weight gain, chest radiography, levels of liver secreted enzymes, cytokines associated with inflammation like TNF and IL-6 established that BEAP is non-toxic and it does not elicit an allergic response. The T cells isolated from BEAP immunized animals’ blood, upon stimulation with M.tb antigen, secreted high levels of IFN-γ, TNF, IL-6 and IL-2. The activated T cells from BEAP group, when co-cultured with M.tb infected macrophages, eliminated largest number of infected macrophages compared to the BCG and control group. This study suggests the safety and efficacy of BEAP in Non-human primate model.
Subject terms: Immunology, Diseases, Medical research
Introduction
BCG (Bacillus Calmette–Guérin) is a live attenuated form of Mycobacterium bovis and it is the only vaccine available and used for tuberculosis worldwide. It has been demonstrated that the BCG vaccine renders protective immunity in infants’ till up to 15 years of age1,2. However, it fails to protect in areas of high TB burden regions3.
There are a few new vaccine candidates in the pipeline4 but so far it has been challenging to provide protection any better than the BCG. In many of the vaccine candidates, mycobacterium similar to BCG have been evaluated3. Alternatively, BCG has been modified genetically to improve its efficacy5–8. New formulations and delivery routes have also been investigated9–11.
In this context, we had previously reported a unique dry powder aerosol (DPA) formulation of BCG12 as a potential vaccine candidate. This formulation is a BCG encapsulated alginate particle (BEAP), which is 2–5 μm in size and to be delivered as an aerosol. The formulation remains viable for 6 months at room temperature.
It is now generally accepted that the immunization by aerosol of BCG provides better protection against M.tb infection than the conventional intradermal immunization10,11. In our previous report, we have demonstrated that the alginate coated DPA formulation of BCG, the BEAP offers enhanced protection as compared to BCG aerosol immunization in mice12. To advance the study towards human applicability, the next obvious step is to investigate the performance of this formulation in a primate model like the Macaca mulatta since these animals manifest the full spectrum of disease conditions as in humans13.
Hence, in this pilot investigation, the dry powder formulation, BEAP was evaluated in Indian rhesus macaques (Macaca mulatta). To maintain similarity with the immunization schedule of BCG in humans, we compared the intratracheal immunization of BEAP in infants’ macaques with conventional intradermal BCG and un-immunized controls.
We assessed the safety of the BEAP formulation in infant macaques by monitoring behaviour and growth, liver function tests and cytokines level in serum for up to 12 months post immunization. The efficacy of the formulation was evaluated by an ex-vivo infection assay with M.tb H37Rv.
Results
Standardization of immunization procedures
The procedure of immunization in infant macaques was first evaluated in two adult macaques. One of the animals was immunized intradermally using a conventional BCG vaccine and the other was immunized intratracheally with DPA of BEAP. The intratracheal intubation and administration of DPA procedures were well tolerated by the animal. They were monitored up to 2 months post immunization during which they remained normal and healthy. Figure 1A shows levels of IFN-γ, IL-6, IL-4 and IL-2 in the serum of BCG/BEAP immunized adult animal on D0 and at 2 months post immunization. Normal levels of these cytokines in these animals confirmed no adverse reaction post immunization.
Figure 1.
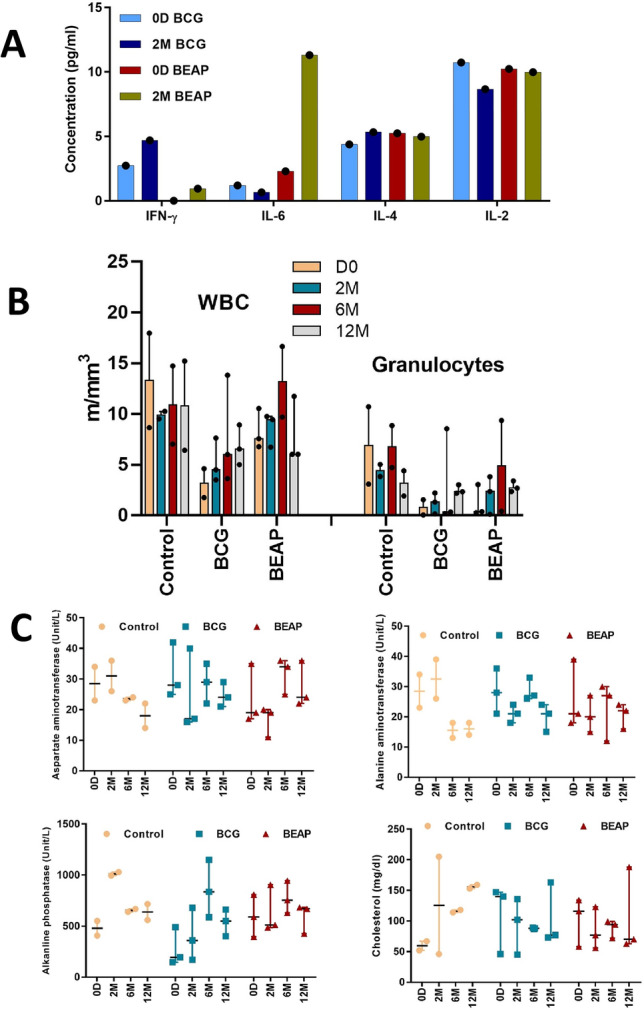
Evaluation of the toxicity or allergenicity of BEAP formulation. (A) Cytokine levels in the serum of BCG/BEAP immunized adult animal on D0 and at 2 months post immunization, (B) The granulocytes and WBC count in the control and post immunized infants. (C) Levels of liver secreted enzymes aspartate aminotransferase (AST), alanine aminotransferase (ALT) alkaline phosphatase (ALP) and cholesterol in the control and BCG/BEAP immunized infants at different time points. Dots indicate data points and bars are median with 95% confidence interval. Graphs were plotted using GraphPad Prism version-7 program (https://www.graphpad.com/).
BEAP is non-toxic and does not elicit an allergic response
The subsequent experiments were done on 8 macaque infants; 2 un-immunized controls, 3 BEAP immunized and 3 BCG immunized infants. Post-immunization all the animals were observed for any adverse effects due to the formulation. The blood and serum were checked for inflammatory or allergic response through the Complete blood count (CBC), cytokine profile and toxicity were checked through Liver Function Tests (LFT).
Granulocytes and WBC counts
CBC test was performed in fresh un-clotted blood of the control and BCG/BEAP immunized infants. The granulocyte and WBC count were in the normal range and suggested no adverse reactions post immunization (Fig. 1B) and Supplementary data Table 2.
LFT
Liver secreted enzymes, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and cholesterol production levels were checked in the serum of the control and BCG/BEAP immunized animals using biochemical assay kits.
The LFT profile revealed that in the majority of the animals, all the enzymes and cholesterol levels were found within the normal reference range (Fig. 1C), suggesting that there was no toxicity due to immunization.
Cytokines in Serum
Cytokines which are typically associated with inflammation like TNF, IL-6, IFN-γ and IL-2 were checked in serum and are shown in Fig. 2A. While only IL-6 was detectable on Day 0, all 4 cytokines showed a gradual increase in levels only after 2 months and detectable on 6 months and 12 months post immunization. The cytokine titres were generally higher in the BEAP immunized group. The m-RNA expression of these cytokine genes in the blood was also checked and is shown in Fig. 2B.
Figure 2.
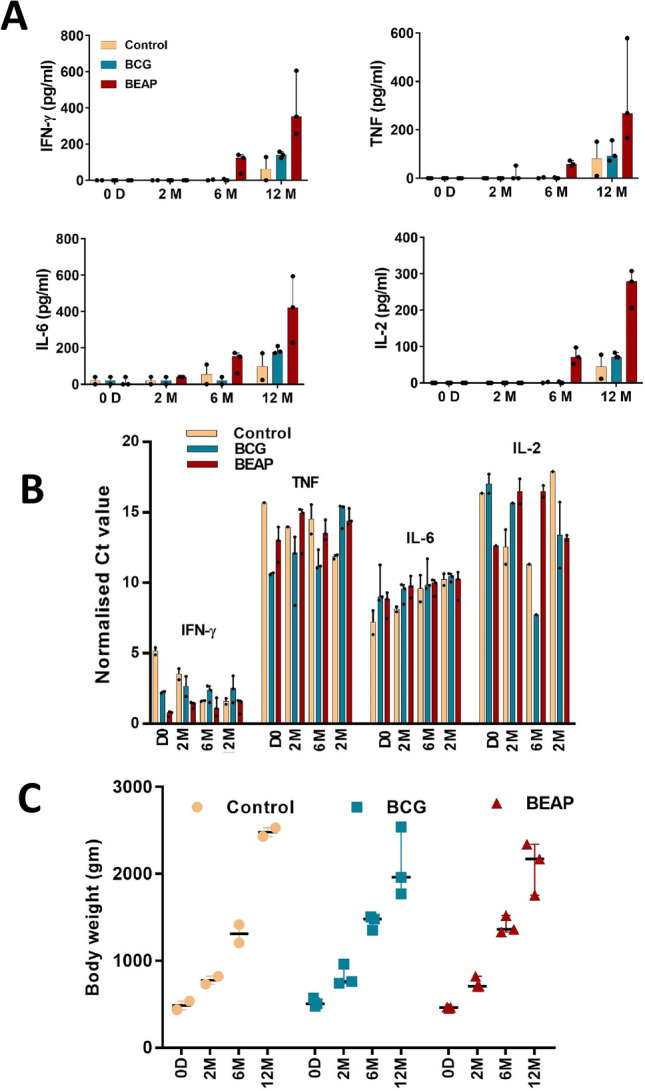
The non-toxic response of BCG/BEAP. (A) Cytokines levels, estimated by the cytometric bead array (BD Biosciences), in serum post immunization. (B) The m-RNA levels of the cytokine genes after normalization with the housekeeping gene GAPDH. (C) The progressive growth in terms of body weight of immunized and control animals up to 12 months. Dots indicate data points and bars are median with 95% confidence interval. Graphs were plotted using GraphPad Prism version-7 program (https://www.graphpad.com/).
CD4 + and CD8 + T cells count
The CD4 + and CD8 + cells were found to be elevated, which also explains the elevated cytokine levels. However, no pattern with respect to age and different time points was apparent. Details are described in the Supplementary data, Figure S1 and S2.
Growth of immunized macaque infants
The bodyweight of the control and the BCG/BEAP immunized infants were measured on the days of blood collection. It was observed that the total body weight of infants was progressive with no symptoms of weakness or weight loss in the immunized animals. The median body weight in grams of all infants on day 0, 2 months, 6 months, and 12 months were 470 interquartile range (IQR) 55 gm, 750 IQR 96 gm, 1387 IQR 141 gm and 2255 IQR 542 gm respectively (Fig. 2C).
Chest radiography
The postero-anterior chest X-ray of the control and immunized infants was done after 12 months of immunization. Both the lung fields and chest were clear in all the X-ray images. No abnormalities were seen.
Behaviour monitoring
The behaviour of the infants was observed after the immunization. It was observed that most of the time the infants were in contact with their mother. Proper milk feeding and food provided externally were also consumed by the infant suggesting no difficulty in swallowing. Moreover, no sign of any external infection or bruises was observed.
Assessment of the efficacy of BEAP
Stimulation of T cells with M.tb antigen
In order to compare the protective efficacy, lymphocytes isolated from blood, primarily comprising of T cells, were stimulated with M.tb antigen for 8 days and levels of INF-γ, TNF, IL-6 and IL-2 were estimated in the culture supernatant.
The difference in cytokine levels between immunized and control groups became more pronounced after 6 months of immunization and at 12 months the levels were the highest in the BEAP group (Fig. 3A), suggesting that in the BEAP immunized group there was enhanced activation of T cells.
Figure 3.
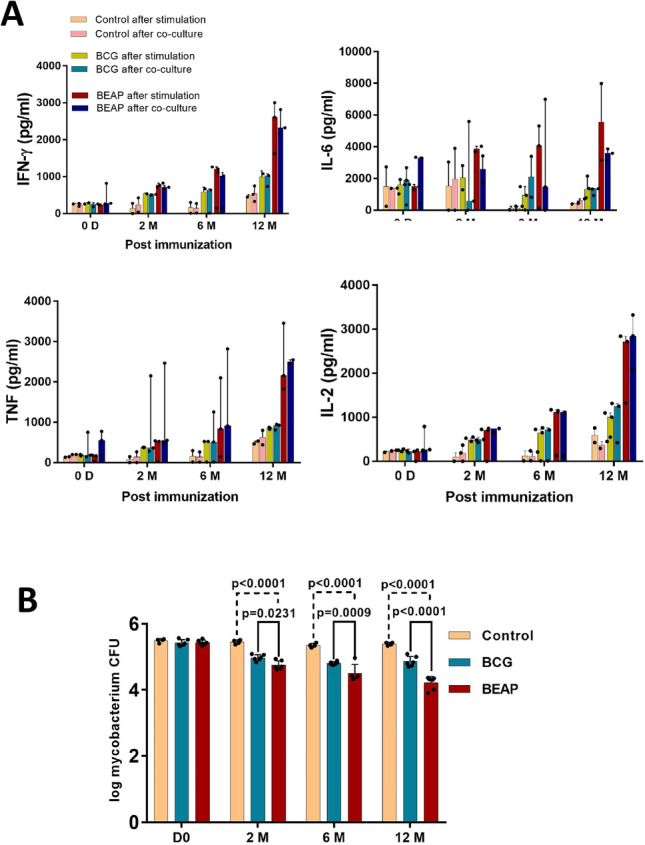
Efficacy and immune response elicited by BCG/BEAP. (A) The cytokine secretion profile in the supernatant of M.tb antigen stimulated T cells and when activated T cells were co-cultured with M.tb infected macrophages from the respective animals. Dots indicate data points and bars are median with 95% confidence interval. (B) Mycobacterium colony-forming units obtained after M.tb infected macrophages and activated T cells were co-cultured. The data demonstrate a substantial decrease in the mycobacterium CFU in the BEAP and BCG immunization compared to control animals. Dots indicate CFU counts calculated from multiple plates and bars are mean ± SD and analysis was done using two-way ANOVA with Tukey’s correction for multiple comparisons. Graphs were plotted using GraphPad Prism version-7 program (https://www.graphpad.com/).
Co-culture of activated T-cells and M.tb infected macrophages
M.tb antigen stimulated effector T cells, when co-cultured with M.tb infected macrophages, generate inflammatory cytokines that enhance the activity of macrophages to eliminate the mycobacteria. As observed in the T cells stimulation by M.tb antigen experiment, in the co-culture experiment too, low levels of cytokines were detected in the control group up to 12 months. Among the BEAP and BCG groups, higher secretion of cytokines was observed in the BEAP group.
After the co-culture, a 1.2 log decrease in the mycobacterium CFU was observed in the BEAP immunized group compared to the BCG group (0.56 log) between 0 day and 12 months (Fig. 3B). Overall, BEAP showed lowest number of mycobacteria CFU followed by the conventional intradermal BCG vaccination.
Discussion
In this study we investigated the safety and efficacy of a new BCG formulation, BEAP, which was delivered intra-tracheally in infant macaques and explored how BEAP could be compared with the conventional intradermal BCG immunization.
Before performing the intratracheal intubation and delivery of the DPA of BEAP in infants, the procedure was evaluated on an adult macaque. The protocol was well tolerated by the adult animal; the whole procedure was completed within a couple of minutes and no adverse reaction was observed for up to two months.
The immunization of infant macaque by BCG or BEAP did not cause an adverse reaction as there were no significant changes observed in the WBC and granulocytes counts post-immunization. Post immunization, the normal growth and behaviour of the animals, clear chest radiography and normal levels14 of liver secreted enzymes indicated the non-toxicity of BCG and BEAP in the macaque model.
The immunized animals had higher levels of INF-γ, TNF, IL-6 and IL-2 in the serum especially in the BEAP immunized group. Further, the genes for these cytokines were expressed in the WBCs (Fig. 2B) even though these cytokines were not detected in serum at 0 to 2 months’ time points after immunization. The increased cytokine levels did not show any adverse effect on the health of the macaques.
So far there is no reported ‘normal range’ of these cytokines in the serum after the BCG immunization in macaque infant. It is likely that the elevated but tolerable higher inflammatory condition caused by TNF and IL-6 post BCG or BEAP immunization would provide ‘non-specific’ innate immune protection against environmental antigens. On the other hand, higher levels of INF-γ and IL-2 post BCG immunization in children is a typically observed phenomena15 and it correlates with the degree of protection against M.tb infection16. Concurrently, we observed higher levels of IL-2 and INF-γ in BCG and BEAP immunized animals indicating the non-specific protection often attributed to BCG immunization17,18. The elevated cytokines indicate vaccine mediated prophylaxis which is imperative for innate immunity activation and subsequent generation of adaptive immunity15,19.
Upon stimulation of T cells with M.tb antigen, the resulting cytokine milieu leads to the proliferation of effector T cells which can effectively eliminate M.tb infected macrophages20,21. Such a response has been considered as an indication of the BCG vaccine generating a protective response22,23.
Our data suggests that upon immunization, animals would be able to generate optimal effector T cell response, wherein IL-2 mediates effector T cell proliferation, IL-6 mediates survival and INF-γ along with IL-6 mediate pleiotropic effect for T cell lineage shift to Th1 cells24–26. Similar findings have been made by Min et al27, where healthy and naturally M.tb infected rhesus monkey’s whole blood was stimulated with a purified protein derivative (PPD). They identified, IL-2, IL-6 and INF-γ among a few others as potential biomarker for tuberculosis.
It was observed that the effector T cells in BEAP and BCG immunized groups were efficient in eliminating M.tb compared to the control group (Fig. 3B). This correlates well with the elevated levels of inflammatory cytokines upon stimulation of T cells with M.tb antigen and co-culture of effector T cells with M.tb infected macrophages.
This assay is typically known as the mycobacterium growth inhibition assay (MGIA). Many reports advocate the advantages of this assay in vaccine testing against TB28–32 and this has enabled monitoring the immunological development in the same animals over time.
The aerosol immunization with BCG had raised some attention in the past33 and there have been a few attempts to evaluate the aerosol immunization with BCG in human subjects34. Barclay et al35 demonstrated higher degree of protection against TB infection in macaques after aerosol immunization with BCG. This was taken up more systematically by White et al.36 and they evaluated the immunogenicity of BCG delivered by the aerosol to the lungs of macaques. They examined the immune response post immunization in detail and demonstrated that after immunization there was an increase in the frequencies of IFN-γ, TNF-α and IL-2 producing cells in the peripheral blood mononuclear cells (PBMCs) and bronchoalveolar lavage (BAL) fluid. The trends of cytokines levels post immunization and overall conclusions of this study corroborate well with earlier findings.
While interpreting the efficacy of BEAP, it is important to be aware that the modulation of cytokine levels with age, post immunization or upon stimulation with antigens, is a complex phenomenon37 and cannot be linked directly to the immunogenicity or efficacy of a vaccine.
It is of considerable interest to compare immunization by the DPA of BEAP with a few alternative BCG candidate vaccines and delivery strategies.
MVA85A is a modified Vaccinia Ankara virus expressing antigen-85A candidate vaccine designed to boost BCG induced immunity38. This vaccine when delivered by the aerosol route was found to be safe and highly immunogenic in macaques39. Aerosol and intradermal booster immunization by MVA85A among BCG immunized people are in the advance stage of clinical trials40,41.
The intravenous (IV) injection of BCG has gained some attention recently but the IV injection of BCG to TB patients has been reported before the invention of antibiotics therapy42 and it is generally accepted that the IV administration of BCG provides enhanced protection against M.tb infection43. This has been confirmed by several investigators; Sharpe et al44 demonstrated that following intravenous immunization by BCG, the M.tb infection resulted in reduced disease pathology due to IFN-γ and TNF-α producing CD4 T cells. However, high frequencies of this cell population lead to increased pathology. More recently the role of systemic and tissue resident T cells after intravenous BCG immunization has also been emphasized45.
While investigating the intravenous routes, so far the major emphasis has been to achieve higher efficacy against M.tb infection and typically a high dose of BCG (around 107 CFUs) is investigated and recommended45. The safety aspect of such a dose is yet to be fully established as the higher frequency of IFN-γ producing CD4 + cells are also known to be responsible for pathogenesis and Tuberculosis-immune reconstitution inflammatory syndrome (TB-IRIS)46.
On the other hand, for the BEAP there are fewer safety concerns as only very few, 1000–1500 BCG bacilli are delivered; this is even fewer than conventional intradermal BCG immunization in which typically 5 × 105 – 5 × 107 BCG bacilli are administered. Moreover, the BEAP DPA delivery system will be free of the needle and a syringe.
Overall, our findings indicate that the BCG encapsulated alginate particle (BEAP) is a promising candidate vaccine for TB among a few others47–50. It would be of interest to investigate how without any genetic modification in the BCG, the BEAP presented enhanced protection. In most likelihood, it is a combination of several factors like the route of administration, alginate encapsulation of BCG which leads to efficient phagocytosis and activation of lung DCs12, size of particles etc., apart from the mechanism of the limited protection provided by the BCG itself which is not yet fully understood51.
This study confirms that when the BEAP is delivered as DPA to infant Rhesus macaques; it is likely to be non-toxic, does not generate a hypersensitive response and elicits an immune response which may provide protection from the TB infection. The ease of delivery and the longer shelf life of at least six months at room temperature are the additional advantages of BEAP12.
Limitation of the study
This pilot study gives encouraging leads to further elaborate the investigation. Since the number of subjects in each group is minimal (2 un-immunized controls, 3 BEAP immunized and 3 BCG immunized macaque infants), this needs to be further extended on more non-human subjects to make statistically significant comparisons of findings among different groups.
Secondly, the TC-MGIA findings are suggestive of expected outcomes; however, confirmation by in-vivo infection would add significantly to establish the pre-clinical safety and efficacy of BEAP vaccine formulation.
Methods
Preparation of BCG encapsulated alginate particle
The BEAP formulation was prepared and characterized as described in Nagpal et al. 201912. Briefly, 1 × 1010 live BCG bacilli was mixed in 50 ml solution of sodium alginate (Fluka, Catalog number: 71238) (1.23%) and trehalose (Sigma Aldrich, Catalog Number: 90210) (8.25%) solution. The mixture was filled in a nebulization assembly and aerosol was generated by a piston-based air pump. The generated aerosol was passed through a 5% calcium chloride solution resulting in the encapsulation of aerosol droplets (HiMedia, Catalog number: GRM3906). As a result, a gel-like solution was formed. It was centrifuged at 350 g for 10 min and washed three times with distilled water to completely remove the remaining calcium chloride. The final pellet was re-suspended in 5 ml distilled water and lyophilized. The lyophilized product was further processed through a jet mill (Sturtevant, USA) to obtain an inhalable powder of 3–5 µm particle size. The BEAP formulation was then characterized for its size, stability, viability and release profile of BCG.
Experimental animals and husbandry
The study was approved by the Institutional Animal Ethics Committee of the National Institute of Immunology (NII) file number (IAEC#316/13). All the methods were performed in accordance with the relevant guidelines and regulations under the supervision of a professional Veterinarian at the primate research facility of NII, New Delhi. All animal experiments and reporting adhere to the ARRIVE guidelines52.
Experiments were performed on two adults and eight infant macaques. One of the adult animals was immunized intradermally using a conventional BCG vaccine and the other was immunized intratracheally with DPA of BEAP. Three infants each were immunized either with BEAP intratracheally or with BCG intradermally. There were two control or unimmunized infants.
The infant mainly feeds on adult mother milk. Lactating mothers were fed with an adequately nutritious diet every day which comprises of soaked gram seed (75–100 gm), bread slices (4–5 slices), dry pellet diet (100 gm), and fresh vegetables/fruits (500–600 gm); vitamins/calcium supplements were given alternatively five times a week. The drinking water was supplied ad libitum to the individual cages. Adult animals were fed similarly except bread slices (3–4 slices), fresh vegetables/fruits (450–500 gm); vitamins/calcium supplements were given alternatively twice in a week. The infants were kept with their mother in temperature-controlled rooms maintained in proper hygiene. For all the procedures, animals were sedated by intramuscular injection with ketamine hydrochloride (Ketajex 50 mg/ml, Claris injectable limited, India) at a dose of 5–10 mg per kg of body weight depending on the experimental needs. None of the animals had previously been used for any other experimental procedures. Before immunization, animals were weighed and physically examined for any gross abnormalities and were monitored for behavioural and clinical changes post immunization.
Immunization
BCG immunization: Two-week old infants or adults were immunized via the intradermal route on the thoracic dorsal area with a single dose of freeze-dried live attenuated BCG Danish strain 1331 vaccine (B.No. 109006; GreenSignal, BioPharma Pvt Ltd, India). The lyophilized vaccine was re-constituted with the diluent provided by the manufacturer and 0.1 ml of the suspension was administered intradermally. The site was monitored for any local reaction after the vaccination.
BEAP immunization: Two-week-old Rhesus macaque infants or adults were immunized via the intratracheal route. The animals were anesthetized using ketamine hydrochloride injection. The animal was rested on a horizontal clean surface after ensuring the animal is fully anesthetized. The mouth of the infant was opened and its tongue was gently pulled out to visualise the epiglottis and tracheal opening of the infant. The salivary secretions were cleaned with sterile cotton gauzes. The intubation was guided and performed by using an otoscope. A sterile Poly vinyl chloride (PVC) tube of outer diameter 1.60 mm and an inner diameter of 0.80 mm was inserted into the trachea of the animal. The tracheal intubation was confirmed by placing a mirror at the outer end of the tube which became foggy due to moisture of warmer exhaled air. The BEAP formulation was administered through the tube into the lung of the animal by a 1 ml syringe using an in-house designed aerosol delivery apparatus12. Briefly, the aerosol of BEAP was generated and filled in a 10 ml syringe. By using a three-way valve, 1 ml of air saturated with BEAP aerosol was withdrawn from the 10 ml aerosol filled syringe and delivered into the infant’s lung.
This procedure was repeated 3 times followed by 3 additional pushes of clean air. Typically, the amount of BEAP transferred contained 1000–1500 BCG CFU. After the delivery of BEAP, the animal was laid on a flat surface until the animal recovered from anaesthesia and subsequently housed with the mother.
Blood and serum collection
The blood was collected from the animals in citrate phosphate dextrose (CPD) anticoagulant containing syringe at zero-day (day of immunization), 2 months, 6 months, and 12 months post immunization. During the standardization of immunization procedures performed on adults, animal’s blood was collected only on the day of immunization and at 2 months post immunization. For serum isolation blood was collected in a vial without CPD. The serum was isolated by spinning the coagulated blood at 12,000 g for 10 min at 4 °C and stored at -80 °C until further analysis.
Cytokine analysis
The cytometric bead array (CBA) (BD Non-Human Primate (NHP) Th1/Th2 Cytokine Kit (Catalog No. 557800) was used to quantify the cytokine levels in the serum and the culture supernatant. The assay was performed as per the manufacturer’s protocol. Briefly, 10 µl of capture beads for each cytokine (IFN-γ, TNF, IL-6, IL-2) per analyte were mixed to prepare a cocktail of capture beads. Similarly, a cocktail of detection antibody for each cytokine was also prepared. All the standards provided with the kit for each cytokine, were mixed in 2 ml assay diluent and incubated at RT for 15 min. Next, in each analyte tube 60 µl of capture beads cocktail and 60 µl of sample/standard, was mixed. Finally, 60 µl of PE detection antibody was added. The mixture was incubated at RT for 3 h in dark. In the last step, the mixture was washed with 1000 µl wash buffer and re-suspended in 300 µl wash buffer. The stained sample was run in the flow cytometer BD FACSCanto. The results were analysed using FCAP array V3 software(https://www.bdbiosciences.com/us/applications/research/bead-based-immunoassays/analysis-software/fcap-array-software-v30/p/652099#).
RNA isolation
RNA was isolated from the WBCs of the infants using the MasterPure Complete DNA and RNA Purification kit (Lucigen, Catalog Number: MC85200) as per the manufacturer’s instruction. The RNA pellet obtained was resuspended in TE buffer and quantified on a nano-drop machine. One µg of RNA was used to synthesize cDNA.
cDNA synthesis and qRT-PCR
RT-PCR was performed to quantitate the mRNA expression level of different cytokine genes. For this cDNA was synthesized from the 1 µg RNA using an iScript cDNA Synthesis kit (Bio-Rad, Catalog number: 1708891) as per the manufacturer’s instructions. The expression of the cytokine genes was confirmed by quantitative PCR using GeneSure SYBR Green qPCR Master Mix (Puregene, Catalog number: PGK025-A). The following PCR program was used: initial denaturation for 10 min (single step) at 95 °C, followed by 40 cycles of, denaturation at 95 °C for 15 s, annealing between 60.2 °C and 65 °C for 15 s, extension at 72 °C for 15 s followed by a single-step melting curve. The primers for different cytokine genes having specificity to Rhesus were designed by Beacon Designer 7.7 software (http://www.premierbiosoft.com/molecular_beacons/). The sequence of the primers is given in Supplementary data Table 1.
T-cell based Mycobacterium growth inhibition assay (TC-MGIA)
The TC-MGIA is an advanced technique similar to PBMC-MGIA used to study vaccine-mediated host immune response ex-vivo and was employed to analyse the efficacy of BCG and BEAP formulation in infants. The assay was performed according to Worku and Hoft20 with some modifications. Briefly, 2 ml of blood from the immunized or control infant was collected in syringes containing CPD anticoagulant. The PBMC was isolated from the blood using HiSep LSM 1077 (HiMedia, Catalog number: LS001) based density gradient centrifugation. Cells from the buffy layer were collected and washed with phosphate buffer saline (PBS) (HiMedia, Catalog number: TS1006) and plated in a TC-25 flasks (Corning, Catalog number: 430639) for 3 h to allow the monocytes to adhere. The non-adherent cells which contain the T cell, was collected and were stimulated with M.tb H37Rv antigen (2 µg/ml) in IMDM media (HiMedia, Catalog number: AL070) containing 10% FBS (Gibco, Catalog number: 10270), 1% antibiotics and anti mycotic cocktail (HiMedia, Catalog number: A0021) for 8 days. The adherent monocytes were harvested by trypsinization and seeded on collagen-coated 96 well plates (Corning, Catalog number: 3599) (0.13 × 106 cells per well) in IMDM media) containing 10% FBS, 1% antibiotics and anti mycotic cocktail, and 30 ng MCSF growth factor (Prospec, Catalog number: CYT-308). Cells were incubated at 37 °C, 5% CO2, and 85–90% humidity for 6 days to differentiate into macrophages. After 6 days, the macrophages were infected with M.tb H37Rv with a multiplicity of infection (MOI) of 1:10 for 6 h. Cells were then washed and incubated for 48 h and co-cultured with M.tb antigen-stimulated T cell for 48 h. Finally, the mycobacterium growth inhibition was checked by lysing the infected macrophages with 0.2% saponin and plating on Middlebrook 7H11 agar plates. Plating was done in triplicate and at least at 3 log dilutions. Plates were incubated at 37 °C for 2–3 weeks and the resulting colonies were counted.
Haematology, LFT and chest X-ray
The haematology and Liver function tests were performed after immunization BCG/BEAP vaccine. Haematology was performed with fresh blood using Haematology Analyzer MS 4e Automated Cell Counter. The LFT and cholesterol were checked in the serum using respective enzymatic assay kits (Coral Clinical system, India) as per the manufacturer’s protocol. A postero-anterior (PA) chest X-ray was performed in-house.
Statistical analysis
All the data were statistically analysed using GraphPad Prism version-7 program (https://www.graphpad.com/). Data was plotted as median with 95% confidence interval, except Fig. 3B in which mean ± standard deviation (SD) was plotted and the ANOVA variance test using Turkey’s correction was made among the groups.
Supplementary Information
Acknowledgements
This work was supported by a grant received from the Department of Science and Technology, Government of India (SB/SO/HS/204/2013) and the core grant received from the Department of Biotechnology, Government of India to National Institute of Immunology, New Delhi. The support by Dr. Subeer Majumdar, then in-charge of Primate Research Centre, NII is gratefully acknowledged. Mr. RK Thapa, Mr. Rajesh Kumar and Mr. JP Bhardwaj assisted in animal experiments.
Author contributions
A.K. and P.U. conceived, conducted experiments and analyzed data. A.K., S.S. and P.U. conducted animal experiments; A.K., Pa.S., K.J., Pr.S. and K.V.M. conducted laboratory experiments; P.S.N. and P.U. standardized BEAP formulation. Manuscript was written and edited by A.K., Pr.S. and P.U. S.S., R.Z., P.N. and P.U. supervised the study. All authors read and approved final manuscript.
Data availability
All data generated or analyzed during this study are included in the manuscript and its supplementary data.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-82614-5.
References
- 1.de Gijsel D, von Reyn CF. A breath of fresh Air: BCG prevents adult pulmonary tuberculosis. Int. J. Infect. Dis. 2019;80S:S6–S8. doi: 10.1016/j.ijid.2019.02.036. [DOI] [PubMed] [Google Scholar]
- 2.Roy P, et al. Potential effect of age of BCG vaccination on global paediatric tuberculosis mortality: a modelling study. Lancet Glob. Health. 2019;7:e1655–e1663. doi: 10.1016/S2214-109X(19)30444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.John TJ. Tuberculosis control in India: Why are we failing? Indian Pediatr. 2014;51:523–527. doi: 10.1007/s13312-014-0440-x. [DOI] [PubMed] [Google Scholar]
- 4.Kaufmann SHE. Vaccination against tuberculosis: revamping BCG by molecular genetics guided by immunology. Front Immunol. 2020;11:316. doi: 10.3389/fimmu.2020.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horwitz MA, Harth G. A new vaccine against tuberculosis affords greater survival after challenge than the current vaccine in the guinea pig model of pulmonary tuberculosis. Infect Immun. 2003;71:1672–1679. doi: 10.1128/iai.71.4.1672-1679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain R, et al. Enhanced and enduring protection against tuberculosis by recombinant BCG-Ag85C and its association with modulation of cytokine profile in lung. PLoS ONE. 2008;3:e3869. doi: 10.1371/journal.pone.0003869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugawara I, Li Z, Sun L, Udagawa T, Taniyama T. Recombinant BCG Tokyo (Ag85A) protects cynomolgus monkeys (Macaca fascicularis) infected with H37Rv Mycobacterium tuberculosis. Tuberculosis (Edinb) 2007;87:518–525. doi: 10.1016/j.tube.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Sun R, et al. Novel recombinant BCG expressing perfringolysin O and the over-expression of key immunodominant antigens; pre-clinical characterization, safety and protection against challenge with Mycobacterium tuberculosis. Vaccine. 2009;27:4412–4423. doi: 10.1016/j.vaccine.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 9.Aguilo N, et al. Pulmonary but not subcutaneous delivery of BCG vaccine confers protection to tuberculosis-susceptible mice by an interleukin 17-dependent mechanism. J Infect. Dis. 2016;213:831–839. doi: 10.1093/infdis/jiv503. [DOI] [PubMed] [Google Scholar]
- 10.Darrah PA, et al. Aerosol vaccination with AERAS-402 elicits robust cellular immune responses in the lungs of rhesus macaques but fails to protect against high-dose Mycobacterium tuberculosis challenge. J. Immunol. 2014;193:1799–1811. doi: 10.4049/jimmunol.1400676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manjaly Thomas ZR, McShane H. Aerosol immunisation for TB: matching route of vaccination to route of infection. Trans. R. Soc. Trop. Med. Hyg. 2015;109:175–181. doi: 10.1093/trstmh/tru206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagpal, P. S., Kesarwani, A., Sahu, P. & Upadhyay, P. Aerosol immunization by alginate coated mycobacterium (BCG/MIP) particles provide enhanced immune response and protective efficacy than aerosol of plain mycobacterium against M.tb. H37Rv infection in mice. BMC Infect. Dis.19, 568, 10.1186/s12879-019-4157-2 (2019). [DOI] [PMC free article] [PubMed]
- 13.Kaushal D, Mehra S, Didier PJ, Lackner AA. The non-human primate model of tuberculosis. J. Med. Primatol. 2012;41:191–201. doi: 10.1111/j.1600-0684.2012.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernie S, Wrenshall E, Malcolm S, Bryce F, Arnold DL. Normative hematologic and serum biochemical values for adult and infant rhesus monkeys (Macaca mulatta) in a controlled laboratory environment. J. Toxicol. Environ. Health. 1994;42:53–72. doi: 10.1080/15287399409531863. [DOI] [PubMed] [Google Scholar]
- 15.Lalor MK, et al. Complex cytokine profiles induced by BCG vaccination in UK infants. Vaccine. 2010;28:1635–1641. doi: 10.1016/j.vaccine.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Q, et al. IL-17 and IFN-gamma production in peripheral blood following BCG vaccination and Mycobacterium tuberculosis infection in human. Eur. Rev. Med. Pharmacol. Sci. 2012;16:2029–2036. [PubMed] [Google Scholar]
- 17.Aaby P, et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J. Infect. Dis. 2011;204:245–252. doi: 10.1093/infdis/jir240. [DOI] [PubMed] [Google Scholar]
- 18.Biering-Sorensen S, et al. Small randomized trial among low-birth-weight children receiving bacillus Calmette-Guerin vaccination at first health center contact. Pediatr. Infect. Dis. J. 2012;31:306–308. doi: 10.1097/INF.0b013e3182458289. [DOI] [PubMed] [Google Scholar]
- 19.Covian C, et al. BCG-Induced Cross-Protection and Development of Trained Immunity: Implication for Vaccine Design. Front Immunol. 2019;10:2806. doi: 10.3389/fimmu.2019.02806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Worku S, Hoft DF. In vitro measurement of protective mycobacterial immunity: antigen-specific expansion of T cells capable of inhibiting intracellular growth of bacille Calmette-Guerin. Clin Infect Dis. 2000;30(Suppl 3):S257–261. doi: 10.1086/313887. [DOI] [PubMed] [Google Scholar]
- 21.Worku S, Hoft DF. Differential effects of control and antigen-specific T cells on intracellular mycobacterial growth. Infect. Immun. 2003;71:1763–1773. doi: 10.1128/iai.71.4.1763-1773.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Black GF, et al. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet. 2002;359:1393–1401. doi: 10.1016/S0140-6736(02)08353-8. [DOI] [PubMed] [Google Scholar]
- 23.Abebe F. Is interferon-gamma the right marker for bacille Calmette-Guerin-induced immune protection? The missing link in our understanding of tuberculosis immunology. Clin. Exp. Immunol. 2012;169:213–219. doi: 10.1111/j.1365-2249.2012.04614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoyer KK, Dooms H, Barron L, Abbas AK. Interleukin-2 in the development and control of inflammatory disease. Immunol. Rev. 2008;226:19–28. doi: 10.1111/j.1600-065X.2008.00697.x. [DOI] [PubMed] [Google Scholar]
- 25.Ladel CH, et al. Lethal tuberculosis in interleukin-6-deficient mutant mice. Infect. Immun. 1997;65:4843–4849. doi: 10.1128/IAI.65.11.4843-4849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez AN, Mehra S, Kaushal D. Role of interleukin 6 in innate immunity to Mycobacterium tuberculosis infection. J. Infect. Dis. 2013;207:1253–1261. doi: 10.1093/infdis/jit037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Min F, Wang J, Huang S, Pan J, Zhang L. In vitro responses of multiple cytokines to purified protein derivative in healthy and naturally Mycobacterium tuberculosis-infected rhesus monkeys (Macaca mulatta) J. Med. Primatol. 2019;48:329–337. doi: 10.1111/jmp.12433. [DOI] [PubMed] [Google Scholar]
- 28.28Brennan, M. J. et al. The Cross-Species Mycobacterial Growth Inhibition Assay (MGIA) Project, 2010–2014. Clin. Vaccine Immunol.10.1128/CVI.00142-17 (2017). [DOI] [PMC free article] [PubMed]
- 29.Kolibab K, et al. A practical in vitro growth inhibition assay for the evaluation of TB vaccines. Vaccine. 2009;28:317–322. doi: 10.1016/j.vaccine.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 30.30Marsay, L. et al. Mycobacterial growth inhibition in murine splenocytes as a surrogate for protection against Mycobacterium tuberculosis (M. tb). Tuberculosis (Edinb)93, 551–557, 10.1016/j.tube.2013.04.007 (2013). [DOI] [PubMed]
- 31.Parra M, et al. Development of a murine mycobacterial growth inhibition assay for evaluating vaccines against Mycobacterium tuberculosis. Clin. Vaccine. Immunol. 2009;16:1025–1032. doi: 10.1128/CVI.00067-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zelmer A, et al. A new tool for tuberculosis vaccine screening: Ex vivo Mycobacterial Growth Inhibition Assay indicates BCG-mediated protection in a murine model of tuberculosis. BMC Infect. Dis. 2016;16:412. doi: 10.1186/s12879-016-1751-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Middlebrook G. Immunological aspects of airborne infection: reactions to inhaled antigens. Bacteriol. Rev. 1961;25:331–346. doi: 10.1128/BR.25.3.331-346.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenthal SR, McEnery JT, Raisys N. Aerogenic BCG vaccination against tuberculosis in animal and human subjects. J. Asthma Res. 1968;5:309–323. doi: 10.3109/02770906809100348. [DOI] [PubMed] [Google Scholar]
- 35.Barclay WR, et al. Protection of monkeys against airborne tuberculosis by aerosol vaccination with bacillus Calmette-Guerin. Am. Rev. Respir. Dis. 1973;107:351–358. doi: 10.1164/arrd.1973.107.3.351. [DOI] [PubMed] [Google Scholar]
- 36.White AD, et al. Evaluation of the immunogenicity of mycobacterium bovis BCG delivered by aerosol to the lungs of macaques. Clin. Vaccine. Immunol. 2015;22:992–1003. doi: 10.1128/CVI.00289-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Decker ML, Grobusch MP, Ritz N. Influence of age and other factors on cytokine expression profiles in healthy children-a systematic review. Front Pediatr. 2017;5:255. doi: 10.3389/fped.2017.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McShane H, Brookes R, Gilbert SC, Hill AV. Enhanced immunogenicity of CD4(+) t-cell responses and protective efficacy of a DNA-modified vaccinia virus Ankara prime-boost vaccination regimen for murine tuberculosis. Infect. Immun. 2001;69:681–686. doi: 10.1128/IAI.69.2.681-686.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White AD, et al. Evaluation of the safety and immunogenicity of a candidate tuberculosis vaccine, MVA85A, delivered by aerosol to the lungs of macaques. Clin. Vaccine Immunol. 2013;20:663–672. doi: 10.1128/CVI.00690-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tameris MD, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013;381:1021–1028. doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manjaly Thomas ZR, et al. Alternate aerosol and systemic immunisation with a recombinant viral vector for tuberculosis, MVA85A: A phase I randomised controlled trial. PLoS Med. 2019;16:e1002790. doi: 10.1371/journal.pmed.1002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.42Holmgren, I. (1936). Employment of BCG, especially in intravenous injection. Acta Medica Scandinavica, 90(S78), 350–361. 10.1111/j.0954-6820.1936.tb15958.x (1936).
- 43.Barclay WR, Anacker RL, Brehmer W, Leif W, Ribi E. Aerosol-induced tuberculosis in subhuman primates and the course of the disease after intravenous BCG vaccination. Infect. Immun. 1970;2:574–582. doi: 10.1128/IAI.2.5.574-582.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharpe S, et al. Alternative BCG delivery strategies improve protection against Mycobacterium tuberculosis in non-human primates: Protection associated with mycobacterial antigen-specific CD4 effector memory T-cell populations. Tuberculosis (Edinb) 2016;101:174–190. doi: 10.1016/j.tube.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Darrah PA, et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature. 2020;577:95–102. doi: 10.1038/s41586-019-1817-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar P. IFNgamma-producing CD4(+) T lymphocytes: the double-edged swords in tuberculosis. Clin. Transl. Med. 2017;6:21. doi: 10.1186/s40169-017-0151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.47Okada, M. Novel vaccines against M. tuberculosis. Kekkaku81, 745–751 (2006). [PubMed]
- 48.48McShane, H. Tuberculosis vaccines: beyond bacille Calmette-Guerin. Philos. Trans. R. Soc. Lond. B Biol. Sci.366, 2782–2789, 10.1098/rstb.2011.0097 (2011). [DOI] [PMC free article] [PubMed]
- 49.Kaushal D, et al. Mucosal vaccination with attenuated Mycobacterium tuberculosis induces strong central memory responses and protects against tuberculosis. Nat. Commun. 2015;6:8533. doi: 10.1038/ncomms9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaushal D. The comeback kid: BCG. J. Infect. Dis. 2020;221:1031–1032. doi: 10.1093/infdis/jiz117. [DOI] [PubMed] [Google Scholar]
- 51.Moliva JI, Turner J, Torrelles JB. Immune responses to bacillus calmette-guerin vaccination: Why do they fail to protect against mycobacterium tuberculosis? Front. Immunol. 2017;8:407. doi: 10.3389/fimmu.2017.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in the manuscript and its supplementary data.


