Abstract
The effect of body mass index (BMI) changes and variability on the risk for Alzheimer’s disease (AD) remains unclear. We analyzed 45,076 participants, whose BMI were measured on phase 1 (2002–2003), phase 2 (2004–2005), and phase 3 (2006–2007), of the Korean National Health Insurance Service-Health Screening Cohort. We evaluated the effect of 2- and 4-year BMI changes and BMI variability on the risk of AD using Cox regression models. In men, association between 2-year BMI changes, BMI variability, and the risk of AD was not significant. Risk of AD was higher in men whose BMI had decreased 10.1–15.0% over 4 years. In women, aHRs and 95% CIs for AD were 1.14 (1.02–1.29), 1.44 (1.17–1.79), and 1.51 (1.09–2.09) when 2-year BMI loss was 5.1–10.0%, 10.1–15.0%, and > 15.0%. The HRs for AD in women significantly increased when 4-year BMI loss was > 5.0%. The aHR and 95% CI for AD was 1.31 (1.17–1.46) in the 4th quartile of average successive variability (ASV) compared with the 1st quartile of ASV in women. BMI loss over 2- and 4-year period was associated with increased risk for AD, and risk increased in women with higher BMI variability. Appropriate body weight management is recommended to prevent AD.
Subject terms: Diseases, Health care, Medical research, Neurology, Risk factors
Introduction
Alzheimer’s disease (AD), the most common subtype of dementia, is a chronic progressive neurodegenerative disorder characterized by cognitive dysfunction, behavioral disturbances, and difficulties in activities of daily living1,2. As the disease progresses, language problems, disorientation, mood swings, and behavioral issues gradually worsen, and ultimately, a patient’s bodily functions are lost1,2, leading to a heavy socioeconomic burden in many countries3,4. Patients at risk for AD can be identified in primary care settings, as they may complain of mild changes in memory and thinking abilities2. Early diagnosis of Alzheimer’s disease leads to appropriate pharmacological intervention and initiation of social support, which could control some of the symptoms5. Therefore, early identification of those at risk of developing AD is essential.
Several modifiable risk factors have been proposed for AD although physiological mechanisms, such as cerebral deposition of the amyloid β–protein and hyperphosphorylation of tau protein, are pivotal in disease development6–8. Despite controversies, numerous studies have investigated the relationship between body mass index (BMI) and the risk of AD. In general, higher midlife BMI was associated with an increased risk of AD, whereas higher late-life BMI was associated with a reduced risk of AD9. The most recent meta-analysis reported that midlife underweight, midlife obesity, and late-life underweight increases overall dementia risk. Particularly, midlife risk for AD increased when BMI > 30 kg/m2, and late-life risk for AD decreased when BMI < 27 kg/m210.
In addition to examining the effect of single point BMI on the risk of AD, few studies have evaluated the association between BMI trajectories and risk of AD. Studies have evaluated either “change” in BMI, which is a difference between measurements at two time points, or “variability”, which is a fluctuation of BMI among three or more measurements. Generally, BMI or weight loss and higher BMI or weight variability were associated with an increased risk for AD11–16. These studies concluded that weight loss precedes AD since weight loss may be a causal factor or a prodromal symptom of AD11–14. Furthermore, recent evidence suggests that a loss of physiological homeostasis can lead to variability in metabolic parameters, ultimately causing disease17,18.
To our knowledge, there is only one study that evaluated the association between BMI change and risk of dementia in the general Korean population. Unlike previous studies, this study reported that both BMI gain and loss were associated with an increased risk for overall dementia19. However, the outcome variable in this study was not restricted to AD, and the definition of dementia solely relied on the International Classification of Diseases, 10th edition (ICD-10), which is subject to false positive. Therefore, in this study, we investigated the association between BMI changes and risk for AD after defining AD based on a combination of the ICD-10 codes and antidementia drug prescription in a nationwide representative sample. Based on three BMI measurements over 4 years, we evaluated 2- and 4-year BMI changes and BMI variability to investigate the effect of BM trajectories on the risk of AD.
Methods
Data source and study population
We used data from the National Health Insurance Service-Health Screening Cohort (NHIS-HEALS). In Korea, NHIS provides compulsory health insurance covering approximately 97% of the entire population20. Furthermore, NHIS provides biennial health screening to all insured individuals. NHIS-HSALS is an anonymized academic database, consisting of a random selection of 10% of all examinees who participated in the biennial national health screening program during 2002 and 2003 (baseline year/phase 1)21. It includes 514,866 participants, aged between 40 and 79 at the baseline year, who were followed up to the year 2015. NHIS-HEALS comprises 4 databases on the participants' insurance eligibility, medical counseling, health screening, and medical care institutions. Insurance eligibility database contains details on demographics, death-related information, type of health insurance, and household income. Medical counseling database offers details on diagnosed diseases based on ICD-10 and prescription-related information. Health screening database provides results of biennial health screening, such as assessment of health behaviors, anthropometric measurements, and laboratory results of blood and urine samples. Medical care institutions database provides information for each medical care facility.
From the 514,866 participants, we excluded participants aged < 60 years at the baseline year (N = 382,013) (Figure S1). Among 132,853 participants aged ≥ 60 years, we excluded 6045 participants diagnosed with any type of dementia; 15,608 participants died before the index date (January 1st, 2011). We further excluded 1811 cancer patients and participants whose BMI was not measured in any of the three consecutive health screenings and whose lifestyle variables were not measurable due to missing responses, leaving 45,076 participants for the final analysis.
Ethics declarations
The study protocol was approved by the Institutional Review Board of Asan Medical Center (IRB number 2018-1524). All research procedures were employed in accordance with the relevant guidelines and regulations. As the NHIS-HEALS database is comprised of de-identified secondary data, obtaining written informed consent from study participants was unnecessary.
Definition of AD
We assessed AD based on the codes from ICD-10. Participants who were prescribed choline acetyltransferase inhibitor (donepezil, galantamine, rivastigmine) or NMDA receptor antagonist (memantine) at least twice with ICD-10 codes pertaining to AD (F00, G30) were determined to have developed AD. In order to fulfill Korean National Health Insurance reimbursement criteria, physicians need to document the evidence for cognitive dysfunction according to the following criteria: (1) Mini Mental State Examination (MMSE) score ≤ 26 and (2) either a Clinical Dementia Rating (CDR) score ≥ 1 or a Global Deterioration Scale (GDS) score ≥ 322. Dementia was diagnosed when participants were prescribed the above antidementia drug at least twice with ICD-10 codes pertaining to dementia (F00, F01, F02, F03, G30, G31), and subjects with dementia were excluded when diagnosed prior to the index date.
Changes in BMI
BMI was calculated by dividing body weight in kilograms by the square of height in meters. BMI was further categorized into four groups: < 18.5 kg/m2, 18.5–22.9 kg/m2, 23.0–24.9 kg/m2, ≥ 25.0 kg/m2. BMI changes over 2 years was defined as percent difference between baseline BMI (phase 1: 2002–2003) and that measured after 2 years (phase 2: 2004–2005). BMI changes over 4 years was defined as the percent difference between BMI measured at baseline and after 4 years (phase 3: 2006–2007). Percent BMI changes were further categorized into the following 7 groups: stable ± 5.0%, decrease of 5.1–10.0%, decrease of 10.1–15.0%, decrease of > 15.0%, increase of 5.1–10.0%, increase of 10.1–15.0%, and increase of > 15.0%.
BMI variability
BMI variability was determined using average successive variability (ASV), which was defined as the average absolute difference between BMI values of the three phases of health screening23. ASV was further divided into quartiles.
Other variables
Covariates were assessed from the baseline year. Sociodemographic variables included age and insurance premium. Lifestyle factors were evaluated, including smoking status, alcohol consumption, and regular exercise. Smoking status was categorized as non-smokers and smokers. Alcohol consumption was categorized as non-, moderate, and heavy drinker, according to the definitions of the National Institute on Alcohol Abuse and Alcoholism24. Regarding regular exercise, participants were asked to provide weekly frequency of exercise. We categorized participants as those who were engaging in exercise ≥ 1 per week (yes) and those who were not (no). We assessed presence of hypertension, diabetes mellitus, and dyslipidemia based on codes from ICD-10. If insurance claims for either outpatient or inpatient treatment were made at least 3 times with ICD-10 codes pertaining to a certain comorbidity, we determined that participant had the comorbidity. The ICD-10 codes were as follows: hypertension (I10, I11, I12, I13, I15), diabetes mellitus (E10, E11, E12, E13, E14), and dyslipidemia (E78).
Statistical analysis
Participants were followed up from the index date to the date AD was diagnosed, date of death, or December 31st, 2015, whichever came first. To reduce the possibility of reverse causation, the index date was set to January 1st, 2011, with 3-year latent period for the diagnosis of AD from the last BMI measurement. We used chi-square test to compare baseline characteristics between men and women. To evaluate the effect of 2- and 4-year BMI changes on the risk of AD, we performed Cox proportional hazards regression analysis after adjusting for age, insurance premium, initial BMI, smoking status, alcohol consumption, physical activity, hypertension, diabetes mellitus, and dyslipidemia. Hazard ratios (HRs) and 95% confidence intervals (CIs) for AD were obtained for each category of BMI changes, considering “stable (± 5.0%)” as a reference group. We plotted a restricted cubic spline of HRs for AD on a logarithmic scale to examine the association using 2- and 4-year BMI changes as continuous variables. To evaluate the effect of BMI variability on the risk of AD, we performed Cox proportional hazards regression analysis, where ASV was computed as both a continuous variable and a categorical variable. We obtained the HRs and 95% CIs for AD for 1 ASV change and in each ASV quartile, considering the 1st quartile as a reference group. Subgroup analyses were performed based on lifestyle factors in order to evaluate the risk for AD according to BMI changes and variability in each category of lifestyle factors. Analyses were separately performed for men and women. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA), and restricted cubic splines were plotted using rms package in R software version 3.3.3 (R Foundation for Statistical Computing, Vienna, Austria). Two-tailed p values < 0.05 were considered statistically significant.
Results
Baseline characteristics, BMI changes, and BMI variability of the study participants
Among the 45,076 study participants (23,298 men and 21,778 women), 4055 cases (1688 men and 2367 women) of AD were observed during the 2.7-year mean follow-up. The cumulative incidence rate for AD was 16.3 cases per 1000 person-years in men and 23.9 cases per 1000 person-years in women. Table 1 shows baseline characteristics, BMI changes, and BMI variability of study participants. Men and women were equally distributed among age groups. The proportion of obese individuals was higher in women, while the proportion of current smokers and heavy drinkers was higher in men. The prevalence of lack of regular exercise, hypertension, diabetes mellitus, and dyslipidemia was higher in women. Approximately 73.3% of men and 68.6% of women maintained stable BMI over 2 years, and 66.1% of men and 62.6% of women maintained stable BMI over 4 years. The proportions of participants were lower in categories with greater BMI changes. ASVs of the BMI for the 3 phases of health screening were 1.44 ± 1.23 kg/m2 in men and 1.63 ± 1.32 kg/m2 in women.
Table 1.
Baseline characteristics, BMI changes, and BMI variability of study participants (n = 45,076).
| Men (N = 23,298) | Women (N = 21,778) | p-value | |
|---|---|---|---|
| N (%) | N (%) | ||
| Age (years) | |||
| 60–69 | 19,222 (82.5) | 17,960 (82.5) | 0.921 |
| 70–79 | 4,076 (17.5) | 3,818 (17.5) | |
| Insurance premium (deciles) | |||
| ≤ 2 (low) | 4437 (19.0) | 4180 (19.2) | 0.008 |
| 3–5 | 5693 (24.4) | 5069 (23.3) | |
| 6–8 | 6543 (28.1) | 6080 (27.9) | |
| 9–10 (high) | 6625 (28.4) | 6449 (29.6) | |
| BMI (kg/m2) | |||
| < 18.5 | 776 (3.3) | 527 (2.4) | < 0.001 |
| 18.5–22.9 | 9063 (38.9) | 6845 (31.4) | |
| 23–24.9 | 6512 (28.0) | 5560 (25.5) | |
| ≥ 25 | 6947 (29.8) | 8846 (40.6) | |
| Smoking status | |||
| Non-smoker | 16,351 (70.2) | 21,194 (97.3) | < 0.001 |
| Current smoker | 6947 (29.8) | 584 (2.7) | |
| Alcohol consumption | |||
| Non-drinker | 10,505 (45.1) | 19,644 (90.2) | < 0.001 |
| Moderate drinker | 9555 (41.0) | 1841 (8.5) | |
| Heavy drinker | 3238 (13.9) | 293 (1.3) | |
| Regular exercise | |||
| No | 12,526 (53.8) | 15,724 (72.2) | < 0.001 |
| Yes | 10,772 (46.2) | 6054 (27.8) | |
| Comorbidities | |||
| Hypertension | 6810 (29.2) | 7475 (34.3) | < 0.001 |
| Diabetes mellitus | 2553 (11.0) | 2587 (11.9) | < 0.001 |
| Dyslipidemia | 1675 (7.2) | 2300 (10.6) | < 0.001 |
| 2-year BMI changes | |||
| Decrease of > 15.0% | 156 (0.7) | 238 (1.1) | < 0.001 |
| Decrease of 10.1–15.0% | 441 (1.9) | 595 (2.7) | |
| Decrease of 5.1–10.0% | 2676 (11.5) | 2808 (12.9) | |
| Stable ± 5.0% | 17,085 (73.3) | 14,941 (68.6) | |
| Increase of 5.1–10.0% | 2251 (9.7) | 2344 (10.8) | |
| Increase of 10.1–15.0% | 455 (2.0) | 558 (2.6) | |
| Increase of > 15.0% | 234 (1.0) | 294 (1.3) | |
| 4-year BMI changes | |||
| Decrease of > 15.0% | 240 (1.0) | 328 (1.5) | < 0.001 |
| Decrease of 10.1–15.0% | 659 (2.8) | 779 (3.6) | |
| Decrease of 5.1–10.0% | 3,075 (13.2) | 3,057 (14.0) | |
| Stable ± 5.0% | 15,394 (66.1) | 13,632 (62.6) | |
| Increase of 5.1–10.0% | 2871 (12.3) | 2780 (12.8) | |
| Increase of 10.1–15.0% | 736 (3.2) | 817 (3.8) | |
| Increase of > 15.0% | 323 (1.4) | 385 (1.8) | |
| BMI variability | |||
| Average successive variabilitya (kg/m2) | 1.44 ± 1.23 | 1.63 ± 1.32 | < 0.001 |
BMI body mass index.
aValues are presented as mean ± standard deviation.
BMI changes and risk for AD
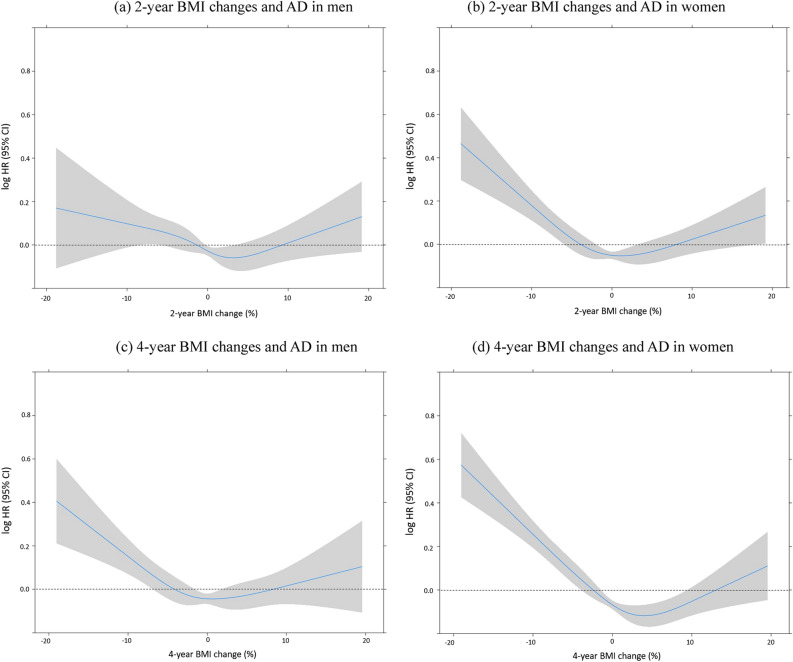
Table 2 shows HRs and 95% CIs for AD in each category of BMI changes in the 2- and 4-year periods in comparison to the stable BMI group. In the multivariate model of men, association between 2-year BMI changes and risk of AD was not significant. Risk of AD was 1.33 (95% CI 1.02–1.72) higher in men whose BMI had decreased 10.1–15.0% over 4 years. In spline representation of logarithmic HR for AD, the association between 2-year BMI changes and risk for AD was not evident (Fig. 1). With the lowest risk in 0–1% gain in BMI, logarithmic HR for AD increased as 4-year BMI changes decreased. Risk of AD was associated with BMI loss in women. Adjusted hazard ratios (aHRs) and 95% CIs for AD were 1.14 (1.02–1.29), 1.44 (1.17–1.79), and 1.51 (1.09–2.09) in women whose 2-year BMI loss was 5.1–10.0%, 10.1–15.0%, and > 15.0%, respectively. The aHRs and 95% CIs for AD were 1.31 (1.17–1.46), 1.60 (1.33–1.92), and 1.68 (1.29–2.20) in women whose 4-year BMI loss was 5.1–10.0%, 10.1–15.0%, and > 15.0%, respectively. In spline representation of logarithmic HR for AD, the association between 2- and 4-year BMI changes and the risk for AD showed a U-shape with the lowest risk in 0–2% and 3–5% BMI gain, respectively (Fig. 1). The increase in the risk for AD was only significant when BMI decreased.
Table 2.
Risk of Alzheimer’s disease according to BMI changes.
| Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Event | Duration | Unadjusted HR (95% CI) |
aAdjusted HR (95% CI) |
Event | Duration | Unadjusted HR (95% CI) |
aAdjusted HR (95% CI) |
|||
| N | Person-years | CIR | N | Person-years | CIR | |||||
| 2-year BMI changes | ||||||||||
| Decrease of > 15.0% | 11 | 670 | 16.4 | 1.04 (0.58–1.89) | 1.00 (0.55–1.82) | 38 | 1034 | 36.8 | 1.64 (1.19–2.26) | 1.51 (1.09–2.09) |
| Decrease of 10.1–15.0% | 39 | 1865 | 20.9 | 1.34 (0.97–1.84) | 1.21 (0.88–1.66) | 91 | 2614 | 34.8 | 1.54 (1.25–1.91) | 1.44 (1.17–1.79) |
| Decrease of 5.1–10.0% | 220 | 11,682 | 18.8 | 1.20 (1.04–1.39) | 1.14 (0.98–1.31) | 335 | 12,653 | 26.5 | 1.17 (1.04–1.32) | 1.14 (1.02–1.29) |
| Stable (± 5.0%) | 1203 | 76,364 | 15.8 | 1.00 | 1.00 | 1,543 | 68,107 | 22.7 | 1.00 | 1.00 |
| Increase of 5.1–10.0% | 162 | 9869 | 16.4 | 1.05 (0.89–1.23) | 0.95 (0.80–1.12) | 240 | 10,728 | 22.4 | 0.99 (0.86–1.13) | 0.94 (0.82–1.08) |
| Increase of 10.1–15.0% | 35 | 1955 | 17.9 | 1.14 (0.82–1.60) | 1.00 (0.71–1.40) | 77 | 2485 | 31.0 | 1.37 (1.09–1.72) | 1.17 (0.93–1.47) |
| Increase of > 15.0% | 18 | 1034 | 17.4 | 1.11 (0.70–1.76) | 0.88 (0.55–1.41) | 43 | 1285 | 33.5 | 1.49 (1.10–2.02) | 1.17 (0.86–1.59) |
| 4-year BMI changes | ||||||||||
| Decrease of > 15.0% | 19 | 1020 | 18.6 | 1.21 (0.77–1.90) | 1.14 (0.73–1.80) | 56 | 1360 | 41.2 | 1.92 (1.47–2.51) | 1.68 (1.29–2.20) |
| Decrease of 10.1–15.0% | 60 | 2782 | 21.6 | 1.39 (1.08–1.81) | 1.33 (1.02–1.72) | 126 | 3409 | 37.0 | 1.72 (1.43–2.06) | 1.60 (1.33–1.92) |
| Decrease of 5.1–10.0% | 245 | 13,426 | 18.2 | 1.18 (1.02–1.35) | 1.13 (0.98–1.30) | 405 | 13,592 | 29.8 | 1.38 (1.23–1.54) | 1.31 (1.17–1.46) |
| Stable (± 5.0%) | 1,071 | 68,805 | 15.6 | 1.00 | 1.00 | 1,354 | 62,410 | 21.7 | 1.00 | 1.00 |
| Increase of 5.1–10.0% | 200 | 12,803 | 15.6 | 1.00 (0.86–1.17) | 0.96 (0.83–1.12) | 284 | 12,723 | 22.3 | 1.03 (0.91–1.17) | 0.98 (0.87–1.12) |
| Increase of 10.1–15.0% | 69 | 3205 | 21.5 | 1.39 (1.09–1.77) | 1.21 (0.94–1.55) | 91 | 3716 | 24.5 | 1.13 (0.91–1.40) | 1.00 (0.80–1.23) |
| Increase of > 15.0% | 24 | 1398 | 17.2 | 1.11 (0.74–1.66) | 0.88 (0.58–1.32) | 51 | 1697 | 30.1 | 1.40 (1.06–1.85) | 1.13 (0.85–1.50) |
BMI body mass index, CIR cumulative incidence rate, HR hazard ratio, CI confidence interval.
aAdjusted for age, insurance premium, initial BMI, smoking status, alcohol consumption, regular exercise, hypertension, diabetes mellitus, and dyslipidemia.
Figure 1.
BMI changes and risk of Alzheimer’s disease.
BMI variability and risk for AD
Table 3 shows risk of AD based on BMI variability in the three phases of health screening. In the multivariate model of men, association between BMI variability and risk of AD was not significant. In women, the risk of AD increased approximately 6% when ASV changed 1 kg/m2. The aHRs and 95% CIs for AD was 1.31 (1.17–1.46) in the 4th quartile of ASV compared with the 1st quartile.
Table 3.
Risk of Alzheimer’s disease according to BMI variability.
| Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Event | Duration | Unadjusted HR (95% CI) |
aAdjusted HR (95% CI) |
Event | Duration | Unadjusted HR (95% CI) |
aAdjusted HR (95% CI) |
|||
| N | Person-years | CIR | N | Person-years | CIR | |||||
| BMI variability | ||||||||||
| ASV | – | – | – | 1.05 (1.03–1.08) | 1.05 (1.02–1.08) | – | – | – | 1.07 (1.05–1.09) | 1.06 (1.03–1.08) |
| BMI variability | ||||||||||
| ASV 1st quartile | 404 | 26,149 | 15.4 | 1.00 | 1.00 | 520 | 24,913 | 20.9 | 1.00 | 1.00 |
| ASV 2nd quartile | 383 | 25,998 | 14.7 | 0.95 (0.83–1.10) | 0.93 (0.81–1.07) | 532 | 25,027 | 21.3 | 1.02 (0.90–1.15) | 1.01 (0.89–1.13) |
| ASV 3rd quartile | 412 | 25,846 | 15.9 | 1.03 (0.90–1.19) | 0.98 (0.86–1.13) | 595 | 24,626 | 24.2 | 1.16 (1.03–1.30) | 1.10 (0.98–1.24) |
| ASV 4th quartile | 489 | 25,446 | 19.2 | 1.25 (1.09–1.42) | 1.13 (0.99–1.29) | 720 | 24,341 | 29.6 | 1.42 (1.27–1.59) | 1.31 (1.17–1.46) |
BMI body mass index, ASV average successive variability, CIR cumulative incidence rate, HR hazard ratio, CI confidence interval.
aAdjusted for age, insurance premium, initial BMI, smoking status, alcohol consumption, regular exercise, hypertension, diabetes mellitus, and dyslipidemia.
Subgroup analysis
Table 4 shows the risk for AD among participants with 2-year BMI loss > 5.0%, 4-year BMI loss > 5.0%, and 4th quartile of ASV in each category of lifestyle factors. In multivariate model of men, 2-year BMI loss did not generally increase the risk for AD; however, the risk for AD increased among current smokers and heavy drinkers when their BMI had decreased > 5.0% over the past 2 years. The risk for AD was 1.16 (95% CI 1.03–1.32) higher when 4-year BMI loss was > 5.0%, and this association was significant among obese men and those who were not doing regular exercise. More than 5.0% of BMI gain over the 2- and 4-year periods did not increase risk for AD in men (data not shown). The risk for AD in the 4th ASV quartile compared with the 1st was higher among obese men and moderate drinkers. In multivariate model of women, the risk for AD increased in those with 2-year BMI loss > 5.0%, 4-year BMI loss > 5.0%, and those whose ASV of BMI was in the highest quartile. The association was significant among normal, overweight, and obese women, as well as non-smokers, non-drinkers, and regardless of regular exercise. More than 5.0% of BMI gain over 2-year and 4-year period did not increase the risk for AD in women (data not shown).
Table 4.
Subgroup analysis for association between 2-year BMI loss, 4-year BMI loss, BMI variability and risk for Alzheimer’s disease according to lifestyle factors.
| Men | Women | |||
|---|---|---|---|---|
| Unadjusted HR (95% CI) |
aAdjusted HR (95% CI) |
Unadjusted HR (95% CI) |
aAdjusted HR (95% CI) |
|
| 2-year BMI loss > 5.0% vs. stable (± 5.0%) BMI | ||||
| All | 1.21 (1.06–1.38) | 1.14 (0.99–1.30) | 1.26 (1.14–1.40) | 1.22 (1.10–1.35) |
| Underweight | 0.67 (0.27–1.69) | 0.49 (0.19–1.24) | 1.25 (0.59–2.68) | 1.26 (0.58–2.70) |
| Normal and overweight | 1.26 (1.07–1.49) | 1.18 (0.99–1.39) | 1.28 (1.11–1.47) | 1.22 (1.05–1.40) |
| Obesity | 1.19 (0.95–1.50) | 1.12 (0.89–1.40) | 1.26 (1.08–1.48) | 1.22 (1.04–1.42) |
| Non-smoker | 1.16 (0.99–1.35) | 1.08 (0.93–1.27) | 1.26 (1.13–1.40) | 1.22 (1.10–1.36) |
| Current smoker | 1.35 (1.06–1.72) | 1.29 (1.01–1.64) | 1.22 (0.71–2.11) | 1.06 (0.61–1.87) |
| Non-drinker | 1.08 (0.89–1.31) | 1.05 (0.87–1.28) | 1.25 (1.12–1.39) | 1.21 (1.08–1.35) |
| Moderate drinker | 1.21 (0.96–1.52) | 1.11 (0.88–1.40) | 1.41 (0.99–2.01) | 1.36 (0.95–1.94) |
| Heavy drinker | 1.50 (1.11–2.01) | 1.43 (1.07–1.93) | 1.19 (0.50–2.85) | 1.12 (0.46–2.76) |
| No regular exercise | 1.22 (1.03–1.44) | 1.18 (0.99–1.39) | 1.25 (1.11–1.40) | 1.21 (1.08–1.36) |
| Regular exercise | 1.17 (0.94–1.44) | 1.08 (0.87–1.33) | 1.24 (0.99–1.55) | 1.26 (1.00–1.57) |
| 4-year BMI loss > 5.0% vs. stable (± 5.0%) BMI | ||||
| All | 1.21 (1.07–1.37) | 1.16 (1.03–1.32) | 1.48 (1.34–1.63) | 1.39 (1.26–1.53) |
| Underweight | 0.59 (0.18–1.91) | 0.50 (0.15–1.63) | 2.23 (1.15–4.30) | 1.86 (0.95–3.65) |
| Normal and overweight | 1.15 (0.98–1.35) | 1.08 (0.92–1.27) | 1.38 (1.21–1.58) | 1.28 (1.12–1.47) |
| Obesity | 1.41 (1.15–1.73) | 1.36 (1.11–1.66) | 1.62 (1.41–1.88) | 1.52 (1.31–1.75) |
| Non-smoker | 1.22 (1.06–1.41) | 1.15 (0.99–1.33) | 1.48 (1.34–1.64) | 1.41 (1.27–1.55) |
| Current smoker | 1.19 (0.94–1.51) | 1.18 (0.93–1.50) | 1.36 (0.79–2.34) | 0.98 (0.55–1.72) |
| Non-drinker | 1.14 (0.96–1.37) | 1.11 (0.92–1.32) | 1.50 (1.35–1.66) | 1.41 (1.27–1.56) |
| Moderate drinker | 1.25 (1.01–1.54) | 1.22 (0.98–1.50) | 1.53 (1.09–2.16) | 1.41 (0.99–1.99) |
| Heavy drinker | 1.33 (0.98–1.80) | 1.22 (0.90–1.66) | 0.55 (0.22–1.37) | 0.44 (0.16–1.20) |
| No regular exercise | 1.30 (1.11–1.53) | 1.26 (1.07–1.48) | 1.41 (1.27–1.58) | 1.34 (1.20–1.50) |
| Regular exercise | 1.10 (0.90–1.33) | 1.04 (0.86–1.27) | 1.66 (1.35–2.04) | 1.59 (1.30–1.96) |
| ASV 4th quartile vs. 1st quartile | ||||
| All | 1.25 (1.09–1.42) | 1.13 (0.99–1.29) | 1.42 (1.27–1.59) | 1.31 (1.17–1.46) |
| Underweight | 0.89 (0.47–1.66) | 0.79 (0.42–1.51) | 1.17 (0.62–2.22) | 0.99 (0.51–1.90) |
| Normal and overweight | 1.19 (1.01–1.40) | 1.06 (0.91–1.25) | 1.44 (1.24–1.67) | 1.31 (1.13–1.52) |
| Obesity | 1.50 (1.16–1.92) | 1.38 (1.07–1.78) | 1.45 (1.21–1.74) | 1.33 (1.11–1.59) |
| Non-smoker | 1.28 (1.09–1.50) | 1.16 (0.99–1.36) | 1.42 (1.27–1.60) | 1.32 (1.17–1.48) |
| Current smoker | 1.17 (0.92–1.49) | 1.07 (0.84–1.36) | 1.30 (0.68–2.48) | 1.00 (0.52–1.95) |
| Non-drinker | 1.17 (0.97–1.41) | 1.10 (0.91–1.33) | 1.41 (1.26–1.59) | 1.30 (1.15–1.47) |
| Moderate drinker | 1.40 (1.12–1.75) | 1.26 (1.01–1.58) | 1.45 (0.99–2.12) | 1.28 (0.87–1.88) |
| Heavy drinker | 1.06 (0.76–1.48) | 0.97 (0.70–1.36) | 2.32 (0.66–8.24) | 2.30 (0.62–8.56) |
| No regular exercise | 1.19 (1.00–1.41) | 1.11 (0.93–1.31) | 1.32 (1.16–1.50) | 1.25 (1.10–1.43) |
| Regular exercise | 1.25 (1.02–1.55) | 1.16 (0.94–1.43) | 1.70 (1.34–2.16) | 1.50 (1.18–1.92) |
BMI body mass index, HR hazard ratio, CI confidence interval, ASV average successive variability.
aAdjusted for age, insurance premium, initial BMI, smoking status, alcohol consumption, regular exercise, hypertension, diabetes mellitus, and dyslipidemia.
Discussion
BMI loss over the 2- and 4-year periods increased the risk for AD. Specifically, the risk increased in women as the amount of BMI loss over both time periods had increased. In men, this association was not clear on multivariate analysis; however, the risk tended to increase when amount of BMI loss over the 4-year period had increased. In contrast, BMI gain was not associated with increased risk for AD. Additionally, higher BMI variability increased the risk for AD in women.
The association between weight loss and the risk of AD can be explained by the following physiological mechanisms. As weight loss is a byproduct of low energy intake, subsequent deficiency in macronutrients and micronutrients may aggravate cognitive function25,26. For instance, deficiency in essential fatty acids comprising neuronal cell membrane results in physiological changes in the neuronal cell membrane, and vitamin deficiency may aggravate oxidative stress26,27. Furthermore, weight loss contributes to cognitive impairment by raising the levels of serum cortisol and free radicals25,28. Moreover, weight loss decreases the amount of leptin produced from subcutaneous and visceral fat tissue29. Leptin is a hormone, which stimulates axonal growth, synaptogenesis, and cell survival, protects against oxidative stress, and associated with hippocampal progenitor cell proliferation29. Thus, decrease in leptin effect could lead to cognitive decline. In the Framingham study, people with the lowest quartile of leptin levels had a four-time greater risk of developing AD after 12 years than those with the highest quartile of leptin level30.
As our analysis is focused on adults ≥ 60 years, bone loss and sarcopenia are critical factors linked to weight loss. Decline in bone mass may increase inflammatory markers, which also increase the risk for AD31. In addition, predisposing conditions for sarcopenia, such as insulin resistance, inflammation, and oxidative stress, are also associated with cognitive dysfunction32. Although some of these mechanisms remain uncertain, studies have shown the link between bone loss, sarcopenia, and cognitive impairment33–35. Therefore, regular physical activity with strength exercise is recommended to prevent cognitive impairment in the elderly population.
Despite the forementioned biologic mechanism suggesting weight loss can be causative for development of AD, weight loss can also be prodromal or early symptoms of AD. Atrophy of the brain areas that play a role in weight control, such as the mesial temporal cortex, may occur during the preclinical phase of AD, thereby leading to weight loss36. Similarly, hypometabolism of the hypothalamus or cingulate gyrus may impair weight control and contribute to weight loss prior to the development of AD37. Furthermore, early symptoms of AD such as reduced olfactory function and predementia apathy may cause weight loss2. Therefore, studies have supported the argument for weight loss presenting as a prodromal symptom of AD rather than a risk factor. However, recent studies have shown that weight loss may even precede mild cognitive impairment, and weight loss was associated with biomarkers for AD in healthy individuals, as well as baseline cortical thinning and accelerated brain atrophy38,39. In our study, BMI was measured three times, in 2002–2003, 2004–2005, and 2006–2007, and the development of AD was measured from January 1, 2011, with a minimum 5 year gap for the association between 2-year BMI change and risk for AD, and at least a 3 year gap for the association between 4-year BMI change and risk for AD. Given the chronic and neurodegenerative nature of AD, the latent period in our study may not be sufficient to exclude the possibility of reverse causation. Nevertheless, it is very clear from our results that BMI loss precedes the diagnosis of AD by several years.
In our analysis, BMI loss over the 2- and 4-year periods increased the risk for AD in women. This association was not significant in men, although an increasing trend for risk of AD was observed from the cubic spline when a 4-year BMI loss was present. Prevalence of AD was higher among women, and clinical progression and neurodegeneration more rapidly manifested in women than in men40. One of possible explanation can be interaction between apolipoprotein E (APOE) ε4 allele polymorphism and sex. APOE ε4 allele, a risk factor for AD, is associated with a greater increase in the probability of developing AD in women than in men41. Cognitive and functional decline were faster in women than in men, and this was greater in APOE ε4 allele carriers42. Furthermore, women experience a sudden decline in estrogen due to menopause in their early 50 s. Estrogen protects against mitochondrial dysfunction, modulates neurogenesis, and decreases the level of amyloid β-protein43–45. Thus, a rapid decrease in estrogen may increase susceptibility for AD in perimenopausal women. Along with genetic and environmental interactions, several risk factors have greater impact on women in the development and progression of AD40,46. Likewise, the sex difference observed in this study could be due to the difference in AD susceptibility.
In the subgroup analyses of women, a BMI loss > 5.0% over a 2-year and 4-year period consistently increased risk for AD in general, except for underweight women, current smokers, moderate drinkers, and heavy drinkers. It is more likely due to the low statistical power in these groups of participants, as the proportion of these participants among the entire group of women participants was 2.4%, 2.7%, 8.5%, and 1.3%, respectively. In the main analyses, association between BMI loss and AD among men was only significant when their BMI had decreased 10.1–15.0% over 4 years. However, when participants with a 4-year BMI loss of > 5.0% were placed into one category, the risk for AD increased by 1.16 (95% CI 1.03–1.22). This result, along with the cubic spline showing the association between 4-year BMI change and logarithmic HR, presents an increased risk for AD among men with BMI loss compared with those with stable BMI. However, inconsistent findings from the subgroup analyses do not support this association, and it is difficult to draw a definite conclusion from our analysis for men.
In our study, higher BMI variability increased the risk for AD in women. Recent studies have demonstrated the association between BMI variability and an increased risk for cardiovascular morbidity and mortality17. Although the underlying mechanism remains unclear, fluctuation in body weight contributes to fluctuations in blood pressure, heart rate, glomerular filtration rate, and glucose and lipid levels, thereby increasing the risk for cardiovascular morbidity and mortality47. Our findings suggest a potential link between weight fluctuation and an increased risk for cognitive decline in addition to cardiovascular risk factors. To our knowledge, there are very few studies, which investigated the association between body weight variability and risk for dementia. Previous studies reported that higher weight variability was associated with an increased risk for dementia15,16. In our study, we used ASV, the average absolute difference between BMI values of each measurement, as a measure of variability. In the first part of our analysis, we reported association between BMI loss and increased risk for AD. An increased risk for AD in those with higher ASV of BMI in the same study population additionally shows that BMI instability may also increase risk for AD regardless of the direction of BMI change.
There are several limitations in this study. First, the diagnosis of AD was not made clinically, using ICD-10 codes instead. To overcome this limitation, we defined AD based on the combination of the ICD-10 codes and antidementia drug prescription as patients must have MMSE score ≤ 26, and either a CDR score ≥ 1 or a GDS score ≥ 3 to necessitate such a prescriptions22, a large number of participants were excluded due to missing data, which could result in a selection bias. The low participation rate for national health screening in the early 2000s (less than 50% of the Korean population) may have caused the large number of missing values in the three phases of health screening. Third, the mechanisms linking BMI declines and AD remain unexplained as we could not obtain bone density, muscle mass, or abdominal circumference from the dataset. The effect of changes in bone density, muscle mass, and adiposity on the risk for AD will provide better insights for the underlying mechanisms. Fourth, we cannot distinguish intentional from unintentional weight loss among study participants. As our study participants were aged ≥ 60, it is likely that a high proportion of observed weight loss was unintentional while some intentional weight loss may present within this cohort. Furthermore, there were no measures of calorie consumption; thus, it is not possible to determine if the weight loss was due to reduced calorie intake or an endogenous metabolic abnormality. In fact, weight loss appears in pre-clinical stages of many neurodegenerative diseases, suggesting the possibility of behavior shifts and/or energy imbalance in neurodegeneration25,48,49. Fifth, we were not able to consider genetic factors or brain functions in this study. Especially, we could not adjust APOE ε4 allele although it is an established genetic risk factor for AD1,2. Future research should focus on the potential role of hypothalamic dysfunction as this brain region is an early target in AD1,2. Lastly, as AD is a slowly progressing neurodegenerative disease, there is a possibility of reverse causation although we established a latent period of at least 3 years from the last BMI measurement to development of AD. Nevertheless, this is the first study to investigate the effect of BMI changes over different time periods, as well as BMI variability, on risk for AD in the same population. It is also meaningful to report the difference in the effect of BMI trajectories and risk for AD in men and women.
In conclusion, BMI loss over 2- and 4-year periods increased the risk for AD, which also increased in those with higher BMI variability. These associations were significant in women. Appropriate body weight management as well as regular monitoring of body weight and BMI is recommended to prevent AD in the elderly population.
Supplementary Information
Author contributions
Y.S.K. is the corresponding author and had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. S.Y.K. and Y.S.K. contributed to the conception and design. Y.J.K. conducted collection and assembly of the data. S.Y.K. and Y.J.K. analyzed the data. S.Y.K. drated the manuscript. S.Y.K., Y.J.K., W.J., K.Y.S., H.S.P., and Y.S.K. contributed to the interpretation of data, critical revision for important intellectual content, and final approval of the article. All authors approved the final copy of the article.
Data availability
The data that support the findings of this study are available from National Health Insurance Service-Health Screening Cohort (NHIS-HEALS) database. To gain access, interested individuals should contact Korea National Health Insurance Service.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-82593-7.
References
- 1.Querfurth HW, LaFerla FM. Alzheimer's disease. N. Engl. J. Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 2.Burns A, Iliffe S. Alzheimer’s disease. BMJ. 2009;338:b158. doi: 10.1136/bmj.b158. [DOI] [PubMed] [Google Scholar]
- 3.Oberg M, Jaakkola MS, Woodward A, Peruga A, Prüss-Ustün A. Worldwide burden of disease from exposure to second-hand smoke: A retrospective analysis of data from 192 countries. Lancet. 2011;377:139–146. doi: 10.1016/S0140-6736(10)61388-8. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Global Health Estimates 2016: Disease Burden by Cause, Age, Sex, by Country and by Region, 2000–2016. https://www.who.int/healthinfo/global_burden_disease/estimates/en/index1.html (2018).
- 5.Briggs, R., Kennelly, S.P. & O’Neill, D. Drug treatments in Alzheimer’s disease. Clin. Med. (Lond).16, 247–253 (2016). [DOI] [PMC free article] [PubMed]
- 6.Xu W, et al. Meta-analysis of modifiable risk factors for Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry. 2015;86:1299–1306. doi: 10.1136/jnnp-2015-310548. [DOI] [PubMed] [Google Scholar]
- 7.Bloom GS. Amyloid-β and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71:505–508. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- 8.Anstey KJ, Ee N, Eramudugolla R, Jagger C, Peters R. A systematic review of meta-analyses that evaluate risk factors for dementia to evaluate the quantity, quality, and global representativeness of evidence. J. Alzheimers Dis. 2019;70:s165–s186. doi: 10.3233/JAD-190181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emmerzaal TL, Kiliaan AJ, Gustafson DR. 2003–2013: A decade of body mass index, Alzheimer's disease, and dementia. J. Alzheimers Dis. 2015;43:739–755. doi: 10.3233/JAD-141086. [DOI] [PubMed] [Google Scholar]
- 10.Qu Y, et al. Association of body mass index with risk of cognitive impairment and dementia: A systematic review and meta-analysis of prospective studies. Neurosci. Biobehav. Rev. 2020;115:189–198. doi: 10.1016/j.neubiorev.2020.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Buchman AS, Wilson RS, Bienias JL, Shah RC, Evans DA, Bennett DA. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005;65:892–897. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- 12.Stewart R, et al. A 32-year prospective study of change in body weight and incident dementia: The Honolulu-Asia Aging Study. Arch. Neurol. 2005;62:55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 13.Tolppanen A, et al. Midlife and late-life body mass index and late-life dementia: Results from a prospective population-based cohort. J. Alzheimers Dis. 2014;38:201–209. doi: 10.3233/JAD-130698. [DOI] [PubMed] [Google Scholar]
- 14.Gu Y, et al. Change in body mass index before and after Alzheimer's disease onset. Curr. Alzheimer Res. 2014;11:349–356. doi: 10.2174/1567205010666131120110930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravona-Springer R, Schnaider-Beeri M, Goldbourt U. Body weight variability in midlife and risk for dementia in old age. Neurology. 2013;80:1677–1683. doi: 10.1212/WNL.0b013e3182904cee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roh, E. et al. Body weight variability increases dementia risk among older adults: A nationwide population-based cohort study. Front. Endocrinol. (Lausanne).11, 291 (2020). [DOI] [PMC free article] [PubMed]
- 17.Zou, H. et al. Body-weight fluctuation was associated with increased risk for cardiovascular disease, all-cause and cardiovascular mortality: A systematic review and meta-analysis. Front. Endocrinol. (Lausanne).10, 728 (2019). [DOI] [PMC free article] [PubMed]
- 18.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 19.Park S, Jeon S, Jung S, Hwang J, Kwon J. Effect of late-life weight change on dementia incidence: A 10-year cohort study using claim data in Korea. BMJ Open. 2019;9:e021739. doi: 10.1136/bmjopen-2018-021739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwon S. Payment system reform for health care providers in Korea. Health Policy Plan. 2003;18:84–92. doi: 10.1093/heapol/18.1.84. [DOI] [PubMed] [Google Scholar]
- 21.Seong SC, et al. Cohort profile: The National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open. 2017;7:e016640. doi: 10.1136/bmjopen-2017-016640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koller D, Hua T, Bynum JPW. Treatment patterns with antidementia drugs in the United States: Medicare cohort study. J Am Geriatr Soc. 2016;64:1540–1548. doi: 10.1111/jgs.14226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mihas C, Kantzanou M. Body-weight fluctuations and outcomes in coronary disease. N. Engl. J. Med. 2017;377:94. doi: 10.1056/NEJMc1705832. [DOI] [PubMed] [Google Scholar]
- 24.U.S. Department of Health & Human Services. National Institutes of Health National Institute on Alcohol Abuse and Alcoholism. Helping Patients Who Drink Too Much: A Clinician’s Guide. https://casatondemand.org/wp-content/uploads/2019/07/helping-patients-clinicians-guide.pdf (2005).
- 25.Sergi G, Rui MD, Coin A, Inelmen EM, Manzato E. Weight loss and Alzheimer's disease: Temporal and aetiologic connections. Proc. Nutr. Soc. 2013;72:160–165. doi: 10.1017/S0029665112002753. [DOI] [PubMed] [Google Scholar]
- 26.Luchsinger JA, Mayeux R. Dietary factors and Alzheimer's disease. Lancet Neurol. 2004;3:579–587. doi: 10.1016/S1474-4422(04)00878-6. [DOI] [PubMed] [Google Scholar]
- 27.Salem N, Niebylski CD. The nervous system has an absolute molecular species requirement for proper function. Mol. Membr. Biol. 1995;12:131–134. doi: 10.3109/09687689509038508. [DOI] [PubMed] [Google Scholar]
- 28.Yen PK. Relationship of dementia and body weight. Geriatr. Nurs. 2005;26:79–80. doi: 10.1016/j.gerinurse.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Morrison CD. Leptin signaling in brain: A link between nutrition and cognition? Biochim. Biophys. Acta. 2009;1792:401–408. doi: 10.1016/j.bbadis.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lieb W, et al. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA. 2009;302:2565–2572. doi: 10.1001/jama.2009.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ershler WB. Interleukin-6: A cytokine for gerontologists. J. Am. Geriatr. Soc. 1993;41:176–181. doi: 10.1111/j.1532-5415.1993.tb02054.x. [DOI] [PubMed] [Google Scholar]
- 32.Cruz-Jentoft AJ, Landi F, Topinková E, Michel JP. Understanding sarcopenia as a geriatric syndrome. Curr. Opin. Clin. Nutr. Metab. Care. 2010;13:1–7. doi: 10.1097/MCO.0b013e328333c1c1. [DOI] [PubMed] [Google Scholar]
- 33.Loskutova N, Watts AS, Burns JM. The cause-effect relationship between bone loss and Alzheimer's disease using statistical modeling. Med. Hypotheses. 2019;122:92–97. doi: 10.1016/j.mehy.2018.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang, H.G., Park, H.Y., Ryu, H.U., & Suk, S.H. Bone mineral loss and cognitive impairment: The PRESENT project. Medicine (Baltimore).97, e12755 (2018). [DOI] [PMC free article] [PubMed]
- 35.Chang KV, Hsu TH, Wu WT, Huang KC, Han DS. Association between sarcopenia and cognitive impairment: A systematic review and meta-analysis. J. Am. Med. Dir. Assoc. 2016;17(1164):e7–1164.e15. doi: 10.1016/j.jamda.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 36.Grundman M, Corey-Bloom J, Jernigan T, Archibald S, Thal LJ. Low body weight in Alzheimer's disease is associated with mesial temporal cortex atrophy. Neurology. 1996;46:1585–1591. doi: 10.1212/WNL.46.6.1585. [DOI] [PubMed] [Google Scholar]
- 37.Hu X, et al. Neuroanatomical correlates of low body weight in Alzheimer's disease: A PET study. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2002;26:1285–1289. doi: 10.1016/S0278-5846(02)00291-9. [DOI] [PubMed] [Google Scholar]
- 38.Alhurani RE, et al. Decline in weight and incident mild cognitive impairment: Mayo Clinic Study of Aging. JAMA Neurol. 2016;73:439–446. doi: 10.1001/jamaneurol.2015.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jimenez A, et al. Weight loss in the healthy elderly might be a non-cognitive sign of preclinical Alzheimer's disease. Oncotarget. 2017;8:104706–104716. doi: 10.18632/oncotarget.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laws KR, Irvine K, Gale TM. Sex differences in cognitive impairment in Alzheimer's disease. World J. Psychiatry. 2016;6:54–65. doi: 10.5498/wjp.v6.i1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Altmann, A., Tian, L., Henderson, VW., Greicius, MD. & Alzheimer's Disease Neuroimaging Initiative Investigators. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann. Neurol. 75, 563–573 (2014). [DOI] [PMC free article] [PubMed]
- 42.Lin KA, et al. Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement. 2015;1:103–110. doi: 10.1016/j.trci.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Long J, He P, Shen Y, Li R. New evidence of mitochondria dysfunction in the female Alzheimer's disease brain: Deficiency of estrogen receptor-β. J. Alzheimers Dis. 2012;30:545–558. doi: 10.3233/JAD-2012-120283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galea LAM, Wainwright SR, Rose MM, Duarte-Guterman P, Chow C, Hamson DK. Sex, hormones and neurogenesis in the hippocampus: Hormonal modulation of neurogenesis and potential functional implications. J. Neuroendocrinol. 2013;25:1039–1061. doi: 10.1111/jne.12070. [DOI] [PubMed] [Google Scholar]
- 45.Anastasi TJ. Exploring the contribution of estrogen to amyloid-beta regulation: A novel multifactorial computational modeling approach. Front. Pharmacol. 2013;4:16. doi: 10.3389/fphar.2013.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mielke MM. Sex and gender differences in Alzheimer’s disease dementia. Psychiatr. Times. 2018;35:14–17. [PMC free article] [PubMed] [Google Scholar]
- 47.Montani, J.P., Viecelli, A.K., Prévot, A. & Dulloo, A.G. Weight cycling during growth and beyond as a risk factor for later cardiovascular diseases: the 'repeated overshoot' theory. Int. J. Obes. (Lond).suppl 4, s58–s66 (2006). [DOI] [PubMed]
- 48.Kashihara, K. Weight loss in Parkinson's disease. J Neurol. 253 Suppl 7, VII38–41 (2006). [DOI] [PubMed]
- 49.Moglia C, et al. Early weight loss in amyotrophic lateral sclerosis: Outcome relevance and clinical correlates in a population-based cohort. J. Neurol. Neurosurg. Psychiatry. 2019;90:666–673. doi: 10.1136/jnnp-2018-319611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from National Health Insurance Service-Health Screening Cohort (NHIS-HEALS) database. To gain access, interested individuals should contact Korea National Health Insurance Service.