Abstract
Probiotic use may be an efficacious treatment option to effectively manage symptoms of prenatal maternal anxiety and depression. Our primary aim was to test feasibility and acceptability for a probiotic randomized controlled trial (RCT) in pregnant women with pre-existing symptoms. This double-blind pilot RCT included 40 pregnant women with low-risk pregnancies and elevated depressive symptoms and/or anxiety. Once daily, participants orally consumed a probiotic (Ecologic Barrier) or a placebo, from 26 to 30 weeks gestation until delivery. A priori key progression criteria for primary outcomes were determined to decide whether or not a full RCT was feasible and acceptable. Secondary outcomes included depressive symptoms, anxiety, stress, and maternal bonding to offspring. In 19 months, 1573 women were screened; following screening, 155 women (10%) were invited for participation, of whom 135 (87%) received study information, and 40 women (30%) were included. Four out of six a priori determined criteria for success on feasibility and acceptability were met. After 8 weeks of intervention, there was no significant difference between the probiotic and placebo groups for secondary outcomes. The pilot trial was feasible and acceptable, but hampered by recruitment method and study design. Secondary endpoints did not reveal differences between the groups for improving maternal mood.
Subject terms: Psychology, Gastroenterology, Medical research, Signs and symptoms
Introduction
Prenatal maternal depression and anxiety are risk factors for adverse outcomes in mothers and children1,2. Simple treatments that effectively manage these symptoms in most women are greatly lacking3. This emphasizes the value of investigating potential efficacious adjuvant treatment options for pregnant women with these symptoms. Increased depressive symptoms and heightened anxiety are suggested to have a complex and multi-factorial etiology with multiple factors interacting and contributing to the development of symptoms4. These factors include personal resources, predisposing demographic factors, antenatal stressors5,6, and socio-cultural beliefs7. Other factors include physiological and epigenetic factors8, and possibly gut microbiota9,10.
The gut microbiota is suggested to communicate with the brain through nervous, endocrine, and immune signaling mechanisms, also referred to as the ‘microbiota-gut-brain-axis’11,12. Via this axis, alterations in the gut microbiota composition may contribute to the development of depressive and anxiety symptomatology13,14. Probiotics can affect gut microbiota and hence may reduce depressive symptoms and/or heightened anxiety in people with pre-existing symptoms9,15,16. Probiotics are defined as “live organisms that, when consumed in adequate amounts, confer a health benefit to the host”17. A randomized double-blind placebo-controlled trial (RCT) in pregnant women found that probiotics ingested during the perinatal period reduced depressive and anxiety symptoms during the postnatal period10. To date, no RCTs exist that investigate the possible efficacy of probiotics to improve maternal mood prenatally.
In psychology and medicine, RCTs are considered “gold standard” to evaluate therapeutic efficacy of new treatments18,19. Success of an RCT is largely determined by adequate recruitment of participants and compliance with the study protocol during a RCT20,21. Inadequate recruitment and non-compliance have negative consequences for participants, investigators, and in some cases public funds, and should therefore be prevented22,23. Pilot trials provide an excellent method to avoid these issues by assessing feasibility (i.e., whether potential participants would be willing to participate in the trial) and acceptability (i.e., whether the design of the trial is acceptable to participants) of a full RCT24.
The primary aim of this pilot RCT was to evaluate the feasibility in terms of recruitment and retention, and acceptability in terms of compliance and participants’ experiences of daily probiotic intake and the collection of data. The secondary aim of the present study was to exploratorily investigate the potential effects of probiotic intake, compared to placebo, on depressive symptoms, anxiety, stress (from here on named ‘psychosocial distress’), and by potentially affecting mood, maternal bonding to the offspring25,26.
Methods
Pilot RCT
The current pilot trial evaluated the feasibility and acceptability of a full RCT (a prenatal intervention on perinatal outcomes). Hence, the study design of the current pilot RCT reflected the design of the potential full RCT. The design reflects an integrative research approach combining both validated questionnaires and biological measures. This approach has been shown successful in many of our previous studies27–31 and previous probiotic trials9. A description of the ethics, participants, in- and exclusion criteria, trial design, randomization, blinding, treatment allocation and sample size of the pilot trial are provided below. Full details about the procedures, methods and outcome measures are described in detail in a separate paper32.
Ethics
The Medical Ethics Committee (METC) of the Radboud University Medical Center in Nijmegen, the Netherlands (NL57780.091.16) approved the study. Study procedures were conducted in accordance with the Helsinki Declaration of 1975 as revised in 1983. The trial was registered under trial number NTR6219 on 28/02/2017 (Netherlands Trial Register).
Participants
Forty healthy pregnant women (≥ 18 years) with at least mild depressive symptoms and/or anxiety in the late second/third trimester of an uncomplicated pregnancy (≥ 26 and ≤ 30 weeks gestation) participated in this study.
Inclusion and exclusion criteria
Inclusion criteria were: (1) elevated levels of depressive symptoms (EPDS ≥ 10) and/or anxiety (STAI-S ≥ 40), (2) start daily probiotic/placebo product intake between 26 and 30 weeks gestational age and continue until delivery. Exclusion criteria include: (1) multiple pregnancy, (2) high suicidal risk according to the suicidality subscale score on the MINI International Neuropsychiatric Interview, (3) illegal drug use, (4) psychiatric history of psychoses or bipolar disorder, (5) inflammatory bowel disease, (6) other autoimmune disorders and/or treatment with immunosuppressive therapy, (7) known pre-existing diabetes mellitus, hyperemesis gravidarum, hypertensive disorder, liver and/or renal disease, (8) malignancy and/or treatment with radiation or chemotherapy, (9) history of major gastro-intestinal surgery, (10) allergy or hypersensitivity to any ingredients in the Ecologic Barrier/placebo product, (11) history of using Ecologic Barrier, (12) presently using food containing probiotics (probiotic intake needed to stop at least 2 weeks prior to the start of the probiotic/placebo product intake), (13) not speaking and/or writing Dutch32.
Trial design
From 26 to 30 weeks gestation until delivery, participants once daily orally consumed a probiotic multispecies mixture (Ecologic Barrier; 2.5 × 109 CFU/g; daily dosage 2 g; Bifidobacterium bifidum W23, Bifidobacterium lactis W51, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24, Lactococcus lactis W19 and Lactococcus lactis W58) or a placebo. The probiotic product and placebo were indistinguishable regarding color, taste, and smell (Winclove Probiotics BV, Amsterdam, the Netherlands). Given that a potential full RCT would not investigate probiotics as a single management option for psychosocial distress, but the potential effectiveness in addition to participants’ current treatment, the use of any kind of co-medication or therapy was allowed.
To assess the potential effect of prenatal probiotic intake compared to placebo on perinatal maternal psychosocial distress women filled in questionnaires at baseline (time point 0 (t0)) and 8 weeks after starting probiotic/placebo intake (time point 1 (t1)). Additionally, to assess the potential long-term effects of prenatal maternal probiotic intake, maternal mood and maternal bonding to offspring were assessed 4 weeks post-partum (time point 2 (t2)).
Randomization, blinding, and treatment allocation
Forty women were randomly allocated to either the probiotic group or placebo group by a computer random number algorithm (1:1 allocation ratio), using blocks of four. Research personnel, who provided the probiotic or placebo and collected data, and participants were blinded to the treatment assignment and uninformed about the randomization sequence. Outcome assessors were also blinded to the treatment assignment. Further details on the intervention, intake, randomization and probiotic/placebo delivery procedure can be found in the protocol manuscript32.
Sample size
A sample size of 40 participants (~ 27% of a full trial) was deemed appropriate to inform about the primary outcomes. Based on a previous study by Steenbergen et al. (2015), which investigated the effect of the same probiotic product on mood in a non-pregnant healthy population, this sample size was also regarded sufficient to exploratorily assess the potential effects of probiotics33.
Primary outcomes of the pilot RCT
The primary outcomes of this pilot RCT were the feasibility and the acceptability. Feasibility was assessed in terms of recruitment (i.e., the proportion of pregnant women with psychosocial distress accepting the invitation to participate in the pilot trial and reasons for (non) participation) and retention (i.e., the number of women completing the study from enrolment to follow-up). Acceptability was measured in terms of compliance (i.e., the proportion of participants adhering to the intervention schedule and the collection of data) and participants’ experiences during the pilot trial. Based on these primary outcomes, six a priori key progression criteria were established, see Table 1. Not meeting one or more key progression criteria indicates that the study protocol needs to be revised before conducting a full RCT.
Table 1.
Primary quantitative outcomes, outcome measures and time point for each outcome (i.e., baseline (t0), 8 weeks after probiotic/placebo intake (t1) and 4 weeks post-partum (t2), (key) progression criteria for a full RCT, and (key) progression criteria that were met (yes/no).
| Primary quantitative outcomes | Outcome measure, time point for each outcome | Progression criteria for full RCT | Progression criteria met (yes/no) |
|---|---|---|---|
| A) Recruitment | 1) The proportion of contacted potential participants meeting the inclusion criteria and who consent to participate in the pilot trial | 30 participants recruited in 6 months | No (40 participants in 19 months)* |
| 2) The number of participants recruited at each recruitment site for the research database | No criteria set | – | |
| 3) Reasons for (non) participation | No criteria set | – | |
| B) Retention rate | 1) The proportion of participants completing the study from enrolment (t0) to follow-up (t2)1 | ≥ 90% of participants | Yes (98% of participants completed the pilot trial)* |
| C) Compliance | 1) The proportion of participants taking ≥ 80% probiotic/placebo product between t0 and t1 | 90% of participants | Yes (90% of participants)* |
| 2) The proportion of participants filling in electronic questionnaires at all time points | 90% of participants | Yes (100% of participants)* | |
| 3) The proportion of participants filling in the cry diary at t2 | 90% of participants | No (85% of participants) | |
| 4) The proportion of participants filling in evaluation form at t2 | 90% of participants | Yes (98% of participants) | |
| 5) The proportion of participants collecting maternal vaginal samples at t0 and t1 | 90% of participants | No (83% of participants) | |
| 6) The proportion of participants collecting maternal microbial samples at t0 and t1 | 90% of participants | No (85% of participants) | |
| 7) The proportion of participants collecting infant microbial samples at t2a and t2b | 90% of participants | No (83% of participants) | |
| 8) The proportion of participants allowing collection of hair samples at t2 | 90% of participants | No (83% of participants) | |
| D) Participants’ experiences | 1) Level of acceptability of electronic questionnaires | No criteria set | - |
| 2) Level of acceptability collection of biological samples2 | No criteria set | - | |
| 3) Level of satisfaction (positive/negative aspect) | No criteria set | - | |
| 4) Level of satisfaction (execution and organization) | No criteria set | - | |
| 5) Level of acceptability of the pilot trial in practice (i.e., perceived burden) | Mean score of ≤ 3 | No (Mean 4; SD 2.2)* | |
| 6) Level of acceptability of the pilot trial in principle (future participation) | Mean score of ≥ 8 | Yes (Mean 8; SD 1.7)* |
Key progression criteria are bold.
1Full completion of the study defined as: the closing home visit has taken place at t2.
2Of the 34 participants who collected biological samples.
*Key progression criteria. If one of more key progression criteria is not met revisions to study protocol should be made prior to conducting a full RCT.
Recruitment
In order to recruit 40 participants, local advertisement to participate in an online questionnaire study on mood and experiences during pregnancy was provided at six sites in the Netherlands: two ultrasound centers, an academic hospital, a lactation consultant practice, and two mental health centers. Participants meeting the criteria for inclusion were invited to participate in the pilot RCT; they were included in the study after providing written informed consent. Additional information of the way participants were identified and consent was obtained can be found in the protocol publication32.
Retention
The proportion of participants completing the study from enrolment (t0) to follow-up (t2) was recorded on Case Report Forms (CRFs) throughout the study.
Compliance
Data on the proportion of participants taking ≥ 80% probiotic/placebo product was collected at t2 by the CI by counting the sachets that were left from the previous weeks.
Participants’ experiences
A self-created evaluation form was used to assess participants’ experiences during the pilot trial. The questionnaire included nine questions in total. Five open questions on experiences of filling in questionnaires during the pilot trial (i.e., length, number of questionnaires, electronic method), the collection of maternal/infant stool and vaginal samples (i.e., number of samples, explanation about the method of collection, potential difficulties in collecting samples), and the collection of the hair sample; and two open questions on the level of satisfaction (“Regarding the study in general, which aspects did you experience as most positive and negative?” “Do you have any general remarks regarding execution and organization of this study?”). Additionally, two closed questions on the level of satisfaction were included (i.e., acceptability of the pilot trial in principle and in practice). To rate the acceptability of the pilot trial in principle, participants indicated the likelihood of participating in a similar trial in the future (0 = ‘no chance of future participation’ to 10 = ‘very high chance of future participation’); for the acceptability of the pilot trial in practice participants rated on a scale of 0 to 10 the perceived burden of the trial (0 = ‘no burden’ to 10 = ‘very large burden’).
Secondary outcomes of the pilot RCT
A brief description of all measurement tools used can be found below; an elaborate description, as well as a description of other measures not used in the analyses for the current manuscript, is provided in the protocol paper32.
Demographic characteristics
Participants filled out questionnaires on demographics, health behavior and lifestyle. Demographic variables were collected during the screening phase (time point -1 (t-1)).
Maternal depressive symptoms
Edinburgh Postnatal Depression Scale
Pre- and postnatal depressive symptoms were measured using the Edinburgh Postnatal Depression Scale (EPDS), which is a validated tool to screen for antenatal and postnatal depressive symptoms34. The EPDS consists of 10-items scored on a 4-point scale with a maximum score of 30. A sum score of 10 or more was considered to reflect the presence of at least mild depressive symptoms35.
Leiden Index of Depression Sensitivity-Revised
Negative thoughts associated with sad mood, as a predictor of depression, was measured with the validated Leiden Index of Depression Sensitivity-Revised (LEIDS-R)36. The questionnaire consists of 34 items for which participants indicate to what extent each statement applies to them on a 5-point Likert scale. A higher total score indicates more cognitive reactivity to sadness, which indicates a potential larger tendency to develop depression.
Maternal anxiety
Pregnancy-Related Anxiety Questionnaire-Revised (Pregnancy-related anxiety)
Pregnancy-specific anxiety was measured using the Pregnancy Related Anxiety Questionnaire-Revised (PRAQ-R), which is a validated 10-item tool scored on 5-point scale that screens for pregnancy-related anxiety in pregnant women37. Two subscales “fear of giving birth” and “fear of bearing a handicapped child” were used. Higher scores on these subscales indicate more pregnancy-related anxiety.
State-trait Anxiety Inventory (general anxiety)
The state version of the State-trait Anxiety Inventory (STAI-S), a validated self-report questionnaire for pregnant women, was used to measure general anxiety38. The STAI-S consists of 20 statements related to current feelings of anxiety, which are scored on a 4-point Likert scale (maximum score of 80). Higher scores indicate more general feelings of anxiety. A cut-off score of 40 has been shown to have high sensitivity and specificity among pregnant women in their third trimester of pregnancy to detect anxiety disorder39.
Maternal stress
Pregnancy Experience Scale (pregnancy-related daily hassles)
The validated Pregnancy Experience Scale (PES) was used to measure maternal appraisal of daily, pregnancy-specific hassles and uplifts40. The questionnaire consists of 43-items on which participants rated the degree to which these specific experiences constituted a hassle and uplift on two separate 5-point Likert scales. To calculate the total intensity score for hassles, a sum of scores for hassles was calculated and divided by the number of endorsed items for hassles. A similar calculation for total intensity score for uplifts was performed. A composite ratio score was computed relating hassles to uplifts (i.e., total intensity score for hassles divided by total intensity score for uplifts). A ratio larger than 1 indicate more hassles than uplifts (i.e., larger negative emotional valence towards pregnancy); a ratio smaller than 1 indicate more uplifts than hassles.
Everyday Problem List (general daily hassles)
The validated 49-item Dutch Everyday Problem List (APL) measured the occurrence and intensity of general daily hassles during pregnancy41. Participants indicated which specific daily hassles they had experienced and scored on a 4-point Likert scale how much each of them had bothered them. The total sum of the Likert scale points was divided by the total number of daily hassles reported. A higher value indicate more perceived stress due to daily hassles.
Mother to infant bonding
The Maternal Antenatal Attachment Scale
The validated Maternal Antenatal Attachment Scale (MAAS) assessed participant’s bonding with her fetus42. The MAAS consists of 19 items scored on a 5-point Likert scale. Higher total sum scores indicate higher preoccupation with the unborn child and higher quality bonding.
The Maternal Postnatal Attachment Scale
The Maternal Postnatal Attachment Scale (MPAS), a validated tool among pregnant women, was used to measure the strength of the mother to infant bonding43. The MPAS questionnaire consists of 19 items that are scored on a 5-point Likert scale. Higher total sum scores reflect higher quality of maternal to infant bonding.
Potential confounders
Previous literature reported several variables that are associated to maternal mood11,44. These include sleep, stressful events, and the use of (non) pharmacological treatments (e.g. yoga-based interventions)45. Data on these variables were collected at baseline (t0) and 8 weeks after treatment (t1) to control for undesired differences between the treatment and placebo groups that may confound potential treatment effects.
Sleep
Sleep (i.e., quality and disturbances during the previous month) was measured using the validated Pittsburgh Sleep Quality Index (PSQI). The questionnaire consists of 19-items, which are scored on a 4-point Likert scale. A total sum score of > 5 indicates poor sleep quality46.
Stressful events
In the current absence of a validated questionnaire on stressful life events for pregnant women, we constructed a 7-item stressful event questionnaire. The items were based on previous literature on predictors of prenatal anxiety and depression4,47. In the questionnaire, participants listed whether they experienced a stressful event (yes/no) during the first eight weeks of probiotic intake. These items included: “sickness of oneself”, “sickness of partner”, “death of a close family member/friend”, “relational/marital problems”, “divorce/separation”, “financial problems”, “domestic violence”, “other” (See Supplementary Fig. 1). Items were summed up so that more reported stressful events were indicative for higher incidence of stressful life events during the probiotic/placebo product intake period.
(Non) Pharmacological treatments
Regarding the use of (non) pharmacological treatments, participants were asked whether they started to use new (non) pharmacological treatments during the intervention period (yes/no).
Side effects and serious adverse events
Side effects (“constipation”, “flatulence”, “nausea”, “diarrhea”, “other”) were recorded at baseline (t0) and 8 weeks after probiotic/placebo intake (t1). Serious adverse events (SAEs) were recorded throughout the study.
Statistical analyses
Qualitative analysis for primary outcomes
Qualitative data were analyzed according to the principles of the Framework approach48. Two researchers (PD and HL; see Acknowledgements) independently developed an analytical framework by reading through all responses on the interview questions and by coding the transcripts separately. The researchers consequently compared the codes and once agreed to a set of codes, grouped them together to form themes and categories to establish an overarching analytical framework. The analytical framework was then used to index all transcripts using an Excel spreadsheet chart (Microsoft Excel, version 14.6.6, 2011).
Quantitative analyses for primary and secondary outcomes
Demographic characteristics of participants were analyzed using descriptive statistics as mean/median and percentages. Following the intention-to-treat principle, data of all participants were included in the statistical analyses. Mean/median and frequencies were compared using t-tests and Fisher’s exact test, respectively. For each questionnaire, mean total scores were initially compared between placebo and probiotic groups by two-sample independent t-tests to detect between-groups effects at baseline (t0), after 8 weeks of probiotic/placebo intake (t1) and 4 weeks post-partum (t2). Data were tested for equal variance and where data did not have equal variance, Welsh’s correction was used. To detect within-groups differences over time, repeated measures ANOVA were performed with time (t0, t1 and t2) as within-subject factor and group (placebo vs. probiotic) as between-subjects factor. Tukey HSD post-hoc test was used to identify significant differences in the means between pairs. A p value of < 0.05 was considered significant. All analyses were performed with the use of R software (R studio, version 1.1.463, 2018; www.r-project.org). Regarding the primary outcome on participants’ experiences, themes and categories were calculated as a percentage of the total number of participants reporting that specific theme or category (see Qualitative analysis for primary outcomes for qualitative analysis method).
Results
Preliminary analyses
The probiotic and placebo groups did not significantly differ with regard to maternal characteristics (t0; M = 27.8 weeks gestation, SD = 1.5) (independent t-test; Fisher’s exact test) (see Table 2). Additionally, there were no significant differences between probiotic and placebo groups for starting new (non) pharmacological treatments between pre-treatment (t0; M = 27.8 weeks gestation, SD = 1.5) and t1 (M = 35.8 weeks gestation, SD = 1.5) (Fisher’s exact test, p > . 05). Furthermore, no significant differences were found for the number of reported stressful life events during the first 8 weeks of probiotic intake for participants in the probiotic versus placebo group [F(1,38) = 0.176, p > 0.05]. There was a significant effect of time on sleep [F(1, 38): 8.72, p < 0.05], with sleep quality worsening over pregnancy for the group as a whole. However, no significant effects of time by group interaction were found [F(1,38) = 0.29, p > 0.05]. In conclusion, new (non) pharmacological treatments, stressful events, and quality of sleep were not regarded as confounding variables for differences between the groups in depressive symptoms, anxiety or stress, and therefore not included in the analyses as confounders.
Table 2.
Pre-treatment demographic characteristics of women receiving probiotic or placebo product.
| Probiotic (N = 20) | Placebo (N = 20) | |
|---|---|---|
| Age (years) [Mean, SD] | 29.65 (3.9) | 31.7 (4) |
| Educational level [n (%)] | ||
| Lower secondary education | 0 (0%) | 2 (10%) |
| Lower secondary vocational education | 4 (20%) | 3 (15%) |
| Higher secondary education | 1 (5%) | 0 (0%) |
| Tertiary education | 15 (75%) | 15 (75%) |
| Number of previous pregnancies [median (IGR)] | 1.5 (1.25) | 2 (1) |
| Marital status [n (%)] | ||
| Married/registered partnership | 15 (75%) | 13 (65%) |
| Unmarried with partner | 5 (25%) | 6 (30%) |
| Single | 0 (0%) | 1 (5%) |
| Employment status [n (%)] | ||
| Employed | 16 (80%) | 19 (95%) |
| Unemployed | 4 (20%) | 1 (5%) |
| Ethnicity [n (%)] | ||
| Dutch | 20 (100%) | 18 (90%) |
| Other | 0 (0%) | 2 (10%) |
| Weeks gestation at start of intervention [median (IGR)] | 28 (2) | 28 (2.25) |
| Psychiatric diagnosis [n (%)] | ||
| Depression | 1 (5%) | 2 (10%) |
| Anxiety disorder | 2 (10%) | 2 (10%) |
| Borderline disorder | 0 (%) | 1 (5%) |
| Posttraumatic stress disorder | 0 (0%) | 1 (5%) |
| Pervasive Developmental Disorder Not Otherwise Specified (PDD-NOS) | 1 (5%) | 0 (0%) |
| History of depression [n (%)]1 | ||
| Prenatal depression | 0 (0%) | 1 (5%) |
| Postpartum depression | 2 (20%) | 6 (30%) |
| Non- pharmacological treatment(s) [n (%)] | 9 (45%) | 6 (30%) |
| Cognitive behavioral therapy | 1 (5%) | 2 (10%) |
| Psychotherapy | 2 (10%) | 0 (0%) |
| Rapid eye-movement therapy | 1 (5%) | 1 (5%) |
| Psychosocial support | 4 (20%) | 2 (10%) |
| Haptotherapy | 1 (5%) | 1 (5%) |
| Pharmacological treatment(s) for depression or anxiety [n (%)] | 1 (5%) | 3 (15%) |
| Food supplement(s) use [n (%)]2 | 6 (30%) | 3 (15%) |
| Vitamin(s) use [n (%)] | 16 (80%) | 18 (90%) |
| Smoking [n (%)] | 0 (0%) | 2 (10%) |
| Alcohol [n (%)] | 0 (0%) | 0 (0%) |
| Diet [n (%)] | ||
| Lactose free | 0 (0%) | 1 (5%) |
| Biological food | 0 (0%) | 1 (5%) |
| Gluten free | 1 (5%) | 0 (0%) |
| Vegetarian (no meat) | 0 (5%) | 2 (10%) |
| Vegan | 0 (0%) | 0 (0%) |
| Baseline mood scores [mean (SD)] | ||
| Depressive symptoms3 | 12.8 (4.3) | 12.5 (3.9) |
| Cognitive reactivity4 | 49.4 (10.4) | 43.45 (10) |
| Pregnancy-related anxiety—fear handicapped child5 | 8.7 (3) | 9.7 (4.5) |
| Pregnancy-related anxiety—fear of birth6 | 7.4 (2.9) | 7.65 (3.2) |
| General anxiety7 | 47.75 (9.1) | 47.55 (9.9) |
| General daily hassles (total score)8 | 0.71 (0.33) | 0.71 (0.31) |
| Pregnancy-related daily hassles (uplifts)9 | 1.50 (0.43) | 1.45 (0.41) |
| Pregnancy-related daily hassles (hassles)9 | 0.96 (0.4) | 0.99 (0.34) |
| Mother to infant bonding10 | 66.8 (8.04) | 65.5 (6.44) |
1Out of 10 women in probiotic group and 13 women in placebo group with previous pregnancies.
2(Homeopathic) iron supplements, bee pollen etc.
3Edinburgh Postnatal Depression Scale (EPDS); scale: 0–3034.
4Leiden index of depression sensitivity-revised (LEIDS-R); scale 0–13651.
5Pregnancy Related Anxiety Questionnaire-Revised (PRAQ-R); scale 3–2037.
6Pregnancy Related Anxiety Questionnaire-Revised (PRAQ-R); scale 3–1537.
7State Trait Anxiety Inventory (STAI-S); scale: 20–8038.
8Everyday Problem Checklist (APL); (-1)–141.
9Pregnancy Experience Scale (PES); scale 0–440.
10Maternal Antenatal Attachment Scale (MAAS); scale 19–9542.
Primary outcomes
Four out of the six key progression criteria were met: 98% of participants completing the study (i.e., the closing home visit took place at t2 to finalize the study), 90% of participants taking ≥ 80% probiotic or placebo product for eight weeks straight, 100% of participants filling in online questionnaires at all time points, and acceptability of the pilot in principle (mean score of 8, SD = 1.7). The progression criterion for recruitment (40 participants recruited in 19 months) and the acceptability of the pilot in practice (mean score of 4, SD = 2.2) were not met (see Table 1).
Recruitment
Participants were recruited from March 2017 to September 2018 for the research database by local staff. During the first three months of recruitment (March–May 2017) it appeared that only 2 of the 14 potential participants consented to participate. In order to improve the recruitment rate, a second echo center (September 2017), an academic hospital (November 2017), a lactational practice (December 2017), and two mental healthcare centers (January 2018) were additionally added as recruitment sites.
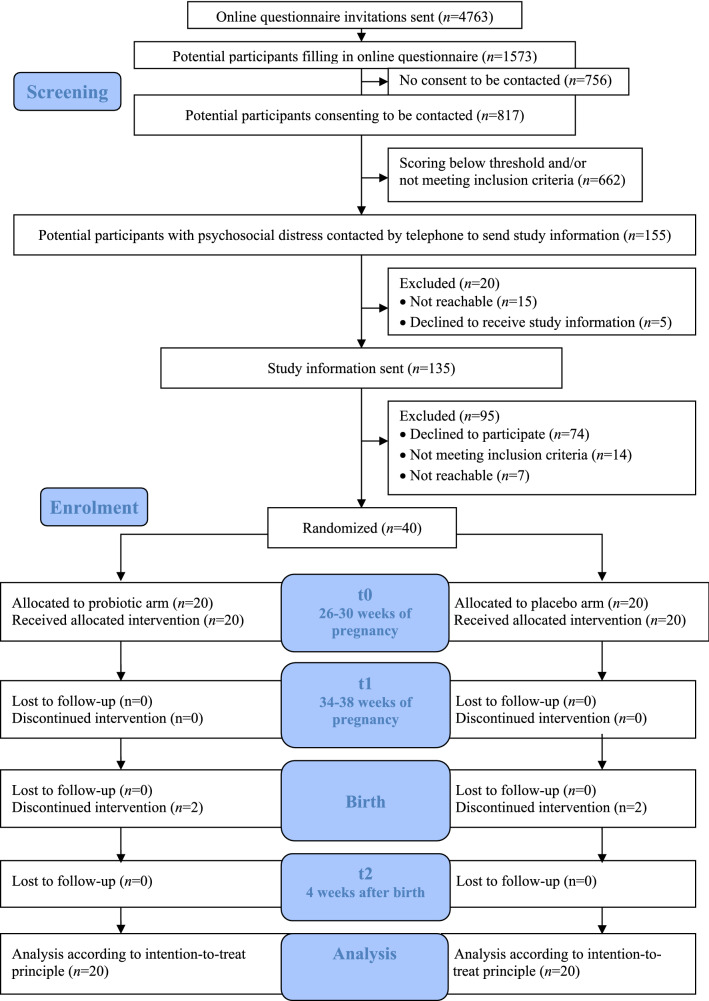
Regarding the recruitment rate, of the 1573 potential participants filling in the online questionnaire, 817 of them consented to be included into the research database to be contacted for other research on psychosocial distress. Of the 817 women, 155 (19%) scored above the threshold for psychosocial distress and met the study inclusion criteria. Most of the 155 women agreed to receive study information (n = 135; 87% of the women contacted by phone) and finally 40 women agreed to participate in the pilot trial (30%) (see Fig. 1).
Figure 1.
Participants’ flow through the study based on the Consolidated Standards of Reporting Trials (CONSORT) flow diagram. From the research database, participants were screened for eligibility and contacted by phone by the coordinating investigator whether they wanted to receive study information. Eligible participants who provided consent were randomized in either the probiotic or placebo group (t0). Measurements took place between baseline (t1) and 4 weeks post-partum (t2). Participants were recruited from March 2017 to September 2018.
With respect to the number of participants recruited at each of the recruitment sites for the research database, Table 3 shows the recruitment numbers per recruitment site. As can be observed in the table, most participants were recruited through the echo centers. At echo center 1, a total of 4147 email invitations were sent to potential participants, of which 1088 women fully completed the questionnaire (response rate 26%). Regarding echo center 2, a total of 596 email invitations were sent and 441 women filled in the questionnaires (response rate 74%). At the academic hospital email addresses from potential participants were collected and a link of the online questionnaire was provided on flyers on site; a total of 20 email invitations were sent to women visiting the academic hospital. At the lactational practice and mental healthcare centers, only a link of the online questionnaire was provided on flyers and therefore the response rate could not be calculated.
Table 3.
Recruitment method, recruitment period, number of potential participants who filled in the online questionnaire, average number of filled in online questionnaires per month, number of potential participants providing consent to be included in the research database rate, and number of included participants per recruitment site.
| Recruitment site | Recruitment method | Recruitment period (Months) | Total number of filled in online questionnaires (N) | Average number filled in online questionnaire per month (N) | Number of women providing consent to be included in the research database (N, %) | Pilot trial participants (N, %) |
|---|---|---|---|---|---|---|
| Echo center 1 | Questionnaire invitation sent1 | 19 | 1088 | 57 | 583 (54%)4 | 27 (68%) |
| Echo center 2 | Questionnaire invitation sent2 | 12 | 441 | 37 | 212 (48%)4 | 12 (30%) |
| Lactational practice | Flyer in waiting room3 | 8 | 11 | 1 | 5 (45%)4 | 0 (0%) |
| Mental healthcare center 1 | Flyer in waiting room3 | 8 | 3 | 0 | 3 (100%)4 | 1 (3%) |
| Mental healthcare center 2 | Flyer in waiting room3 | 8 | 0 | 0 | 0 (0%) | 0 (0%) |
| Academic hospital | Flyer in waiting room3 | 10 | 30 | 3 | 14 (48%)4 | 0 (0%) |
| Total | – | 1573 | – | 817 (52%) | 40 (3%) |
1The online questionnaire invitation was sent to potential participants 3 weeks after the 20-week ultrasound.
2When women visited echo centre 2 for their 20-week ultrasound, personal email addresses were collected; 3 weeks thereafter the online questionnaire invitation was sent per email.
3Flyer with link to online questionnaire was located in the waiting room.
4Percentage of total number of filled in online questionnaires per recruitment site.
Regarding reasons for (non) participation, there were four specific moments when participants made the decision to join the study: after speaking with researcher by phone, after speaking to midwife, after speaking to partner, and after reading study information. For some participants, there was no specific moment when they made the decision to join (see Table 4). Four main reasons concerning reasons why participants participated in the pilot trial were identified: potential benefits, study topic, familiarity with research, and moral obligation. The most widely reported reason to join the study was the potential benefit that participation might bring participants, and that participation might help other women, healthcare and science (see Table 5).
Table 4.
Description of reported moments of decision to participate in the pilot trial.
| Moment | Specific elements | Representative comments from participants |
|---|---|---|
| 1. After speaking with researcher by phone | Personal interaction with the researcher, personal approach | “After filling in the online questionnaire, I was positive about joining a study, and when I heard you on the phone I decided to join” |
| Safety reassurance | “I decided to join the study after I was able to ask you my remaining questions regarding safety” | |
| Discussing protocol compliance | “When I heard that I could leave out the collection of some samples, if it would be too much of a burden for me” | |
| 2. After speaking to midwife | Midwife’s reassurance and encouragement | “After I spoke with you on the phone, I discussed the study with my husband and midwife. The fact that my midwife said that she had also thought about advising me to sign up was especially reassuring.” |
| 3. After speaking to partner | Partner’s agreement and encouragement | “After discussing the study with my husband; he was very enthusiastic about the study” |
| 4. After reading study information | “After reading the study information, I directly emailed and signed up” | |
| 5. No specific moment | “There was no specific moment. I first had to let the information sink in, because at first I was afraid that it would cost too much time” |
Table 5.
Description of reasons for participation in the pilot trial.
| Reason | Specific elements | Representative comments from participants |
|---|---|---|
| 1. Potential benefits | Personal yield | “In my situation it is appealing to me to receive something that might help me, even if it is a placebo effect” |
| Potential benefit for other people | “I’m joining the study because it eventually might help other people to reduce or prevent symptoms” | |
| Potential benefit for healthcare | “I had depressive symptoms after my first pregnancy. It would be nice if there was an alternative to medication to reduce depressive symptoms” | |
| Potential benefit for science | “I believe it is important to help scientific research, and with that the health of mothers and babies” | |
| Cost/benefit analysis | “I like to contribute to studies which pose no burden on my child and which contribute to something I find useful. Given that your study included both, I directly signed up to join. To me, research is useful when it is more focused on healthy life style and eating patterns than on medication” | |
| 2. Study topic | Personal interest in (gut) microbiota, mind–body interaction, diet, mood | “I choose to join because the research topic appeals to me: the link between the brain/mood/mental wellbeing, the gut and diet” |
| Prenatal anxiety and depression being a taboo subject | “Prenatal anxiety and depression are important themes, but they’re a taboo” | |
| Recognizing oneself in the study population | “I am familiar with having depressive symptoms during pregnancy” | |
| Through friends/family being familiar with food supplements, or anxiety and depression | “I have a brother who mentally felt much better after taking vitamin D (..) and a sister-in-law who was suicidal during her pregnancy” | |
| 3. Familiarity with research |
Probiotic product Previous participation in research |
“I’m joining because this study includes a safe and natural product (I would not have participated if a medication would have been tested)” “In the past, I joined a scientific research study on gut microbiota” |
| 4. Moral obligation | Important to participate in scientific research to generate more knowledge | “I see it as a moral obligation to join: this research is important and therefore important to contribute to it” |
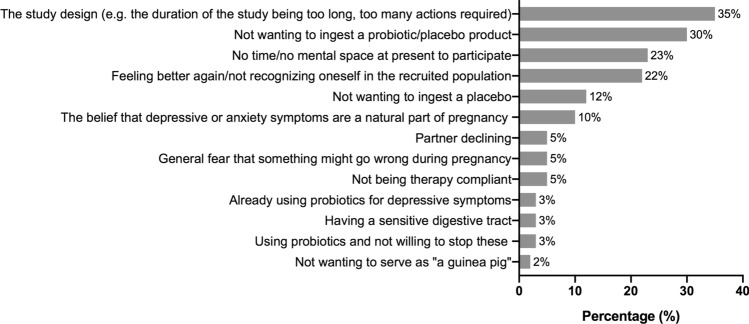
Sixty women provided reasons why they did not want to participate after receiving study information. The main reason to decline participation was the study design (e.g. the duration of the study being too long, too many actions required) (Fig. 2).
Figure 2.
Reasons for declining study participation as a percentage of the total number of women who were invited to join the pilot trial and provided reasons for declining participation (N = 60).
Retention
Ninety-eight percent of recruited participants completed the pilot trial. One participant was unreachable to schedule the closing visit (see Fig. 1).
Compliance
Thirty-nine participants (98%) complied with the intervention regime of > 80% of probiotic and placebo intake during the first 8 weeks. For one participant, compliance was unknown during the first eight weeks because of the participant being unreachable to schedule a home visit. Thirty-six women (90% of the total population) adhered to probiotic or placebo ingestion of > 80% of probiotic intake between t1 up to delivery. Several reasons were reported for non-adherence between t1 and delivery: daily intake posed too much burden on daily life (n = 1), not observing an effect on mood (n = 1), receiving new medication and not wanting to ingest the sachets and medication in parallel (n = 1). One participant was unreachable at the end of the study. Furthermore, the compliance criterion for the evaluation form was met with 98% of women filling in the evaluation form. Other compliance criteria that were not met included: filling in the cry diary and collecting vaginal and microbial samples (83–85% compliance rate) (see Table 1). Participants who did not collect these data indicated that it posed too much burden on their daily life to fill in the cry diary and/or to collect biological samples.
Participants’ experiences
The progression criterion for level of acceptability of the pilot in principle was met (i.e., likelihood of future participation in a trial; mean score of 8, SD = 1.7; scale range = 1–10), but not the acceptability of the pilot in practice (mean score of 4, SD = 2.2; scale range = 1–10, lower score indicating lower perceived burden) (see Table 1). Regarding the level of acceptability of electronic questionnaires and collection of biological samples, the majority of participants indicated that they found the number of online questionnaires during the pilot trial appropriate (95%) and the online method of filling in questionnaires convenient (87%). With respect to the collection of maternal stool and vaginal samples, most women indicated that clear user guides were provided (88% and 91%, respectively) and that the number of samples to be collected was doable/appropriate (88% and 76%, respectively). Regarding the collection of infant stool samples, 33% of women indicated that the collection guide was clear and 30% found the number of infant samples to be collected appropriate. Most women indicated that they had no problem with collection of hair (88%), however some women (9%) found it a little scary to have hair collected (see Supplementary Fig. 2A–E). Most positive experienced aspects of the pilot trial were the personal guidance and interaction with the researcher (37%) and the home visits (24%). Most negative experienced aspects were the collection of biological samples (32%) and the ingestion of the probiotic/placebo product (24%) (see Supplementary Fig. 3A,B).
Secondary outcomes
Maternal psychosocial distress (i.e., depressive symptoms, anxiety, stress) was scored both pre-treatment (t0; M = 27.8 weeks gestation, SD = 1.5), after 8 weeks of probiotic/placebo intake (t1; M = 35.8 weeks gestation, SD = 1.5) and 4 weeks post-partum (t2; M = 4.35 weeks postpartum, SD = 0.8). Note that depression/cognitive reactivity to sad mood (LEIDS-R), pregnancy-related anxiety (PRAQ-R), pregnancy-related hassles (PES), general daily hassles (APL), and antenatal attachment (MAAS) questionnaires were only filled in at t0 (M = 27.8 weeks gestation, SD = 1.5) and t1 (M = 35.8 weeks gestation, SD = 1.5). The Maternal Postnatal Attachment Scale (MPAS) was filled in at t2 (M = 4.35 weeks postpartum, SD = 0.8). For the secondary analyses, all 40 participants were included in the originally assigned groups.
Depression (EPDS)
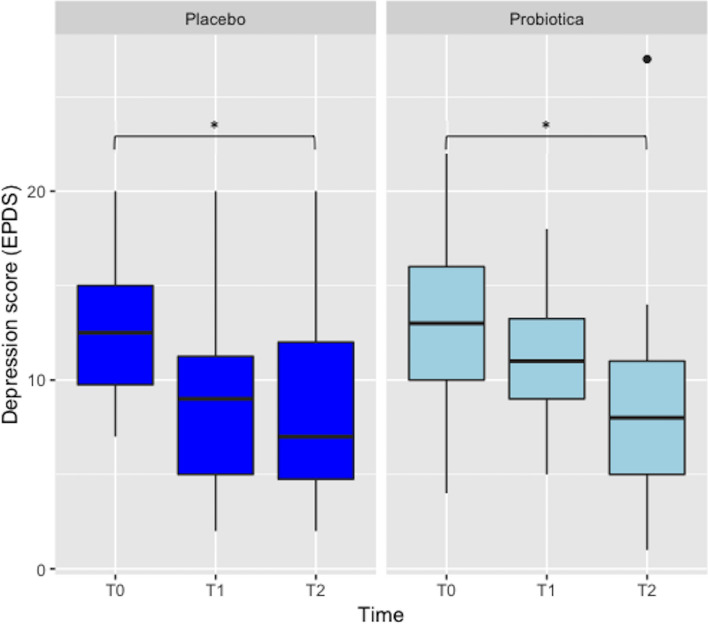
ANOVAs performed on the EPDS score revealed a main negative effect of time [F(2,76) = 11.2, p < 0.05]. No effect was observed for group [F(2,38) = 0.8, p > 0.05] nor for a time by group interaction [F(2.76) = 0.84, p > 0.05]. Tukey HSD post-hoc tests were performed and revealed that participants in the probiotic group had a significant decrease in depressive symptoms between baseline (t0) and 4 weeks postpartum (t2) (p = 0.04). Similarly, depressive symptoms of women in the placebo group significantly decreased between baseline (t0) and 4 weeks postpartum (t2) (p = 0.04). Thus, depressive symptoms decreased in the group as a whole from baseline to after the intervention, irrespective of study group (see Fig. 3).
Figure 3.
Depressive symptom scores of women in the probiotic (N = 20) and placebo groups (N = 20) measured at baseline (t0), 8 weeks after probiotic/placebo intake (t1) and 4 weeks postpartum (t2). *p < 0.05.
Depression/cognitive reactivity to sad mood (LEIDS-R)
ANOVAs on LEIDS-R total score showed a significant negative effect of time [F(1,38) = 12.55, p = 0.02] and group [F(1,38) = 5.82, p < 0.001], but no time by group interaction [F(1,38) = 0.92, p > 0.05]. Tukey HSD post-hoc test showed that women in the placebo group had significantly lower total scores on cognitive reactivity at t0 than women in the probiotic group at t1 (p = 0.001).
General anxiety (STAI-S)
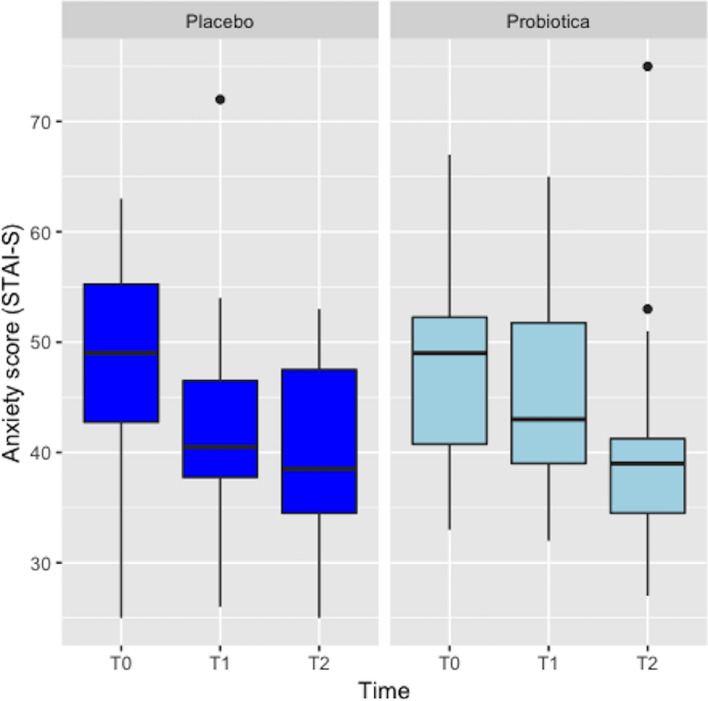
Symptoms of general anxiety decreased in both groups. ANOVAs revealed a significant negative effect of time on general anxiety [F(1,38) = 10.79, p < 0.05], but not for group [F(1,38) = 0.34, p > 0.05] and time by group interaction [F(1,38) = 0.47, p > 0.05]. Tukey HSD post-hoc test revealed that the decrease in anxiety was only significant for the total population (p < 0.001) (Fig. 4).
Figure 4.
Anxiety symptom scores of women in the probiotic (N = 20) and placebo groups (N = 20) measured at baseline (t0), 8 weeks after probiotic/placebo intake (t1) and 4 weeks postpartum (t2).
Pregnancy-related anxiety (PRAQ-R)
A significant negative effect of time on pregnancy-related anxiety was found for the subscale fear of bearing a handicapped child [F(1,38) = 6.37, p < 0.05], but no effect was observed for time by group interaction [F(1,38) = 0.7, p > 0.05]. For the subscale fear of giving birth, no significant effects of time [F(1,38) = 2.5, p > 0.05], group [F(1,38) = 0.2, p > 0.05], nor time by group interaction were found [F(1,38) = 0.28, p > 0.05].
Pregnancy-related daily hassles and general daily hassles (PES and APL)
There was a significant negative effect of time on pregnancy-related daily hassles (PES composite ratio score; [F(1,38) = 14.6, p < 0.05]) and general daily hassles (APL stress score; [F(1,38) = 13.9, p < 0.05]). Tukey HSD post-hoc showed no significant decrease in pregnancy-related daily hassles from baseline to t1 in the probiotic group (p = 0.18) or placebo group (p = 0.19). Similarly, the decrease in general daily hassles was not significant in neither probiotic nor placebo group (p = 0.37, p = 0.38, respectively).
Maternal Antenatal Attachment Scale (MAAS)
ANOVAs yielded a main positive effect for time on maternal attachment score, with maternal attachment increasing for the group as a whole [F(1,38) = 27.8, p < 0.05]. Tukey post-hoc test showed no significant time by group interaction (p = 1).
Maternal Postnatal Attachment Scale (MPAS)
Women in the probiotic group (M = 67.35, SD = 8.42) and women in the placebo group (M = 68.85, SD = 7.08) had comparable attachment scores postpartum (t38 = 0.6, p = 0.55).
Side effects and serious adverse events
No significant differences were found in the number of side effects and SAEs between the probiotic and placebo groups (Fisher’s exact test, p = 0.16, p = 1, respectively). Seventeen participants in the probiotic group and 12 participants in the placebo group reported side effects during intake of the probiotic/placebo product. Most reported side effects were constipation (10 probiotic group vs. 5 placebo group), flatulence (6 probiotic group vs. 9 placebo group), nausea (7 probiotic group vs. 4 placebo group), and diarrhea (5 probiotic group vs. 3 placebo group) during the first 8 weeks of probiotic intake. Four SAEs occurred: in the probiotic group, two participants were admitted to the hospital, one for pregnancy induced hypertension and the second for preterm delivery (34 weeks gestational age). In the placebo group, one participant was admitted due to severe headache and another participant had placenta praevia. No SAEs were related to study participation.
Discussion
The PIP pilot trial was generally feasible and acceptable. Women were positive about future participation in a similar trial, dropout rate was minimal, and compliance to the study protocol high. However, the recruitment and acceptability of the trial in practice criteria (i.e., the perceived burden of the study protocol) were not met. Secondary data analyses revealed a decrease in pregnancy psychosocial distress, but there was no significant difference between the probiotic and placebo groups for these outcomes. Similarly, there were no significant differences between the probiotic and placebo groups for the long-term outcomes.
While retention was very high with minimal dropout, recruitment was problematic in this study. Of the 1573 women screened, 3% were randomized. This randomization rate is similar to the Milgrom et al. (2011) study on the effectiveness of a behavioral intervention for reducing depressive symptoms among pregnant women49. However, the participation rate of this study (30%) was lower than the Milgrom et al. (2011) study participation rate (56%). While women in the Milgrom et al. (2011) study were screened and offered participation at clinical centers by midwives, recruitment in the current study was conducted through telephone invitation by a researcher, possibly explaining the difference in participation rates between the studies. Clinical staff are indeed known good partners to facilitate recruitment by identifying and speaking directly to eligible pregnant women about study protocols50. In light of our results, these approaches might be even more essential for pregnant women with psychosocial distress. Future studies investigating food supplements in this population may hence consider having midwives or mental healthcare professionals personally introduce the trial to pregnant women. In addition, to ensure constant and effective recruitment, a future full trial should invest in strong collaboration between researchers, midwives, and mental healthcare professionals51. Additionally, the results of our qualitative analyses showed that the main reason of women to decline study participation was the burden of the study. One method to reduce the burden of the study and improve recruitment rates would be meeting with women after regular pregnancy care visits and guiding and supporting them through the study’s various steps. Another would be to shorten the duration of the study by limiting it to pregnancy (i.e. leaving out child outcome measures). Finally, for a larger trial focused on clinical outcomes, the collection of biological samples could be left out. Alternatively, recruitment staff should invest more time in better explaining the value of collecting biological samples to potential participants.
Although measured in a small population, this study found no effect of probiotics on psychosocial distress after 8 weeks of probiotic intake. Note that in our study we investigated group effects of probiotics, while it is known that effects can vary from one individual to another. Factors that can influence probiotic effects are host diet52, genetics, age, medication use53, severity of symptoms (e.g. mild, moderate, severe depression)9, and gut microbiota composition54. In future studies, these factors could be included to assess whether they would predict who would benefit from probiotic supplementation. For instance, baseline gut bacterial samples can be collected and bacterial profiles used as predictors of probiotic response55.
The lack of effect of 8 weeks of probiotic intake on psychosocial distress is in contrast with the Slykerman et al. (2017) study, which reported a significant positive effect of perinatal maternal probiotic intake on postpartum depressive and anxiety symptoms10. The difference in outcomes may be explained by methodological differences between the studies. The Slykerman et al. (2017) study included a substantially larger sample than the current study (n = 423) and used Lactobacillus rhamnosus HN001, whereas our sample size was relatively small (n = 40) and participants received a probiotic mixture without this strain. A recent meta-analysis indicated that probiotic effects are strain and species-specific56. Furthermore, differences in the timing and length of probiotic intake may, in part, explain the differences in outcomes57.
The general reduction of psychosocial distress after 8 weeks of probiotic intake may indicate a natural decrease of symptoms. Studies report that anxiety (i.e., state and trait) naturally decreases over gestation58–60 and spontaneous improvement of symptoms often occurs in RCTs that include participants with high depression symptom scores61. However, it is possible that two other factors played a role in reduction of symptoms: participants’ expectancy to respond to the intervention product62–64 and strong engagement between research staff and participants65,66. In support of the former, the qualitative results of this study showed that most women indicated that their main reason for participation was the potential benefit it might bring them. Regarding research staff and participant engagement, a recent RCT investigating the same probiotic product also reported a reduction in depressive symptoms after 8 weeks of probiotic and placebo intake, but no significant difference between the probiotic and placebo groups. Similar to the current study, research staff was in close contact with participants throughout the study65. Thus, at this point, it is unclear whether the decrease in symptoms after 8 weeks of product intake was a natural phenomenon that resulted from participants’ expectancy to respond to the intervention product, was due to strong engagement between with the research staff, or a combination of all factors. Future RCTs with blinded assessment should account for the possible effects of these factors on psychosocial distress by including a third control arm receiving no intervention.
The strength of this pilot study is the mixed methods approach of combining both qualitative and quantitative measures. This method provided an in depth portrayal of reasons for joining and declining study participation in women with psychosocial distress. Other strengths of the study are the inclusion of confounders and the high compliance rate of probiotic/placebo intake and mood questionnaires, which provided a realistic and unbiased preliminary result of the probiotic/placebo intervention for the secondary outcomes.
Several limitations should be mentioned regarding the study results. First, the study included a relatively small sample size to explore potential efficacy of a probiotic product, and therefore these secondary outcomes should be interpreted with caution. Second, given that this study did not include a third control arm, the possibility of a natural decrease of symptoms should be acknowledged. Third, the study population included a relatively large number of women of higher socio-economic status, which hampers the generalizability of the study results. Finally, the study included a relatively large number of women with subclinical depressive and anxiety symptoms (i.e. without a diagnosis of depression or anxiety disorder). Previous systematic reviews and meta-analyses have shown that lower depressive symptom severity is positively associated with a higher placebo response, and thus it is possible that the placebo response was relatively high in the current pilot trial67–69.
The PIP pilot trial was generally feasible and acceptable, but suffered from low recruitment rates. Alternative methods of recruitment, including active recruitment by healthcare professionals, might be considered in a future definitive trial. Although evaluated in a relatively small population, analyses of secondary endpoints did not reveal differences between probiotic and placebo groups. In light of the increased risk of adverse maternal and infant outcomes for women suffering from prenatal depression and anxiety, future studies should explore the potential efficacy of other study designs or other (non) pharmacological treatments. Possibilities are, for example, to reduce the number of study measures and to use the same probiotic supplement starting in the first trimester of pregnancy, when natural pregnancy changes to the maternal gut microbiota have not yet taken place70 and the probiotic may have a larger effect. Alternatively, the use of other probiotics or combinations of probiotics and dietary fibers that promote the activity of the probiotic bacteria should be explored71,72.
Supplementary Information
Acknowledgements
The authors gratefully thank Christy Bowerman (CB), Carola Groen (CG), Emma Baljet (EB), Gea Vogelzang (GV), Henrike Haugke (HH), Hellen Lustermans (HL), Hilal Sekban (HS), Ilse van de Ven (IvV), Loes Maurits (LM), Martine Hollander (MH), Monique van Tooren (MvT), Olga van den Berg (OvB), Petra Kuiper (PK), Petra Schermers de Bruijne (PSB), Sabine Speel (SB), Siegrid Hoekstra (SH) for their contributions for recruitment, data collection, and data management. The authors thank Winclove Probiotics B.V. for providing Ecologic Barrier in kind.
Abbreviations
- APL
Dutch Everyday Problem List
- CRF
Case Report Form
- EPDS
Edinburgh Postnatal Depression Scale
- CI
Coordinating investigator
- LEIDS-R
Leiden Index of Depression Sensitivity-Revised
- MAAS
Maternal Antenatal Attachment Scale
- ETC
Medical Ethics Committee
- MPAS
Maternal Postnatal Attachment Scale
- PSQI
Pittsburgh Sleep Quality Index
- PES
Pregnancy Experience Scale
- PRAQ-R
Pregnancy-Related Anxiety Questionnaire-Revised
- RCTs
Randomized clinical trials
- STAI-S
State-trait Anxiety Inventory
- AEs
Serious Adverse Events
Author contributions
P.B.: Conceptualization, Methodology, Software, Formal Analysis, Investigation, Data Curation, Writing – Original Draft, Vizualization, Project Administration; A.B.: Conceptualization, Methodology, Writing – Review & Editing; I.V.: Conceptualization, Resources, Review & Editing; E.C.: Conceptualization, Funding Acquisition, Writing – Review & Editing; Cd.W.: Conceptualization, Methodology, Resources, Writing – Review & Editing, Supervision, Project Administration, Funding Acquisition.
Funding
This research was supported by Radboud University, Clinical Research Organization Rotterdam (CR2O), a Netherlands Organization for Scientific Research VICI Grant (016.Vici.185.038-to de Weerth), and a Jacobs Foundation Advanced Research Fellowship (to de Weerth). The probiotic product was provided in kind by Winclove probiotics B.V.; sample collection material was provided in kind by Public Health Service of Amsterdam (GGD Amsterdam). The sponsor (Radboud University) was involved in the study design, collection, analysis and interpretation of data, writing of the report, and the decision to submit the article for publication. Winclove probiotics B.V. prepared and provided the probiotic/placebo product and reviewed the manuscript prior to publication. Winclove probiotics B.V. was not involved in collection or analyses of the data or decision to submit the article for publication. CR2O, GGD Amsterdam, Jacobs Foundation, and the Netherlands Organization for Scientific Research were not involved in the study design, collection, analyses of data, writing of the report, and decision to submit the article for publication.
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
Eric Claassen is a consultant for several commercial parties in the field of probiotics; none of his advising practices are in direct conflict with the content of this pilot trial. Isolde Besseling-Van der Vaart is employed at Winclove Probiotics B.V. fulfilling the function of Manager Research Partnerships. The other authors have no conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Pamela D. Browne, Email: Pamela.Browne@radboudumc.nl
Carolina de Weerth, Email: Carolina.deWeerth@radboudumc.nl.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-81204-9.
References
- 1.Gelaye B, Rondon MB, Araya R, Williams MA. Epidemiology of maternal depression, risk factors, and child outcomes in low-income and middle-income countries. The Lancet Psychiatry. 2016;3:973–982. doi: 10.1016/S2215-0366(16)30284-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Accortt EE, Cheadle ACD, Dunkel Schetter C. Prenatal depression and adverse birth outcomes an updated systematic review. Matern. Child Health J. 2015;19:1306–1337. doi: 10.1007/s10995-014-1637-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Ravesteyn LM, van den Lambregtse Berg MP, Hoogendijk WJG, Kamperman AM. Interventions to treat mental disorders during pregnancy: a systematic review and multiple treatment meta-analysis. PLoS One. 2017;12:0173397. doi: 10.1371/journal.pone.0173397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biaggi A, Conroy S, Pawlby S, Pariante CM. Identifying the women at risk of antenatal anxiety and depression: a systematic review. J. Affect. Disord. 2016;191:62–77. doi: 10.1016/j.jad.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leigh B, Milgrom J. Risk factors for antenatal depression, postnatal depression and parenting stress. BMC Psychiatry. 2008;8:24. doi: 10.1186/1471-244X-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martini J, Petzoldt J, Einsle F, Beesdo-Baum K, Höfler M, Wittchen HU. Risk factors and course patterns of anxiety and depressive disorders during pregnancy and after delivery: a prospective-longitudinal study. J. Affect. Disord. 2015;175:385–395. doi: 10.1016/j.jad.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Higgins A, et al. Barriers to midwives and nurses addressing mental health issues with women during the perinatal period: The Mind Mothers study. J. Clin. Nurs. 2018;27:1872–1883. doi: 10.1111/jocn.14252. [DOI] [PubMed] [Google Scholar]
- 8.Osborne L, et al. Replication of epigenetic postpartum depression biomarkers and variation with hormone levels. Neuropsychopharmacology. 2016;41:1648–1658. doi: 10.1038/npp.2015.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng QX, Peters C, Ho CYX, Lim DY, Yeo W-S. A meta-analysis of the use of probiotics to alleviate depressive symptoms. J. Affect. Disord. 2018;228:13–19. doi: 10.1016/j.jad.2017.11.063. [DOI] [PubMed] [Google Scholar]
- 10.Slykerman RF, et al. Effect of Lactobacillus rhamnosus HN001 in pregnancy on postpartum symptoms of depression and anxiety: a randomised double-blind placebo-controlled trial. EBioMedicine. 2017;24:159–165. doi: 10.1016/j.ebiom.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Weerth C. Do bacteria shape our development? Crosstalk between intestinal microbiota and HPA axis. Neurosci. Biobehav. Rev. 2017;83:458–471. doi: 10.1016/j.neubiorev.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Dinan TG, Cryan JF. Brain–gut–microbiota axis—mood, metabolism and behaviour. Nat. Rev. Gastroenterol. Hepatol. 2017;14:69–70. doi: 10.1038/nrgastro.2016.200. [DOI] [PubMed] [Google Scholar]
- 13.Stevens BR, et al. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut. 2018;67:1555–1557. doi: 10.1136/gutjnl-2017-314759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang H, et al. Altered gut microbiota profile in patients with generalized anxiety disorder. J. Psychiatr. Res. 2018;104:130–136. doi: 10.1016/j.jpsychires.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Kazemi A, Noorbala AA, Azam K, Eskandari MH, Djafarian K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: a randomized clinical trial. Clin. Nutr. 2019;38:522–528. doi: 10.1016/j.clnu.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Vaghef-Mehrabany E, Maleki V, Behrooz M, Ranjbar F, Ebrahimi-Mameghani M. Can psychobiotics “mood” ify gut? An update systematic review of randomized controlled trials in healthy and clinical subjects, on anti-depressant effects of probiotics, prebiotics, and synbiotics. Clin. Nutr. 2020;39(5):1395–1410. doi: 10.1016/j.clnu.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Hill C, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 18.Bothwell LE, Greene JA, Podolsky SH, Jones DS. Assessing the Gold Standard — Lessons from the History of RCTs. N. Engl. J. Med. 2016;374:2175–2181. doi: 10.1056/NEJMms1604593. [DOI] [PubMed] [Google Scholar]
- 19.Gold SM, Enck P, Hasselmann H, Friede T, Hegerl U, Mohr DC, Otte C. Control conditions for randomised trials of behavioural interventions in psychiatry: a decision framework. The Lancet Psychiatry. 2017;4:725–732. doi: 10.1016/S2215-0366(17)30153-0. [DOI] [PubMed] [Google Scholar]
- 20.Czobor P, Skolnick P. The secrets of a successful clinical trial: compliance, compliance, and compliance. Mol. Interv. 2011;11:107–110. doi: 10.1124/mi.11.2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Zande IS, van der Graaf R, Hooft L, van Delden JJ. Facilitators and barriers to pregnant women’s participation in research: a systematic review. Women Birth. 2018;31:350–361. doi: 10.1016/j.wombi.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Chauhan C, Smith ML. Closing an on-going clinical trial: When is it betrayal of participants? Breast Cancer Res. Treat. 2013;142:461–2. doi: 10.1007/s10549-013-2736-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amstutz A, et al. Discontinuation and non-publication of randomised clinical trials supported by the main public funding body in Switzerland: a retrospective cohort study. BMJ Open. 2017;7:e016216. doi: 10.1136/bmjopen-2017-016216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eldridge SM, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355:i5239. doi: 10.1136/bmj.i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alhusen JL, Gross D, Hayat MJ, Rose L, Sharps P. The role of mental health on maternal-fetal attachment in low-income women. J. Obstet. Gynecol. Neonatal Nurs. 2012;41:E71–E81. doi: 10.1111/j.1552-6909.2012.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goecke TW, et al. The association of prenatal attachment and perinatal factors with pre- and postpartum depression in first-time mothers. Arch. Gynecol. Obstet. 2012;286:309–316. doi: 10.1007/s00404-012-2286-6. [DOI] [PubMed] [Google Scholar]
- 27.Hechler C, et al. Association between psychosocial stress and fecal microbiota in pregnant women. Sci. Rep. 2019;9:4463. doi: 10.1038/s41598-019-40434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hechler C, Beijers R, Riksen-Walraven JM, de Weerth C. Are cortisol concentrations in human breast milk associated with infant crying? Dev. Psychobiol. 2018;60:639–650. doi: 10.1002/dev.21761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zijlmans MAC, Korpela K, Riksen-Walraven JM, de Vos WM, de Weerth C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology. 2015;53:233–245. doi: 10.1016/j.psyneuen.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 30.de Weerth C, Fuentes S, Puylaert P, de Vos WM. Intestinal microbiota of infants with colic: development and specific signatures. Pediatrics. 2013;131:e550–e558. doi: 10.1542/peds.2012-1449. [DOI] [PubMed] [Google Scholar]
- 31.Cooijmans KHM, Beijers R, Rovers AC, de Weerth C. Effectiveness of skin-to-skin contact versus care-as-usual in mothers and their full-term infants: study protocol for a parallel-group randomized controlled trial. BMC Pediatr. 2017;17:154. doi: 10.1186/s12887-017-0906-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Browne PD, Bolte A, Claassen E, de Weerth C. Probiotics in pregnancy: protocol of a double-blind randomized controlled pilot trial for pregnant women with depression and anxiety (PIP pilot trial) Trials. 2019;20:440. doi: 10.1186/s13063-019-3389-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steenbergen L, Sellaro R, van Hemert S, Bosch JA, Colzato LS. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 2015;48:258–264. doi: 10.1016/j.bbi.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry. 1987;150:782–6. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 35.van Santen A, et al. Psychological traits and the cortisol awakening response: results from the Netherlands Study of Depression and Anxiety. Psychoneuroendocrinology. 2011;36:240–248. doi: 10.1016/j.psyneuen.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 36.Van der Does AJW. Cognitive reactivity to sad mood: structure and validity of a new measure. Behav. Res. Ther. 2002;40:105–120. doi: 10.1016/S0005-7967(00)00111-X. [DOI] [PubMed] [Google Scholar]
- 37.Huizink AC, Mulder EJH, Robles de Medina PG, Visser GHA, Buitelaar JK. Is pregnancy anxiety a distinctive syndrome? Early Hum. Dev. 2004;79:81–91. doi: 10.1016/j.earlhumdev.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 38.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press; 1983. [Google Scholar]
- 39.Grant K-A, McMahon C, Austin M-P. Maternal anxiety during the transition to parenthood: a prospective study. J. Affect. Disord. 2008;108:101–111. doi: 10.1016/j.jad.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 40.DiPietro JA, Ghera MM, Costigan K, Hawkins M. Measuring the ups and downs of pregnancy stress. J. Psychosom. Obstet. Gynaecol. 2004;25:189–201. doi: 10.1080/01674820400017830. [DOI] [PubMed] [Google Scholar]
- 41.Vingerhoets AJJM, Jeninga AJ, Menges LJ. The measurement of daily hassles and chronic stressors-the development of the everyday problem checklist (EPCL, Dutch-APL) Gedrag Gezond. 1989;1:10–17. [Google Scholar]
- 42.van Bussel JCH, Spitz B, Demyttenaere K. Reliability and validity of the Dutch version of the maternal antenatal attachment scale. Arch. Womens. Ment. Health. 2010;13:267–277. doi: 10.1007/s00737-009-0127-9. [DOI] [PubMed] [Google Scholar]
- 43.van Bussel JCH, Spitz B, Demyttenaere K. Three self-report questionnaires of the early mother-to-infant bond: reliability and validity of the Dutch version of the MPAS, PBQ and MIBS. Arch. Womens. Ment. Health. 2010;13:373–384. doi: 10.1007/s00737-009-0140-z. [DOI] [PubMed] [Google Scholar]
- 44.Anderson JR, et al. A preliminary examination of gut microbiota, sleep, and cognitive flexibility in healthy older adults. Sleep Med. 2017;38:104–107. doi: 10.1016/j.sleep.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ng QX, et al. A meta-analysis of the effectiveness of yoga-based interventions for maternal depression during pregnancy. Complement. Ther. Clin. Pract. 2019;34:8–12. doi: 10.1016/j.ctcp.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 46.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 47.Lancaster CA, et al. Risk factors for depressive symptoms during pregnancy: a systematic review. Am. J. Obstet. Gynecol. 2010;202:5–14. doi: 10.1016/j.ajog.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med. Res. Methodol. 2013;13:117. doi: 10.1186/1471-2288-13-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Milgrom J, Schembri C, Ericksen J, Ross J, Gemmill AW. Towards parenthood: An antenatal intervention to reduce depression, anxiety and parenting difficulties. J. Affect. Disord. 2011;130:385–394. doi: 10.1016/j.jad.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 50.Frew PM, et al. Recruitment and Retention of Pregnant Women Into Clinical Research Trials: An Overview of Challenges, Facilitators, and Best Practices. Clin. Infect. Dis. 2014;59:S400–S407. doi: 10.1093/cid/ciu726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moore J, Crozier K, Kite K. An action research approach for developing research and innovation in nursing and midwifery practice: building research capacity in one NHS foundation trust. Nurse Educ. Today. 2012;32:39–45. doi: 10.1016/j.nedt.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 52.Bennet SMP, et al. Multivariate modelling of faecal bacterial profiles of patients with IBS predicts responsiveness to a diet low in FODMAPs. Gut. 2018;67:872–881. doi: 10.1136/gutjnl-2016-313128. [DOI] [PubMed] [Google Scholar]
- 53.Zhernakova A, et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016;352:565–569. doi: 10.1126/science.aad3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abildgaard A, et al. The antidepressant-like effect of probiotics and their faecal abundance may be modulated by the cohabiting gut microbiota in rats. Eur. Neuropsychopharmacol. 2019;29:98–110. doi: 10.1016/j.euroneuro.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 55.Hod K, et al. The effect of a multispecies probiotic on microbiota composition in a clinical trial of patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol. Motil. 2018;30:e13456. doi: 10.1111/nmo.13456. [DOI] [PubMed] [Google Scholar]
- 56.Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2018;48:1044–1060. doi: 10.1111/apt.15001. [DOI] [PubMed] [Google Scholar]
- 57.Nordqvist M, et al. Timing of probiotic milk consumption during pregnancy and effects on the incidence of preeclampsia and preterm delivery: a prospective observational cohort study in Norway. BMJ Open. 2018;8:e018021. doi: 10.1136/bmjopen-2017-018021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huizink AC, et al. The interrelationship between pregnancy-specific anxiety and general anxiety across pregnancy: a longitudinal study. J. Psychosom. Obstet. Gynecol. 2014;35:92–100. doi: 10.3109/0167482X.2014.944498. [DOI] [PubMed] [Google Scholar]
- 59.Leach LS, Christensen H, Mackinnon A. Pregnancy and levels of depression and anxiety: a prospective cohort study of Australian women. Aust. New Zeal. J. Psychiatry. 2014;48:944–951. doi: 10.1177/0004867414533013. [DOI] [PubMed] [Google Scholar]
- 60.Buist A, Gotman N, Yonkers KA. Generalized anxiety disorder: course and risk factors in pregnancy. J. Affect. Disord. 2011;131:277. doi: 10.1016/j.jad.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krogsbøll LT, Hróbjartsson A, Gøtzsche PC. Spontaneous improvement in randomised clinical trials: meta-analysis of three-armed trials comparing no treatment, placebo and active intervention. BMC Med. Res. Methodol. 2009;9:1. doi: 10.1186/1471-2288-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pu J, Hou H, Ma R. Direct and indirect effects of self-efficacy on depression: the mediating role of dispositional optimism. Curr. Psychol. 2017;36:410–416. doi: 10.1007/s12144-016-9429-z. [DOI] [Google Scholar]
- 63.Wampold BE, Imel ZE, Imel ZE. The Great Psychotherapy Debate. Abingdon: Routledge; 2015. [Google Scholar]
- 64.Rutherford BR, et al. Patient expectancy as a mediator of placebo effects in antidepressant clinical trials. Am. J. Psychiatry. 2017;174:135–142. doi: 10.1176/appi.ajp.2016.16020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chahwan B, et al. Gut feelings: a randomised, triple-blind, placebo-controlled trial of probiotics for depressive symptoms. J. Affect. Disord. 2019;253:317–326. doi: 10.1016/j.jad.2019.04.097. [DOI] [PubMed] [Google Scholar]
- 66.Martin DJ, Garske JP, Davis MK. Relation of the therapeutic alliance with outcome and other variables: a meta-analytic review. J. Consult. Clin. Psychol. 2000;68:438–450. doi: 10.1037/0022-006X.68.3.438. [DOI] [PubMed] [Google Scholar]
- 67.Weimer K, Colloca L, Enck P. Placebo effects in psychiatry: mediators and moderators. The Lancet Psychiatry. 2015;2:246–257. doi: 10.1016/S2215-0366(14)00092-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brunoni AR, Lopes M, Kaptchuk TJ, Fregni F. Placebo response of non-pharmacological and pharmacological trials in major depression: a systematic review and meta-analysis. PLoS One. 2009;4(3):e4824. doi: 10.1371/journal.pone.0004824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sinyor M, et al. Does inclusion of a placebo arm influence response to active antidepressant treatment in randomized controlled trials? Results from pooled and meta-analyses. J. Clin. Psychiatry. 2010;71:270–279. doi: 10.4088/JCP.08r04516blu. [DOI] [PubMed] [Google Scholar]
- 70.Koren O, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150:470–480. doi: 10.1016/j.cell.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jacka FN, et al. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial) BMC Med. 2017;15:23. doi: 10.1186/s12916-017-0791-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miki T, et al. Dietary fiber intake and depressive symptoms in Japanese employees: The Furukawa Nutrition and Health Study. Nutrition. 2016;32:584–589. doi: 10.1016/j.nut.2015.11.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.