Abstract
Background:
Multiple strategies have been used to evaluate minimal important change (MIC) of Eczema Area and Severity Index (EASI) and Scoring Atopic Dermatitis (SCORAD). The meaningfulness of these MIC is not well established across all AD severities.
Objectives:
Determine the MIC of % and absolute improvement of EASI and SCORAD scores in adults and children with AD.
Methods:
We performed a prospective dermatology practice-based study using questionnaires and evaluation by a dermatologist (n=826). An anchor-based approach was used to determine thresholds for % and absolute MIC of EASI, SCORAD and O-SCORAD at follow-up from baseline.
Results:
One-grade improvements of Physician’s Global Assessment (PGA) and Validated Investigator’s Global Assessment of AD (vIGA-AD) were associated with 50%, 35%, and 35% decreases of EASI, SCORAD and objective-SCORAD, respectively. The thresholds for % MIC of EASI (Kruskal-Wallis test, P=0.61), SCORAD (P=0.07) and O-SCORAD (P=0.09) were similar across baseline AD severity. One-grade improvements of PGA and vIGA-AD were associated with 14.0 and 14.9-point decreases of EASI; 19.9 and 14.9-point decreases of SCORAD; 15.5 and 17.4-point decreases of O-SCORAD. The thresholds for absolute MIC of EASI (P<0.001), SCORAD (P=0.0001) and O-SCORAD (P<0.0001) significantly differed by baseline AD severity. Percent and absolute MIC for EASI and SCORAD were associated with improvements of AD symptoms and quality of life.
Conclusions:
EASI50, SCORAD35 and O-SCORAD35 were meaningful % MIC regardless of baseline AD severity. Absolute MIC for EASI, SCORAD and O-SCORAD varied by baseline AD severity.
Introduction
Atopic dermatitis (AD) is a heterogeneous disease with variable morphology and intensity of lesions, including erythema, edema/papulation, excoriation, lichenification, and xerosis, as well variable body surface area and distribution of lesions1–5. There are numerous clinician-reported outcome measures for AD severity, each encompassing different aspects of lesional morphology and/or extent.
Eczema Area and Severity Index (EASI) and Scoring AD (SCORAD) are complex measures that assess a weighted average of the severity of AD signs and extent of lesions 6–10. EASI was previously found to be valid and was recommended by the Harmonizing Outcome Measures in Eczema (HOME) international consensus group to assess AD signs in clinical trials11. SCORAD was also found to be valid for assessing AD severity and is used to classify AD severity in the European consensus guidelines for AD.
Many different approaches have been used analyze improvement of EASI and SCORAD in clinical trials and other research. One commonly used approach has been to examine the relative or percent improvement of these scores using somewhat arbitrary cutoffs of 50%, 75% and 90% improvement 12. This approach has similarly been used to assess the Psoriasis Area and Severity Index in psoriasis research 13. However, the meaningfulness of these EASI and/or SCORAD thresholds is not well established.
In addition, thresholds for minimal important change (MIC) were established for absolute improvements of EASI (6.6) and SCORAD (8.2) using data from clinical trial cohorts with moderate-severe AD14. However, it is unclear whether such MIC values are appropriate when assessing clinical improvement of patients with mild AD. For example, patients with mild EASI scores, e.g. 5, cannot achieve a 6.6-point improvement. Moreover, there is a wide-range of EASI and SCORAD scores that have previously been found to encompass moderate to severe AD15,16. Thus, we sought to determine whether the previously established MIC values perform consistently among patients with moderate and severe AD. We hypothesized that different thresholds for MIC are more appropriate across different patient populations and target severities. The smallest detectable change (SDC) is the minimal change of a scale. SDC is required to properly evaluate whether the observed change of scores over time is not just due to measurement error 17.
In this study, we sought to evaluate the thresholds for meaningful absolute and percent improvement and SDC of EASI and SCORAD scores in adults and children with AD. In addition, we sought to compare whether these thresholds performed equally well across mild, moderate and severe AD. Finally, we sought to determine whether observed MIC for EASI and SCORAD were clinically meaningful to patients.
Methods
Study design
A prospective, dermatology practice-based study of children and adults was performed with AD as defined by the Hanifin-Rajka diagnostic criteria18. Exclusion criteria included those without a definite diagnosis of AD or being unwilling or unable to complete assessments. Virtually all (>99%) patients who were invited agreed to participate. Patients received standard of care follow-up and treatment, including emollients, prescription topical, systemic and/or phototherapy, where appropriate.
Self-administered electronic surveys were completed by adult patients and parents of pediatric patients of the eczema clinic at an academic medical center prior to their encounter. Questionnaires were completed in the following order: Patient-reported Global Assessment of AD severity (PtGA; “Would you describe your (child’s) atopic dermatitis or eczema as clear, almost clear, mild, moderate, or severe?”)19, Numerical Rating Scale (NRS) for worst-itch in the past 7 days (1 question each, range 0-10), Visual Analog Scale (VAS) itch and sleep for Scoring AD (SCORAD) (1 question each, range 0-10), Patient Oriented Eczema Measure (POEM) (7 questions, range: 0-28)20–25, and Dermatology Life Quality Index (DLQI) (10 questions, range: 0-30, only for age ≥17 years)22,26–28.
Patients were assessed with full body skin examination by a dermatologist (JS). Validated Investigator Global Assessment scale for Atopic Dermatitis (vIGA-AD™) 29 and a gestalt Physician’s Global Assessment (PGA)(range: 0=clear, 1=almost clear, 2=mild, 3=moderate, 4=severe), Eczema Area and Severity Index (EASI; 4 signs [erythema, excoriation, swelling, lichenification] on 4 body sites, range: 0-72)16,30–32, and objective component of the SCORAD (O-SCORAD; 6 signs [erythema, excoriation, swelling, oozing/crusting, lichenification, dryness] on 8 body sites, no symptoms; range: 0-83)6,16,31,32 were the clinically reported outcomes (ClinROs) examined.
Patients were enrolled sequentially between January, 2018 and September, 2019. The study was approved by the institutional review boards of Northwestern University. Informed consent was obtained electronically.
Statistical analysis
Summary statistics were estimated for baseline population characteristics. An anchor-based approach was used to determine thresholds for MIC of EASI, SCORAD and O-SCORAD at follow-up from baseline. The mean percent and absolute improvement of EASI, SCORAD and O-SCORAD were calculated among patients with a 1-grade, 2-grade, and ≥3-grade improvement of PtGA and vIGA-AD. The mean percent improvement of EASI, SCORAD and O-SCORAD were also calculated in patients achieving vIGA-AD or PGA scores of clear or almost clear with ≥2-grade improvement. Analyses were conducted overall and stratified by baseline vIGA-AD or PGA scores of mild, moderate or severe. Analysis of variance was used to compare whether the MIC significantly differed between patients with baseline severities. In addition, the above analyses were performed using PtGA as the anchor to determine whether the observed thresholds were clinically meaningful to patients.
The smallest detectable difference (SDC), i.e. the smallest change that can be detected above measurement error, was determined in patients with unchanged physician-reported global AD severity scores at follow-up using: 1.96 * √2 * standard error of measurement (SEM), where SEM = SDpooled * √(1-ICC agreement)33,34.
Floor- or ceiling-effects arise when an assessment has a lower or upper limit to the values it can reliably measure. Floor effect occurs when responses on a measure, questionnaire or scale cluster at the more negative health state end of the scale. Ceiling effect occurs when responses on a measure or questionnaire cluster at the more positive health state end of the scale. Floor or ceiling effects of total scores were considered present if 15% of responses fell in the lowest or highest scores35,36.
Finally, mean improvement of the PROMs POEM, SCORAD-sleep, NRS-itch and DLQI were examined in those achieving 25-49%, 50-74%, 75-89% and 90% improvement and with absolute improvement ≥MIC identified for EASI, SCORAD and O-SCORAD. T-tests and analysis of variance were used to determine whether there were significant differences of the PROMs in those achieving vs. not achieving these levels of improvement in EASI, SCORAD and O-SCORAD.
The above statistical analyses were performed in SAS version 9.4.3 (SAS Institute, Cary, IN). Complete case analysis was performed, i.e. missing values were excluded. A two-sided P-value of 0.05 was considered statistically significant.
Results
Patient characteristics
Overall, 826 patients (ages 8.8-96.8 years) with AD were assessed at follow-up (mean±std. dev. time to follow-up: 4.1±5.2 months), including 434 females (52.5%), 457 self-reported Caucasians/whites (55.3%), with a mean±std. dev. age at enrollment was 42.6±19.3 years. Baseline characteristics of AD severity are presented in Table 1.
Table 1.
Subject characteristics (n=826).
| Variable | Value |
|---|---|
| Demographics | |
| Age (yr) | |
| mean ± std. dev. | 42.6 ± 19.3 |
| Min – max | 8.8 – 96.8 |
| Freq (%) | |
| <12 | 37 (4.5%) |
| 12-17 | 59 (7.1%) |
| ≥18 | 730 (88.4%) |
| Female sex – freq (%) | 434 (52.5%) |
| Race/ethnicity – freq (%) | |
| Caucasian/white | 457 (55.3%) |
| African-American/black | 71 (8.6%) |
| Hispanic | 119 (14.4%) |
| Asian | 179 (21.7%) |
| Level of education – freq (%) | |
| High school or less | 105 (12.7%) |
| Greater than high school | 721 (87.3%) |
| Patient-reported outcomes | |
| NRS worst-itch – median (min, max) | 6 (0, 10) |
| NRS average-itch – median (min, max) | 5 (0, 10) |
| POEM – median (min, max) | 11 (0, 28) |
| Patient-reported global AD severity – freq (%) | |
| Clear | 4 (0.6%) |
| Almost clear | 33 (4.6%) |
| Mild | 244 (33.9%) |
| Moderate | 225 (31.3%) |
| Severe | 213 (29.6%) |
| Clinical-reported outcomes | |
| EASI – median (min, max) | 5.8 (0.0, 66.7) |
| Objective-SCORAD – median (min, max) | 25.0 (0.0, 82.8) |
| SCORAD – median (min, max) | 32.7 (0.0, 103.0) |
| vIGA-AD© – freq (%) | |
| Clear | 30 (3.7%) |
| Almost clear | 175 (21.5%) |
| Mild | 112 (13.7%) |
| Moderate | 393 (48.2%) |
| Severe | 105 (12.9%) |
| Physician-reported global AD severity – freq (%) | |
| Clear | 0 (0.0%) |
| Almost clear | 34 (4.4%) |
| Mild | 329 (42.5%) |
| Moderate | 235 (30.4%) |
| Severe | 176 (22.7%) |
MIC for % improvement of EASI, SCORAD and O-SCORAD
The threshold for MIC of % improvement of EASI (29.9% to 93.4%), SCORAD (27.2% to 75.1%) and O-SCORAD (26.2% to 78.9%) varied by the anchor and definition used (Figure 1A–C). For each anchor (PtGA, PGA and vIGA-AD), the thresholds for MIC were higher with each level of improvement of the anchor. One-grade improvements of PGA and vIGA-AD were associated with approximately 50%, 35%, and 35% decreases of EASI, SCORAD and O-SCORAD, respectively. Two-grade improvements of PGA / vIGA-AD were associated with 75.0 / 83.2%, 53.0 / 63.2%, and 56.0 / 61.6% decreases of EASI, SCORAD and O-SCORAD. Three-grade or more improvements of PGA / vIGA-AD were associated with 93.4% / 93.2%, 69.6 / 75.1%, and 65.3 / 78.9% decreases of EASI, SCORAD and O-SCORAD.
Figure 1. Meaningful % and absolute improvement Eczema Area and Severity Index (EASI), Scoring Atopic Dermatitis (SCORAD) and objective component of SCORAD (O-SCORAD).
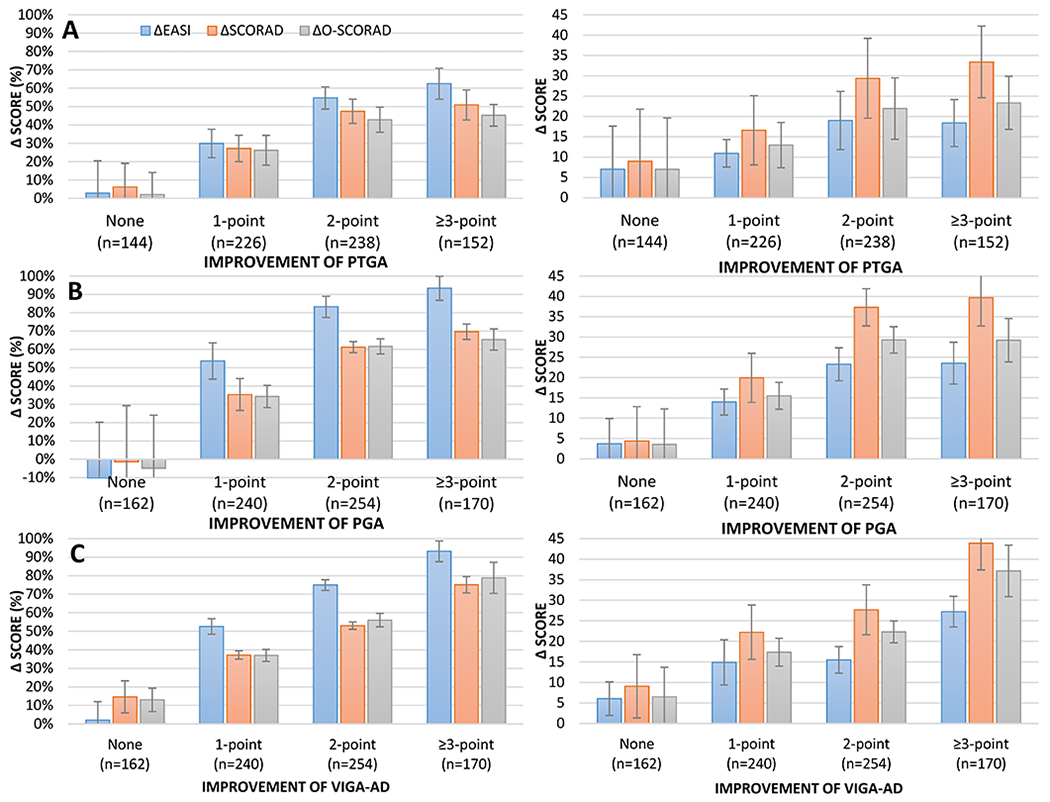
The percent (A, B, C) and absolute (D, E, F) improvement EASI (blue), SCORAD (orange) and O-SCORAD (gray) stratified by 1-point, 2-point or ≥3-point improvements of Patient-Reported Global Assessment (PtGA) (A, D), Physician’s Global Assessment (PGA) (B, E) and Validated Investigator’s Global Assessment (vIGA-AD) (C, F).
However, the MIC for % improvement of EASI (1-grade / 2-grade / ≥3-grade improvement: 29.9% / 54.7% / 62.5%), SCORAD (27.2% / 47.4% / 50.9%) and O-SCORAD (26.2% / 42.9% / 45.2%) were considerably lower when using improvement of PtGA as the anchor.
Patients who achieved a PtGA / PGA / vIGA-AD scores of clear or almost clear with ≥2-grade improvement also achieved 41.0% / 92.2% / 81.2% decrease of EASI, 46.3% / 67.7% / 59.5% decrease of SCORAD, and 39.2% / 65.9% / 62.9% decrease of O-SCORAD.
The thresholds for % MIC of EASI (P=0.61), SCORAD (P=0.07) and O-SCORAD (P=0.09) were similar among patients with mild, moderate and severe baseline AD severity.
Improvement of PROMs with % improvement of EASI, SCORAD and O-SCORAD
There were stepwise and significant improvements of POEM, NRS-sleep, NRS-itch and DLQI with increasing % improvement of EASI, SCORAD and O-SCORAD (P<0.0001 for all) (Figure 2A–C). EASI90 was associated with greater improvement of PROMs than EASI75. However, SCORAD90 and O-SCORAD90 were not associated with greater improvement of PROMS compared to SCORAD75 and O-SCORAD75, respectively.
Figure 2. Improvement of patient-reported outcome measures in patients achieving different thresholds of % and absolute improvement Eczema Area and Severity Index (EASI), Scoring Atopic Dermatitis (SCORAD) and objective component of SCORAD (O-SCORAD).
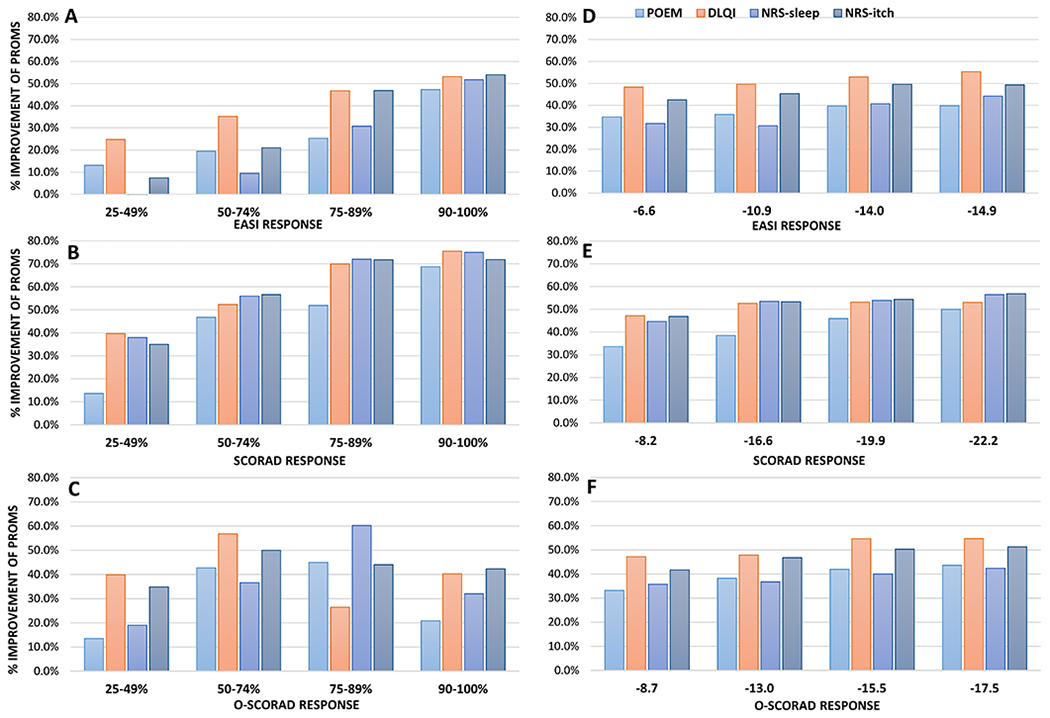
% improvement of Patient-Oriented Eczema Measure (POEM), Dermatology Life Quality Index (DLQI), Numeric Rating Scale (NRS)-sleep and itch were stratified by % improvement (A-C) or absolute improvement (D-F) of EASI (A, D), SCORAD (B, E) and O-SCORAD (C, F).
SDC and MIC for absolute change of EASI, SCORAD and O-SCORAD
The SDC for absolute change of EASI, SCORAD and O-SCORAD were 8.0, 11.6 and 9.9. The threshold for absolute MIC of EASI (1.1 to 24.6), SCORAD (27.2 to 75.1) and O-SCORAD (26.2 to 78.9) varied by the anchor and definition used (Figure 1D–F). For each anchor (PtGA, PGA and vIGA-AD), the thresholds for MIC were higher with each level of improvement of the anchor. One-grade improvements of PtGA, PGA and vIGA-AD were associated with approximately 10.9, 14.0 and 14.9-point decreases of EASI; 16.6, 19.9 and 14.9-point decreases of SCORAD; 13.0, 15.5 and 17.4-point decreases of O-SCORAD, respectively. Two- and ≥3-grade improvements of PtGA, PGA and vIGA-AD were associated with even greater decreases of EASI, SCORAD and O-SCORAD.
The thresholds for absolute MIC of EASI (P<0.001), SCORAD (P=0.0001) and O-SCORAD (P<0.0001) were significantly different between patients with mild, moderate and severe baseline AD severity (Figure 3). The absolute MIC for EASI ranged from 1.1 to 7.9 in patients with mild AD, 8.7 to 13.5 in patients with moderate AD, and 15.4 to 24.2 in patients with severe AD. The MIC for SCORAD ranged from 2.7 to 15.8 in patients with mild AD, 17.5 to 23.3 in patients with moderate AD, and 22.3 to 29.2 in patients with severe AD. Finally, the MIC for O-SCORAD ranged from 1.5 to 11.7 in patients with mild AD, 13.1 to 24.2 in patients with moderate AD, and 17.9 to 23.2 in patients with severe AD.
Figure 3. Meaningful absolute improvement Eczema Area and Severity Index (EASI), Scoring Atopic Dermatitis (SCORAD) and objective component of SCORAD (O-SCORAD) differs by baseline AD severity.
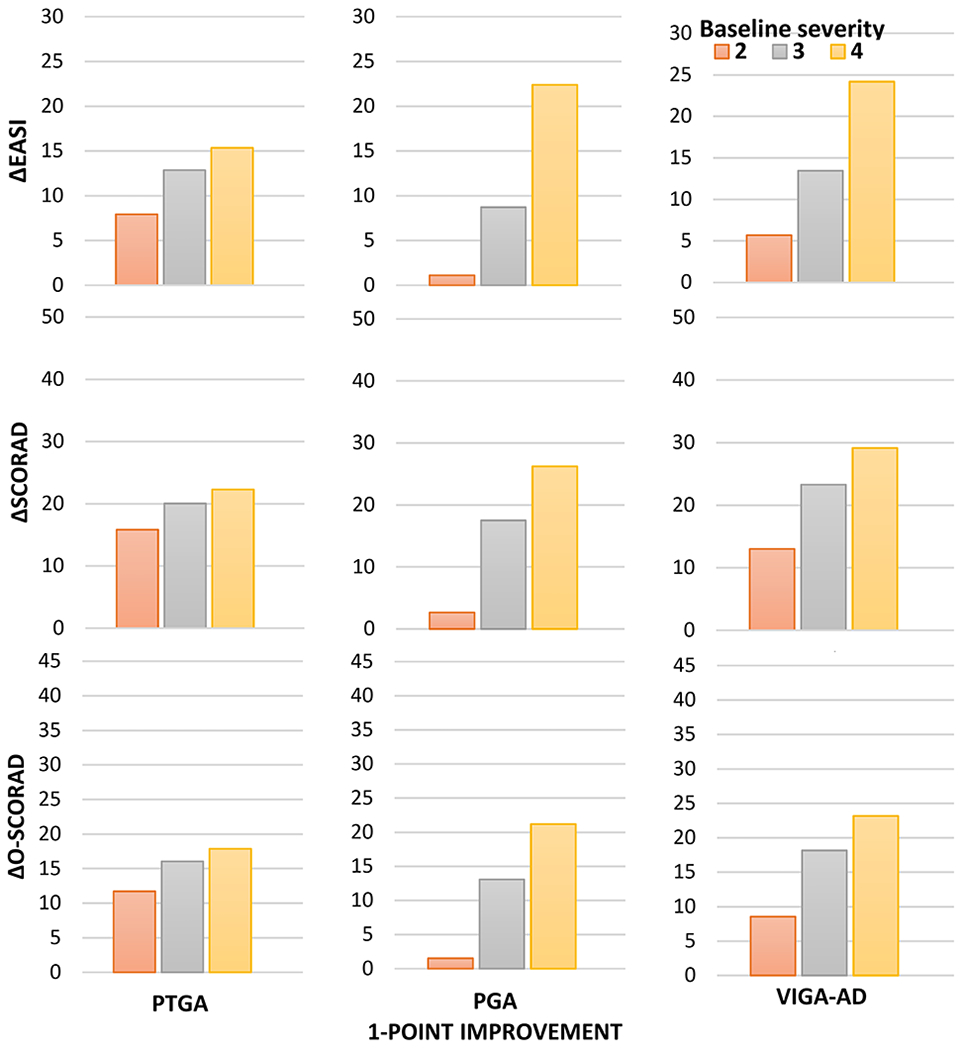
The absolute improvement of EASI (top row), SCORAD (middle row) and O-SCORAD (bottom row) among patients achieving a 1-point improvement of Patient-Reported Global Assessment (PtGA) (left column), Physician’s Global Assessment (PGA) (middle column) and Validated Investigator’s Global Assessment (vIGA-AD) (right column) are presented stratified by the baseline severity of the respective scores.
Floor or ceiling effects
The proportions of patients with lowest / highest values for EASI (6.1% / 0.3%), SCORAD (0.8% / 0.2%) and O-SCORAD (2.3% / 0.2%) were below 15%, indicating there were no floor- or ceiling-effects. However, in patients with baseline mild AD, floor-effects were observed for EASI (20.0%), but not SCORAD (0.0%) or O-SCORAD (0.0%); there were no ceiling-effects for these measures (0.0% for all). There were no floor- or ceiling-effects for EASI, SCORAD or O-SCORAD among those with baseline moderate or severe AD.
Improvement of PROMs with absolute improvement of EASI, SCORAD and O-SCORAD
Achieving absolute MIC Improvement of EASI, SCORAD and O-SCORAD was associated with significant decreases of POEM, SCORAD-sleep, NRS-itch and DLQI for all MIC thresholds tested (P<0.0001 for all). Greater numerical improvements of one or more PROMs were observed at higher MIC thresholds for EASI, SCORAD and O-SCORAD in this study compared with previously reported MIC (Figure 2E–F).
Discussion
This study found that several different MIC for EASI, SCORAD and O-SCORAD. EASI50 appears to be the most meaningful MIC for EASI, reflecting an approximately 1-grade improvement of PGA and vIGA-AD and 2-grade improvement of PtGA. EASI75 reflects a 2-grade improvement of PGA and vIGA-AD. Whereas, EASI90 reflects a ≥3-grade improvement and score of clear or almost clear with ≥2-grade improvement for PGA and vIGA-AD. The thresholds for % MIC of SCORAD and O-SCORAD are lower than EASI. SCORAD35 and O-SCORAD35 appear to be the most meaningful MIC for SCORAD and O-SCORAD, reflecting a 1-grade improvement of PGA, vIGA-AD and PtGA. SCORAD50 and O-SCORAD50 reflect an approximately 2-grade improvement of PGA and PtGA. SCORAD75 and O-SCORAD75 reflect a 3-grade improvement of PGA and vIGA-AD, and score of clear or almost clear with ≥2-grade improvement for PGA, vIGA-AD and PtGA.
The SDC for absolute change of EASI, SCORAD and O-SCORAD were lower than most of the respective MIC estimates, though some MIC estimates were lower than the SDC particularly among patients with mild AD. The SDC must not be larger than the MIC in order to determine whether a change of scores is clinically important and not merely due to measurement error17. Those MIC that are below the SDC may not be valid and should not be used. All thresholds for percent and absolute MIC of EASI, SCORAD and O-SCORAD demonstrated clinical meaningfulness with significant improvements of AD symptoms and QOL.
There were no floor- or ceiling-effects overall for EASI, SCORAD or O-SCORAD. However, floor-effects were observed for EASI in patients with baseline mild AD, suggesting that EASI may be less sensitive than SCORAD or O-SCORAD at detecting mild AD; this is likely due to SCORAD and O-SCORAD including two additional signs (xerosis and oozing/weeping) and SCORAD including two additional symptoms (itch and sleep loss). These results are consistent with a previous study that found EASI to be a poorer measurer than oSCORAD when assessing patients with more limited disease32. Consequently, EASI may be less responsive than SCORAD and O-SCORAD particularly in mild AD patients.
The thresholds identified for absolute MIC of EASI, SCORAD and O-SCORAD were all higher than −6.6, −8.2 and −8.7 previously reported from clinical trial data of moderate-severe AD in children and/or adults14. In addition, thresholds for absolute MIC of EASI, SCORAD and O-SCORAD were significantly higher in more severe AD at baseline. Together, these results suggest that different MIC thresholds may be warranted to correctly evaluate absolute improvement of EASI, SCORAD and O-SCORAD in different target populations. Whereas, MIC thresholds based on % improvement of EASI, SCORAD and O-SCORAD were similar in patients with mild, moderate and severe baseline disease. That is, EASI50, EASI75 and EASI90 appear to have similar interpretations across patients with mild to severe AD.
This study has several strengths, including large sample size, good representation across gender, age, race/ethnicity and AD severity, testing of multiple multiple ClinROs and PROs for AD, itch and QOL impact. There are some limitations. Patients were recruited from a single academic center, which may limit generalizability. Additional studies are needed to confirm these findings in a population-based and/or multicenter cohort.
In conclusion, improvements of 50%, 35% and 35% appear to be the most meaningful MIC for EASI, SCORAD and O-SCORAD, respectively, and performed similarly among patients with mild, moderate and severe AD at baseline. However, thresholds for absolute MIC of EASI, SCORAD and O-SCORAD varied by baseline AD severity. As such, baseline AD severity should be considered when selecting an absolute MIC threshold for analysis.
What is already known about this topic?
Most existing clinician-reported outcomes for atopic dermatitis severity are not feasible or valid for use in clinical practice.
What does this study add?
This study demonstrated that EASI50, SCORAD35 and O-SCORAD35 appear to be the most meaningful endpoints for EASI, SCORAD and O-SCORAD in clinical practice. Whereas, minimal important change (MIC) for absolute improvement of EASI, SCORAD and O-SCORAD varied by baseline AD severity and may need to be selected based on the target population.
EASI50, SCORAD35 and O-SCORAD35 should be evaluated in future studies of AD that use EASI and SCORAD.
Acknowledgments
Northwestern Medicine Enterprise Data Warehouse (NMEDW) was supported, in part, by the Northwestern University Clinical and Translational Science Institute, funded, in part, by Grant Number UL1TR000150 from the National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The Clinical and Translational Science Award (CTSA) is a registered trademark of DHHS.
Funding Support: This publication was made possible with support from the Agency for Healthcare Research and Quality (AHRQ), grant number K12 HS023011, the Dermatology Foundation, and a research grant from Galderma.
Abbreviations used:
- AD
atopic dermatitis
- POEM
Patient Oriented Eczema Measure
- NRS
Numeric Rating Scale
- DLQI
Dermatology Life Quality Index
- SCORAD
SCORing Atopic Dermatitis
- EASI
Eczema Area and Severity Index
- QOL
quality of life
- IGA
Investigator’s Global Assessment
- BSA
Body Surface Area
- vIGA-AD
validated Investigator’s Global Assessment
Footnotes
Conflicts of interest: None
References
- 1.Yew YW, Thyssen JP, Silverberg JI. A systematic review and meta-analysis of the regional and age-related differences in atopic dermatitis clinical characteristics. Journal of the American Academy of Dermatology 2019; 80: 390–401. [DOI] [PubMed] [Google Scholar]
- 2.Silverberg JI, Margolis DJ, Boguniewicz M et al. Distribution of atopic dermatitis lesions in United States adults. Journal of the European Academy of Dermatology and Venereology : JEADV 2019; 33: 1341–8. [DOI] [PubMed] [Google Scholar]
- 3.Chiesa Fuxench ZC, Block JK, Boguniewicz M et al. Atopic Dermatitis in America Study: A Cross-Sectional Study Examining the Prevalence and Disease Burden of Atopic Dermatitis in the US Adult Population. The Journal of investigative dermatology 2019; 139: 583–90. [DOI] [PubMed] [Google Scholar]
- 4.Silverberg JI, Gelfand JM, Margolis DJ et al. Health Utility Scores of Atopic Dermatitis in US Adults. The journal of allergy and clinical immunology. In practice 2019; 7: 1246–52 e1. [DOI] [PubMed] [Google Scholar]
- 5.Silverberg JI, Gelfand JM, Margolis DJ et al. Patient burden and quality of life in atopic dermatitis in US adults: A population-based cross-sectional study. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology 2018; 121: 340–7. [DOI] [PubMed] [Google Scholar]
- 6.Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology 1993; 186: 23–31. [DOI] [PubMed] [Google Scholar]
- 7.Schmitt J, Langan S, Deckert S et al. Assessment of clinical signs of atopic dermatitis: a systematic review and recommendation. The Journal of allergy and clinical immunology 2013; 132: 1337–47. [DOI] [PubMed] [Google Scholar]
- 8.Hill MK, Kheirandish Pishkenari A, Braunberger TL et al. Recent trends in disease severity and quality of life instruments for patients with atopic dermatitis: A systematic review. Journal of the American Academy of Dermatology 2016; 75: 906–17. [DOI] [PubMed] [Google Scholar]
- 9.Rehal B, Armstrong AW. Health outcome measures in atopic dermatitis: a systematic review of trends in disease severity and quality-of-life instruments 1985–2010. PloS one 2011; 6: e17520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wollenberg A, Barbarot S, Bieber T et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. Journal of the European Academy of Dermatology and Venereology : JEADV 2018; 32: 657–82. [DOI] [PubMed] [Google Scholar]
- 11.Chalmers JR, Schmitt J, Apfelbacher C et al. Report from the third international consensus meeting to harmonise core outcome measures for atopic eczema/dermatitis clinical trials (HOME). The British journal of dermatology 2014; 171: 1318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snast I, Reiter O, Hodak E et al. Are Biologics Efficacious in Atopic Dermatitis? A Systematic Review and Meta-Analysis. American journal of clinical dermatology 2018; 19: 145–65. [DOI] [PubMed] [Google Scholar]
- 13.Puig L, Lopez A, Vilarrasa E et al. Efficacy of biologics in the treatment of moderate-to-severe plaque psoriasis: a systematic review and meta-analysis of randomized controlled trials with different time points. Journal of the European Academy of Dermatology and Venereology : JEADV 2014; 28: 1633–53. [DOI] [PubMed] [Google Scholar]
- 14.Schram ME, Spuls PI, Leeflang MM et al. EASI, (objective) SCORAD and POEM for atopic eczema: responsiveness and minimal clinically important difference. Allergy 2012; 67: 99–106. [DOI] [PubMed] [Google Scholar]
- 15.Leshem YA, Hajar T, Hanifin JM et al. What the Eczema Area and Severity Index score tells us about the severity of atopic dermatitis: an interpretability study. The British journal of dermatology 2015; 172: 1353–7. [DOI] [PubMed] [Google Scholar]
- 16.Chopra R, Vakharia PP, Sacotte R et al. Severity strata for Eczema Area and Severity Index (EASI), modified EASI, Scoring Atopic Dermatitis (SCORAD), objective SCORAD, Atopic Dermatitis Severity Index and body surface area in adolescents and adults with atopic dermatitis. The British journal of dermatology 2017; 177: 1316–21. [DOI] [PubMed] [Google Scholar]
- 17.Terwee CB, Roorda LD, Knol DL et al. Linking measurement error to minimal important change of patient-reported outcomes. Journal of clinical epidemiology 2009; 62: 1062–7. [DOI] [PubMed] [Google Scholar]
- 18.Hanifin J, Rajka G. Diagnostic features of atopic eczema. Acta dermato-venereologica 1980; 92: 44–7. [Google Scholar]
- 19.Vakharia PP, Chopra R, Sacotte R et al. Validation of patient-reported global severity of atopic dermatitis in adults. Allergy 2017. [DOI] [PubMed] [Google Scholar]
- 20.Gerbens LA, Prinsen CA, Chalmers JR et al. Evaluation of the measurement properties of symptom measurement instruments for atopic eczema: a systematic review. Allergy 2017; 72: 146–63. [DOI] [PubMed] [Google Scholar]
- 21.Spuls PI, Gerbens LAA, Simpson E et al. Patient-Oriented Eczema Measure (POEM), a core instrument to measure symptoms in clinical trials: a Harmonising Outcome Measures for Eczema (HOME) statement. The British journal of dermatology 2017; 176: 979–84. [DOI] [PubMed] [Google Scholar]
- 22.Silverberg JI, Gelfand JM, Margolis DJ et al. Severity strata for POEM, PO-SCORAD, and DLQI in US adults with atopic dermatitis. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology 2018; 121: 464–71. [DOI] [PubMed] [Google Scholar]
- 23.Silverberg JI, Margolis DJ, Boguniewicz M et al. Validation of five patient-reported outcomes for atopic dermatitis severity in adults. The British journal of dermatology 2019. [DOI] [PubMed] [Google Scholar]
- 24.Vakharia PP, Cella D, Silverberg JI. Patient-reported outcomes and quality of life measures in atopic dermatitis. Clin Dermatol 2018; 36: 616–30. [DOI] [PubMed] [Google Scholar]
- 25.Vakharia PP, Chopra R, Sacotte R et al. Severity strata for five patient-reported outcomes in adults with atopic dermatitis. The British journal of dermatology 2018; 178: 925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basra MK, Fenech R, Gatt RM et al. The Dermatology Life Quality Index 1994–2007: a comprehensive review of validation data and clinical results. The British journal of dermatology 2008; 159: 997–1035. [DOI] [PubMed] [Google Scholar]
- 27.Patel KR, Singam V, Vakharia PP et al. Measurement properties of three assessments of burden used in atopic dermatitis in adults. The British journal of dermatology 2019; 180: 1083–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silverberg JI, Gelfand JM, Margolis DJ et al. Validation and Interpretation of Short Form 12 and Comparison with Dermatology Life Quality Index in Atopic Dermatitis in Adults. The Journal of investigative dermatology 2019; 139: 2090–7 e3. [DOI] [PubMed] [Google Scholar]
- 29.Simpson E, Bissonnette R, Eichenfield L et al. The validated Investigator Global Assessment for atopic dermatitis (vIGA-AD™): A novel clinical outcome measurement instrument for the severity of atopic dermatitis. In: Oral Presentation at: The European Academy of Dermatology and Venereology Congress. Paris, France. 2018. [Google Scholar]
- 30.Hanifin JM, Thurston M, Omoto M et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Experimental dermatology 2001; 10: 11–8. [DOI] [PubMed] [Google Scholar]
- 31.Chopra R, Silverberg JI. Assessing the severity of atopic dermatitis in clinical trials and practice. Clin Dermatol 2018; 36: 606–15. [DOI] [PubMed] [Google Scholar]
- 32.Chopra R, Vakharia PP, Sacotte R et al. Relationship between EASI and SCORAD severity assessments for atopic dermatitis. The Journal of allergy and clinical immunology 2017; 140: 1708–10 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruynesteyn K, Boers M, Kostense P et al. Deciding on progression of joint damage in paired films of individual patients: smallest detectable difference or change. Annals of the rheumatic diseases 2005; 64: 179–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oosterhaven JAF, Ofenloch RF, Schuttelaar MLA. Interpretability of the Quality Of Life in Hand Eczema Questionnaire (QOLHEQ). The Journal of investigative dermatology 2019: S0022-202X(19)33297-X. [DOI] [PubMed] [Google Scholar]
- 35.Terwee CB, Bot SD, de Boer MR et al. Quality criteria were proposed for measurement properties of health status questionnaires. Journal of clinical epidemiology 2007; 60: 34–42. [DOI] [PubMed] [Google Scholar]
- 36.Lim CR, Harris K, Dawson J et al. Floor and ceiling effects in the OHS: an analysis of the NHS PROMs data set. BMJ open 2015; 5: e007765. [DOI] [PMC free article] [PubMed] [Google Scholar]


