Although lung cancer remains the leading cause of cancer-related death in the United States, the past decade has seen continuous declines in mortality, with age-adjusted death rates falling on average 3.3% each year over 2008–2017.1 Further progress in these encouraging trends will in part be tied to continued advances in identifying oncogenic genomic alterations and the development of targeted therapies for non-small cell lung cancer (NSCLC), particularly nonsquamous NSCLC (ie, mostly adenocarcinoma). Indeed, the month of May 2020 alone saw an unprecedented 7 new approvals by the US Food and Drug Administration (FDA) for NSCLC therapies!2 These therapeutic advances have implications for the pulmonary pathology and cytology community, namely the ever-increasing need for biomarker testing in NSCLC. In short, we need to identify these actionable genomic alterations before the oncologists can treat them.
Most cytologists are familiar with the molecular testing guidelines for NSCLC testing put forth by the College of American Pathologists (CAP), the International Association for the Study of Lung Cancer (IASLC), and the Association for Molecular Pathology (AMP), which recommend that all advanced-stage lung adenocarcinomas be tested for EGFR mutations as well as ALK and ROS1 gene rearrangements.3,4 Soon thereafter, the American Society of Clinical Oncology endorsement statement for these guidelines added BRAF testing to the must-test list.5 However, the rapid pace of drug development with efficacy proven in large clinical trials has quickly rendered these guideline recommendations somewhat outdated. As such, the practicing cytologist should become familiar with the most detailed and frequently updated clinical practice guidelines available: the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology for NSCLC, which reflect the current standard of care for managing patients with NSCLC.6,7 The NCCN guidelines are also used by the largest health care payers in the United States, including the Centers for Medicare and Medicaid Services and United Healthcare (among other private insurance companies), to determine their oncology coverage policies; these decisions have implications for pathology laboratories with respect to the financial feasibility of NSCLC testing practices.7,8
The list of biomarkers that need to be assessed for NSCLC has grown to now include at least 8 targets, with MET exon 14 skipping mutations, RET and NTRK gene rearrangements, and PD-L1 expression in tumor cells and/or tumor-infiltrating immune cells being added to the 4 previously mentioned.6 Apart from PD-L1, which currently can be assessed only via immunohistochemistry, as extensively covered elsewhere,9 the NCCN guidelines strongly advise broader molecular profiling testing to capture targetable alterations in this ever-growing list of biomarkers. This shift from single-gene assays to larger next-generation sequencing (NGS)-based panels is the most effective and efficient way to identify rare driver mutations in tumors that can be treated with FDA-approved therapies or alternatively can direct patients to clinical trials. In addition, larger NGS comprehensive molecular testing panels can provide information on other genomic alterations that have FDA-approved therapies, such as those for microsatellite instability-high tumors2; emerging biomarkers such as the tumor mutational burden that may help to select patients suited for immune checkpoint inhibitor therapy, especially if a high tumor mutational burden (≥10 mutations per megabase) is present2; and the tumoral co-mutational landscape, which is increasingly being shown to harbor prognostic information.10 These targets, therapies, and other emerging and evolving biomarkers in NSCLC are summarized in Figure 1.
FIGURE 1.
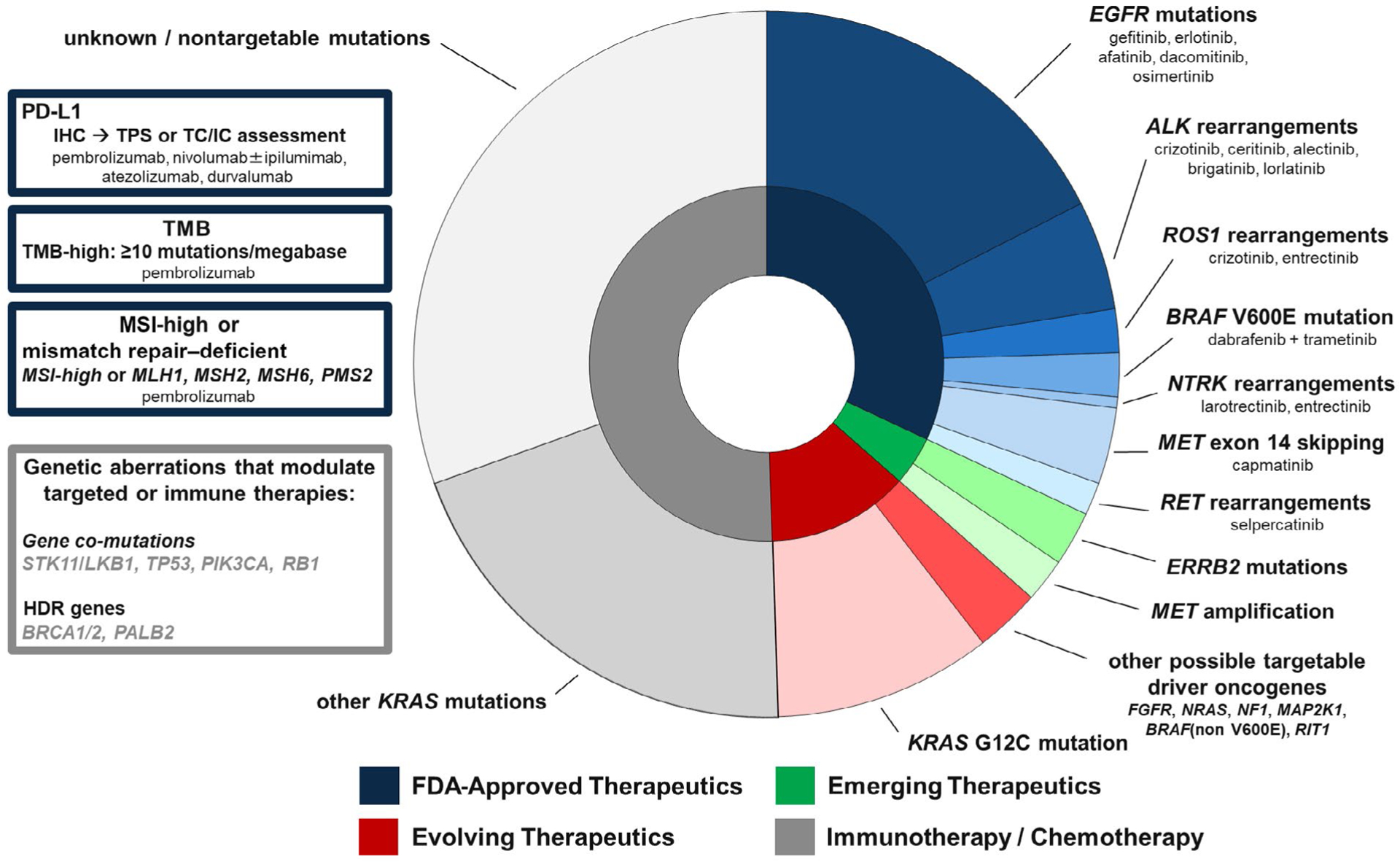
Landscape of genomic alterations in NSCLC with corresponding FDA-approved therapeutic options. The shading of the concentric pie chart rings and text box borders reflect the current state of FDA-approved NSCLC therapies as of June 2020. The inset boxes also highlight additional immune and genomic changes that can have therapeutic and/or prognostic implications in the setting of NSCLC. FDA indicates Food and Drug Administration; HDR, homology-directed repair; IHC, immunohistochemistry; MSI, microsatellite instability; NSCLC, non-small cell lung cancer; TC/IC, Tumor cells/Tumor-infiltrating immune cells; TMB, tumor mutation burden; TPS, tumor proportion score.
In light of this ever-changing testing landscape, the cytologist may ask why so many biomarkers need to be assessed. The answer is both simple and complex. In short, optimal therapeutic selection for patients with advanced-stage NSCLC is critically dependent on it, because patients who receive biomarker-driven care live both better and longer when appropriate treatments are selected according to the tested biomarkers. In a sense, this is the holy grail of personalized medicine. The long-term outcomes of patients treated with a biomarker-driven strategy include previously unheard-of milestones in NSCLC, such as the unprecedented 5-year survival rates seen with metastatic ALK-rearranged NSCLC treated with first-line alectinib11 and metastatic PD-L1-positive NSCLC treated with pembrolizumab.12 The first decade of biomarker-driven care for NSCLC, from the discovery of EGFR mutations in 2004 to the first CAP/IASLC/AMP guidelines recommending EGFR mutation and ALK rearrangement testing in 2013,3 has been painstakingly slow. However, it has allowed the interdisciplinary oncology community at large time to develop the necessary scientific knowledge and clinical/operational infrastructure that now support cutting-edge, more optimal care for patients with lung cancer. As this effort has evolved, 2 themes have emerged: 1) an expanding number of requisite biomarkers of significant clinical and therapeutic relevance and 2) an expansion of the precision oncology effort from advanced-stage disease to earlier in the disease course.
As mentioned previously, currently approved targeted therapies include those directed at tumors with actionable alterations in EGFR, ALK, ROS1, BRAF, MET, RET, and NTRK. The list of therapeutic options for actionable alterations has expanded exponentially in recent years, with novel oral targeted inhibitors, antibody-drug conjugates, and combination therapies showing promising efficacy and tolerability and with approvals being anticipated for an ever-growing list of additional alterations, such as mutations in ERRB2, KRAS-G12C, and others (Fig. 1). Currently, the majority of new drug approvals for the management of recurrent/metastatic NSCLC are linked to companion diagnostic assays that evaluate either the tumor PD-L1 status or an expanding number of specific driver oncogene alterations that are considered clinically relevant (Fig. 1). Although the bevy of biomarker-driven drug approvals to date has been for patients with advanced-stage disease, the practice of precision oncology promises to move shortly into the armamentarium for the treatment of patients with early-stage disease as well.13
Thus, a comprehensive assessment of the tumor histopathology and genomic profile has become increasingly necessary for the treating oncologist to make an optimal therapeutic selection for any given patient about to receive first-line palliative systemic therapy for advanced NSCLC: one that maximizes efficacy and quality of life while minimizing the risk of toxicity without clinical benefit. Therapeutic stratification in the first-line setting has additional importance because roughly one-third of all patients with advanced-stage disease may not remain well enough to tolerate or benefit from second-line therapy when the initial therapy does not prove to be effective/tolerable.14
In the real-world setting, there is the added complexity surrounding how to provide comprehensive tumor profiling in a timely fashion that aligns with the clinical needs and biology of disease without creating delays in care with unintended adverse consequences. How can this be done without making perfect the enemy of good? Current CAP/IASLC/AMP guidelines recommend a maximum 10-day interval between receipt of the specimen for testing and delivery of genomic testing results3,4; this is an ideal target that is not always readily achieved in routine clinical practice. Optimization of the pre- and postanalytical phases of biomarker testing remains a practical challenge in many care settings.15 An increased number of viable assays and vendors have allowed for optimization of testing turnaround times and processes to ensure that testing is done in an accurate and timely manner. Commercial genomic profiling assays now have robust gene panels with FDA approval as companion diagnostic assays (eg, the FoundationOne CDx and the Oncomine Dx Target Test), and many academic centers have additionally vetted and validated their own in-house, proprietary NGS platforms (eg, MSK-IMPACT, Memorial Sloan Kettering Integrated Mutation Profiling of Actionable Cancer Targets).
For the cytologist and the pathologist, exhaustion of the tissue specimen during the diagnostic workup before ancillary testing has become an additional pragmatic concern because the number of required biomarkers for upfront testing has continued to expand. With recent rapid advances in these technologies, increased use of comprehensive genomic (rather than single-gene) testing platforms offers an overall timelier and more cost- and tissue-conscious approach; however, not all clinical specimens are adequate to successfully complete NGS assays. Although most molecular laboratories have optimized and validated testing on traditional formalin-fixed, paraffin-embedded specimens (eg, the cytology cell block), a growing body of evidence shows that other cytology specimens such as direct smears, liquid-based preparations, and even specimen supernatants can be used for NGS testing if properly validated.16 From a clinical standpoint, not all patients may be amenable/appropriate for rebiopsy or other efforts needed to obtain adequate tissue both for histopathologic diagnosis and for tumor genomic profiling. As a result, there has been mounting interest in the use of liquid biopsies that use NGS technology to evaluate circulating tumor DNA as a reflection of the tumoral genomic profile. Currently, blood-based biomarker testing should be considered not a replacement for tissue-based testing but rather a complementary approach. That said, it is foreseeable that the use of liquid biopsies will be better defined in future iterations of the guidelines and more commonly applied in day-to-day clinical practice for management of both early- and advanced-stage disease.17
Despite the many scientific, clinical, and technological advances in the field of NSCLC and the clear demonstration of benefit to the patients for whom this progress is intended, it remains a sobering reality that only a fraction of eligible NSCLC cases worldwide currently achieve guideline-specified standards for mandatory upfront testing as described here.18 Therefore, maximizing the value of precision oncology for all those who stand to benefit will require continued intensive efforts and interdisciplinary collaboration between all members of the oncology care team, including those of us in the cytopathology community. To be sure, the onus to guarantee evidence-based tumor biomarker testing lies with us all.
FUNDING SUPPORT
Daniel B. Costa was supported in part by funds from National Institutes of Health grant R37 CA218707.
Biographies

Paul A. VanderLaan, MD, PhD
Paul A. VanderLaan serves as the director of cytopathology and the director of thoracic pathology at the Beth Israel Deaconess Medical Center, and is an associate professor of pathology at Harvard Medical School. His clinical interests lie at the intersection of cytopathology and pulmonary pathology in the setting of both neoplastic and nonneoplastic lung disease. He sits on the editorial boards of multiple medical journals, enjoys teaching residents and fellows, and consistently has an eye out for optimizing specimen processing and ongoing quality improvement measures in the cytopathology laboratory.

Deepa Rangachari, MD
Deepa Rangachari is a member of the Thoracic Oncology Group at Beth Israel Deaconess Medical Center and a member of the Lung Cancer Program at the Dana-Farber/Harvard Cancer Center as well as an assistant professor of medicine at Harvard Medical School. She additionally serves as the medical director of inpatient medical oncology and the associate director of the Hematology/Oncology Fellowship Program. Her clinical and investigational interests are in optimizing clinical care and outcomes for patients with advanced lung cancer as well as curriculum development and leadership in postgraduate medical education.

Daniel B. Costa, MD, PhD
Daniel B. Costa serves as the medical director of the Cancer Clinical Trials Office and as leader of the Thoracic Oncology Group within the Division of Medical Oncology at Beth Israel Deaconess Medical Center. He is a coleader of the Lung Cancer Program at the Dana-Farber/Harvard Cancer Center and is an associate professor of medicine at Harvard Medical School. His research and clinical interests lie at the intersection of precision oncology and thoracic oncology. He is funded by multiple governmental and industry sources, sits on the editorial board of lung cancer-specific journals, and is engaged in fellow teaching and junior faculty development.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
PVL reports consulting fees from Gala Therapeutics, Foundation Medicine, Caris Life Sciences, Intuitive Surgical, Flatiron Health, and Clearview Healthcare Partners outside the submitted work. DR reports institutional research support from Bristol-Myers Squibb, Novocure, and AbbVie/Stemcentrx and personal fees from Advance Medical/TeleDoc Health, DynaMed, and AstraZeneca outside the submitted work. DBC reports consulting fees and honoraria and institutional research support from Takeda/Millennium Pharmaceuticals, AstraZeneca, and Pfizer and institutional research support from Merck Sharp & Dohme Corp, Merrimack Pharmaceuticals, Bristol-Myers Squibb, Clovis Oncology, Spectrum Pharmaceuticals, and Tesaro outside the submitted work.
Contributor Information
Paul A. VanderLaan, Beth Israel Deaconess Medical Center,; Harvard Medical School,
Deepa Rangachari, Beth Israel Deaconess Medical Center,; Dana-Farber/Harvard Cancer Center, Harvard Medical School,
Daniel B. Costa, Beth Israel Deaconess Medical Center,; Dana-Farber/Harvard Cancer Center, Harvard Medical School,
References
- 1.Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Lung and Bronchus Cancer. Statistics at a Glance. Accessed July 1, 2020. http://seer.cancer.gov/statfacts/html/lungb.html [Google Scholar]
- 2.US Food and Drug Administration. Hematology/Oncology (Cancer) Approvals & Safety Notifications. Accessed July 1, 2020. https://www.fda.gov/drugs/resources-information-approved-drugs/hematologyoncology-cancer-approvals-safety-notifications
- 3.Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. Arch Pathol Lab Med. 2013;137:828–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindeman NI, Cagle PT, Aisner DL, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch Pathol Lab Med. 2018;142:321–346. [DOI] [PubMed] [Google Scholar]
- 5.Kalemkerian GP, Narula N, Kennedy EB, et al. Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology clinical practice guideline update. J Clin Oncol. 2018;36:911–919. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology (NCCN Guidelines): Non-Small Cell Lung Cancer, Version 6.2020. Accessed July 1, 2020. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- 7.Pluchino LA, D’Amico TA. National Comprehensive Cancer Network Guidelines: who makes them? What are they? Why are they important? Ann Thorac Surg Published online April 13, 2020. doi: 10.1016/j.athoracsur.2020.03.022 [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network. NCCN at a Glance. Accessed July 1, 2020. https://www.nccn.org/about/pdf/NCCN_Fact_Sheet.pdf
- 9.Lantuejoul S, Sound-Tsao M, Cooper WA, et al. PD-L1 testing for lung cancer in 2019: perspective from the IASLC Pathology Committee. J Thorac Oncol. 2020;15:499–519. [DOI] [PubMed] [Google Scholar]
- 10.Skoulidis F, Heymach JV. Co-occurring genomic alterations in non-small-cell lung cancer biology and therapy. Nat Rev Cancer. 2019;19:495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters S, Mok TSK, Gadgeel SM, et al. Updated overall survival (OS) and safety data from the randomized, phase III ALEX study of alectinib (ALC) versus crizotinib (CRZ) in untreated advanced ALK+ NSCLC [abstract 9518]. J Clin Oncol. 2020;38(15 suppl):9518. [Google Scholar]
- 12.Rangachari D, Costa DB. From hope to reality: durable overall survival with immune checkpoint inhibitors for advanced lung cancer. J Clin Oncol. 2019;37:2511–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herbst RS, Tsuboi M, John T, et al. Osimertinib as adjuvant therapy in patients (pts) with stage IB-IIIA EGFR mutation positive (EGFRm) NSCLC after complete tumor resection: ADAURA [abstract LBA5]. J Clin Oncol. 2020;38(18 suppl):LBA5. [Google Scholar]
- 14.Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382:41–50. [DOI] [PubMed] [Google Scholar]
- 15.DiStasio M, Chen Y, Rangachari D, Costa DB, Heher YK, VanderLaan PA. Molecular testing turnaround time for non-small cell lung cancer in routine clinical practice confirms feasibility of CAP/IASLC/AMP guideline recommendations: a single-center analysis. Clin Lung Cancer. 2017;18:e349–e356. [DOI] [PubMed] [Google Scholar]
- 16.Roy-Chowdhuri S, Pisapia P, Salto-Tellez M, et al. Invited review-next-generation sequencing: a modern tool in cytopathology. Virchows Arch. 2019;475:3–11. [DOI] [PubMed] [Google Scholar]
- 17.Leighl NB, Page RD, Raymond VM, et al. Clinical utility of comprehensive cell-free DNA analysis to identify genomic biomarkers in patients with newly diagnosed metastatic non-small cell lung cancer. Clin Cancer Res. 2019;25:4691–4700. [DOI] [PubMed] [Google Scholar]
- 18.Smeltzer MP, Wynes MW, Lantuejoul S, et al. The International Association for the Study of Lung Cancer (IASLC) Global Survey on Molecular Testing in Lung Cancer. J Thorac Oncol. Published online May 14, 2020. doi: 10.1016/j.jtho.2020.05.002 [DOI] [PubMed] [Google Scholar]


