Abstract
The most important and greatest source in the body for regenerative cells is fat tissue. Obtaining regenerative cells from adipose tissue can be done in two ways: Enzymatic and mechanical. The regenerative cell cocktail obtained by the enzymatic method, including stem cells, is called Stromal vascular fracture (SVF). In the literature, there is no clear definition of regenerative cells obtained by mechanical method. We systematically searched the techniques and definitions for stromal cells obtained from adipose tissue by scanning different databases. To evaluate the mechanical stromal-cell isolation techniques and end products from adipose tissue. Systematic review of English and non-English articles using Embase, PubMed, Web of Science and Google scholar databases. Search terms included Nanofat, fragmented fat, mechanical stromal / stem cell, mechanical SVF, SVF gel. We screened all peer-reviewed articles related with mechanical stromal-cell isolation. Author performed a literature query with the aforementioned key words and databases. A total of 276 publications containing the keywords we searched were reached. In these publications, there are 46 different definitions used to obtain mechanical stromal cells. The term SVF is only suitable for enzymatic methods. A different definition is required for mechanical. The most used term nanofat is also not suitable because the product is not in both "fat" and in "nanoscale". We think that the term total stromal-cells would be the most appropriate definition since both extracellular matrix and all stromal cells are protected in mechanical methods.
Keywords: SVF, Nanofat, Stromal cells, Regeneration, Mechanical isolation, Total stromal cells
Introduction
Regenerative medicine is the most popular subject of recent years in aesthetic plastic surgery and regenerative medicine. There are a growing number of sources in literature regarding regenerative cells, stem/stromal cell applications for different indications in several disciplines. Although cell therapies are very important and promising a valuable future, De Francesco, one of the most important authors on this subject, explains the reasons why it still cannot find the place it deserves: restrictive worldwide measures, which fundamentally entail a common operating protocol, toxin- and xeno-free reagents, replacing enzymes and enabling a rapid monitoring of quality standards to ensure an adequate cell identity and the efficiency of the donor tissue [1] According to our classical knowledge, there are two different types of cells in all our organs that make up our body—parenchymal cells, responsible for the function of the organ and stromal cells, that support them. Stromal cells provide tissue repair and renewal following stress, injury, illness, or aging, that is, from both intrinsic and extrinsic causes [2]. In fact, one of the most important features of adipose tissue, in addition to its thermoregulation, shock absorption, and being the body’s energy storage, is that it is the largest and most important regenerative cell source in the body [3]. This tissue is a type of loose connective tissue that contains an eclectic reservoir of cells, including immune cells, erythrocytes, progenitors, and stromal components [4]. The stroma is the area with support cells; there are also adipose derived stem cells (ASCs) in the perivascular area of the stroma [5]. The use of regenerative cells from adipose tissue is not a new subject. The first applications which promoting the healing, by transferring the fat tissue particles into the wounds of the injured soldiers were made by Morrestin in World War 1 [6]. Thus, it actually used the regenerative effect of fat tissue. Later, although in 1986 Jarrell presented the effect of microvascular endothelial cells from adipose tissue in his study [7] this issue was mainly popularized by Zuk et al. [8]. They presented that, fat tissue is a very important and the largest mesenchymal stem / stromal cell source of body. Stromal cells from the adipose tissue for regenerative purposes are obtained by two methods. Enzymatic and mechanical. Until recently, the gold standard was the use of enzymes in the extraction of stromal cells from adipose tissue. The enzyme destroyed the dense bonds in the adipose tissue, and stromal cells were obtained by centrifugation [9]. This stromal-cell cocktail, including adipose stem cells, was called stromal vascular fraction (SVF). The SVF contains different kinds of cells, including ASCs, mesenchymal and endothelial progenitor cells, leukocytes, and pericytes. Although the enzymatic method is the most effective for SVF isolation, it is an expensive and time-consuming open system, which requires a further step of enzyme purification. In addition, it destroys the stem-cell niche, known as the microenvironment, which surrounds the stem cell, allowing interactions with neighboring cells that promote cell survival, proliferation, and differentiation [10] Regulatory authorities evaluated SVF production as biological drug production and required that current good manufacturing practice (cGMP) and current good laboratory practice (cGLP) standards be met [11]. More importantly, in the production of SVF by enzyme, the enzyme destroys not only bonds but also extra cellular matrix (ECM) and regenerative cells [12]. For these reasons, mechanical stromal-cell production has become increasingly popular [13]. Recently, Tonnard et al., who changed our perspective on fat grafts and stromal cell isolations from adipose tissue, offered a definition of nanofat in their 2013 study which can be accepted as milestone in mechanical stromal cell isolation [5] To work even more superficially with still finer sharp needles (27 gauge), the harvested fat was mechanically emulsified and filtered until a liquid suspension was obtained. They called this “nanofat”. Although the term nanofat has become popular since 2013, this new concept has been criticized in two aspects: First, in terms of the concept of “nano”, a nanometer is one-billionth of a meter, and dimensions between approximately 1 and 100 nm are known as the nanoscale, but in general, nanofat is thought of as fat parcel sizes of 600 microns ( https://en.wikipedia.org/wiki/Nanotechnology). Second, in terms of “fat”, Stuzin, who discussed Tonnard’s paper, stated that the most intriguing point in the article is that the substance the authors term nanofat is not fat at all [14]. After histologic examination of their suspension, the authors realized that in processing the nanofat, the normal fat structure was destroyed. Also, they preferred to using of “emulsification” term for the process for the mechanical isolation of stromal cells from adipose tissue in the original study of Tonnard in 2013 [5]. But, emulsification is to force two immiscible liquids to combine in a suspension—substances like oil and water, which cannot dissolve in each other to form a uniform, homogenous solution. On the other hand, The term SVF is used for a fat tissue-derived regenerative cell cocktail obtained using enzymes such as collagenase [11]. The term SVF is not suitable for mechanically derived stromal cells for many reasons. Although mechanically, regenerative cells originating from adipose tissue are referred to by the term "nanofat", many alternatives are also presented in the literature. We systematically searched the techniques and definitions for stromal cells obtained from adipose tissue by scanning different databases. As a result of this systematic research, we proposed a new term for mechanically derived stromal cells.
Methods
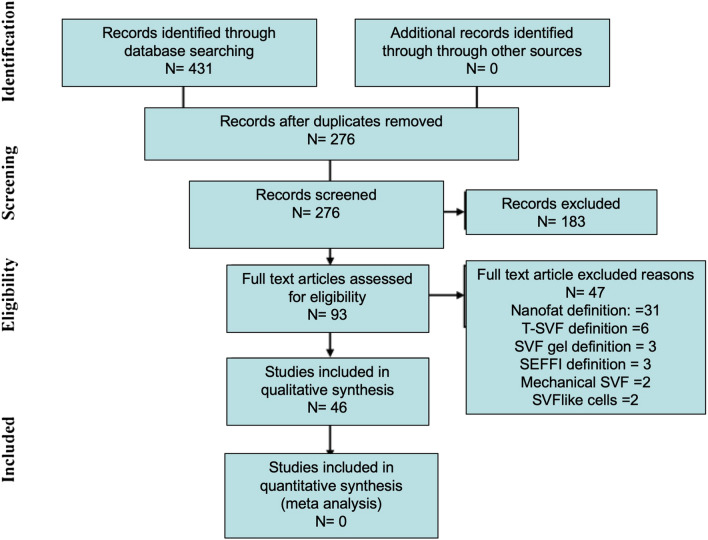
A systematic electronic search was performed according to the guidelines and recommendations from preferred reporting items for systematic reviews and meta-analysis checklist (PRISMA) in databases of Embase, PubMed, Web of Science and Google scholar (Fig. 1). The following search terms and synonyms were used: nanofat, fragmented fat, mechanical stromal / stem cell, mechanical SVF, SVF gel. All abstracts/proceedings were screened according to search strategies.
Fig. 1.
Flow of information through the different phases of a systematic review
Articles reporting on the analysis or defined the final product of mechanically isolated adipose derived stromal cells were included. Abstracts were included when sufficient data – defined isolation procedure and outcomes were provided. When necessary, authors of non-English language studies were contacted to provide an English language summary of their findings. All studies using the term of SVF or only nanofat were excluded.
Systematic literature search results
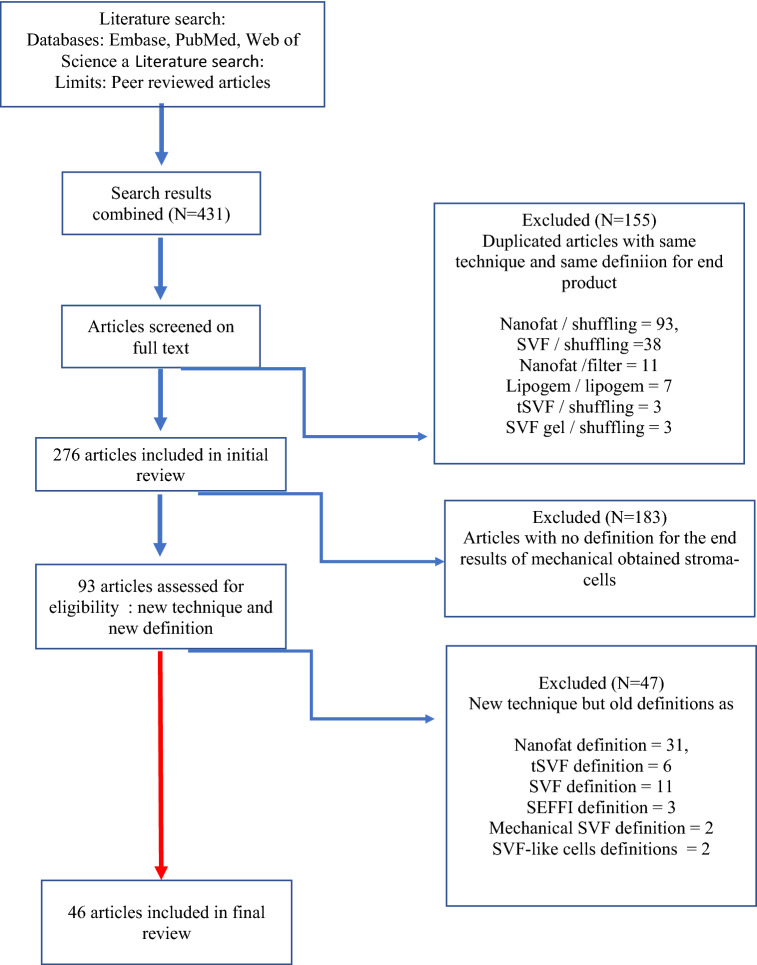
Overall, 431 articles were retrieved from the aforementioned databases. After deduplication, 276 articles were screened based on full text manner. Duplicated articles with same technique and same definition for end product and 155 publications were excluded from the study. The most used term in publications as a new technique or protocol was nanofat and was repeated 93 times. In the second place, the term SVF comes and was repeated 38 times. In each term, a protocol was created between 2 injectors as shuffling. In the third row, the term Lipogem comes and the Lipogem-specific tool and protocol developed by Tremolada was used [12] tSVF and SVF gel were repeated 3 times and shuffling was applied to both. When the remaining 276 publications were examined in detail, 183 publications were again excluded. Because in these publications, a new definition or any definition was not made after mechanical extraction for stromal regenerative cells. Finally, 47 of the remaining 93 publications were excluded from the study. Because, although these publications describe a new technique/protocol/tool, they did not make a new definition for the stromal cells obtained, they repeated the old definitions. Again, the most repeated definition was "nanofat" and it was defined with a new approach 30 times in total. The second was SVF and 11 times in total, then tSVF 6 times, SEFFI 3 times, mechanical SVF 2 times and SVF-like cells 2 times, respectively. All details of systematic review according to the PRISMA was presented in Fig. 1.There were a total of 46 different terms used for the end product in mechanical stromal cell production from adipose tissue. These are presented in Fig. 2.
Fig. 2.
Selection of included studies
Discussion
In the light of the results of the existing techniques, after a systematic literature search in this study, both a new technique has been defined and a new term has been defined for the final product containing the stromal cells obtained. Stromal cell isolation from adipose tissue mechanically has an increasing popularity. The main reason for this is not only the difficulties in using enzymes, but also the advantages of mechanical methods. Unfortunately, unlike the enzyme, there are no established standards for the mechanical methods. This concerns both the specifications used and the presentation of the results. First, when the enzyme is used, the final product is SVF. The end product obtained mechanically has a different composition than SVF obtained by enzyme [15]; therefore, a different definition is needed. A total of 47 different definitions have been made so far in mechanical stromal cell production. In this regard, there is no consensus not only in terms of definition, but also in terms of quantity and quality of the product isolated. The reasons for this are that mechanical approaches are much easier and simpler than the enzyme, and many methods have been described, and unfortunately there are no protocols like the enzyme. Since the ECM and cell–cell connections are maintained, a nearly solid product is obtained, both in liquid and dense mass. However, in the enzyme, SVF, the final product, is always in the same liquid form. The most recent publication on stromal cell extraction from adipose tissue mechanically was published by Copcu and Oztan [16]. In their study, these authors used an ultra-sharp knife called Adinizer to cut the ligaments/bonds in order to free the stromal cells in the adipose tissue without creating excessive blunt pressure. As a result of their study, they obtained more cells than other methods and showed the characterization of these stromal cells. The greatest advantage of mechanical methods over enzymatic methods is the protection of both ECM and stromal cells during the procedure. Since the main purpose in mechanical methods is to protect the stromal cells as much as we can, we think the final product should be called total stromal-cell (TOST) as described by Copcu and Oztan [16]. These authors also showed cell characterizations in their study and regeneratively in the final product containing the stromal cells obtained, vascular endothelial growth factor (VEGF)-A, EGF-A, fibroblast growth factor (FGF)-2, platelet-derived growth factor (PDFG), nerve growth factor (NGF), transforming growth factor-β1 (TGFβ1); showed in detail the values of interleukin (IL)-10, IL1a as anti-inflammatory and interferon gamma (IFNg), IL-1b, IL6 and tumor necrosis factor alpha (TNFa) as pg/mL. Mechanical disaggregation of human tissues into living micro-grafts, in 2015, Travato et al. reported by as a new medical device [17]. And very recently, Astarita C. et al. examined tissue regeneration from stem cell to micrografts in their review [18]. They reported that the viability of the cells obtained by micrograft varied between 70 and 90% and showed that they have a very high regenerative potential. In vitro studies have shown that micrografts obtained mechanically by selecting particles with a cut-off of 80 mm are positive for MSC markers such as CD73, CD90, CD115, and CD146 and negative for hematopoietic markers such as CD34 and CD45 [20–22]
As presented in Table 1, there are 47 different definitions and a confusion for stromal / regenerative cells obtained by mechanical method in the literature. The disadvantages of the enzymatic methods, especially the limitations of the application rules, directed the researchers from the adipose tissue to stromal cell extraction with an increasing number of mechanical methods. Therefore, an increasing number of new methods, new devices and protocols are described in the literature and unfortunately a common language is not used. The purpose of this systematic review is to systematically scan all publications in the literature on the acquisition of stromal cells by mechanical method, both to suggest a the most suitable name for the cell population obtained and to reveal the differences of existing techniques, protocols and devices. With 47 different definitions, it's not just about name; The physical character of the product obtained is also different. Although there is no "living" adipose tissue for "nanofat" defined by Tonnard, physical structure is defined as "emulsified" adipose tissue [5]. However, Bernardini defined the more intensive "nanofat" format as "fat paste" and used it [23]. Again, Bernardini has defined ultra-micro fat so that it does not create viscosity or lumpiness in the highly superficial and especially periocular and perioral areas it. Gennai et al., on the other hand, tried to make the stromal cell community even more liquid and presented the concept of M-SEFF, micro superficial enhanced fluid fat [24]. Bernardini preferred to use stromal cells and the concept of "fluid fat" by putting it in liquid form only in 2016 [25]. Van Dongen proposed the term cSVF, cellular stromal vascular fraction, as a single cell in a pellet [26]. Lo Furno, has skipped of final filtration and squeezing in classical nanofat production and he provided “more dense” nanofat and called it as “Nanofat 2.0” [27]. Yao et al. introduced the resulting stromal cell population into gel formation and described it as hypercondensed fat [28]. In 2018, Yu et al. bought cellular components in the final product and ensured that only lipid remnant remained and the product obtained was called fat extract [29]. The first SVF gel term was used by Wang et al. [30]. SVF gel has defined as follows: mechanical processing method that removes most lipids and fluid from lipoaspirates and leaves only SVF cells and fractionated extracellular matrix [30]. In the mechanical cell extraction process, Sesé et al., although there are regenerative cells in the lowest liquid layer, they have thrown this liquid layer out and used the cell layer above it and called this layer nanofat cell aggregates [15]. De Francesco emphasized the importance of mechanical methods as follows: Currently, the use of mechanical procedures for the isolation of the SVF from adipose tissue has become of vital importance. Especially for the requirements of minimal tissue manipulation and the impossibility of using collagenase [31].
Table 1.
Definitions related with mechanically stromal cell isolations
| No. | Definition | Explanation | Year | Author |
|---|---|---|---|---|
| 1 | Nanofat | Mechanically emulsified adipose tissue which passing easily from 27 gauge needle | 2013 | Tonnard et al. [5] |
| 2 | Super micro-fat | New definition for "nanofat" | 2014 | Friiji et al. [50] |
| 3 | NE-SVF | Non-enzymatic stromal vascular fraction | 2015 | Nguyen et al. [51] |
| 4 | SEFF | Superficial enhanced fluid fat | 2015 | Bernardini et al. [24] |
| 5 | Fat paste | Dense lower layer of end product | 2015 | Bernardini et al. [24] |
| 6 | Ultra-micro fat | Very superficial implant in the periocular and perioral areas | 2015 | Bernardini et al. [24] |
| 7 | SVF-like cellular components | An alternative to nanofat term | 2015 | Pu et al. [52] |
| 8 | Enzyme free vascular fraction | SVF without enzyme | 2016 | Chaput et al. [53] |
| 9 | Adipose extracellular matrix/SVF GEL | Eliminating of the lipid and other unwanted components, leaving only extracellular matrix and stromal vascular fraction cells | 2016 | Yao et al. [42] |
| 10 | siSVF | Stress induced stromal vascular fraction | 2016 | Banyard et al. [42] |
| 11 | M-SEFF | Micro superficial enhanced fluid fat | 2016 | Gennai et al. [25] |
| 12 | Lipogem | Small adipose tissue clusters (0.2–0.8 mm) | 2016 | Boureaux et al. [54] |
| 13 | Non-enzymatic isolated SVF | For the end product of all mechanical techniques | 2016 | Conde-Green et al. [29] |
| 14 | MI-SVCs | Mechanically isolated stromal vascular cells | 2016 | Conde-Green et al. [41] |
| 15 | Fluid fat | New definition for the SEFF | 2016 | Bernardini et al. [23] |
| 16 | hASCS | Human adipose-derived stromal/stem cells | 2016 | Rossi et al. [55] |
| 17 | cSVF | Cellular stromal vascular fraction: single cell in a pellet | 2017 | van Dongen et al. [13] |
| 18 | tSVF | Tissue stromal vascular fraction: intact cell to cell communication | 2017 | van Dongen et al. [13] |
| 19 | Nonofat 2.0 | Skipping of final filtration and squeezing in classical Nanofat production | 2017 | Lo Furno et al. [27] |
| 20 | Tissue like SVF | Mechanically isolated stromal vascular fraction, that still has a stromal tissue-like structure | 2017 | van Dongen et al. [13] |
| 21 | Hypercondensed fat | A new term for SVF gel | 2017 | Yao et al. [28] |
| 22 | Mechanical SVF | Stromal vascular fraction by mechanically | 2017 | Cohen et al. [36] |
| 23 | Super-charged modified nanofat | SVF enrichment of nanofat | 2017 | Gentile et al. [56] |
| 24 | Evo-modified nanofat | Nanofat performed by slow centrifuge | 2017 | Gentile et al. [56] |
| 25 | Centrifuged-nanofat | Nanofat performed by regular centrifuge | 2017 | Gentile et al. [56] |
| 26 | NFSC | Nanofat derived stem cell | 2017 | Wei et al. [57] |
| 27 | SVF gel | Mixture generated by a simple mechanical process and particularly rich in ASCs, vascular endothelial cells (ECs), and native adipose extracellular matrix | 2017 | Yao et al. [28] |
| 28 | NFSCs | Nanofat derived stem cells | 2018 | Liang et al. [58] |
| 29 | Fractofat | Fractionalization of fat by emulsification | 2018 | Rohrich et al. [59] |
| 30 | Vivo-nanofat | Adding enzyme to nanofat | 2018 | Bi et al. [60] |
| 31 | Stem-like cells | Isolate of cells with stem-like potential derived from adipose tissue | 2018 | Glass et al. [61] |
| 32 | Fat extract | To remove the cellular components and the lipid remnants by mechanically | 2018 | Yu et al. [29] |
| 33 | Cell aggregates | Nanofat can be defined as an injectable product composed of cell aggregates | 2019 | Sese et al. [15] |
| 34 | Fat press | Nanofat procedure without washing the initial lipoaspirate | 2019 | Verpaele et al. [62] |
| 35 | t-SVF | Tissue stromal vascular fraction | 2019 | Jan et al. [63] |
| 36 | Micronized tSVF | Tissue SVF by mechanically ways | 2019 | Trivisonno et al. [32] |
| 37 | SVFgel | Mechanical processing method that removes most lipids and fluid from lipoaspirates and leaves only SVF cells and fractionated extracellular matrix | 2019 | Wang et al. [30] |
| 38 | FAT | Fractionation of adipose tissue | 2019 | Stevens [64] |
| 39 | Picograft | Stem cell quantity is high and there is no inflammatory reaction to the oil | 2019 | Ramirez [65] |
| 40 | MM-SVF | Mechanical micronized fat | 2019 | Zhu et al. [66] |
| 41 | ME-SVF | Microfat enriched stromal vascular fraction | 2019 | Grimaud et al. [67] |
| 42 | Human adipose liquid extract | Adipose tissue derived liquid portion | 2019 | He et al. [68] |
| 43 | Lipid-devoid adipose tissue | A definition for extracellular matrix/stromal vascular fraction gel (SVF-gel) | 2019 | He et al. [68] |
| 44 | Cell optimized, matrix rich nanofat | Nanofat with higher cells | 2019 | Cohen et al. [37] |
| 45 | CEFFE | Cell-free fat extract | 2019 | Xu et al. [69] |
| 46 | CFSCs | Chyle fat-derived stem cells | 2019 | Chen et al. [70] |
| 47 | TOST | Total stromal-cell transfer | 2020 | Copcu and Oztan [16] |
Copcu and Oztan, on the other hand, added a completely different dimension to the subject and for the first time they defined a clear protocol for obtaining stromal cells by mechanical method: IP's (Indication based) protocols [16]. This was a very important approach for the mechanical stromal cell extraction. Because, in order to obtain stromal cells by mechanical means, which is a very new subject, to be a valid, acceptable and reliable method, obtaining the same result in repetitive samples in accordance with a certain protocol, and the result is sufficient number and variety of stromal cells and sufficient viability is required. Predilution leads to better cutting of fat tissue and better isolation of stromal cells. We speculate that these effects can be explained by not only the difference in density but also the interaction and polarity between adipose tissue/saline, but further studies are needed to confirm this. However, with the predilution method, just as with enzymatic methods, stromal cells in solution form were obtained providing ease of application.
At the same time, with the IPS they have defined, TOST can be obtained in any quantity and volume that is suitable for the desired indication, and it is a great advantage, especially since its liquid form is SVF-like. In this protocol, they developed varying amounts of stromal cells for different indications, especially in the solution form. Four separate IPs were defined. The aim is the isolation of the bottom stromal-cell solution and the cell aggregate, and the final product, obtained by mixing these two layers together, is called the TOST. Adipose tissue was prediluted with saline in 50% ratio using 10 or 20 cc injectors in IPs 1 and 2, while condensed adipose tissue was used directly in IPs 3 and 4. In accordance with the selected IPs, 10 or 20 cc luer-lock injectors were used. An injector with condensed fat was placed on one end of the adinizer disc, an empty injector was placed on the other end, and the fat tissues were cut with sharp blades in the adinizer under minimal pressure with back and forth movements. The back and forth movement was made 25 times on average, at least 20 and up to 30. While starting the process of mechanically cutting the fat, pressure can be felt in the injectors between the researcher’s fingers, and this pressure subsides after approximately 20 passes. The relief of the pressure indicates that sufficient cuts have been made, and the process is terminated with a small cut having been made. Adinizing was first performed with a 4,000-micron adinizer; after approximately 25 passes, the cutting process was continued with the next-smaller diameter disc.
The term adinizing, which they defined above, was used for the first time in the literature; it defined the process of cutting fat tissue without blunt pressure, with sharp blades for reducing grafts to the desired diameter and separating the parenchymal and stromal cells in adipose tissue without using enzymes. The separation process with sharp blades will not only ensure the separation of stromal cells but also ASCs [12]. When this work is done with an enzyme, such as collagenase, destruction in both ECM and stromal regenerative cells occurs. Obtaining stromal cells from adipose tissue mechanically has been very popular recently; there are studies in the literature related to many devices [12, 15, 32, 33].
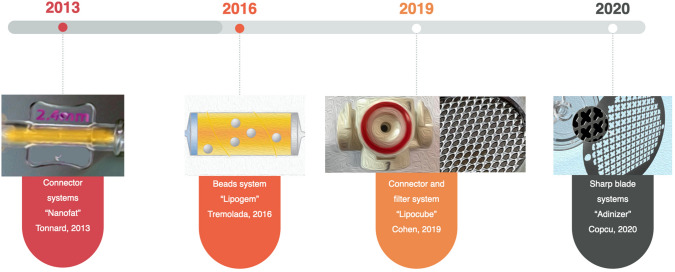
Cell extraction from adipose tissue showed evolutionary improvement as in all other methods in medicine and many approaches have been defined. Veronese et al. compared the mechanical processing techniques and they stated that the first type mechanical processing used to isolate ASCs was centrifugation only, and in 1987, Coleman was the first author [34]. When all the mechanical methods described so far are evaluated, the evolution of these methods can be classified under 4 separate headings (Fig. 3). As like Veronesse et al., we accept that Coleman’s study should be accept of milestone in evaluation of mechanical stromal cell extraction devices [35] 1. The connector system defined by Tonnard, which was Milestone in 2013. This system is a system with classic nanofat definitions, and it has been reported in Tonnard's original publication that 1 975 000 cells are obtained from 100 mL of adipose tissue [5]. 2. The isolation of stromal cells using “bead” popularized by Tremolada. Although there are many studies in the literature on Lipogem, no data showing the number of stromal cells obtained has been found [12]. 3. Connector and filter system combination was popularized by Cohen: Lipocube. He found that LipocubeNano produced a cell count of 2,240,000 cells per cc and cell viability of 96.75% [36]. 4. Finally, Copcu and Oztan defined the system using sharp blades without excessive blunt pressure: Adinizer lowest rate was found to be 28,660,000 and the highest 88,880,000 in their study [16]. In many other studies, very different numbers were presented; [5, 15, 33] even in several studies using the same product [37–40].Dai Pre et al., described the features of “ideal” mechanical devices as: fast, safe, standardized, and autologous [10].
Fig. 3.
Evolution of mechanical stromal cell isolation techniques
To our knowledge, this is the first systematic literature searching about the final product of mechanically isolated stromal cells from adipose tissue. We recommend using the term TOST, for mechanical stromal cells, instead of SVF or nanofat. Unlike the decomposing enzyme, stromal cells and ECM in mechanical methods are not destroyed and are protected to the maximum. Very different results have been reported regarding the viability and number of cells in mechanical stromal-cell production [5, 15, 37]. De Francesco concluded that mechanical methods allow the isolation of adipose-derived stromal cells with stemness and immunosuppressive properties similar to those obtained after collagenase digestion [1].
Mechanical methods have many advantages over enzymatic methods [13, 41]. The enzymatic disruption of adipose tissue results in a single-cell suspension in which all cell–cell communications are fully disrupted, and the extracellular matrix is digested; adipocytes are destroyed too. After mechanical isolation, however, adipocytes are also destroyed, but intercellular connections and cell–extracellular matrix connections remain intact [26]. Since sharp blades were used in Copcu and Oztan’s study, the structure of adipocytes was also preserved and alive adipocytes have been proven histopathologically [16]. They speculate that this is due to the effect of the sharp blade system used. Even after the last centrifuge, there is still intact adipose tissue, which is used for soft tissue augmentation. Moreover, the extracellular matrix, which is an important reservoir of growth factors and acts as an instructive scaffold in the regenerative processes, also remains intact, in contrast to enzymatically dissociated lipoaspirate. When adipose tissue is enzymatically digested, the architecture and instructive capacity of the stromal tissue are fully destroyed, although the isolated stromal tissue cells will survive [26]. More effective stromal cells can be obtained by mechanical methods in greater numbers and with more compositions for wound healing and regeneration [15]. Mechanical shear stress is always created in these techniques, which may lead to the upregulation of multipotent and pluripotent markers that connote regenerative capacity [42]. Mechanical disaggregation requires 10 times less fat tissue as the starting material to provide a similar or even higher cell dose, compared with conventional enzymatic stromal vascular fraction isolation [15]. In addition to enhanced cell yield performance, mechanically disrupted cell aggregates (nanofat) remain attached to their natural matrix niche, which has been shown to promote cell viability, proliferation, and differentiation. Moreover, obtaining SVF enzymatically is time-consuming and expensive, requiring equipment and personnel, and above all, it is an application that is classified as a biological drug by authorities, such as the FDA and EMA, meaning that conditions of cGMP, cGLP must be met, which is impossible for many hospitals and physicians [11]. The major disadvantage of mechanical methods and the resistance against them is the resulting low blunt pressure resulting in the death of the cells, resulting in low viability and few stromal cells [43].The most detailed study on the clinical uses of stromal cells obtained mechanically was recently published by Ghiasloo et al. [44]. Researchers have scanned 4505 articles and created a database of 1458 diseases. One of the most interesting results of this study is that it is mentioned in 10 different modifications besides nanofat concept for mechanical stromal acquisition. As clinical applications of stromal cells obtained mechanically: They found 8 separate studies for the quality and regeneration of the skin, 6 studies for chronic wound, diabetic foot and complications, 5 studies for scar treatment. Also, knee OA, achilles tendinopathy, TMJ dysfunction, perineal fistula They also reported their use in the treatment of vocal cord scars and paralysis, critical limb ischemia, androgenic, and migraine. Clinical applications of mechanically isolated stromal cells will be more with time, for now, the volume and number of cells used in the same SVF applications can be referenced. TOST will offer more types of stromal cells than SVF. For example, the concept accepted in knee OA is that 6 ml and one million cells for each knee [45]. With IPs 2, this volume and cell amount can be achieved in a very short time under local anesthesia with MEST technique using only 10 ml of condensed fat. However, TOST to be applied for vocal cord has to be in denser form and much less volume. In the treatment of Peyronie's disease, when using SVF, 50 cc fat harvesting and stromal cell isolation using enzyme in GMP and GLP standards are required [46]. However, the same process can be done with IPS1 using only 5 cc of condensed fat in a very short time and quite simply under standard conditions. Similarly, in the treatment of erectile dysfunction, SVF is applied intra-cavernous route with the amount of 8.4 – 37.2 million regenerative cells [47]. This can be done very easily with IPs 2 under local anesthesia with higher amount of cells. IPs allow different approaches for both the desired number of cells and the desired end product form.
In the study of Copcu and Oztan [16], for the first time in the literature, indication-based protocols were defined, and stromal cells were obtained in different cell numbers in different volumes for different indications. Differences between cell numbers can be explained by predilution with saline. In their study, Sesé et al. [15] proved that the main stromal cells are in the adipose tissue excreted in enzymatic methods. However, this fat tissue had to be discarded because it contained tissue enzymes. It is unclear how many stromal cells should be given to which tissue, but Sesé et al. described the cell dose. They reported that the constitutive cell density of adipose tissue is 10.5 ± 0.7 million cells per gram by mechanical technique but same does is 0.68 ± 0.04 million cells per gram of lipoaspirate in enzymatic technique [15] Compared to enzymatic dissociation of stromal vascular fraction, their findings revealed that emulsified nanofat yielded 10-times more cells by reaching 6.6 ± 0.4 million cells per gram of processed fat tissue. IPs allow different approaches for both the desired number of cells and the desired end product form. For example, if the stromal cell will be applied to the face or hair with needling only in the solution form, IP 1 or 2 is preferred, depending on the amount of fluid required, while IP 3 or 4 is preferred if the fat will be used to increase tissue retention. Stromal cell solutions may have great potential use in tissue repair and regeneration, indeed for site-specific intralesional delivery [48] Preferring IPS 1 and IPS 2 will also prevent the risk of oil cyst formation and tissue necrosis due to the fat they contain.
Our results show that by cutting the adipose tissue very gently with sharp blades, without creating blunt pressure, without killing adipose tissue, in the same session, both the desired size and the fat graft can be prepared; in many ways, superior stromal cells can be obtained.
than with enzymatic methods. Senesi et, al compared the mechanical and enzymatic isolation of stromal cells form adipose tissue and concluded that mechanical techniques are effective in stromal cell isolations form adipose tissue [49] Those cells were in low differentiative capacity but high immunomodulatory effect through cytokins and growth factor release. They concluded that, mechanical systems provides minimization of tissue manipulation and protection of ECM and tissue integrity. We believe that the superiority of mechanical techniques over enzymatic techniques can be explained by the maximum protection of stromal cells. Therefore, we speculate that the definition of TOST will be a more appropriate definition than SVF.
When the stromal cell extraction by mechanical method has become very popular in recent years, many new medical devices that provide this process have entered the market. The latest study of the methods used by these devices was presented by Veronesse et al. in September 2020 [34]. The latest study of the methods used by these devices was presented by Veronesse et al. in September 2020. A total of 13 different devices were analyzed and 9 different methods were found to obtain stromal cells mechanically. These: centrifugation, emulsification, filtration, mincing, purreing, sedimentation, squeezing, telfa-rolling and washing. However, the process of cutting fat tissue with sharp knives, which Copcu and Öztan defined as "adinizing", is completely different from all these 9 methods in terms of both the procedure and the philosophy [16]. Because adipose tissue is one of the most delicate tissues in the body, it is extremely sensitive to trauma. Separating the fat tissue into very fine pieces with sharp blades under very little blunt pressure is an entirely new and innovative approach.
Conclusion
In this study, nanofat was defined and published in 2013 as milestone and the techniques and definitions used in mechanical stromal cell isolation until May 2020 were systematically researched according to PRISMA. As a result of these studies, 2 definitions were made. SVF and nanofat, the two most used terms, are unable to precisely define the end product obtained mechanically, a clear definition has been made for the first time for mechanical stromal cell extraction from adipose tissue. We recommend using the term TOST, for mechanical stromal cells, instead of SVF or nanofat. Because, although the mechanical methods offer many advantages over enzymatic methods, the most important advantage is that no dissolving chemical is used, such as an enzyme, so the integrity and presence of stromal cells are maximized, just like ECM. This ensures that the stromal cells are most protected and obtained. It is absolutely necessary to show that all stromal cells are protected by further studies. However, we believe that the most appropriate identification for mechanical stromal cells is neither nanofat nor SVF, but TOST.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
H. Eray Copcu, Email: ecopcu@gmail.com.
Sule Oztan, Email: sule@mest.co.
References
- 1.De Francesco F, Mannucci S, Conti G, Dai Prè E, Sbarbati A, Riccio M. A non-enzymatic method to obtain a fat tissue derivative highly enriched in adipose stem cells (ASCs) from human lipoaspirates: preliminary results. Int J Mol Sci. 2018;19:2061. doi: 10.3390/ijms19072061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schildberg FA, Donneberg VS. Stromal cells in health and disease. Cytometry A. 2018;93:871–875. doi: 10.1002/cyto.a.23600. [DOI] [PubMed] [Google Scholar]
- 3.Alexander RW. Understanding mechanical emulsification (nanofat) versus enzymatic isolation of tissue stromal vascular fraction (tSVF) cells from adipose tissue: Potential uses in biocellular regenerative medicine. J Prolotherapy. 2016;8:e947–e960. [Google Scholar]
- 4.Guo J, Widgerow AD, Banyard D, Toranto J, Wirth GA, Paydar K, et al. Strategic sequences in fat graft survival. Ann Plast Surg. 2015;74:376–382. doi: 10.1097/SAP.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 5.Tonnard P, Verpaele A, Peeters G, Hamdi M, Cornelissen M, Declercq H. Nanofat grafting: Basic research and clinical applications. Plast Reconstr Surg. 2013;132:1017–1026. doi: 10.1097/PRS.0b013e31829fe1b0. [DOI] [PubMed] [Google Scholar]
- 6.Morestin H. Quelques cas de greffes graisseuse appliquees aIa chirurgie reparatrice. Bull Mem Soc Chir (Paris) 1915;41:1631. [Google Scholar]
- 7.Jarrell BE, Williams SK, Stokes G, Hubbard FA, Carabasi RA, Koolpe E, et al. Use of freshly isolated capillary endothelial cells for the immediate establishment of a monolayer on a vascular graft at surgery. Surgery. 1986;100:392–399. [PubMed] [Google Scholar]
- 8.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 9.Aronowitz JA, Lockhart RA, Hakakian CS. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. Springerplus. 2015;4:713. doi: 10.1186/s40064-015-1509-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai Prè E, Busato A, Mannucci S, Vurro F, De Francesco F, Riccio V. In vitro characterization of adipose stem cells non-enzymatically extracted from the thigh and abdomen. Int J Mol Sci. 2020;21:3081. doi: 10.3390/ijms21093081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rohrich RJ, Wan D. Making sense of stem cells and fat grafting in plastic surgery: the hype, evidence, and evolving U.S. Food and Drug Administration Regulations. Plast Reconstr Surg. 2019;143:417e–424. doi: 10.1097/PRS.0000000000005207. [DOI] [PubMed] [Google Scholar]
- 12.Tremolada C, Colombo V, Ventura C. Adipose tissue and mesenchymal stem cells: state of the art and Lipogems® technology development. Curr Stem Cell Rep. 2016;2:304–312. doi: 10.1007/s40778-016-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Dongen JA, Stevens HP, Harmsen MC, van der Lei B. Mechanical micronization of lipoaspirates: squeeze and emulsification techniques. Plast Reconstr Surg. 2017;139:1369e–1370. doi: 10.1097/PRS.0000000000003372. [DOI] [PubMed] [Google Scholar]
- 14.Stuzin JM. Discussion: nanofat grafting: basic research and clinical applications. Plast Reconstr Surg. 2013;132:1027–1028. doi: 10.1097/PRS.0b013e31829fe246. [DOI] [PubMed] [Google Scholar]
- 15.Sesé B, Sanmartín JM, Ortega B, Matas-Palau A, Llull R. Nanofat Cell aggregates: a nearly constitutive stromal cell inoculum for regenerative site-specific therapies. Plast Reconstr Surg. 2019;144:1079–1088. doi: 10.1097/PRS.0000000000006155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Copcu HE, Oztan S. New mechanical fat separation technique: adjustable regenerative adipose-tissue transfer (ARAT) and mechanical stromal cell transfer (MEST). Aesthet Surg J Open Forum. 2020;2:ojaa035. 10.1093/asjof/ojaa035. [DOI] [PMC free article] [PubMed]
- 17.Trovato L, Monti M, Del Fante C, Cervio M, Lampinen M, Ambrosio L. A new medical device rigeneracons allows to obtain viable micro-grafts from mechanical disaggregation of human tissues. J Cell Physiol. 2015;230(10):2299–2303. doi: 10.1002/jcp.24973. [DOI] [PubMed] [Google Scholar]
- 18.Astarita C, Arora CL, Trovato L. Tissue regeneration: an overview from stem cells to micrografts. J Int Med Res. 2020;48:300060520914794. doi: 10.1177/0300060520914794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becker AJ, McCulloch EA, Tıll JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452–454. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- 20.Zanzottera F, Lavezzari E, Trovato L, Icardi A, Graziano A. Adipose derived stem cells and growth factors applied on hair transplantation. follow-up of clinical outcome. J Cosmet Dermatol Sci Appl. 2014;4:268–274. [Google Scholar]
- 21.Purpura V, Bondioli E, Graziano A, Trovato L, Melandri D, Ghetti M, et al. Tissue Characterization after a New Disaggregation Method for Skin Micro-Grafts Generation. J Vis Exp. 2016 doi: 10.3791/53579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monti M, Graziano A, Rizzo S, Perotti C, Del Fante C, d'Aquino R, et al. In vitro and in vivo differentiation of progenitor stem cells obtained after mechanical digestion of human dental pulp. J Cell Physiol. 2017;232(3):548–555. doi: 10.1002/jcp.25452. [DOI] [PubMed] [Google Scholar]
- 23.Bernardini FP, Gennai A, Izzo L, Zambelli A, Repaci E, Baldelli I, et al. Superficial enhanced fluid fat injection (SEFFI) to correct volume defects and skin aging of the face and periocular region. Aesthetic Surg J. 2015;5:504–515 . doi: 10.1093/asj/sjv001. [DOI] [PubMed] [Google Scholar]
- 24.Gennai A, Zambelli A, Repaci E, Quarto R, Baldelli I, Fraternali G, et al. Skin rejuvenation and volume enhancement with the micro superficial enhanced fluid fat injection (M-SEFFI) for skin aging of the periocular and perioral regions. Aesthet Surg J. 2017;37:14–23. doi: 10.1093/asj/sjw084. [DOI] [PubMed] [Google Scholar]
- 25.Bernardini FP, Gennai A. Fluid fat injection for volume restoration and skin regeneration of the periocular aesthetic unit. JAMA Facial Plast Surg. 2016;18:68–70. doi: 10.1001/jamafacial.2015.1146. [DOI] [PubMed] [Google Scholar]
- 26.van Dongen JA, Tuin AJ, Spiekman M, Jansma J, van der Lei B, Harmsen MC. Comparison of intraoperative procedures for isolation of clinical grade stromal vascular fraction for regenerative purposes: a systematic review. J Tissue Eng Regen Med. 2018;12:e261–274. doi: 10.1002/term.2407. [DOI] [PubMed] [Google Scholar]
- 27.Lo Furno D, Tamburino S, Mannino G, Gili E, Lombardo G, Tarico MS, et al. Nanofat experimental evidence for a fat grafting rich in mesenchymal stem cells. Physiol Res. 2017;66:663–671. doi: 10.33549/physiolres.933451. [DOI] [PubMed] [Google Scholar]
- 28.Yao Y, Cai J, Zhang P, Liao Y, Yuan Y, Dong Z, et al. Adipose stromal vascular fraction gel grafting: a new method for tissue volumization and rejuvenation. Dermatol Surg. 2018;44:1278–1286. doi: 10.1097/DSS.0000000000001556. [DOI] [PubMed] [Google Scholar]
- 29.Yu Z, Cai Y, Deng M, Li D, Wang X, Zheng H, et al. Fat extract promotes angiogenesis in a murine model of limb ischemia: a novel cell-free therapeutic strategy. Stem Cell Res Ther. 2018;9:294. doi: 10.1186/s13287-018-1014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Liao Y, Xia J, Wang Z, Mo X, Feng J, et al. Mechanical micronization of lipoaspirates for the treatment of hypertrophic scars. Stem Cell Res Ther. 2019;10:42. doi: 10.1186/s13287-019-1140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Francesco F. Editorial: mesenchymal stem cells and ınteractions with scaffolds — biomaterials in regenerative medicine: from research to translational applications. Front Cell Dev Biol. 2019;12:193. doi: 10.3389/fcell.2019.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trivisonno A, Alexander RW, Baldari S, Cohen SR, Di Rocco G, Gentile P, et al. Intraoperative strategies for minimal manipulation of autologous adipose tissue for cell- and tissue-based therapies: concise review. Stem Cells Transl Med. 2019;8:1265–1271. doi: 10.1002/sctm.19-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Dongen JA, Gostelie OFE, Vonk LA, De Bruijn JJ, Van Der Lei B, Harmsen MC, et al. Fractionation of Adipose Tissue Procedure With a Disposable One-Hole Fractionator. Aesthet Surg J. 2020;40:NP194–NP201. doi: 10.1093/asj/sjz223. [DOI] [PubMed] [Google Scholar]
- 34.Veronese S, Dai Prè E, Conti G, Busato A, Mannucci S, Sbarbati A. Comparative technical analysis of lipoaspirate mechanical processing devices. J Tissue Eng Regen Med. 2020;14:1213–1226. doi: 10.1002/term.3093. [DOI] [PubMed] [Google Scholar]
- 35.Coleman SR. Structural fat grafting. Aesthet Surg J. 1998;18:386–388. doi: 10.1016/S1090-820X(98)70098-6. [DOI] [PubMed] [Google Scholar]
- 36.Cohen SR, Tiryaki T, Womack HA, Canikyan S, Schlaudraff KU, Scheflan M. Cellular optimization of nanofat: comparison of two nanofat processing devices in terms of cell count and viability. Aesthet Surg J Open Forum. 2014;1:ojz028. doi: 10.1093/asjof/ojz028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen SR, Womack H, Ghanem A. Fat grafting for facial rejuvenation through injectable tissue replacement and regeneration: a differential, standardized, anatomic approach. Clin Plast Surg. 2020;47:31–41. doi: 10.1016/j.cps.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Cohen SR, Womack H. Injectable tissue replacement and regeneration: anatomic fat grafting to restore decayed facial tissues. Plast Reconstr Surg Glob Open. 2019;7:e2293. doi: 10.1097/GOX.0000000000002293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen SR, Hewett S, Ross L, Delaunay F, Goodacre A, Ramos C, et al. Regenerative cells for facial surgery: biofilling and biocontouring. Aesthet Surg J. 2017;37:S16–S32. doi: 10.1093/asj/sjx078. [DOI] [PubMed] [Google Scholar]
- 40.Tiryaki K. Mekanik ve enzimatik yöntem ile izole edilen stromal vasküler fraksiyonun yara iyileşmesi üzerine etkisisnin in vitro incelenmesi. J Istanbul Fac Med. 2020;1:28–29. [Google Scholar]
- 41.Condé-Green A, Kotamarti VS, Sherman LS, Keith JD, Lee ES, Granick MS, et al. Shift toward mechanical isolation of adipose-derived stromal vascular fraction: review of upcoming techniques. Plast Reconstr Surg Glob Open. 2016;4:e1017. doi: 10.1097/GOX.0000000000001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banyard DA, Sarantopoulos CN, Borovikova AA, Qiu X, Wirth GA, Paydar KZ, et al. Phenotypic analysis of stromal vascular fraction after mechanical shear reveals stress-induced progenitor populations. Plast Reconstr Surg. 2016;138:237e–247. doi: 10.1097/PRS.0000000000002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mashiko T, Wu SH, Feng J, Kanayama K, Kinoshita K, Sunaga A, et al. Mechanical micronization of lipoaspirates: squeeze and emulsification techniques. Plast Reconstr Surg. 2017;139:79–90. doi: 10.1097/PRS.0000000000002920. [DOI] [PubMed] [Google Scholar]
- 44.Ghiasloo M, Lobato RC, Díaz JM, Singh K, Verpaele A, Tonnard P. Expanding clinical indications of mechanically isolated stromal vascular fraction: A systematic review. Aesthet Surg J. 2020;40:NP546–NP560. doi: 10.1093/asj/sjaa111. [DOI] [PubMed] [Google Scholar]
- 45.Jones IA, Wilson M, Togashi R, Han B, Mircheff AK, Thomas Vangsness C., Jr. A randomized, controlled study to evaluate the efficacy of intra-articular, autologous adipose tissue injections for the treatment of mild-to-moderate knee osteoarthritis compared to hyaluronic acid: a study protocol. BMC Musculoskelet Disord. 2018;19:383. doi: 10.1186/s12891-018-2300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lander EB, Berman MH, See JR. Stromal vascular fraction combined with shock wave for the treatment of Peyronie’s disease. Plast Reconstr Surg Glob Open. 2016;4:e631. doi: 10.1097/GOX.0000000000000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sesé B, Sanmartín JM, Ortega B, Llull R. Human Stromal Cell Aggregates Concentrate Adipose Tissue Constitutive Cell Population by In-Vitro DNA Quantification Analysis. Plast Reconstr Surg. 2020 doi: 10.1097/PRS.0000000000007342. [DOI] [PubMed] [Google Scholar]
- 48.Senesi L, De Francesco F, Farinelli L, Manzotti S, Gagliardi G, Papalia GF. Mechanical and enzymatic procedures to isolate the stromal vascular fraction from adipose tissue: preliminary results. Front Cell Dev Biol. 2019;7:88. doi: 10.3389/fcell.2019.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haahr MK, Jensen CH, Toyserkani NM, Andersen DC, Damkier P, Sørensen JA, et al. Safety and potential effect of a single intracavernous injection of autologous adipose-derived regenerative cells in patients with erectile dysfunction following radical prostatectomy: an open-label phase I clinical trial. EBioMedicine. 2016;5:204–210. doi: 10.1016/j.ebiom.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friji MT. Nanofat grafting: basic research and clinical applications. Plast Reconstr Surg. 2014;134:333e–334. doi: 10.1097/PRS.0000000000000333. [DOI] [PubMed] [Google Scholar]
- 51.Nguyen A, Guo J, Banyard DA, Fadavi D, Toranto JD, Wirth GA, et al. Stromal vascular fraction: A regenerative reality? Part 1: Current concepts and review of the literature. J Plast Reconst Aesthet Surg. 2016;69:170–179. doi: 10.1016/j.bjps.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 52.Pu LL, Yoshimura K, Coleman SR. Future perspectives of fat grafting. Clin Plast Surg. 2015;42:389–394. doi: 10.1016/j.cps.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 53.Chaput B, Bertheuil N, Escubes M, Grolleau JL, Garrido I, Laloze J, et al. Mechanically isolated stromal vascular fraction provides a valid and useful collagenase-free alternative technique: a comparative study. Plast Reconstr Surg. 2016;138:807–819. doi: 10.1097/PRS.0000000000002494. [DOI] [PubMed] [Google Scholar]
- 54.Boureaux E, Chaput B, Bannani S, Herlin C, De Runz A, Carloni R, et al. Eyelid fat grafting: Indications, operative technique and complications; a systematic review. J Craniomaxillofac Surg. 2016;44:374–380. doi: 10.1016/j.jcms.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 55.Rossi M, Alviano F, Ricci F, Vignoli F, Marchionni C, Valente S, et al. In vitro multilineage potential and immunomodulatory properties of adipose derived stromal/stem cells obtained from nanofat lipoaspirates. CellR4. 2016;4e2212.
- 56.Gentile P, Scioli MG, Bielli A, Orlandi A, Cervelli V. Comparing different nanofat procedures on scars: role of the stromal vascular fraction and its clinical implications. Regen Med. 2017;12:939–952. doi: 10.2217/rme-2017-0076. [DOI] [PubMed] [Google Scholar]
- 57.Wei H, Gu SX, Liang YD, Liang ZJ, Chen H, Zhu MG, et al. Nanofat-derived stem cells with platelet-rich fibrin improve facial contour remodeling and skin rejuvenation after autologous structural fat transplantation. Oncotarget. 2017;8:68542–68556. doi: 10.18632/oncotarget.19721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liang ZJ, Lu X, Li DQ, Liang YD, Zhu DD, Wu FX, et al. Precise intradermal injection of nanofat-derived stromal cells combined with platelet-rich fibrin improves the efficacy of facial skin rejuvenation. Cell Physiol Biochem. 2018;47:316–329. doi: 10.1159/000489809. [DOI] [PubMed] [Google Scholar]
- 59.Rohrich RJ, Mahedia M, Shah N, Afrooz P, Vishvanath L, Gupta RK. Role of fractionated fat in blending the lid-cheek junction. Plast Reconstr Surg. 2018;142:56–65. doi: 10.1097/PRS.0000000000004526. [DOI] [PubMed] [Google Scholar]
- 60.Bi HS, Zhang C, Nie FF, Pan BL, Xiao E. Basic and clinical evidence of an alternative method to produce vivo nanofat. Chin Med J. 2018;131:588–593. doi: 10.4103/0366-6999.226074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Glass GE, Ferretti P. Adipose-derived stem cells in aesthetic surgery. Aesthetic Surg J. 2019;39:423–438. doi: 10.1093/asj/sjy160. [DOI] [PubMed] [Google Scholar]
- 62.Verpaele A, Tonnard P. Discussion: Nanofat cell aggregates: A nearly constitutive stromal cell inoculum for regenerative site-specific therapies. Plast Reconstr Surg. 2019;144:1089–1090. doi: 10.1097/PRS.0000000000006186. [DOI] [PubMed] [Google Scholar]
- 63.Jan SN, Bashir MM, Khan FA, Hidayat Z, Ansari HH, Sohail M, et al. Unfiltered nanofat injections rejuvenate postburn scars of face. Ann Plast Surg. 2019;82:28–33. doi: 10.1097/SAP.0000000000001631. [DOI] [PubMed] [Google Scholar]
- 64.Stevens HR. ACA-Technik: Stromal vascular fraction, platelet-rich plasma und Mikrofett zur körpereigenen Regeneration und Hautverjüngung. Ästhet Chir. 2019;12:77–83. doi: 10.1007/s12631-018-0151-6. [DOI] [Google Scholar]
- 65.Ramirez O. Treating the aging face: “high-definition—high-tech” comprehensive facial rejuvenation. MKG-Chirurg. 2019;12:68–77. doi: 10.1007/s12285-019-0194-5. [DOI] [Google Scholar]
- 66.Zhu H, Ge J, Chen X, Lu F, Cai J. Mechanical micronization of lipoaspirates for regenerative therapy. J Vis Exp. 2019 doi: 10.3791/58765. [DOI] [PubMed] [Google Scholar]
- 67.Grimaud F, Serrero M, Magalon J. Adipose-derived therapeutic products for the management of refractory Crohn’s fistula. Gastroenterology. 2019;157:1690–1691. doi: 10.1053/j.gastro.2019.05.078. [DOI] [PubMed] [Google Scholar]
- 68.He Y, Xia J, Chen H, Wang L, Deng C, Lu F. Human adipose liquid extract induces angiogenesis and adipogenesis: a novel cell-free therapeutic agent. Stem Cell Res Ther. 2019;10:252. doi: 10.1186/s13287-019-1356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu Y, Deng M, Cai Y, Zheng H, Wang X, Yu Z, et al. Cell-free fat extract increases dermal thickness by enhancing angiogenesis and extracellular matrix production in nude mice. Aesthet Surg J. 2020;40:904–13. doi: 10.1093/asj/sjz306. [DOI] [PubMed] [Google Scholar]
- 70.Chen J, Li Z, Huang Z, Liang L, Chen M. Chyle fat-derived stem cells conditioned medium inhibits hypertrophic scar fibroblast activity. Ann Plast Surg. 2019;83:271–277. doi: 10.1097/SAP.0000000000001932. [DOI] [PubMed] [Google Scholar]