Abstract
Background:
Sufficient blood supply through neo-vasculature is a major challenge in cell therapy and tissue engineering in order to support the growth, function, and viability of implanted cells. However, depending on the implant size and cell types, the natural process of angiogenesis may not provide enough blood supply for long term survival of the implants, requiring supplementary strategy to prevent local ischemia. Many researchers have reported the methodologies to form pre-vasculatures that mimic in vivo microvessels for implantation to promote angiogenesis. These approaches successfully showed significant enhancement in long-term survival and regenerative functions of implanted cells, yet there remains room for improvement.
Methods:
This paper suggests a proof-of-concept strategy to utilize novel scaffolds of dimpled/hollow electrospun fibers that enable the formation of highly mature pre-vasculatures with adequate dimensions and fast degrading in the tissue.
Result:
Higher surface roughness improved the maturity of endothelial cells mediated by increased cell-scaffold affinity. The degradation of scaffold material for functional restoration of the neo-vasculatures was also expedited by employing the hollow scaffold design based on co-axial electrospinning techniques.
Conclusion:
This unique scaffold-based pre-vasculature can hold implanted cells and tissue constructs for a prolonged time while minimizing the cellular loss, manifesting as a gold standard design for transplantable scaffolds.
Keywords: Vascular tissue engineering, Human umbilical vein endothelial cells (HUVECs), Electrospinning
Introduction
Blood transports nutrition and oxygen in the vascular network throughout the entire body [1]. Therefore, continuous blood supply is essential for maintaining the viability and metabolism of functional tissues [2]. This physiological significance of blood circulation demonstrates why blood vessel formation, called angiogenesis, is critical during the regeneration and restoration process. However, the natural angiogenesis is inherently limited by the rate at which new vessels are formed [3], and thus the damages that exceed the regenerative capacity of the natural tissue would result in ischemic necrosis/ulcer. Therefore, sufficient blood supply through neo-vasculature is the major challenge in regenerative medicine to support the growth, integration, function, and viability of grafted cells and tissues [4–7].
Recent advances in the field of regenerative medicine have utilized the pre-vascular structures to assist the natural angiogenesis. The pre-vasculatures are constructed by utilizing various techniques such as 3D printing, cotton candy machine, and electrospinning [8–10]. Material-wise, a wide range of degradable biomaterials, including collagen, synthetic polymers, and temperature-responsive polymers, have been used [11]. One of the key parameters to consider in the selection of the scaffold design is the degradation time of the scaffold structures after implantation, such that only microvessel-like hollow structures would remain for further integration with the newly sprouting vessels in the tissue [12]. Furthermore, there have been reports on the implantation of in vitro cultured compact endothelial layer on scaffolds [13]. This endothelial layer, pre-formed in vitro, was integrated well with neighboring microvessel when implanted in the tissue, effectively increasing the rate at which angiogenesis occurred [14, 15]. However, the in vivo micro-vessels feature a complex network, consisting of 10–200 μm diameter vessels, and it remains a challenge to mimic these microvessel network structures with well-matured endothelial cells [16].
This study suggests a proof-of-concept strategy for novel dimpled electrospun fiber scaffolds that enable the formation of highly matured pre-vasculatures of human umbilical vein endothelial cells (HUVECs) with adequate dimensions while degrading fast in the tissue. Based on the phase separation principle with the co-axial nozzle utilization [17], our scaffolds were fabricated to feature dimples on the surface with a hollow core. The dimpled topology increases the area for cell attachments on the electrospun fibers, promoting cell-substrate affinity for HUVECs. These endothelial cells can later be connected to pre-existing vessels in the tissue, enabling a rapid vascular network restoration. Also, the scaffolds’ hollow core provides a significant advantage in polymeric body degradation after the complete formation of the vessel network, expanding the range of applications in the field of regenerative medicine.
Materials and methods
Electrospinning
Polycaprolactone (PCL) fibrous scaffolds were fabricated using an electrospinning device with or without dimples. For smooth surface scaffolds, PCL pellets were dissolved in the mixture of 7.5 mL of dichloromethane (DM, Junsei Chemical Co., Ltd., Tokyo, Japan) and 2.5 mL of N,N-dimethylformamide (NDF, Junsei Chemical Co., Ltd.) with 20% (w/v). For the scaffolds with dimpled surfaces, a mixture of 9 mL of DM and 1 mL of dimethyl sulfoxide (DMSO, D8418, Sigma-Aldrich, St. Louis, MO, USA) was used at 20% (w/v) PCL concentration (Table 1). DMSO is a volatile chemical, triggering phase separation during the electrospinning process. DMSO droplets formed on the fibers evaporate after fiber collection, creating micro-size pores on the surface of PCL fibers. PCL solution was loaded in a 6-mL disposable syringe and injected through the nozzle at the feeding rate of 3 mL/h. The spinning height was set to be 275 mm from the collector, and the fabricated fibers were collected on aluminum collectors for 3 min. A DC power supply generated 13 kV voltage. To fabricate hollow fibers, the co-axial nozzle was utilized. The mineral oil was used as the core solution at the feed rate of 0.1 mL/h, and the applied voltage was adjusted to 14 kV. The fabricated hollow fibers were then immersed in surfactant (ES 7x, MP, Santa Ana, CA, USA) for 12 h. The humidity of the room was kept below 40% for all electrospinning process.
Table 1.
The names, purposes and composition of each polymer solutions
| Name | Purpose | Composition | |
|---|---|---|---|
| Chemical | Amount | ||
| Normal solution |
Smooth solid fibers Smooth hollow fibers |
Dichloromethane | 7.5 mL |
| N,N-dimethylformamide | 2.5 mL | ||
| PCL | 2 g | ||
| Phase separated solution |
Dimpled solid fibers Dimpled hollow fibers |
Dichloromethane | 9 mL |
| Dimethyl sulfoxide | 1 mL | ||
| PCL | 2 g | ||
| Mineral oil | Core part of the hollow fibers | – | |
Scaffold preparation and surface modification
A direct polymer melt deposition (DPMD) device was used to make the 2 × 1 cm2 rectangular grips on the collected fibers. PCL pellets were melted in a metal syringe at 110 °C by heating coil, and ejected by air pressure at 3 atm through a syringe tip (ID: 0.5 mm). All fibers were immersed in 70% ethanol solution for 24 h under UV light for sterilization. The sterilized fibers were exposed to oxygen plasma for 1 min at 6.5 × 10−1 torr using the O2 plasma generator and coated with 2 mg/mL Matrigel (Corning, NY, USA) for 1 h at 37 °C.
Scaffold characterization
The scanning electron microscope (SEM, SU5000, Hitachi, Japan) was used to identify each scaffold’s structural characteristics. PCL fibrous scaffolds were coated with platinum using a sputter coater. The coating was initiated with 1 kV for 30 s. The diameter and porosity were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA) for identifying the spatial uniformity and the morphological characteristics.
Scaffold degradation
The scaffolds were immersed and incubated for 24 days in culture media to verify the time taken to degrade the fibers. The fibers’ characteristics were observed using SEM (SU5000, Hitachi, Tokyo, Japan), and the degradation rate was analyzed using ImageJ software (NIH) by measuring the change in diameter of the fibers.
Cell culture
Human endothelial umbilical vein cells (HUVECs, C2517A, Lonza, Switzerland) were cultured using endothelial cell growth media (EGM-2, Lonza, Switzerland) in an incubator at 37 °C and 5% CO2. Cells were seeded onto scaffolds at the seeding density of 4 × 104 cells/cm2 and expanded for 48 h.
Immunofluorescence
HUVEC cells were fixed using 3.7% paraformaldehyde (P6148, Sigma-Aldrich) for 20 min. After the fixation, the cells were treated with 0.2% Triton X-100 (Samchun pure chemicals, Seoul, South Korea) for 15 min, and 5% bovine serum albumin (BSA, Melbourne, Australia) was added for 1 h. The cells were then treated with anti-FAK antibody (1:200, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-VE cadherin antibody (1:200, Abcam, Cambridge, UK), and anti-β catenin antibody (1:250, BD Biosciences, Franklin Lakes, NJ, USA) following the blocking process for 24 h and incubated with Alex Fluor 488 anti-rabbit and anti-mouse (1:200) for 12 h. Also, actin fibers were fluorescently labeled using Alex Fluor 568 Phalloidin antibody for 20 min, and nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) for 3 min. Between each step, the cell-containing scaffolds were washed with 1 × phosphate-buffered saline (PBS) 3 times. Stained cells were observed using a fluorescence microscope (ZEISS, Oberkochen, Germany).
Quantitative polymerase chain reaction (qPCR)
Before extracting mRNA, HUVECs on the electrospun scaffold were washed 3 times with 1x PBS. HUVECs and scaffold were dissolved completely by adding 700 μL Trizol (Takara Bio Inc., Shiga, Japan) to the cell containing scaffolds and gently mixing the solution by pipetting up and down. The dissolved solution was collected in a 1.5 mL tube and vortexed for 5–10 s. 200 μL of chloroform (Sigma Aldrich) was then added, and the solution was spun down at 4 °C, 12,000 rpm for 5 min to purify the mRNA. Isolated mRNA and debris were separated into two layers, and the only mRNA was collected from the top layer. Then, isopropanol (Merck, Darmstadt, Germany) was added to the mRNA solution at a ratio of 1:1 to be stored at room temperature for 20 min. The mRNA was then spun down at 4 °C, 12,000 rpm for 10 min to form an mRNA pellet. All the solution was removed except for the pellet. The mRNA pellet was rinsed with 1 mL of 70% ethanol (Merck) and centrifuged for 5 min at 4 °C, 7500 rpm. Extracted mRNA was dissolved using 20 μL of RNase-free water (Welgene, Kyungsan, South Korea). The concentration of mRNA was quantitated using a spectrophotometer (Wilmington). cDNA synthesis was done using the iScripts TM kit (Bio-Rad). 1000 ng of mRNA was mixed with 4 μL buffer and 1 μL of the reverse-transcribed mixture to synthesize the cDNA in 20 μL volume for each sample. Biometra T-personal thermal cycler was used for the cDNA synthesis with the following protocol: Initially started at 4 °C for 30 s, activated the primer binding for 5 min at 25 °C, incubated the cDNA reaction for 1 h at 42 °C, and inactivated the enzymes for 5 min at 85 °C. Synthesized cDNA was stored at − 20 °C. Real-time qPCR was used for the cDNA amplification first by initial denaturation at 95 °C for 5 min followed by 45 cycles of cDNA amplification (denaturation for 10 s at 95 °C, annealing for 30 s at 60 °C, an extension for 15 s at 72 °C) on iQ SYBR green supermix (Bio-Rad, Hercules, CA, USA). A Bio-Rad CFX96, real-time detection system. The gene expression levels were normalized to Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and calculated as the fold increase compared to the control. The following primers were used: GAPDH, Angiopoietin 1 (Ang1), vascular endothelial growth factor A (VEGFA), vascular endothelial growth factor receptor (VEGFR), and vascular endothelial growth factor receptor (VEGFR2). The sequence of each primer was shown in Table 2.
Table 2.
Primer sequence for qPCR
| Target gene name | Primer sequence |
|---|---|
| GAPDH | F: ACAACTTTGGTATCGTGGAAGG |
| R: GCCATCACGCCACAGTTTC | |
| Ang1 | F: AGAACCTTCAAGGCTTGGTTAC |
| R: GGTGGTAGCTCTGTTTAATTGCT | |
| VEGFA | F: AGGGCAGAATCATCACGAAGT |
| R: AGGGTCTCGATTGGATGGCA | |
| VEGFR | F: TGCCGGGTTACGTCACCTA |
| R: GTCCCAGATTATGCGTTTTCCAT | |
| VEGFR2 | F: AGGGCAGAATCATCACGAAGT |
| R: AGGGTCTCGATTGGATGGCA |
Vasculature sprouting assay
The in vitro vasculature sprouting assay was performed to demonstrate the interconnection between pre-existence vessel and engineered micro-vasculature. To mimic pre-formed vasculature, tubular vessel formation assay was utilized. 10 mg/mL Matrigel was coated on the 12 well cell culture dish, and HUVECs were seeded onto the Matrigel with 4 × 104 cells/cm2 concentration. For the formation of a tubular vessel structure for the in vitro tissue model, the dish was incubated for 24 h. After incubation, the engineered vasculature sheet was gently patched on the Matrigel surface, and 20 μL of additional Matrigel was gently dropped to avoid detachment. 20 ng/mL VEGF (V7259, Sigma-Aldrich) was gently mixed with EGM-2 media and promote the sprouting of the pre-formed vessel toward the scaffolds. To verify the connection between the engineered vasculature and pre-formed vasculature, cell viability tests were performed after 2 days from starting of the incubation.
Statistical analysis
Statistical significance between mean values was determined by a one-way analysis of variance using GraphPad QuickCalcs. p Values < 0.05 were considered statistically significant.
Results and discussion
Integrating surface dimples on PCL fibers
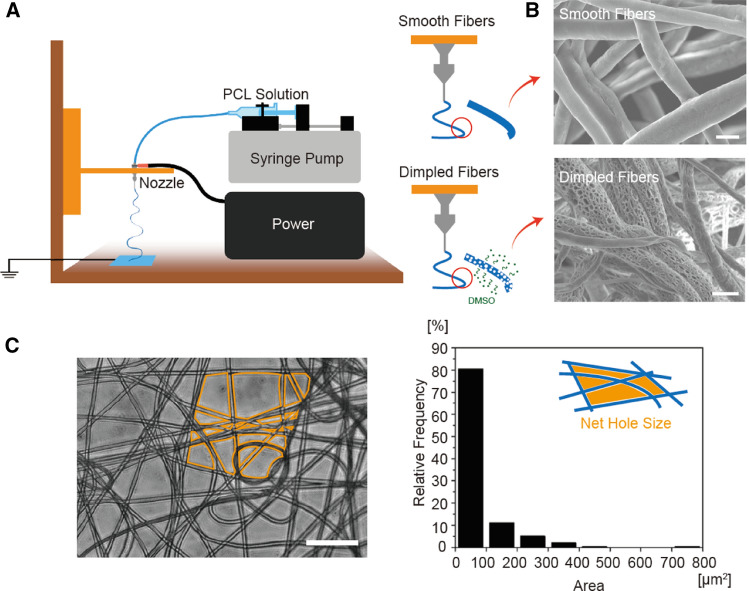
Microvasculature scaffolds were fabricated by electrospinning with/without dimples on the fiber surfaces (Fig. 1A). For the dimpled fibers, 10% DMSO was mixed in the initial PCL solution. Because DMSO is an amphiphilic molecule, DMSO formed microdroplets in solvents, creating micro-size dimples on fiber surfaces. The geometric features of smooth and dimpled fiber, including the dimple size and density of these fibers, were confirmed by SEM imaging (Fig. 1B). Based on the image analysis, smooth and dimpled fiber diameters were 3.93 ± 0.99 μm and 3.65 ± 1.38 μm, respectively, with no statistical difference between two samples. And the dimpled surfaces exhibited high-density holes whose long axis was shorter than 2 μm, whereas the smooth fibers featured entirely smooth surfaces. These fabricated fibers were collected as fibrous sheets, suitable as carriers for cells, spheroids, and tissue constructs. The density of fibers in the sheets was controlled by varying the collection time. The fiber density correlated directly with the effective mesh pore sizes in the fiber sheets. We aimed ~ 80% void area between fibers with < 100 μm2 size to prevent the cell loss at the transplant site, and it took 3 min collection on grounded flat aluminum surface (Fig. 1C).
Fig. 1.
The characterization of microvasculature scaffolds. A The illustration of electrospinning methods for smooth and dimpled fibers. The dimpled surface was created by phase separation of DMSO in the PCL solution. B SEM images of the electrospun fiber surface. Dimpled fiber exhibited micropores on the fiber surface, but smooth fiber did not. (scale bars: 5 μm). C The phase image of the fibrous mat. Electrospun fibers were collected as a sheet form with appropriated fiber density. The void area between fiber meshes was quantified using ImageJ software. (scale bar: 50 μm)
Development of mature endothelium on dimpled surfaces
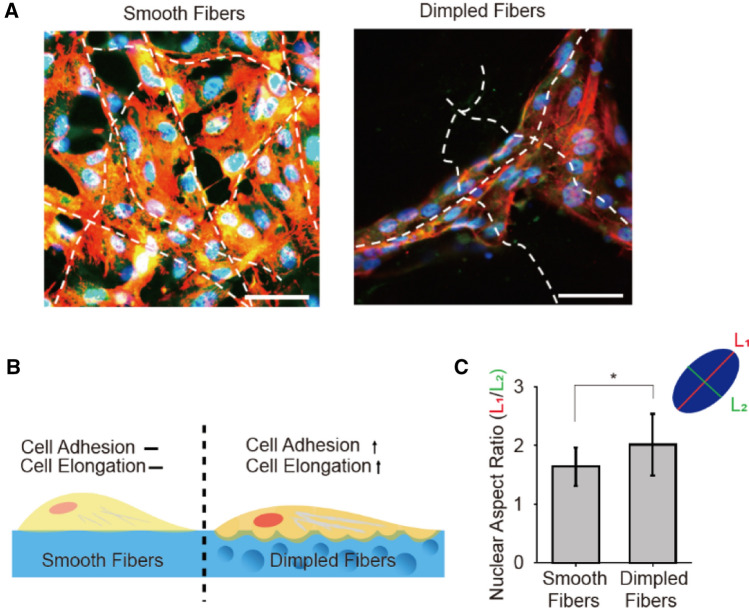
HUVECs were cultured on both smooth and dimpled fibers and were compared to investigate the effects of the surface roughness on cell adhesion affinity and microvascular formation. As shown in Fig. 2, on smooth fibers, HUVECs spread over multiple fibers without any directional preferences. In contrast, HUVECs stretched along the dimpled fibers to align in the fiber direction. The cellular elongation difference was quantified by cell nuclei aspect ratio (length of the major axis L1/length of minor axis L2) (Fig. 2C). HUVECs on dimpled fibers exhibited 2.02 ± 0.52 aspect ratio value, whereas 1.64 ± 0.33 on smooth fibers. Also, the overall vascular wall formation was enhanced on the dimpled surfaces. As shown in Fig. 3A, we compared expressions of focal adhesion kinase (FAK), vascular endothelial cadherin (VE-cadherin), and β-catenin of HUVECs on smooth versus dimpled fibers using a fluorescence microscope. The FAK, often used as a marker representing the strength of the cell-substrate adhesion, was significantly enhanced on the dimpled fibers. This result confirms the positive correlation between surface roughness and adhesion affinity [18]. Culturing cells on the dimpled fibers also promoted VE-cadherin and β-catenin expression, both of which are known to correlate with the vessel formation and maturation [19, 20]. In particular, the pronounced VE-cadherin expression along with the cell–cell junctions indicated high maturity of intercellular junctions in HUVECs cultured on the dimpled surface. The dimpled surface had an effect not only on protein expressions but also on gene expressions. The expression levels of the genes related to vessel formation, including Ang1, VEGFA, VEGFR, and VEGFR2, were dramatically increased on dimpled fibers, and all genes except VEGFR2 exhibited the statistically significant difference compared to ones cultured on TCPs. In contrast, no significant changes were noted in cells cultured on smooth fibers (Fig. 3B). These results indicated that synthetic microvasculature formed around dimpled fibers offer considerable advantages to creating neo-vessels and connecting them with the existing vessel when transplanted in the tissue (Fig. 4).
Fig. 2.
Change of cell adhesion properties by the surface modification of fibers. A Immunofluorescence images of HUVECs, cultured on smooth and dimpled fibers. (green: FAK, red: actin, blue: cell nuclei, scale bars: 100 μm). B Illustration of cell adhesion morphology difference between smooth and dimpled fibers substrate. C Aspect ratio (L1/L2) of cell nuclei on smooth and dimpled fibers. (*p < 0.05)
Fig. 3.
The difference of HUVECs maturation on smooth and dimpled surface. A Immunofluorescence images of HUVECs, cultured on S, D fiber types. (Green: target protein—FAK, VE-Cadherin, β-Catenin respectively, red: actin, blue: cell nuclei, scale bars: 100 μm) The images in the yellow line represent magnified images of each result. (Scale bar: 25 μm). B Real-time qPCR results of HUVECs on TCPs, smooth fibers, dimpled fibers. The expression levels of Ang1, VEGFA, VEGFR1, and VEGFR2 for each condition were normalized by samples from the TCPs condition (*p< 0.05, **p < 0.005)
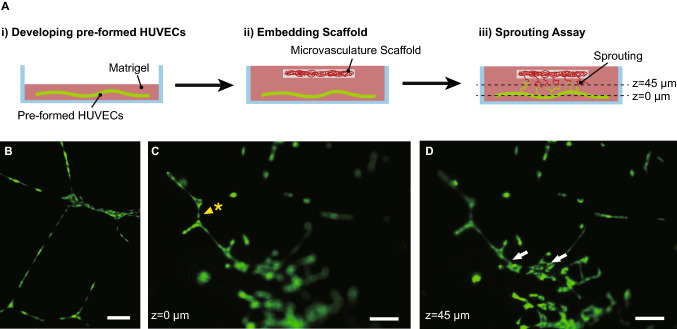
Fig. 4.
Fluorescence images of the HUVECs during the vasculature sprouting assay. A Illustration of sprouting assay experiment process. HUVECs were developed pre-formed vessel in Matrigel at (i), and embedded the HUVEC-seeded microvasculature scaffold at (ii). Culture the HUVECs 2 days more and observed the sprouting vessels between the pre-formed vessel (bottom) and HUVECs on microvasculature scaffold (top) at (iii). B Pre-formed HUVECs vasculature in the Matrigel. C, D The microvascular scaffold with HUVECs was placed on the matrigel to observe the sprouting and connection with pre-formed HUVECs. After 2 days from the assay started, (C) and (D) were taken. (Green: live cells, Scale bar: 100 μm). C Bottom (h = 0 μm) of the vascular sprouting assay. The yellow arrowhead with an asterisk was used to point the tubular structure of HUVECs regarded as pre-formed vasculature. (Green: live cells, Scale bar: 100 μm). D Top (h = 42.9 μm) of the vascular sprouting assay. The white arrows were used to point the successful sprouting between the pre-formed vasculature and the engineered vasculature. (Green: live cells, Scale bar: 100 μm)
Development of hollow fibers for fast degradation
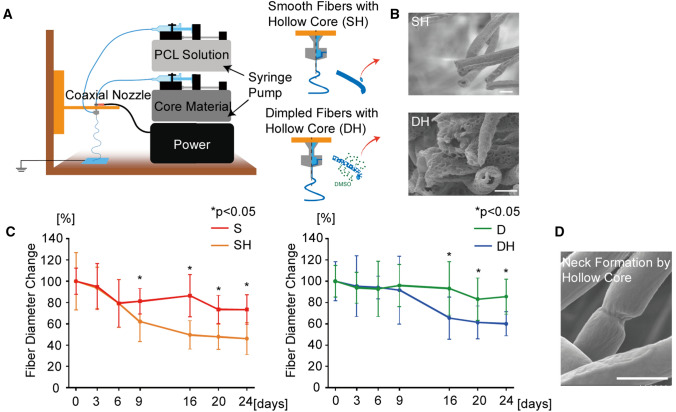
The developed microvasculature must degenerate as a complete endothelial layer is formed at the transplant site for proper blood perfusion. Therefore, it is critical to design the fiber structures that degrade within a reasonable time frame. Here we employed the co-axial nozzle to create the hollow fibers to expedite the degeneration rate. Mineral oil was used as a core material, and PCL solution with and without DMSO was injected for the outer shell of fibers to form dimpled and smooth fibers, respectively (Fig. 5A). Mineral oil was washed during the surfactant immersion process to generate the hollow core. The diameters of the hollow-core smooth and dimpled fibers were quantified by SEM images (Fig. 5B). The average diameters of smooth and dimpled fibers with hollow-core were 3.24 ± 0.68 μm and 4.75 ± 1.67 μm, respectively. The discrepancy in different sets of samples is most likely due to the different solvent ratios in PCL solutions to generate dimpled surface on hollow fibers. To quantify the fibers’ degradation rate, the entire scaffolds (1.5 × 1.5 cm fibrous sheet) were immersed in culture medium for 24 days and analyzed the changes in the average diameter of the fibers over time (Fig. 5C). The results showed that the hollow-core fibers dramatically increased the degradation rate by more than 30% over 24 days compared to the solid counterparts in both smooth and dimpled fibers. The degradation slowed down after 16 days, possibly due to the saturating effects of the dissolved substances in the media. Also, we noted that dimpled surfaces exhibited slower degradation than the smooth surfaces because it is likely that air pockets are formed in the dimples, not fully wetting the surface for proper degradation. SEM images of the hollow-core fibers taken on the 14th day showed neck formation (Fig. 5D), suggesting fiber dissolution on the inner side as well.
Fig. 5.
Hollow-core fiber fabrication and degradation analysis. A The illustration of co-axial electrospinning methods for smooth and dimpled fibers with hollow-core. Mineral oil was used as the sacrificial core material. B SEM images of smooth and dimpled fiber. Hollow-core was exhibited in the center of fiber cross-section. (Scale bars: 5 μm). C Average fiber diameter changes during degradation process. (S: smooth fiber, SH: smooth fiber with hollow-core, D: dimpled fiber, DH: dimpled fiber with hollow-core). D SEM image of neck formation of SH during degradation process. (Scale bar: 5 μm)
Conclusion
This paper presents the proof-of-concept strategy for utilizing novel electrospun fiber scaffolds with surface dimples to enable the formation of highly matured pre-vasculatures with fast degradation. We demonstrated that the proposed sheet of dimpled fibers could serve as a scaffold to develop endothelial cell-based pre-vasculature before transplantation. The high surface roughness of dimpled fiber enhanced the cell-substrate adhesion affinity, leading to better endothelial adhesion and elongation. Elongated endothelial cells with strong adhesion on the dimpled fibers resulted in vessel-like networks of HUVECs that expressed the enhanced level of vessel-related proteins and genes. These pre-formed vessel networks are expected to reduce the time for angiogenesis and neo-vascular network formation at the transplant site, eventually increasing the viability of the implanted cells and tissues. Having the hollow structure, enabled by co-axial fabrication, expedited the degradation process, minimizing the undesired effects of remaining fibers after transplantation. The hollow structure reduced the total PCL volume of fibers by eliminating the core. It also allowed the exposure of the inner surface to facilitate the faster degradation rate. The complete fiber degradation would help the vessels perform the normal blood vessel perfusion in the tissue.
Our study showed that PCL fiber degradation would take a few weeks, implying that nutrition supplements would be required for the first few weeks following the implantation to warrant adequate cell survival. To expedite the degradation process while maintaining other functionality of the fiber-based scaffolds, natural biomaterial like collagen or gelatin can be used to form composites instead of 100% PCL fibers. Furthermore, one can consider utilizing the hollow core as a reservoir of nutrition or growth factors to improve cell survival and vascularization. In conclusion, our pre-vasculature scaffolds were shown to improve angiogenesis by providing two key features: surface dimples and a hollow core that allowed better cellular adhesion and fast scaffold degradation. These key features in our electrospun platform are expected to pave a way in future clinical applications of transplantable pre-vasculature scaffolds in tissue engineering.
Acknowledgements
This research was supported by National Research Fundation granted by the Korean Government (NRF-2015M3A9B3028685). We also thank the contribution of Mr. Eunmin Ko for technical help during qPCR and gene expression analysis.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
Footnotes
Je-Hyun Han and Ung Hyun Ko are co-first authors.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hamasaki N, Yamamoto M. Red blood cell function and blood storage. Vox Sang. 2000;79:191–197. doi: 10.1046/j.1423-0410.2000.7940191.x. [DOI] [PubMed] [Google Scholar]
- 2.Hardy JD. Landmark perspective. Transplantation of blood vessels, organs, and limbs. JAMA. 1983;250:954–957. doi: 10.1001/jama.1983.03340070060030. [DOI] [PubMed] [Google Scholar]
- 3.Norton KA, Popel AS. Effects of endothelial cell proliferation and migration rates in a computational model of sprouting angiogenesis. Sci Rep. 2016;6:36992. doi: 10.1038/srep36992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain RK, Au P, Tam J, Duda DG, Fukumura D. Engineering vascularized tissue. Nat Biotechnol. 2005;23:821–823. doi: 10.1038/nbt0705-821. [DOI] [PubMed] [Google Scholar]
- 5.Lovett M, Lee K, Edwards A, Kaplan DL. Vascularization strategies for tissue engineering. Tissue Eng Part B Rev. 2009;15:353–70. doi: 10.1089/ten.teb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulsen SJ, Miller JS. Tissue vascularization through 3D printing: will technology bring us flow? Dev Dyn. 2015;244:629–640. doi: 10.1002/dvdy.24254. [DOI] [PubMed] [Google Scholar]
- 7.Kaully T, Kaufman-Francis K, Lesman A, Levenberg S. Vascularization—the conduit to viable engineered tissues. Tissue Eng Part B Rev. 2009;15:159–169. doi: 10.1089/ten.teb.2008.0193. [DOI] [PubMed] [Google Scholar]
- 8.Kinstlinger IS, Miller JS. 3D-printed fluidic networks as vasculature for engineered tissue. Lab Chip. 2016;16:2025–43. doi: 10.1039/C6LC00193A. [DOI] [PubMed] [Google Scholar]
- 9.Lee JB, Wang X, Faley S, Baer B, Balikov DA, Sung HJ, et al. Development of 3D microvascular networks within gelatin hydrogels using thermoresponsive sacrificial microfibers. Adv Healthc Mater. 2016;5:781–785. doi: 10.1002/adhm.201500792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Awad NK, Niu H, Ali U, Morsi YS, Lin T. Electrospun fibrous scaffolds for small-diameter blood vessels: a review. Membranes (Basel) 2018;8:15. doi: 10.3390/membranes8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravi S, Chaikof EL. Biomaterials for vascular tissue engineering. Regen Med. 2010;5:107–20. doi: 10.2217/rme.09.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thottappillil N, Nair PD. Scaffolds in vascular regeneration: current status. Vasc Health Risk Manag. 2015;11:79–91. doi: 10.2147/VHRM.S50536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costa-Almeida R, Granja PL, Soares R, Guerreiro SG. Cellular strategies to promote vasculaisation in tissue engineering applications. Eur Cells Mater. 2014;28:51–67. doi: 10.22203/eCM.v028a05. [DOI] [PubMed] [Google Scholar]
- 14.Inomata K, Honda M. Co-culture of osteoblasts and endothelial cells on a microfiber scaffold to construct bone-like tissue with vascular networks. Materials (Basel) 2019;12:2869. doi: 10.3390/ma12182869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perry L, Flugelman MY, Levenberg S. Elderly patient-derived endothelial cells for vascularization of engineered muscle. Mol Ther. 2017;25:935–948. doi: 10.1016/j.ymthe.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song HG, Rumma RT, Ozaki CK, Edelman ER, Chen CS. Vascular tissue engineering: progress, challenges, and clinical promise. Cell Stem Cell. 2018;22:340–354. doi: 10.1016/j.stem.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katsogiannis KAG, Vladisavljević GT, Georgiadou S. Porous electrospun polycaprolactone (PCL) fibres by phase separation. Eur Polym J. 2015;69:284–295. doi: 10.1016/j.eurpolymj.2015.01.028. [DOI] [Google Scholar]
- 18.Chung TW, Liu DZ, Wang SY, Wang SS. Enhancement of the growth of human endothelial cells by surfaceroughness at nanometer sca. Biomaterials. 2003;24:4655–4661. doi: 10.1016/S0142-9612(03)00361-2. [DOI] [PubMed] [Google Scholar]
- 19.Birdsey GM, Shah AV, Dufton N, Reynolds LE, Almagro LO, Yang Y, et al. The endothelial transcription factor ERG promotes vascular stability and growth through Wnt/b-catenin signaling. Dev Cell. 2015;32:82–96. doi: 10.1016/j.devcel.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferreri DM, Minnear FL, Yin T, Kowalczyk AP, Vincent PA. N-cadherin levels in endothelial cells are regulated by monolayer maturity and p120 availability. Cell Commun Adhes. 2008;15:333–349. doi: 10.1080/15419060802440377. [DOI] [PMC free article] [PubMed] [Google Scholar]