Abstract
Background:
Tissue engineering centers on creating a niche similar to the natural one, with a purpose of developing an organ construct. A natural scaffold can replace none while creating a scaffold unique to each tissue in composition, architecture and cues that regulate the character of cells.
Methods:
Whole pancreas from mouse was decellularized using detergent and enzymes, followed by recellularizing with MSC from human placenta. This construct was transplanted in streptozotocin induced diabetic mice. Histopathology of both decellularized and recellularized transplanted pancreas and qPCR analysis were performed to assess its recovery.
Results:
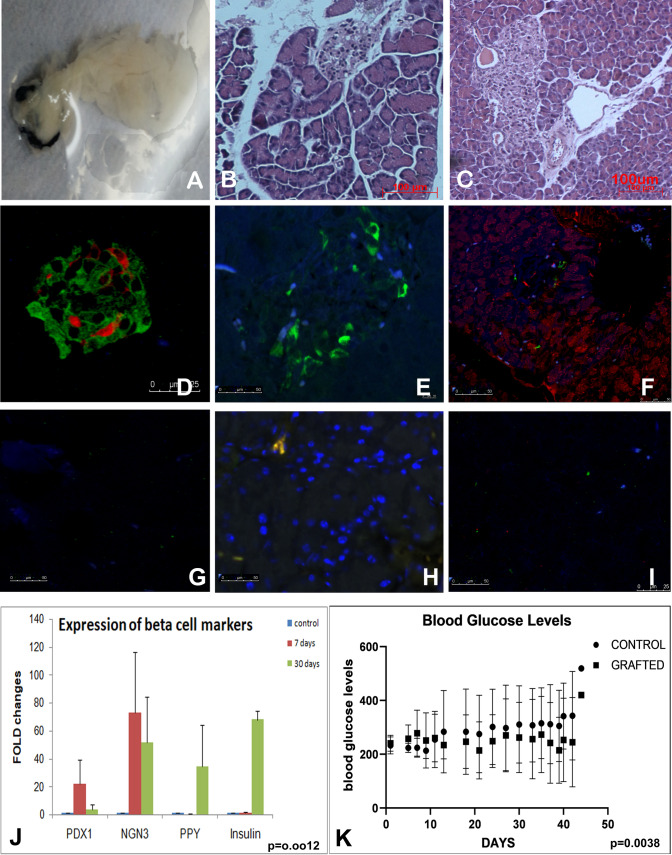
Decellularization removes the cells leaving behind extracellular matrix rich natural scaffold. After reseeding with mesenchymal stem cells, these cells differentiate into pancreas specific cells. Upon transplantation in streptozotocin induced diabetic mice, this organ was capable of restoring its histomorphology and functioning. Restoration of endocrine (islets), the exocrine region (acinar) and vascular network was seen in transplanted pancreas. The process of functional recovery of endocrine system took about 20 days when the mice start showing blood glucose reduction, though none achieved gluconormalization.
Conclusion:
Natural decellularized scaffolds of soft organs can be refunctionalized using recipient’s mesenchymal stem cells to restore structure and function; and counter immune problems arising during transplantation.
Electronic supplementary material
The online version of this article (10.1007/s13770-020-00296-y) contains supplementary material, which is available to authorized users.
Keywords: Extracellular matrix, Scaffolds, Mesenchymal stem cells, Endocrine, Exocrine
Introduction
Transplantation is the only hope for scores of patients with irreversible or end organ failure. Organ supply remains the first hurdle, followed by the immune-complications and rejection failures. The reason behind the hype of tissue engineering has been its potential to manage the debilitating diseases involving organ damage/failures. The usual approach of tissue engineering has been to create a construct that would mimic an organ functionally to attenuate and ultimately overcome the dysfunctionality.
There are a whole slew of approaches with variety of materials (synthetic, natural, combinations) and manifold techniques to create scaffolds that can be seeded with cells to create a biomimetic tissue constructs [1, 2]. Most of these have shown partial success. Attempts have been made to derive scaffolds from the whole organs/tissues by decellularization [3–7]. Decellularization is a process by which organs are denuded of cells, leaving behind the entire blueprint in the form of an architectural scaffold and cues for cell preservation and homeostasis. With the final goal to form a remodeled functional organ, the extracellular matrix obtained with close to ‘intact’ details and composition is seeded with cells. Successful recellularization of organ scaffolds has been reported in liver [8]; lungs [9]; tendons [10]; bladder [11], pancreas [12, 13], heart and valves [14, 15]; kidney [16].
Tissue decellularization has been attempted by flushing the organ through vascular channels with mild detergents, enzymatic solutions, chemicals or mechanical, or a combination of few of these [17–19]. A fine balance is sought between preserving the tissue architecture with essential structural proteins, growth stimulating signals/factors and complete removal of cellular, DNA or any immunogenic content. Various reagents like SDS, Triton X, per-acetic acid, EDTA and trypsin have been reported in various studies for different organ decellularization [19].
Diabetes remains one of the most inadequately managed disease; the population ending up with morbidity and mortality due to hyperglycemia related organ damage. Long term benefits are sought with whole pancreas transplantations or islets but as usual poor availability and use of immune suppressants are some of the limitations. Islets are encased in semisynthetic, semipermeable membrane to avoid immune surveillance of host but cell survival and functionality still remains an issue [20]. Addition of ECM components affects survival and insulin release and also forms the background for using the decellularized ECM for refunctionalization [13, 21–24].
Managing a disease with stem cells has been priority in regenerative medicine. For therapeutics, stem cells from embryonic sources or iPS (induced pluripotent stem cells) are debatable as these cells are highly tumorogenic [25]. Mesenchymal stem cells (MSC) available from a variety of sources; are one class of cells that have been on the radar for their potential clinical applications. These can be obtained in a large number without much manipulation and have been used in a few clinical applications [26–28]. Apart from the immune advantage, these cells ‘may’ offer actual regenerative advantage [27, 29]. Few problems with these cells are their diminishing proliferating potential in culture conditions due to ageing and actual implantation rate once injected at the site or systemically. Owing to these, long term benefits of these procedures have been questionable. In this study, we have explored the possibility of use of human MSC in the scaffold obtained by decellularization to repopulate and functionalize the organ.
Decellularization of pancreas has been attempted for different species [30] the pancreatic scaffold thus obtained was generally recellularized with beta cells, or stem cells or islets with focus on islet cells [12, 13, 21–24]. For the first time, we have looked at overall structural and functional recovery using the full pancreas recellularized with MSC. We additionally show that it is the infused MSC that functionalize the pancreas.
In this paper, we present our preclinical study dealing with decellularization to create an acellular scaffold, reseeding with mesenchymal stem cells for recellularization of pancreas. Implantation of this construct restores the histology and glucoregulation in a streptozotocin induced diabetic mice.
Materials and methods
Mouse pancreas harvest and perfusion decellularization
Balb/c mice about 6–8 weeks of age were used for the experiment. The animals were maintained at standard environmental conditions (temperature 22–25 °C, humidity 40–70% with 12:12 dark/light photoperiod) approved by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) whereas all the experimental protocols were approved by Institutional Animal Ethics Committee (IAEC), CCMB (IAEC-04/2011, IAEC93/2014, IAEC 44/2017).
Female Balb/c mice, between the ages of 6–8 weeks were euthanized by cervical dislocation. Sterile conditions were observed during the whole procedure. The pancreas was carefully detached free from all adjacent structures including the stomach, intestine, spleen while retaining the pancreatic and spleenic vessels intact. Insulin needle was inserted into the hepatic portal vein to enable perfusion of the organ.
The isolated pancreas with the cannulated needle was connected to a perfusion system to allow retrograde perfusion at 5 ml/min. Phosphate buffered saline (PBS) was perfused in through the hepatic portal vein and throughout the vasculature of the pancreas and exited through the spleenic vein. After complete removal of blood, non-ionic detergent, 1% Triton X-100, and 0.1% Ammonia (Sigma Aldrich) in deionized water [22] was used for perfusion to detach cells and cell debris from the pancreas. After the tissues became translucent (roughly around 12 h), subsequent steps of perfusion were using DNase 2000U/ml in (PBS) (~ 4 h) perfusion followed by a final washing step with PBS containing Pencillin/Streptomycin (100U/ml). The pancreas was perfused with PBS for additional 48 h to clear remaining cellular debris. The entire operation was carried out in a sterile chamber. The same protocol of decellularization was used for rabbit pancreas.
Cell culture
The human placenta derived MSC (hPL-MSC) [28, 29] were maintained in IMDM (Iscove’s Modified Dulbecco’s medium; Gibco, Grand Island, NY, USA) containing 15% FBS with 100U/ml Pencillin-Streptomycin (Life Technologies, Waltham, MA, USA) at 37 °C and in a 95% air/5% CO2 atmosphere. MSC between passages 3–6 were used for recellularizing the tissue scaffold. MSC were characterized to be CD44+, CD73+, CD90+, CD105+, CD34−, and CD45− [29].
Recellularization
Confluent cultures of MSC were trypsinized and 6–10 × 105 cells were collected in 3 ml of CMRL 1640 medium. The cell suspension was introduced into the decellularized pancreas by means of perfusion via the hepatic portal vein in 3 infusion steps. Each time 1 ml cell suspension containing 2 × 105 cells was perfused for over 20 min. The next infusion of cells was passed after 2 h. Throughout the process, pancreata was continuously perfused with CMRL 1640 (Gibco) culture medium for 5 days. Cannula to the portal vein was kept inserted to allow perfusion feeding of media continuously at the rate of 200ul/min. Rabbit pancreas were seeded using the same protocol using about a total of 12–15 × 105 cells.
Induction of diabetes in mice
Balb-C mice about 6–8 weeks of age were used for the experiment (IAEC-04/2011, IAEC-93/2014, IAEC-44/2017). The animals were injected with a single dose of 160 mg/kg bodyweight streptozotocin [31]. Glucose levels were checked by tail vein puncture with a glucometer till the animal showed higher glucose levels confirming its diabetic status. The animals were maintained at standard environmental conditions (temperature 22–25 °C, humidity 40–70% with 12:12 dark/light photoperiod). The diabetic animals were grouped into three groups; one group received the recellularized pancreatic graft (n = 10), one group was implanted with acellular graft (n = 10) and the third group received no graft (n = 10).
In vivo implantation of recellularized pancreas
The mouse was anaesthetized by intraperitoneal administration of ketamine (50 mg/kg body weight) and xylazine (5 mg/kg body weight). The surgical site was prepared in sterile fashion using 70% isopropyl alcohol followed by the placement of sterile drapes. Using a scalpel a skin incision was made near right abdominal region, the two layer of abdominal muscle were cut open to expose the pancreas. After rinsing with normal saline, the recellularized scaffold was placed in the abdominal cavity near the host pancreas. Single simple interrupted suture was made at anterior end of scaffold near anterior end of pancreas by using absorbable suture (3-0 prolene) material. Then the muscle was opposed with simple interrupted suture using absorbable suture material. The skin was opposed with simple interrupted suture using silk thread [6-0; non absorbable]. Mice was administered antibiotic (enterocin 100 mg/l of water) and analgesic (meloxicam − 1 mg/kg body weight) for the period of 5 days post surgery.
The animals that survived the surgical procedure were monitored for fasting blood glucose level every alternate day by bleeding through tail vein. Our survival rate was 12/15. The animals were sacrificed on day 15, 25 and 40.
Histopathology and immunohistochemistry (IHC)
Native, decellularized and recellularized pancreata (n = 3) were processed for histochemical analysis. All the resected pancreatic samples were immediately fixed in neutral formalin, processed for histology and sectioned. Routine haematoxylin–eosin staining was done and sections were examined in axioimager-2 (Zeiss, Oberkochen, Germany). Immunofluorescence staining was performed using primary antibodies as listed in supplementary Table S2 and counter stained with appropriate fluorescent tagged second antibody. After de-parafinization the slides were treated with citrate buffer (96 °C for 20 min) for antigen retrieval, blocked with 3% BSA and processed for immunohistochemistry. The slides were examined in LEICA TCS SP5 with appropriate filters after counter staining with DAPI for nuclear staining. Analysis was performed using LEICA Application suite X version 2.0. 2.15022
For qualitative determination of presence of glycosaminglycans (GAG), staining was carried out on decellularized pancreas sections. Briefly, after de-parafinization and dehydration, sections were directly stained for 5 min with Safranine O (SRL) stain at room temperature. The slides were washed in running tap water for 5 min followed by a distilled water rinse and mounted with glycerol. Slides were examined in Axioimager-2 microscope.
Proteomics analysis
The decellularized Balb/c mouse pancreas scaffolds were washed in PBS and suspended in 8 M urea with 10 mM DTT with continuous agitation for ~ 2 h at 37 °C followed by alkylation using 25 mM Iodoacetamide. After diluting to 2 M urea with ammonium bicarbonate, samples were incubated with Lys-C Peptide overnight at 37 °C and trypsin for 2 h. Samples were acidified with 50% tri-fluoro acetic acid and centrifuged. The supernatant is transferred to low retention tubes and desalted. The peptides eluted in the digests were analyzed by Q Exactive™ MS (Thermo Fisher Scientific, Waltham, MA, USA) using a 90 min gradient. Protein identification was performed with Proteome Discoverer 1.4.0.288 using the Sequest-HT search engine of proteome discoverer (Thermo Scientific Version 1.4) against uniprot 2018 Mus musculus. Settings used for protein ID mapping were for a full Lys C-Trypsin digest, with two missed cleavages, one static modification (cysteine carbamidomethylation), two dynamic modifications (oxidized methionines) Deamidated N and Q, mass tolerance of 5 ppm for precursor mass and 0.05 Da for fragment masses. Percolator; a post-processing software using a target/decoy database approach, was used to evaluate the accuracy of peptide identifications. Peptide identifications were filtered with a q-value cutoff of 0.01 (1% global False Discovery Rate, FDR). Proteins were grouped using the maximum parsimony principle and compared with the Uniprot mouse database for statistical analysis. Further analysis of the protein data was carried out using online STRING Software.
Western blot analysis
Decellularized scaffolds (n = 5) were homogenized for 10 min in SDS gel sample buffer. The ECM proteins were analyzed on SDS-PAGE. The proteins were transferred on to a membrane (Millipore IVD HYBOND–C Extra membrane) by slot transfer under vacuum. The membrane was blocked using milk proteins and incubated overnight with primary antibodies against collagen type I, type VI, laminin and fibronectin. After washing, the membranes were treated with HRP conjugated secondary antibodies and visualized by ECL.
DNA quantification
The decellularized and native pancreata (n = 5) were used for total DNA isolation. After digestion with proteinase K and 0.1% SDS solution and TEN buffer at 55 °C for 2 h. DNA was isolated by phenol chloroform extraction method. DNA was analyzed by agarose gel electrophoresis and the absolute amount of DNA (ng/ml) was quantified by Nanodrop spectrophotometry.
Real time analysis
RNA was isolated from the grafted pancreas (n = 3) directly using Trizol reagent and converted to cDNA using superscript II. Glucose-6 phosphate dehydrogenase (Gapdh), 18S ribosomal RNA and β-2 microglobulin (B2M) served as internal controls. Real-time PCR was carried out for PDX1; NGN3, INS and PPY genes. The primers were custom synthesized by Bioserve India Ltd. and optimized using a crosswise combination matrix (Supplementary Table S3: primer sequences). A total of 25 ng cDNA was used for real-time PCR with SYBR® Green as indicator using the Applied Biosystem (7900) HT fast real-time PCR system. Fold changes in gene expression were calculated by DDCT method. Statistical analysis was carried out using GraphPad prism by two way ANOVA analysis for all the genes analyzed.
In situ hybridization
In situ hybridization was carried out on sections of implanted grafts re-cellularized with mouse adipose and human placental cells as described in Steck et al. [32]. Hybridization was carried out using digoxigenin labeled (DIG) mouse specific probes for mouse SINE/B1 and SINE/B2 repetitive elements/and human specific probes for Human Specific Alu repeats. In brief, probes were prepared by using specific primers using DIG labeling PCR kit (Roche, Basel, Swiss). DIG labeled PCR products were purified using the nucleospin gel and PCR cleanup kit (Macherey–Nagel).
Sections were deparaffinized; rehydrated and washed three times in PBS containing 0.1% Tween at RT. After treatment with 20 µg/ml proteinase K (Sigma-Aldrich, St. Louis, MO, USA), sections were treated with 0.25% acetic acid containing 0.1 M triethanolamine (pH 8.0) and prehybridized. Hybridization was carried out using specific probes overnight at 42 °C. After washing, signals were detected using anti-DIG labeled alkaline phosphatase antibody by chromogen method using NBT/BCIP (Roche). Sections were counter stained with nuclear fast red and mounted after dehydration. Sections were examined in AxioImager II (Zeiss).
Estimation of insulin and C-peptide
Blood was collected from animals used in transplantation experiments and serum was isolated. Serum was used for estimation of Insulin and c peptide using commercial kits following the procedure. Human c-peptide ELISA kit (cat # EZHCP-20K, Millipore, MA, USA), Human Insulin ELISA kit (cat # ELH-Insulin, RayBiotech, GA, USA), mouse insulin ELISA kit (cat # ELM insulin, RayBiotech) and rat/mouse c-peptide ELISA kit (cat # EZRMCP 2-21K, Millipore, MA, USA).
Statistical analysis
All the quantitative data were analyzed using GraphPad Prism software 8.4.2/3, using paired T test or ANOVA as suitable to data. p values of less than 0.5 were considered significant.
Results
Perfusion decellularization of mouse pancreas
Isolated mouse pancreata were cannulated and retrograde perfusion was carried out via the hepatic portal vein. After flushing the organ completely of blood, the cellular contents were removed with 1% Triton X-100, a non-ionic surfactant based detergent. Triton X-100 would help in lysis of the cells resulting in removal of cell debris. Following perfusion, the whole pancreas turned completely translucent in about 12 h (Fig. 1). The organ was later perfused with DNAse solution to rid the scaffolds of remnants of nucleic acids and finally washed free of lysate material with a thorough perfusion with buffer solution till the whole pancreas turned completely transparent (Fig. 1C). Histological examination by H&E staining showed no traces of cells remaining after the completion of decellularization. The histology showed a scaffold of fine fibrous network left behind. The scaffold sections were stained with DAPI as a quick check to look for cell’s nuclear content and were found to be negative (Fig. 2A–C). To further assess the extent of DNA removal, DNA quantification was performed using phenol–chloroform method. This method showed that DNA content recovered from decellularized structures was about 20–50 ng/sample (n = 5).The agarose gel analysis showed presence of fragmented DNA from the decellularized pancreas as opposed to high molecular weight DNA obtained from a normal un-processed pancreas (Fig. 2D). Feasibility of this perfusion decellularization technique in rabbit pancreas was also demonstrated by following the same protocol of decellularization. At the end of the process, organ scaffold appeared transparent in each case (Fig. 1C). Perfusion mediated decellularization of pancreas via the vascular route efficiently removed cellular components leaving behind the relatively insoluble scaffold.
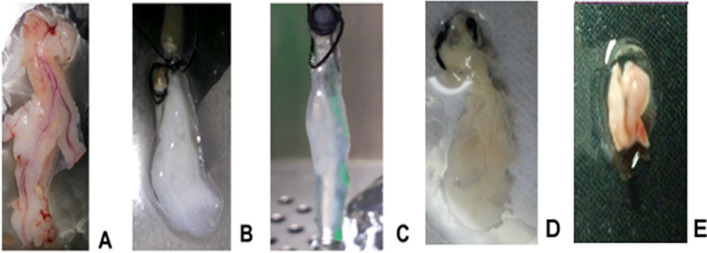
Fig. 1.
Decellularization of the pancreas. A Whole pancreas excised from the animal. B and C The pancreas after perfusion decellularization using detergent and enzymes. D Recellularized pancreas infused with mouse adipose MSC before implantation in diabetic mouse, E recellularized mouse pancreas grafted in mouse excised after 40 days
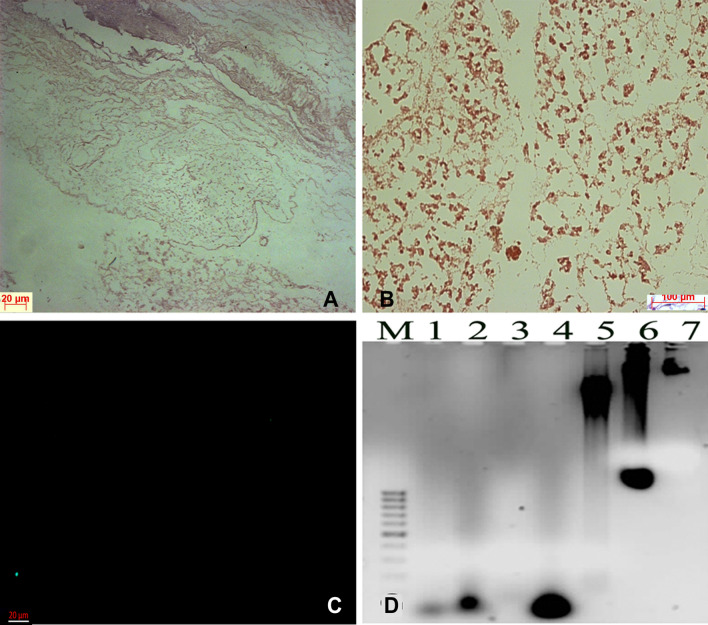
Fig. 2.
Histology of the decellularized pancreas. A Fine fibrillar network of the extracellular matrix without cells. B Scaffold section stained with safranin G for proteoglycans. C Scaffold section stained with DAPI to show lack of nuclear material. D Agarose gel picture showing degraded DNA in lane 1–4 for decellularized pancreas and lane 5–7 normal mouse pancreas
ECM characterization
Immunostaining of the decellularized pancreas stained with antibodies against some of the ECM proteins like Collagen type 1 and VI, fibronectin, laminin showed positive reaction (Fig. 3A). All these matrix proteins showed their presence in both the normal and decellularized sections. Western analysis of the proteins obtained from the scaffolds confirmed the presence of collagen type I, II, VI and Laminin. Western analysis of decellularized, normal and recellularized pancreas showed there was slight loss of these matrix proteins during the process of decellularization (Fig. 3B). The sections were stained for Glycosaminoglycans (GAGs) using safaranin O showed a glycan rich scaffold (Fig. 2B).
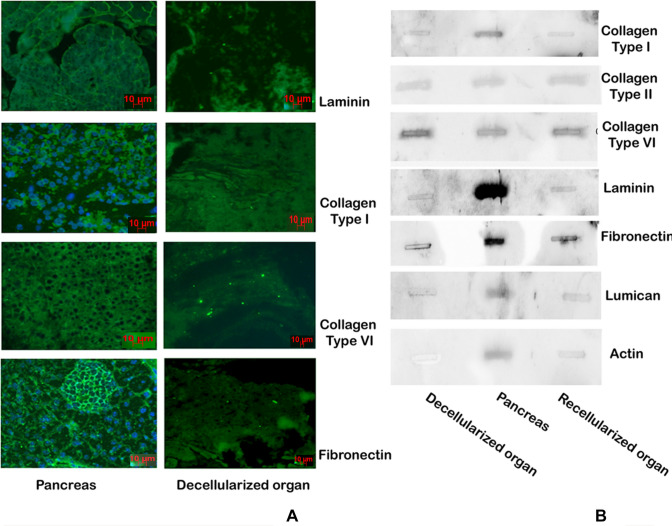
Fig. 3.
Characterization of decellularized scaffold. A Immunochemistry of the decellularized section versus normal pancreas emphasizing the presence of ECM proteins in decellularized scaffolds. B Western analysis of blotted proteins showing the presence of the major ECM proteins those remain in the scaffold though there is some loss of proteins during the process of decellularization
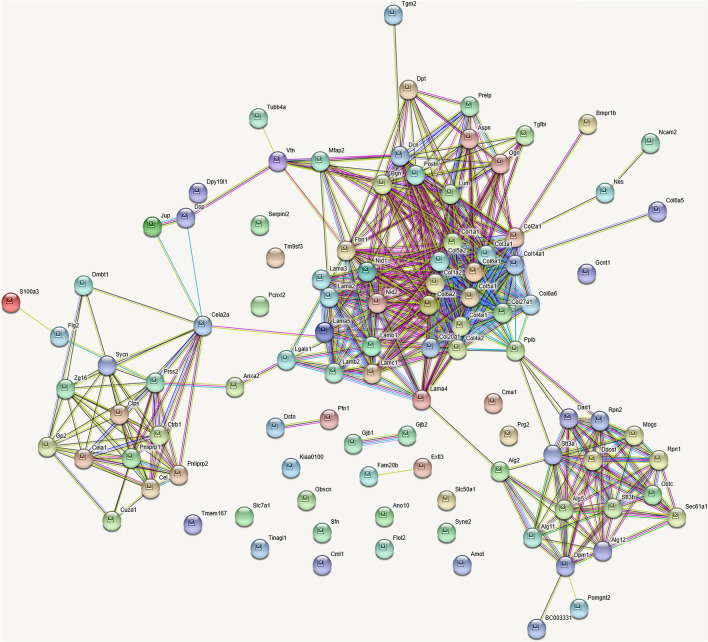
The scaffold thus obtained by decellularization was analyzed by Mass spectrometry (n = 4). The total number of proteins scored was around 100 (Supplementary Table S1, Fig. 4). About 75% were associated with matrix. The major proteins obtained in the scaffold were major ECM protein collagens, glycoproteins, glycans, few matrix regulating and associated proteins, and secreted proteins. Many forms of collagen about 19, including fibril forming like type I, II, III, V, and XXVII, type VI of beaded filaments; fibril associated types XIV and XX; basement membrane associated type IV, along with multiple isoforms of laminins, nidogens and proteoglycans, all with higher number of peptide spectral counts, in every replicate accounted for their abundance. Some of the proteins were matrix synthesizing and glycating membrane associated enzymes. Analysis of the proteome using STRING accounted for about 56 proteins in the extracellular region as structural and secreted proteins and about 31 associated with membranes involved in glycation etc. or junctional proteins.
Fig. 4.
Proteomics. Proteomic analysis of the scaffold obtained after decellularization. About 100 proteins are scored. Majority get clustered as ECM associated proteins. Few belong to pancreas specific exosome proteins and few transmembrane proteins involved in matrix synthesis
Recellularization of scaffolds
After a thorough perfusion with PBS, the extracellular matrix rich scaffold obtained was seeded with cells using the same perfusion set up. The choice of cells was MSC from human placentas that were well characterized and archived in the laboratory (29). MSC were used within 3–6 passages. The organ scaffold was perfused with a total of 6–10 × 105 cells in three rounds and followed with perfusion with DMEM medium for a period of 5–10 days. The organs appeared less translucent at this stage (Fig. 1D). Histology of organs at this stage showed repopulation of the scaffold, though the histology does not appear distinctly pancreatic. Cells appear rounded and just settled (Supplementary Fig. S1A and B). Similarly with rabbit pancreas, seeding with MSC resulted in complete recovery of organ histology, both the islets and exocrine pancreas showed normal histology (Supplementary Fig. S1C). Most of the islets were in the vicinity of vascular structures.
Transplant experiments
The animals injected with a single dose of 160 mg/Kg bodyweight streptozotocin developed high glucose levels mostly by day 4. Streptozotocin treated diabetic mice were implanted with the recellularized pancreas intraperitoneally at a site near the host pancreas holding one end near the C–loop while sutured to the peritoneal muscle layer.
About 80% mice survived the surgery (12/15). The grafted pancreas developed vascular anastomoses at the graft site (Supplementary Fig. S2). Grafted and native pancreata of the mice were excised from these animals and processed separately. Histology of the grafted pancreas in experimental group was similar to the histology of the native pancreas. The islets were developed and so were the acinar cells. The vasculature was well developed and the blood cells were seen in the capillaries. Immunohistochemistry of the recellularized grafted pancreas showed a pancreas with cells positive for endocrine markers like insulin, somatostatin, glucagon, amylin, pancreatic poly peptide (PPY) in the islets and exocrine pancreas markers like carboxypeptidase in acinar cells (Fig. 5A–I). Control slides without primary antibody showed no staining. qPCR analysis for expression of islet specific markers revealed manifold enhancement of PDX1, NGN3, insulin and PPY genes in grafted pancreas at day 7 and 30 as compared to MSC cells. PDX1 and NGN3 showed a decline of expression after an initial rise at day 7 (PDX1 p = 0.001, NGN3 p = 0.09). Enhanced Insulin and PPY expression was seen by day 30 (INS p = 0.002, PPY p = 0.028) (Fig. 5J). Two way ANOVA analysis for all the genes showed that the expression of all these genes had significantly changed (p = 0.0012). The mice that received the recellularized pancreas were able to reduce blood glucose level by day 20–25 as compared to control animals (Fig. 5K). Sham operated mice and mice that received acellular graft showed high levels of glucose up to experimental period, i.e. 40 days post surgery. In the control animals where an acellular pancreas was grafted, histology was not restored. The grafted acellular pancreas was not easy to locate, it did not get cellularized even up to 40 days nor did the animal regain gluco-regulation. The acellular graft was invaded by granulocytes.
Fig. 5.
Regeneration of the pancreas. Recellularized pancreas infused with human placental MSC before implantation in diabetic mouse A Histology of the grafted pancreas after day 15 B and day 40 C show well developed acinar cells, islets along with ducts and vasculature. D–F Immunohistochemistry of recellularized pancreas grafted in mouse for 40 days. Insulin (green), somatostatin (red) and glucagon (blue) in the grafted pancreas (D). Recellularized pancreas section stained with Polypeptide Y (E) and section stained for carboxypeptidase (acinar cells-red) and NGN3 (green) for beta cells (F). G–I are corresponding antibody controls of D–F. J Real time PCR analysis of beta cell markers in harvested recellularized pancreatic graft at days 7 and 30, shows enhanced expression of beta cell specific markers when compared to control MSC from placenta (p = 0.0012). K Blood glucose profile of 5 mice grafted with recellularized pancreas show reduction of blood glucose levels. None of the mice with graft become normoglycemic but started to maintain lower glucose levels compared to diabetic mice. Paired T test analysis of the glucose levels among the two groups showed a significant change (p = 0.0038)
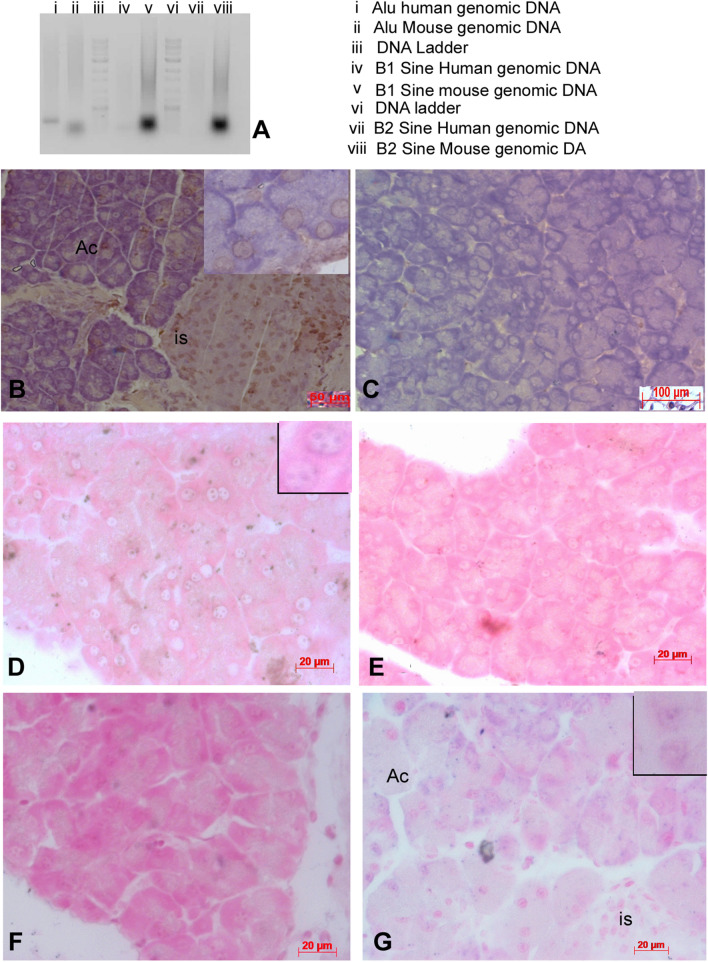
The mouse pancreas recellularized with human MSC had regenerated both islets and the exocrine pancreas. The histology of pancreas was restored and these pancreata were functional in the mice when transplanted. These cells could be additionally tracked with human nuclear antigen in the pancreas when recovered even up to 40 days (Fig. 6). Pancreas infused with cells containing red tracker dye could be imaged up to 15 days (Supplementary Fig. S3). No adverse immune rejection was seen and the recovered pancreas stained with human nuclear antigen showed the presence of human cells also (Fig. 6B, C). The presence of human cells up to 40 days was also confirmed by using human specific Alu repeat probes on sections of pancreas recellularized with human placental MSC cells. Acinar and islet cells stained positive for the human specific Alu repeats. Pancreas recellularized with mouse adipose as control remained unstained with Alu repeat probes and showed reaction with mouse specific B1/B2 Sine repeats (Fig. 6D–G). There was no cross-reactivity. The rabbit pancreas recellularized with human placental cells also showed a positive reaction to Alu repeats (Supplementary Fig. S1J–K).
Fig. 6.
Cell tracking of human cells. Decellularized pancreas recellularized with human placental MSC and implanted in diabetic mice show recovery of normal histology and tendency to achieve blood glucose reduction. A PCR products amplified using Alu primers and B1/B2 Sine primers. Genomic DNA from mouse and human were used as template. Section of the pancreas recovered after 40 days were stained with HNA antigen and also by in situ hybridization using Alu (Human) and sine (Mouse) repeat sequences to show presence of human cells. B Recellularized pancreas with human cells grafted in mouse for 40 days stained for human nuclear antigen (HNA) show the presence of human cells differentiated into islets and acinar cells. C The pancreas recellularized with mouse adipose MSC does not show HNA staining. D and E sections of pancreas recellularized with human cells hybridized/stained with human Alu probe {inset showing nuclear staining of Alu probes} (D) and Mouse b1 and B2 sine probes (E). F and G pancreas recellularized with mouse adipose cells stained with Human Alu probes (F) and B1& B2 mouse sine probes {inset showing nuclear staining of mouse Sine repeats probes}. Human specific Alu repeats could only be localized in human cells and there was no cross reaction
The mouse that received recellularized grafts showed reduction of glucose levels compared to parallel control though none achieved gluconormalcy (Fig. 5K). The animals that received the recellularized pancreas started showing improved levels of human insulin and c-peptide by day 30 (p = 0.0014) (Table 1). Initially, the animals continued to show reduced levels of mouse Insulin and c-peptide up to day 10. Human insulin and c-peptide were undetected on day 10 and day 15 but one could detect higher levels of Human insulin and c peptide in fed animals at day 30. Pancreas recellularized with mouse adipose MSC, the animals did not show human insulin and C-peptide detection. The mouse insulin and mouse C-peptide did show improved levels significantly (p = 0033).
Table 1.
Insulin and C-peptide levels in serum of mouse implanted with recellularized pancreas
| Model system | Human Insulin (U/ml) | Human C peptide (ng/ml) | Mouse Insulin (U/ml) | Mouse C peptide (ng/ml) | Blood glucose levels (mg/dl) |
|---|---|---|---|---|---|
| Normal mice n = 2 | – | 0.004+ | 29.25 + 3.93 | 1.92 ± 0.083 | 98 ± 1.2 |
| Diabetic mice n = 2 | – | – | 14.08 ± 1.8 | 0.031 ± 0.01 | 341 ± 184 |
| Mice with recellularized pancreas hPl-MSC n = 3 | 59.64 ± 9.43 | 6.39 ± 0.9 | – | – | 189 ± 78 |
| Mice with recellularized pancreas mAD n = 2 | – | 0.002 + 0.0005 | 40.21 ± 5.02 | 1.69 ± 0.08 | 372 ± 64 |
| Mice with acellular pancreas n = 2 | – | – | 16.16 ± 3.17 | 0.22 ± 0.11 | 350 ± 86 |
| p values | p = 0.0004 | p = 0.0014 | p = 0.0033 | p < 0.05 |
The serum collected was analyzed for human insulin and human C-peptide as well mouse insulin and mouse C peptide. – indicates values in negative or undetected
We did not attempt grafting rabbit recellularized pancreas.
Discussion
This study reports the recovery of mouse pancreas after decellularization and seeding with MSC cells; and applicability of the protocol to pancreas from rabbit. The process of decellularization and recellularization works well for soft organs and organs with complex cell types like pancreas. Recellularization with adult mesenchymal stem cells can successfully regenerate the structure both endocrine and exocrine; and function of the organ. Recipient animals are able to reduce blood glucose levels and restore the normal histology of the organ. So far there was no immunogenicity reported in animals with intra-peritoneal implants up to 40 days.
The main considerations for the protocol were as follows (i) removal of cellular material and debris including nucleic acid residues/components; (ii) preservation of ECM structure and composition; (iii) functional restoration of the organ.
There are a number of strategies available to decellularize organs; we chose to use continuous flow perfusion of Triton X-100, a non-ionic detergent followed by enzyme DNase to remove even traces of nucleic acids. Triton X 100 is able to disrupt lipid–lipid and lipid protein interactions, at the same time leaving protein–protein interactions undisturbed [33]. This action would help loosen the cellular material and the perfusion process per se may help in removal of the cell debris. Our protocol resulted in removal of cellular material as also reported by [13, 22]. We have used the perfusion via the hepatic portal vein to get a complete decellularized pancreas.
Proteomic analysis of the scaffolds obtained revealed the ECM proteins and associated proteins to be the major components left behind including Collagens, laminins, proteoglycans and other matrix associated proteins. 19 isoforms of collagen were identified and almost matched a similar study [34]. Isoforms of laminins and nidogens along with other glycoproteins, proteoglycans that make the basement membrane components were also identified. These proteins have an important role in regulating survival, differentiation and maintenance of cell types [35]. The immunochemistry confirmed their architectural organization. The proteoglycans, an important constituent of the ECM—that gives the required stiffness to the matrices, were also shown to be present both in proteomic analyses and by the staining of the sections. There are conflicting reports on use of Triton X-100; as it is known to remove GAGs in Aortic valve decellularization protocol [36], our protocol confirms presence of the glycans. Also, decellularization using Triton X-100 was shown to be the most suitable for getting a clinical grade scaffold [18, 37]. Our studies confirm Triton X-100 to be suitable in all respects as the scaffolds obtained are rich in both, GAGs and matrix proteins as seen by staining and proteomics. Our decellularization protocol seems to take care of the two important considerations for obtaining an acellular scaffold; removal of cell debris and maintenance of ECM composition and structure. Triton X-100 has yielded a scaffold without much cell debris as proteomic analysis scored about 100 proteins and majority of these were ECM related. There were some membrane associated proteins too that were scored but most of these were associated with junctions or with matrix metabolism. Western analysis of the ECM obtained after decellularization, native pancreas and recellularized pancreas shows that there is not much loss of the matrix proteins during process of decellularization.
Another point of concern was nucleic acid residues left behind in the scaffold; residual DNA fragments in the scaffolds could cause immune-compatibility issues or be virus carriers. The goal is set to have DNA below 50 ng ds DNA per scaffold and the fragment length should not be more than 200 bases [17]. Our preparations also conform to these standards; the amount of DNA recovered per scaffold was around 20–50 ng and the gel analysis revealed it to be fragmented.
One also looks for minimal loss of active components for growth and differentiation. The scaffold obtained is checked for its biocompatibility. The ECM components are known to support the very nature of cells residing therein [35, 38, 39]. Our concern was that when we seed the recovered scaffold with MSC; these MSC should be able to home in; attach and differentiate into the functional cells within the exocrine and endocrine units of pancreas. Our proteomics studies did reveal retention of TGFb1, PDGF like growth factors/cytokines therein and possibly the architectural/compositional or 3D ECM cue (guides) that the stem cells infused into the pancreas were indeed able to differentiate into islets, acinar and other cell types to form a functional pancreas. We did not require any additional growth factors for the cells to differentiate into the two types of zone specific cell types.
Pancreatic cells have great deal of plasticity inherently as shown earlier (40–41); where extreme loss of beta cells upon exposure to diphtheria toxin was overcome by α cells giving rise to beta cells. It was important to show that the cell implanted during the recellularization makes the pancreas functional not cells left behind by any chance. Human cells infused in the scaffolds could be identified by human nuclear antigen staining and by in situ hybridization using human specific Alu repeat probes up to 40 days; or by tracking the graft for up to 3 weeks post transplant (Supplementary Fig. S3). Scaffolds without reseeding cells, when implanted (n = 10) did not function alike or survive.
The histology of pancreas distinctly shows the formation of islets and the acinar cells over a period of time. The histology and immune fluorescence studies using antibodies against pancreatic exo- and endocrine markers clearly show distinct expression of region specific proteins. Expression of islet specific markers as estimated by qPCR was seen to increase manifold in grafted pancreas. Expression of PDX1 and NGN3 seems to follow the developmental pattern as the levels are higher in the early stages and are lower comparatively by day 30 when the pancreas seems to be fully recovered as seen by histology. PDX1 and NGN3 show a peak in early pancreatic development and are restricted to few cell types towards maturity [41–43]. PPY and insulin expression can be seen only in later stages when the pancreas seems to be functional by day 30. The gene expression pattern also points to de novo development of pancreatic cells.
Grafted pancreas developed vascular anastomoses in surrounding adipose tissue in the region of implantation. Vascular net work was intact and was seen to be fully developed in histology preparations of grafted pancreas of mouse and rabbit. The vessels in fact can be seen loaded with blood cells in the transplanted pancreas. In rabbit recellularized pancreas, the vessels were well formed with some eosinophilic stains in it.
The functionality of constructs was confirmed in the transplantation studies where the recellularized scaffold was implanted in the peritoneal cavity near the pancreas. The streptozotocin treated mice regain glucose reduction within a period of 25 days. Complete gluconormalization was not achieved but most of the animals started maintaining lower glucose levels compared to the sham operated diabetic mice. Analysis of insulin and c peptide levels in the serum of the transplanted animals also confirmed the recovery of the function as the increased serum levels of human insulin and c peptide coincide with the decline in glucose levels. Importance of ECM–cell interaction has been demonstrated by earlier studies [23, 35]. Islet survival over the ECM and increased insulin secretion when grown in contact with ECM both in vitro and in vivo were also demonstrated in the study of De Carlo et al. [17, 24]. ECM indeed forms a dynamic complex structure that can control cellular morphogenesis, proliferation, differentiation, lineage maintenance and ultimately function of the cells. Lineage restriction has been demonstrated when the ECM from liver was seeded with hepatocyte stem cells and kidney was seeded with ES cells [44, 45]. The control animals that are streptozotocin treated but did not receive the graft continue to remain hyperglycemic, substantiating functional recovery of the implanted mass. The exocrine function was not demonstrated but one can see expression of carboxypeptidase in acinar cells. Another indirect proof of exocrine activity was difficulty in RNA isolation due to the proteolytic activity of recellularized constructs.
Our studies clearly establish that extracellular matrix rich organ scaffolds prepared by decellularization can dictate differentiation of stem cells to pancreatic cell types in a region specific manner; the same procedure can be used for creating artificial pancreas for transplantation; we had successfully decellularized human fetal pancreas with the same procedure (not reported). Upon seeding with patient specific MSCs one can expect to develop a functional pancreas. This would take care of rejection if recipient’s own cells are used. So in fact cadaveric pancreas can be used to generate an effective bioscaffold and that can be created into a functional organ. The trials with a full-fledged adult human pancreas would require preclinical standardization in bigger animals.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (DOCX 17139 kb)
Acknowledgements
Authors wish to thank Indian council of Medical Sciences New Delhi for financial support to carry out this work. Grant No. 2011-00060. Authors also wish to thank Dr. Archana B. Siva for carefully reading and editing the MS.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical statement
All the animal studies were carried out after obtaining permission from IAEC ((IAEC-04/2012, IAEC-93/2014, IAEC-44/2017). Human Placental cells were used from the cryostocks maintained in laboratory after obtaining ethical permission from the Institutional ethical committee (IEC34/2015) and Institutional committee for stem cell research (IC-SCR-10/2015).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Karp JM, Langer R. Development and therapeutic applications of advanced biomaterials. Curr Opin Biotechnol. 2007;18:454–459. doi: 10.1016/j.copbio.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 3.Scarritt ME, Pashos NC, Bunnell BA. A review of cellularization strategies for tissue engineering of whole organs. Front Bioeng Biotechnol. 2015;3:43. doi: 10.3389/fbioe.2015.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spector M. Decellularized tissues and organs: an historical perspective and prospects for the future. Biomed Mater. 2016;11:020201. doi: 10.1088/1748-6041/11/2/020201. [DOI] [PubMed] [Google Scholar]
- 5.Baiguera S, Birchall MA, Macchiarini P. Tissue-engineered tracheal transplantation. Transplantation. 2010;89:485–491. doi: 10.1097/TP.0b013e3181cd4ad3. [DOI] [PubMed] [Google Scholar]
- 6.Hinderer S, Schenke-Layland K. Tracheal tissue engineering: building on a strong foundation. Expert Rev Med Devices. 2013;10:33–35. doi: 10.1586/erd.12.74. [DOI] [PubMed] [Google Scholar]
- 7.Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 8.Baptista PM, Siddiqui MM, Lozier G, Rodriguez SR, Atala A, Soker S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 2011;53:604–617. doi: 10.1002/hep.24067. [DOI] [PubMed] [Google Scholar]
- 9.Gilpin SE, Ott HC. Using nature’s platform to engineer bio-artificial lungs. Ann Am Thorac Soc. 2015;12:S45–S49. doi: 10.1513/AnnalsATS.201408-366MG. [DOI] [PubMed] [Google Scholar]
- 10.Martinello T, Bronzini I, Volpin A, Vindigni V, Maccatrozzo L, Caporale G, et al. Successful recellularization of human tendon scaffolds using adipose-derived mesenchymal stem cells and collagen gel. J Tissue Eng Regen Med. 2014;8:612–619. doi: 10.1002/term.1557. [DOI] [PubMed] [Google Scholar]
- 11.Loai Y, Yeger H, Coz C, Antoon R, Islam SS, Moore K, et al. Bladder tissue engineering: tissue regeneration and neovascularization of HA-VEGF-incorporated bladder acellular constructs in mouse and porcine animal models. J Biomed Mater Res A. 2010;94:1205–1215. doi: 10.1002/jbm.a.32777. [DOI] [PubMed] [Google Scholar]
- 12.Goh SK, Bertera S, Olsen P, Candiello JE, Halfter W, Uechi G, et al. Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials. 2013;34:6760–6772. doi: 10.1016/j.biomaterials.2013.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peloso A, Urbani L, Cravedi P, Katari R, Maghsoudlou P, Fallas ME, et al. The human pancreas as a source of protolerogenic extracellular matrix scaffold for a new-generation bioartificial endocrine pancreas. Ann Surg. 2016;264:169–179. doi: 10.1097/SLA.0000000000001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 15.Zhou J, Fritze O, Schleicher M, Wendel HP, Schenke-Layland K, Harasztosi C, et al. Impact of heart valve decellularization on 3-D ultrastructure, immunogenicity and thrombogenicity. Biomaterials. 2010;31:2549–2554. doi: 10.1016/j.biomaterials.2009.11.088. [DOI] [PubMed] [Google Scholar]
- 16.Schmitt A, Csiki R, Tron A, Saldamli B, Tübel J, Florian K, et al. Optimized protocol for whole organ decellularization. Eur J Med Res. 2017;22:31. doi: 10.1186/s40001-017-0272-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peloso A, Dhal A, Zambon JP, Li P, Orlando G, Atala A, et al. Current achievements and future perspectives in whole-organ bioengineering. Stem Cell Res Ther. 2015;6:107. doi: 10.1186/s13287-015-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hillebrandt KH, Everwuen H, Haep N, Keshi E, Pratschke J, Sauer IM. Strategies based on organ decellularization and recellularization. Transpl Int. 2019;32:517–585. doi: 10.1111/tri.13462. [DOI] [PubMed] [Google Scholar]
- 20.Qi M. Transplantation of encapsulated pancreatic islets as a treatment for patients with type 1 diabetes mellitus. Adv Med. 2014;2014:429710. doi: 10.1155/2014/429710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Carlo E, Baiguera S, Conconi MT, Vigolo S, Grandi C, Lora S, et al. Pancreatic acellular matrix supports islet survival and function in a synthetic tubular device: in vitro and vivo studies. Int J Mol Med. 2010;25:195–202. [PubMed] [Google Scholar]
- 22.Mirmalek-Sani SH, Orlando G, McQuilling JP, Pareta R, Mack DL, Salvatori M, et al. Porcine pancreas extracellular matrix as a platform for endocrine pancreas bioengineering. Biomaterials. 2013;34:5488–5495. doi: 10.1016/j.biomaterials.2013.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salg GA, Giese NA, Schenk M, Hüttner FJ, Felix K, Probst P, et al. The emerging field of pancreatic tissue engineering: a systematic review and evidence map of scaffold materials and scaffolding techniques for insulin-secreting cells. J Tissue Eng. 2019;10:2041731419884708. doi: 10.1177/2041731419884708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guruswamy Damodaran R, Vermette P. Decellularized pancreas as a native extracellular matrix scaffold for pancreatic islet seeding and culture. J Tissue Eng Regen Med. 2018;12:1230–1237. doi: 10.1002/term.2655. [DOI] [PubMed] [Google Scholar]
- 25.Herberts CA, Kwa MS, Hermsen HP. Risk factors in the development of stem cell therapy. J Transl Med. 2011;9:29. doi: 10.1186/1479-5876-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikebe C, Hermsen HP. Risk factors in the development of stem cell therapy. J Transl Med. 2014;2014:951512. doi: 10.1186/1479-5876-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 2016;25:829–848. doi: 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]
- 28.Thomsen GM, Gowing G, Svendsen S, Svendsen CN. The past, present and future of stem cell clinical trials for ALS. Exp Neurol. 2014;262:127–137. doi: 10.1016/j.expneurol.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 29.Thejaswi K, Amarnath M, Srinivas G, Jerald MK, Raj TA, Singh S. Immune modulatory responses of mesenchymal stem cells from different sources in cultures and in vivo. Cell Tissue Transpl Ther. 2012;4:1–13. [Google Scholar]
- 30.Salvatori M, Katari R, Patel T, Peloso A, Mugweru J, Owusu K, et al. Extracellular matrix scaffold technology for bioartificial pancreas engineering: state of the art and future challenges. J Diabetes Sci Technol. 2014;8:159–169. doi: 10.1177/1932296813519558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graham ML, Janecek JL, Kittredge JA, Hering BJ, Schuurman HJ. The streptozotocin-induced diabetic nude mouse model: differences between animals from different sources. Comp Med. 2011;61:356–360. [PMC free article] [PubMed] [Google Scholar]
- 32.Steck E, Burkhardt M, Ehrlich H, Richter W. Discrimination between cells of murine and human origin in xenotransplants by species specific genomic in situ hybridization. Xenotransplantation. 2010;17:153–159. doi: 10.1111/j.1399-3089.2010.00577.x. [DOI] [PubMed] [Google Scholar]
- 33.Seddon AM, Curnow P, Booth PJ. Membrane proteins, lipids and detergents: not just a soap opera. Biochim Biophys Acta. 2004;1666:105–117. doi: 10.1016/j.bbamem.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Naba A, Clauser KR, Mani DR, Carr SA, Hynes RO. Quantitative proteomic profiling of the extracellular matrix of pancreatic islets during the angiogenic switch and insulinoma progression. Sci Rep. 2017;7:40495. doi: 10.1038/srep40495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Townsend SE, Gannon M. Extracellular matrix-associated factors play critical roles in regulating pancreatic β-cell proliferation and survival. Endocrinology. 2019;160:1885–1894. doi: 10.1210/en.2019-00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grauss RW, Hazekamp MG, Oppenhuizen F, van Munsteren CJ, Gittenberger-de Groot AC, DeRuiter MC. Histological evaluation of decellularised porcine aortic valves: matrix changes due to different decellularisation methods. Eur J Cardiothorac Surg. 2005;27:566–571. doi: 10.1016/j.ejcts.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 37.Vavken P, Joshi S, Murray MM. Triton-x is most effective among three decellularization agents for acl tissue engineering. J Orthop Res. 2009;27:1612–1618. doi: 10.1002/jor.20932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Juhl K, Bonner-Weir S, Sharma A. Regenerating pancreatic beta-cells: plasticity of adult pancreatic cells and the feasibility of in vivo neo-genesis. Curr Opin Organ Transplant. 2010;15:79–85. doi: 10.1097/MOT.0b013e3283344932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thorel F, Népote V, Avril I, Kohno K, Desgraz R, Chera S, et al. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464:1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson ME, Scheel D, German MS. Gene expression cascades in pancreatic development. Mech Dev. 2003;120:65–80. doi: 10.1016/S0925-4773(02)00333-7. [DOI] [PubMed] [Google Scholar]
- 42.Miyatsuka T, Kaneto H, Shiraiwa T, Matsuoka TA, Yamamoto K, Kato K, et al. Persistent expression of PDX-1 in the pancreas causes acinar-to-ductal metaplasia through Stat3 activation. Genes Dev. 2006;20:1435–1440. doi: 10.1101/gad.1412806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyttle BM, Li J, Krishnamurthy M, Fellows F, Wheeler MB, Goodye CG, et al. Transcription factor expression in the developing human fetal endocrine pancreas. Diabetologia. 2008;51:1169–1180. doi: 10.1007/s00125-008-1006-z. [DOI] [PubMed] [Google Scholar]
- 44.Ross EA, Williams MJ, Hamazaki T, Terada N, Clapp WL, Adin C, et al. Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J Am Soc Nephrol. 2009;20:2338–2347. doi: 10.1681/ASN.2008111196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Cui CB, Yamauchi M, Miguez P, Roach M, Malavarca R, et al. Lineage restriction of human hepatic stem cells to mature fates is made efficient by tissue-specific biomatrix scaffolds. Hepatology. 2011;53:293–305. doi: 10.1002/hep.24012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (DOCX 17139 kb)