Abstract
Background:
Corneal scarring or disease may lead to severe corneal opacification and consequently, severe loss of vision due to the complete loss of corneal epithelial cells. We studied the use of epithelial cell sheets differentiated from fetal cartilage-derived stem cells (FCSC) to resurface damaged cornea.
Methods:
The FCSC were isolated from the femoral head of immature cartilage tissue. The ability of the FCSCs to differentiate into corneal epithelial cells was evaluated using differentiation media at 2 days and 7 days post-seeding. A sheet fabricated of FCSCs was also used for the differentiation assay. The results of the in vitro studies were evaluated by immunocytochemistry and Western blots for corneal epithelial cell markers (CK3/12 and Pax6) and limbal epithelial stem cell markers (ABCG2 and p63). To test the material in vivo, an FCSC-sheet was applied as a treatment in a chemically burned rabbit model. The healing ability was observed histologically one week after treatment.
Results:
The in vitro experiments showed morphological changes in the FCSCs at two and seven days of culture. The differentiated cells from the FCSCs or the FCSC-sheet expressed corneal epithelial cells markers. FCSC were create cell sheet that successfully differentiated into corneal epithelial cells and had sufficient adhesion so that it could be fused to host tissue after suture to the ocular surface with silk suture. The implanted cell sheet maintained its transparency and the cells were alive a week after implantation.
Conclusion:
These results suggest that carrier-free sheets fabricated of FCSCs have the potential to repair damaged corneal surfaces.
Electronic supplementary material
The online version of this article (10.1007/s13770-020-00317-w) contains supplementary material, which is available to authorized users.
Keywords: Corneal epithelial repair, Cell sheets, Fetal cartilage-derived stem cells
Introduction
Corneal ulcer are a wide variety of causes of corneal ulcers, including infection, physical and chemical trauma, corneal drying and exposure, and contact lens overwear and misuse. Corneal ulcers are a serious problem and may result in loss of vision or blindness [1–3]. If corneal epithelial cells are completely absent because of limbal disorders from burns or eye diseases, then the sources of corneal epithelial cells have been exhausted and the peripheral conjunctival epithelium invades inwardly [4]. Eventually, the corneal surface becomes enveloped by vascularized conjunctival scar tissue, resulting in corneal opacification that leads to severe visual impairment [5].
Autologous corneal limbal epithelial stem cell (LESC) transplantation is a method whereby the surface of the cornea is reconstructed [6]. This procedure, however, requires a large LESC graft from the healthy eye, incurring the risk of causing corneal stem-cell deficiency in the healthy eye. In the last decades, novel techniques such as cultivated limbal epithelial transplantation (CLET) have been proposed in order to reduce the damage of the healthy fellow eye [7–9]. Clinical and experimental evidence showed that CLET is effective in inducing long-term regeneration of a healthy corneal epithelium in patients with Limbal stem cell deficiency (LSCD) with a success rate of 70%–80%. Current limitations for the treatment of LSCD are bilateral LSCD which requires other sources of stem cells for ocular surface reconstruction. Accordingly, conjunctival flaps and grafts (e.g., cultured autologous oral mucosa epithelial cell sheets and limbal stem cells sheets) have been commonly used to treat corneal ulcerations [10]. However, a conjunctival flap or graft may leave opaque areas and blood vessels on the cornea, even if the grafted tissue is removed after corneal epithelial regeneration.
In efforts to address these hurdles, we first aimed to develop a scaffold-free corneal epithelial cell sheet using fetal cartilage stem cells (FCSC). Fetal cells were isolated from fetal tissue such as liver, bone marrow, blood, lung, kidney, pancreas, placenta, brain, and spinal cord and differentiated into a variety of mesenchymal stem cell (MSC) lineage cells, such as osteoblasts, adipocytes, and chondrocytes [11–14]. They have been shown to have high multi-potentiality and proliferation capacity, which are sometimes better than adult stem cells isolated from aged donors [11, 15, 16]. Moreover, MSCs from fetal tissue have been shown to elicit no alloreactive T-cell response, which suggests that fetal stem cells do not cause an allogenic immune response [17, 18]. Thus, FCSCs are regarded as an attractive cell source in regenerative medicine, such as cell therapies and tissue engineering.
We studied a new method of transplantation involving a carrier-free cell sheet. All carriers or scaffolds were excluded from the graft. We investigated a new method of transplantation involving the carrier-free epithelial cell sheet by culturing harvested cells for seven days on cell culture surfaces that had been treated with mimicked-medium. In addition, we fabricated FCSC-sheets for in vivo application and confirmed that FCSCs were differentiated into corneal epithelial cells in a chemically burned rabbit model.
Materials and methods
Cell isolation and culture
The study was approved by the institutional review board (IRB) of the Ajou University Medical Center (AJIRB-CRO-07-139) and was carried out with the informed consent of all donors. All experiments were performed in accordance with relevant guidelines and regulations. Human fetal cartilage tissues (n = 2, F12w-c, M11w) were obtained from patients following elective termination at 12 weeks after gestation, and cells were isolated from the femoral head of the cartilage tissue. Cartilage tissues were cut into small pieces and treated with 0.1% collagenase type II (Worthington Biochemical Corp, Freehold, NJ, USA) in high-glucose Dulbecco's modified Eagle medium (DMEM; Hyclone, Logan, UT, USA) containing 1% fetal bovine serum (FBS; Biotechnics research, Inc.) at 37 °C under 5% CO2. After 12 h, isolated cells were cultured in DMEM supplemented with 10% FBS, Penicillin streptomycin [100 U/ml penicillin G (Gibco BRL, Grand Island, NY, USA), 100 μg/ml streptomyocin (Gibco BRL)], 5 ng/ml basic FGF (R&D systems, Recombinant human FGF basic146aa, USA). Cells were passaged at 80% confluence, where the plating density was approximately 8 × 103 cells/cm2.
Immortalized SV40 human CEC line was obtained from Dr. Tae-im Kim (Yonsei University College of Medicine, Seoul) [19]. Cells were cultured in Dulbeco’s modified ragle’s media (DMEM)/F12 (Invitrogen, Carlsbad, CA, USA) supplemented with human recombinant EGF (10 ng/mL) (Upstate Biotechnology, Lake Placid, NY, USA) and 10% fetal bovine serum. Cells were incubated in a CO2-regulated incubator and the medium was renewed every 2 days.
Label with PKH26
FCSCs were labeled with the red fluorescent dye PKH26 (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s protocol. Briefly, the detached FCSCs were washed by a serum-free medium and resuspended in 1 mL of dilution buffer from the manufacturer’s labeling kit. The cells suspension was mixed with an equal volume of the labeling solution containing 4 × 106 M PKH26 in the dilution buffer and incubated for 5 min at room temperature (RT). After the reaction was ended by adding 2 mL fetal bovine serum (FBS), cells were washed 3 times with the Dulbecco’s modified Eagle’s medium (DMEM)/F12 and observed by fluorescent microscopy.
Differentiation of corneal epithelial-like cells
FCSCs (3 × 105 cells/cm2) were cultured in Differentiation-medium of low-glucose Dulbecco’s modified Eagle medium (DMEM; HyClone, Logan, UT, USA) containing 2% KnockOut™ Serum Replacement (KnockOutTM SR, Thermo Fisher Scientific, Waltham, MA, USA), 10 ng/ml Keratinocyte Growth Factor (KGF, Wako Pure Chemical, Osaka, Japan), 10 ng/ml Hepatocyte Growth Factor (HGF, Wako Pure Chemical), 20 ng/ml Epidermal Growth Factor (EGF, Wako Pure Chemical, Japan), 0.5 ug/ml Hydrocortisone (Sigma-Aldrich), 5 uM Retinoic acid (Sigma-Aldrich) at 37 °C with 5% CO2. The medium was replaced every 2 days.
Fabrication of FCSCs-sheet
FCSCs populations were increased through the repeated passages of the cells by trypsinization (4 to 5 passages). Non-adherent cells were then washed during 2–3 fresh medium changes. These FCSCs (3 × 105, and 6 × 105 cell/cm2) were then cultured in the Sheet-medium, which consisted of high-glucose DMEM supplemented with 100 U/ml penicillin G, 100 μg/ml streptomycin (Pen-Strep; HyClone), insulin-transferrin-selenium (ITS; Gibco BRL), 50 μg/ml ascorbate-2 phosphate, 100 nM dexamethasone, 40 μg/ml proline, 1.25 mg/ml bovine serum albumin (BSA), 100 μg/ml sodium pyruvate (all from Sigma-Aldrich), and incubated at 37 ºC with 5% CO2 for 2 weeks until FCSCs was detached from the 12-Well cell culture flat-bottom plate as a monolayer cell sheet and medium change of differentiation-medium.
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde for 20 min and incubated in 0.1% Triton X-100 for 15 min and 5% BSA for 1 h. The primary antibodies included Anti-PAX6, Anti-BCRP/ABCG2, Anti-p63, Anti-CK3/12 (1: 200; All product, Abcam, Cambridge, UK). Cells were incubated with the primary antibodies 2 h at room temperature. Cells were washed with phosphate-buffered saline (PBS; Welgene, Daegu, Korea) and then incubated with goat anti-mouse IgG H&L and goat anti-rabbit IgG H&L (1: 1000; Alexa Fluor® 488, Abcam) for 1 h at room temperature. The cells were then stained with 4, 6-diamidino-2-phenylindole (DAPI) to visualize the nuclei. Fluorescence was observed using a fluorescence microscope (model: Mi8, Leica Microsystems, Wetzlar, Germany).
Flow cytometry analysis
Cells at passages 5 were analyzed for the expression of corneal epithelial stem markers and corneal epithelial differentiation marker on the cell surface or cyto-plasma. Cells in the suspension were incubated with anti-CD34-FITC (BD Biosciences, San Jose, CA, USA), anti-CD105 (BD Biosciences), Anti-PAX6, Anti-BCRP/ABCG2, Anti-p63, Anti-CK3/12 (1: 500; Abcam) antibodies for 1 h at room temperature. Cells were washed with phosphate-buffered saline (PBS; Welgene, Daegu, Korea) and then incubated with goat anti-mouse IgG H&L and goat anti-rabbit IgG H&L (1: 1000; Alexa Fluor® 488, Abcam) for 1 h at room temperature. The dilution factors of each antibody were determined according to the manufacturers’ instructions. Stained cells were analyzed by flow cytometry (Becton Dickinson FACS avantage).
Western blot analysis
Cells were collected and lysed by shaking at 4 °C for 30 min in RIPA buffer that contained protease inhibitors (Rockland Immunochemicals, Pottstown, PA, USA). The cell lysates were centrifuged at 12,000 g for 15 min at 4 °C. Appropriate volumes of the samples (20 µg/lane), were mixed with equal volumes of sample buffer (100 mM Tris–HCl, pH 6.8, 4% SDS, 20% glycerol, 10% 2-mercaptoethanol, and 0.02% bromophenolblue), heated at 100 °C for 10 min, and then subjected to SDS–PAGE using 4–20% Mini-PROTEAN TGXTM Gel (Bio-Rad, Hercules, CA, USA). The proteins were transferred by means of a semidry electroblotting system from the gels to polyvinylidene difluoride membranes for 2 h. The blots were then blocked with the primary antibodies for Actin (1: 1000) (GeneTex Inc., Irvine, CA, USA), Anti-PAX6, Anti- BCRP/ABCG2, Anti-p63, and Anti-CK3 (1: 200; Abcam) for 2 h at room temperature. Skim milk powder was used for blocking buffer. Next, the blots were incubated with an appropriate second antibody, horseradish peroxidase-conjugated goat anti-rabbit IgG and goat anti-mouse IgG (1: 1000; GeneTex Inc.), for 1 h. Immunoreactive bands were visualized using enhanced chemiluminescence (ECL kit; Bio-Rad). Imaging was performed using Chemiluminescence system (model: Fusion SL2, VILBER LOURMAT, Collégien, France) and image analysis using ImageJ (NIH, Bethesda, MD, USA).
Histological and immunohistochemistry analysis
The samples were fixed with 4% formaldehyde (Duksan Chemical, Ansan, Korea) and embedded in paraffin wax (Merck, Darmstadt, Germany). Sections 8 mm thick were stained with H&E to confirm of FCSCs-sheet and cell morphology in vivo, respectively. For an immunohistochemical analysis of CK3 and Human nucleus, sections were treated with 3% hydrogen peroxide (Duksan Chemical) in methanol for 10 min and reacted with a pepsin solution (Golden Bridge International, Inc., Mukilteo, WA, USA) for 10 min. After blocking the sections with 1% BSA in PBS, they were incubated with anti-CK3 antibody (1:100; Abcam) or anti-human nucleus antibody (1:100; Millipore) for 1.5 h at room temperature. The sections were then incubated with a biotinylated secondary antibody against mouse IgG (SPlink HRP Detection Kit; Golden Bridge International, Inc.) for 30 min and with a peroxidase-conjugated streptavidin solution (SPlink HRP Detection Kit; Golden Bridge International, Inc.) for 30 min. Finally, the sections were reacted with a 3,3′-diaminobenzidine (DAB) solution (Golden Bridge International, Inc.) and counterstained with Mayer’s hematoxylin (YD Diagnostics, Seoul, Korea) before mounting.
Animal model of alkali-burned cornea epithelium
The study was approved by Institutional Animal Care and Use Committee of the Laboratory Animal Research Centre, Ajou University. Experiments involving rabbit were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Limbal stem cell deficiency (LSCD) was generated in the right eye of each rabbit (18 rabbits). Rabbits were anesthetized by intramuscular injection of a mixture of ketamine and zoletile. A filter paper with a diameter of 8 mm was saturated with l M NaOH and was placed on the corneal limbus for 30 s, followed by rinsing with saline for 1 min and 3 times. Antibiotic drops were applied to the injured eyes three times a day. To confirm that the animal model of the alkaline burncorneal epithelium was well made, the changes in the anterior part were periodically observed using a slit lamp. Gross images showed that the rabbit eyes were cloudy.
Surgical procedure for transplantation
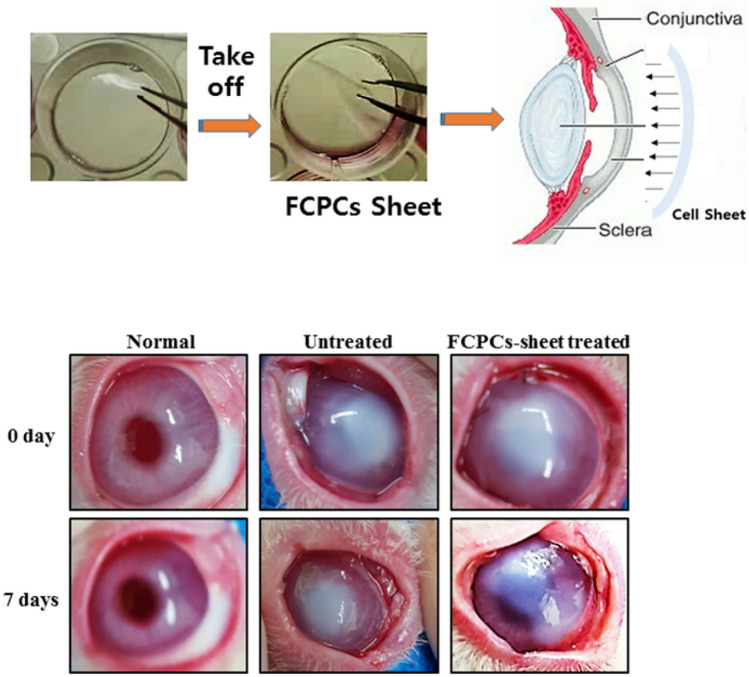
FCSCs-sheets transplanted on the 1 day after the creation of the corneal burn. The damaged corneal epithelium was carefully keratectomized under anesthesia and then rinsed with normal saline. The FCSCs-sheet and PKH26 labeled FCSCs-sheet was placed on the cornea with forceps, and the suture was performed on the upper, lower, left, and right sides with 6–0 black silk suture. After transplantation, a 0.0 5% dexamethasone gentamicin solution was applied. Finally, the lids were sutured and the sutures were removed seven days later. Dexamethasone gentamicin was applied to the right eye of each rabbit three times a day. The ocular surface of each rabbit was evaluated for 1 weeks after surgery. The rabbits were sacrificed and the corneas were extracted and processed for H&E and immunofluorescence staining.
Statistical analyses
GraphPad Prism 7 version 7.00 (GraphPad, San Diego, CA, USA) was used to produce graphic images and perform statistical analysis. Data were expressed as the mean ± standard deviation (SD) from at least three independent experiments. Statistical significance was analyzed by a one-way analysis of variance (ANOVA) followed by a Tukey–Kramer post-hoc test. A value of p < 0.05 was considered to be statistically significant (*p < 0.05, **p < 0.01, and ***p < 0.001).
Results
Morphological features and changes in undifferentiated and differentiated FCSCs
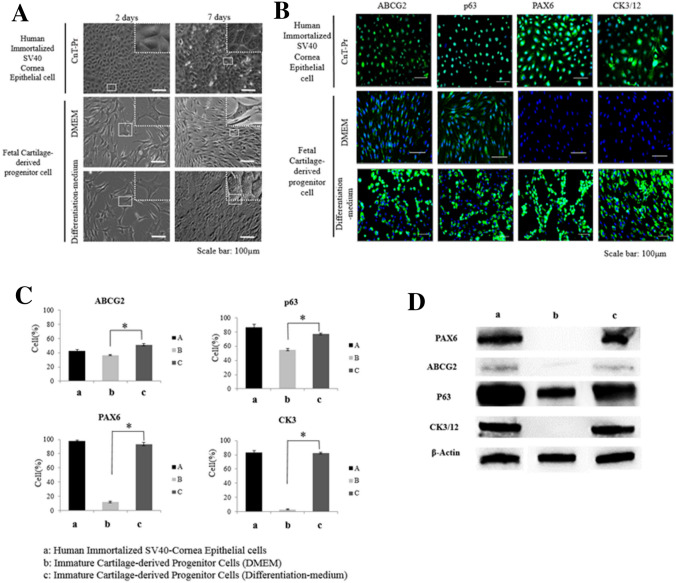
Within one day after seeding, human immortalized SV40-corneal epithelial cells attached to the culture dish and started to spread. The human immortalized SV40-corneal epithelial cell morphology appeared to be compact, uniform, and small polygons in shape (Fig. 1A). The FCSCs also began to spread rapidly on the culture dish within one day after seeding. The cells were positive for human MSC markers CD29 (β1 integrin; 92.4%), CD90 (Thy-1; 98.2%) and CD105 (endoglin; 72.5%). However, the cells were negative for human endothelial cell markers CD34 in flow cytometry analyses (Supplementary Table 1). They displayed the spindle-like appearance typical of FCSCs. As the incubation times increased, the shape of the FCSCs cultured on the DMEM grew radial and later became elongated and fibroblast-like. However, the shape of the FCSCs grown on the differentiation medium was uniform and polygonal, like that of the corneal epithelial cells (Fig. 1A).
Fig. 1.
Phenotypes of the corneal epithelial cells. A The effect of differentiation medium on FCSC morphology compared to human immortalized SV40 corneal epithelial cells. Scale bar = 100 µm. B The effect of differentiation medium on FCSC expression of corneal epithelial stem cell markers (ABCG2 and p63) and corneal epithelial differentiation markers (PAX6 and CK3/12) compared to human immortalized SV40 corneal epithelial cells at 7 days in culture. Scale bar = 100 µm. C Representative FACS analysis showing the effect of differentiation medium on the FCSC expression of corneal epithelial stem cell markers (ABCG2 and p63), and corneal epithelial differentiation markers (PAX6 and CK3) compared to human immortalized SV40 corneal epithelial cells at 7 days in culture. D The effect of differentiation medium on FCSC expression of corneal epithelial stem cell markers (ABCG2 and p63) and corneal epithelial differentiation markers (PAX6 and CK3/12) compared to human immortalized SV40 corneal epithelial cells at 7 days in culture
Differentiation of FCSCs into corneal epithelial cells
To confirm and characterize the differentiated FCSCs, we investigated the expression of PAX6, p63, ABCG2, CK3/12 by immunocytochemistry (Fig. 1B). The human immortalized SV40-corneal epithelial cells expressed of PAX6, p63, ABCG2, and CK3/12, the markers of stem cells and differentiated corneal cells. The proteins were strongly expressed in the cytomembrane, nucleus, and cytoplasm. The FCSCs cultured on DMEM medium showed weak expression of ABCG2, associated with corneal stem cells but did not express PAX6 and CK3/12 associated with ocular development and corneal epithelium differentiation. However, the FCSCs cultured on defined medium showed higher expression levels of PAX6 and CK3/12, as well as higher expression of ABCG2 and p63 associated with corneal stem cells than did the FCSCs cultured in DMEM medium (Fig. 1B).
To further confirm and characterize the differentiated FCSCs, we investigated the expression of PAX6, p63, ABCG2, CK3/12 on the cells by FACS analysis (Fig. 1C). Human immortalized SV40-corneal epithelial cells expressed PAX6 (98%), p63 (86.69%), ABCG2 (42.2%), and CK3 (83.3%), which are markers of stem cells and differentiated corneal cells. The FCSCs cultured in DMEM weakly expressed ABCG2 (36.7%) and p63 (55%) associated with corneal stem cells but did not express PAX6 (11.65%) or CK3 (3%) associated with ocular development and corneal epithelium differentiation. However, the FCSCs cultured in differentiation medium showed higher expression levels of PAX6 (93.26%) and CK3 (82.28%), which are associated with ocular development and corneal epithelial differentiation, as well as higher expression of ABCG2 (51.24%) and p63 (76.95%) associated with corneal stem cells, than did the FCSCs cultured in DMEM.
The Western blots were quantified to confirm the differentiation of FCSCs into corneal epithelial cells (Fig. 1D). The two groups of human immortalized SV40-corneal epithelial cells and FCSCs cultured in differentiation medium expressed PAX6, ABCG2, p63, and CK3/12. However, the FCSCs cultured in DMEM strongly expressed p63 but weakly or did not express PAX6, ABCG2, and CK3/12 (Fig. 1D).
Fabrication of the FCSC sheet
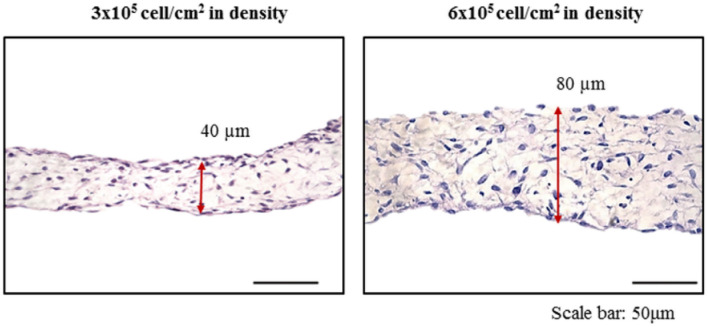
To facilitate cell transplantation for cell therapy, FCSC-based sheets were fabricated. FCSCs were seeded at cell densities of 3 × 105, and 6 × 105 cell/cm2 in culture dishes and cultured in sheet medium for seven days. Cell sheets could not be fabricated at cell density of 1.5 × 105 cell/cm2 (data not shown) but cell sheets could be produced at cell densities of 3 × 105 and 6 × 105 cell/cm2. The cell sheets were collected and examined by H & E staining (Fig. 2). The results showed that the cells were randomly arranged and surrounded by an extracellular matrix. At 3 × 105 cell/cm2 density, the thickness of the FCSC-sheet measured about 40 µm. And 6 × 105 cell/cm2 density, the thickness of FCSC-sheet measured about 80 µm. These results confirmed that the thickness of the cell sheet could be controlled by adjusting the cell density (Fig. 2).
Fig. 2.
The effect of cell density on the thickness of FCSC-sheets made from FCSCs. A At 3 × 105 cell/cm2 density, the thickness of the FCSC-sheet was about 40 µm and B at 6 × 105 cell/cm2 density, the thickness of the FCSC-sheet was about 80 µm. Scale bar = 50 µm
Differentiation ability of the FCSC-sheet
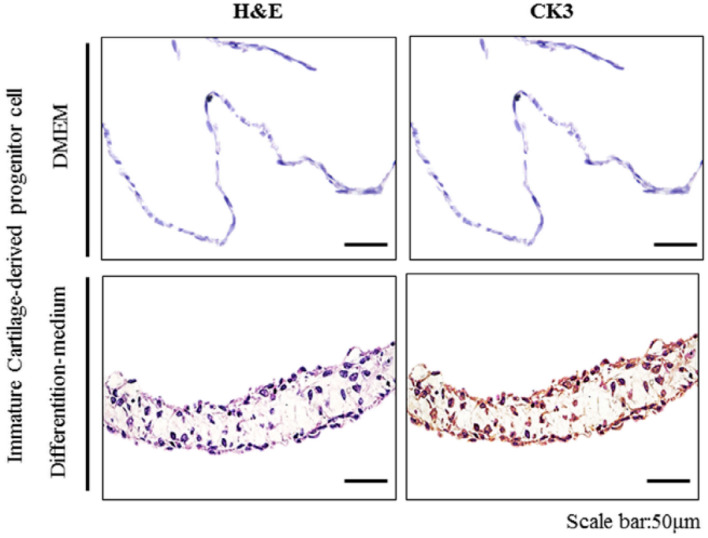
To determine whether the FCSCs in the FCSC-sheet were differentiated into corneal epithelial cells, the sheet medium was changed to differentiation medium during the production of the FCSC-sheet. As a result of changing the culture medium, immunohistochemical analysis showed that the FCSC-sheet strongly expressed CK3, a corneal epithelial differentiation marker (Fig. 3).
Fig. 3.
The effect of differentiation medium on the FCSC-sheet (3 × 105 cell/cm2 density) expression of a corneal epithelial differentiation marker (CK3) compared to native cornea. Scale bar = 50 µm
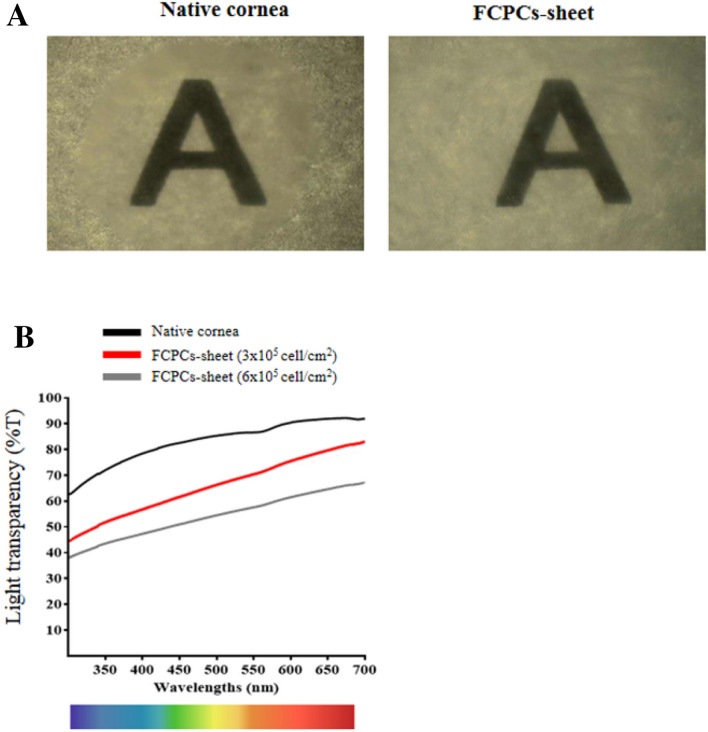
Transparency of the FCSCs sheet
When the FCSC-sheet was overlaid on a paper on which the alphabet was written to check the transparency of the FCSC-sheet, both the corneal tissue and the cell sheet could be identified (Fig. 4A). In addition, when the light transmittance was evaluated by UV-spectrometry, the results showed that the thicker the FCSC-sheet was, the lower the light transmittance. The FCSC-sheet fabricated using 3 × 105 cell/cm2 was found to have a light transmittance of about 76% that of native corneal tissue (Fig. 4B).
Fig. 4.
The effect of cell density on light transparency of FCSC-sheets made from FCSCs compared to the native cornea. A Light transparency of the native cornea, FCSC-sheet at 3 × 105 cell/cm2 density density are shown by gross images. B The transparency of the native cornea, FCSC-sheet at 3 × 105 cell/cm2 density, and FCSCs-sheet at 6 × 105 cell/cm2 density measured by UV-spectrometry
Rabbit eyes before and after transplantation of the FCSC-sheet
A chemically burned model was made by applying 1 N NaOH to rabbit eyes. Gross images showed that the rabbit eyes were cloudy. The untreated burned group still showed strong corneal clouding after seven days but the cornea was significantly improved clear after seven days in the FCSC-sheet treated group (Fig. 5).
Fig. 5.
The effect of an FCSC-sheet (3 × 105 cell/cm2 density) on the chemically burned rabbit model was observed by gross image. Corneal epithelium was observed at 0 and 7 days
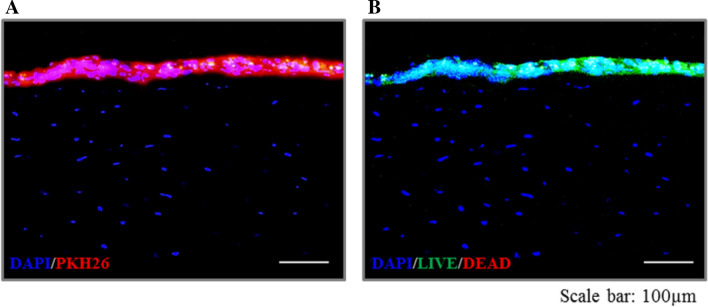
Evaluation of the FCSC-sheet location and viability in vivo
To investigate the location of the FCSCs-sheets in the defect area in vivo, FCSCs-sheets were prepared using PKH26-labeled FCSCs and in vivo experiments were conducted (Fig. 6). In order to confirm the viability of the FCSCs in the attached FCSC-sheet, a live/dead assay was performed after the in vivo experiments. The PKH26 experiments showed that the FCSC-sheet was still attached to the rabbit eyes more than seven days after in vivo placement. In addition, the live/dead assay results showed that most FCSCs survived well in the attached FCSC-sheets (Fig. 6).
Fig. 6.
Evaluation of FCSC-sheet (3 × 105 cell/cm2 density) location and viability in the chemically burned rabbit model 7 days post-transplantation. A The location of the PKH26 labeled FCSCs-sheet in the defect area. B Live/dead assay for viability of FCSCs in the defect area. Scale bar = 100 µm
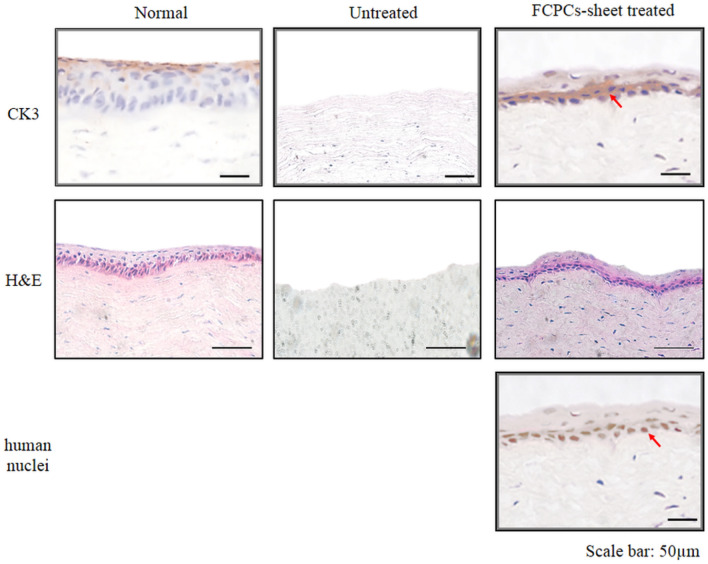
Histology and immunohistochemical analyses
To investigate the cellular characteristics of the FCSCs-sheet in vivo, samples were collected and immunohistochemistry was performed after the in vivo experiment (Fig. 7). In the normal eye group, the corneal epithelial cells were regularly and compactly arranged and CK3, a corneal epithelium differentiation marker, was expressed. In the untreated group, corneal epithelium was not present because of the NaOH burns and the analyses confirmed that the stroma was damaged by the corneal epithelial burn. However, in the FCSC-sheet-treated group, there was a portion of the corneal epithelium that was regularly arranged, similar to normal corneal epithelial cells. In addition, human immunohistochemical nuclear staining was performed to confirm the origin of the cells present in this region. The staining confirmed that the FCSCs of FCSC-sheet moved to the interface between the sheet and the stroma and were rearranged (Fig. 7).
Fig. 7.
The healing ability of the chemically burned rabbit model was evaluated by CK3, a corneal epithelial differentiation marker 7 days post-transplantation. The location of the FCSCs (red arrow) in the defect area was observed by anti-human nuclei antibody. Scale bar = 25 µm. The cell arrangement was confirmed by H and E staining. Scale bar = 50 µm
Discussion
Multipotent MSCs isolated from different tissues of an adult organism are attractive alternatives for regeneration of injured and/or deficient cells and tissues [20]. These cells have been reported to be immunocompetent, as well as immunosuppressive [21–23]. Hence, these cells may represent ideal candidates for transplantation. MSC isolated from human bone marrow have already been used to reconstruct the ocular surface of an animal model of limbal stem cell deficiency induced by a chemical burn [24–28]. Although these cells were detected in the grafted area after transplantation, their differentiation into corneal epithelium was not confirmed. However, only a small number of injected cells remained in the corneal injury site and this has been a limitation of cell supplementation by injection for a long time [29].
Previous studies have shown that FCSCs are novel stem-like cells with greater proliferation, differentiation (chondrogenesis, adipogenesis, osteogenesis), and anti-inflammation activity [15, 30]. This study showed that FCSCs can be differentiated to corneal epithelial cells (Fig. 1). During the differentiation process, identifiable cell morphology changes were seen (Fig. 1A). From the original morphology, spindle-like shaped FCSCs were changed to polygonal shaped corneal epithelial cell morphology at seven days in differentiation medium. In addition, protein expression was used to identify the ability of FCSCs to differentiate. Western blots indicated that the FCSCs in defined medium expressed ABCG2, p63, PAX6, and CK3 (Fig. 1D), indicating that the FCSCs differentiated to ectodermal lineages from mesodermal lineages [24–28].
Many studies of tissue engineered sheet transplantation reported a requirement for additional procedures to harvest the membrane and to implant the stem cells into the membrane as cell carriers [31]. In addition, severe tear-film and lid abnormalities often associated with these diseases continue to be a challenge, since immunologically driven inflammation of the ocular surface persists chronically in these patients [32]. Compared to these complex and cumbersome methods, FCSCs formed into sheets by themselves, depending on the culture conditions (Figs. 2, 3). FCSC-sheet fabrication is a novel approach that can improve corneal regeneration.
Our study showed that tissue cell sheets engineered from FCSCs may serve as effective substitutes for allografts of limbal tissue in the reconstruction of corneal and limbal surfaces. The transparency of the carrier-free sheets of tissue-engineered epithelial cells fabricated from FCSC was found to have a light transmission approximately equivalent to 76% of normal rabbit corneal tissues (Fig. 4). During the follow-up period, all corneal surfaces remained transparent and there were no serious complications. According to previous studies, conjunctival epithelial cells invade the cornea after allogeneic transplantation because of the gradual depletion of allogeneic corneal epithelial cells due to epithelial rejection or stem-cell depletion [33, 34]. In our study, stromal vascularization was observed only in the defect group. Cartilage is a typical avascular tissue that exhibits powerful resistance to angiogenesis or vascular invasion [35].
We observed that the transplanted cell sheets became more transparent and achieved smoother, integrated surfaces on the corneal stroma, further resembling normal corneal epithelium in in vivo rabbit models (Fig. 5). Ultimately, the adhesive ability and corneal epithelial cell differentiation of the FCSC-sheet significantly improved eye injury healing (Figs. 6, 7). Thus, the FCSC-sheet could be a useful treatment for corneal epithelium repair of chemical burns. To the best of our knowledge, this is the first study to demonstrate the inherent adhesion ability and ability of FCSC cells to differentiate into corneal epithelial cells, which can be used to treat chemically burned corneas.
In conclusion, FCSC was successfully able to differentiate into corneal epithelial cells. The implanted cell sheet maintained its transparency and the cells were shown to be viable after one week of implantation. As a result, it is thought that FCSC-sheet improves repair of damaged corneal surface by producing an efficient cell sheet. Long-term follow-up and additional experience are needed to further assess the benefits and risks of this method, which offers the potential to treat severe ocular diseases that are resistant to standard approaches.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by a Grant of the Korea Health Technology R&D Project funded by the Ministry of Health & Welfare, Republic of Korea (HI17C2191).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical statement
The study was approved by the institutional review board (IRB) of the Ajou University Medical Center (AJIRB-CRO-07-139) and was carried out with the informed consent of all donors. All applicable international, national and/or institutional guidelines for the care and use of animals were followed. All animal studies were performed in compliance with the regulations and guidelines of the Ajou University institutional animal care committee and conducted according to AAALAC and IACUC guidelines. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
In Su Park and Byeong Kook Kim contributed equally to this work.
References
- 1.Belknap EB. Corneal emergencies. Top Companion Anim Med. 2015;30:74–80. doi: 10.1053/j.tcam.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Mahdavi SS, Abdekhodaie MJ, Mashayekhan S, Baradaran-Rafii A, Djalilian AR. Bioengineering approaches for corneal regenerative medicine. Tissue Eng Regen Med. 2020;17:567–593. doi: 10.1007/s13770-020-00262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venugopal B, Mohan S, Kumary TV, Anil Kumar PR. Peripheral blood as a source of stem cells for regenerative medicine: emphasis towards corneal epithelial reconstruction: an in vitro study. Tissue Eng Regen Med. 2020;17:495–510. doi: 10.1007/s13770-020-00273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couture C, Zaniolo K, Carrier P, Lake J, Patenaude J, Germain L, et al. The tissue-engineered human cornea as a model to study expression of matrix metalloproteinases during corneal wound healing. Biomaterials. 2016;78:86–101. doi: 10.1016/j.biomaterials.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Yin J, Jurkunas U. Limbal stem cell transplantation and complications. Semin Ophthalmol. 2018;33:134–141. doi: 10.1080/08820538.2017.1353834. [DOI] [PubMed] [Google Scholar]
- 6.Albert R, Veréb Z, Csomós K, Moe MC, Johnsen EO, Olstad OK, et al. Cultivation and characterization of cornea limbal epithelial stem cells on lens capsule in animal material-free medium. PLoS One. 2012;7:e47187. doi: 10.1371/journal.pone.0047187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behaegel J, Zakaria N, Tassignon MJ, Leysen I, Bock F, Koppen C, et al. Short- and long-term results of xenogeneic-free cultivated autologous and allogeneic limbal epithelial stem cell transplantations. Cornea. 2019;38:1543–1549. doi: 10.1097/ICO.0000000000002153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramírez BE, Sánchez A, Herreras JM, Fernández I, García-Sancho J, Nieto-Miguel T, et al. Stem cell therapy for corneal epithelium regeneration following good manufacturing and clinical procedures. Biomed Res Int. 2015;2015:408495. doi: 10.1155/2015/408495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 10.Krishnan S, Iyer GK, Subramanian K. Culture and characterisation of limbal epithelial cells and oral mucosal cells. Indian J Med Res. 2010;131:422–428. [PubMed] [Google Scholar]
- 11.Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–2402. doi: 10.1182/blood.V98.8.2396. [DOI] [PubMed] [Google Scholar]
- 12.Fan CG, Tang FW, Zhang QJ, Lu SH, Liu HY, Zhao ZM, et al. Characterization and neural differentiation of fetal lung mesenchymal stem cells. Cell Transplant. 2005;14:311–321. doi: 10.3727/000000005783983070. [DOI] [PubMed] [Google Scholar]
- 13.Jo CH, Kim OS, Park EY, Kim BJ, Lee JH, Kang SB, et al. Fetal mesenchymal stem cells derived from human umbilical cord sustain primitive characteristics during extensive expansion. Cell Tissue Res. 2008;334:423–433. doi: 10.1007/s00441-008-0696-3. [DOI] [PubMed] [Google Scholar]
- 14.Tamaki S, Eckert K, He D, Sutton R, Doshe M, Jain G, et al. Engraftment of sorted/expanded human central nervous system stem cells from fetal brain. J Neurosci Res. 2002;69:976–986. doi: 10.1002/jnr.10412. [DOI] [PubMed] [Google Scholar]
- 15.Choi WH, Kim HR, Lee SJ, Jeong N, Park SR, Choi BH, et al. Fetal cartilage-derived cells have stem cell properties and are a highly potent cell source for cartilage regeneration. Cell Transplant. 2016;25:449–461. doi: 10.3727/096368915X688641. [DOI] [PubMed] [Google Scholar]
- 16.Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 2000;113:1161–1166. doi: 10.1242/jcs.113.7.1161. [DOI] [PubMed] [Google Scholar]
- 17.Götherström C, Ringdén O, Tammik C, Zetterberg E, Westgren M, Le Blanc K. Immunologic properties of human fetal mesenchymal stem cells. Am J Obstet Gynecol. 2004;190:239–245. doi: 10.1016/j.ajog.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 18.Götherström C, Ringdén O, Westgren M, Tammik C, Le Blanc K. Immunomodulatory effects of human foetal liver-derived mesenchymal stem cells. Bone Marrow Transplant. 2003;32:265–272. doi: 10.1038/sj.bmt.1704111. [DOI] [PubMed] [Google Scholar]
- 19.Maeng YS, Lee B, Choi SI, Kim TI, Kim EK. Role of TGFBIp in wound healing and mucin expression in corneal epithelial cells. Yonsei Med J. 2017;58:423–431. doi: 10.3349/ymj.2017.58.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li F, Zhao SZ. Mesenchymal stem cells: potential role in corneal wound repair and transplantation. World J Stem Cells. 2014;6:296–304. doi: 10.4252/wjsc.v6.i3.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32:252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrachina L, Remacha AR, Romero A, Vázquez FJ, Albareda J, Prades M, et al. Priming equine bone marrow-derived mesenchymal stem cells with proinflammatory cytokines: implications in immunomodulation-immunogenicity balance, cell viability, and differentiation potential. Stem Cells Dev. 2017;26:15–24. doi: 10.1089/scd.2016.0209. [DOI] [PubMed] [Google Scholar]
- 23.García-Sancho J, Sánchez A, Vega A, Noriega DC, Nocito M. Influence of HLA matching on the efficacy of allogeneic mesenchymal stromal cell therapies for osteoarthritis and degenerative disc disease. Transplant Direct. 2017;3:e205. doi: 10.1097/TXD.0000000000000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu S, Xing C, Han J, Tso MO, Hong J. Differentiation of rabbit bone marrow mesenchymal stem cells into corneal epithelial cells in vivo and ex vivo. Mol Vis. 2009;15:99–107. [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang TS, Cai L, Ji WY, Hui YN, Wang YS, Hu D, et al. Reconstruction of the corneal epithelium with induced marrow mesenchymal stem cells in rats. Mol Vis. 2010;16:1304–1316. [PMC free article] [PubMed] [Google Scholar]
- 26.Katikireddy KR, Dana R, Jurkunas UV. Differentiation potential of limbal fibroblasts and bone marrow mesenchymal stem cells to corneal epithelial cells. Stem Cells. 2014;32:717–729. doi: 10.1002/stem.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nieto-Miguel T, Galindo S, Reinoso R, Corell A, Martino M, Pérez-Simón JA, et al. In vitro simulation of corneal epithelium microenvironment induces a corneal epithelial-like cell phenotype from human adipose tissue mesenchymal stem cells. Curr Eye Res. 2013;38:933–944. doi: 10.3109/02713683.2013.802809. [DOI] [PubMed] [Google Scholar]
- 28.Zhu J, Zhang K, Sun Y, Gao X, Li Y, Chen Z, et al. Reconstruction of functional ocular surface by acellular porcine cornea matrix scaffold and limbal stem cells derived from human embryonic stem cells. Tissue Eng Part A. 2013;19:2412–2425. doi: 10.1089/ten.tea.2013.0097. [DOI] [PubMed] [Google Scholar]
- 29.Fernandes-Cunha GM, Na KS, Putra I, Lee HJ, Hull S, Cheng YC. Corneal wound healing effects of mesenchymal stem cell secretome delivered within a viscoelastic gel carrier. Stem Cells Transl Med. 2019;8:478–489. doi: 10.1002/sctm.18-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sardesai VS, Shafiee A, Fisk NM, Pelekanos RA. Avoidance of maternal cell contamination and overgrowth in isolating fetal chorionic villi mesenchymal stem cells from human term placenta. Stem Cells Transl Med. 2017;6:1070–1084. doi: 10.1002/sctm.15-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan BP, Leong KW. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur Spine J. 2008;17 Suppl 4:467–79. [DOI] [PMC free article] [PubMed]
- 32.Gomes JA, Geraldes Monteiro B, Melo GB, Smith RL, Cavenaghi Pereira da M, Lizier NF, et al. Corneal reconstruction with tissue-engineered cell sheets composed of human immature dental pulp stem cells. Invest Ophthalmol Vis Sci. 2010;51:1408–1414. doi: 10.1167/iovs.09-4029. [DOI] [PubMed] [Google Scholar]
- 33.Hanson C, Hardarson T, Ellerström C, Nordberg M, Caisander G, Rao M, et al. Transplantation of human embryonic stem cells onto a partially wounded human cornea in vitro. Acta Ophthalmol. 2013;91:127–130. doi: 10.1111/j.1755-3768.2011.02358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shortt AJ, Secker GA, Rajan MS, Meligonis G, Dart JK, Tuft SJ, et al. Ex vivo expansion and transplantation of limbal epithelial stem cells. Ophthalmology. 2008;115:1989–1997. doi: 10.1016/j.ophtha.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 35.Shukunami C, Hiraki Y. Role of cartilage-derived anti-angiogenic factor, chondromodulin-I, during endochondral bone formation. Osteoarthritis Cartilage. 2001;9 Suppl A:S91-101. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.