Abstract
BACKGROUND:
Different methods have been used to inject stem cells into the eye for research. We previously explored the intravitreal route. Here, we investigate the efficacy of intravenous and subretinal-transplanted human dental pulp stem cells (DPSCs) in rescuing the photoreceptors of a sodium iodate-induced retinal degeneration model.
Methods:
Three groups of Sprague Dawley rats were used: intervention, vehicle group and negative control groups (n = 6 in each). Intravenous injection of 60 mg/kg sodium iodate (day 0) induced retinal degeneration. On day 4 post-injection of sodium iodate, the rats in the intervention group received intravenous DPSC and subretinal DPSC in the right eye; rats in the vehicle group received subretinal Hank’s balance salt solution and intravenous normal saline; while negative control group received nothing. Electroretinogram (ERG) was performed to assess the retinal function at day 0 (baseline), day 4, day 11, day 18, day 26, and day 32. By the end of the study at day 32, the rats were euthanized, and both their enucleated eyes were sent for histology.
Results:
No significant difference in maximal ERG a-wave (p = 0.107) and b-wave, (p = 0.153) amplitude was seen amongst the experimental groups. However, photopic 30 Hz flicker amplitude of the study eye showed significant differences in the 3 groups (p = 0.032). Within the intervention group, there was an improvement in 30 Hz flicker ERG response of all 6 treated right eyes, which was injected with subretinal DPSC; while the 30 Hz flicker ERG of the non-treated left eyes remained flat. Histology showed improved outer nuclear layer thickness in intervention group; however, findings were not significant compared to the negative and vehicle groups.
Conclusion:
Combination of subretinal and intravenous injection of DPSCs may have potential to rescue cone function from a NaIO3-induced retinal injury model.
Keywords: Dental pulp, Mesenchymal stem cell, Sodium iodate, Sprague-Dawley rats, Electroretinography, Degenerated retina
Introduction
Dystrophic diseases of the retinal pigment epithelium (RPE) and photoreceptors, for instance retinitis pigmentosa (RP), can cause severe visual impairment and ultimately blindness. Up till now, there has been no effective treatment to prevent or cure a majority of these diseases.
Stem cell therapy has recently emerged as a promising therapy for retinal degeneration or dystrophy. Stem cells are biological cells which are undifferentiated. They, have the capability to differentiate into cells with specialised functions such as new photoreceptors or RPE [1, 2] which may restore the visual function in these diseases. Stem cells can be found in several tissues, including bone marrow [2–4], adipose tissue [5, 6], umbilical cord tissue [1], and also in dental pulp tissue [7–9]. Various types of stem cells have been investigated. Both bone marrow-derived (BMSCs) and adipose tissue-derived stem cells (ADSCs) did not show any differentiation into photoreceptors and RPE when transplanted into the vitreous or subretinal space [2–6]. Embryonic stem cells (ESCs) have shown great potential for cell replacement therapy in retinal diseases. Previous studies reported that the ESCs were able to differentiate into photoreceptors and RPE and transplant into the eye [10–13]. However, the transplanted cells have short survival periods and failed to show integration into retina [10, 13]. The paracrine effect of ESCs has yet to be determined [7]. Furthermore, ESCs may undergo genetic changes which increase the risk of malignant transformation and thus, undifferentiated ESCs cannot be simply transplanted into the eye [14]. Therefore, a better source of these stem cells becomes necessary. Dental pulp stem cells (DPSCs) were selected to investigate their utility as a stem cell source. In our previous study, we injected DPSCs into the vitreous space of Sprague-Dawley rats and measured the ERG b waves, which revealed visual improvements in the sodium iodate-treated rats [8].
DPSCs are retrieved from dental pulp of third adult molars (wisdom tooth) collected during routine dental procedures. DPSCs represent a source of mesenchymal stem cells which are easy to collect albeit with the loss of one tooth. They also represent a form of autologous stem cell source. DPSCs have high proliferating capacity with wide variety of differentiation potential. They can differentiate into odontoblast, osteoblast, chondrocytes, adipocytes, endotheliocytes, melanocytes and many other cell lines [9]. Recent studies have shown neural-regeneration properties of DPSCs. For instance, Sakai et al. have demonstrated some functional recovery in the rat with complete transection of spinal cord after transplanting human DPSC, and are more potent than bone marrow-derived mesenchymal stem cells (BMSCs) [15]. Leong et al. had demonstrated significant improvements in forelimb sensorimotor functions four weeks after intracerebral transplantation of DPSCs following focal cerebral ischemia in rodents [16]. Fang et al. also had demonstrated significant improvements in behavioural test following intraventricular transplantation of DPSCs in hypoxic-ischemic brain damaged neonatal rats [17]. Mead et al. have shown that DPSCs enhance the survival and neuritogenesis of retinal cells in an in-vitro culture assay with retinal primary cultures from adult rats, and axongenesis of retinal ganglion cells in an in-vivo model of optic nerve crush, which is more effective compared to BMSCs [18]. Mead et al. also showed that intravitreal DPSCs preserve the retinal ganglion cells (RGC) function by slowing the RGC cell loss in a rodent model of glaucoma [19]. Studies have suggested that DPSCs mostly arise from cranial neural crest, as Nestin, S-100, P75, HNK-1 and GFAP, which are markers for neural crest stem cells are expressed [20, 21]. This may explain the neuro-regeneration properties of DPSC and, therefore, their role in treatment of neurodegenerative diseases.
DPSCs are known to express and secrete a number of neurotrophic factors. These include nerve growth factor (NGF), neurotrophin-3 (NT-3), glial cell-line derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) [15, 18, 22, 23]. Recent studies have even shown that these trophic factors are two to three folds higher than levels present in BMSC cultures [18, 22] and are responsible for neuronal protection and neuronal differentiation. BDNF is a neuroprotective protein which binds to Trkb receptors which stimulates intracellular signalling and promotes synaptic localization, synaptic stabilization, dendritogenesis, and neurogenesis. BDNF, therefore, has anti-apoptotic effects and neuroprotective effects [3, 4].
A study by Bray et al. was able to show that DPSCs in-vitro had the ability to respond to environmental cues from rat retina and differentiate into retinal neuronal markers. DPSCs exposed to conditioned medium of damaged retina exhibited immunopositivity for rhodopsin [23]. Despite many studies in animal models on various nerve tissues [15–19], there is no in-vivo evidence of DPSCs-mediated retinal stem-cell therapy. In our study, the potential rescue effect of human DPSCs in a sodium iodate (NaIO3)-induced retinal degeneration model was investigated.
Materials and Methods
Animal models
The Sprague Dawley (SD) rats were housed at facilities of the Department of Ophthalmology, Universiti Kebangsaan Malaysia Medical Centre (UKMMC) with standard animal care conditions which followed the regulations of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The ethics approval was obtained from UKM Animal Ethical Committee (Reference code: UKM FPR.4/244/FF-2017-096). The SD rats were obtained from the animal house facility in UKM Kuala Lumpur campus at around 5 weeks of age. They were caged in Individually Ventilated Cages (IVC) of the animal biobubble facility at the Tissue Engineering Department, UKMMC. All the rats used in the study were males, 6–8 weeks of age and weighed 150 to 200 g. They were tagged with numbers and their weight and activity were monitored and recorded every other day. Eighteen rats were allocated for the study.
Sample size was calculated using the crude method based on the law of diminishing returns [24].
-
E = Number of animals (total) − Number of groups (total)
(number of groups = 3 groups, 6 rats per group)
E = 18 – 3 = 15 (10 number per group);
E = should be between 10–20.
Thus 18 rats with 6 rats in each group were calculated.
Several previous similar studies used Royal College of Surgeons (RCS) rats [1, 25]. RCS rats inherited a tyrosine kinase gene Mertk receptor mutation which results in inability of the RPE to phagocytise the photoreceptor outer segment and subsequently causes pigment corpuscles to accumulate in subretinal space [26]. NaIO3 on the other hand triggers caspase-dependent narcotic apoptosis in RPE, and concomitant photoreceptor degeneration [27]. Both these animal models share similar pathology with defects in the RPE to phagocytise the photoreceptor outer segment and lead to photoreceptor degeneration. This made the animal model with NaIO3-induced retinal degeneration an easily available alternative animal model for this current study. Therefore, NaIO3-induced retinal degeneration SD rats were used in this study. The concentration of 60 mg was selected for this study. This was based on work by Koh et al. who investigated the ERG responses following various concentrations of NaIO3 in SD rats recently [28].
Electroretinogram (ERG) examination
All ERG recordings were performed using animal ERG machine RETI-port Roland-consult GmBH of Brandenburg, Germany. Dark adaptation of the rats was performed for 1 h prior to the test. This was followed by administration of rat intramuscular ketamine (150 mg/kg) (Bioketan, Vetoquinol Biowet, Poland) and xylazine (10 mg/kg) (Xylazil-20 Troy Laboratories Australia Pty Ltd., Glendenning NSW, Australia) for anaesthesia. Their eyes were dilated with tropicamide 0.5% (Alcon, Fort Worth, TX, USA). Topical proparacaine hydrochloride 0.5% eye drops (Alcon) were instilled into both eyes to anesthetize the cornea and reduce blinking reflex. In order to maintain a body temperature of 36 °C, a w warm blanket was applied as they lay on the table. Electrodes were attached to the tail as ground, ear as reference and both corneas with a loop wire. The room had to be completely dark for the ERG recordings. Dim red lights were used as a guide. A full-field ERG recording was obtained with stimulation produced by LED light source. The intensity of the light source ranged from 0.0003 to 3.0 cd.s/m2 which allowed a general retinal response to be obtained. An average of 8 to 12 responses were taken to obtain a consistent response. A light adapted 30 Hz flicker stimulation at 3 cd.s/m2 was used to obtain a cone response.
The ERG examinations were performed on day 0 prior to chemical-induction of retinal degeneration, day 4 to confirm retinal degeneration prior to stem cell transplantation and at days-11, 18, 26 and 32 to determine retinal function following transplantation of cells. Subsequently, the animals were euthanized, and the eyes enucleated for histological examination.
Chemically-induced retinal degeneration in SD rats using sodium iodate
There were 3 groups namely the negative control, vehicle group and treatment group) with six rats in each group (n). Negative control rats received sodium iodate at day 0 without any further subretinal or intravenous injection. Vehicle group rats received sodium iodate at day 0, followed by subretinal Hank’s balance salt solution (HBSS) injection at day 4 and intravenous normal saline injection at day 4 and day 18. In the intervention group, the rats received sodium iodate at day 0, followed by subretinal injection of human DPSCs at day 4 and intravenous injection of human DPSCs at day 4 and day 18.
Sodium iodate (Alfa Aesar, Heysham, UK) was injected intravenously through the lateral tail vein with a 24 G intravenous catheter. An intravenous catheter was used to ensure a precise dose of sodium iodate is injected. The compound which is in powder form is first diluted in normal saline to obtain a 3% concentration. This is then administered at a dosage of 60 mg/kg to cause retinal degeneration [28].
Injection of stem cells was done on day 4 as suggested by Koh et al. in which injection of sodium iodate would attain a gross retinal degeneration with diminished ERG responses by day 4 [28].
Human Dental Pulp Stem Cells (DPSCs) preparation for transplantation
A privately-owned stem cell bank in Malaysia (Cryocord™, Selangor, Malaysia) provided the single vial of primary DPSCs. This was placed in liquid nitrogen for preservation until use. In order to expand the cell population, the DPSC vial was first placed in a water bath at 37 °C for 1 min to thaw it. Next 9 ml of fresh culture media was added to the 1 ml cell suspension at 37 °C to dilute it in a 15 ml centrifuge tube. The culture media consisted of Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 High Glucose (DMEM/F12, Gibco, Grand Island, NY, USA) which was supplemented with 10% foetal bovine serum (FBS) (Thermo Fisher Scientific, Waltham, MA, USA) and 1% antibiotic (penicillin, streptomycin) (Thermo Fisher Scientific). Centrifugation of the mixture was performed at 1200 rpm for 5 to 10 min. The supernatant was then collected with an aspirator and this pellet was then re-suspended in 1 ml of fresh culture media. In order to count the cells manually, the cells were stained with Trypan-blue and placed on a scientific counting chamber. Subsequently, DPSCs were seeded at an approximate concentration of 3000 cells per cm2 into T75 culture flasks. A change of culture medium was performed every 3–4 days until adherent DPSCs achieved confluency of 80%. When this percentage was reached, a standard protocol pf cell passage was carried out. In short, the used media was removed, and phosphate buffered saline (PBS) (Gibco) was added to rinse the cells followed with 0.025% trypsin (Gibco). Incubation of this preparation for 5 to 10 min in a humidified cell incubator was performed at a temperature of 37 °C with 5% CO2, and the flask of contents was softly tapped to separate cells adherent to the flask from it. The cell count was repeated to ascertain cell numbers following trypsinization. Lastly, seeding of cells into T75 culture flasks at a concentration of 3,000 cells per cm2 occurred. Prior characterization was performed and reported in our previous study [8].
Transplantation of human DPSC
In the intervention group, the SD rats are injected with DPSCs intravenously twice on day-4 and day 18, as well as subretinally on day 4. Intramuscular injection of ketamine (150 mg/kg) and xylazine (10 mg/kg) was used for anaesthesia prior to transplantation. Subretinal injection was performed under an operating microscope (ZEISS, Oberkochen, Germany). Only right eyes received the injection. Anaesthesia of the eye with proparacaine hydrochloride 0.5% eye drops was given to suppress the corneal reflex. The skin around the eye was then stretched gently and pulled backward to proptose the eye for injection. A drop of normal saline was applied to the injection site on the supero-temporal conjunctiva to wash off any debris. The conjunctiva 1 mm behind the limbus was gripped with forceps to make a “tent” and a cut on the “tent” was made with scissors. This exposed the underlying sclera and subsequently, a small trans-scleral cut was performed with a 30 G insulin needle (BD Biosciences, Bedford, MA, USA) to breach the scleral-choroidal complex for subretinal injection. A total of 3 μL of DPSCs cells (1 × 105/μL) was carefully delivered into the subretinal space with a 30 G, 10 μL Hamilton syringe (Hamilton Co., Reno, NV, USA) to avoid retinal detachment. The conjunctival incision was then sutured. Fundus examination was done following the injection, which revealed an area of retinal detachment which corresponded to the site of injection. Complications such as vitreous bleeding, total retinal detachment or collapsed globe following injection resulted in rats being excluded from the study. There were no complications in this study and no rats were excluded. Post-injection, dexamethasone/neomycin (Maxitrol™, Alcon) was applied on the injection site.
At the same time, the rats were injected with 200 μL of DPSC cells (1 × 106/μL) intravenously through the lateral tail vein with a 24 G intravenous catheter. The intravenous injection of human DPSCs was repeated on day 18 with the same amount of DPSCs as a booster. The dose of the DPSCs injected was selected because of results from a previous study conducted by Bakondi et al. in which a combination of subretinal and intravenous mesenchymal stem cells injections were adopted in an RCS rat [29]. To avoid rejection, all rats were injected with dexamethasone (1 mg/kg) (CCM, Duopharma Biotech Berhad, Kuala Lumpur, Malaysia) intraperitoneally, commencing with the day of the surgery, 3 times per week for the first 2 weeks. They also received cyclosporine-A (Bedford Labs, Bedford, MA, USA) added to their drinking water (210 mg/l; blood concentration range: 250–300 μg/l) starting from one day prior to the injection until euthanasia.
Euthanasia and enucleation for histology
On day 32 the rats were sacrificed, and the study eyes were enucleated. The enucleated eyes were prepared for histology. In more detail, ketamine (150 mg/kg) (Bioketan, Vetoquinol Biowet, Poland) and xylazine (10 mg/kg) (Xylazil-20 Troy Lab Australia) were used for anaesthesia. Subsequently the rats were euthanized with intravenous injection of sodium phenobarbital (15 mg/100 g body weight) (Sandgate, VT, USA). Enucleation was then performed with the globe separated from the optic nerve. Then enucleated eyes were placed immediately in optimal cutting temperature (OCT) and frozen at −20 °C. The frozen specimens were cut in horizontal sections of 4 μm thick with a cryostat and mounted on glass slides. The frozen sections were stained with haematoxylin and eosin. Image J software (National Institutes of Health, Bethesda, MD, USA) was used to measure the thickness of the outer nuclear layer (ONL) thickness. This was measured at three different sites. The three different sites were identified as upper periphery (200 μm from upper ora serrata), lower periphery (200 μm from lower ora serrata) and centre (200 μm from the optic disc).
Statistical analysis
This was performed using SPSS 23.0 and median and interquartile range (IQR) was used to present all the data. Given the small sample size and non-normally distributed data, non-parametric tests were used. Mann-Whitney test was performed to analyse the ERG readings in comparing the different groups. Kruskal-Wallis test was used to analyse ERG readings within groups. Statistical difference was considered significant when p value was less than 0.05.
Results
All 18 transplanted animals survived throughout the whole study with no complications noted as mentioned above.
In the negative control group, there was significant reduction of 3 cd.s/m2 (maximal) ERG a-wave amplitude (p = 0.004) from 135.5 (IQR 93.2–186.0) μV to 9.3 (IQR 6.6–12.0) μV and b-wave amplitude (p = 0.004) from 312.0 (IQR 250.0–383.0) μV to 24.2 (IQR 2.4–42.3) μV at day-4 post-injection of sodium iodate 60 mg/kg. There was no statistically significant difference in a-wave amplitude (p = 0.204) and b-wave amplitude (p = 0.258) in the sham group from day 4 to day 32, which indicated that the retina remained degenerated throughout the study after sodium iodate injection.
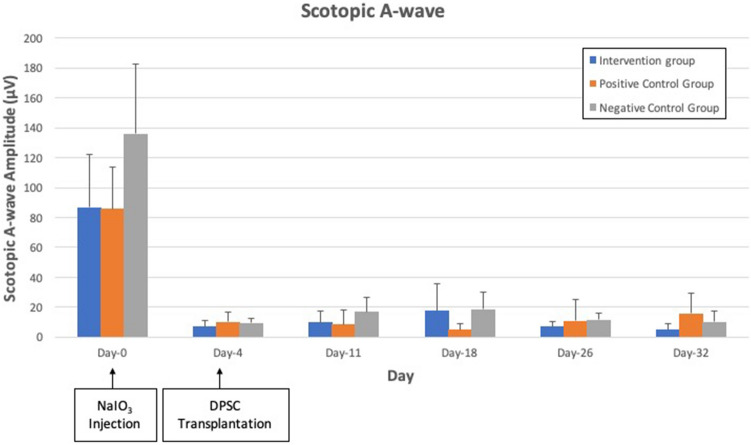
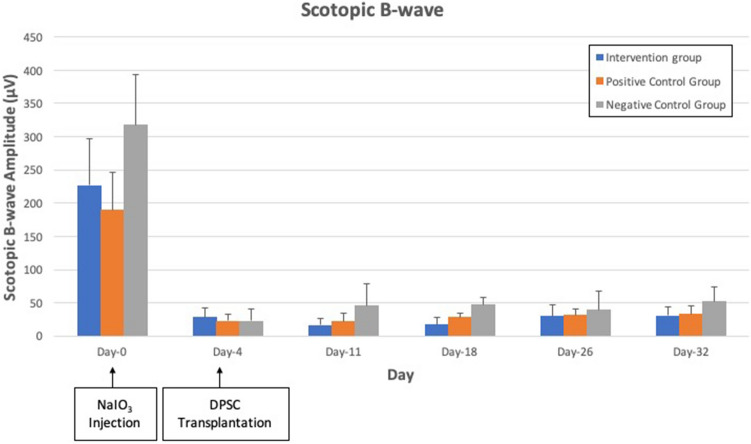
There was no significant difference in both maximal ERG a-wave (p = 0.107) and b-wave amplitude (p = 0.153) in all 3 groups. ERG responses of the three experimental groups over the study period are shown in Figs. 1, 2 and 3. Scotopic a-wave and b-wave amplitudes were similar in all 3 groups at all study time points.
Fig. 1.
Scotopic 3 cd.s/m2 a-wave amplitude of intervention group, vehicle group group and negative control group. There was reduction of the amplitude from day 4 to day 32 after NaIO3 injection in all 3 groups. No significant difference is seen in a-wave amplitude amongst the experimental groups at all points in time (p = 0.107)
Fig. 2.
Scotopic 3 cd.s/m2 b-wave amplitude in the intervention group, vehicle group group and negative control groups. There was reduction of the amplitude from day 4 to day 32 after NaIO3 injection in all 3 groups with no significant differences (p = 0.153)
Fig. 3.
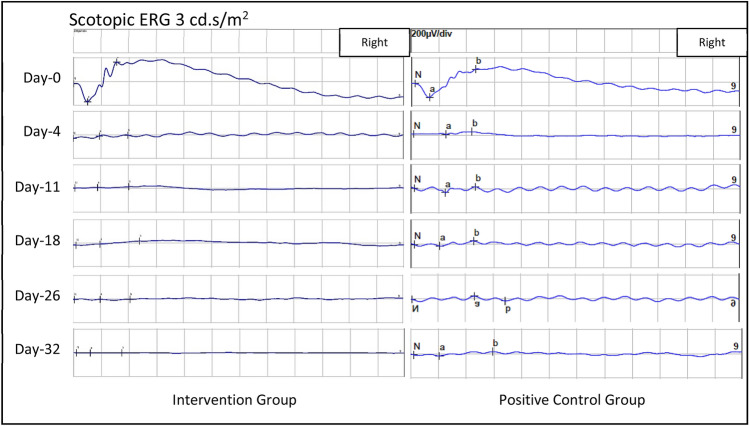
Scotopic 3 cd.s/m2 ERG strip of both the intervention group and negative control group throughout the study, showing similar responses at all study points
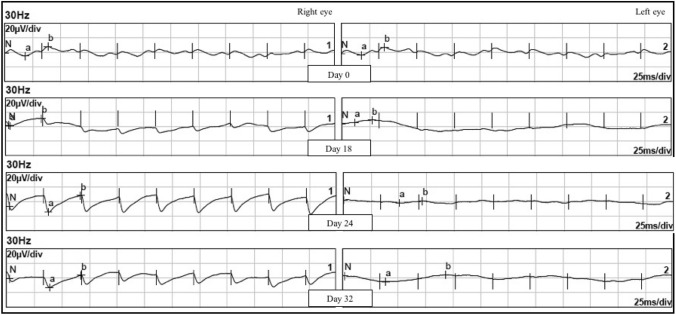
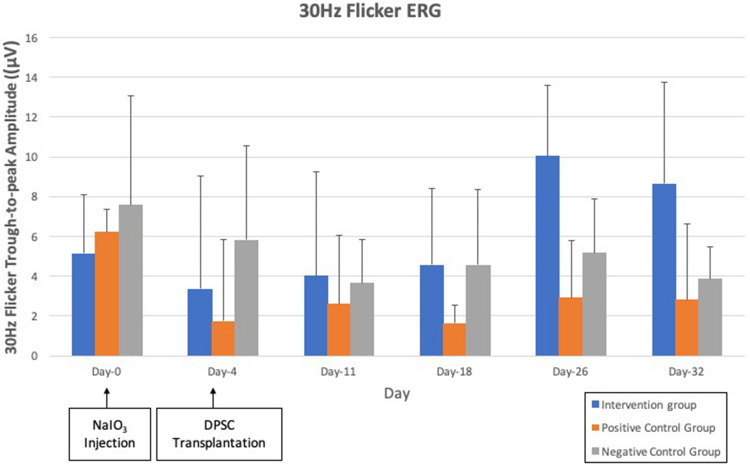
As for 30 Hz flicker ERG, interestingly, all 6 rats from the intervention group exhibited regular flicker waves upon day 18 to day 32 in the right eye only, which is the eye injected with subretinal DPSC, as shown in Fig. 4. The left eye which did not receive subretinal DPSC, in contrast, did not show any flicker ERG response throughout the study. Similarly, 30 Hz flicker ERG of both negative control group and vehicle group remained flat with no regular waveform throughout the study after injection of NaIO3. When the 3 groups were compared, there was a significant difference in trough-to-peak amplitude of the 30 Hz flicker ERG (p = 0.032) in Fig. 5. Post-hoc tests revealed significant differences in the intervention group and vehicle group (p = 0.000), as well as intervention and negative control group (p = 0.016).
Fig. 4.
30 Hz Flicker ERG of right eye and left eye of SD rat in intervention group. There were regular flicker waves appearing from day 18 onwards to day 32 over right eye only which is the eye injected with subretinal DPSC. However, the waveform was not similar to those at baseline on day 0. The left eye, on the other hand, remains flat throughout the study
Fig. 5.
30 Hz flicker ERG trough-to-peak amplitude of intervention group, vehicle group and negative control group with significant difference (p = 0.032)
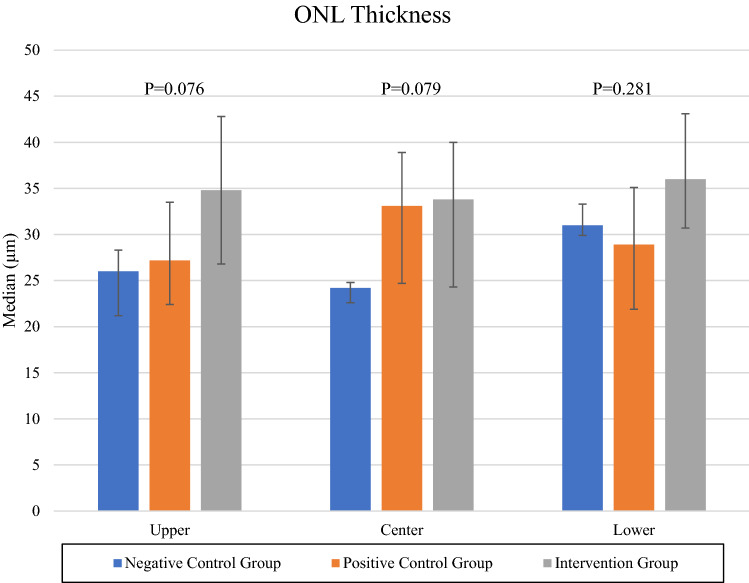
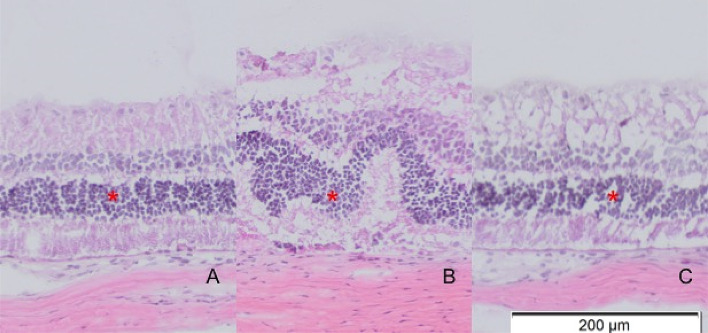
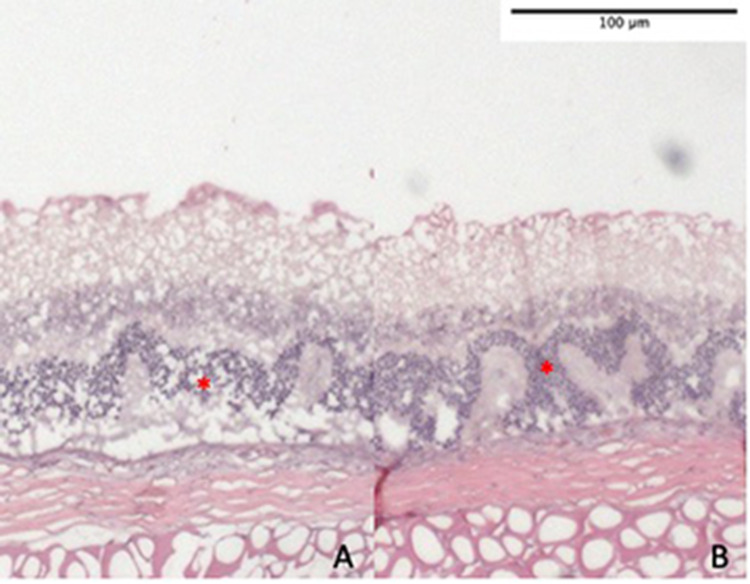
On histological examination, all 6 rats with transplanted DPSC showed thicker ONL at all three sites (upper periphery, centre and lower periphery) as shown in Fig. 6. Nevertheless, this was not statistically significant; p = 0.076 for upper periphery, p = 0.079 for centre, and p = 0.281 for lower periphery. In transplanted eyes, the peripheral retina showed better morphology and improvement in thickness compared to central retina as shown in Figs. 6 and 7, in which there was preservation of retinal ONL in peripheral retina as opposed to corrugation and disruption of the ONL in central retina. A similar corrugation and disruption of the ONL was seen throughout the retina in eyes from both the negative control group and vehicle group in Fig. 8.
Fig. 6.
ONL thickness of upper peripheral retina, central retina, and lower peripheral retina of both intervention group and negative control group
Fig. 7.
A–C Histology of intervention group showing some preservation of ONL thickness in upper peripheral retina (A) and lower peripheral retina (C) in comparison to central retina (B) with less corrugation and disruption of ONL indicated by red asterisks
Fig. 8.
A, B Histology of the central retina from negative controls (A) and vehicle groups (B) showing corrugation and thinning of ONL due to NaIO3 toxicity. ONL is indicated by red asterisks
Discussion
In our previous study, a single intravitreal transplantation of 1.0 × 105 cell/uL dental pulp stem cells was performed on the sodium iodate-induced rat model [8]. This was sufficient enough to reduce the degree of retinal degeneration up to a certain degree. However, this improvement was only temporary, and the rat visual function eventually deteriorated to the same level of the control. Hence, we hypothesized that a multimodal (subretinal and intravenous) delivery method, as shown by Bakondi et al. (2016), would lead to improved results [30]. In this study, the scotopic 3 cd.s/m2 ERG did not show response improvements in the negative controls, vehicle groups and intervention groups, illustrating that there is no significant recovery in general retinal function despite injection of DPSCs (Figs. 1, 2 and 3). Similarly, no statistically significant increase in ONL thickness in intervention group compared to negative controls was found (Fig. 6). In this study, only limited beneficial effects were observed as compared to our previous study. This could be attributed to several factors. The subretinal injection adopted in this study can only deliver limited amount of stem cells that are confined to the small space. This limits the regenerative properties of the stem cells to a localized area of the retina. The other possible factors involved in the limited beneficial effects observed here are low graft survival and blood-retinal barrier breach [31]. Due to the subretinal injection of a high number of DPSCs, there was a risk of breaching the blood retinal barrier. This could have resulted in the immunogenic activation of resident microglia that acted on the DPSCs and caused cell loss. In the case of intravenous infusion, it has been shown by several studies that systemically-administered stem cells are actively distributed in the liver, spleen, and even the lungs, thereby reducing the number of stem cells that can successfully home to a tissue of interest [29, 32]. Conversely, our previous study showed that intravitreal injection of DPSCs showed some improvements in ERG responses. The intravitreal route is not restricted by the space limitation of subretinal injections, and it may explain the differences observed [8]. Apart from that, the key difference between our study and Bakondi et al. (2016) is type of stem cells used [30]. Their study used native MSCs from the rats as allogeneic transplants, while our human derived DPSCs are xenogeneic. The species barrier can impede the effectiveness of our treatment. Still, other studies have shown stem cell competency through xenogeneic transplants of human cells into animal models of retinal disorders with varying rates of success [33, 34]. This is a possible limitation in our study. In our previous study, only the b waves were observed, whereas the current study is more in depth, involving the a waves as well. Leow et al. were able to show through the use of gold-loaded mesenchymal stem cells injected subretinally that there was no further migration of the gold-loaded mesenchymal stem cells to the rest of retina upon tracking with micro-computed tomography [1]. Bakondi et al. has likewise demonstrated the localized effects of subretinal mesenchymal stem cells injection with focal improvement in thickness of retina by histology examination [30]. Both the studies utilised a similar amount of stem cells as this current study. In our previous study, we were able to track DPSCs that were transplanted into the eye [8]. The current study, however, was more focused on the electrophysiological readings after DPSC transplantation. The inadequate amount of stem cells delivered and lack of migration of the stem cells may have led to the poor response. Furthermore, using human stem cells in this rat model instead of the rats’ own stem cells may not be effective due to the interspecies incompatibility, however, harvesting rat DPSCs poses another great challenge.
Despite previous research demonstrating that systemic transplantation of stem cells alone has limited or no migration into retina to exert its recovery effects [30], in this study, we introduce both intravenous and subretinal injections of DPSCs. The cells were characterized in our previous study [8]. As suggested by Bakondi et al., the combination of subretinal and intravenous injection of stem cells exerts a synergistic effect and produces a wider area of rescue and preservation of retina function [30]. Unfortunately, the results in this study is imperfect. The short study period may have contributed to the insignificant result as the retina may take longer than expected time to attain recovery. A longer study period may achieve a better result, as previous studies demonstrated a significant result for at least 60 days’ post-therapy [1, 25, 30]. Guan et al. had also demonstrated a significant ERG result only after 5 weeks’ post subretinal injection of mesenchymal stem cells upon weekly ERG examination up to 8 weeks [35]. The insignificant results may also be contributed by the small sample size.
Full-field ERG represents the gold-standard test in assessing the retina function, and it reflects the total retinal response to light stimulation [36]. In view of the focal area of recovery as suggested by previous studies with subretinal stem cell injection [1, 25], full field ERG may not be able to detect a small area of functional improvement. A focal or multifocal ERG may allow the assessment of a localized retina area for functional change. However, focal or multifocal ERG is more challenging to perform in view of the small rat eye, light scattering effect within the retina and self-adapting nature of the stimulus [37]. This type of ERG requires a longer time to perform and more detailed procedures as compared to full-field type.
In our previous study, the photopic 30 Hz flicker ERG response was not explored since rats have a dominant rod vision. Interestingly in this study, there was a significant recovery in the photopic 30 Hz flicker ERG response of the intervention group, in which there was presence of regular flicker waves with significant results in the photopic 30 Hz flicker trough-to-peak amplitude. This is newly reported in the current study. The flicker waves were only detected in their right eyes which received the DPSCs subretinally, while the left eye which did not receive subretinal DPSCs did not show any regular flicker waves. The flicker waves signify a cone pathway response. However, the waveforms were different from those at baseline. Previous studies mainly focused on scotopic ERG as it represents the whole retina response and the rat retina is predominantly occupied by rods [1, 25, 38]. The significant results reported may suggest that there were some changes in retinal circuitry with some cone function recovery following subretinal DPSC injections. The neurotrophic factors that were secreted by DPSCs may have exerted their neuroprotective effects on the cones and preserved their function. The cellular proliferation of limited amounts of DPSCs may not be enough to allow the restoration in function of a large population of rods. Another possible explanation is that some cones were probably spared from NaIO3 toxicity and DPSCs may have managed to revive the cone function. The rod damage, on the other hand, was irreversible. Similar findings have been reported by Girman et al. in which cone function was rescued upon subretinal RPE and Schwann cell grafting in RCS rats, while rod function remained compromised. The mechanism of cone rescue was otherwise unclear [39]. This interesting observation warrants further studies. We postulate that the recovery will be more apparent if observed longer. Previously, this recovery was not reported in our intravitreal DPSC injection study [8]. Thus, we advocate a longer study duration to ensure a more conclusive outcome in future.
Thickness of the ONL has been used to examine the recovery of the photoreceptors in retinal degeneration as this has been shown by several studies to be a useful measure of retinal function [1, 25, 38]. ONL thickness of the intervention group showed rise in thickness although this was not statistically significant. Among the negative controls and vehicle groups, there is gross disruption of outer retina with corrugation and loss of outer nuclear layer due to toxicity of NaIO3, which is consistent with previous findings [40–42]. In the intervention group, the corrugation pattern of the outer nuclear layer was less obvious especially at the peripheral retina. These morphological changes may indicate that DPSCs are able to differentiate into photoreceptors and RPE as well as preserve ONL. However, the non-significant findings may be due to the short follow up and small sample size as explained earlier. Despite the improvements in ONL thickness especially over the peripheral retina, the effect did not translate into improvements in scotopic ERG responses. This may be due to differentiated neuronal cells that have not integrated or synapsed with the remaining retinal cells, and therefore, the differentiated neuronal cells may not be functional. The integration and synaptic process may require a longer time; given the short duration of study, the process may have not been mature yet. Philips et al. conducted a laboratory study which showed that most genes critical for synaptic transmission were expressed significantly from day 78 onwards with pluripotent stem cells-derived retinal neurons induced from a human source [43].
Subretinal route of DPSC transplantation was performed in this study as we expected the stem cells could readily penetrate the outer retinal layer and salvage the damaged area. Previous studies have shown successful integration of differentiated stem cells following subretinal transplantation. However, it is technically more challenging with higher risk of retinal injury and therefore most studies adopt an intravitreal route. In intravitreal route, however, the stem cells have to penetrate the inner limiting membrane in order to migrate into the inner retina and subsequently outer retina [44, 45]. In spite of that, Qu et al. had shown a low transplantation rate into inner retina following intravitreal injection of human mesenchymal stem cells derived from the bone marrow [25]. The recovery of 30 Hz flicker ERG response in the right eye suggests that the subretinal route of DPSC transplantation plays a significant role in retinal regeneration due to its direct and localized effects on the outer retina.
In the intervention group, the SD rats are injected with DPSCs intravenously twice on day 4 and day 18, as well as subretinally on day 4. All rats survived to the end of the study without any obvious adverse effects, were able to maintain reasonable weight gain, and displayed no abnormal behaviour, similar to a previous study [23]. Therefore, DPSCs appear safe when administered systemically and locally with co-administration of immunosuppressants. NaIO3-induced retinal degeneration in SD rats can mimic retinal degeneration and act as a suitable animal model in research with stem cell therapy in retinal degeneration diseases as shown by the ERG responses and histology. This agrees with previous studies in which administration of high dose NAIO3 rendered both scotopic and photopic visual suppression and wide areas of retina damage [40, 42]. This again signifies that NaIO3 is an effective model of retinal degeneration and stem cell therapies. Previous studies also suggest a higher dose is required for intravenous administration compared to retro orbital venous-plexus administration [40–42]. Yang et al. and Yamashita et al. have suggested a dose between 50 mg/kg to 75 mg/kg via intravenous route to avoid systemic side effects and optimal retinal degeneration [36, 46]. In this study, 60 mg/kg of NAIO3 was injected intravenously through lateral tail vein [31].
One main limitation of this preliminary study is its short duration and small sample size. The study also lacks a control group for which normal saline is injected. Further studies with longer duration and larger sample size is therefore justified, and a few issues are required to be addressed. Immunohistochemistry may be helpful in identifying the in-vivo differentiation of the transplanted cells, particularly the rod and cone photoreceptors. The integration and synaptic connections of the differentiated cells with host retina also need to be determined. Subretinal injection may be performed at more than one site to achieve wider areas of treatment.
In conclusion, this study has found no significant improvements in maximal ERG. However significant results in 30 Hz flicker ERG amplitude was seen following administration of subretinal and intravenous DPSCs to SD rats who had received NaIO3 at doses shown to result in retinal degeneration. There was emergence of regular flicker in 30 Hz ERG in intervention groups only after 18 days of DPSC administration. This may suggest that a combination of subretinal and intravenous injection of DPSC has the potential to rescue cone function from a NaIO3-induced retinal injury model. The ONL thickness did not significantly increase after DPSC administration in this study.
Acknowledgement
The authors would like to acknowledge the funding provided by UKM through the Fundamental Grant awarded to Drs Mae-Lynn Catherine Bastion and Lam Chen Shen (Project Code FF 2017-096) and the Publication Grant awarded to Dr Mae-Lynn Catherine Bastion (Project Code GP-K009894). The authors also extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number 375213500. Last but not least, the authors acknowledge CryoCord Sdn Bhd for providing the DPSC.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
The ethics approval was obtained from UKM Animal Ethical Committee (Reference code: UKM FPR.4/244/FF-2017-096).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
References
- 1.Leow SN, Luu CD, Hairul Nizam MH, Mok PL, Ruhaslizan R, Wong HS, et al. Safety and efficacy of human Wharton’s Jelly-derived mesenchymal stem cells therapy for retinal degeneration. PLoS One. 2015;10:e0128973. doi: 10.1371/journal.pone.0128973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang Y, Zhang Y, Zhang L, Wang M, Zhang X, Li X. Therapeutic effect of bone marrow mesenchymal stem cells on laser-induced retinal injury in mice. Int J Mol Sci. 2014;15:9372–9385. doi: 10.3390/ijms15069372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Machalinska A, Roginska D, Pius-Sadowska E, et al. Neuroprotective and antiapoptotic activity of lineage-negative bone marrow cells after intravitreal injection in a mouse model of acute retinal injury. Stem Cells Int. 2015;2015:620364. doi: 10.1155/2015/620364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Wang W. Effects of bone marrow mesenchymal stem cell transplantation on light-damaged retina. Invest Ophthalmol Vis Sci. 2010;51:3742–3748. doi: 10.1167/iovs.08-3314. [DOI] [PubMed] [Google Scholar]
- 5.Haddad-Mashadrizeh A, Bahrami AR, Matin MM, Edalatmanesh MA, Zomorodipour A, Gardaneh M, et al. Human adipose-derived mesenchymal stem cells can survive and integrate into the adult rat eye following xenotransplantation. Xenotransplantation. 2013;20:165–176. doi: 10.1111/xen.12033. [DOI] [PubMed] [Google Scholar]
- 6.Hambright D, Park KY, Brooks M, McKay R, Swaroop A, Nasonkin IO. Long-term survival and differentiation of retinal neurons derived from human embryonic stem cell lines in un-immunosuppressed mouse retina. Mol Vis. 2012;18:920–936. [PMC free article] [PubMed] [Google Scholar]
- 7.Tucker BA, Park IH, Qi SD, Klassen HJ, Jiang C, Yao J, et al. Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice. PLoS One. 2011;6:e18992. doi: 10.1371/journal.pone.0018992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alsaeedi HA, Koh AE, Lam C, Rashid MBA, Harun MHN, Saleh MFBM, et al. Dental pulp stem cells therapy overcome photoreceptor cell death and protects the retina in a rat model of sodium iodate-induced retinal degeneration. J Photochem Photobiol B. 2019;198:111561. doi: 10.1016/j.jphotobiol.2019.111561. [DOI] [PubMed] [Google Scholar]
- 9.Banin E, Obolensky A, Idelson M, Hemo I, Reinhardtz E, Pikarsky E, et al. Retinal incorporation and differentiation of neural precursors derived from human embryonic stem cells. Stem Cells. 2006;24:246–257. doi: 10.1634/stemcells.2005-0009. [DOI] [PubMed] [Google Scholar]
- 10.Arnhold S, Klein H, Semkova I, Addicks K, Schraermeyer U. Neurally selected embryonic stem cells induce tumor formation after long-term survival following engraftment into the subretinal space. Invest Ophthalmol Vis Sci. 2004;45:4251–4255. doi: 10.1167/iovs.03-1108. [DOI] [PubMed] [Google Scholar]
- 11.Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Concise review: dental pulp stem cells: a novel cell therapy for retinal and central nervous system repair. Stem Cells. 2017;35:61–67. doi: 10.1002/stem.2398. [DOI] [PubMed] [Google Scholar]
- 12.Kawashima N. Characterisation of dental pulp stem cells: a new horizon for tissue regeneration? Arch Oral Biol. 2012;57:1439–1458. doi: 10.1016/j.archoralbio.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Sakai K, Yamamoto A, Matsubara K, Nakamura S, Naruse M, Yamagata M, et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest. 2012;122:80–90. doi: 10.1172/JCI59251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leong WK, Henshall TL, Arthur A, Kremer KL, Lewis MD, Helps SC, et al. Human adult dental pulp stem cells enhance post stroke functional recovery through non-neural replacement mechanisms. Stem Cells Transl Med. 2012;1:177–187. doi: 10.5966/sctm.2011-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang CZ, Yang YJ, Wang QH, et al. Intraventricular injection of human dental pulp stem cells improves hypoxic-ischemic brain damage in neonatal rats. PLoS One. 2013;8:e66748. doi: 10.1371/journal.pone.0066748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Invest Ophthalmol Vis Sci. 2013;54:7544–56. doi: 10.1167/iovs.13-13045. [DOI] [PubMed] [Google Scholar]
- 17.Mead B, Hill LJ, Blanch RJ, Ward K, Logan A, Berry M, et al. Mesenchymal stromal cell-mediated neuroprotection and functional preservation of retinal ganglion cells in a rodent model of glaucoma. Cytotherapy. 2016;18:487–96. doi: 10.1016/j.jcyt.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg M, Smith AJ. Cells and extracellular matrices of dentin and pulp: a biological basis for repair and tissue engineering. Crit Rev Oral Biol Med. 2004;15:13–27. doi: 10.1177/154411130401500103. [DOI] [PubMed] [Google Scholar]
- 19.Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, et al. A fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- 20.Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: comparison with human bone marrow and adipose-derived mesenchymal stem cells. PLoS One. 2014;9:e109305. doi: 10.1371/journal.pone.0109305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nosrat IV, Widenfalk J, Olson L, Nosrat CA. Dental pulp cells produce neurotrophic factors, interact with trigeminal neurons in vitro, and rescue motoneurons after spinal cord injury. Dev Biol. 2001;238:120–132. doi: 10.1006/dbio.2001.0400. [DOI] [PubMed] [Google Scholar]
- 22.Harper MM, Grozdanic SD, Blits B, Kuehn MH, Zamzow D, Buss JE, et al. Transplantation of BDNF-secreting mesenchymal stem cells provides neuroprotection in chronically hypertensive rat eyes. Invest Ophthalmol Vis Sci. 2011;52:4506–4515. doi: 10.1167/iovs.11-7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bray AF, Cevallos RR, Gazarian K, Lamas M. Human dental pulp stem cells respond to cues from the rat retina and differentiate to express the retinal neuronal marker rhodopsin. Neuroscience. 2014;280:142–155. doi: 10.1016/j.neuroscience.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 24.Charan J, Kantharia ND. How to calculate sample size in animal studies? J Pharmacol Pharmacother. 2013;4:303–306. doi: 10.4103/0976-500X.119726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qu L, Gao L, Xu H, Duan P, Zeng Y, Liu Y, et al. Combined transplantation of human mesenchymal stem cells and human retinal progenitor cells into the subretinal space of RCS rats. Sci Rep. 2017;7:199. doi: 10.1038/s41598-017-00241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strauss O, Stumpff F, Mergler S, Wienrich M, Wienrich M. The Royal College of Surgeons rat: an animal model for inherited retinal degeneration with a still unknown genetic defect. Acta Anat (Basel) 1998;162:101–111. doi: 10.1159/000046474. [DOI] [PubMed] [Google Scholar]
- 27.Balmer J, Zulliger R, Roberti S, Enzmann V. Retinal cell death caused by sodium iodate involves multiple caspase-dependent and caspase-independent cell-death pathways. Int J Mol Sci. 2015;16:15086–15103. doi: 10.3390/ijms160715086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koh AE, Alsaeedi HA, Rashid MBA, Lam C, Harun MHN, Saleh MFBM, et al. Retinal degeneration rat model: A study on the structural and functional changes in the retina following injection of sodium iodate. J Photochem Photobiol B. 2019;196:111514. doi: 10.1016/j.jphotobiol.2019.111514. [DOI] [PubMed] [Google Scholar]
- 29.Traverse JH. Is there a role for intravenous stem cell delivery in nonischemic cardiomyopathy? Circ Res. 2017;120:256–8. doi: 10.1161/CIRCRESAHA.116.310342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakondi B, Girman S, Lu B, Wang S. Multimodal delivery of isogenic mesenchymal stem cells yields synergistic protection from retinal degeneration and vision loss. Stem Cells Transl Med. 2017;6:444–457. doi: 10.5966/sctm.2016-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xian B, Huang B. The immune response of stem cells in subretinal transplantation. Stem Cell Res Ther. 2015;6:161. doi: 10.1186/s13287-015-0167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kraitchman DL, Tatsumi M, Gilson WD, Ishimori T, Kedziorek D, Walczak P, et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112:1451–1461. doi: 10.1161/CIRCULATIONAHA.105.537480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan D, Chang X, Xu M, Zhang M, Zhang S, Wang Y, et al. UMSC-derived exosomes promote retinal ganglion cells survival in a rat model of optic nerve crush. J Chem Neuroanat. 2019;96:134–139. doi: 10.1016/j.jchemneu.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Tu HY, Watanabe T, Shirai H, Yamasaki S, Kinoshita M, Matsushita K, et al. Medium- to long-term survival and functional examination of human iPSC-derived retinas in rat and primate models of retinal degeneration. EBioMedicine. 2019;39:562–574. doi: 10.1016/j.ebiom.2018.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guan Y, Cui L, Qu Z, Lu L, Wang F, Wu Y, et al. Subretinal transplantation of rat MSCs and erythropoietin gene modified rat MSCs for protecting and rescuing degenerative retina in rats. Curr Mol Med. 2013;13:1419–31. doi: 10.2174/15665240113139990071. [DOI] [PubMed] [Google Scholar]
- 36.Sothilingam V, Mühlfriedel R, Tanimoto N, Seeliger MW. In-depth functional analysis of rodents by full-field electroretinography. Methods Mol Biol. 2018;2018:207–213. doi: 10.1007/978-1-4939-7522-8_14. [DOI] [PubMed] [Google Scholar]
- 37.Ball SL, Petry HM. Noninvasive assessment of retinal function in rats using multifocal electroretinography. Invest Ophthalmol Vis Sci. 2000;41:610–617. [PubMed] [Google Scholar]
- 38.Johnson TV, Bull ND, Hunt DP, Marina N, Tomarev SI, Martin KR. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2010;51:2051–2059. doi: 10.1167/iovs.09-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Girman SV, Wang S, Lund RD. Time course of deterioration of rod and cone function in RCS rat and the effects of subretinal cell grafting: a light- and dark-adaptation study. Vision Res. 2005;45:343–354. doi: 10.1016/j.visres.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y, Ng TK, Ye C, Yip YW, Law K, Chan SO, et al. Assessing sodium iodate–induced outer retinal changes in rats using confocal scanning laser ophthalmoscopy and optical coherence tomography. Invest Ophthalmol Vis Sci. 2014;55:1696–705. doi: 10.1167/iovs.13-12477. [DOI] [PubMed] [Google Scholar]
- 41.Machalińska A, Lejkowska R, Duchnik M, Kawa M, Rogińska D, Wiszniewska B, et al. Dose-dependent retinal changes following sodium iodate administration: application of spectral-domain optical coherence tomography for monitoring of retinal injury and endogenous regeneration. Curr Eye Res. 2014;39:1033–41. doi: 10.3109/02713683.2014.892996. [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Iacovelli J, Spencer C, Saint-Geniez M. Direct effect of sodium iodate on neurosensory retina. Invest Ophthalmol Vis Sci. 2014;55:1941–53. doi: 10.1167/iovs.13-13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phillips MJ, Wallace KA, Dickerson SJ, Miller MJ, Verhoeven AD, Martin JM, et al. Blood-derived human iPS cells generate optic vesicle–like structures with the capacity to form retinal laminae and develop synapses. Invest Ophthalmol Vis Sci. 2012;53:2007–2019. doi: 10.1167/iovs.11-9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X, Zhou Y, Li H, Wang R, Yang D, Li B, et al. Intravenous administration of DPSCs and BDNF improves neurological performance in rats with focal cerebral ischemia. Int J Mol Med. 2018;41:3185–3194. doi: 10.3892/ijmm.2018.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Machalińska A, Lubiński W, Kłos P, Kawa M, Baumert B, Penkala K, et al. Sodium iodate selectively injuries the posterior pole of the retina in a dose-dependent manner: morphological and electrophysiological study. Neurochem Res. 2010;35:1819–1827. doi: 10.1007/s11064-010-0248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamashita H, Yamasaki K, Sugihara K, Miyata H, Tsutsumi S, Iwaki Y. Full-field electroretinography obtained using a contact lens electrode with built-in high-intensity white-light-emitting diodes can be utilized in toxicological assessments in rats. Ophthalmic Res. 2009;42:15–20. doi: 10.1159/000219680. [DOI] [PubMed] [Google Scholar]