Abstract
Background:
Extracellular vesicles (EVs) exhibit potential as functional biomolecules for tissue regeneration and immunomodulation as they play important roles in the physiological communication between cells. EV internal cargo contains miRNAs, proteins, lipids, and so on. Osteoarthritis (OA) is a common joint disease causing disability owing to impaired joint function and pain. EVs originating from animal cells and tissue matrices are also being considered for OA, in addition to research involving non-steroidal therapeutic agents. However, there are no studies on EVs from marine organisms. Hence, we focused on sea cucumber-derived EVs and conducted experiments to set up an extraction protocol and to demonstrate their efficacy to modulate the inflammatory environment.
Methods:
Sea cucumber extracellular matrices (SECMs) were prepared by a decellularization process. Lyophilized SECMs were treated with collagenase and filtered to isolate sea cucumber extracellular vesicles (SEVs). After isolation, we conducted physical characterization and cell activation studies including cytotoxicity, proliferation, and anti-inflammation effect assays.
Results:
The physical characterization results showed circular SEVs in the size range of 66–480 nm. These SEVs contained large amounts of protein cargo, infiltrated the synoviocyte membrane without damage, and had a suppressive effect on inflammatory cytokines.
Conclusion:
This study established an extraction process for EVs from sea cucumber and reported the anti-inflammatory ability of SEVs. Isolated SEVs can be further utilized for tissue regeneration studies and can be compared to various marine or animal-derived EVs.
Keywords: Extracellular matrix, Extracellular vesicles, Sea cucumber, Marine organism, Anti-inflammation
Introduction
Inflammation is a strategy evolved to protect the body against microbial infections, tissue damage, and harmful substances. This immune response is an essential process to remove harmful stimuli and enable healing of damaged tissue [1]. Inflammation occurs due to acute or chronic infections, with acute conditions like fractures, seizures being serious and inflammation occurring suddenly at the beginning. In contrast, chronic conditions like cancer, diabetes, stroke, asthma, and arthritis are long-term conditions [2]. Osteoarthritis (OA) is the most common chronic disease, and the underlying pathology progresses through interaction with surrounding tissues. Early stage OA has an inflammatory microenvironment controlled by complex factors, with the most important factor being inflammatory cytokines [3–5]. Various treatment methods for OA are being used, and the development of non-steroidal treatments is being actively conducted, including a treatment method using extracellular vesicles (EVs) from mesenchymal stem cells (MSCs) [6–10].
Most cells release nano or micro vesicles consisting of a lipid bilayer to the extracellular environment [11]. In the early days of discovery, these vesicles were considered as dead cells [12]. Later it was found that EVs released outside of cells play a role in cell-to-cell communication [13], such as maintaining homeostasis and regulating immune responses [14]. EVs are generally found in biological fluids like blood, saliva, urine, or in the supernatant after cell culture. EVs are also known to play an important role in the physiological activity of cells as they contain numerous miRNAs and proteins. Hence, many studies have applied EVs to the treatment of degenerative diseases such as atopic dermatitis [15], osteoarthritis, cancer [16], myocardial infarction [17], and stroke [18, 19]. Although EV treatments do not use stem cells, many basic and clinical studies are being conducted as an alternate to stem cell therapy, and the advantage of eliminating problems associated with the use of stem cells [20].
The extracellular matrix (ECM) is defined as the composite accumulation of structural and functional molecules that are secreted by cells of all tissues and are arranged in a tissue-specific niche [21]. Tissue ECMs have the potential to control biological activity [22] by cell recruitment, immune modulation, and angiogenesis, and is involved in regenerating injured tissue. The biological functions of ECMs may be dependent on the characteristics of the tissue source [23] and therefore, it is essential to study the characteristics of specific tissues in regenerative medicine research. Nowadays, pure ECM is obtained through decellularization of xenogenic or allogenic tissues to take advantage of the structural and functional molecules [24, 25], and is applied to regeneration treatments [26]. Several studies have shown potential application of ECM in a variety of tissues as EVs are bound in decellularized ECM. These ECM-bound EV molecules were able to protect the internal nucleic acid and protein cargos that are involved in cellular, organismal development, nerve regeneration, and immune regulation [27–30].
Marine organisms are recently being considered as alternative biomaterial sources for terrestrial vertebrates. Bovine-derived biomaterials have a risk of mad cow disease and porcine-derived materials have religious restrictions [31]. Marine derived substances produced by metabolism in the marine organisms have unique structures and strong physiological activities [32, 33]. Regenerative medicine and biomedical applications using marine derived substances are actively being considered for implantable polymers, polysaccharides, and ceramics. Marine polymers show anti-inflammation, anti-microbial, and anti-cancer properties, and marine ceramics are reported to play an important role in osteogenesis for bone regeneration [34]. Marine organism-derived components are applied to OA treatment [35], with echinoderm-derived biomaterials being especially utilized for inflammation control [36].
Echinoderms are a group of marine invertebrates. The term echinoderm is derived from the spiny skin, and sea cucumbers, sea urchins, and starfish are common examples. Echinoderms have an incredible regenerative capacity, with many species routinely regenerating their arms and organs [37]. Sea cucumbers often drain some of their internal organs when threatened, and the tissue is regenerated over several months. Sea urchins and starfish also constantly regenerate arms and thorns lost due to damage [38–41]. In the present study, sea cucumbers were the echinoderms selected, as sea cucumbers are composed of bioactive components such as saponin, chondroitin sulfate, collagen, amino acids, and phenols [42]. Research on the application of sea cucumber components to cosmetics, anti-cancer, anti-inflammatory, and wound healing treatments are being actively conducted [43–46].
Therefore, this study intends to expand from studies on terrestrial animal-derived EVs to study on marine organ-derived EVs. Physical characterization, cell proliferation, and anti-inflammation effects of sea cucumber ECM-anchored EVs (SEVs) were evaluated.
Materials and methods
Experimental marine animal
A sea cucumber (Stichopus japonicus) weighing over 400 g was used. The intestines were completely removed, washed with deionized water, and stored at − 80 °C in an ultralow temperature refrigerator (ULT freezer; Thermo Fisher Scientific, Waltham, MA, USA).
Marine ECM preparation
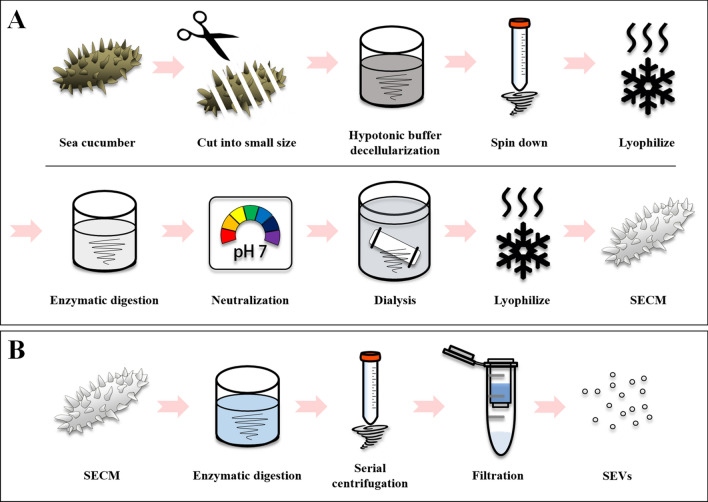
The sea cucumber body wall collagen was isolated as previously described [31], with modifications to simplify the preparation step. Briefly, body walls (200 g by net) of the frozen sea cucumber were cut in 1–2 cm size pieces and then agitated with 1000 mL hypotonic buffer containing 0.1 M tris-base (Sigma-Aldrich, St. Louis, MO, USA) at 4 °C for 4 days. The sea cucumber tissue residue was filtered using a sieve, as it did not dissolve completely, followed by centrifugation (8000 g, 60 min) of the highly viscous sea cucumber solution. The centrifuged pellet was dried through a freeze-drying process. Enzymatic digestion was performed by treating 0.3 M HCl with pepsin for 1 day at room temperature. After digestion, 10 M NaOH was used to block the activity of pepsin, and then the salt was removed through dialysis with a 2 kDa molecular weight cut-off (MWCO) membrane (Spectra/Por7, Spectrum Laboratories, Rancho Dominguez, CA, USA). Finally, the sea cucumber ECM (SECM) was harvested and freeze-dried (Fig. 1A).
Fig. 1.
Schematic diagram. A Sea cucumber-derived ECM (SECM) isolation protocol. B Sea cucumber-derived EVs (SEVs) isolation protocol
Marine ECM-anchored EVs isolation
To isolate the matrix anchored EVs, lyophilized SECM was treated with a disaggregating solution containing phosphate buffer saline (PBS) and collagenase (Worthington, OH, USA) for 2 days at room temperature. Enzymatically digested SECM was subjected to a continuous centrifugation process such as 500 g for 10 min, 2500 g for 20 min, and 10,000 g for 30 min to remove the ECM debris. A 0.45, 0.2 μm filter was used to obtain high-purity EVs, and to remove insoluble collagen fiber debris. The filtered supernatant was concentrated in a 100 kDa filter and ExoQuick-TC (System Biosciences, Palo Alto, CA, USA) precipitation solution (Fig. 1B).
Marine EVs morphology
Electron microscope imaging analysis was conducted using a transmission electron microscope (TEM) (H-7500; HITACHI, Tokyo, Japan) and scanning electron microscopy (SEM) (JEM-2100F; JEOL, Tokyo, Japan). Sea cucumber-derived EVs (SEVs) were fixed in 2.5% glutaraldehyde (JUNSEI, Tokyo, Japan) for 24 h, and a small amount of SEV mixture was dropped on the carbon-coated grid (TED PELLA, Redding, CA, USA) or cover glass and then dried at room temperature.
Size distribution of marine EVs
The SEVs were diluted in 0.2 μm filtrated deionized water (DW), and the nanoparticle suspension’s Brownian motion was determined using dynamic light scattering (DLS) (ELS-8000; Ozuka-Electronics, Osaka, Japan).
Cell culture
Synovium-derived cells (SW-982; ATCC, Manassas, VA, USA) were used. Synoviocytes were cultured in DMEM (GE Healthcare, Boston, MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA) and 1% Antibiotic-Antimyotic (Gibco) at 37 °C in a 5% CO2 incubator. The FBS was centrifuged at 120,000 g using an ultra-centrifuge for 6 h to remove EVs. Except for cell culture, exosome-depleted FBS was used in other experiments.
Marine EVs fluorescence labeling
The SEV membrane was labeled with PKH-26 red fluorescent cell linker (Sigma-Aldrich) for 24 h at 4 °C. Labeled SEVs were washed with 0.2 μm filtered PBS and concentrated again using a 100 kDa filter, and further diluted in DMEM (GE Healthcare, MA, USA) containing 10% exosome-depleted FBS (Gibco) and 1% Antibiotic-Antimyotic (Gibco) to treat the SW-982 cells, followed by treatment with the DAPI fluorescent reagent after 6 h. Endocytosis into the cells was observed using a fluorescence microscope (Axio-Observer 5; Carl Zeiss, Oberkochen, Germany).
Measurement of SEVs implied proteins
SEV protein cargos were measured by destroying the membrane with lysis buffer (RIPA; Rockland, PA, USA) and then diluting with distilled water filtered through a 0.2 μm filter. Diluted normal SEVs and destroyed SEVs were measured using Bradford assay (Bio-Rad, Hercules, CA, USA).
Cell viability and proliferation
The effect of SEVs on the viability and proliferation of synoviocytes was evaluated by the water soluble tetrazolium salt (WST) assay (EZ-cytox, DoGenBio, Seoul, South Korea). To detect cell cytotoxicity, cells were seeded in 48-well plates at 2 × 104 cells per well and incubated at 37 °C with 5% CO2. After 24 h, the culture medium was changed with a new medium containing SEVs at 0, 1, 5, 10, and 20 μg/mL. The assay was performed after 6 h, according to the manufacturer’s protocol. Proliferation assays were performed under the same conditions except for the use of 5 × 103 cell density, and were measured after 0, 1, 3, 5, and 7 days.
Anti-inflammation assay
To test the anti-inflammatory effect of SEVs, an in vitro OA model was used. Synoviocytes were seeded 6-well plates and treated with tumor necrosis factor-α (TNF-α) (25 ng/mL) and interleukin-1β (IL-1β) (10 ng/mL) (Peprotech, Cranbury, NJ, USA) after 24 h. The in vitro OA models were treated with 10 μg/mL SEVs for 7 days. Fresh media was replaced once every 2–3 days. RNA was collected after 7 days for Real-Time PCR (RT-qPCR) analysis.
Gene expression analysis
RNA was extracted using an isolation kit (Bioneer, Deajeon, South Korea) and the total quantity was detected using a SpectraDrop Micro-Volume Microplate (Molecular Devices, San Jose, CA, USA). RNA (1 μg) was reverse transcribed using a cDNA synthesis kit (Cellsafe, Yongin, South Korea). RT-qPCR was performed using specific primers and 1X SYBR Green Reaction Mix (Thermo Fisher Scientific). The relative gene expression levels of the samples were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal control and calculated by the comparative method.
Statistical analysis
GraphPad Prism version 7.0 (GraphPad, San Diego, CA, USA) was used to produce graphic images and perform statistical analysis. Data were expressed as mean ± standard deviation (SD) from at least three independent experiments. Statistical significance was analyzed by using Student’s t test and one-way analysis of variance (ANOVA) followed by a Tukey–Kramer post-hoc test. A value of p < 0.05 was considered as statistically significant (*,#p < 0.05, **,##p < 0.01, and ***,###p < 0.001).
Results
Physical characterization of SEVs
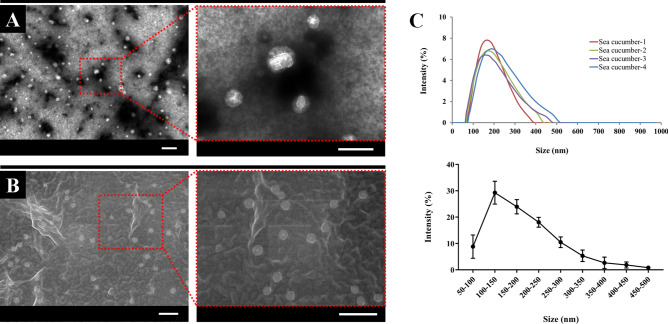
Specific analytical methods were used to explore the physical properties and biological effects of SEVs. SEV morphology was studied using TEM and SEM, and large amounts of circular material 100–200 nm in size was observed from the electron microscopy results (Fig. 2A, B). The similarity of their appearance to exosomes or microvesicles derived from cells of terrestrial animals was confirmed. The size of the SEVs was measured using DLS, and presence of a size of 100–200 nm and size distribution of 66–479 nm was confirmed. Moreover, micrometer-sized debris were not observed (Fig. 2C).
Fig. 2.
Physical characterization. A SEVs morphologies using transmission electron microscope. Scale bar 0.5 μm. B SEVs morphologies using scanning electron microscope. Scale bar 0.5 μm. C Size distribution measured by dynamic light scattering. Data are presented from four independent experiments
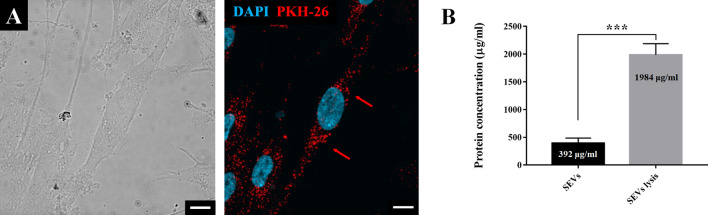
The SEV internalization assay was conducted by staining them with PKH-26 to track the location, and results demonstrated absorption of the stained SEVs into the cell membrane of synoviocytes without changes in cell morphology (Fig. 3A). To confirm whether SEVs contained proteins as protein cargo, the SEV membrane was destroyed. The confined protein amount in SEV cargo was measured as 5.06 times higher than the amount before treatment (Fig. 3B).
Fig. 3.
A PKH-26 labeled SEVs (red) endocytosis into SW-982 counterstained with DAPI (blue), the cells were observed using fluorescence microscope at 630× magnification. Scale bar 10 μm. B The amount of SEVs protein cargo measured using Bradford assay. Red arrows point out representative PHK-26 labeled SEVs. Data are presented as the mean ± SD from three independent experiments. (***p < 0.001 by Student’s t test)
Cytotoxicity and proliferation
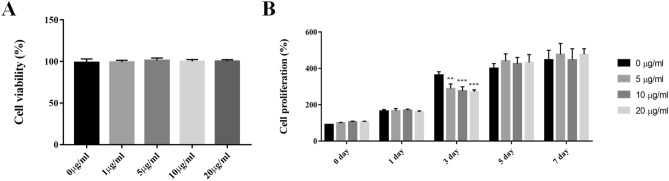
Various concentrations of SEVs (0, 1, 5, 10, and 20 μg/mL) were added to the synoviocytes to test cell cytotoxicity and proliferation. Results for mitochondrial activity by WST assay confirmed no change in cell viability at all concentrations of SEV treatment from 1 to 20 μg/mL. These results indicate that SEVs did not induce cytotoxicity during cell culture (Fig. 4A).
Fig. 4.
Basic cell activation. A Effect of SEVs cytotoxicity on SW-982. B Effect of SEVs for 7 days using cell proliferation assay. Data are presented as mean ± SD from four independent experiments. (**p < 0.01 and ***p < 0.001 by one-way ANOVA)
Cell growth rates were compared between the same treated groups, and the synoviocyte proliferation rate decreased to 74.6% at 20 μg/mL SEV concentration on day 3. However, no statistical difference was observed after one week between the groups (Fig. 4B).
Analysis of inflammatory cytokines
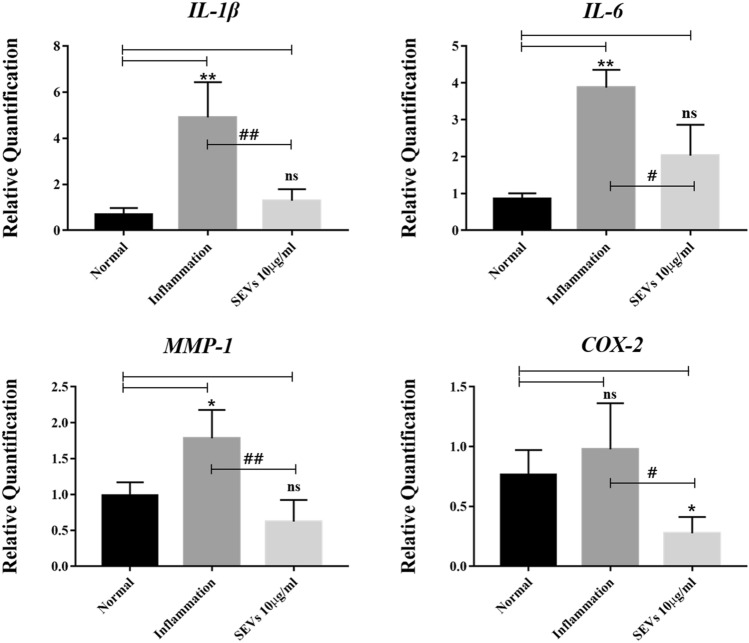
The relative amount of mRNA after treatment with SEVs was measured to determine whether SEVs were able to suppress the production of inflammatory cytokines related to early stage OA. The SEVs-treated group showed suppression of inflammatory cytokine at the gene level. IL-1β gene expression in the SEV-treated group was less than 26.4% compared to the inflammation group. However, there was no statistical difference between the SEV-treated and normal groups (Table 1).
Table 1.
Primer sequences for real time polymerase chain reaction
| Target | Forward sequences (5′-3′) | Reverse sequences (5′-3′) |
|---|---|---|
| IL-1β | AGCTCGCCAGTGAAATGATG | GGAGCACTTCATCTGTTTAGGG |
| IL-6 | AGCATCCCTCCACTGCAAA | AGCATCCCTCCACTGCAAA |
| MMP-1 | GGCCACAAAGTTGATGCAGT | TGCTACGGCAATGAAATGGAG |
| COX-2 | GTTGTATCTCTGTCTTCATCGCC | TCTGGCCGAGGCTTTTCTAC |
| GAPDH | TATGGACACGCTCCCCTGA | CATTCCCCAGCTCTCATACCA |
Expression of IL-6 gene and matrix metalloproteinase-1 (MMP-1) were 1.9 times (52.4%) and 2.8 times (34.9%) less than that in the inflammation group, respectively. Moreover, cyclooxygenase-2 (COX-2) involved in pain, was expressed less than 3.5 times (28.2%) (Fig. 5).
Fig. 5.
Anti-inflammatory effects of SEVs on infected SW-982. Real-Time PCR results showed that stimulation of SW-982 with or without IL-1β and TNF-α successfully induced inflammation. After SEVs treatment in an in vitro early stage OA model, mRNA expression levels of IL-1β, IL-6, MMP-1, and COX-2 decreased significantly. Data are presented as the mean ± SD from three independent experiments. (*,#p < 0.05 and **,##p < 0.01, versus untreated control (*) or inflammatory cytokines treated (#) by one-way ANOVA)
Discussion
Marine biomaterials have been widely utilized for biological applications; particularly, the regenerative ability of echinoderm has been of interest. In this study, we focused on sea cucumbers among the echinoderms [43–45], as sea cucumber-derived components have been utilized as pharmaceutical products for the prevention or treatment of cancer [47] and modulation of macrophage inflammatory response [48]. However, no studies have been reported to extract EVs from marine organisms. To date, research on marine EVs has mainly focused on vesicles secreted from bacteria to investigate ecosystems [49–51]. The present study establishes an isolation method for marine organs-derived EVs to apply to regenerative medicine. We demonstrated the extraction of EVs from sea cucumber, and the chemical reagents suggested in the isolation process were minimized to prevent degeneration of EVs anchored to the ECM of sea cucumber. Finally, SEVs isolated in this study showed physical characteristics similar to EVs derived from terrestrial vertebrates.
Circular particles were observed by TEM and SEM, and the molecular SEVs showed a shape similar to the commonly known EVs [52]. The smallest of the SEVs was 66 nm and the largest measured 479 nm in the DLS results. The size of isolated SEVs also indicated that the SEVs included a range of exosomes and microvesicles. However, additional experiments were needed to verify that the isolated molecules were not calcareous matters of the sea cucumber. The isolated molecules were stained with the PKH-26 cell tracking dye and were composed of a lipid layer. The PKH-26 labeled SEVs also easily entered the cells without changes in cell morphology. Based on these results, we confirm that the isolated SEVs were types of exosomes and microvesicles. We further determined whether the isolated SEVs contained several proteins as a protein cargo, and in the protein concentration assay, large amounts of protein were detected after SEV lysis, suggesting presence of various proteins as protein cargo in isolated SEVs.
Cytotoxicity experiments are important for establishing an isolation protocol, and the study analyzed the residual enzyme and contamination factor. The SECM anchored EV extraction process involved enzymatic digestion using pepsin and collagenase, and the isolated SEVs did not change cell viability even after treatment with high SEVs concentrations.
A previous study reported that SECM could promote skin wound healing [31]. Therefore, acceleration of cell proliferation by SEV treatment was expected; however, SEVs did not affect the proliferation of synoviocytes. As the study focused only on the synovium tissue related to OA, the response of different cells to SEVs should be tested to clarify the effects of SEVs on tissue regeneration.
EVs are high-purity factors that cells release outside to maintain homeostasis or resolve a threatening environment [14]. EVs have been applied in various diseases [15–19], with particular interest on the effects of EV treatment on regulating inflammation and alleviating OA [6–10]. However, the effect of EVs on inflammation and immune regulation need to be elucidated further.
Although SEVs did not affect cell proliferation in our study, they are expected to change the inflammatory environment because of the reported anti-inflammatory effects of SECM, and hence, SEVs were treated in an OA in vitro model and expression of genes related to OA progression like IL-1β, IL-6, MMP-1, and COX-2 were analyzed. The expression levels of IL-1β, MMP-1, and COX-2 cytokine genes in the SEV-treated group were significantly reduced compared to the levels in the inflammation group. Although IL-6 mRNA expression was not as high as in the normal control group, it was statistically lower as compared to the inflammation group. IL-1β suppresses type II collagen and aggrecan (ACAN) synthesis, but also induces pain. IL-6 upregulates MMP expression and reduces the expression of type II collagen. COX-2 enzyme is involved in the synthesis of prostaglandin-2, which induces inflammation, fever, and pain, and plays an important role in pain related to chronic diseases such as osteoarthritis [3–5, 53]. Taken together, the present study demonstrates the anti-inflammatory effects of SEVs.
It is expected that proteins and miRNAs inside SEVs [54, 55] may suppress the amount of inflammatory cytokines. However, the factors in SEVs associated with reduction in OA inflammation are unknown. There is a need for future studies to identify the mechanism of inflammation and to determine the efficacy of the protein and miRNA cargos.
In conclusion, we have successfully set a SEV isolation protocol and identified anti-inflammatory effects of SEVs isolated from sea cucumber. Although an early stage research, this novel trial using marine-derived EVs can be used to further expand marine biomaterial research for regenerative medicine.
Acknowledgement
This research was supported by the National Research Foundation Grant (NRF- 2019M3E5D1A02070861.)
Compliance with ethical standards
Conflict of interest
The authors have no financial conflicts of interest.
Ethical statement
There are no animal experiments carried out for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ahmed AU. An overview of inflammation: mechanism and consequences. Front Biol. 2011;6:274. doi: 10.1007/s11515-011-1123-9. [DOI] [Google Scholar]
- 2.Chen Y, Jiang W, Yong H, He M, Yang Y, Deng Z, et al. Macrophages in osteoarthritis: pathophysiology and therapeutics. Am J Transl Res. 2020;12:261–268. [PMC free article] [PubMed] [Google Scholar]
- 3.Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. doi: 10.1155/2014/561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 5.Miller RE, Miller RJ, Malfait AM. Osteoarthritis joint pain: the cytokine connection. Cytokine. 2014;70:185–193. doi: 10.1016/j.cyto.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noël D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 2017;7:16214. doi: 10.1038/s41598-017-15376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toh WS, Lai RC, Hui JHP, Lim SK. MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin Cell Dev Biol. 2017;67:56–64. doi: 10.1016/j.semcdb.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Zhang S, Chuah SJ, Lai RC, Hui JHP, Lim SK, Toh WS. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27. doi: 10.1016/j.biomaterials.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Yu D, Liu Z, Zhou F, Dai J, Wu B, et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Ther. 2017;8:189. doi: 10.1186/s13287-017-0632-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Zou R, Wang Z, Wen C, Zhang F, Lin F. Exosomal KLF3-AS1 from hMSCs promoted cartilage repair and chondrocyte proliferation in osteoarthritis. Biochem J. 2018;475:3629–3638. doi: 10.1042/BCJ20180675. [DOI] [PubMed] [Google Scholar]
- 11.Edgar JR. Q&A: What are exosomes, exactly? BMC Biol. 2016;14:46. doi: 10.1186/s12915-016-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Broe ME, Wieme RJ, Logghe GN, Roels F. Spontaneous shedding of plasma membrane fragments by human cells in vivo and in vitro. Clin Chim Acta. 1977;81:237–245. doi: 10.1016/0009-8981(77)90054-7. [DOI] [PubMed] [Google Scholar]
- 13.Buzas EI, György B, Nagy G, Falus A, Gay S. Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol. 2014;10:356–364. doi: 10.1038/nrrheum.2014.19. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi A, Okada R, Nagao K, Kawamata Y, Hanyu A, Yoshimoto S, et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat Commun. 2017;8:15287. doi: 10.1038/ncomms15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho BS, Kim JO, Ha DH, Yi YW. Exosomes derived from human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis. Stem Cell Res Ther. 2018;9:187. doi: 10.1186/s13287-018-0939-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim W, Kim HS. Exosomes as therapeutic vehicles for cancer. Tissue Eng Regen Med. 2019;16:213–223. doi: 10.1007/s13770-019-00190-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahoo S, Losordo DW. Exosomes and cardiac repair after myocardial infarction. Circ Res. 2014;114:333–344. doi: 10.1161/CIRCRESAHA.114.300639. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Chopp M. Exosome therapy for stroke. Stroke. 2018;49:1083–1090. doi: 10.1161/STROKEAHA.117.018292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang ZG, Chopp M. Exosomes in stroke pathogenesis and therapy. J Clin Invest. 2016;126:1190–1197. doi: 10.1172/JCI81133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2017;35:851–858. doi: 10.1002/stem.2575. [DOI] [PubMed] [Google Scholar]
- 21.Hussey GS, Keane TJ, Badylak SF. The extracellular matrix of the gastrointestinal tract: a regenerative medicine platform. Nat Rev Gastroenterol Hepatol. 2017;14:540–552. doi: 10.1038/nrgastro.2017.76. [DOI] [PubMed] [Google Scholar]
- 22.Kim S, Kim BS. Control of adult stem cell behavior with biomaterials. Tissue Eng Regen Med. 2014;11:423–430. doi: 10.1007/s13770-014-0068-x. [DOI] [Google Scholar]
- 23.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 25.Badylak SF. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl Immunol. 2004;12:367–377. doi: 10.1016/j.trim.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Park DY, Yun HW, Lim S, Truong MD, Yin XY, Park J, et al. Cross-linked cartilage acellular matrix film decreases postsurgical peritendinous adhesions. Artif Organs. 2020;44:E136–E149. doi: 10.1111/aor.13591. [DOI] [PubMed] [Google Scholar]
- 27.Huleihel L, Bartolacci JG, Dziki JL, Vorobyov T, Arnold B, Scarritt ME, et al. Matrix-bound nanovesicles recapitulate extracellular matrix effects on macrophage phenotype. Tissue Eng Part A. 2017;23:1283–1294. doi: 10.1089/ten.tea.2017.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huleihel L, Hussey GS, Naranjo JD, Zhang L, Dziki JL, Turner NJ, et al. Matrix-bound nanovesicles within ECM bioscaffolds. Sci Adv. 2016;2:e1600502. doi: 10.1126/sciadv.1600502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Merwe Y, Faust AE, Sakalli ET, Westrick CC, Hussey G, Chan KC, et al. Matrix-bound nanovesicles prevent ischemia-induced retinal ganglion cell axon degeneration and death and preserve visual function. Sci Rep. 2019;9:1–15. doi: 10.1038/s41598-018-37186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.An M, Kwon K, Park J, Ryu DR, Shin JA, Lee Kang J, et al. Extracellular matrix-derived extracellular vesicles promote cardiomyocyte growth and electrical activity in engineered cardiac atria. Biomaterials. 2017;146:49–59. doi: 10.1016/j.biomaterials.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Park SY, Lim HK, Lee S, Hwang HC, Cho SK, Cho M. Pepsin-solubilised collagen (PSC) from Red Sea cucumber (Stichopus japonicus) regulates cell cycle and the fibronectin synthesis in HaCaT cell migration. Food Chem. 2012;132:487–492. doi: 10.1016/j.foodchem.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 32.Silva TH, Moreira-Silva J, Marques AL, Domingues A, Bayon Y, Reis RL. Marine origin collagens and its potential applications. Mar Drugs. 2014;12:5881–5901. doi: 10.3390/md12125881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Binnewerg B, Schubert M, Voronkina A, Muzychka L, Wysokowski M, Petrenko I, et al. Marine biomaterials: biomimetic and pharmacological potential of cultivated Aplysina aerophoba marine demosponge. Mater Sci Eng C Mater Biol Appl. 2020;109:110566. doi: 10.1016/j.msec.2019.110566. [DOI] [PubMed] [Google Scholar]
- 34.Kim SK, Ngo DH, Vo TS, Ryu B. Industry perspectives of marine-derived proteins as biomaterials. In: Kim SK, editor. Marine biomaterials: characterization, isolation and applications. Boca Raton, FL, USA: CRC Press; 2013. [Google Scholar]
- 35.Ohta N, Sato M, Ushida K, Kokubo M, Baba T, Taniguchi K, et al. Jellyfish mucin may have potential disease-modifying effects on osteoarthritis. BMC Biotechnol. 2009;9:98. doi: 10.1186/1472-6750-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomes AR, Freitas AC, Duarte AC, Rocha-Santos TA. Echinoderms: a review of bioactive compounds with potential health effects. Amsterdam: Elsevier; 2016. pp. 1–54. [Google Scholar]
- 37.Carnevali MC. Regeneration in echinoderms: repair, regrowth, cloning. Invertebrate Surviv J. 2006;3:64–76. [Google Scholar]
- 38.San Miguel-Ruiz JE, García-Arrarás JE. Common cellular events occur during wound healing and organ regeneration in the sea cucumber Holothuria glaberrima. BMC Dev Biol. 2007;7:115. doi: 10.1186/1471-213X-7-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun L, Chen M, Yang H, Wang T, Liu B, Shu C, et al. Large scale gene expression profiling during intestine and body wall regeneration in the sea cucumber Apostichopus japonicus. Comp Biochem Physiol Part D Genomics Proteomics. 2011;6:195–205. doi: 10.1016/j.cbd.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 40.García-Arrarás JE, Dolmatov IY. Echinoderms: potential model systems for studies on muscle regeneration. Curr Pharm Des. 2010;16:942–955. doi: 10.2174/138161210790883426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith GN., Jr Regeneration in the sea cucumber Leptosynapta. II. The regenerative capacity. J Exp Zool. 1971;177:331–342. doi: 10.1002/jez.1401770307. [DOI] [PubMed] [Google Scholar]
- 42.Siahaan EA, Pangestuti R, Munandar H, Kim SK. Cosmeceuticals properties of sea cucumbers: prospects and trends. Cosmetics. 2017;4:26. doi: 10.3390/cosmetics4030026. [DOI] [Google Scholar]
- 43.Kijjoa A, Sawangwong P. Drugs and cosmetics from the sea. Mar Drugs. 2004;2:73–82. doi: 10.3390/md202073. [DOI] [Google Scholar]
- 44.Li X, Roginsky AB, Ding XZ, Woodward C, Collin P, Newman RA, et al. Review of the apoptosis pathways in pancreatic cancer and the anti-apoptotic effects of the novel sea cucumber compound, frondoside a. Ann N Y Acad Sci. 2008;1138:181–198. doi: 10.1196/annals.1414.025. [DOI] [PubMed] [Google Scholar]
- 45.Oh GW, Ko SC, Lee DH, Heo SJ, Jung WK. Biological activities and biomedical potential of sea cucumber (Stichopus japonicus): a review. J Fish Aquat Sci. 2017;20:28. doi: 10.1186/s41240-017-0071-y. [DOI] [Google Scholar]
- 46.Janakiram NB, Mohammed A, Rao CV. Sea cucumbers metabolites as potent anti-cancer agents. Mar Drugs. 2015;13:2909–2923. doi: 10.3390/md13052909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim JL, Park SH, Jeong S, Kim BR, Na YJ, Jo MJ, et al. Sea cucumber (Stichopus japonicas) F2 enhanced TRAIL-induced apoptosis via XIAP ubiquitination and ER stress in colorectal cancer cells. Nutrients. 2019;11:1061. doi: 10.3390/nu11051061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Himaya SW, Ryu B, Qian ZJ, Kim SK. Sea cucumber, Stichopus japonicus ethyl acetate fraction modulates the lipopolysaccharide induced iNOS and COX-2 via MAPK signaling pathway in murine macrophages. Environ Toxicol Pharmacol. 2010;30:68–75. doi: 10.1016/j.etap.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 49.Biller SJ, Schubotz F, Roggensack SE, Thompson AW, Summons RE, Chisholm SW. Bacterial vesicles in marine ecosystems. Science. 2014;343:183–186. doi: 10.1126/science.1243457. [DOI] [PubMed] [Google Scholar]
- 50.Soler N, Krupovic M, Marguet E, Forterre P. Membrane vesicles in natural environments: a major challenge in viral ecology. ISME J. 2015;9:793–796. doi: 10.1038/ismej.2014.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toyofuku M, Morinaga K, Hashimoto Y, Uhl J, Shimamura H, Inaba H, et al. Membrane vesicle-mediated bacterial communication. ISME J. 2017;11:1504–1509. doi: 10.1038/ismej.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shah R, Patel T, Freedman JE. Circulating extracellular vesicles in human disease. N Engl J Med. 2018;379:958–966. doi: 10.1056/NEJMra1704286. [DOI] [PubMed] [Google Scholar]
- 53.Cook AD, Christensen AD, Tewari D, McMahon SB, Hamilton JA. Immune cytokines and their receptors in inflammatory pain. Trends Immunol. 2018;39:240–255. doi: 10.1016/j.it.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Zhang P, Li C, Zhang R, Zhang W, Jin C, Wang L, et al. The roles of two miRNAs in regulating the immune response of sea cucumber. Genetics. 2015;201:1397–1410. doi: 10.1534/genetics.115.178871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhan Y, Liu L, Zhao T, Sun J, Cui D, Li Y, et al. MicroRNAs involved in innate immunity regulation in the sea cucumber: a review. Fish Shellfish Immunol. 2019;95:297–304. doi: 10.1016/j.fsi.2019.10.049. [DOI] [PubMed] [Google Scholar]