Abstract
It is largely unknown how post-translational protein modifications, including glycosylation, impacts recognition of self and non-self T cell epitopes presented by HLA molecules. Data in the literature indicate that O- and N-linked glycosylation can survive epitope processing and influence antigen presentation and T cell recognition. In this perspective, we hypothesize that glycosylation of viral proteins and processed epitopes contribute to the T cell response to HIV. Although there is some evidence for T cell responses to glycosylated epitopes (glyco-epitopes) during viral infections in the literature, this aspect has been largely neglected for HIV. To explore the role of glyco-epitope specific T cell responses in HIV infection we conducted in silico and ex vivo immune studies in individuals with chronic HIV infection. We found that in silico viral protein segments with potentially glycosylable epitopes were less frequently targeted by T cells. Ex vivo synthetically added glycosylation moieties generally masked T cell recognition of HIV derived peptides. Nonetheless, in some cases, addition of simple glycosylation moieties produced neo-epitopes that were recognized by T cells from HIV infected individuals. Herein, we discuss the potential importance of these observations and compare limitations of the employed technology with new methodologies that may have the potential to provide a more accurate assessment of glyco-epitope specific T cell immunity. Overall, this perspective is aimed to support future research on T cells recognizing glycosylated epitopes in order to expand our understanding on how glycosylation of viral proteins could alter host T cell immunity against viral infections.
Keywords: virus, human immunodeficiency virus, T cell, cytotoxic T lymphocyte, epitope, glycosylation
Introduction
Since the early years of the human immunodeficiency virus (HIV) pandemic, it has been noticed that not all HIV-1 infected individuals show equally fast disease progression to acquired immunodeficiency syndrome (AIDS) (1). It is now well-recognized that a small proportion of HIV infected people can maintain low or even undetectable levels of plasma viremia for a long time in the absence of antiretroviral treatment. This population of long term non-progressors has been extensively studied, with the intention to identify immune correlates of controlled HIV replication and to develop an effective HIV vaccine. The immunological mechanisms that allow superior HIV infection control are not fully understood (2–4), but some HIV-specific CD8+ cytotoxic T lymphocyte (CTL) responses have been consistently associated with HIV viral set point. In line with this, different human leukocyte antigen (HLA) alleles have been related to HIV virus control or disease progression (4–6) and major efforts have been made to fine map HLA-restricted epitopes targeted by virus-specific CTL responses across the entire viral proteome (7). However, recent studies indicate that this impressive amount of information is possibly still lacking a significant portion of the full HIV epitope landscape (8, 9). One potential gap in the current knowledge of the CTL response to HIV is the potential existence of HLA class I restricted epitopes containing post-translational modifications (PTM) derived from HIV proteins. There are many types of PTM, we will focus herein on the most abundant, glycosylation, and the existence of HIV-specific T cell responses to glycosylated epitopes (“glyco-epitopes”). T cell responses to such glyco-epitopes have been described in tumors (10–14), tuberculosis (15) and other viruses (16–18), but to our knowledge only in two studies for HIV (19–21).
Does Glycosylation Have an Impact on Epitope Presentation?
Eukaryotic cell proteins can undergo two main types of protein glycosylation: i) N-glycosylation of asparagine residues and ii) O-glycosylation of serine and threonine residues, which can be α- or β-O-linked (22). Glycosylation enzymes are thought to be highly compartmentalized which explains why the cellular localization of a protein can determine its glycosylation profile. N- and α-O-glycosylation are thought to occur predominantly on secreted proteins, whereas β-O-glycosylation affects nuclear and cytosolic proteins (23, 24). Of importance for T cell reactivity to glyco-epitopes, several studies have documented that glycans can survive the antigen processing and presenting process. In the late 1990s, CD4+ and CD8+ T cells that specifically recognized peptides carrying mono- or disaccharides where isolated (19, 25–32). The existence of HLA class I presented epitopes was further supported when it was shown that 0.1% of all peptides bound to HLA class I carried O-linked GlcNAc residues (33, 34). However, this number could be an underestimation since the epitope elution process can cause the stripped glyco-epitopes to lose their sugar moieties (35).
Despite this evidence of HLA-presented glyco-epitopes, the in vitro demonstration of reactive T cell responses has been limited, since most research on T cell responses and epitope mapping has employed only synthetic peptides to stimulate T cells. Such synthetic peptides do not carry any PTM and T cells targeting glyco-epitopes will thus not be detected. Alternatively, epitope mapping studies have used recombinant proteins or viral vectors expressing the antigen of interest (e.g. adenovirus or vaccinia virus). In recombinant proteins, glycosylation could be present if eukaryotic systems were used to produce them, while antigens expressed off viral vectors could be glycosylated by the host cells. In both cases the sugar residues added could possibly match the ones seen in the native protein, but this may only be the case if the same intracellular protein trafficking pathways are targeted (36).
These considerations indicate that in theory, protein glycosylation could affect antigen-specific T cell responses in several ways: i) epitope residues modified by glycosylation could be loaded onto HLA class I and recognized by the T cell receptor (TCR), but would be missed when using non-glycosylated peptide stimulations in vitro. ii) Glycosylation of epitope residues could mask proteolytic sites from proteasome digestion, HLA anchor residues or TCR binding residues, offering viruses an escape strategy to avoid CTL immune recognition.
Indeed, crystal structure analyses of MHC/glyco-peptide/TCR complexes indicate that MHC binding is mediated by the peptide backbone, while the glycan moieties interact with the TCR variable sites (17, 25, 37, 38). In a report by Avci et al. (39, 40), a carbohydrate CD4+ T cell epitope derived from a streptococcal glyco-conjugate was found to significantly increase vaccine-induced T cell responses. Also, the presence of a sugar moiety was tolerated by T cells, except when the glycosylation affected the epitope anchor residues (27–30). Still, Apostolopoulos et al. showed that in some cases, the MHC class I binding pocket itself could also accommodate an α-O-linked GalNAc (41). This glyco-epitope elicited CTL responses and was capable of cross-reacting with the non-glycosylated counterpart as its structure could be superimposed with a peptide showing a canonical anchor (42). Indeed, some data indicate that the smaller O-glycans may be more readily tolerated by T cell receptors than the larger N-glycans and that the central CDR3 region of αβTCR cannot accommodate more than four sugars (43). Whether this is rather the exception than the rule and which HLA complexes could accommodate glycosylated anchor residues on presented epitope remains to be clarified (31, 44). It is however interesting to note that several HLA class I alleles use anchor residues that could potentially be glycosylated, including: A*01, A*26, A*30:04, A*34:02; A*66, A*68, A*69, B*15:16, B*15:17, B*40, B*57:01, B*57:02, B*58:01, and B*58:02, (http://www.syfpeithi.de/bin/MHCServer.dll/FindYourMotif.htm). Six of these alleles (B*15’s, B*57’s, and B*58’s) have been associated with superior control of HIV infection in vivo (4). Yet, no study has addressed how and whether glycosylation at anchor residues could affect epitope binding and recognition by HIV-specific T cells.
To date, most studies on HIV protein glycosylation have been focused on the envelope protein (Env) and its relationship with viral escape from humoral immunity (45). As other viruses, HIV is highly dependent on the host cellular machinery and extensive glycosylation of viral proteins has been documented (18, 23, 46, 47). In addition, there are several reports that suggest that HIV could interfere with the host glycosylation machinery (45, 48–54), but only two (19, 21) describe T cell responses to glycosylated epitopes.
Together, the available studies indicate that glycosylated epitopes can be presented by HLA molecules and that T cell responses directed against glyco-epitopes can be induced in vivo (35, 55, 56). It is tempting to speculate that, if HIV glyco-epitope specific T cell responses exist and are restricted by HLA class I alleles that are associated with superior HIV control in vivo, they could contribute to HIV control. Strikingly, they would have been largely missed by the use of synthetic non-glycosylated peptides to screen for T cell responses.
Are HIV Peptides Containing Predicted Glycosylation Sites Less Frequently Targeted by T Cells?
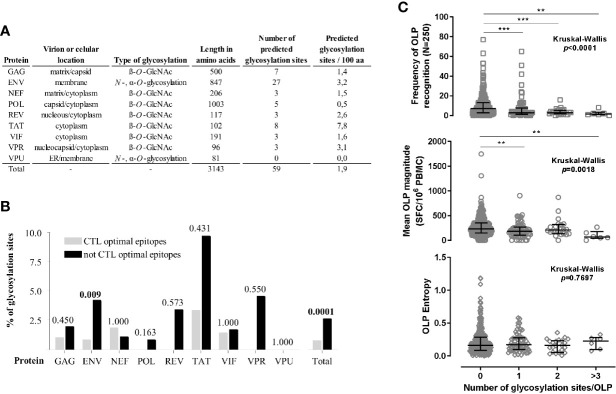
To establish evidence for potential effects of glycosylation on T cell recognition of HIV derived epitopes, we assessed whether HIV protein fragments containing predicted glycosylable positions were less frequently targeted by T cells than the rest of the viral proteome. N-linked oligosaccharides are covalently attached to glycoproteins on asparagine residues within the Asn-X-Ser/Thr sequence motif (where X is any amino acid residue except proline) (57) and can be fairly well predicted in silico. In contrast, O-glycans have no single consensus sequence, although most frequently occur on serine or threonine residues. Current prediction tools use artificial neural networks that examine the sequence context of glycosylable amino acids to predict them (23, 58, 59). We used NetNGlyc (N-glycosylation sites), NetOGlyc (mucin type GalNAc O-glycosylation) and YinOYang (O-β-GlcNAc attachment sites) in the CBS website (http://www.cbs.dtu.dk/services/), to predict 87 glycosylation sites in the HIV clade B 2001 consensus protein sequences (https://www.hiv.lanl.gov/content/sequence/NEWALIGN/align.html). Since the type of glycosylation that a protein can undergo is highly depended on cellular location, we used protein location to reduce these predictions to a total of 59 glycosylation sites ( Figure 1A ). The distribution of these 59 sites could affect T cell epitopes distributed over a range of approximately 1000 amino acids of the virus and affect thus T cell immunity to a third of the viral proteome. Overall, Gag and Tat proteins showed the highest number of glycosylable positions, with Tat containing the highest density (8 glycosylable positions/100 amino acid, Figure 1A ).
Figure 1.
Potential impact of glycosylation on T cell responses to HIV. (A) Predicted glycosylated positions in HIV proteins taking into account cellular location of viral proteins. (B) Percentage of predicted glycosylated sites in known, optimally defined CTL epitopes (9) in comparison to regions for which no optimal CTL epitopes have been defined. Frequency differences were analyzed by the Fisher exact test, p<0.05 are highlighted in bold numbers. (C) Frequency of recognition and magnitude of the response to HIV OLP in 250 HIV infected individuals stratified by the number of potential glycosylations in each OLP. The existence of statistically significant differences was analyzed using the Kruskal-Wallis test, Mann-Whitney p values are indicated by *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
To investigate whether these sites could indeed be involved in the T cell response to HIV infection, we reanalyzed existing T cell response data (2, 60), adding the predicted glycosylation sites across all HIV proteins into the analysis. We asked whether the potential glycosylation sites fell within previously described CTL epitopes or in regions in which screenings using non-glycosylated synthetic peptides have shown little T cell reactivity. For this, we used the Optimal HIV CTL epitope list at the Los Alamos HIV Immunology Database (http://www.hiv.lanl.gov/) curated by our laboratory to define epitope-rich regions (9). We found a statistically significant underrepresentation of glycosylation sites among all the optimally defined CTL epitopes compared to the rest of the viral proteome (Fisher exact test p=0.0001, Figure 1B ). This trend still held true for all viral proteins analyzed individually (except Nef), with statistical significance maintained for the Env protein (p=0.009).
In a second step we used the frequency of recognition and the magnitude of response to 410 overlapping peptides (OLP), spanning the entire viral proteome, in a cohort of 250 clade B HIV chronically infected subjects previously tested in our laboratory (2, 61). The OLP reactivity data were stratified by the presence of one or more predicted glycosylation positions within each OLP and compared to the frequency of OLP recognition. This analysis showed a strong inverse relationship between the presence of glycosylation site(s) in a given OLP and the frequency at which the OLP was targeted ( Figure 1C , Kruskal-Wallis p<0.0001). In addition, when OLP containing glycosylation sites were targeted, they elicited responses of reduced magnitude ( Figure 1C , Kruskal-Wallis p= 0.018). Together, these results indicate that fragments of the viral proteome containing potentially glycosylated positions are less frequently targeted by T cells - in studies using non-glycosylated synthetic peptides as stimuli. These observations suggest two different scenarios: (i) glycosylation sites are inherently poorly immunogenic because of the PTM or (ii) epitopes can contain glycosylated residues and are able to induce a T cell responses, but they have not been detected because essentially all T cell epitope screenings have been performed using non-glycosylated synthetic peptides. There are several arguments that give strong support to the second scenario: OLP with potential glycosylation sites do not differ in entropy from the ones not containing these sites ( Figure 1C ). This indicates that the frequency of response to these regions was not underestimated because of increased sequence divergence between autologous virus and the OLP sequences used as recall antigen (62). In addition, there are studies that have shown different results when T cell responses to HIV were assessed using synthetic peptides or viral antigens expressed by a vaccinia virus, further supporting the hypothesis that some responses are detected only when the antigen is produced in a eukaryotic cell system, which allows PTM to occur, but not when using PTM-free synthetic peptides (63).
Technical Limitations of Synthetic Glycopeptide Screens
Based on the previously referenced literature and the above in silico data, an INFγ ELISPOT or intracellular staining (ICS) screen, using synthetically glycosylated OLP as stimulus, would be the first-choice option to screen for HIV derived glyco-epitopes. Such an approach has been successful in detecting T cell responses to regular peptides but, in our hands poses a number of technical limitations when attempting to detect responses to glycosylated peptides. In particular, α-O- and N-glycosylation of proteins includes complex, highly branched, sugars that are challenging to approach by chemically synthesis of the corresponding glycopeptides. Moreover, glycoproteins suffer extensive de-glycosylation in the cytosol before entering the proteasome, making difficult to predict which α-O- or N-linked glycans will be present in the peptides eventually presented by an HLA class I molecule. This severely limits glycosylated peptide design, discouraging the use of α-O- or N-glycosylated peptides in T cell screens. However, β-O-GlcNAc glycosylation, involving the addition of a single N-acetylglucosamine (GlcNAc) to a Ser or Thr residue, is comparably much easier to approach experimentally. For this reason, we attempted to evaluate the effect of glycosylation on T cell responses using β-O-glycosylated synthetic peptides. Additionally, since N-glycosylation followed by complete N-de-glycosylation causes a change of an Asn (N) residue to Asp (D), we also tested OLP sequence variants that contained N to D substitutions to account for potential responses to N-de-glycosylated neo-epitopes (64).
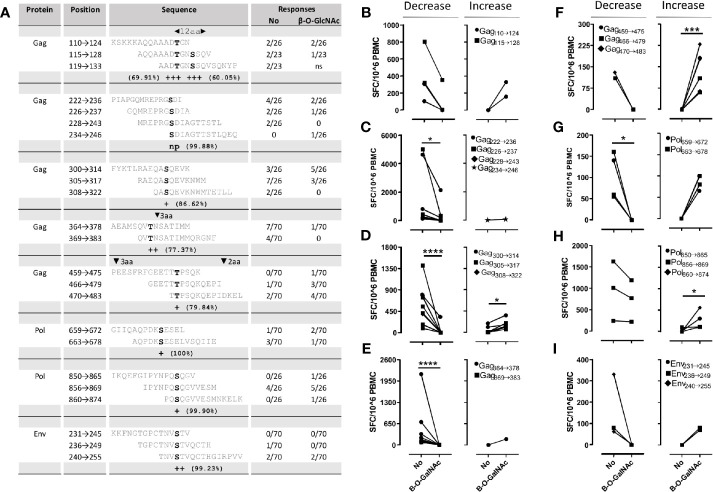
To perform this proof-of-concept analysis, OLP covering potentially glycosylated regions were designed using the 2010 compendium alignment of Gag, Pol, Env, and Nef from the Los Alamos HIV Sequence Database (https://www.hiv.lanl.gov/content/sequence/NEWALIGN/align.html). Seven regions in Gag, Env and Pol containing predicted β-O-glycosylation sites were selected for glycopeptide synthesis. Consensus sequence based OLP and the corresponding O-GlcNAc glycopeptides covering these regions ( Figure 2 ) were synthesized using stepwise solid-phase peptide synthesis following standard Fmoc protocols using glycosylated serine and threonine building blocks (see Supporting information). Additionally, we synthesized OLP covering three potential N-glycosylation sites in Env and Gag with the original N residue or the D substitution, which can be caused by de-glycosylation of N-glycosylated positions during antigen processing. Two shorter (9mer), already described, epitopes containing N-glycosylation sites in Env, together with their D modifications were also produced (19). Four out of the five potentially N-glycosylated positions in these peptides (positions 88, 156, 160, and 301 in Uniprot entry P04578) have been demonstrated to be N-glycosylated experimentally (65–67). These peptides were used to screen HIV infected individuals for INFγ-producing T cell responses in peripheral blood mononuclear cells (PBMC) to potentially β-O-glycosylated or de-N-glycosylated epitopes using an INFγ-ELISPOT (Mabtech). We used samples from a total of 71 individuals (supporting information) including individuals with different HIV-infection status and representing 55 different HLA-A, -B, and -C alleles.
Figure 2.
INFγ-ELISPOT screening with β-O-GlcNAc OLP and its non-glycosylated counterparts. (A) Frequency of responders to OLP with and without glycosylation modification. Presence of insertions or deletions is indicated in upper grey bars. Prediction of O-β-glycosylated positions by YingOYang is based on the server output (http://www.cbs.dtu.dk/services/). The frequency of the potential glycosylation sites in the HIV sequence database alignments is also indicated. (B–I) Magnitude of the response to β-O-glycosylated peptides and their non-glycosylated equivalents. OLP covering the same region are shown in the same graph, individuals who decreased their OLP response to the glycosylated OLP version are shown in the left panels while individuals with increased responses to the glycosylation containing OLP are shown in the right hand panels. Mann-Whitney p values are indicated by *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Globally, the frequency of responses to these peptides was low and precluded drawing of strong conclusions from the results. This low frequency of responses could be because glycosylated sequences are inherently poorly immunogenic, but also by a poor representation in the samples used of the HLA alleles capable of presenting glycosylated epitopes. Since these HLA alleles have not been identified and taking into account the vast number of different HLA alleles described, they could have been largely missed in this study. Overall, responses were more frequent (range: 0%–26%, Figure 2A ) and of higher magnitude (range: 0–2715 SFC/106 PBMC, Mann-Whitney p=0.0054) when targeting non-glycosylated peptides compared to reactions to their O-β-glycosylated counterparts (0%–19% and 0-560 SFC/106 PBMC, respectively). Despite its low frequency, these results provide evidence that O-β-glycosylation can interfere negatively with epitope recognition. Indeed, analyzing each potentially glycosylable region individually, the magnitude of the response to O-β-GlcNAc modified peptides was usually lower or zero (p<0.05 in Figures 2C–G ). However, responses to two regions in Gag and one in Pol showed a significant increase after glycosylation in some individuals (p<0.05 in Figures 2D, F, H ). This suggested that, although they are rare, responses to O-β-glycosylated epitopes can be detected. We also screened 22 HIV infected individuals with the OLP containing potentially N-glycosylable positions (N) and its deaminated counterparts (D). We found only four responders and were unable to detect differences among the different versions of the peptides (data not shown).
Future Perspectives
Our preliminary data, indicate that O-β-GlcNAc glycosylation usually reduces peptide recognition and suggest that sugar moieties in synthetic glyco-peptides often interfere with peptide binding to HLA or with TCR recognition. In the context of HIV infection, this would be in line with a mechanism where HIV could use glycosylation to escape from the T cell response, similarly to what happens with antibody recognition of the viral Env protein. Still, it seems that specific T cell responses to O-β-glycosylated peptides could exist, although they would be relatively rare and weak. It remains to be addressed what physiological role these responses may have in in vivo HIV control. As shown recently, inhibition of glycosylation in HIV producing cells leads to massive increase in virus replication; suggesting that the glycosylation of viral proteins comes at some fitness costs while possibly protecting from immune surveillance (46, 68). It will be interesting to assess the balance between such reduced replication fitness and the ability to use glycosylation as an escape strategy to avoid T cell immunity (if at all occurring in vivo) in future research. However, future studies will need more refined methodologies to identify glycosylated peptides that can be accommodated in the HLA-class I groove, the specific HLA alleles that can bind them and measure glyco-epitope specific T cell responses. The use of HLA peptide elution methodologies in combination with lectin columns will allow to specifically capture glyco-peptides, while identifying the specific HLA alleles that can present them. The exact molecular nature of the captured glyco-peptides can be then characterized by mass-spectrometry (13, 69–74). Independent synthesis of these newly identified HLA-binding glycopeptides, containing the specific N- or O-linked glycans characterized by mass-spectrometry, should allow identifying specific T cell responses in individuals bearing the HLA class I allele from where the glycosylated peptides were eluted from. Additional structural analyses will be needed as well, to better define the molecular structure of glyco-epitope glycan moieties, to identify carbohydrates that can block recognition by specific TCRs, but also to identify TCR that can accommodate such (complex) sugars. This improved focus should permit to accurately characterize glyco-epitope specific T cell responses and to identify interference with CTL recognition due to epitope glycosylation and relate both with HIV control. Filling this gap of knowledge might contribute to the development of CTL based vaccines, which may all depend on the proper glycosylation profiles of vaccine-delivered antigens. This may require adequate cellular expression and trafficking and may be especially important for the induction of vaccine-encoded antigens. However, our emerging data and the presented consideration also highlight an urgent need to better understand the impact of glycosylation on natural and vaccine-induced antiviral T cell responses.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of the Hospital Universitari Germans Trias i Pujol, Badalona, Spain (PI-13-017). All participants provided written informed consent in accordance with the Declaration of Helsinki.
Author Contributions
AO and CB performed data analysis and first manuscript writing. GA assessed O- and N-glycosylation metabolic routes and synthesized the O-GlcNAc peptides. AO, AL, and SC designed and performed INFγ-ELISPOT assays. JS and BM coordinated HIV-infected subject cohort. All authors contributed to the article and approved the submitted version.
Funding
The present study was supported by grant PI12/00529 (AO) from the Instituto de Salud Carlos III, co-financed by the Fondo Europeo de Desarrollo Regional (FEDER) “Una manera de hacer Europa” and funding from the European Union’s Horizon 2020 research and innovation program under grant European AIDS Vaccine Initiative 2020 (EAVI2020) #GA681137 (CB). CB is a senior ICREA research professor. The work was also partly supported by the HIVACAT program, the Fondation Dormeur, Vaduz, (Liechtenstein) and an unrestricted gift by Rafael Punter.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Gregorio Valencia for critically reviewing this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2020.573928/full#supplementary-material
References
- 1. Sheppard HW, Lang W, Ascher MS, Vittinghoff E, Winkelstein W. The characterization of non-progressors: long-term HIV-1 infection with stable CD4+ T-cell levels. AIDS (1993) 7:1159–66. 10.1097/00002030-199309000-00002 [DOI] [PubMed] [Google Scholar]
- 2. Mothe B, Llano A, Ibarrondo J, Daniels M, Miranda C, Zamarreño J, et al. Definition of the viral targets of protective HIV-1-specific T cell responses. J Transl Med (2011) 9:208. 10.1186/1479-5876-9-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tomaras GD, Plotkin SA. Complex immune correlates of protection in HIV-1 vaccine efficacy trials. Immunol Rev (2017) 275:245–61. 10.1111/imr.12514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Naranbhai V, Carrington M. Host genetic variation and HIV disease: from mapping to mechanism. Immunogenetics (2017) 69:489–98. 10.1007/s00251-017-1000-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mothe B, Ibarrondo J, Llano A, Brander C. Virological, immune and host genetics markers in the control of HIV infection. Dis Markers (2009) 27:105–20. 10.3233/DMA-2009-0655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McLaren PJ, Coulonges C, Bartha I, Lenz TL, Deutsch AJ, Bashirova A, et al. Polymorphisms of large effect explain the majority of the host genetic contribution to variation of HIV-1 virus load. Proc Natl Acad Sci (2015) 112:14658–63. 10.1073/pnas.1514867112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yusim K, Korber BTM, Brander C, Barouch D, de Boer R, Haynes BF, et al. eds. HIV Molecular Immunology. Los Alamos, New Mexico: Los Alamos National Laboratory, Theoretical Biology and Biophysics; (2017). [Google Scholar]
- 8. Arora J, McLaren PJ, Chaturvedi N, Carrington M, Fellay J, Lenz TL. HIV peptidome-wide association study reveals patient-specific epitope repertoires associated with HIV control. Proc Natl Acad Sci (2019) 116:944–9. 10.1073/pnas.1812548116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Llano A, Cedeño S, Silva Arrieta S, Brander C. The 2019 Optimal HIV CTL epitopes update: Growing diversity in epitope length and HLA restriction. In: Yusim B, Korber K, Brander B, Barouch C, de Boer D, Haynes R, Koup BF, Moore R, Walker JP, editors. HIV Molecular Immunology. Los Alamos: Theoretical Biology and Biophysics Group, Los Alamos National Laboratory; (2019). [Google Scholar]
- 10. Pathangey L, Lakshminarayanan V, Suman V, Pockaj B, Mukherjee P, Gendler S. Aberrant Glycosylation of Anchor-Optimized MUC1 Peptides Can Enhance Antigen Binding Affinity and Reverse Tolerance to Cytotoxic T Lymphocytes. Biomolecules (2016) 6:31. 10.3390/biom6030031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stepensky D, Tzehoval E, Vadai E, Eisenbach L. O-glycosylated versus non-glycosylated MUC1-derived peptides as potential targets for cytotoxic immunotherapy of carcinoma. Clin Exp Immunol (2006) 143:139–49. 10.1111/j.1365-2249.2005.02965.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ninkovic T, Hanisch FG. O-glycosylated human MUC1 repeats are processed in vitro by immunoproteasomes. J Immunol (2007) 179:2380–8. 10.4049/jimmunol.179.4.2380. [DOI] [PubMed] [Google Scholar]
- 13. Malaker SA, Ferracane MJ. Mass Spectrometric Identification and Molecular Modeling of Glycopeptides Presented by MHC Class I and II Processing Pathways. In: Methods in Molecular Biology. New York, NY: Humana Press Inc; (2019). p. 269–85. 10.1007/978-1-4939-9597-4_17 [DOI] [PubMed] [Google Scholar]
- 14. Malaker SA, Penny SA, Steadman LG, Myers PT, Loke JC, Raghavan M, et al. Identification of glycopeptides as posttranslationally modified neoantigens in Leukemia. Cancer Immunol Res (2017) 5:376–84. 10.1158/2326-6066.CIR-16-0280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harriff MJ, Wolfe LM, Swarbrick G, Null M, Cansler ME, Canfield ET, et al. HLA-E Presents Glycopeptides from the Mycobacterium tuberculosis Protein MPT32 to Human CD8+ T cells. Sci Rep (2017) 7:4622. 10.1038/s41598-017-04894-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carbone FR, Gleeson PA. Carbohydrates and antigen recognition by T cells. Glycobiology (1997) 7:725–30. 10.1093/glycob/7.6.725-d [DOI] [PubMed] [Google Scholar]
- 17. Hafstrand I, Badia-Martinez D, Josey BJ, Norström M, Buratto J, Pellegrino S, et al. Crystal structures of H-2Db in complex with the LCMV-derived peptides GP92 and GP392 explain pleiotropic effects of glycosylation on antigen presentation and immunogenicity. PLoS One (2017) 12:e0189584. 10.1371/journal.pone.0189584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bagdonaite I, Wandall HH. Global aspects of viral glycosylation. Glycobiology (2018) 28:443–67. 10.1093/glycob/cwy021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ferris RL, Buck C, Hammond SA, Woods AS, Cotter RJ, Takiguchi M, et al. Class I-restricted presentation of an HIV-1 gp41 epitope containing an N-linked glycosylation site. Implications for the mechanism of processing of viral envelope proteins. J Immunol (1996) 156:834–40. [PubMed] [Google Scholar]
- 20. Ferris RL, Hall C, Sipsas NV, Safrit JT, Trocha A, Koup RA, et al. Processing of HIV-1 envelope glycoprotein for class I-restricted recognition: dependence on TAP1/2 and mechanisms for cytosolic localization. J Immunol (1999) 162:1324–32. [PubMed] [Google Scholar]
- 21. Sun L, Paschall AV, Middleton DR, Ishihara M, Ozdilek A, Wantuch PL, et al. Glycopeptide epitope facilitates HIV-1 envelope specific humoral immune responses by eliciting T cell help. Nat Commun (2020) 11:1–14. 10.1038/s41467-020-16319-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dwek RA. Glycobiology: Toward understanding the function of sugars. Chem Rev (1996) 96:683–720. 10.1021/cr940283b [DOI] [PubMed] [Google Scholar]
- 23. Varki A (Executive Editor), Cummings RD, Esko JD, Stanley PGWH, Aebi M, Darvill AG, et al. eds. Essentials of glycobiology. 3rd New York: Cold Spring Harbor Laboratory Press; (2017). [PubMed] [Google Scholar]
- 24. Yang X, Qian K. Protein O-GlcNAcylation: Emerging mechanisms and functions. Nat Rev Mol Cell Biol (2017) 18:452–65. 10.1038/nrm.2017.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abdel-Motal UM, Dahmen J, Liu T, Ljunggren HG, Jondal M. External glycopeptide binding to MHC class-I in relation to expression of TAP transporters, beta 2-microglobulin and to pH. Immunol Lett (1996) 54:31–5. 10.1016/s0165-2478(96)02637-5 [DOI] [PubMed] [Google Scholar]
- 26. Deck B, Elofsson M, Kihlberg J, Unanue ER. Specificity of glycopeptide-specific T cells. J Immunol (1995) 155:1074–8. [PubMed] [Google Scholar]
- 27. Harding CV, Kihlberg J, Elofsson M, Magnusson G, Unanue ER. Glycopeptides bind MHC molecules and elicit specific T cell responses. J Immunol (1993) 151:2419–25. [PubMed] [Google Scholar]
- 28. Haurum JS, Arsequell G, Lellouch AC, Wong SY, Dwek RA, McMichael AJ, et al. Recognition of carbohydrate by major histocompatibility complex class I-restricted, glycopeptide-specific cytotoxic T lymphocytes. J Exp Med (1994) 180:739–44. 10.1084/jem.180.2.739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ishioka GY, Lamont AG, Thomson D, Bulbow N, Gaeta FC, Sette A, et al. MHC interaction and T cell recognition of carbohydrates and glycopeptides. J Immunol (1992) 148:2446–51. [PubMed] [Google Scholar]
- 30. Jensen T, Galli-Stampino L, Mouritsen S, Frische K, Peters S, Meldal M, et al. T cell recognition of Tn-glycosylated peptide antigens. Eur J Immunol (1996) 26:1342–9. 10.1002/eji.1830260625 [DOI] [PubMed] [Google Scholar]
- 31. Mouritsen S, Meldal M, Christiansen-Brams I, Elsner H, Werdelin O. Attachment of oligosaccharides to peptide antigen profoundly affects binding to major histocompatibility complex class II molecules and peptide immunogenicity. Eur J Immunol (1994) 24:1066–72. 10.1002/eji.1830240509 [DOI] [PubMed] [Google Scholar]
- 32. Abdel-Motal UM, Berg L, Rosen A, Bengtsson M, Thorpe CJ, Kihlberg J, et al. Immunization with glycosylated Kb-binding peptides generates carbohydrate-specific, unrestricted cytotoxic T cells. Eur J Immunol (1996) 26:544–51. 10.1002/eji.1830260307 [DOI] [PubMed] [Google Scholar]
- 33. Haurum JS, Hoier IB, Arsequell G, Neisig A, Valencia G, Zeuthen J, et al. Presentation of cytosolic glycosylated peptides by human class I major histocompatibility complex molecules in vivo. J Exp Med (1999) 190:145–50. 10.1084/jem.190.1.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kastrup IB, Stevanovic S, Arsequell G, Valencia G, Zeuthen J, Rammensee HG, et al. Lectin purified human class I MHC-derived peptides: evidence for presentation of glycopeptides in vivo. Tissue Antigens (2000) 56:129–35. 10.1034/j.1399-0039.2000.560203.x [DOI] [PubMed] [Google Scholar]
- 35. Hudrisier D, Riond J, Mazarguil H, Gairin JE. Pleiotropic effects of post-translational modifications on the fate of viral glycopeptides as cytotoxic T cell epitopes. J Biol Chem (2001) 276:38255–60. 10.1074/jbc.M105974200M105974200 [DOI] [PubMed] [Google Scholar]
- 36. Wang S, Voronin Y, Zhao P, Ishihara M, Mehta N, Porterfield M, et al. Glycan Profiles of gp120 Protein Vaccines from Four Major HIV-1 Subtypes Produced from Different Host Cell Lines under Non-GMP or GMP Conditions. J Virol (2020) 94:e01968-19. 10.1128/jvi.01968-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Glithero A, Tormo J, Haurum JS, Arsequell G, Valencia G, Edwards J, et al. Crystal structures of two H-2Db/glycopeptide complexes suggest a molecular basis for CTL cross-reactivity. Immunity (1999) 10:63–74. 10.1016/s1074-7613(00)80007-2 [DOI] [PubMed] [Google Scholar]
- 38. Speir JA, Abdel-Motal UM, Jondal M, Wilson IA. Crystal structure of an MHC class I presented glycopeptide that generates carbohydrate-specific CTL. Immunity (1999) 10:51–61. 10.1016/s1074-7613(00)80006-0 [DOI] [PubMed] [Google Scholar]
- 39. Avci FY, Li X, Tsuji M, Kasper DL. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat Med (2011) 17:1602–9. 10.1038/nm.2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Flight MH. Vaccines: Enhancing glycan presentation increases vaccine efficacy. Nat Rev Drug Discovery (2012) 11:21. 10.1038/nrd3637 [DOI] [PubMed] [Google Scholar]
- 41. Tang CK, Apostolopoulos V. Strategies used for MUC1 immunotherapy: preclinical studies. Expert Rev Vaccines (2008) 7:951–62. 10.1586/14760584.7.7.951 [DOI] [PubMed] [Google Scholar]
- 42. Apostolopoulos V, Yuriev E, Ramsland PA, Halton J, Osinski C, Li W, et al. A glycopeptide in complex with MHC class I uses the GalNAc residue as an anchor. Proc Natl Acad Sci USA (2003) 100:15029–34. 10.1073/pnas.2432220100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Werdelin O, Meldal M, Jensen T. Processing of glycans on glycoprotein and glycopeptide antigens in antigen-presenting cells. Proc Natl Acad Sci U.S.A. (2002) 99:9611–3. 10.1073/pnas.152345899152345899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Haurum JS, Tan L, Arsequell G, Frodsham P, Lellouch AC, Moss PA, et al. Peptide anchor residue glycosylation: effect on class I major histocompatibility complex binding and cytotoxic T lymphocyte recognition. Eur J Immunol (1995) 25:3270–6. 10.1002/eji.1830251211 [DOI] [PubMed] [Google Scholar]
- 45. Colomb F, Giron LB, Trbojevic-Akmacic I, Lauc G, Abdel-Mohsen M. Breaking the Glyco-Code of HIV Persistence and Immunopathogenesis. Curr HIV/AIDS Rep (2019) 16:151–68. 10.1007/s11904-019-00433-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Silver ZA, Antonopoulos A, Haslam SM, Dell A, Dickinson GM, Seaman MS, et al. Discovery of O-Linked Carbohydrate on HIV-1 Envelope and Its Role in Shielding against One Category of Broadly Neutralizing Antibodies. Cell Rep (2020) 30:1862–9.e4. 10.1016/j.celrep.2020.01.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Watanabe Y, Bowden TA, Wilson IA, Crispin M. Exploitation of glycosylation in enveloped virus pathobiology. Biochim Biophys Acta Gen Subj (2019) 1863:1480–97. 10.1016/j.bbagen.2019.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lefebvre JC, Giordanengo V, Limouse M, Doglio A, Cucchiarini M, Monpoux F, et al. Altered glycosylation of leukosialin, CD43, in HIV-1-infected cells of the CEM line. J Exp Med (1994) 180:1609–17. 10.1084/jem.180.5.1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hiyoshi M, Takahashi-Makise N, Yoshidomi Y, Chutiwitoonchai N, Chihara T, Okada M, et al. HIV-1 Nef perturbs the function, structure, and signaling of the golgi through the Src kinase Hck. J Cell Physiol (2011) 227(3):1090–7. 10.1002/jcp.22825 [DOI] [PubMed] [Google Scholar]
- 50. Rayne F, Debaisieux S, Bonhoure A, Beaumelle B. HIV-1 Tat is unconventionally secreted through the plasma membrane. Cell Biol Int (2009) 34:409–13. 10.1042/CBI20090376 [DOI] [PubMed] [Google Scholar]
- 51. Pinter A, Honnen WJ, Revesz K, Herz R. Identification of large glycosylated proteins recognized by monoclonal antibodies against HIV-1 gag proteins. AIDS Res Hum Retroviruses (1992) 8:1341–4. 10.1089/aid.1992.8.1341 [DOI] [PubMed] [Google Scholar]
- 52. Berger CT, Carlson JM, Brumme CJ, Hartman KL, Brumme ZL, Henry LM, et al. Viral adaptation to immune selection pressure by HLA class I-restricted CTL responses targeting epitopes in HIV frameshift sequences. J Exp Med (2010) 207:61–75. 10.1084/jem.20091808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Saunders PM, van Endert P. Running the gauntlet: from peptide generation to antigen presentation by MHC class I. Tissue Antigens (2011) 78:161–70. 10.1111/j.1399-0039.2011.01735.x [DOI] [PubMed] [Google Scholar]
- 54. Nitta T, Tam R, Kim JW, Fan H. The cellular protein La functions in enhancement of virus release through lipid rafts facilitated by murine leukemia virus glycosylated Gag. MBio (2011) 2:e00341–10. 10.1128/mBio.00341-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hudrisier D, Riond J, Mazarguil H, Oldstone MB, Gairin JE. Genetically encoded and post-translationally modified forms of a major histocompatibility complex class I-restricted antigen bearing a glycosylation motif are independently processed and co-presented to cytotoxic T lymphocytes. J Biol Chem (1999) 274:36274–80. 10.1074/jbc.274.51.36274 [DOI] [PubMed] [Google Scholar]
- 56. Selby M, Erickson A, Dong C, Cooper S, Parham P, Houghton M, et al. Hepatitis C virus envelope glycoprotein E1 originates in the endoplasmic reticulum and requires cytoplasmic processing for presentation by class I MHC molecules. J Immunol (1999) 162:669–76. [PubMed] [Google Scholar]
- 57. Helenius A, Aebi M. Intracellular functions of N-linked glycans. Sci (80 ) (2001) 291:2364–9. 10.1126/science.291.5512.2364 [DOI] [PubMed] [Google Scholar]
- 58. Steentoft C, Vakhrushev SY, Joshi HJ, Kong Y, Vester-Christensen MB, Schjoldager KT-BG, et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J (2013) 32:1478–88. 10.1038/emboj.2013.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Blom N, Sicheritz-Pontén T, Gupta R, Gammeltoft S, Brunak S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics (2004) 4:1633–49. 10.1002/pmic.200300771 [DOI] [PubMed] [Google Scholar]
- 60. Mothe B, Llano A, Ibarrondo J, Zamarreno J, Schiaulini M, Miranda C, et al. CTL responses of high functional avidity and broad variant cross-reactivity are associated with HIV control. PLoS One (2012) 7:e29717. 10.1371/journal.pone.0029717PONE-D-11-17091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Olvera A, Pérez-Álvarez S, Ibarrondo J, Ganoza C, Lama JR, Lucchetti A, et al. The HLA-C∗04:01/KIR2DS4 gene combination and human leukocyte antigen alleles with high population frequency drive rate of HIV disease progression. AIDS (2015) 29:507–17. 10.1097/QAD.0000000000000574 [DOI] [PubMed] [Google Scholar]
- 62. Frahm N, Korber BT, Adams CM, Szinger JJ, Draenert R, Addo MM, et al. Consistent cytotoxic-T-lymphocyte targeting of immunodominant regions in human immunodeficiency virus across multiple ethnicities. J Virol (2004) 78:2187–200. 10.1128/JVI.78.5.2187-2200.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Assarsson E, Sidney J, Oseroff C, Pasquetto V, Bui H-H, Frahm N, et al. A Quantitative Analysis of the Variables Affecting the Repertoire of T Cell Specificities Recognized after Vaccinia Virus Infection. J Immunol (2007) 178:7890–901. 10.4049/jimmunol.178.12.7890 [DOI] [PubMed] [Google Scholar]
- 64. Mei S, Ayala R, Ramarathinam SH, Illing PT, Faridi P, Song J, et al. Immunopeptidomic analysis reveals that deamidated HLA-bound peptides arise predominantly from deglycosylated precursors. Mol Cell Proteomics (2020) 19(7):1236–47. 10.1074/mcp.ra119.001846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Behrens AJ, Vasiljevic S, Pritchard LK, Harvey DJ, Andev RS, Krumm SA, et al. Composition and Antigenic Effects of Individual Glycan Sites of a Trimeric HIV-1 Envelope Glycoprotein. Cell Rep (2016) 14:2695–706. 10.1016/j.celrep.2016.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chen L, Do Kwon Y, Zhou T, Wu X, O’Dell S, Cavacini L, et al. Structural basis of immune evasion at the site of CD4 attachment on HIV-1 gp120. Sci (80-) (2009) 326:1123–7. 10.1126/science.1175868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Leonard CK, Spellman MW, Riddle L, Harris RJ, Thomas JN, Gregory TJ. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J Biol Chem (1990) 265:10373–82. [PubMed] [Google Scholar]
- 68. Olvera A, Martinez JP, Casadellà M, Llano A, Rosás M, Mothe B, et al. Benzyl-2-acetamido-2-deoxy-α-d-galactopyranoside increases human immunodeficiency virus replication and viral outgrowth efficacy in vitro. Front Immunol (2018) 8:2010. 10.3389/fimmu.2017.02010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bagdonaite I, Vakhrushev SY, Joshi HJ, Wandall HH. Viral glycoproteomes: technologies for characterization and outlook for vaccine design. FEBS Lett (2018) 592:3898–920. 10.1002/1873-3468.13177 [DOI] [PubMed] [Google Scholar]
- 70. Xiao H, Sun F, Suttapitugsakul S, Wu R. Global and site-specific analysis of protein glycosylation in complex biological systems with Mass Spectrometry. Mass Spectrom Rev (2019) 38:356–79. 10.1002/mas.21586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Xiao H, Suttapitugsakul S, Sun F, Wu R. Mass Spectrometry-Based Chemical and Enzymatic Methods for Global Analysis of Protein Glycosylation. Acc Chem Res (2018) 51:1796–806. 10.1021/acs.accounts.8b00200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Huang J, Jiang B, Zhao H, Wu M, Kong S, Liu M, et al. Development of a Computational Tool for Automated Interpretation of Intact O -Glycopeptide Tandem Mass Spectra from Single Proteins. Anal Chem (2020) 92:6777–84. 10.1021/acs.analchem.0c01091 [DOI] [PubMed] [Google Scholar]
- 73. Wang S, Qin H, Mao J, Fang Z, Chen Y, Zhang X, et al. Profiling of Endogenously Intact N-Linked and O-Linked Glycopeptides from Human Serum Using an Integrated Platform. J Proteome Res (2020) 19:1423–34. 10.1021/acs.jproteome.9b00592 [DOI] [PubMed] [Google Scholar]
- 74. Ye Z, Mao Y, Clausen H, Vakhrushev SY. Glyco-DIA: a method for quantitative O-glycoproteomics with in silico-boosted glycopeptide libraries. Nat Methods (2019) 16:902–10. 10.1038/s41592-019-0504-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.